Abstract
Background
Elevated TNFα likely contributes to the excess cardiovascular risk observed in rheumatoid arthritis. We compared the cardiovascular risk in rheumatoid arthritis patients starting a TNFα blocking agent versus a non-biologic disease-modifying anti-rheumatic drug (nbDMARD).
Methods
Subjects with rheumatoid arthritis participating in several different US insurance programs between 1998-2007 who received methotrexate were eligible. Those who added a TNFα blocking agent were compared with subjects who added a nbDMARD in Cox regression models stratified by propensity score decile and adjusted for oral glucocorticoid dosage. We examined the composite cardiovascular endpoint of myocardial infarction, stroke, or coronary re-vascularization after six months.
Results
We compared 8,656 new users of a nbDMARD with 11,587 new users of a TNFα blocking agent with similar baseline covariates. Incidence rates per 100 person-years for the composite cardiovascular endpoint were 3.05 (95% CI 2.54 – 3.65) for nbDMARDs and 2.52 (95% CI 2.12-2.98) for TNFα blocking agents. The hazard ratio (HR) for the TNFα blocking agent compared with nbDMARD carrying the first exposure forward was 0.80 (95% CI 0.62 - 1.04), while the HR for the as-treated analysis was 0.71 (95% CI 0.52 - 0.97). The potential cardiovascular benefit of TNFα blocking agents was strongest among persons ≥ 65 years of age (HR 0.52, 95% CI 0.34 – 0.77; p for interaction = 0.075).
Conclusion
Among subjects with rheumatoid arthritis, TNFα blocking agents may be associated with a reduced risk of cardiovascular events compared to a nbDMARD. Randomized controlled clinical trials should be considered to test this hypothesis.
Keywords: rheumatoid arthritis, TNFα blocking agents, cardiovascular disease
INTRODUCTION
Among many cytokines, TNFα appears to play an important role in mediating cardiovascular disease. Multiple studies suggest that TNFα is involved in atherosclerotic plaque formation and rupture, endothelial dysfunction and post-infarct remodeling. 1, 2 Moreover, rheumatoid arthritis, in which TNFα appears to play a major role driving the disease process,3, 4 is associated with an elevation in cardiovascular risk.5, 6 The increased risk of cardiovascular events among persons with rheumatoid arthritis is thought to be related to both traditional risk factors, as well as the inflammation that underpins rheumatoid arthritis.7-9
The known role of TNFα in cardiovascular disease in the general population and the elevated cardiovascular risk in RA suggests that blocking TNFα may reduce cardiovascular risk. The results of at least four prior studies suggest a reduced risk of cardiovascular events among users of TNFα blocking agents.10-13 However, other studies have found no difference in risk for TNFα blocking agent users,14-16 and one concluded that there may be an increased cardiovascular risk.17 While many prior studies were well conducted, limitations noted include: heterogeneous exposure definitions, lack of differentiation between current and past TNFα blocking agent users; mixing incident and prevalent users of TNFα blocking agents; various cardiovascular outcome definitions; small sample sizes; comparing TNFα blocking agents to a heterogeneous group of non-users rather than to a specific and well-defined reference group; and the lack of consideration of glucocorticoid exposure.
We aggregated several administrative and health plan datasets to form a large cohort of patients with RA to compare the risk of cardiovascular events among methotrexate users adding or switching to a TNFα blocking agent versus a non-biologic disease modifying anti-rheumatic drug (nbDMARD).
METHODS
Design and Study Cohort
This study was part of a larger study collaborative (SABER: The Safety Assessment of Biologic Therapy).18 The collaborative study shared limited datasets across institutions to facilitate large-scale comparative effectiveness studies, while maintaining de-identified analytic cohorts.19 The datasets that were combined include the US Medicaid Analytic Extract (MAX) linked to national U.S. Medicare data for people with both (so-called ‘dual eligibles’ with Medicare and Medicaid eligibility), the Tennessee Medicaid file (TennCare), two US states Medicare population databases, and Kaiser-Permanente of Northern California's administrative database. Information contained in these separate databases includes limited sociodemographic data (age, gender, race), enrollment dates, inpatient and outpatient health care encounter insurance claims with diagnoses, procedures, and all pharmacy claims. The datasets included information from 1998-2007.
From the total potentially eligible study populations, we selected persons with at least one encounter associated with a diagnosis of rheumatoid arthritis (ICD 714, excluding 714.3) and > 16 years of age at the diagnosis date. Patients with a diagnosis of ankylosing spondylitis or psoriatic arthritis were excluded. To further ensure the consistency of the study population's disease severity at baseline, we required our cohort to have been users of methotrexate. Requiring a diagnosis and a DMARD has a positive predictive value for rheumatoid arthritis of over 86%.20 Persons then qualified for the analytic cohort if they added or switched to an available TNFα blocking agent (adalimumab, etanercept, or infliximab; certolizumab pegol and golimumab were not yet available) or a non-methotrexate nbDMARD (hydroxychloroquine, leflunomide, sulfasalazine).
Analyses were carried out according to a pre-specified analytic plan. The study protocol was reviewed and approved by the responsible Institutional Review Boards.
TNFα Blocking and Non-biologic DMARD Exposures
We started following subjects from the date that each person switched from methotrexate to a TNFα blocking agent or nbDMARD, or added one of those drugs to their methotrexate regimen. No specific disease activity requirements for a TNFα blocking agent were in place in any of the health insurance programs studied. We pre-specified two analyses: (1) a primary analysis considered subjects in their initial treatment group at the start of follow-up for 6 months (“first exposure carried forward”) regardless of any subsequent change in treatment, and (2) a secondary analysis that followed subjects while on the first treatment (“as treated”). In the as-treated analysis, follow-up ended upon stopping the specified treatment plus 30 days, switching to the other treatment group, experiencing a cardiovascular outcome, or death. We allowed subjects to switch agents within a class of drugs (i.e., between nbDMARDs or TNFα blocking agents). We allowed subjects in the TNFα blocking agent group to concurrently use nbDMARDs but not the reverse. In our primary analysis, we chose 6 months of follow-up to minimize exposure misclassification as a result of subjects’ switching between therapies. Sensitivity analyses also considered 12 months of follow-up.
Cardiovascular Outcomes
Ischemic cardiovascular outcomes have been studied previously using health care encounter insurance claims data, in identical databases or those very similar to what we included, and found to be accurately defined (see eTable I).21-23 We considered the composite of myocardial infarction, stroke or coronary re-vascularization the primary endpoint and each of the components as secondary outcomes. The same definitions were used across each of the databases. It is debatable whether sudden death can be accurately defined in insurance claims data; thus it was not included as part of the outcome.24, 25
Potential Confounders
We used a propensity score to control for confounding. A propensity score is the estimated probability of receiving one treatment versus another -- TNFα blocking agent versus nbDMARD.26 The propensity score was estimated using a multivariate logistic regression model, predicting the use of TNFα blocking agent versus a nbDMARD (the reference group). The propensity scores were estimated based on potential confounders that we measured in computerized data assembled for administrative or clinical care purposes, such as demographics (age, gender and race), relevant diagnoses, surgical procedures, and pharmacy dispensings (see eTable II for a complete listing of variables). These variables were determined over the 365 days before the start of follow-up. The propensity score models had adequate discrimination (c-statistic 0.68 – 0.77). No information was available on several potential confounders, such as aspirin use, tobacco use, body mass index, rheumatoid arthritis severity, serologic status, and educational attainment. However, we did include tobacco-related illness and obesity-related illness and procedures in the propensity score. The use of oral glucocorticoids was included as a separate covariate in the outcome model and not in the propensity score.
Statistical Analyses
In the primary analyses, subjects were split into ten equal size groups based on their propensity score. To minimize non-overlap in covariates, we excluded (“trimmed”) subjects with the top and bottom 5% of propensity scores.27 We examined the distribution of baseline propensity score in both exposure groups after trimming and there was complete overlap (see eFigure I).
The cardiovascular outcomes were defined and incidence rates with 95% confidence intervals (CI) calculated for each exposure group separately. Kaplan-Meier cardiovascular event free survival curves were compared for the groups exposed to a TNFα blocking agent or nbDMARDs, and the log-rank test p-values calculated after 6 and 12 months of follow-up
We constructed Cox regression models comparing the risk of the composite cardiovascular outcome over 180-days among those exposed to TNFα blocking agents or nbDMARDs. The 180-day prior cumulative oral glucocorticoid dosage was the only covariate included. The Cox regression models were stratified by propensity score decile and the source of data. A secondary analysis compared risks over 365 days.
The proportional hazards assumption was tested using the Kolmogorov supremum test of Lin, Wei, and Ying and was not violated in either the first exposure carried forward (p= 0.57) or the as-treated (p = 0.26) analyses.28 All analyses were pre-specified in a statistical analysis plan and no adjustment was made for multiple comparisons.29 Statistical significance was inferred from 95% confidence intervals excluding one.
RESULTS
The study cohort was assembled from four databases that provided 139,611 potentially eligible rheumatoid arthritis patients (see Figure 1). Among this group, 22,907 persons had used methotrexate and then added or switched to one of the agents of interest – 9,964 added or switched to a nbDMARD and 12,943 to a TNFα blocking agent. After excluding subjects with the highest and lowest 5% of propensity scores, we compared 8,656 new users of a nbDMARD with 11,587 new users of a TNFα blocking agent.
Figure 1.
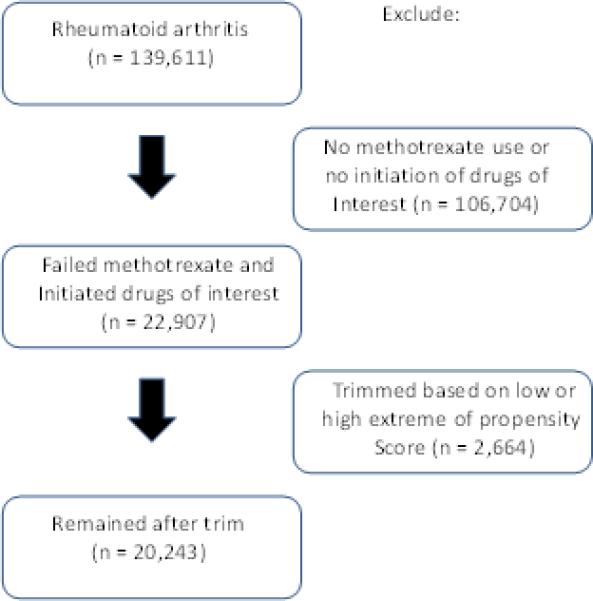
demonstrates the cohort assembly.
Baseline characteristics of the untrimmed and trimmed cohorts are shown in Table 1. The trimmed treatment groups had a mean age of 56 years with 86% women. In both treatment groups, similar percentages of subjects had experienced a prior myocardial infarction (1.8-1.9%), stroke (1.7%), or coronary re-vascularization (0.7%). As well, cardiovascular risk factors were similarly distributed across trimmed treatment groups: diabetes (22.9-23.5%), hypertension (41.6-42.2%), and hyperlipidemia (52.3-53.5%).
Table 1.
Baseline characteristics of untrimmed and trimmed cohorts using a TNFα blocking agent or a non-biologic disease modifying anti-rheumatic drug
| Untrimmed* | Trimmed* | |||
|---|---|---|---|---|
| TNFα | nbDMARD | TNFα | nbDMARD | |
| N (%) except where noted | ||||
| Number | 12943 | 9964 | 11587 | 8656 |
| Sociodemographic characteristics | ||||
| Female gender | 11149 (86.1) | 8562 (85.9) | 10021 (86.5) | 7434 (85.9) |
| Age, years (mean ± SD) | 55.8 ± 14.3 | 56.2 ± 14.4 | 55.4 ± 14.4 | 56.2 ± 14.3 |
| Race | ||||
| White | 8088 (62.5) | 6114 (61.4) | 7232 (62.4) | 5332 (61.6) |
| Black | 2019 (15.6) | 1581 (15.9) | 1799 (15.5) | 1343 (15.5) |
| Other | 2836 (21.9) | 2269 (22.8) | 2556 (22.1) | 1981 (22.9) |
| Nursing home residence | 459 (3.6) | 390 (3.9) | 415 (3.6) | 331 (3.8) |
| Rheumatoid arthritis characteristics | ||||
| Extra-articular manifestation | 622 (4.8) | 446 (4.5) | 541 (4.7) | 381 (4.4) |
| Naproxen use | 1894 (14.6) | 1769 (17.8) | 1742 (15.0) | 1490 (17.2) |
| Non-naproxen NSAID use | 8964 (69.2) | 6791 (68.2) | 8032 (69.3) | 5884 (68.0) |
| Cox-2 selective inhibitor use | 5135 (39.7) | 3477 (34.9) | 4542 (39.2) | 3039 (35.1) |
| Oral glucocorticoid use | 10562 (81.6) | 7846 (78.7) | 9351 (80.7) | 6855 (79.2) |
| Cardiovascular factors | ||||
| Prior myocardial infarction | 243 (1.9) | 177 (1.8) | 224 (1.9) | 153 (1.8) |
| Peripheral vascular disease | 516 (4.0) | 386 (3.9) | 457 (3.9) | 333 (3.9) |
| Heart failure | 480 (3.7) | 431 (4.3) | 432 (3.7) | 359 (4.2) |
| Hypertension | 5352 (41.4) | 4181 (42.0) | 4818 (41.6) | 3654 (42.2) |
| Diabetes mellitus | 3017 (23.3) | 2262 (22.7) | 2722 (23.5) | 1982 (22.9) |
| Angina | 255 (2.0) | 219 (2.2) | 228 (2.0) | 177 (2.0) |
| Statin treatment | 2848 (22.0) | 2241 (22.5) | 2618 (22.6) | 2000 (23.1) |
| Transient ischemic attack | 119 (0.9) | 106 (1.06) | 104 (0.9) | 89 (1.0) |
| Stroke | 216 (1.7) | 181 (1.8) | 195 (1.7) | 150 (1.7) |
| Coronary revascularization | 84 (0.7) | 70 (0.7) | 75 (0.7) | 57 (0.7) |
| Hyperlipidemia | 6714 (51.9) | 5197 (52.2) | 6054 (52.3) | 4632 (53.5) |
| Beta-blocker treatment | 2859 (22.1) | 2287 (23.0) | 2584 (22.3) | 2077 (23.2) |
Note:
Untrimmed refers to the entire study cohort and trimmed to the cohort after excluding the top and bottom 5%, based on the propensity scores. Abbreviations: nbDMARD, non-biologic disease modifying anti-rheumatic drug; NSAID, non-steroidal anti-inflammatory drug use
Over the first six months of follow-up, the incidence rate for the composite cardiovascular outcome was numerically lower among the users of TNFα blocking agents compared with nbDMARDs (see Table 2). The component cardiovascular outcomes followed a similar pattern, except stroke where the incidence rates were similar across exposures. The Kaplan-Meier cardiovascular event free survival curves (see Figure 2) demonstrated a similar trend for the composite outcome. In both the first exposure carried forward (see Figure 2a) and the as-treated analyses (see Figure 2b), the cardiovascular event free survival curves diverged over the first six months. The hazard ratio (HR) for the TNFα blocking agent compared with nbDMARD in the first exposure carried forward was 0.80 (95% CI 0.62 – 1.04), and the as-treated analysis at 6 months was 0.71 (95% CI 0.52 – 0.97). However, by 12 months the curves appeared to converge with the HRs approaching the null (first exposure carried forward: HR 0.95, 95% CI 0.78 – 1.17; as-treated: HR 0.87, 95% CI 0.67 – 1.12). Less than 1% of subjects died over the first six months of follow-up (73, 0.84%, in the nbDMARD group and 98, 0.85%, in the TNFα blocking agent group).
Table 2.
Incidence rates (per 100 person-years) of composite cardiovascular outcomes and each component through six months of follow-up
| Type of analysis | nbDMARD | TNFα blocking agent | ||
|---|---|---|---|---|
| First Exposure Carried Forward | Events | Rate | Events | Rate |
| Composite CV endpoint | 116 | 3.05 (2.54 – 3.65) | 133 | 2.52 (2.12 – 2.98) |
| Myocardial infarction | 38 | 1.00 (0.72 – 1.37) | 39 | 0.74 (0.54 – 1.01) |
| Stroke | 41 | 1.07 (0.79 – 1.46) | 59 | 1.12 (0.86 – 1.44) |
| Coronary re-vascularization | 56 | 1.47 (1.13 – 1.91) | 55 | 1.04 (0.80 – 1.35) |
| As-Treated | ||||
| Composite CV endpoint | 82 | 3.07 (2.47 – 3.81) | 103 | 2.31 (1.90 – 2.80) |
| Myocardial infarction | 28 | 1.04 (0.72 – 1.51) | 30 | 0.67 (0.47 – 0.96) |
| Stroke | 30 | 1.12 (0.78 – 1.60) | 49 | 1.09 (0.83 – 1.45) |
| Coronary re-vascularization | 36 | 1.34 (0.97 – 1.86) | 44 | 0.98 (0.73 – 1.32) |
Notes: See text for definition of the analysis types. The number of events of each of the component cardiovascular outcomes does not add to the total events in the composite outcome since subjects were censored at their first event in the composite (primary) analysis.
Abbreviations: nbDMARD, non-biologic disease modifying anti-rheumatic drug.
Figure 2.
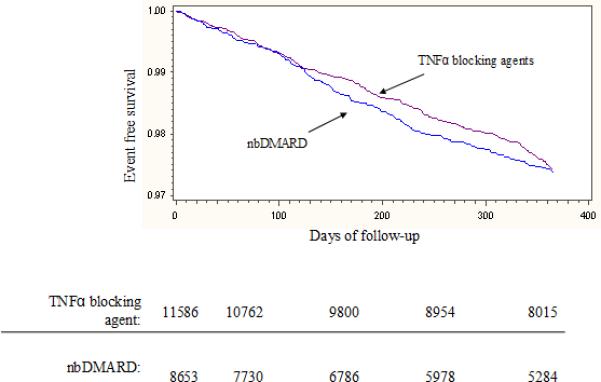
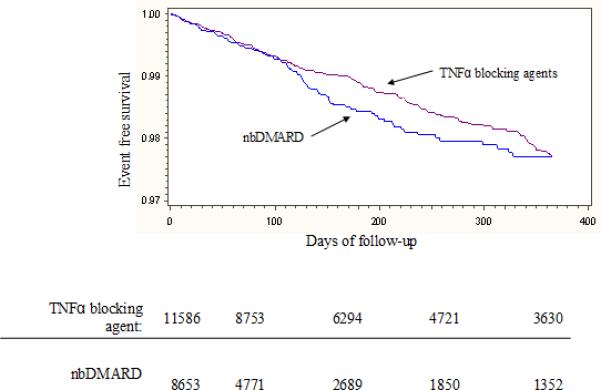
illustrates the event free survival curves through 12 months of follow-up. Panel A uses a first exposure carried forward analysis and Panel B an as-treated analysis. The tables below the panels represent the number of subjects at risk for the composite cardiovascular endpoint at 90 day intervals throughout the first year. For Panel A, the log rank p-value at 180 days was 0.22 and at 365 days was 0.67. For Panel B, the log rank p-value at 180 days was 0.08 and at 365 days was 0.84.
We observed a numerically lower risk of cardiovascular outcomes associated with TNFα blocking agents compared with nbDMARDs using alternative analytic approaches, examining secondary outcomes, and focusing on specific subgroups (see Figures 3 and 4). However, the only HR in the sensitivity analysis that was significant was for persons ≥ 65 years of age (p for interaction between treatment and age = 0.075). In fact, for persons < 65, there was no apparent reduction in cardiovascular risk associated with the use of TNFα blocking agents.
Figure 3.
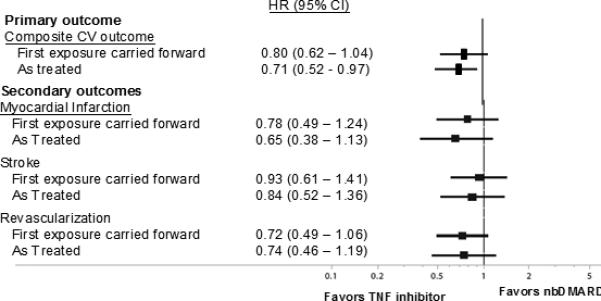
represents the adjusted hazard ratios for the primary and secondary cardiovascular outcomes calculated in Cox proportional hazards regression models.
Figure 4.
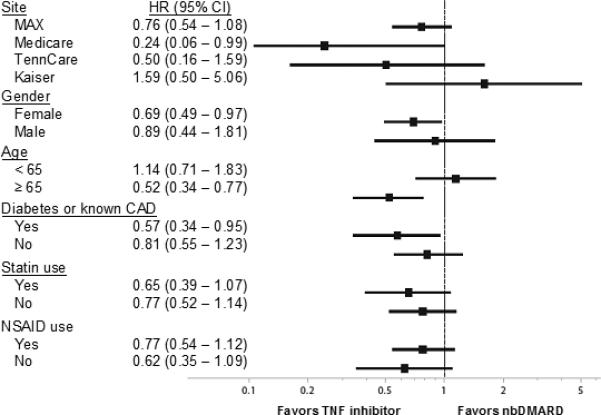
represents the hazard ratios for the sensitivity analyses with the study cohort stratified by database, age, gender, diabetes or cardiovascular disease, NSAID use, and statin use. These analyses were conducted using the as-treated approach.
During the first six months of follow-up in the first exposure carried forward analysis, we found that 24.8% of the cohort switched medications, and that 8% actually added or switched to a medication that would put them in the opposite exposure category.
DISCUSSION
As greater evidence accumulates for the role of inflammation in atherosclerosis, consideration has been given to the use of immunosuppressive treatment regimens in cardiovascular disease. While statins and aspirin may reduce cardiovascular risk in part through their anti-inflammatory properties,30, 31 targeting cytokines known to be part of cardiovascular disease is an attractive therapeutic option. Since the use of potent immunosuppressive agents is common in a systemic inflammatory condition, such as rheumatoid arthritis, studying the effect of these agents on cardiovascular disease may provide important insights into the potential role of this strategy in the general population. We examined the effect of TNFα blocking agents compared with nbDMARDs on cardiovascular risk in a large rheumatoid arthritis population. As with several prior studies, 10-13 our findings provide suggestive evidence that TNFα blocking agents were associated with a reduced risk of composite cardiovascular outcomes. However, any possible cardiovascular benefit of TNFα blocking agents waned by 12 months. The apparent benefit was most dramatic in persons 65 years or older. Furthermore, the apparent reduction in risk appeared consistent across myocardial infarction, stroke and coronary re-vascularization.
These results are subject to all the potential biases well-described for observational drug studies.32 However, we have taken a number of significant steps to limit these biases. First, we used rigorous comparative effectiveness methods, including propensity scores and a new user design.26, 33-36 The propensity score deciles with trimming created well balanced cohorts. We did not pursue marginal structural models that can help account for time-varying confounding because our follow-up period was relatively brief. We carefully considered exposure status and tested variable definitions in sensitivity analyses. Endpoints were defined using well characterized algorithms that are known to be accurate for the outcomes of interest. 21, 22 We did not have reliable information about sudden death, but there were almost identical rates of death across the two exposure groups during follow-up (see Results above). The cohorts were large in size permitting relevant secondary and subgroup analyses. Moreover, follow-up is complete for insurance claims during the period of insurance coverage.
This type of observational drug study has important potential limitations, including residual confounding, exposure and endpoint misclassification, and surveillance bias. Neither validated markers of rheumatoid arthritis disease severity nor serologic status were contained in the study database. While Table 1 suggests that the cohorts were well balanced with respect to measured covariates, it is possible that one of the two groups had worse disease severity and different seropositive prevalence. We did not include an untreated group of subjects with rheumatoid arthritis, since their disease severity would be very different. Worse disease severity predisposes patients to receive a TNFα blocking agent and also may be associated with an increased risk of cardiovascular disease.7 This unmeasured confounder would likely bias our findings toward an increased risk associated with TNFα blocking agent use, the opposite of what we observed. On the other hand, patients with more comorbid medical conditions may be less likely to receive a TNFα blocking agent and more likely to experience a cardiovascular outcome. Other unmeasured factors may contribute to drug selection, such as socioeconomics and supplemental insurance. There is likely some misclassification of rheumatoid arthritis and the cardiovascular outcomes, however it is not likely to be substantial nor differential based on prior work validating these algorithms.21, 22 Finally, several potentially important variables were unmeasured, including over-the-counter non-steroidal anti-inflammatory drug use, lipid levels, smoking, educational status, body mass index, physical activity, and aspirin use. In other cohorts with rheumatoid arthritis followed in the US, these variables are well balanced across patients using TNFα blocking agents or an nbDMARD.13
We found small differences in HRs using different analytic methods, as-treated versus first exposure carried forward. The first exposure carried forward analyses were pre-specified as the primary analysis because of concerns that there may be selective discontinuation of the two treatments, possibly related to the outcome. However, as we noted above, one-quarter of the study cohort switched medications during the first 6 months of follow-up and approximately one in ten switched to a DMARD that would put them in the opposite treatment group. This degree of switching introduces substantial exposure misclassification, higher than we anticipated before starting the study. While either analytic option – first exposure carried forward or as-treated – has potential limitations, it is clear to us post-hoc that the as-treated is less likely to be biased. Both analytic techniques gave similar results.
Our research findings may have clinical implications. The results generally agree with prior work suggesting a reduced risk of cardiovascular outcomes among users of TNFα blocking agents. TNFα appears to affect several aspects of cardiovascular disease, such as plaque stabilization, endothelial function, and post-infarct remodeling.37-41 Thus, one would anticipate that blockade of TNFα would reduce ischemic cardiovascular outcomes. This finding supports the inflammatory underpinnings of cardiovascular disease and highlights a potential role for immunosuppression in cardiovascular risk reduction. At least one randomized controlled trial of an immunomodulator is enrolling post-myocardial infarction patients without a systemic rheumatic disease to determine potential benefits.42 The findings of our study support randomized controlled clinical trials testing targeted immunosuppression, perhaps with agents other than TNFα blocking agents, to reduce cardiovascular risk. While this may be a difficult trial in a chronic systemic inflammatory condition, such as rheumatoid arthritis, where cross-over would be common, it is likely possible in the general population with cardiovascular disease.
In conclusion, we found that persons starting a TNFα blocking agent may have a reduced risk of cardiovascular outcomes over the first six months of use compared with those starting a nbDMARD. These epidemiologic data are in line with prior studies and support a possible role for targeted immunosuppression in the treatment of cardiovascular disease.
Acknowledgement
This work was supported by the Agency for Healthcare Research and Quality (AHRQ) and the Food and Drug Administration (FDA) US Department of Health and Human Services (DHHS) as part of a grant (No. 1U18 HSO17919-0) administered through the AHRQ CERTs Program. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by AHRQ, FDA, or DHHS. This work was part of a larger collaborative, the Safety Assessment of Biologic Therapy (SABER). This collaboration includes: Agency for Healthcare Research and Quality, Parivash Nourjah; Brigham and Women's Hospital, Robert Glynn, Joyce Lii, Jeremy Rassen, Sebastian Schneeweiss, Daniel Solomon; Food and Drug Administration, Jane Gilbert, David Graham, Carolyn McCloskey, Rita Ouellet-Hellstrom, Kristin Phucas, James Williams; Kaiser Permanente Northern California, Lisa Herrinton, Liyan Liu, Marcia Raebel; University of Alabama at Birmingham, Lang Chen, Jeffrey Curtis, Elizabeth Delzell, Nivedita Patkar, Kenneth Saag, Fenglong Xie; University of Pennsylvania, Kevin Haynes, James Lewis, Vanderbilt University, Marie Griffin, Carlos Grijalva, Ed Mitchell.
Potential Conflicts of Interest
Dr. Solomon has received research grants from Abbott, Amgen and Lilly, has served in unpaid roles on two Pfizer trials not related to rheumatoid arthritis, has directed an educational course supported by Bristol Myers Squibb, and serves as a consultant to CORRONA. Dr. Curtis has received research grants and/or consulting with Amgen, Abbott, BMS, Genentech/Roche, Janssen, UCB, and CORRONA. Dr. Harrold serves as a consultant to CORRONA. Dr. Lewis has received research grant from Centocor and served as a paid consultant to Amgen and Pfizer.
Footnotes
All authors had access to the data and a role in writing the manuscript.
REFERENCES
- 1.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108(19):2317–22. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101(18):2149–53. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 3.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358(9285):903–11. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 4.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annual review of immunology. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 5.Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis.[see comment]. Circulation. 2003;107(9):1303–7. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 6.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59(12):1690–7. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 7.del Rincon I, Freeman GL, Haas RW, O'Leary DH, Escalante A. Relative contribution of cardiovascular risk factors and rheumatoid arthritis clinical manifestations to atherosclerosis. Arthritis Rheum. 2005;52(11):3413–23. doi: 10.1002/art.21397. [DOI] [PubMed] [Google Scholar]
- 8.Solomon DH, Kremer J, Curtis JR, et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis. 2010;69(11):1920–5. doi: 10.1136/ard.2009.122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitas GD, Gabriel SE. Cardiovascular disease in rheumatoid arthritis: state of the art and future perspectives. Ann Rheum Dis. 2011;70(1):8–14. doi: 10.1136/ard.2010.142133. [DOI] [PubMed] [Google Scholar]
- 10.Naranjo A, Sokka T, Descalzo MA, et al. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther. 2008;10(2):R30. doi: 10.1186/ar2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobsson LT, Turesson C, Gulfe A, et al. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. The Journal of rheumatology. 2005;32(7):1213–8. [PubMed] [Google Scholar]
- 12.Carmona L, Descalzo MA, Perez-Pampin E, et al. All-cause and cause-specific mortality in rheumatoid arthritis are not greater than expected when treated with tumour necrosis factor antagonists. Ann Rheum Dis. 2007;66(7):880–5. doi: 10.1136/ard.2006.067660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(4):576–82. doi: 10.1136/ard.2010.129916. [DOI] [PubMed] [Google Scholar]
- 14.Weisman MH, Paulus HE, Burch FX, et al. A placebo-controlled, randomized, double-blinded study evaluating the safety of etanercept in patients with rheumatoid arthritis and concomitant comorbid diseases. Rheumatology (Oxford, England) 2007;46(7):1122–5. doi: 10.1093/rheumatology/kem033. [DOI] [PubMed] [Google Scholar]
- 15.Dixon WG, Watson KD, Lunt M, Hyrich KL, Silman AJ, Symmons DP. Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2007;56(9):2905–12. doi: 10.1002/art.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon DH, Avorn J, Katz JN, et al. Immunosuppressive medications and hospitalization for cardiovascular events in patients with rheumatoid arthritis. Arthritis & Rheumatism. 2006;54(12):3790–8. doi: 10.1002/art.22255. [DOI] [PubMed] [Google Scholar]
- 17.Suissa S, Bernatsky S, Hudson M. Antirheumatic drug use and the risk of acute myocardial infarction. Arthritis Rheum. 2006;55(4):531–6. doi: 10.1002/art.22094. [DOI] [PubMed] [Google Scholar]
- 18.Herrinton LJ, Curtis JR, Chen L, et al. Study design for a comprehensive assessment of biologic safety using multiple healthcare data systems. Pharmacoepidemiology and drug safety. 2011;20(11):1199–209. doi: 10.1002/pds.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rassen JA, Solomon DH, Curtis JR, Herrinton L, Schneeweiss S. Privacy-maintaining propensity score-based pooling of multiple databases applied to a study of biologics. Med Care. 2010;48(6 Suppl):S83–9. doi: 10.1097/MLR.0b013e3181d59541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SY, Servi A, Polinski JM, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13(1):R32. doi: 10.1186/ar3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. American Heart Journal. 2004;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Medical Care. 2005;43(5):480–5. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 23.Choma NN, Griffin MR, Huang RL, et al. An algorithm to identify incident myocardial infarction using Medicaid data. Pharmacoepidemiology and drug safety. 2009;18(11):1064–71. doi: 10.1002/pds.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox CS, Evans JC, Larson MG, et al. A comparison of death certificate out-of-hospital coronary heart disease death with physician-adjudicated sudden cardiac death. The American journal of cardiology. 2005;95(7):856–9. doi: 10.1016/j.amjcard.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Chung CP, Murray KT, Stein CM, Hall K, Ray WA. A computer case definition for sudden cardiac death. Pharmacoepidemiology and drug safety. 2009;19(6):563–72. doi: 10.1002/pds.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 27.Johnson ML, Crown W, Martin BC, Dormuth CR, Siebert U. Good research practices for comparative effectiveness research: analytic methods to improve causal inference from nonrandomized studies of treatment effects using secondary data sources: the ISPOR Good Research Practices for Retrospective Database Analysis Task Force Report--Part III. Value Health. 2009;12(8):1062–73. doi: 10.1111/j.1524-4733.2009.00602.x. [DOI] [PubMed] [Google Scholar]
- 28.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of Martingale-based residuals. Biometrika. 1993;80:557–72. [Google Scholar]
- 29.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–6. [PubMed] [Google Scholar]
- 30.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 32.Schneeweiss S. Developments in post-marketing comparative effectiveness research. Clinical pharmacology and therapeutics. 2007;82(2):143–56. doi: 10.1038/sj.clpt.6100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol. 172(9):1092–7. doi: 10.1093/aje/kwq224. 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biometrical journal. 2009;51(1):171–84. doi: 10.1002/bimj.200810488. [DOI] [PubMed] [Google Scholar]
- 35. [August 1, 2011];Pharamcoepidemiology Toolbox version 2. 2011 (at http://www.hdpharmacoepi.org.)
- 36.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. American Journal of Epidemiology. 2003;158(9):915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 37.Kleinbongard P, Heusch G, Schulz R. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacology & therapeutics. 2010;127(3):295–314. doi: 10.1016/j.pharmthera.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Kurrelmeyer KM, Michael LH, Baumgarten G, et al. Endogenous tumor necrosis factor protects the adult cardiac myocyte against ischemic-induced apoptosis in a murine model of acute myocardial infarction. Proc Natl Acad Sci U S A. 2000;97(10):5456–61. doi: 10.1073/pnas.070036297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hurlimann D, Forster A, Noll G, et al. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002;106(17):2184–7. doi: 10.1161/01.cir.0000037521.71373.44. [DOI] [PubMed] [Google Scholar]
- 40.Rayment NB, Moss E, Faulkner L, et al. Synthesis of TNF alpha and TGF beta mRNA in the different micro-environments within atheromatous plaques. Cardiovasc Res. 1996;32(6):1123–30. doi: 10.1016/s0008-6363(96)00145-9. [DOI] [PubMed] [Google Scholar]
- 41.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 42.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: Rationale and Design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J. 2011;162(4):597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]


