Acquired von Willebrand Syndrome (AVWS) is a bleeding disorder resulting from an acquired deficiency or dysfunction of von Willebrand factor (VWF). It is a rare condition in children with only 113 pediatric cases published in the literature between 1968 and 2009 [1]. Recent data suggest that pediatric AVWS is most frequently found in association with acquired or congenital heart defects [2].
The main reported pathogenic mechanism of the disease in patients with cardiac defects is the loss of high molecular weight VWF multimers (HMWM) caused by a shear stress-induced increase in VWF proteolysis [2].
Diagnosing AVWS can be challenging. Laboratory assessment requires documentation of an abnormality in the patient’s VWF. However, several individual and pre-analytical factors can affect the sensitivity of the tests used for diagnosing the condition. Moreover, the variation in assay techniques and thresholds used for interpreting test results adds to the complexity of diagnosing AVWS. In addition, because of the rarity of the condition and the challenges involved in the laboratory assessment, a high degree of clinical suspicion is required by health care providers who encounter such cases.
Here, we describe the diagnosis and disease management-related difficulties associated with two cases of AVWS followed up at The Hospital for Sick Children in Canada.
Case Reports
Case 1
A 9 year-old Caucasian male was referred to the Bleeding Disorders’ Clinic due to recurrent epistaxis and easy bruising for the previous 2 years. He was scheduled to undergo surgical repair of a small restrictive perimembranous VSD, which was complicated by a history of endocarditis and concomitant aortic valve regurgitation. He had no previous hemostatic challenges. There was no family history of bleeding disorders.
Laboratory investigations showed normal hemoglobin and platelet studies (count, size, morphology, and aggregation). Shown in the Table are his Factor VIII functional (FVIII:C), VWF antigen (VWF:Ag), VWF ristocetin cofactor (VWF:RCo) levels and platelet function analyzer (PFA)-100 test results.
Table.
Hemostatic Parameters in Case 1 and Case 2
| Case 1 | Case 2 | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Pre-surgery | Post-surgery | Pre-surgery | Post-surgery | ||||
| 2 weeks | 2 months | 2 years | 3 weeks | 5 months | |||
|
| |||||||
| Hemoglobin g/L | 130 | 105 | 128 | 124 | 140 | 110 | 133 |
|
| |||||||
| Platelets × 109/L | 253 | 528 | 246 | 226 | 133 | 295 | 195 |
|
| |||||||
| aPTT (s) | 36 | 34 | 38 | 34 | 28 | 25 | 26 |
|
| |||||||
| FVIII:C IU/mL# | 0.55 | 0.90 | 0.73 | 1.20 | 1.46 | 2.03 | 1.91 |
|
| |||||||
| VWF:Ag IU/mL## | 0.64 | 0.71 | 0.64 | 0.85 | 0.80 | 0.83 | nd |
|
| |||||||
| VWF:RCo IU/mL### | 0.35 | 0.43 | 0.47 | 0.89 | 0.62 | 0.68 | 0.73 |
|
| |||||||
| VWF:RCo/VWF:Ag | 55% | 60% | 73% | 104% | 77% | 82% | - |
|
| |||||||
| Multimers | Normal | - | - | - | Normal | - | - |
|
| |||||||
| Platelet aggregation | Normal | - | - | - | Normal | - | Normal |
|
| |||||||
| PFA: Col/Epi*(s) | 274–282 | 130 | 152 | 106 | 300->300 | 158 | 128 |
|
| |||||||
| PFA: Col/ADP**(s) | 230–251 | 122 | 107 | 123 | 153–224 | nd | 79 |
|
| |||||||
| Blood type | O+ | B+ | |||||
Institutional normal range: 0.56–1.72 IU/mL
Institutional normal range for patients > 3 months of age: 0.47–1.39 IU/mL for blood group 0, 0.84–1.92 IU/mL for non blood group 0
Institutional normal range for patients > 3 months of age: 0.38–1.22 IU/mL for blood group 0, 0.73–1.81 IU/mL for non blood group 0)
Collagen/epinephrine cartridge: normal <168 seconds
Collagen/ADP cartridge: normal <123 seconds
aPTT, activated partial thromboplastin time; nd, not done
His parents were also tested. Whereas the maternal results were normal, the paternal results were suggestive of type 1 von Willebrand disease (VWD) and included a slightly prolonged PFA-100 closure time (CT) with the collagen/epinephrine cartridge (182s) but a normal CT with the collagen/ADP cartridge (113s). His VWF:Ag level was 69% while his VWF:RCo level was 45%; he is blood type O negative.
The combination of the patient’s normal platelet studies and abnormal PFA-100 CTs and the VWF:RCo/VWF:Ag ratio of 55% was suggestive of AVWS. However, the paternal VWF panel results made it unclear whether the patient had congenital VWD or AVWS. The patient underwent surgical repair of the VSD under the cover of a FVIII/VWF concentrate. No excessive bleeding was noted peri-operatively. His PFA-100 CTs completely corrected two weeks post-surgery. VWF:Ag and VWF:RCo levels improved slightly. The VWF:RCo/VWF:Ag ratio was now 61%. Blood work was repeated several times over the following 2 years, showing completely normal PFA-100 CTs and VWF levels. Episodes of epistaxis became less frequent and less severe.
Case 2
A male Caucasian patient with un-operated moderate aortic valve stenosis and regurgitation was referred to the Bleeding Disorders’ Clinic at the age of 11 years for investigation of a 4-year history of recurrent epistaxis. He had undergone circumcision without bleeding as a neonate. Episodes affected either or both nostrils, occurred 2–3 times per month, and were worse in the winter. He had nasal cautery performed twice with no improvement before being referred to the Bleeding Disorders’ Clinic for investigations. There was no family history of bleeding disorders.
Laboratory results disclosed normal hemoglobin, as well as normal platelets studies (count, morphology, electron microscopy, and aggregation studies) and VWF levels. The only detected abnormality were prolonged PFA-100 CTs (Table). VWF multimer studies were normal.
Given his cardiac condition and his laboratory investigations, AVWS was suspected. However, surgical correction of the cardiac defect was not indicated from a cardiac standpoint and, given the mildness of his bleeding manifestations; no corrective surgery at the time was undertaken.
Five years after the initial consult, the patient presented with appendicitis and underwent laparoscopic appendectomy under desmopressin coverage, without excessive bleeding. Of note, the patient had previously undergone a desmopressin challenge and had a good response: VWF:RCo had risen from 63% (pre-desmopressin) to 134% (1 hour post desmopressin) and at 4 hours post desmopressin was still 190%. At age 16 years he had an episode of hematemesis requiring hospitalization. An upper gastrointestinal endoscopy performed to rule out angiodysplasia showed only mild chronic duodenitis and esophagitis. At the age of 17 years, the patient had a severe episode of epistaxis with a 30-g/L fall in his hemoglobin level. The epistaxis was unresponsive to desmopressin and to balloon packing, and required local fibrin sealant, FVIII/VWF concentrates, and tranexamic acid. The aortic valve stenosis mean gradient was 45 mmHg and there was moderate aortic regurgitation with normal left ventricular function and size for which cardiac surgery was not absolutely indicated. Given that the basis for correcting his aortic stenosis were his bleeding episodes, which were presumed to be secondary to AVWD, the patient underwent a Ross procedure which consists of placing the patient’s own pulmonary valve in the aortic position (i.e., autograft), and implanting a homograft conduit in the pulmonary position. He received peri-operative hemostatic coverage with FVIII/VWF concentrates and showed no excessive bleeding. Three weeks later his PFA-100 CTs results had completely normalized (Table) and he denied any bleeding symptoms. Five months later he has not had any further bleeding issues and his PFA-100 CTs continue to be normal.
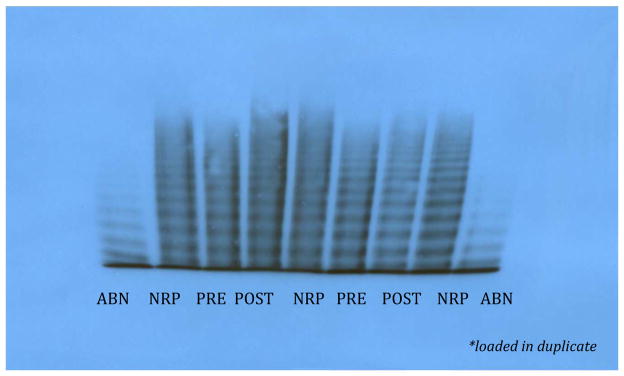
A very interesting point is the interpretation of the results of multimer analyses, performed in a specialized center following a previously published protocol [3]. The analyses showed that even though the percentage of HMWM was within normal limits before the surgery (71%, Figure), it increased to 92% 3 weeks after surgical repair of the cardiac defect (Figure).
Figure. Multimer Analysis in Case 2, Pre- and Post Surgery (provided by A. Tuttle and P. James).
ABN refers to abnormal plasma control; NRP to normal plasma control, PRE and POST refer to the patient test
Note: Multimer patterns were analyzed by 1-D densitometry using AlphaEaseFC version 3.1.2. Lanes were tracked manually and the area of intensity under the peaks was quantitated in comparison to the normal control. Bands 1–5 are defined as low molecular weight, 6–10 as medium molecular weight and bands >10 as high molecular weight.
Owing to the lack of substantive prospective data or epidemiologic studies, the true incidence of AVWS is uncertain [2, 4]. Because milder cases are only recognized upon hemostatic challenges, and because of a lack of awareness, this disorder is likely underestimated.
The association between aortic stenosis and gastrointestinal bleeding in adults was recognized and reported by Edward Heyde in 1958 [5]. Vascular lesions in the colonic mucosa and submucosa were subsequently identified as the source of bleeding and later termed “angiodysplasias”. The combination of aortic stenosis and gastrointestinal bleeding from angiodysplasias represent a particular type of AVWS, known as Heyde’s syndrome.
The first study reporting loss of HMWM in association with any type of cardiovascular disorder was published in 1984. In the study, Gill et al described 12 children with congenital heart disease and loss of HMWM; 7/12 patients had a history of mucocutaneous bleeding [6]. Four of the five patients who underwent surgical repair of their cardiac condition showed resolution of the VWF defect. The remaining patient, who did not show normalization of multimers, had a persistent cardiac defect [6]. The normalization of laboratory defects following surgical correction of cardiac defects was similarly reported by Rauch et al [7] in infants with patent ductus arteriosus. Arslan et al prospectively followed up a cohort of 49 children with congenital heart disease [8]. Six patients met laboratory criteria for AVWS chosen by the researchers, having low VWF:Ag and VWF:RCo levels prior to surgery. One week after surgery, laboratory results normalized in all patients and remained normal 6 months after surgery.
Definition of cases of AVWS can be difficult: some authors argue that diagnosis should only be made in the presence of bleeding symptoms [4]. However, it has been pointed out that some patients may not have had enough time to manifest bleeding symptoms from the moment the condition develops [9].
Functional assays, such as VWF:RCo and VWF:collagen binding (VWF:CB) activity, are recommended for laboratory diagnosis of AVWS, since other assays, like activated partial thromboplastin time (aPTT), VWF:Ag, and FVIII levels can be normal. Low VWF:RCo/Ag or low VWF:CB/Ag ratios are indicative of structural or functional alterations of VWF [2]. It has been reported that the combination of VWF:Ag <50 IU/dl, VWF:RCo/Ag ratio <70% and VWF:CB/Ag ratio of <80% has an 86% sensitivity for the diagnosis of AVWS [10].
Multimer analysis can be a sensitive test for detection of VWF abnormalities in AVWS in association with cardiac defects. However, as mentioned above, the test is also very susceptible to pre-analytical variables [2]. PFA-100 CTs are sensitive to the presence of VWD, though many other factors can influence results. In consequence, diagnosis of AVWS can be difficult.
The first of the cases presented in this report showed a reduced VWF:RCo/Ag ratio (55%) and prolonged PFA-100 CTs. The even more challenging second case only showed prolonged PFA-100 CTs. In this second case, the disappearance of bleeding symptoms, correction of PFA-100 CTs and improvement of HMWM percentage post surgical repair of the cardiac defect all support the diagnosis of AVWS.
The optimal management of AVWS in cardiac disease is also unclear, and both desmopressin and FVIII/VWF concentrates have been used in this context, with a wide reported success range [10, 11]. The theoretical advantage of using desmopressin over FVIII/VWF concentrates is that the former promotes local secretion of the more hemostatically active ultralarge HMWM at the site of injury, whereas the latter lack these ultralarge complexes [2]. However, there is no clear indication of which agent should be used to treat and to prevent bleeding in these circumstances, which adds to the complexity of AVWS, and decisions are usually tailored to each case according to the experience of the physician.
Following the recommendations for children with new diagnosis of VWD, performing a desmopressin challenge should be considered in patients with confirmed or suspected AVWS [12].
The limitations in laboratory assessment highlighted in these two patients reflect a major challenge in the identification of cases. Further advances in laboratory diagnosis are required, as well as further prospective studies in pediatric patients with cardiac disorders. Such studies will allow a better understanding of the natural history of the disease, and will serve as a basis for a precise and accurate definition of the disorder.
Until such studies are available, a high degree of clinical suspicion should be combined with thorough laboratory testing in order to determine the likelihood of a patient having the disease as well as to rule out other potential disorders, such as platelet defects.
Footnotes
The authors have no competing interests.
References
- 1.Will AM. Acquired von Willebrand Syndrome in Childhood and Adolescence. Journal of Coagulation Disorders. 2009;1(1):1–10. [Google Scholar]
- 2.Federici AB, et al. Current diagnostic and therapeutic approaches to patients with acquired von Willebrand syndrome: a 2013 update. Semin Thromb Hemost. 2013;39(2):191–201. doi: 10.1055/s-0033-1334867. [DOI] [PubMed] [Google Scholar]
- 3.Stakiw J, et al. The effect of exercise on von Willebrand factor and ADAMTS-13 in individuals with type 1 and type 2B von Willebrand disease. J Thromb Haemost. 2008;6(1):90–6. doi: 10.1111/j.1538-7836.2007.02790.x. [DOI] [PubMed] [Google Scholar]
- 4.Sucker C, Michiels JJ, Zotz RB. Causes, etiology and diagnosis of acquired von Willebrand disease: a prospective diagnostic workup to establish the most effective therapeutic strategies. Acta Haematol. 2009;121(2–3):177–82. doi: 10.1159/000214858. [DOI] [PubMed] [Google Scholar]
- 5.Heyde E. Gastrointestinal bleeding in aortic stenosis. N Engl J Med. 1958;259:196. [Google Scholar]
- 6.Gill JC, et al. Loss of the largest von Willebrand factor multimers from the plasma of patients with congenital cardiac defects. Blood. 1986;67(3):758–61. [PubMed] [Google Scholar]
- 7.Rauch R, et al. Acquired von Willebrand syndrome in children with patent ductus arteriosus. Heart. 2002;88(1):87–8. doi: 10.1136/heart.88.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arslan MT, et al. Frequency of acquired von Willebrand’s disease in children with congenital heart disease. Acta Cardiol. 2007;62(4):403–8. doi: 10.2143/AC.62.4.2022285. [DOI] [PubMed] [Google Scholar]
- 9.Tiede A, et al. How I treat the acquired von Willebrand syndrome. Blood. 2011;117(25):6777–85. doi: 10.1182/blood-2010-11-297580. [DOI] [PubMed] [Google Scholar]
- 10.Tiede A, et al. Diagnostic workup of patients with acquired von Willebrand syndrome: a retrospective single-centre cohort study. J Thromb Haemost. 2008;6(4):569–76. doi: 10.1111/j.1538-7836.2008.02909.x. [DOI] [PubMed] [Google Scholar]
- 11.Federici AB, et al. Acquired von Willebrand syndrome: data from an international registry. Thromb Haemost. 2000;84(2):345–9. [PubMed] [Google Scholar]
- 12.Revel-Vilk S, et al. Desmopressin (DDAVP) responsiveness in children with von Willebrand disease. Journal of Pediatric Hematology/Oncology. 2003;25(11):874–9. doi: 10.1097/00043426-200311000-00010. [DOI] [PubMed] [Google Scholar]