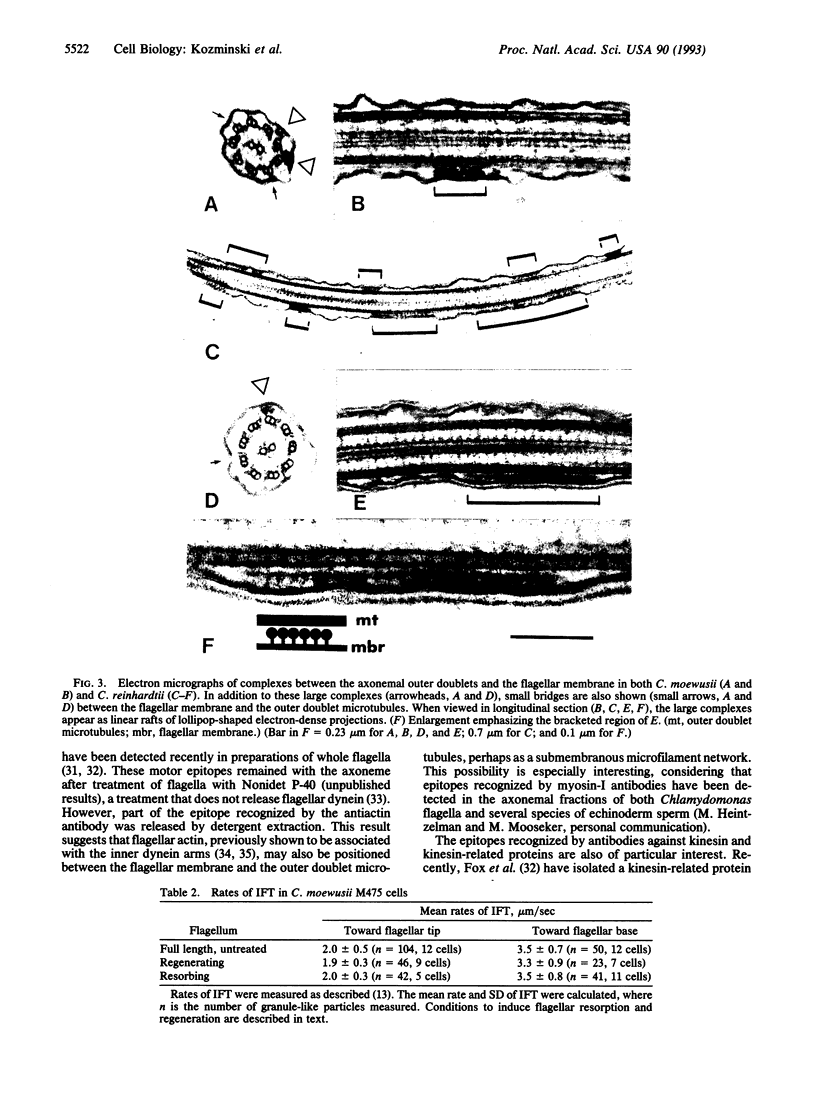
Abstract
We report a motility in the flagella of the green alga Chlamydomonas that is unrelated to dynein-based flagellar beating. This motility, referred to as intraflagellar transport, was observed as the rapid bidirectional movement of granule-like particles along the length of the flagella. Intraflagellar transport could be experimentally separated from other, previously reported, nonbeat flagellar motilities. EM of flagella showed groups of nonvesicular, lollipop-shaped structures positioned between the outer doublet microtubules and the flagellar membrane. Movement of these complexes along the length of the flagella may be responsible for intraflagellar transport.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Lee K. S., Brown A. M. The calcium current of Helix neuron. J Gen Physiol. 1978 May;71(5):509–531. doi: 10.1085/jgp.71.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood R. A. Directed movements of ciliary and flagellar membrane components: a review. Biol Cell. 1992;76(3):291–301. doi: 10.1016/0248-4900(92)90431-y. [DOI] [PubMed] [Google Scholar]
- Bloodgood R. A., Leffler E. M., Bojczuk A. T. Reversible inhibition of Chlamydomonas flagellar surface motility. J Cell Biol. 1979 Sep;82(3):664–674. doi: 10.1083/jcb.82.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood R. A. Motility occurring in association with the surface of the Chlamydomonas flagellum. J Cell Biol. 1977 Dec;75(3):983–989. doi: 10.1083/jcb.75.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentler W. L., Pratt M. M., Stephens R. E. Microtubule-membrane interactions in cilia. II. Photochemical cross-linking of bridge structures and the identification of a membrane-associated dynein-like ATPase. J Cell Biol. 1980 Feb;84(2):381–403. doi: 10.1083/jcb.84.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euteneuer U., Koonce M. P., Pfister K. K., Schliwa M. An ATPase with properties expected for the organelle motor of the giant amoeba, Reticulomyxa. Nature. 1988 Mar 10;332(6160):176–178. doi: 10.1038/332176a0. [DOI] [PubMed] [Google Scholar]
- Fennikoh K. B., Hirshfield H. I., Kneip T. J. Cadmium toxicity in planktonic organisms of a freshwater food web. Environ Res. 1978 Jun;15(3):357–367. doi: 10.1016/0013-9351(78)90117-2. [DOI] [PubMed] [Google Scholar]
- Forscher P., Lin C. H., Thompson C. Novel form of growth cone motility involving site-directed actin filament assembly. Nature. 1992 Jun 11;357(6378):515–518. doi: 10.1038/357515a0. [DOI] [PubMed] [Google Scholar]
- Forscher P., Smith S. J. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J Cell Biol. 1988 Oct;107(4):1505–1516. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough U. W., Jurivich D. Tipping and mating-structure activation induced in Chlamydomonas gametes by flagellar membrane antisera. J Cell Biol. 1978 Dec;79(3):680–693. doi: 10.1083/jcb.79.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. A., Rosenbaum J. L. Polarity of flagellar assembly in Chlamydomonas. J Cell Biol. 1992 Dec;119(6):1605–1611. doi: 10.1083/jcb.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya R., Kurimoto E., Muto E. Two types of Chlamydomonas flagellar mutants missing different components of inner-arm dynein. J Cell Biol. 1991 Feb;112(3):441–447. doi: 10.1083/jcb.112.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P. A., Nordstrom S. A., Moulder J. E., Rosenbaum J. L. Flagellar elongation and shortening in Chlamydomonas. IV. Effects of flagellar detachment, regeneration, and resorption on the induction of flagellar protein synthesis. J Cell Biol. 1978 Jul;78(1):8–27. doi: 10.1083/jcb.78.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P. A., Silflow C. D., Wieben E. D., Rosenbaum J. L. Increased levels of mRNAs for tubulin and other flagellar proteins after amputation or shortening of Chlamydomonas flagella. Cell. 1980 Jun;20(2):469–477. doi: 10.1016/0092-8674(80)90633-9. [DOI] [PubMed] [Google Scholar]
- Mesland D. A., Hoffman J. L., Caligor E., Goodenough U. W. Flagellar tip activation stimulated by membrane adhesions in Chlamydomonas gametes. J Cell Biol. 1980 Mar;84(3):599–617. doi: 10.1083/jcb.84.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. R., Rosenbaum J. L. A motile Chlamydomonas flagellar mutant that lacks outer dynein arms. J Cell Biol. 1985 Apr;100(4):1228–1234. doi: 10.1083/jcb.100.4.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., Luck D. J. An actin-like protein is a component of axonemes from Chlamydomonas flagella. J Biol Chem. 1979 Apr 10;254(7):2187–2190. [PubMed] [Google Scholar]
- Piperno G., Luck D. J. Axonemal adenosine triphosphatases from flagella of Chlamydomonas reinhardtii. Purification of two dyneins. J Biol Chem. 1979 Apr 25;254(8):3084–3090. [PubMed] [Google Scholar]
- Piperno G., Mead K., Shestak W. The inner dynein arms I2 interact with a "dynein regulatory complex" in Chlamydomonas flagella. J Cell Biol. 1992 Sep;118(6):1455–1463. doi: 10.1083/jcb.118.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. L., Child F. M. Flagellar regeneration in protozoan flagellates. J Cell Biol. 1967 Jul;34(1):345–364. doi: 10.1083/jcb.34.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. L., Moulder J. E., Ringo D. L. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol. 1969 May;41(2):600–619. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGER R., GRANICK S. Nutritional studies with Chlamydomonas reinhardi. Ann N Y Acad Sci. 1953 Oct 14;56(5):831–838. doi: 10.1111/j.1749-6632.1953.tb30261.x. [DOI] [PubMed] [Google Scholar]
- Sattler C. A., Staehelin L. A. Ciliary membrane differentiations in Tetrahymena pyriformis. Tetrahymena has four types of cilia. J Cell Biol. 1974 Aug;62(2):473–490. doi: 10.1083/jcb.62.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M., Shimizu T., Vale R. D., Euteneuer U. Nucleotide specificities of anterograde and retrograde organelle transport in Reticulomyxa are indistinguishable. J Cell Biol. 1991 Mar;112(6):1199–1203. doi: 10.1083/jcb.112.6.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman G. B., Carlson K., Berliner J., Rosenbaum J. L. Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes. J Cell Biol. 1972 Sep;54(3):507–539. doi: 10.1083/jcb.54.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]