Abstract
Reported herein is the effect of cyclodextrins on the rates of aqueous Diels Alder reactions of 9-anthracenemethanol with a variety of N-substituted maleimides. These reactions occurred under mild reaction conditions (aqueous solvent, 40 °C), and were most efficient for the reaction of N-cyclohexylmaleimide with a methyl-β-cyclodextrin additive (94% conversion in 24 hours). These results can be explained on the basis of a model wherein the cyclodextrins bind the hydrophobic substituents on the maleimides and activate the dienophile via electronic modulation of the maleimide double bond. The results reported herein represent a new mechanism for cyclodextrin-promoted Diels Alder reactions, and have significant potential applications in the development of other cyclodextrin-promoted organic transformations. Moreover, the ability to deplanarize polycyclic aromatic hydrocarbons (PAHs) under mild conditions, as demonstrated herein, has significant applications for PAH detoxification.
Keywords: cyclodextrin, supramolecular catalysis, Diels-Alder, maleimide, polycyclic aromatic hydrocarbons
Cyclodextrins are torus shaped cyclic oligoamyloses, with the size of the interior cavity determined by the number of repeating amylose units. The ability of cyclodextrins to form host-guest complexes with hydrophobic guests occurs as a result of their hydrophobic interiors, whereas their relatively hydrophilic exteriors enable them to be used in mostly aqueous environments.1 Once host-guest complexes form, the guests can undergo cyclodextrin-mediated catalysis;2 such catalysis has been reported for sigmatropic rearrangements,3 for Diels-Alder reactions,4 and for a variety of other organic transformations.5 Cyclodextrins have also been used for a number of applications based on their ability to form host-guest complexes, including the solubilization of pharmaceutically active compounds,6 the extraction of polycyclic aromatic hydrocarbons (PAHs) from contaminated sediments,7 soil,8 and water,9 and the promotion of proximity-induced energy transfer.10
Previous research in our group has focused on the development of cyclodextrin-based systems for the detection of a wide variety of aromatic toxicants in multiple complex environments via cyclodextrin-promoted energy transfer from the toxicants to high quantum yield fluorophores.11 We have also reported the ability of cyclodextrins to extract aromatic toxicants, in particular PAHs, from complex oils, including motor oil, vegetable oil, and vacuum pump oil, as well as oil collected directly from an oil spill site.12 This dual function system of extraction followed by detection has significant applications in oil spill remediation efforts.
Much of the toxicity of PAHs is related to their highly planar structures, which enable the PAHs to intercalate in DNA and form covalent, carcinogenic adducts.13 Converting the PAHs to non-planar products using chemical transformations disrupts this facile intercalation and limits their ability to form carcinogenic adducts. Reported herein is the ability of cyclodextrins to promote such transformations for one PAH, 9-anthracenemethanol (compound 1), via its Diels-Alder reactions with N-substituted maleimides. Mechanistic investigations demonstrate that the rate enhancements achieved in the presence of cyclodextrin rely on cyclodextrin-induced activation of the maleimide double bond via binding of the hydrophobic substituents to promote the reaction and achieve substantial rate accelerations.
The conversion of compound 1 to its corresponding Diels Alder adduct 3 was calculated after various time intervals under standard reaction conditions (5 mM aqueous cyclodextrin, 40 °C) (Equation 1). The percent conversion of each reaction was calculated based on the following equation:
| (Equation 2) |
Equation 1.
Cyclodextrin-catalyzed aqueous Diels Alder reactions of 9-anthracenemethanol 1 with N-substituted maleimides 2.
The starting material NMR peak used in this equation corresponds to 3 aromatic protons of the 9-anthracenemethanol and the product peak used for this equation corresponds to 1 proton at the bridgehead of the Diels-Alder adduct 3 (Figure 1). The integration of the NMR peaks were the relative areas under the curve measured against a calibrated internal standard corresponding to the residual CHCl3 peak at 7.26 ppm.
Figure 1.
1H NMR peaks of protons (marked in red) integrated for the starting material 1 and product 3.
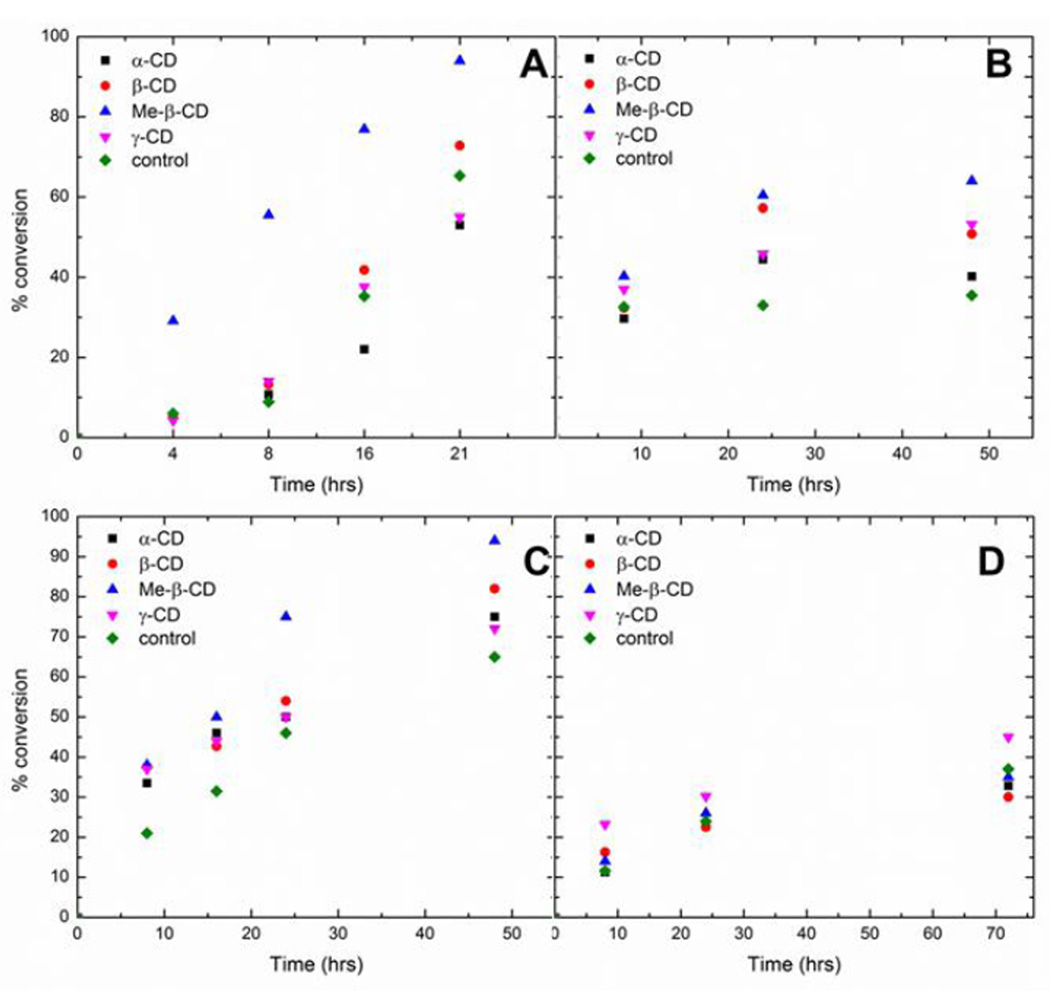
Methyl-β-cyclodextrin yielded the highest percent conversion to product for any of the various reactions studied (94% for the conversion of 2a to 3a after 24 hours, compared to 65% for the cyclodextrin-free reaction under analogous conditions) (Figure 2). The beneficial effect of methyl-β-cyclodextrin (as well as all of the cyclodextrin derivatives) diminished for the less bulky maleimides. For example, the conversion of 2d to 3d in the presence of methyl-β-cyclodextrin was 26% after 24 hours, compared to 24% conversion in the cyclodextrin-free solution under analogous conditions. The dependence of the cyclodextrin efficacy on the structure of the maleimide dienophile indicates that binding of the hydrophobic N-substituent in the cyclodextrin cavity is necessary for achieving optimal rate accelerations.
Figure 2.
Percent conversion of compounds 1 and 2 to product 3 in various cyclodextrins for (A) compound 2a; (B) compound 2b; (C) compound 2c; (D) compound 2d.
The dependence of the rate enhancements on the structures of the dienophiles (and in particular on the size and hydrophobicity of the nitrogen substituent) was further probed by 1H NMR spectroscopy of the maleimide-cyclodextrin binary complexes. These studies revealed that all of the substituted maleimides (compounds 2a–2c) demonstrated significant changes in the 1H NMR shifts of the maleimide alkene protons (Table 1), indicating strong binding to the cyclodextrin hosts (approximately 300 M−1 for compound 2a, and 8 M−1 for compound 2c).14 The changes in the protons’ chemical shifts were directly proportional to the hydrophobicity of the nitrogen substituent, with the largest changes and the strongest binding observed for N-cyclohexylmaleimide (compound 2a). Virtually no shift in the alkene protons was observed for maleimide 2d, which lacks a hydrophobic N-substituent.
Table 1.
Changes in the alkene proton chemical shifts in 5 mM cyclodextrin solutionsa
| Dienophile | α-CD | β-CD | Me-β-CD | γ-CD |
|---|---|---|---|---|
| 2a | 0.018 | 0.047 | 0.078 | 0.033 |
| 2b | 0.014 | 0.014 | 0.029 | 0.001 |
| 2c | 0.004 | 0.016 | 0.013 | 0.003 |
| 2d | 0.005 | 0.004 | 0.001 | 0.000 |
Δ ppm is defined as ∂(CD solution) − ∂(control)
Analysis of the conversion efficiencies with different dienophiles reveals that the dienophiles that bound most strongly in the methyl-β-cyclodextrin cavity (as indicated by greatest changes in the 1H NMR chemical shifts) were also the most reactive (Figure 3). This binding strength in turn depends largely on the hydrophobicity of the N-substituent of the maleimide, with compound 2a demonstrating the greatest binding affinities and fastest reaction rate.
Figure 3.
Graphical representation of the average conversion after 24 hours for various N-substituted maleimides in the presence of cyclodextrins.
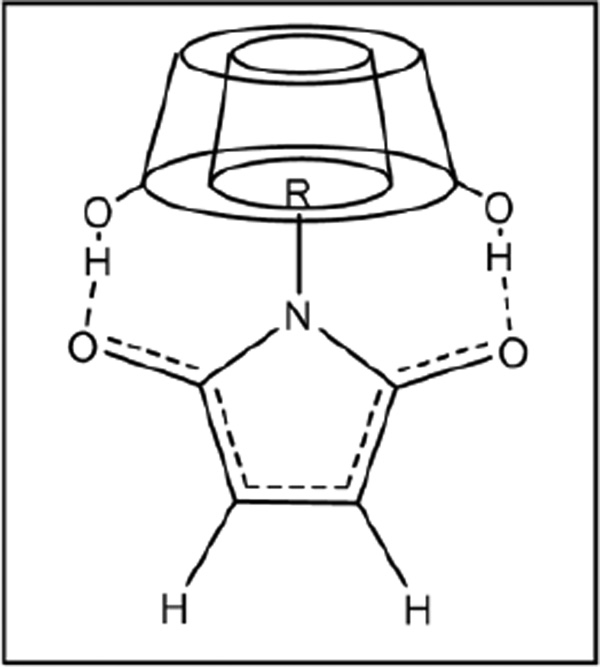
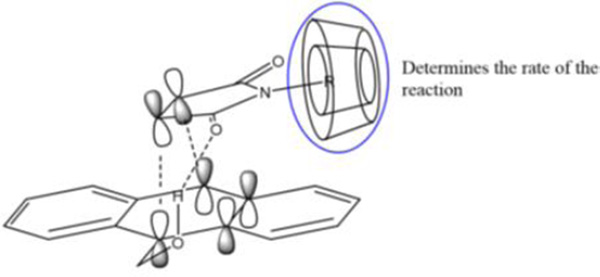
The proposed mechanism by which the cyclodextrin derivatives promote the Diels-Alder reaction of compounds 1 and 2 likely involves the binding of hydrophobic N-substituted maleimides 2 in the hydrophobic cyclodextrin cavity, with additional stabilization provided by hydrogen bonding between the cyclodextrin hydroxyl groups and the carbonyl groups of the maleimide (Figure 4).
Figure 4.
Schematic illustration of how cyclodextrin complexation activates the dienophile through a combination of hydrophobic binding and electronic perturbation of the π-bond.
This additional binding withdraws electron density from the π-bond, activating the alkene for the resultant cycloaddition reaction. This effect was maximal for the binding of 2a in methyl-β-cyclodextrin, due to the highly hydrophobic nature of the cyclohexyl substituent15 and the optimal size match between the cyclohexyl and the methyl-β-cyclodextrin cavity.16 A similar phenomenon has been reported by Ritter and co-workers, wherein cyclodextrin binding of N-substituted maleimides led to enhanced reactivity in free radical polymerization reactions.17 However, the mechanism by which such binding led to activation of the alkene bond in the N-substituted maleimides was not explicitly discussed.
Interestingly, methyl-β-cyclodextrin was significantly more efficient than β-cyclodextrin at promoting this Diels-Alder reaction, despite the fact that methyl-β-cyclodextrin and β-cyclodextrin have similar cavity dimensions. This trend is likely a result of the fact that methyl-β-cyclodextrin is both more flexible and has a more non-polar cavity than β-cyclodextrin, a fact that has been reported in the literature but has been rarely exploited in organic reactions.18
A closer look at the reaction conversions (Figure 3) reveals that as the N-substituent decreases in bulk, the conversions obtained with γ-cyclodextrin approach those observed with methyl-β-cyclodextrin. For example, the difference between the conversions achieved with methyl-β-cyclodextrin compared to γ-cyclodextrin was 39% for substrate 2a; this difference drops to 25% for substrate 2c and to 4% in favor of γ-cyclodextrin for substrate 2d. The less bulky substrates can form ternary complexes in γ-cyclodextrin, with both the diene and dienophile binding simultaneously in the cavity interior. γ-Cyclodextrin is known to form ternary complexes,19 and such ternary complexes have already been used in γ-cyclodextrin mediated dimerization reactions.20 This ternary complexation binding mode is distinct from the binding mode proposed in Figure 4, which is expected to be the dominant mechanism for methyl-β-cyclodextrin binding of bulky N-substituents.
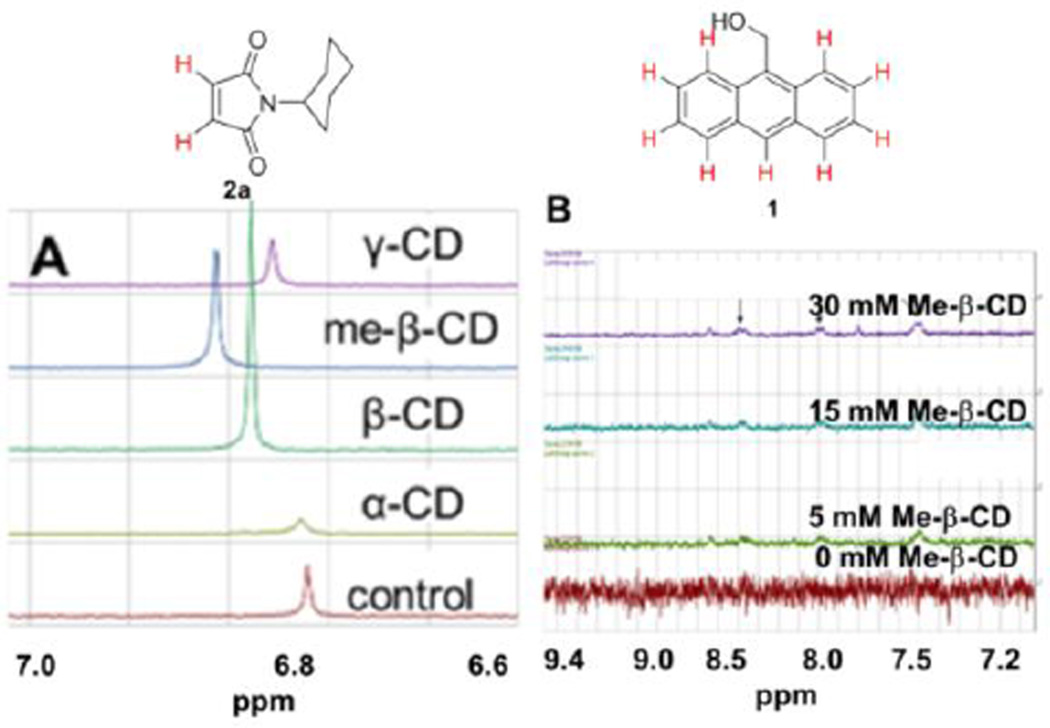
Interestingly, 1H NMR investigations of the binary complexes indicate that the binding of alkene 2a in cyclodextrin leads to shifts in the alkene protons, with the magnitude of the shift greatest for binding in methyl-β-cyclodextrin (Figure 5A). Binding of compound 1 in methyl-β-cyclodextrin led to increasing intensity in the signals of the aromatic protons as a result of the increased solubilization conferred through complex formation (Figure 5B).
Figure 5.
(A) Illustration of the chemical shifts for alkene protons of 2a in binary cyclodextrin complexes (5 mM cyclodextrin); (B) Illustration of the increasing intensity of aromatic protons for compound 1 in the presence of increasing concentrations of methyl-β-cyclodextrin.
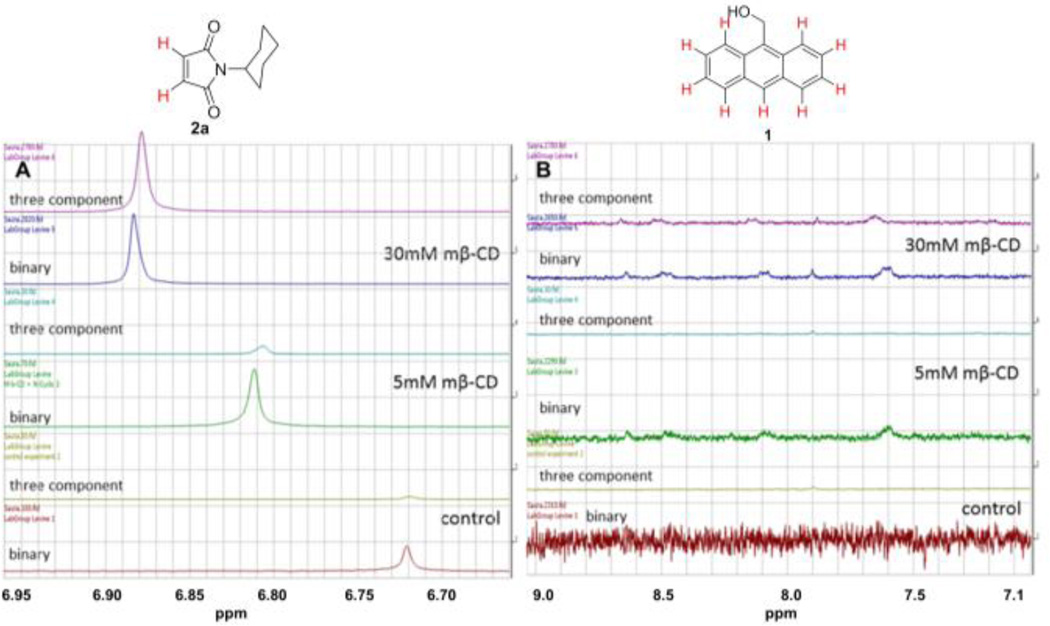
1H NMR analysis of the three component mixture (diene 1, dienophile 2a, and cyclodextrin host) indicated that binding of the diene led to a slight upfield shift in the 1H NMR spectrum of the dienophile compared to its binding in binary complexes (1:1 ratio of maleimide 2a and diene 1). The aromatic protons of compound 1 shifted downfield in the three component mixture (compared to their chemical shifts in a binary cyclodextrin:1 complex) (Figure 6). The 1H NMR peak shifts for the three component complexes can be explained based on the orbital interaction between the HOMO of diene 1 and the LUMO of dienophile 2a.21 As the π-electron cloud of the electron rich diene redistributes along the electron deficient dienophile, the alkene protons get shielded whereas the aromatic protons get deshielded. Moreover, the magnitude of these chemical shifts depends on the concentration of the methyl-β-cyclodextrin, with a 30 mM cyclodextrin solution leading to more pronounced chemical shifts compared to a 5 mM cyclodextrin solution.
Figure 6.
(A) Comparison of the 1H NMR shifts of the alkene protons in a three component mixture and in the 2a:methyl-β-cyclodextrin binary complex; (B) Comparison of the 1H NMR shifts of the protons of compound 1 in a three component mixture and in the 1:methyl-β-cyclodextrin binary complex.
As a whole, the data reported herein suggests a supramolecular assembly of the type shown in Figure 7, wherein a hydrophobically bound dienophile is linked by hydrogen bonding to the diene. The hydroxyl group in the diene 1 is believed to contribute to the solubility of the diene in the aqueous medium (an important criteria for the reaction, since unsubstituted anthracene failed to react under similar conditions).
Figure 7.
The hypothesized supramolecular assembly involving HOMO and LUMO interactions between an uncomplexed 1 and cyclodextrin-complexed 2.
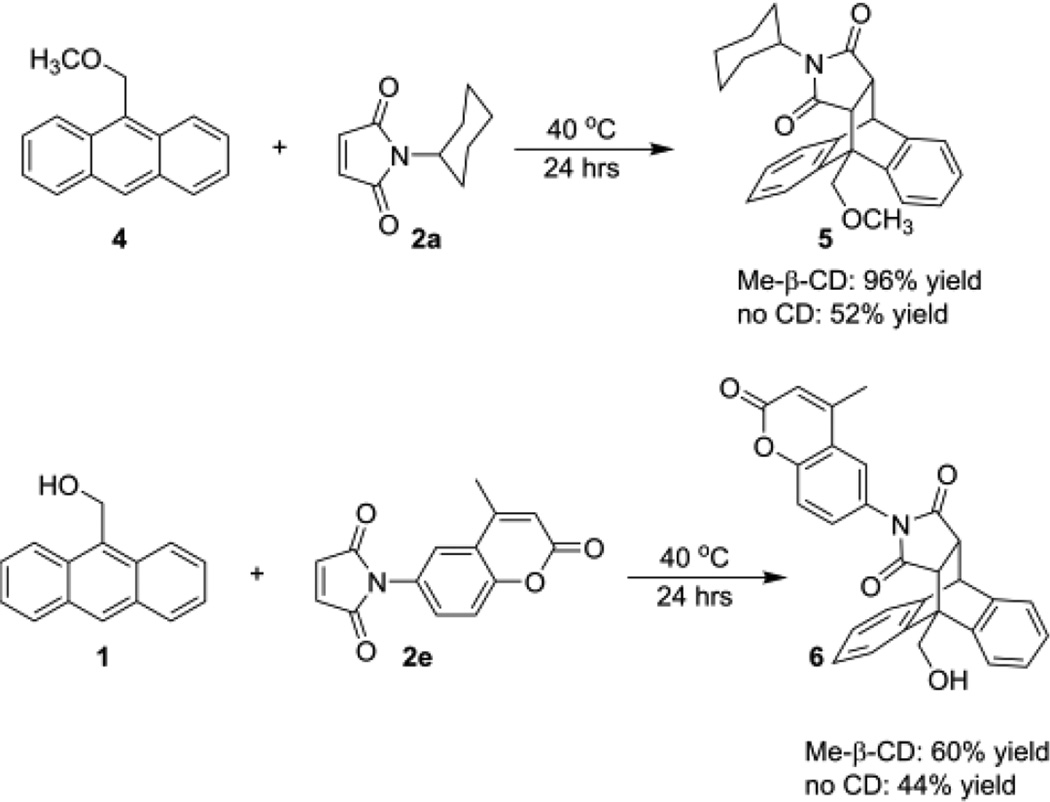
Preliminary efforts to expand the scope of this cyclodextrin-mediated Diels-Alder reaction have demonstrated that other anthracene dienes and other maleimide dienophiles also participate in this reaction efficiently, including compounds 4 and 2e (Scheme 1). Compound 2e is of particular interest, as both the maleimide 2e and the product 6 are photophysically active, which provides a facile tool for tracking in complex environments. Moreover, the methyl-β-cyclodextrin promoted reaction of compounds 1 and 2a (Equation 1) proceeded in up to 60% yield when run in unpurified seawater (compared to 15% for the cyclodextrin-free reaction), which indicates the ability to run these Diels-Alder reactions in real world environments for environmental detoxification applications.
Scheme 1.
Diels-Alder reactions of other dienes and dienophiles under analogous conditions.
In summary, these experiments demonstrate the ability of methyl-β-cyclodextrin to catalyze the conversion of a PAH to non-planar hydrophobic adducts under mild reaction conditions. This rate enhancement is primarily due to the superior hydrophobic binding of methyl-β-cyclodextrin to hydrophobic substituents on the N-substituted maleimides, which in turn enhances the alkene reactivity. The resulting adducts 3 are both less planar and more hydrophobic than the starting PAH, which will help to mitigate toxicity by reducing the degree of PAH intercalation in the DNA as well as the mobility of the PAH adduct in highly polar biological environments. Current efforts are focused on expanding the scope of this Diels-Alder reaction to include other hydrophobically-substituted dienophiles and other aromatic dienes, as well as investigations of other cyclodextrin-promoted organic transformations. Results of these and other investigations will be reported in due course.
Supplementary Material
Acknowledgments
This research was funded by a grant from the Gulf of Mexico Research Initiative (GOMRI) and by a grant from the National Cancer Institute (1R21CA185435-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Materials and methods, general procedures for 1H NMR experiments, full result tables for all 1H NMR chemical shifts and percent conversion efficiencies. This material is available free of charge via the Internet.
References
- 1.(a) Breslow R. Acc. Chem. Res. 1991;24:317–324. [Google Scholar]; (b) Harada A. Acta Polym. 1998;49:3–17. [Google Scholar]; (c) Takahashi K, Hattori K. J. Inclusion Phenom. Molec. Recognition Chem. 1994;17:1–24. [Google Scholar]
- 2.Marchetti L, Levine M. ACS Catal. 2011;1:1090–1118. [Google Scholar]
- 3.Harano K, Kiyonaga H, Hisano T. Tetrahedron Lett. 1991;32:7557–7558. [Google Scholar]
- 4.(a) Breslow R, Maitra U, Rideout D. Tetrahedron Lett. 1983;24:1901–1904. [Google Scholar]; (b) Gonzalez A, Holt SL. J. Org. Chem. 1982;47:3186–3188. [Google Scholar]; (c) Schneider HJ, Sangwan NK. J. Chem. Soc. Chem. Commun. 1986:1787–1789. [Google Scholar]; (d) Sternbach DD, Rossana DM. J. Am. Chem. Soc. 1982;104:5853–5854. [Google Scholar]
- 5.(a) Hapiot F, Bricout H, Tilloy S, Monflier E. Eur. J. Inorg. Chem. 2012:1571–1578. 2012. [Google Scholar]; (b) Hromadova M, Sokolova R. Curr. Org. Chem. 2011;15:2950–2956. [Google Scholar]
- 6.Agrawal R, Gupta V. Int. J. Pharma. Frontier Res. 2012;2:95–112. [Google Scholar]
- 7.Gao H, Ma J, Xu L, Jia L. Environ. Sci. Pollution Res. 2014;21:8620–8630. doi: 10.1007/s11356-014-2701-6. [DOI] [PubMed] [Google Scholar]
- 8.Petitgirard A, Djehiche M, Persello J, Fievet P, Fatin-Rouge N. Chemosphere. 2009;75:714–718. doi: 10.1016/j.chemosphere.2009.01.072. [DOI] [PubMed] [Google Scholar]
- 9.Gao H, Miles MS, Meyer BM, Wong RL, Overton EB. J. Environ. Monitoring. 2012;14:2164–2169. doi: 10.1039/c2em30223c. [DOI] [PubMed] [Google Scholar]
- 10.(a) Sforazzini G, Kahnt A, Wykes M, Sprafke JK, Brovelli S, Montarnal D, Meinardi F, Cacialli F, Beljonne D, Albinsson B, Anderson HL. J. Phys. Chem. C. 2014;118:4553–4566. [Google Scholar]; (b) Klotz EJF, Claridge TDW, Anderson HL. J. Am. Chem. Soc. 2006;128:15374–15375. doi: 10.1021/ja0665139. [DOI] [PubMed] [Google Scholar]
- 11.(a) Mako T, Marks P, Cook N, Levine M. Supramol. Chem. 2012;24:743–747. [Google Scholar]; (b) Serio N, Miller K, Levine M. Chem. Commun. 2013;49:4821–4823. doi: 10.1039/c3cc40534f. [DOI] [PubMed] [Google Scholar]; (c) Serio N, Chanthalyma C, Prignano L, Levine M. Supramol. Chem. 2014;26:714–721. doi: 10.1080/10610278.2013.860226. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Serio N, Prignano L, Peters S, Levine M. Polycyclic Aromatic Compounds. 2014;34:561–572. doi: 10.1080/10406638.2014.918889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Serio N, Chanthalyma C, Peters S, Levine D, Levine M. J. Inclusion Phenom. Macrocyclic Chem. 2014 Ahead of Print; [Google Scholar]; (b) Serio N, Chanthalyma C, Prignano L, Levine M. ACS Appl. Mater. Interfaces. 2013;5:11951–11957. doi: 10.1021/am403702n. [DOI] [PubMed] [Google Scholar]
- 13.Tang Y, Liu Z, Ding S, Lin CH, Cai Y, Rodriguez FA, Sayer JM, Jerina DM, Amin S, Broyde S, Geacintov NE. Biochem. 2012;51:9751–9762. doi: 10.1021/bi3013577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Efforts to measure the binding affinity of compound 2b were unsuccessful to date.
- 15.Sahin-Toth M, Gunawan P, Lawrence MC, Toyokuni T, Kaback HR. Biochem. 2002;41:13039–13045. doi: 10.1021/bi0203076. [DOI] [PubMed] [Google Scholar]
- 16.Chau NTT, Handjani S, Guegan J-P, Guerrero M, Monflier E, Philippot K, Denicourt-Nowicki A, Roucoux A. ChemCatChem. 2013;5:1497–1503. [Google Scholar]
- 17.(a) Bernhardt S, Gloeckner P, Theis A, Ritter H. Macromolecules. 2001;34:1647–1649. [Google Scholar]; (b) Bernhardt S, Glockner P, Ritter H. Polym. Bull. 2001;46:153–157. [Google Scholar]; (c) Born M, Koch Th, Ritter H. Acta Polym. 1994;45:68–72. [Google Scholar]; (d) Born M, Ritter H. Angew. Chem. Int. Ed. 1995;34:309–311. [Google Scholar]
- 18.(a) Zoppi A, Delrivo A, Aiassa V, Longhi MR. AAPS PharmSciTech. 2013;14:727–735. doi: 10.1208/s12249-013-9958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yang C, Castelvetro V, Scalarone D, Bianchi S, Zhang Y. J. Polym. Sci. A Polym. Chem. 2011;49:4518–4530. [Google Scholar]
- 19.(a) Hamai S. Bull. Chem. Soc. Japan. 2007;80:1527–1533. [Google Scholar]; (b) Hamai S. J. Inclusion Phenom. Macrocyclic Chem. 2010;67:471–481. [Google Scholar]
- 20.(a) Tamaki T, Kokubu T, Ichimura K. Tetrahedron. 1987;43:1485–1494. [Google Scholar]; (b) Tamaki T, Kawanishi Y, Seki T, Sakuragi M. J. Photochem. Photobiol. A Chem. 1992;65:313–320. [Google Scholar]
- 21.(a) Jensen KL, Dickmeiss G, Jiang H, Albrecht L, Jorgensen KA. Acc. Chem. Res. 2012;45:248–264. doi: 10.1021/ar200149w. [DOI] [PubMed] [Google Scholar]; (b) Li J-L, Liu T-Y, Chen Y-C. Acc. Chem. Res. 2012;45:1491–1500. doi: 10.1021/ar3000822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.