Abstract
Purpose
Prescription drug overdose (PDO) deaths are a critical public health problem in the United States. This study aims to assess the association between emergency department (ED) utilization patterns in a cohort of ED patients and the risk of subsequent unintentional PDO mortality.
Methods
Using data from the New York Statewide Planning and Research Cooperative System for 2006–2010, a nested case-control design was used to examine the relationship between ED utilization patterns in New York State residents of age 18–64 years and subsequent PDO death.
Results
The study sample consisted of 2732 case patients who died of PDO and 2732 control ED patients who were selected through incidence density sampling. With adjustment for demographic characteristics, and diagnoses of pain, substance abuse, and psychiatric disorders, the estimated odds ratios of PDO death relative to one ED visit or less in the previous year were 4.90 (95% confidence interval [CI]: 4.50–5.34) for those with two ED visits, 16.61 (95% CI: 14.72–18.75) for those with three ED visits, and 48.24 (95% CI: 43.23–53.83) for those with four ED visits or more.
Conclusions
Frequency of ED visits is strongly associated with the risk of subsequent PDO death. Intervention programs targeting frequent ED users are warranted to reduce PDO mortality.
Keywords: Drug overdose; Emergency service, hospital; Prescription drugs; Prevention and control
Introduction
Increases in drug overdose death in the United States are a critical public health problem. Nearly 60% of drug overdose deaths involved prescription drugs, and in 2011, 1.4 million emergency department (ED) visits involved nonmedical use of prescription drugs [1,2]. Frequent ED utilization has been shown to be a marker for substance use—narcotic use, especially [3,4], and is associated with nonmedical opioid use, drug diversion, and poorly controlled pain [3–5]. Therefore, frequent ED utilization may be a marker for increased risk for prescription drug overdose (PDO), and this point of clinician contact may serve as a setting to launch preventive intervention efforts [6–15]. Preventive interventions targeted at ED patients at high risk for PDO death, may include advice such as not mixing opioids with sedatives or alcohol [16], or targeted patient education initiatives [17]. Furthermore, identifying high risk populations can help medical practitioners decide whom to target with screening, intervention (e.g., take-home naloxone), and treatment program referrals [18–24].
Despite the importance of the ED as a key clinical entry point for patients at high risk of PDO, the relationship between ED utilization patterns and subsequent drug overdose death is understudied. In the present study, we used a nested case-control design to examine the relationship between ED utilization in a cohort of ED patients and the risk of subsequent unintentional PDO death. Characterizing risk markers for fatal PDO available in administrative databases can be useful for identifying individuals at increased likelihood of PDO death. The goal of the analysis was to determine whether increased ED utilization in a cohort of ED patients was associated with subsequent unintentional PDO death.
Materials and methods
Data sources
Data for this study came from the New York Statewide Planning and Research Cooperative System (SPARCS) for the years 2006–2010. SPARCS is a data reporting system that collects ED visit data from nonfederal hospitals in New York State (NYS) [25]. SPARCS contains patient-level data including patient characteristics, discharge diagnoses, procedures received, and charges for ED visits [25]. To ensure data quality, SPARCS visit data are examined for proper formatting upon submission format [26]. Data completeness and accuracy are assessed for each facility. Data are reviewed monthly and compared with other benchmarks from Department of Health data [26,27]. To ascertain dates of death, and underlying and contributing causes of death for patients who visited the ED during the study period, SPARCS ED data were linked to NYS and New York City vital statistics records by a SPARCS analyst. Data were linked based on the patient’s date of birth, residential address, first two characters of the patient’s first name, the first and last two characters of the patient’s last name, and the last four digits of the patient’s social security number. PDO mortality within one year of the most recent ED visit within the study period 2006–2010 was evaluated. The study protocol was reviewed and approved by the Institutional Review Board of Columbia University Medical Center (IRB-AAAK6304 New York, NY).
ED utilization
ED utilization (1, 2, 3, and ≥4 visits) in last year of follow-up was calculated with respect to date of death for cases. The choice of these time frames was based on research by the Agency for Healthcare Research and Quality, which proposed two ED visits or more in 3 days, three ED visits or more in 90 days, and four ED visits or more in a year as metrics to track suboptimal quality of care [28].
Model evaluation for which ED utilization time frame best fit the data was based on Akaike information criterion statistics [29]. Time-varying 365-day ED utilization was operationalized using three separate indicator variables: two, three, or four or more ED visits in a 365-day time window. When calculating these ED utilization measures, admissions for patients who were transferred on the day of discharge to other acute care hospitals, including patients admitted to hospital specialty units, inpatient rehabilitation facilities, skilled nursing facilities, and long-term care hospitals, were collapsed and treated as one continuous admission. If a patient died at the ED, this visit was not counted. Same-day ED revisits were included in the analyses.
Other risk markers
Other risk markers that were evaluated included age, sex, and race (white, black, Asian/Pacific Islander/Native American, and other). To help improve model fit, a discharge against medical advice (AMA) at the initial ED visit and several other diagnoses were examined as potential risk markers for drug overdose death given that discharges AMA are associated with drug abuse [30]. Previous studies have noted a rise in nontraumatic dental condition-related ED visits that may result in an opioid analgesic prescription (Online Appendix A) [31–33]. Studies of drug overdose have noted that substance use disorders, mental health disorders, and chronic pain conditions are associated with drug overdose [34–42]. Accordingly, these conditions were evaluated as potential risk markers of PDO death. In accordance with the State Personal Privacy Protection Law, individual characteristics involving less than six patients may not be reported.
Outcome measurements
Classification codes from the International Classification of Diseases and Related Health Problems, 10th Revision were used to classify PDO deaths. Unintentional and undetermined intent overdose deaths (X40–X44, Y10–Y14), where there was also an indication that a prescription drug was a contributing cause of death (T40.2, T40.3, T40.4 [opioid analgesics], T40.6 [Other and unspecified narcotics], T42.2 [Succinimides and oxazolidinediones], T42.3 [Barbiturates], T42.4 [Benzodiazepines], T43.0 [Tricyclic and tetracyclic antidepressants], T43.1 [Monoamine-oxidase-inhibitor antidepressants], T43.2 [other and unspecified antidepressants], T50.9 [other and unspecified drugs]), were evaluated as the primary outcome of interest [43,44].
Study design
A nested case-control study design was used. ED patients who subsequently died of PDO during the follow-up were considered cases. Using incidence density sampling, one control was randomly selected from the same base population from which the cases arose and was matched to the corresponding case on follow-up time [45]. The first visit date in the study period was considered the entry visit. The end of follow-up for cases was the date when PDO death occurred. For controls, the end of follow-up time corresponded to elapsed time from study entry to the time when death occurred for the matched case. To be eligible to be a control the individual must be alive when the case occurred. All case patients were able to be matched with control patients. Incidence density sampling was used because ED utilization is time-varying. Time-dependent covariates may be accurately assessed in nested case-control study by using incidence density-based sampling to create risk sets [45].
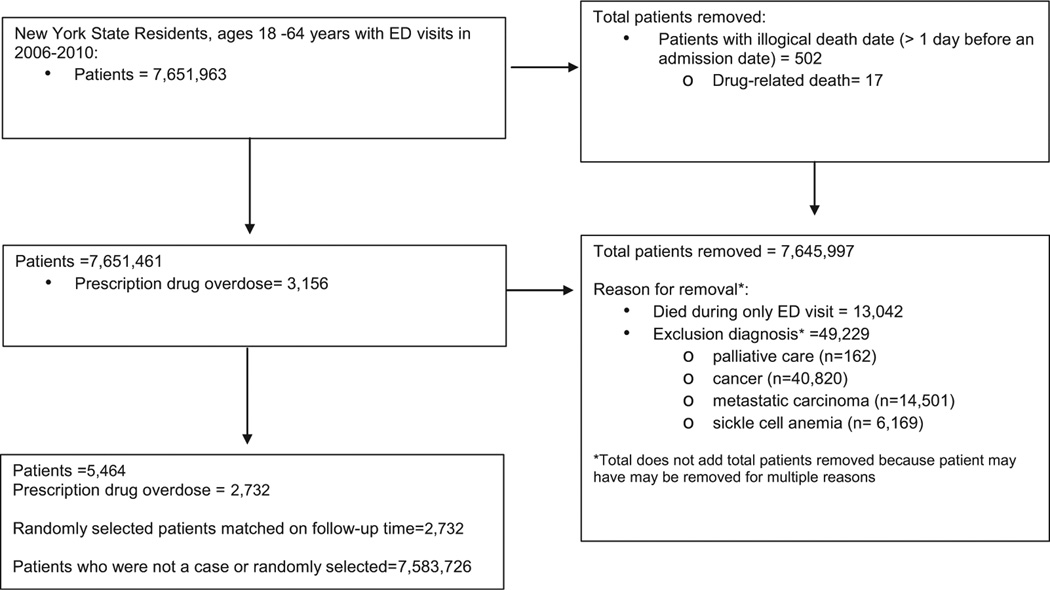
The cohort from which the cases arose included patients who were NYS residents, were 18–64 years of age, and who visited the ED in NYS from 2006–2010 (Fig. 1). Patients with any discharge diagnoses indicating palliative care, cancer, metastatic carcinoma, or sickle cell anemia at any ED visit were excluded from the cohort because they may have different ED utilization patterns and may receive higher doses of opioids than other patients. Patients without a previous ED visit who died during the index ED visit were excluded from the cohort because there was no pattern of prior ED visits to predict the study outcome. Of the remaining patients who visited the ED, all individuals who subsequently died of a PDO death were used as cases.
Fig. 1.
Flowchart of ED data study population, New York SPARCS, New York State, 2006–2010.
Statistical analysis
To better understand the attributes of the individuals included in this study, the frequency distribution of patient characteristics at the initial ED visit from 2006–2010 who subsequently died of a PDO was compared with those of patients who did not. Differences between groups were compared using χ2 tests. A P-value of .05 or less was considered statistically significant. Baseline characteristics were examined in unadjusted models to estimate the odds ratio (OR) and 95% confidence interval (CI) for PDO death. Conditional logistic regression was used to estimate ORs [46,47]. Because this study is based on the nested case-control design, the measure of association is the OR. With incidence density sampling, the OR approximates the incidence rate ratio [48]. To prevent over-adjustment in multivariable models, demographic characteristics (age, sex, and composite independent variables of diagnoses at study entry) were created and included in the final multivariable model. Four binary composite independent variables of diagnoses at entry were created, including presence or absence of the following: (1) Diagnosis of alcohol abuse, alcohol drug seeking, drug dependence, opioid abuse, other drug abuse, nonfatal opioid overdose, or sedative anxiolytic abuse at the initial ED visit; (2) Diagnosis of anxiety disorder, depression, episodic mood disorder, major depressive disorder, personality disorder, post-traumatic stress disorder, psychosis or agitation, or schizophrenia at their initial ED visit; (3) Diagnosis of back pain, fibromyalgia, or musculoskeletal pain at their initial ED visit; (4) Diagnosis of general symptoms, soft tissue disorders, nontraumatic dental conditions, or vertebrae disorders at their initial ED visit. The multivariable models included the following independent variables: time independent age at study entry, sex, race, AMA status, composite independent variables of diagnoses at study entry, and time-varying ED utilization. Analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
A total of 2732 PDO deaths were recorded during the follow-up and were used as cases in this study (Fig. 1). Characteristics of cases and controls at study entry showed that patients who subsequently died of a PDO were disproportionately 35–54 years of age (59.1% vs. 42.2%, χ2 = 170.5, P < .0001), male (65.2% vs. 44.2%, χ2 = 243.3, P <.0001), and identified as white race (74.4% vs. 51.6%, χ2 = 319.3, P <.0001) compared with patients that did not die of PDO (Table 1). Additionally, patients who subsequently died of a PDO were more likely to be discharged AMA at the initial visit (3.9% vs. 2.1%, χ2 = 15.2, P < .0001, Table 1) than those that did not die. In unadjusted models, patients who were discharged AMA at the initial ED visit were more likely to subsequently die of PDO (Table 2) than patients were not discharged AMA. Having a psychiatric disorder diagnosis, or any substance abuse, or dependence diagnosis was also associated with increased risk of PDO death (Table 2).
Table 1.
ED utilization and demographic and clinical characteristics by case-control status, SPARCS emergency department data, New York State, 2006–2010
| Characteristics* | Cases | Controls | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| ED visits in the last year of follow-up | ||||
| ≤1 | 1388 | 50.8 | 2312 | 84.6 |
| 2 | 464 | 17.0 | 244 | 8.9 |
| 3 | 278 | 10.2 | 81 | 3.0 |
| ≥4 | 602 | 22.0 | 95 | 3.5 |
| Demographics | ||||
| Age† (y) | ||||
| 18–24 | 351 | 12.9 | 592 | 21.7 |
| 25–34 | 541 | 19.8 | 655 | 24.0 |
| 35–44 | 796 | 29.1 | 596 | 21.8 |
| 45–54 | 818 | 29.9 | 558 | 20.4 |
| 55–64 | 226 | 8.3 | 331 | 12.1 |
| Sex† | ||||
| Female | 951 | 34.8 | 1525 | 55.8 |
| Male | 1781 | 65.2 | 1207 | 44.2 |
| Race† | ||||
| White | 2033 | 74.4 | 1410 | 51.6 |
| Black or African American | 313 | 11.5 | 679 | 24.9 |
| Native American/Asian/Pacific Islander | 26 | 1.0 | 76 | 2.8 |
| Other or unknown | 360 | 13.2 | 567 | 20.8 |
| Clinical characteristics | ||||
| Discharged AMA† | ||||
| Yes | 106 | 3.9 | 57 | 2.1 |
| No | 2626 | 96.1 | 2675 | 97.9 |
| Psychiatric diagnosis | ||||
| Anxiety disorder† | ||||
| Yes | 125 | 4.6 | 51 | 1.9 |
| No | 2607 | 95.4 | 2681 | 98.1 |
| Depression† | ||||
| Yes | 125 | 4.6 | 35 | 1.3 |
| No | 2607 | 95.4 | 2697 | 98.7 |
| Episodic mood disorder† | ||||
| Yes | 100 | 3.7 | 25 | 0.9 |
| No | 2632 | 96.3 | 2707 | 99.1 |
| Major depressive disorder | ||||
| Yes | 18 | 0.7 | 11 | 0.4 |
| No | 2714 | 99.3 | 2721 | 99.6 |
| Personality disorder† | ||||
| Yes | 107 | 3.9 | 26 | 1.0 |
| No | 2625 | 96.1 | 2706 | 99.1 |
| Psychosis or agitation† | ||||
| Yes | 95 | 3.5 | 12 | 0.4 |
| No | 2637 | 96.5 | 2720 | 99.6 |
| Schizophrenia† | ||||
| Yes | 42 | 1.5 | 7 | 0.3 |
| No | 2690 | 98.5 | 2725 | 99.7 |
| Substance use diagnosis | ||||
| Alcohol abuse† | ||||
| Yes | 215 | 7.9 | 68 | 2.5 |
| No | 2517 | 92.1 | 2664 | 97.5 |
| Alcohol drug seeking† | ||||
| Yes | 486 | 17.8 | 97 | 3.6 |
| No | 2246 | 82.2 | 2635 | 96.4 |
| Drug dependence† | ||||
| Yes | 125 | 4.6 | 13 | 0.5 |
| No | 2607 | 95.4 | 2719 | 99.5 |
| Opioid abuse† | ||||
| Yes | 48 | 1.8 | 7 | 0.3 |
| No | 2684 | 98.2 | 2725 | 99.7 |
| Other drug abuse† | ||||
| Yes | 137 | 5.0 | 12 | 0.4 |
| No | 2595 | 95.0 | 2720 | 99.6 |
| Tobacco use disorder† | ||||
| Yes | 104 | 3.8 | 69 | 2.5 |
| No | 2628 | 96.2 | 2663 | 97.5 |
| Pain-related diagnosis | ||||
| Arthritis | ||||
| Yes | 99 | 3.6 | 88 | 3.2 |
| No | 2633 | 96.4 | 2644 | 96.8 |
| Back pain† | ||||
| Yes | 244 | 8.9 | 134 | 4.9 |
| No | 2488 | 91.1 | 2598 | 95.1 |
| Fibromyalgia | ||||
| Yes | 30 | 1.1 | 26 | 1.0 |
| No | 2702 | 98.9 | 2706 | 99.1 |
| Headaches | ||||
| Yes | 73 | 2.7 | 92 | 3.4 |
| No | 2659 | 97.3 | 2640 | 96.6 |
| Joint disorder | ||||
| Yes | 78 | 2.9 | 69 | 2.5 |
| No | 2654 | 97.1 | 2663 | 97.5 |
| Musculoskeletal pain | ||||
| Yes | 306 | 11.2 | 301 | 11.0 |
| No | 2426 | 88.8 | 2431 | 89.0 |
| Neuropathy | ||||
| Yes | 8 | 0.3 | 10 | 0.4 |
| No | 2724 | 99.7 | 2722 | 99.6 |
| Visceral pain† | ||||
| Yes | 24 | 0.9 | 66 | 2.4 |
| No | 2708 | 99.1 | 2666 | 97.6 |
| Other conditions | ||||
| Abdominal symptoms† | ||||
| Yes | 132 | 4.8 | 170 | 6.2 |
| No | 2600 | 95.2 | 2562 | 93.8 |
| General symptoms† | ||||
| Yes | 189 | 6.9 | 146 | 5.3 |
| No | 2543 | 93.1 | 2586 | 94.7 |
| Nontraumatic dental | ||||
| Yes | 65 | 2.4 | 65 | 2.4 |
| No | 2667 | 97.6 | 2667 | 97.6 |
| Respiratory symptoms† | ||||
| Yes | 135 | 4.9 | 204 | 7.5 |
| No | 2597 | 95.1 | 2528 | 92.5 |
| Soft tissue disorder | ||||
| Yes | 94 | 3.4 | 79 | 2.9 |
| No | 2638 | 96.6 | 2653 | 97.1 |
Data for prior nonfatal opioid overdose, sedative abuse, post-traumatic stress disorder, central sleep apnea, vertebrae disorders not shown because cell counts less than 6.
P < 0.05.
Table 2.
Unadjusted ORs and 95% CIs for risk of PDO death by ED utilization and demographic and clinical characteristics from nested case-control analysis, SPARCS ED data, New York State, 2006–2010
| Characteristics | OR (95% CI) |
|---|---|
| ED visits in the past year | |
| ≤1 | |
| 2 | 3.2 (2.7–3.7) |
| 3 | 5.7 (4.4–7.4) |
| ≥4 | 10.6 (8.4–13.2) |
| Demographics | |
| Age (y) | |
| 18–24 | 0.63 (0.60–0.67) |
| 25–34 | 1.00 |
| 35–44 | 2.26 (2.15–2.37) |
| 45–54 | 1.74 (1.65–1.83) |
| 55–64 | 0.69 (0.65–0.74) |
| Sex | |
| Female | 1.00 |
| Male | 2.01 (1.94–2.08) |
| Race | |
| White | 1.00 |
| Black or African American | 0.29 (0.27–0.30) |
| Native American/Asian/Pacific Islander | 0.27 (0.23–0.31) |
| Other or unknown | 0.47 (0.45–0.50) |
| Clinical characteristics | |
| Discharged AMA | 2.89 (2.74–3.04) |
| Psychiatric diagnoses | |
| Anxiety disorder | 4.16 (3.72–4.64) |
| Depression | 2.82 (2.54–3.14) |
| Episodic mood disorder | 4.27 (3.70–4.91) |
| Major depressive disorder | 1.60 (1.26–2.04) |
| Personality disorder | 4.76 (2.8–8.04) |
| Post-traumatic stress disorder | 2.91 (1.81–4.67) |
| Psychosis or agitation | 7.25 (5.99–8.78) |
| Schizophrenia | 5.18 (4.07–6.59) |
| Substance use diagnoses | |
| Alcohol abuse | 3.23 (2.96–3.51) |
| Alcohol drug seeking | 5.03 (4.68–5.39) |
| Drug dependence | 8.63 (7.16–10.41) |
| Opioid abuse | 13.71 (10.13–18.55) |
| Other drug abuse | 6.81 (5.86–7.93) |
| Prior nonfatal opioid overdose | 13.40 (7.93–22.64) |
| Sedative anxiolytic abuse | 8.57 (5.02–14.61) |
| Tobacco use disorder | 1.46 (1.33–1.61) |
| Pain-related diagnoses | |
| Arthritis | 1.02 (0.94–1.12) |
| Back pain | 2.20 (2.06–2.35) |
| Fibromyalgia | 1.34 (1.10–1.62) |
| Headaches | 0.98 (0.89–1.07) |
| Joint disorder | 1.09 (0.99–1.22) |
| Musculoskeletal pain | 1.15 (1.09–1.21) |
| Neuropathy | 0.64 (0.48–0.86) |
| Visceral pain | 0.39 (0.33–0.45) |
| Other conditions | |
| Abdominal symptoms | 0.89 (0.83–0.95) |
| Central sleep apnea | 0.18 (0.05–0.60) |
| General symptoms | 1.09 (1.02–1.17) |
| Nontraumatic dental | 1.13 (1.02–1.26) |
| Respiratory symptoms | 0.72 (0.67–0.77) |
| Soft tissue disorder | 1.38 (1.25–1.51) |
| Vertebrae disorders | 2.22 (1.78–2.78) |
Multivariable models adjusted for age, race, and sex show that drug, or alcohol abuse, or dependence diagnoses at an initial ED visit were associated with five times greater risk of PDO death (adjusted odds ratio [aOR]: 5.40, 95% CI: 4.84–6.03, Table 3). Having a psychiatric disorder diagnosis at the initial ED visit was associated with a three times higher risk of subsequent PDO death compared with patients without a psychiatric disorder diagnoses at the initial ED visit (aOR: 3.71, 95% CI: 3.23–4.14). Having a pain-related diagnosis at the initial ED visit was associated with a 73% higher risk of PDO death (aOR: 1.88, 95% CI: 1.60–1.86). Other diagnoses, including general symptoms, soft tissue, nontraumatic dental, or vertebrae disorders at an initial ED visit were associated with 40% higher risk of PDO death (aOR 1.38, 95% CI 1.28–1.63). A discharge AMA at the initial visit was also associated with subsequent PDO death (aOR: 1.38, 95% CI: 1.16–1.58).
Table 3.
Adjusted ORs and 95% CIs for risk of PDO death by ED utilization, demographic and clinical characteristics, SPARCS ED data, New York State, 2006–2010
| Characteristic | OR (95% CI) |
|---|---|
| ED visits in the past year | |
| 2 | 4.90 (4.50–5.34) |
| 3 | 16.61 (14.72–18.75) |
| ≥4 | 48.24 (43.23–53.83) |
| Age, y | |
| 18–24 | 0.49 (0.44–0.54) |
| 35–44 | 1.86 (1.71–2.01) |
| 45–54 | 1.74 (1.60–1.90) |
| 55–64 | 0.82 (0.74–0.92) |
| Race | |
| Black or African American | 0.27 (0.25–0.29) |
| Native American/Asian/Pacific Islander | 0.27 (0.21–0.34) |
| Other or Unknown | 0.42 (0.39–0.45) |
| Sex | |
| Male | 2.35 (2.22–2.50) |
| Discharged AMA at initial ED visit | 1.38 (1.16–1.63) |
| Drug or alcohol abuse, or dependence diagnosis | 5.40 (4.84–6.03) |
| Mental health diagnosis | 3.71 (3.23–4.14) |
| Pain-related diagnosis | 1.73 (1.60–1.86) |
| Other diagnosis | 1.38 (1.26–1.50) |
At the end of follow-up, about 50% of the cases and 15% of the controls had two or more visits in the prior year. Relative to one or less visit in the previous year, the aORs of PDO death were 4.90 (95% CI: 4.50–5.34) for those with two visits, 16.61 (95% CI: 14.72–18.75) for those with three visits, and 48.24 (95% CI: 43.23–53.83) for those with four or more visits (Table 3).
Discussion
This study is among one of the first studies to evaluate the relationship between patterns of ED utilization and PDO death, as opposed to the more well-defined literature on “doctor shopping,” the practice of visiting multiple health care providers to obtain controlled substances [49–53]. ED utilization patterns are thought to be related to poorly controlled pain and in some instances substance use [3,54,55]. This study indicates that ED utilization patterns are also important predictors of PDO death.
ED visits are common among people who abuse drugs and alcohol [6,56–58]. An alcohol intervention trial of college students found that 30% of men and 27% women visited the ED at least once in the 2 years of follow-up [6]. Another study of adolescents using ED at the University of Michigan Medical Center found that 35% of past-year self-reported nonmedical prescription opioid users had an ED visit in the prior year, compared with 25% of respondents not reporting past-year nonmedical prescription opioid users [58]. Compared with individuals not reporting past-year nonmedical sedative use, self-reported past-year nonmedical sedative users were more likely to have had an ED visit in the past year (25% vs. 40%) [58].
Additionally, this investigation demonstrates that patients who were discharged AMA were more likely to die of PDO death than patients who were not discharged AMA; previous literature has shown that leaving AMA is associated with substance use [30]. Although it is generally believed that drug-seeking patients have increased ED utilization and that patients discharged AMA are more likely to abuse drugs and alcohol, examination of ED utilization patterns with respect to overdose is scant [30,54,55].
This study confirms prior findings that patients who are male, ages 35–54 years, and are identified as white race are at increased risk for PDO compared with females, other age groups, and races [34,39,40,43,52,59–63], and it mirrors previous research on the association of clinical risk markers for overdose, such as psychiatric comorbidities, drug and alcohol abuse, previous ED visits for opioid overdose and chronic pain conditions [34–36,39,40,64]. Of note, at study entry, chronic pain and other conditions, such as visceral pain and headaches, which previous studies have found to be frequently occurring in patients who overdose [36,55], were more frequently noted for patients who did not subsequently die of PDO.
ED visits may serve as an opportunity to launch a preventive intervention, as to date findings suggest that prescription drug monitoring programs have had limited or mixed effects on reducing drug overdose deaths and opioid or other controlled substance prescribing [65–69]. Risk assessment and stratification can help identify patients most vulnerable to overdose who are using ED services [40,62]. Based on all 2006–2010 SPARCS ED data, about 40% of patients who subsequently die of overdose, who were 18–64 years, and NYS residents had multiple ED encounters (data not shown). About 19% of people who died of PDO did not visit the ED during the study period (data not shown).
These repeated visits represent opportunities to intervene, such as referral to substance abuse treatment, or access to rescue treatments, including take-home naloxone for reversing opioid overdose. A Canadian study found that by monitoring frequent ED users, it was possible to reduce ED visits and improve patient care by using medical and social interventions, such as denying narcotic and benzodiazepine prescriptions, restricting patients to using one pharmacy, providing supportive counseling, and linking patients with community mental health services [70]. Although there is debate regarding the appropriateness of interventions in already overburdened EDs, reports show that individuals with four ED visits or more in a year make up 28% of all ED visits [28], suggesting interventions targeted at high ED utilizers adequate care outside the ED may help alleviate strain on EDs.
Limitations
This study has several notable limitations. First, this study examines whether ED utilization patterns can predict overdose death. Preventive interventions launched in the ED will not directly affect drug user populations who do not use ED services, and only 40% of all overdose victims 18–64 years of age in NYS had multiple ED visits. If patients who died at their only ED visit had been included, effect estimates would have been slightly attenuated. Second, increased health care utilization is a marker of illness and sicker patients who are more likely to receive more pharmacologic treatments and are at increased risk for all-cause mortality. Third, this investigation is based on currently coded diagnoses and not necessarily conditions present in individuals; some conditions may not be recorded in administrative data. It is likely that only the most severe comorbidities of patients are recorded, and that the findings of this study may not be generalizable to patients with less severe disease. Fourth, this study does not evaluate provider prescribing or its appropriateness. Because SPARCS does not collect prescription drug data, it is not possible to determine if patients were prescribed prescription opioids or other drugs at their ED visit. Similarly, other important characteristics cannot be accounted for such as, drugs taken, drug dosages, the setting in which drugs were taken or the mode of drug administration (e.g., oral or intravenous), factors associated with opioid adverse events, and overdose death [39,60,62,71–73]. Fifth, there have been conflicting studies regarding the accuracy of drug overdose death certification and coding [74–77]. If drug overdose deaths are not ascertained and indiscriminately not recorded on death certificates, this study may underestimate effect estimates. Additionally, if a death to a NYS resident occurred out of state, the death would not be captured in this analysis.
Conclusions
ED utilization patterns are a strong predictor of subsequent overdose death. Understanding the timing of overdose death in relation to ED utilization is essential to recognizing which patients to target with overdose prevention interventions. Identifying markers for increased risk is paramount for optimizing the effectiveness of preventive intervention efforts, such as providing high-risk patients, their friends and families with take-home naloxone, or drug treatment referral. Future studies should focus on identifying other health encounter patterns and alternative approaches to aid vulnerable individuals who do not use ED services.
Supplementary Material
Acknowledgment
This work was supported in part by the National Center for Injury Prevention and Control of the Centers for Disease Control and Prevention (Grant 1 R49 CE002096) and the National Institutes of Health (NIAAA: K01 AA021511, Keyes). The contents of the manuscript are solely the responsibility of the authors and do not necessarily reflect the official views of the funding agency.
The authors thank Barbara Lang and Kristin Wong for their editorial and administrative assistance. Data for this manuscript were obtained from the New York Statewide Planning and Research Cooperative System (SPARCS), New York State Department of Health and the New York Department of Vital Statistics, New York State Department of Health. The information contained herein was derived from data provided in part by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene.
References
- 1.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657–659. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration (SAMHSA) Highlights of the 2011 Drug Abuse Warning Network (DAWN) findings on drug-related emergency department visits. Rockville, MD: DSHS Health and Recovery Services Administration and Research and Data Analysis Division; 2013. [PubMed] [Google Scholar]
- 3.Thompson JCP, Dowler D, Mancuso D. Overview of new DSHS drug and alcohol programs, PRR program, and ER initiative—what physicians, hospitals & communities need to know. [Accessed December 8, 2013];2006 Available from: casat.unr.edu/docs/AddictionsInstitute_7_06_2006_DD.ppt. [Google Scholar]
- 4.Mancuso D, Nordlund D, Felver B. Services WSDoSH. Olympia, WA: Research & Data Analysis Division; 2004. Frequent emergency room visits signal substance abuse and mental illness. [Google Scholar]
- 5.Wisniewski AM, Purdy CH, Blondell RD. The epidemiologic association between opioid prescribing, non-medical use, and emergency department visits. J Addict Dis. 2008;27(1):1–11. doi: 10.1300/J069v27n01_01. [DOI] [PubMed] [Google Scholar]
- 6.Mundt MP, Zakletskaia LI. Prevention for college students who suffer alcohol-induced blackouts could deter high-cost emergency department visits. Health Aff (Millwood) 2012;31(4):863–870. doi: 10.1377/hlthaff.2010.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelman EJ, Dinh A, Radulescu R, Lurie B, D’Onofrio G, Tetrault JM, et al. Combining rapid HIV testing and a brief alcohol intervention in young unhealthy drinkers in the emergency department: a pilot study. Am J Drug Alcohol Abuse. 2012;38(6):539–543. doi: 10.3109/00952990.2012.701359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke PJ, O’Sullivan J, Vaughan BL. Adolescent substance use: brief interventions by emergency care providers. Pediatr Emerg Care. 2005;21(11):770–776. doi: 10.1097/01.pec.0000186435.66838.b3. [DOI] [PubMed] [Google Scholar]
- 9.Walton MA, Goldstein AL, Chermack ST, McCammon RJ, Cunningham RM, Barry KL, et al. Brief alcohol intervention in the emergency department: moderators of effectiveness. J Stud Alcohol Drugs. 2008;69(4):550–560. doi: 10.15288/jsad.2008.69.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernstein SL, Bijur P, Cooperman N, Jearld S, Arnsten JH, Moadel A, et al. Efficacy of an emergency department-based multicomponent intervention for smokers with substance use disorders. J Subst Abuse Treat. 2013;44(1):139–142. doi: 10.1016/j.jsat.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy MK, Bijur PE, Rosenbloom D, Bernstein SL, Gallagher EJ. Feasibility of a computer-assisted alcohol SBIRT program in an urban emergency department: patient and research staff perspectives. Addict Sci Clin Pract. 2013;8(1):2. doi: 10.1186/1940-0640-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babor TF, Kadden RM. Screening and interventions for alcohol and drug problems in medical settings: what works? J Trauma. 2005;59(3 Suppl):S80–S87. doi: 10.1097/01.ta.0000174664.88603.21. discussion S94–S100. [DOI] [PubMed] [Google Scholar]
- 13.Woolard R, Baird J, Longabaugh R, Nirenberg T, Lee CS, Mello MJ, et al. Project reduce: reducing alcohol and marijuana misuse: effects of a brief intervention in the emergency department. Addict Behav. 2013;38(3):1732–1739. doi: 10.1016/j.addbeh.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu LT, Swartz MS, Wu Z, Mannelli P, Yang C, Blazer DG. Alcohol and drug use disorders among adults in emergency department settings in the United States. Ann Emerg Med. 2012;60(2):172–180. e5. doi: 10.1016/j.annemergmed.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Onofrio G, Fiellin DA, Pantalon MV, Chawarski MC, Owens PH, Degutis LC, et al. A brief intervention reduces hazardous and harmful drinking in emergency department patients. Ann Emerg Med. 2012;60(2):181–192. doi: 10.1016/j.annemergmed.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad Med. 2013;125(4):115–130. doi: 10.3810/pgm.2013.07.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gugelmann H, Shofer FS, Meisel ZF, Perrone J. Multidisciplinary intervention decreases the use of opioid medication discharge packs from 2 urban EDs. Am J Emerg Med. 2013;31(9):1343–1348. doi: 10.1016/j.ajem.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Sherman SG, Gann DS, Tobin KE, Latkin CA, Welsh C, Bielenson P. “The life they save may be mine”: diffusion of overdose prevention information from a city sponsored programme. Int J Drug Policy. 2009;20(2):137–142. doi: 10.1016/j.drugpo.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Albert S, Brason FW, 2nd, Sanford CK, Dasgupta N, Graham J, Lovette B. Project Lazarus: community-based overdose prevention in rural North Carolina. Pain Med. 2011;12(Suppl 2):S77–S85. doi: 10.1111/j.1526-4637.2011.01128.x. [DOI] [PubMed] [Google Scholar]
- 20.Strang J. Take-home naloxone: the next steps. Addiction. 1999;94(2):207. doi: 10.1046/j.1360-0443.1999.9421993.x. [DOI] [PubMed] [Google Scholar]
- 21.Strang J, Best D, Man L, Noble A, Gossop M. Peer-initiated overdose resuscitation: fellow drug users could be mobilised to implement resuscitation. Int J Drug Policy. 2000;11(6):437–445. doi: 10.1016/s0955-3959(00)00070-0. [DOI] [PubMed] [Google Scholar]
- 22.Dettmer K, Saunders B, Strang J. Take home naloxone and the prevention of deaths from opiate overdose: two pilot schemes. BMJ. 2001;322(7291):895–896. doi: 10.1136/bmj.322.7291.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wermeling DP. A response to the opioid overdose epidemic: naloxone nasal spray. Drug Deliv Transl Res. 2013;3(1):63–74. doi: 10.1007/s13346-012-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agerwala SM, McCance-Katz EF. Integrating screening, brief intervention, and referral to treatment (SBIRT) into clinical practice settings: a brief review. J Psychoactive Drugs. 2012;44(4):307–317. doi: 10.1080/02791072.2012.720169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(SPARCS) SPaRCS. SPARCS overview. [Accessed December 8, 2013];2007 Available from: http://www.health.ny.gov/statistics/sparcs/operations/overview.htm.
- 26.SPARCS Operations. SPARCS frequently asked questions (FAQs) [Accessed December 8, 2013];2013 Available from: http://www.health.ny.gov/statistics/sparcs/faqs/#SP.
- 27.Statewide Planning and Research Cooperative System (SPARCS) Data quality initiatives. [Accessed January 12, 2014];2005 Available from: http://www.health.ny.gov/statistics/sparcs/operations/dataqual.htm.
- 28.Agency for Healthcare Research and Quality (AHRQ) Agency information collection. Fed Regist. 2013;78(529):52925–52927. [Google Scholar]
- 29.Allison PD. Survival analysis using SAS: a practical guide. Cary, NC, USA: SAS Institute Inc.; 2010. [Google Scholar]
- 30.Alfandre DJ. “I’m going home”: discharges against medical advice. Mayo Clin Proc. 2009;84(3):255–260. doi: 10.4065/84.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okunseri C, Okunseri E, Fischer MC, Sadeghi SN, Xiang Q, Szabo A. Nontraumatic dental condition-related visits to emergency departments on weekdays, weekends and night hours: findings from the National Hospital Ambulatory Medical Care survey. Clin Cosmet Investig Dent. 2013;5:69–76. doi: 10.2147/CCIDE.S49191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okunseri C, Okunseri E, Thorpe JM, Xiang Q, Szabo A. Medications prescribed in emergency departments for nontraumatic dental condition visits in the United States. Med Care. 2012;50(6):508–512. doi: 10.1097/MLR.0b013e318245a575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pajewski NM, Okunseri C. Patterns of dental service utilization following nontraumatic dental condition visits to the emergency department in Wisconsin Medicaid. J Public Health Dent. 2014;74(1):34–41. doi: 10.1111/j.1752-7325.2012.00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitmire T, Adams G. Unintentional overdose deaths in the North Carolina Medicaid population: prevalence, prescription drug use, and medical care services. SCHS Studies. 2010;162:1–11. [Google Scholar]
- 35.Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686–691. doi: 10.1001/archinternmed.2011.117. [DOI] [PubMed] [Google Scholar]
- 36.Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940–947. doi: 10.1001/jama.2012.234. [Erratum appears in JAMA. 2012 Jun 20;307(23):2489]. [DOI] [PubMed] [Google Scholar]
- 37.Silva K, Schrager SM, Kecojevic A, Lankenau SE. Factors associated with history of non-fatal overdose among young nonmedical users of prescription drugs. Drug and Alcohol Dependence. 2013;128(1–2):104–110. doi: 10.1016/j.drugalcdep.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartung DM, Middleton L, Haxby DG, Koder M, Ketchum KL, Chou R. Rates of adverse events of long-acting opioids in a state Medicaid program. Ann Pharmacother. 2007;41(6):921–928. doi: 10.1345/aph.1K066. [Erratum appears in Ann Pharmacother. 2007 Sep;41(9):1552]. [DOI] [PubMed] [Google Scholar]
- 39.Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 40.Bohnert AS, Ilgen MA, Ignacio RV, McCarthy JF, Valenstein M, Blow FC. Risk of death from accidental overdose associated with psychiatric and substance use disorders. Am J Psychiatry. 2012;169(1):64–70. doi: 10.1176/appi.ajp.2011.10101476. [Erratum appears in Am J Psychiatry. 2012 Jan;169(1):99]. [DOI] [PubMed] [Google Scholar]
- 41.Havens JR, Young AM, Havens CE. Nonmedical prescription drug use in a nationally representative sample of adolescents: evidence of greater use among rural adolescents. Arch Pediatr Adolesc Med. 2011;165(3):250–255. doi: 10.1001/archpediatrics.2010.217. [DOI] [PubMed] [Google Scholar]
- 42.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487–1492. [PubMed] [Google Scholar]
- 44.Warner M, Chen L. Office of Analysis, Epidemiology. Atlanta, GA: Centers for Disease Control and Prevention; 2013. Drug poisoning mortality: scope of the problem. [Google Scholar]
- 45.Richardson DB. An incidence density sampling program for nested case-control analyses. Occup Environ Med. 2004;61(12):e59. doi: 10.1136/oem.2004.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Essebag V, Platt R, Abrahamowicz M, Pilote L. Comparison of nested case-control and survival analysis methodologies for analysis of time-dependent exposure. BMC Med Res Methodol. 2005;5(1):1–6. doi: 10.1186/1471-2288-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keogh RH, Cox D. Case-control studies. New York: Cambridge University Press; 2014. [Google Scholar]
- 48.Rothman KJ, Greenland S. Modern epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 49.Martyres RF, Clode D, Burns JM. Seeking drugs or seeking help? Escalating “doctor shopping” by young heroin users before fatal overdose. Med J Aust. 2004;180(5):211–214. doi: 10.5694/j.1326-5377.2004.tb05887.x. [DOI] [PubMed] [Google Scholar]
- 50.Peirce GL, Smith MJ, Abate MA, Halverson J. Doctor and pharmacy shopping for controlled substances. Med Care. 2012;50(6):494–500. doi: 10.1097/MLR.0b013e31824ebd81. [DOI] [PubMed] [Google Scholar]
- 51.Nordmann S, Pradel V, Lapeyre-Mestre M, Frauger E, Pauly V, Thirion X, et al. Doctor shopping reveals geographical variations in opioid abuse. Pain physician. 2013;16(1):89–100. [PubMed] [Google Scholar]
- 52.Hall AJ, Logan JE, Toblin RL, Kaplan JA, Kraner JC, Bixler D, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 53.Gunnarsdottir OS, Rafnsson V. Risk of suicide and fatal drug poisoning after discharge from the emergency department: a nested case-control study. Emerg Med J. 2010;27(2):93–96. doi: 10.1136/emj.2008.065060. [DOI] [PubMed] [Google Scholar]
- 54.Liu SW, Nagurney JT, Chang Y, Parry BA, Smulowitz P, Atlas SJ. Frequent ED users: are most visits for mental health, alcohol, and drug-related complaints? Am J Emerg Med. 2013;31(10):1512–1515. doi: 10.1016/j.ajem.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Braden JB, Russo J, Fan M-Y, Edlund MJ, Martin BC, DeVries A, et al. Emergency department visits among recipients of chronic opioid therapy. Arch Intern Med. 2010;170(16):1425–1432. doi: 10.1001/archinternmed.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones AL, Dargan PI. Advances, challenges, and controversies in poisoning. Emerg Med J. 2002;19(3):190–192. doi: 10.1136/emj.19.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marshall BD, Grafstein E, Buxton JA, Qi J, Wood E, Shoveller JA, et al. Frequent methamphetamine injection predicts emergency department utilization among street-involved youth. Public Health. 2012;126(1):47–53. doi: 10.1016/j.puhe.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whiteside LK, Walton MA, Bohnert AS, Blow FC, Bonar EE, Ehrlich P, et al. Nonmedical prescription opioid and sedative use among adolescents in the emergency department. Pediatrics. 2013;132(5):825–832. doi: 10.1542/peds.2013-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cerdá M, Ransome Y, Keyes KM, Koenen KC, Vlahov D, Galea S. Revisiting the role of the urban environment in substance use. The case of analgesic overdose fatalities. Am J Public Health. 2013;103(12):2252–2260. doi: 10.2105/AJPH.2013.301347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lanier WA, Johnson EM, Rolfs RT, Friedrichs MD, Grey TC. Risk factors for prescription opioid-related death, Utah, 2008–2009. Pain Med. 2012;13(12):1580–1589. doi: 10.1111/j.1526-4637.2012.01518.x. [DOI] [PubMed] [Google Scholar]
- 61.Piercefield E, Archer P, Kemp P, Mallonee S. Increase in unintentional medication overdose deaths: Oklahoma, 1994–2006. Am J Prev Med. 2010;39(4):357–363. doi: 10.1016/j.amepre.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 62.Paulozzi LJ, Kilbourne EM, Shah NG, Nolte KB, Desai HA, Landen MG, et al. A history of being prescribed controlled substances and risk of drug overdose death. Pain Med. 2012;13(1):87–95. doi: 10.1111/j.1526-4637.2011.01260.x. [DOI] [PubMed] [Google Scholar]
- 63.Centers for Disease Control and Prevention grand rounds: prescription drug Overdoses—a U.S. epidemic. MMWR. 2012;61:10–13. [PubMed] [Google Scholar]
- 64.Hasegawa K, Brown DF, Tsugawa Y, Camargo CA., Jr Epidemiology of emergency department visits for opioid overdose: a population-based study. Mayo Clin Proc. 2014;89(4):462–471. doi: 10.1016/j.mayocp.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 65.Brady JE, Wunsch H, DiMaggio C, Lang BH, Giglio J, Li G. Prescription drug monitoring and dispensing of prescription opioids. Public Health Rep. 2014;129(2):139–147. doi: 10.1177/003335491412900207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li G, Brady J, Lang BH, Giglio J, Wunsch H, DiMaggio C. Prescription drug monitoring and drug overdose mortality. Inj Epidemiol. 2014;1:9. doi: 10.1186/2197-1714-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 2011;12(5):747–754. doi: 10.1111/j.1526-4637.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- 68.Ringwalt C, Garrettson M, Alexandridis A. The Effects of North Carolina’s Prescription Drug Monitoring Program on the Prescribing Behaviors of the State’s Providers. J Prim Prev. 2015;36(2):131–137. doi: 10.1007/s10935-014-0381-0. [DOI] [PubMed] [Google Scholar]
- 69.Haegerich TM, Paulozzi LJ, Manns BJ, Jones CM. What we know, and don’t know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend. 2014;145:34–47. doi: 10.1016/j.drugalcdep.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pope D, Fernandes CM, Bouthillette F, Etherington J. Frequent users of the emergency department: a program to improve care and reduce visits. CMAJ. 2000;162(7):1017–1020. [PMC free article] [PubMed] [Google Scholar]
- 71.Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73–81. doi: 10.1002/jhm.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lucas CE. The impact of street drugs on trauma care. J Trauma. 2005;59(3 Suppl):S57–S60. doi: 10.1097/01.ta.0000176044.21200.b0. discussion S7–S75. [DOI] [PubMed] [Google Scholar]
- 73.Gerevich J, Bacskai E, Farkas L, Danics Z. A case report: Pavlovian conditioning as a risk factor of heroin ’overdose’ death. Harm Reduct J. 2005;2:11. doi: 10.1186/1477-7517-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Warner M, Paulozzi L, Nolte KB, Davis GG, Nelson LS. State variation in certifying manner of death and drugs involved in drug intoxication deaths. Acad Forensic Pathol. 2013;3(2):231–237. [Google Scholar]
- 75.Landen MG, Castle S, Nolte KB, Gonzales M, Escobedo LG, Chatterjee BF, et al. Methodological issues in the surveillance of poisoning, illicit drug overdose, and heroin overdose deaths in New Mexico. Am J Epidemiol. 2003;157(3):273–278. doi: 10.1093/aje/kwf196. [DOI] [PubMed] [Google Scholar]
- 76.Manini AF, Nelson LS, Olsen D, Vlahov D, Hoffman RS. Medical examiner and medical toxicologist agreement on cause of death. Forensic Sci Int. 2011;206(1–3):71–76. doi: 10.1016/j.forsciint.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ossiander EM. Using textual cause-of-death data to study drug poisoning deaths. Am J Epidemiol. 2014;179(7):884–894. doi: 10.1093/aje/kwt333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.