Abstract
Flow-induced K+ secretion in the aldosterone-sensitive distal nephron is mediated by high-conductance Ca2+-activated K+ (BK) channels. Familial hyperkalemic hypertension (pseudohypoaldosteronism type II) is an inherited form of hypertension with decreased K+ secretion and increased Na+ reabsorption. This disorder is linked to mutations in genes encoding with-no-lysine kinase 1 (WNK1), WNK4, and Kelch-like 3/Cullin 3, two components of an E3 ubiquitin ligase complex that degrades WNKs. We examined whether the full-length (or “long”) form of WNK1 (L-WNK1) affected the expression of BK α-subunits in HEK cells. Overexpression of L-WNK1 promoted a significant increase in BK α-subunit whole cell abundance and functional channel expression. BK α-subunit abundance also increased with coexpression of a kinase dead L-WNK1 mutant (K233M) and with kidney-specific WNK1 (KS-WNK1), suggesting that the catalytic activity of L-WNK1 was not required to increase BK expression. We examined whether dietary K+ intake affected L-WNK1 expression in the aldosterone-sensitive distal nephron. We found a paucity of L-WNK1 labeling in cortical collecting ducts (CCDs) from rabbits on a low-K+ diet but observed robust staining for L-WNK1 primarily in intercalated cells when rabbits were fed a high-K+ diet. Our results and previous findings suggest that L-WNK1 exerts different effects on renal K+ secretory channels, inhibiting renal outer medullary K+ channels and activating BK channels. A high-K+ diet induced an increase in L-WNK1 expression selectively in intercalated cells and may contribute to enhanced BK channel expression and K+ secretion in CCDs.
Keywords: high-conductance calcium-activated potassium channels, potassium adaptation, kidney-specific with-no-lysine kinase 1, pseudohypoaldosteronism, with-no-lysine kinase 1, long with-no-lysine kinase 1
total body k+ content depends on the balance between K+ intake and excretion, the latter regulated primarily by renal K+ secretion in the aldosterone-sensitive distal nephron (ASDN) (16, 22–24, 35, 52, 54, 73). High tubular flow rates, the consumption of diets with high-K+ content, and elevated aldosterone levels stimulate K+ secretion in the ASDN (16, 24, 38, 53, 73). K+ secretion within this segment is mediated by two distinct K+-permeable channels: 1) renal outer medullary K+ channel (ROMK), a low-conductance ATP-sensitive K+ channel (4, 19, 21, 25, 30, 76, 94, 103, 107), and 2) BK, a high-conductance K+ channel that is activated by membrane depolarization, increases in intracellular Ca2+ concentration, hypoosmotic stress, and membrane stretch (19, 20, 34, 44, 62, 63, 77, 79, 82, 83, 87). BK channels are formed by pore-forming α-subunits and, in most cells, regulatory β-subunits (2, 14, 40, 50). ROMK channels are thought to mediate basal K+ secretion, whereas BK channels mediate flow-induced K+ secretion (FIKS), which is Ca2+ dependent and selectively blocked by iberiotoxin (IBTX) in rabbit (74, 99).
The ASDN is comprised of the distal convoluted tubule (DCT), connecting tubule (CNT), and cortical collecting duct (CCD). The last two segments contain two major cell types, principal cells (PCs) that mediate Na+ absorption via epithelial Na+ channel (ENaC) and K+ secretion via ROMK and intercalated cells (ICs) that secrete H+ via an apical vacuolar H+-ATPase (α-cells) or HCO3− via apical pendrin (β-cells). Intercalated cells can also reabsorb K+ via an apical H+-K+-ATPase and reabsorb Na+ via an Na+-dependent Cl−/HCO3− exchanger that operates in tandem with pendrin (10, 43, 51, 75, 81). Non-A non-B ICs express both apical H+-ATPase and pendrin (15, 39). Whereas ENaC and ROMK channels are restricted to PCs in the ASDN, apical BK channels are functionally expressed in both PCs and ICs (44, 62, 63, 77).
The identity of the specific cells in the ASDN responsible for BK-mediated K+ secretion has yet to be determined. Whereas PCs possess robust basolateral Na+-K+-ATPase activity (3, 8, 63, 71, 78), the density of conducting and immunoreactive apical BK channels in these cells is low compared with ICs (17, 27, 44, 59, 62, 63, 68, 77, 89, 100), suggesting that PCs might not be responsible for FIKS. In contrast to PCs, functional and immunoreactive BK channels are abundant in the apical membrane of ICs (17, 27, 44, 59, 62, 63, 68, 77, 100). These cells presumably sustain FIKS via basolateral Na+-K+-Cl− cotransporter-mediated K+ uptake (46), since they possess little basolateral Na+-K+-ATPase activity (3, 8, 63, 71, 78).
In animals fed a high-K+ diet, net K+ secretion in the ASDN is markedly enhanced, along with an increase in ROMK expression in PCs and BK expression in ICs (44, 59, 64, 94). Some of the factors that translate a high-K+ diet to an increase in these secretory K+ channels are known. For example, aldosterone likely has a role in increasing ROMK expression in this setting (92, 105). However, it is still unclear whether aldosterone, as well as other factors, have roles in translating an increase in dietary K+ to an increase in BK expression in ICs (12, 17, 97).
With-no-lysine (WNK) kinases are a group of serine/threonine kinases that regulate the expression of ion channels, transporters, and tight junction-associated proteins in the ASDN (1). Mutations in WNK1, WNK4, or their cognate E3 ubiquitin ligase complex Kelch-like 3/Cullin 3 cause familial hyperkalemic hypertension (FHHt; also referred to as pseudohyopaldosteronism type II) (5, 49, 98). FHHt is a hereditary disease characterized by hypertension, hyperkalemia, and hyperchloremic metabolic acidosis with normal or slightly elevated aldosterone levels (65, 98). A growing body of evidence implicates WNK kinases in differentiating between the state of volume depletion, where the aldosterone-sensitive distal nephron must enhance the reabsorption of both Na+ and Cl−, from that of hyperkalemia, where the primary need is to enhance renal K+ secretion, facilitated by Na+ absorption via ENaC. Both the full-length (or long) form of WNK1 (L-WNK1) and WNK4 activate NaCl cotransporters (NCC) in the DCT. FHHt-associated mutations in genes encoding these kinases increase their expression, further enhancing NCC activation (9, 29, 32, 37, 41, 69, 84, 90, 98, 102, 104). Because FHHt is associated with hyperkalemia and impaired renal K+ secretion, it is not surprising that both L-WNK1 and WNK4 inhibit the expression of ROMK channels (11, 28, 37, 42, 69, 91). While WNK4 also inhibits the expression of BK channels (95, 106, 108), the effects of L-WNK1 on BK channels have only recently begun to be addressed (48).
We examined the effects of L-WNK1 on BK channel expression. In contrast to the inhibitory effect of WNK4 on BK channel expression, we found that L-WNK1 increased BK α-subunit whole cell and BK functional expression in HEK cells. The WNK1-dependent increase in BK whole cell expression was modestly blunted if a L-WNK1 mutant bearing a substitution in the catalytic domain was coexpressed with BK and was not altered if BK was coexpressed with a kidney-specific WNK1 splice variant (KS-WNK1) lacking the WNK1 NH2-terminus and catalytic domain.
Our results and previous observations suggest that L-WNK1 has different effects on the two key secretory K+ channels in the ASDN, whose expression is increased in the setting of a high-K+ diet (11, 28, 42, 48, 91). Because the expression of BK channels appears to be selectively increased in ICs in the setting of a high-K+ diet (44, 59), we examined whether expression of L-WNK1 is also increased in ICs in this setting. We found that a high-K+ diet promotes a selective increase in the expression of L-WNK1 in ICs. In summary, our studies are consistent with a role of L-WNK1 in the regulation of K+ secretory channels by dietary K+ intake in a cell-specific manner in the ASDN.
MATERIALS AND METHODS
Molecular biology.
The mouse BK α-subunit encoding an NH2-terminal Myc-tagged QEERL isoform of SloI was cloned in a bicistronic vector (pIRES-hrGFP II; Clontech). Hemagglutinin (HA)-tagged L-WNK1 (101) and KS-WNK1 (85) were cloned in pcDNA 3.1 (Invitrogen). L-WNK1 corresponds to the original rat isoform lacking exons 11–12 cloned and characterized by Xu et al. (101). Site-directed mutagenesis was performed with QuickChange XL (Agilent Technologies) according to the manufacturer's instructions. Mutations were verified by direct sequencing.
Cell culture and transient transfection.
HEK293-H cells were cultured with 5% CO2 at 37°C in DMEM supplemented with 10% fetal calf serum, 1% penicillin/streptomycin, and 1% minimal essential medium nonessential amino acids. For patch-clamp, HEK293-H cells were seeded on 8-mm-diameter round glass cover slips coated with poly-l-lysine. HEK293-T L-WNK1 knockout (KO) cells were grown as previously described (70). Transient transfections were performed using 293fectin (Invitrogen) according to the manufacturers' recommendations. Cells were transfected with 1.5 μg of BK α-subunit and 1.5 μg of either mouse L-WNK1, KS-WNK1, L-WNK1, and KS-WNK1 (combined), the L-WNK1 K233M mutant, or an empty vector [green fluorescent protein (GFP) alone]. The total amount of plasmid DNA was held constant at 3 μg.
Patch-clamp studies.
Transiently transfected HEK293-H cells grown on glass cover slips were transferred to a chamber mounted on the stage of a Nikon inverted microscope equipped with light-emitting diodes (Thorlabs) for identification of GFP-expressing cells. Voltage-clamp experiments were performed with a PC-505B patch-clamp amplifier (Warner Instruments), as described previously (95). Whole cell recordings from HEK293-H cells were obtained at room temperature with the amphotericin B perforated patch-clamp technique or by mechanical rupture of the cell membrane in the cell-attached mode (conventional whole cell technique). Amphotericin B was added in the patch pipette to a final concentration of 120 μg/ml. The bath solution was composed of (in mM) 138 NaCl, 5 KCl, 0.5 MgCl2, 1.5 CaCl2, 2 EGTA, and 10 HEPES, pH 7.4. The pipette solution was composed of (in mM) 138 KCl, 4 MgCl2, 0.955 CaCl2, 1 EGTA, and 5 HEPES (pH 7.2). For current recordings in transfected cells with the amphotericin B perforated patch-clamp technique, the membrane potential was initially held at −60 mV, and whole cell currents were evoked by 10-mV depolarizing steps from −80 to +100 mV with 200 ms duration. BK currents were defined as the IBTX (Alomone)-sensitive component of the whole cell current. IBTX was used at a final concentration of 100 nM. To examine BK channel Ca2+ and voltage dependence, we generated steady-state conductance-voltage (G–V) relationships using the conventional whole cell technique with a pipette solution containing 10 μM free Ca2+ concentration ([Ca2+]). The pipette solution was composed of (in mM) 138 KCl, 4 MgCl2, 0.982 CaCl2, 1 EGTA, and 5 HEPES (pH 7.2). The amount of CaCl2 necessary to achieve a free [Ca2+] of 10 μM was calculated using Ca-EGTA Calculator software, version 1.2. The membrane potential was initially held at −80 mV, and whole cell currents were evoked by 20-mV depolarizing steps from −100 to +200 mV with 200 ms duration. The voltage of half-maximal activation (V50) was determined by fitting normalized G–V curves to the Boltzmann function G/Gmax = 1/[1 + e−(V −V50)QF/RT], where G is the chord conductance at the command potential (V) assuming an K+ equilibrium potential (EK) of −85 mV, Gmax is the maximal conductance, Q is the equivalent gating charge (slope of the G–V relationship or “voltage dependence”), and F, R, and T have their usual meanings. Currents were low-pass filtered at 1 KHz (4-pole Bessel filter) and digitized with a Digidata 1440A interface at 5 kHz (Molecular Devices). Command protocols and data acquisition were controlled by pClamp 10 (Molecular Devices). Capacitance of the cell membrane was measured using the cell test in pClamp 10. The whole cell capacitance was then compensated with the amplifier.
BK and WNK1 whole cell expression.
HEK293-H cells or HEK293-T L-WNK1 KO cells were plated at 50% confluency on plastic six-well plates (Corning) the day before transfection. Three days after transfection, cells were washed four times with ice-cold PBS for 5 min. To extract proteins, six-well plates containing transfected cells were incubated for 20 min at room temperature on a rotating shaker with 250 μl of detergent buffer [50 mM Tris·HCl, 4 mg/ml deoxycholate, 1% Nonidet P-40, Protease Inhibitor Cocktail Set III (EMD Bioscience), pH 8]. Cell debris was removed by centrifugation at 20,800 g for 10 min at 4°C. Supernatants were recovered and saved for whole cell immunoblotting. Total protein concentration before Western blot analysis was measured using the BCA protein assay (Pierce). To assess whole cell BK α-subunit, L-WNK1, KS-WNK1 or actin expression, cell lysates were diluted in Laemmli sample buffer supplement with 0.277 M SDS, 1.420 M β-mercaptoethanol, and 0.050 M dithiothreitol (DTT). Equal amounts of protein were loaded on SDS-PAGE for separation based on molecular weight. Proteins were transferred to nitrocellulose membranes and subjected to immunoblotting with an anti-myc antibody (Cell Signaling) at a 1:1,000 dilution (to detect BK α-subunit), an anti-HA antibody (Covance) at a 1:2,000 dilution (to detect L-WNK1 or KS-WNK1), a mouse anti-actin antibody (Sigma-Aldrich) at a 1:20,000 dilution, or a rabbit monoclonal anti-GAPDH antibody (Cell Signaling) at a 1:1,000 dilution, followed by a goat anti-mouse or goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (Jackson ImmunoResearch) at a 1:5,000 dilution. Bands were visualized using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer) and quantified with ImageJ (National Institutes of Health).
Analysis of L-WNK1 expression in KO cells.
HEK293-H and L-WNK1 KO HEK293-T cells (70) were plated on plastic six-well Costar clusters. One day after plating, cells in six-well plates were washed two times with ice-cold PBS and then scraped on ice-cold PBS. Cell suspensions were centrifuged at 2,460 g for 5 min at 4°C, and the supernatants were discarded. Pellets containing the cells were resuspended in 100 μl of detergent buffer [50 mM Tris·HCl, 0.4% deoxycholate, 1% Nonidet P-40, Protease Inhibitor Cocktail III (Roche), 1 mM phenylmethylsulfonyl fluoride, and 10 μg/ml pepstatin, pH 8] and placed on ice for 20 min. Cell debris was removed by centrifugation at 20,800 g for 10 min at 4°C. The supernatant was recovered and saved for whole cell immunoblotting. To assess whole cell L-WNK1 expression, cell lysates were diluted in Laemmli sample buffer supplemented with (in M) 0.277 SDS, 1.420 β-mercaptoethanol, and 0.050 DTT. Samples were subjected to SDS-PAGE and immunoblotting with an anti-L-WNK1 antibody (Sigma-Aldrich) at a 1:1,000 dilution and a goat anti-rabbit secondary antibody at a 1:5,000 dilution. Bands were visualized using Western Lightning Chemiluminescence Reagent Plus.
Immunofluorescence analysis of perfused CCDs.
Adult (>6 wk) female New Zealand White rabbits obtained from Covance (Denver, PA) were housed in the Center for Comparative Medicine at the Icahn School of Medicine at Mount Sinai. All animals were allowed free access to water and chow. Animals were killed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Animal protocols were approved by the Institutional Animal Care and Use Committee at the Icahn School of Medicine at Mount Sinai. Single CCDs isolated from New Zealand White rabbits, fed a high (1.56%)- or a low (0.13%)-K+ diet for 10 days, were transferred to a specimen chamber, mounted on concentric glass pipettes for in vitro microperfusion, and bathed with Burg's solution containing (in mM) 120 NaCl, 25 NaHCO3, 2.5 K2HPO4, 2.0 CaCl2, 1.2 MgSO4, 4.0 sodium lactate, 1.0 Na3 citrate, 6.0 l-alanine, and 5.5 d-glucose, pH 7.4, 290 ± 2 mosmol/kgH2O at 37°C for a 45-min equilibration period (99). Tubules were then fixed on the perfusion rig with 2.5% paraformaldehyde added to the bathing solution for 1 h at room temperature. Tubules were permeabilized with 0.3% Triton X-100 in PBS, blocked with PBS containing 0.1% glycine and 1% bovine serum albumin (BSA) and subsequently with PBS supplemented with 1% BSA and 10% normal goat serum. After incubation by luminal perfusion with the primary antibody [IgG rabbit anti-L-WNK1, NH2-terminal (Sigma Aldrich), 1:100 dilution] for 2 h and secondary antibody [Alexa488-conjugated goat anti-rabbit IgG Ab (Molecular Probes), 1:500 dilution] for 1 h, the tubules were mounted on a glass slide with Vectashield mounting medium with DAPI for visualization by confocal microscopy. Thereafter, some tubules from high-K+ rabbits were additionally perfused with rhodamine-conjugated peanut agglutinin (PNA; 5 μg/ml in PBS, 30 min). To confirm the specificity of the anti-L-WNK1 antibody in the rabbit CCD, some tubules isolated from high-K+-fed rabbits were immunoperfused with the anti-WNK1 antibody (1:100) that was preincubated with 3 μg/ml of a human WNK1 protein fragment (ab132905; Abcam), which includes the epitope for the primary antibody, for 2 h at room temperature. CCDs were visualized by confocal microscopy using a ×63 oil immersion plan-Apochromat objective.
Quantitation of L-WNK1 signal intensity.
L-WNK1 signal intensity was determined using LAS AF Lite software from Leica Microsystems. Stacks of confocal sections were collected from immunoperfused CCDs isolated from high-K+-fed rabbits with a ×63 oil immersion plan-Apochromat objective using a laser-scanning Leica SP5 DM at the following confocal settings: pinhole = 80.0 μm, pinhole (airy) = 0.84, step size = 1.01 μm. Randomly identified single PNA-positive (PNA+) and -negative (PNA−) cells in the wall of the tubule were outlined using the freehand tool in the software to define the whole cell and apical-subapical areas. Regions of the tubular lumen with no visual signal were selected for background correction. The mean value of the gray scale (pixel sum/pixel count) for the single channel corresponding to the WNK1 signal was obtained from five stacks. A total of 20 whole cells (PNA+, PNA−, and background areas) and 29 apical-subapical regions (PNA+, PNA−, and background areas) were analyzed in four CCDs isolated from high-K+-fed rabbits. The background average was calculated and subtracted from the individual intensity values for each region/tubule to calculate an average fluorescent intensity value for each group (PNA+ and PNA−). Individual data points were normalized to the average fluorescent intensity of PNA+ cells. Data are reported as a ratio of mean ± SE fluorescent intensity.
Statistical analyses.
Data are presented as means ± SE (n), where n indicates the number of independent experiments analyzed. For Western blot analysis, n indicates the number of separate transfections performed for each set of experiments. A P value <0.05 was considered statistically significant. For statistical analyses, the normality and equality of the data were tested. Based on these results, a parametric or a nonparametric test was used to make statistical comparisons. Calculated V50 values from patch-clamp recordings are expressed as means ± SE with a 95% confidence interval (CI). Fitting and statistical comparisons were performed with Clampfit (Molecular Devices), Sigmaplot 11.0 (Systat Software, Chicago, IL), and GraphPad 5.03 (GraphPad Software, San Diego, CA).
RESULTS
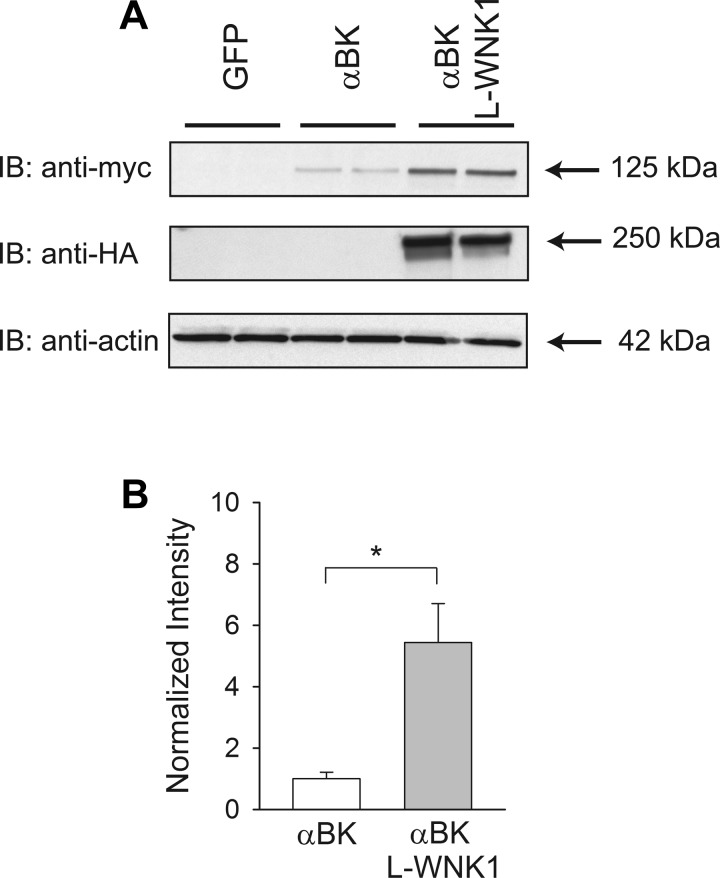
To determine whether L-WNK1 regulates BK α-subunit protein expression, we performed transient transfections in HEK cells with a myc-tagged BK α-subunit construct and a HA-tagged L-WNK1 construct. Whole cell BK α-subunit expression was assessed by immunoblotting and was normalized to actin expression. We observed a significant 5.4-fold increase in BK α-subunit expression in response to L-WNK1 coexpression compared with cells that were transfected with BK α-subunit alone (Fig. 1).
Fig. 1.
Long (L) with-no-lysine kinase 1 (WNK1) expression increases high-conductance Ca2+-activated K+ (BK) α-subunit abundance. HEK293 cells were transfected with green fluorescent protein (GFP, control), BK α-subunit, or BK α-subunit and L-WNK1. A: L-WNK1 expression increases whole cell BK α-subunit abundance. Whole cell lysates were subjected to immunoblot with an anti-myc antibody to detect BK α-subunit, an anti-hemagglutinin (HA) antibody to detect L-WNK1, or an anti-actin antibody. B: summary of analyses of BK α-subunit whole cell expression. Immunoblots were quantified as described in materials and methods (n = 11, *P < 0.001) (Student's t-test). Results were normalized to BK α-subunit expression in the absence of exogenous L-WNK1.
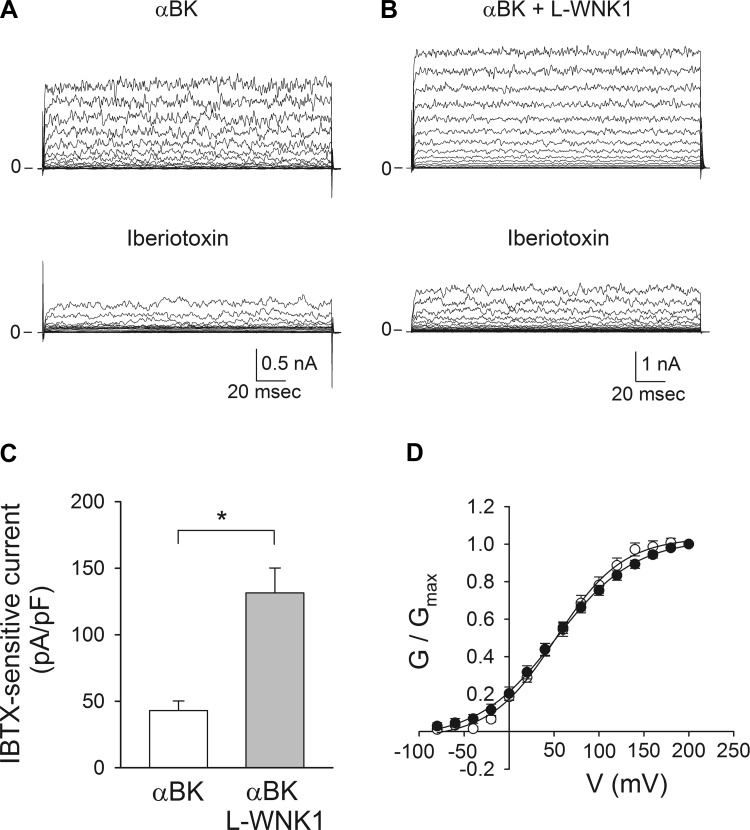
To examine whether the effect of L-WNK1 in BK α-subunit whole cell expression translates to an increase in functional BK channel activity at the plasma membrane, we performed electrophysiological measurements in cells expressing the BK α-subunit with or without L-WNK1. We employed the amphotericin B perforated patch-clamp technique in the voltage-clamp mode, which has the advantage of maintaining cytosolic constituents without altering endogenous signaling molecules. IBTX, a toxin purified from the Eastern Indian red scorpion Buthus tamulus that selectively binds to the BK α-subunit and inhibits current by decreasing channel open probability, was used to define the component of the K+ current mediated by BK (7). Currents were measured by applying 200-ms voltage steps from −80 to +100 mV in 10-mV increments from a holding potential of −60 mV. Representative tracings obtained from cells expressing the BK α-subunit alone or with L-WNK1 before and after the addition of 100 nM IBTX are shown in Fig. 2, A and B. Consistent with the observed effect of L-WNK1 on BK α-subunit abundance, the IBTX-sensitive whole cell conductance for cells transfected with BK α-subunit and L-WNK1 was 2.01 ± 0.33 nS/pF, which was significantly greater than that observed in cells expressing the BK α-subunit alone (0.88 ± 0.13 nS/pF). Figure 2C shows a summary of the IBTX-sensitive currents at +80 mV. BK channels open when the membrane is depolarized or in response to increases in the intracellular [Ca2+] (33, 96). The voltage dependence of channel activation is allosterically modulated by the intracellular [Ca2+], such that less positive voltages are required for activation as the intracellular [Ca2+] increases (72, 96). To determine whether L-WNK1 modifies the biophysical properties of BK channels, we conducted experiments with the conventional whole cell patch-clamp technique with a pipette solution containing 10 μM free [Ca2+] on cells transfected with the BK α-subunit alone or with L-WNK1. The normalized conductance (G/Gmax) plotted as a function of the holding potential (V) was fitted to a Boltzman sigmoidal equation to determine V50. The estimated values for V50 were 52.4 ± 3.2 mV (CI 46.1–58.6) for cells transfected with the BK α-subunit alone and 51.8 ± 3.6 mV (CI 44.6–59.0) for cells transfected with BK α-subunit and L-WNK1 (Fig. 2D). These values were not significantly different (Student's t-test), suggesting that L-WNK1 expression does not alter the voltage dependence or Ca2+ sensitivity of BK α-subunit channels. Taken together, our results indicate that L-WNK1 expression promotes an increase in BK α-subunit whole cell protein abundance and a concomitant increase in functional channels at the cell surface.
Fig. 2.
L-WNK1 expression increases whole cell BK channel activity. HEK293 cells were transfected with BK α-subunit alone or with L-WNK1. Patch-clamp studies were performed with the amphotericin B perforated whole cell technique (A–C) or with conventional whole cell patch-clamp (D), as described in materials and methods. BK currents were evoked by voltage steps from −80 to 100 mV in 10-mV increments from a holding potential of −60 mV (A–C) or by voltage steps from −80 to 200 mV in 20-mV increments from a holding potential of −80 mV (D). A and B: representative tracings obtained in the absence or presence of 100 nM iberiotoxin (IBTX) from cells transfected with myc-tagged BK α-subunit alone (A) or with L-WNK1 (B). C: summary of the effect of L-WNK1 on IBTX-sensitive whole cell currents measured at +80 mV (n = 6–7, *P < 0.01) (Student's t-test). D: normalized steady-state conductance-voltage (G–V) relationships for cells transfected with BK α-subunit alone (open circles) or with L-WNK1 (closed circles) (n = 9–11). Data were fitted to a Boltzmann function as described in materials and methods.
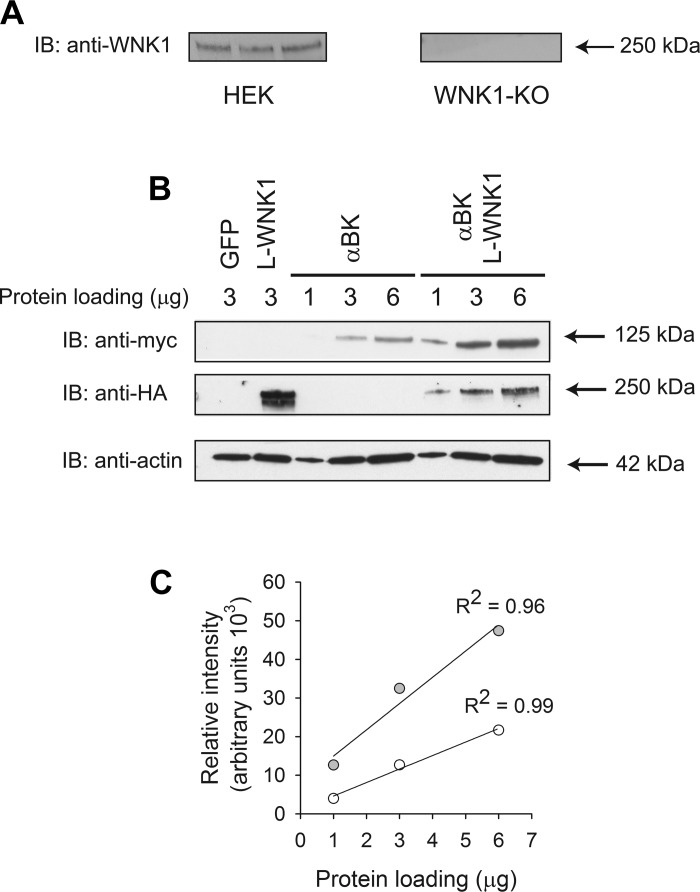
HEK293-H cells constitutively express L-WNK1 (Fig. 3A and Ref. 70). To assess the role of wild-type, catalytically inactive L-WNK1 mutant (K233M) and KS-WNK1 on BK α-subunit expression, we conducted a series of experiments using HEK293 L-WNK1-KO cells that lacked endogenous L-WNK1 expression. In these cells, L-WNK1 expression was selectively knocked out using RNA-guided CRISPR/Cas9-mediated genome editing (70) (Fig. 3A). Roy and colleagues reported that L-WNK1 deletion in HEK293 cells also resulted in a reduction of WNK2, WNK4, OSR1, and pSPAK/pOSR1 protein abundance and an increase in Kelch-like 3 and Cullin 3 protein abundance (70). To determine whether the signal detected with the myc antibody was directly proportional to the BK α-subunit abundance in our samples, we loaded increasing amounts of protein homogenate from cells transfected with the BK α-subunit alone or with L-WNK1 and performed a Western blot analysis (Fig. 3B). Figure 3C shows the relative intensity of the bands as a function of the amount of protein loaded in each lane for homogenates from cells transfected with BK α-subunit alone or with L-WNK1. Regression analysis revealed that our detection system is capable of producing a linear response over a large range of BK α-subunit abundance.
Fig. 3.
L-WNK1 increases whole cell BK expression in HEK293 L-WNK1 knockout (KO) cells. A: lack of endogenous L-WNK1 expression in HEK293 L-WNK1 KO cells. Lysates obtained from HEK293 and HEK293 L-WNK1 KO cells were subjected to immunoblot with an anti-L-WNK1 antibody as described in materials and methods. Each lane was loaded with 12 μg of protein. B: L-WNK1 increases whole cell BK expression in HEK293 L-WNK1 KO cells. Whole cell lysates from HEK293 L-WNK1 KO cells transfected with GFP (control), L-WNK1, BK α-subunit, or BK α-subunit with L-WNK1 were subjected to immunoblot with an anti-HA antibody to detect L-WNK1, an anti-myc antibody to detect BK α-subunit, or an anti-actin antibody. Lanes were loaded with 1–6 μg of protein. Representative of 4 independent experiments. C: linearity of the Western blot detection system for BK α-subunit abundance. The relative intensity of the bands in the immunoblot shown in B was calculated with ImageJ. Data from cells transfected with BK α-subunit alone are shown as open symbols while those from cells transfected with the BK α-subunit and L-WNK1 are shown as gray symbols. Data were analyzed by linear regression.
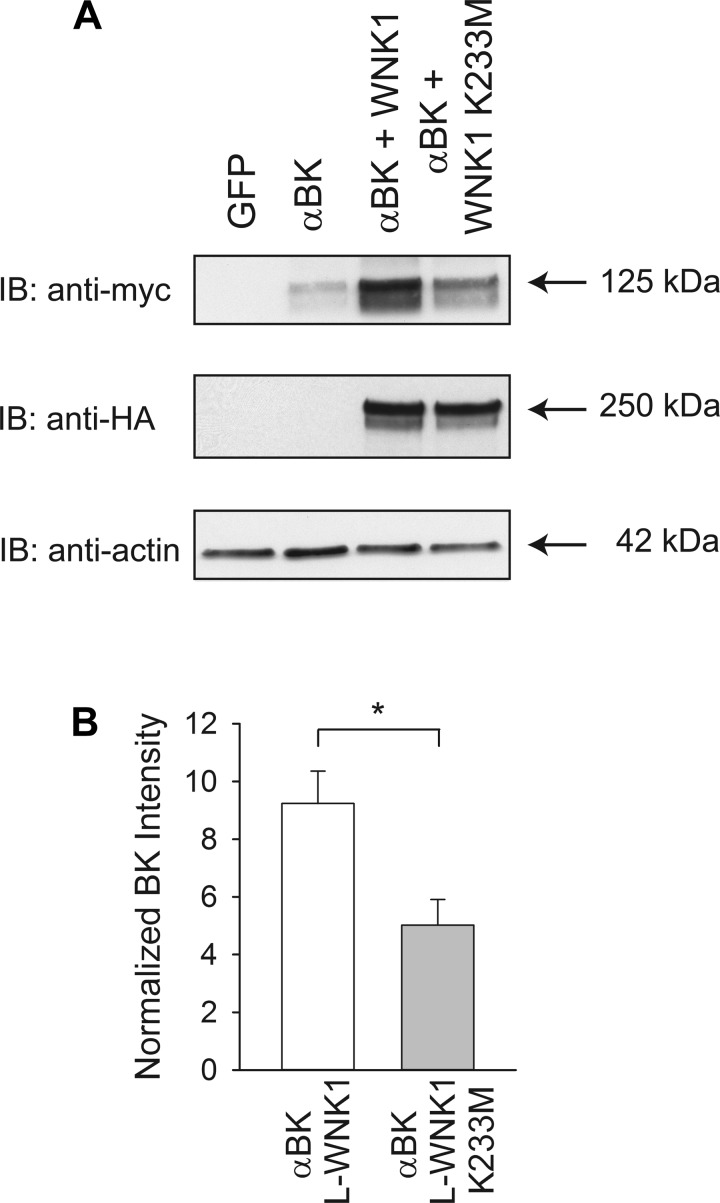
To determine whether the L-WNK-1-mediated regulation of BK α-subunit abundance requires its kinase activity, we coexpressed the BK α-subunit with a L-WNK1 mutant bearing a substitution in a catalytic residue within the kinase domain (K233M) (58, 101). Consistent with the studies conducted with the HEK293-H cells, the results obtained with HEK293 L-WNK1-KO cells showed a significant 9.3-fold increase in BK α-subunit whole cell abundance in response to L-WNK1 expression (Fig. 4). We found that the L-WNK1 K233M mutant significantly increased BK α-subunit abundance by 5.1-fold compared with cells only expressing the BK α-subunit (Fig. 4). However, the magnitude of the increase in BK α-subunit expression with wild-type L-WNK1 was significantly greater than the increase we observed with the L-WNK1 K233M mutant (Fig. 4B).
Fig. 4.
L-WNK1 increases whole cell BK expression in the absence of kinase activity. A: kinase dead L-WNK1 (K233M) increases whole cell BK α-subunit abundance. Whole cell lysates from HEK293 L-WNK1 KO cells transfected with GFP (control), BK α-subunit, or BK α-subunit with either wild-type L-WNK1 or L-WNK1 K233M were subjected to immunoblot with an anti-HA antibody to detect L-WNK1, an anti-myc antibody to detect BK α-subunit, or an anti-actin antibody. B: summary of analyses of BK α-subunit whole cell expression. Immunoblots were quantified as described in materials and methods (n = 8, *P < 0.01) (Student's t-test). Results were normalized to BK α-subunit expression in the absence of exogenous L-WNK1 or L-WNK1 K233M. Levels of expression of BK α-subunit were significantly increased by coexpression of L-WNK1 (P < 0.001) or by coexpression of the K233M mutant (P < 0.05) (Kruskal-Wallis test followed by Dunn's multiple-comparisons test).
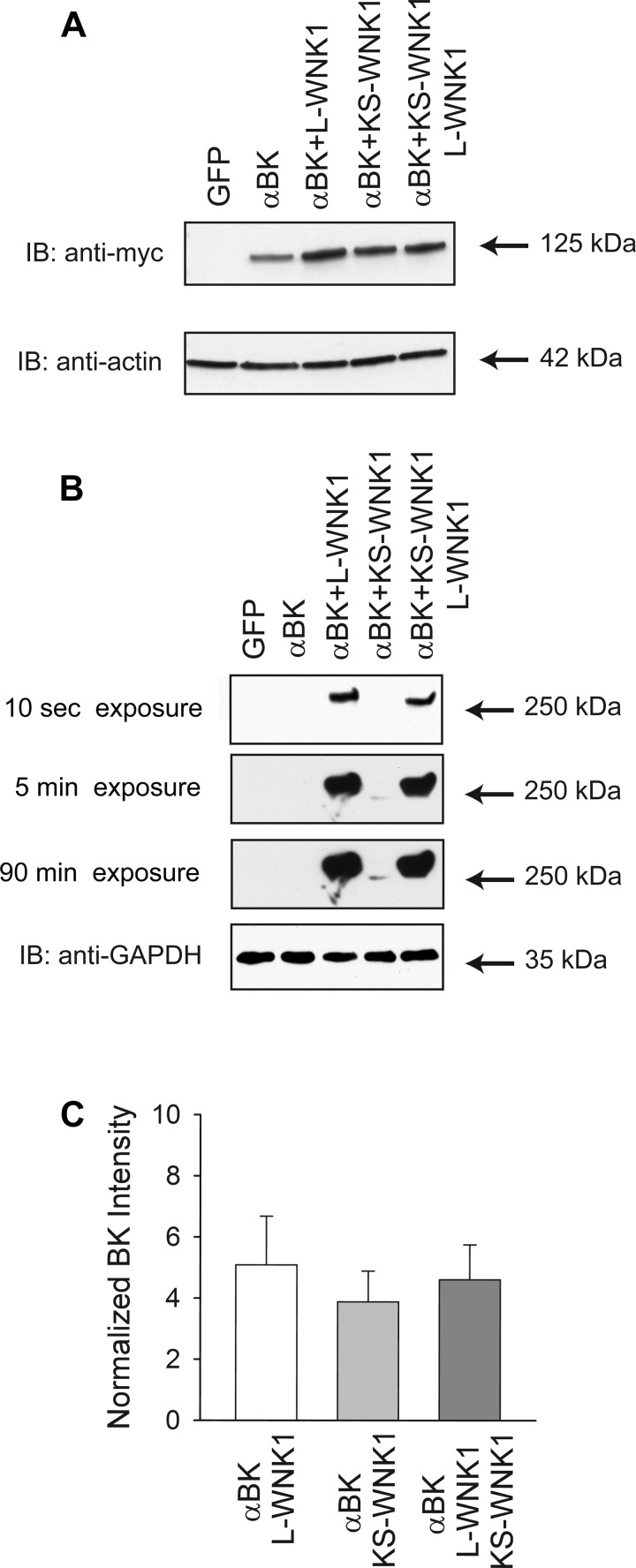
KS-WNK1 lacks a portion of the NH2-terminus of L-WNK1, including the kinase domain, and has been reported to function in a dominant negative manner (85, 91). We examined whether coexpression of KS-WNK1 with the BK α-subunit increased channel expression in HEK293 L-WNK1-KO cells. We found that both L-WNK1 and KS-WNK1 increased BK α-subunit expression to a similar extent (Fig. 5, A and C). Unexpectedly, KS-WNK1 protein abundance was significantly lower than L-WNK1 abundance assessed by Western blot (Fig. 5B), suggesting that KS-WNK1 is less stable than L-WNK1. In contrast to other mediators of renal ion transport such as NCC and ROMK, our observations suggest that KS-WNK1 does not antagonize the effect of L-WNK1 on BK α-subunit expression.
Fig. 5.
kidney-specific (KS) WNK1 increases whole cell BK expression. A: both L-WNK1 and KS-WNK1 increase whole cell BK α-subunit expression. Lysates from HEK293 L-WNK1 KO cells transfected with GFP (control), BK α-subunit, or BK α-subunit with either wild-type L-WNK1, KS-WNK1, or both L-WNK1 and KS-WNK1 were subjected to immunoblot with an anti-myc antibody to detect BK α-subunit, an anti-HA antibody to detect L-WNK1 or KS-WNK1, or an anti-actin antibody. B: reduced levels of KS-WNK1 compared with levels of L-WNK1 in HEK293 L-WNK1 knockout cells. Whole cell lysates from cells transfected as indicated above were subjected to immunoblot with an anti-HA antibody to detect L-WNK1 or KS-WNK1 or an anti-GAPDH antibody. Note that the detection of KS-WNK1 required significantly longer exposure times than were required to detect L-WNK1. For this experiment, cells were harvested 24 h posttransfection. C: summary of analyses of BK α-subunit whole cell expression. Immunoblots were quantified as described in materials and methods (n = 9). Results were normalized to BK α-subunit expression in the absence of exogenous L-WNK1 or KS-WNK1. Levels of expression of BK α-subunit were significantly increased by coexpression of L-WNK1 (P < 0.001), KS-WNK1 (P < 0.01), or both L-WNK1 and KS-WNK1 (P < 0.001) (Kruskal-Wallis test followed by Dunn's multiple-comparisons test).
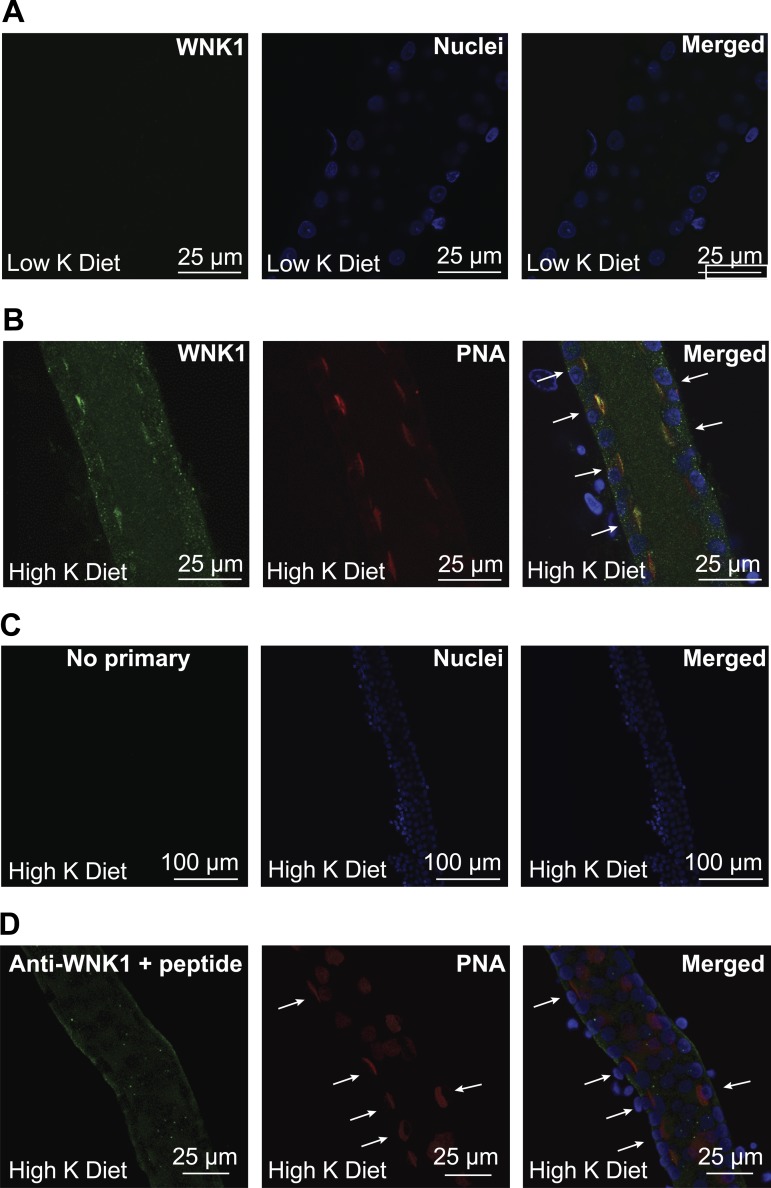
Both L-WNK1 and WNK4 inhibit ROMK expression in vitro (37, 42, 91). FHHt-associated mutations that increase L-WNK1 and WNK4 expression further reduce ROMK expression and likely contribute to the hyperkalemia observed in this disorder (37, 90). These kinases may also have a role in the renal adaptation to a high-K+ diet (42, 88, 91). We previously found that BK α-subunit expression was significantly increased in ICs in response to a high-K+ diet (59). If L-WNK1 contributes to the adaptive regulation of BK channel expression by dietary K+, we speculated that L-WNK1 expression should be selectively increased in ICs in the setting of a high-K+ diet, since an increase of L-WNK1 in PCs would inhibit ROMK expression. We examined L-WNK1 expression in isolated CCDs from rabbits fed with a low- or a high-K+ diet by confocal microscopy (Fig. 6). We observed that L-WNK1 was predominantly localized to a subset of cells in the CCDs from rabbits maintained in a high-K+ diet (Fig. 6B) but was difficult to detect in CCDs isolated from rabbits fed a low-K+ diet (Fig. 6A). Fluorescent staining was not observed in rabbit CCDs incubated only with secondary antibody (Fig. 6C), and only modest basal membrane staining was seen when the anti-L-WNK1 antibody was preincubated with a peptide fragment containing the peptide antigen that was used to raise the antibody. To identify the cells expressing L-WNK1, CCDs were colabeled with the L-WNK1-antibody and PNA conjugated to rhodamine. This lectin binds specifically to β-type intercalated cells in the rabbit CCD (73, 80). As shown in Fig. 6B, L-WNK1 is prominently expressed in cells that bind PNA in CCDs harvested from animals maintained on a high-K+ diet. We quantified the extent of cell-specific L-WNK1 labeling (Fig. 7). Whole cell L-WNK1 immunofluorescence intensity in PNA+ cells was 2.3-fold greater than that of PNA− cells. The L-WNK1 immunofluorescence intensity at the apical membrane and subapical region of PNA+ cells was 7.8-fold greater than that of PNA− cells. Together, our results indicate that L-WNK1 expression is regulated by dietary K+ intake in the distal nephron in a cell type-specific manner.
Fig. 6.
High-K+ (HK) diet increases L-WNK1 expression in rabbit cortical collecting ducts (CCDs). Single CCDs isolated from New Zealand White (NZW) rabbits fed a HK or a low-K+ (LK) diet for 10 days were microperfused and fixed for immunofluorescence microscopy. Immunolabeling was performed as indicated in materials and methods. A: L-WNK1 expression in CCDs from LK-fed animals. Immunostaining for L-WNK1 was difficult to visualize in CCDs from rabbits fed a LK diet. B: expression of L-WNK1 in rabbit CCD intercalated cells after 10 days on a HK diet. Intercalated cells (arrows) were identified by their apical labeling with rhodamine-conjugated peanut agglutinin (PNA). C: negative control incubated with Alexa488-conjugated goat anti-rabbit IgG antibody but not with primary antibody. D: antigen competition. Only modest basal membrane labeling was seen when the anti-L-WNK1 antibody was preincubated with a WNK1 peptide fragment (arrows indicate PNA+ cells).
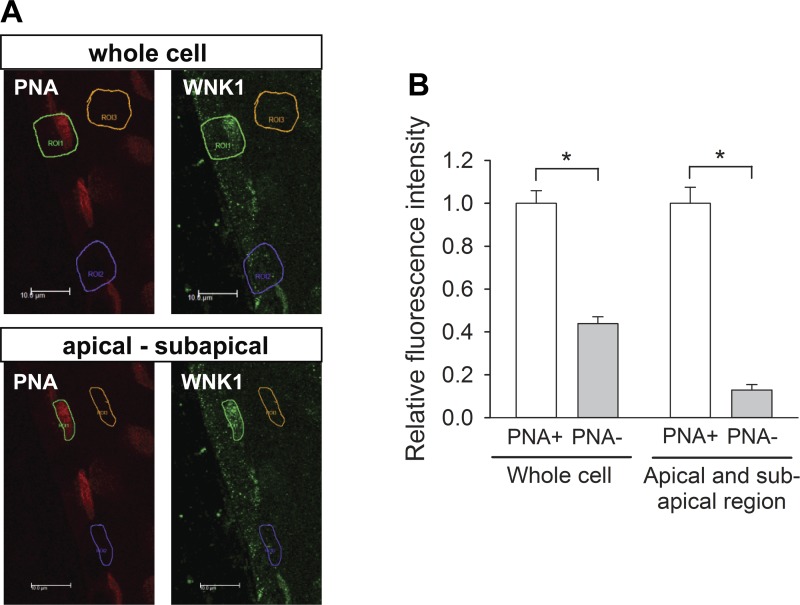
Fig. 7.
Quantitation of L-WNK1 expression in the apical and subapical regions of PNA-positive (PNA+) and PNA-negative (PNA−) cells. A: representative confocal photomicrographs of L-WNK1 immunolabeling (green) in immunoperfused CCD isolated from a HK-fed rabbit. Type B intercalated cells are labeled with apical PNA (red). Rectangles identify PNA-positive β-intercalated cells (green), PNA-negative cells (purple), and lumen (background, yellow). B: relative fluorescence intensity of PNA+ and PNA− cells. The L-WNK1 immunofluorescence intensity of PNA+ cells (1.00 ± 0.06) was 2.3-fold greater than that of PNA− cells (0.44 ± 0.03). The relative fluorescence intensity of the apical and subapical region of PNA+ cells (1.00 ± 0.07) was 7.8-fold greater than that of PNA− cells (0.13 ± 0.02). Fluorescence intensity was normalized to the average signal in PNA+ cells. Median comparisons between cell types made using a Mann-Whitney test. *P ≤ 0.001; n = 20 (whole cell) and n = 29 (apical-subapical region) (PNA+ and PNA−, each) in 4 tubules.
DISCUSSION
Specific mutations in WNK1 or WNK4 cause FHHt, a hereditary disease characterized by hypertension, and hyperkalemia (65, 98). Both kinases have important roles in regulating Na+ transporters in the ASDN, including NCC and ENaC (29, 32, 37, 41, 69, 84, 98, 102, 104). Because the mutations in this disorder are predicted to increase expression of these kinases (55), it is not surprising that these kinases regulate K+ secretory channels. It is well established that L-WNK1 and WNK4 inhibit ROMK expression by enhancing channel endocytosis (11, 28, 37, 42, 69, 91). FHHt-causing WNK4 mutations enhance the inhibition of ROMK and are associated with a further reduction in ROMK surface density and K+ secretion (37). This enhanced inhibitory effect is likely a consequence of increased WNK4 protein expression, since FHHt-causing WNK4 mutations are missense mutations that reduce binding to Kelch-like 3/Cullin 3 (60). The mechanism by which WNK1 regulates ROMK is more complex, since the alternatively spliced KS-WNK1 isoform lacking the NH2-terminus and kinase domain has been reported to function in a dominant interfering mode (13, 42, 61, 85, 91).
Emerging evidence indicates that WNK kinases regulate BK channel activity. WNK4 reduces whole cell and surface expression of the BK α-subunit in HEK293 cells by a process that is kinase dependent and sensitive to inhibitors of lysosomal degradation, consistent with the notion that WNK4 enhances the routing of BK channels to lysosomes in a dynamin-independent manner (108) that is dependent on activation of MAP kinases (106). We have reported that WNK4 enhances ubiquitination of the BK α-subunit, consistent with a role for WNK4 in targeting the channel for internalization and/or degradation via a ubiquitin-dependent pathway (95). Recent published work (48) and our studies described in this manuscript examined the effects of L-WNK1 on BK channel expression. In contrast to its inhibitory effect on ROMK, L-WNK1 significantly increases BK α-subunit whole cell and functional expression in HEK293 cells. Liu et al. found that L-WNK1 expression reduced ERK phosphorylation, whereas knockdown of WNK1 increased the ubiquitination of BK α-subunit, effects that are opposite to the effects seen with WNK4 (48).
We found that both catalytically inactive L-WNK1 K233M, as well as KS-WNK1 lacking the kinase domain, increased BK α-subunit expression. However, the increase in BK expression with wild-type L-WNK1 was significantly greater than that observed with L-WNK1 K233M. These results suggest there are mechanisms by which L-WNK1 increases BK α-subunit expression that are independent of its catalytic activity, as well as mechanisms that require L-WNK1 catalytic activity.
While we observed that L-WNK1 and KS-WNK1 increased BK α-subunit whole cell expression to a similar extent, levels of expression of KS-WNK1 were notably lower than that of L-WNK1. These results, and our observation that coexpression of L-WNK1 and KS-WNK1 did not have an additive effect on BK α-subunit expression, suggest that both isoforms use a common signaling pathway to regulate BK α-subunit expression. Previous studies reported reduced stability for KS-WNK1 in Xenopus oocytes but not to the extent that we observed in HEK293-T L-WNK1 KO cells (85, 91). We speculate that differences in degradation pathways, expression of chaperones, and incubation temperature may account for these differences in KS-WNK1 stability in HEK293 cells and Xenopus oocytes. In this regard Roy and colleagues (70) recently reported that WNK1 knockdown resulted in a reduction of WNK2, WNK4, OSR1, and pSPAK/pOSR1 protein abundance and an increase in Kelch-like 3 and Cullin 3 protein abundance.
Whereas BK channels are generally comprised of pore-forming α-subunits and regulatory β-subunits (2, 14, 40, 50), and mice with genetic ablation of β1 (66–68) or β4 (31) exhibit a markedly attenuated kaliuresis in response to increased distal tubular flow rate, the specific β-isoform mediating FIKS, a physiologically relevant readout of BK channel activity in the ASDN, is still unclear. Immunodetectable BKα β1 has been identified in rabbit in a cortical segment that is most likely initial CCD (66–68) but is not expressed (at the level of message) in the rabbit midcortical CCD (17, 59), a segment that consistently exhibits robust IBTX-sensitive FIKS (45, 47). Immunodetectable β4 is found with the BK α-subunit in the thick ascending limb of Henle's loop, DCT, and intercalated cells (but not CNT/principal cells) of the mouse distal CNT and CCD (26), but this subunit renders the BK channel relatively insensitive to IBTX (6, 36, 57, 93). We thus elected to focus the current study on the regulation of the pore-forming α-subunit. Future efforts must be directed toward examining whether coexpression of BKα with β-subunits impacts WNK-dependent regulation. Without such studies, it remains uncertain as to whether L-WNK1 modulates the BK channel in vivo.
Although PCs are responsible for K+ secretion mediated by ROMK, there is increasing evidence that BK channels in ICs are primarily responsible for FIKS (17, 27, 44, 46, 59, 62, 63, 68, 77, 89, 100). The findings that L-WNK1 and WNK4 both inhibit ROMK expression and activity, yet L-WNK1 and WNK4 exert different effects on BK channel expression and activity, present a conundrum. In the setting of hyperkalemia or a high-K+ diet, a reduction in expression of WNK4 would increase ROMK and BK channel activity. However, a reduction in L-WNK1 in this setting would increase ROMK but inhibit BK channel expression. A potential explanation that could reconcile these findings is that hyperkalemia alters the expression of WNK kinases in a cell type-specific manner in the ASDN. In this model, a reduction of L-WNK1 expression in PCs should enhance ROMK channel expression, whereas enhanced L-WNK1 expression in ICs should enhance BK channel expression. To test this hypothesis, we performed immunostaining with an L-WNK1-specific antibody in isolated perfused CCDs of rabbits subjected to high- or low-K+ diet. Our results demonstrate a selective increase in the expression of L-WNK1 in ICs vs. PCs in response to a high-K+ diet.
FHHt-associated mutations in WNK1 result in increased L-WNK1 expression (90, 98). Recent studies suggest that FHHt-associated mutations in Cullin 3 or Kelch-like 3 also increase L-WNK1 expression and are associated with hyperkalemia (56, 86). In these settings, our results suggest that there should be increased BK channel expression in ICs. In FHHt caused by mutations in WNK4, the associated hyperkalemia should enhance L-WNK1 and BK channel expression in ICs. In the setting of increased L-WNK1 expression in ICs, one would predict an increase in BK channel expression that should protect against hyperkalemia. However, there is presumably enhanced NaCl absorption in the early DCT in FHHt, leading to reduced distal Na delivery and tubular flow that would limit FIKS. Because thiazide diuretics correct hyperkalemia in FHHt (18), likely reflecting BK channel-mediated FIKS, we suggest that CCD BK channel expression in ICs is maintained or increased in FHHt in association with increased WNK1 expression in these cells.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants, including R01-DK-038470 (L. M. Satlin and T. R. Kleyman), R01-DK-084184 (M. D. Carattino), R01-DK-098145 (A. R. Subramanya), R37-DK-051391 (T. R. Kleyman and L. M. Satlin), T32-DK-091202, and P30-DK-079307 (Pittsburgh Center for Kidney Research). R. Carrisoza-Gaytan was supported, in part, by a CONACYT postdoctoral scholarship (232526).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.N.W., A.R.S., L.M.S., T.R.K., and M.D.C. conception and design of research; T.N.W., R.C.-G., N.M., A.C.R., A.R., A.M.S., and M.D.C. performed experiments; T.N.W., R.C.-G., N.M., A.C.R., A.R., L.M.S., T.R.K., and M.D.C. analyzed data; T.N.W., R.C.-G., A.R.S., L.M.S., T.R.K., and M.D.C. interpreted results of experiments; T.N.W., R.C.-G., L.M.S., T.R.K., and M.D.C. drafted manuscript; T.N.W., R.C.-G., A.R.S., L.M.S., T.R.K., and M.D.C. edited and revised manuscript; T.N.W., R.C.-G., N.M., A.C.R., A.R., A.M.S., A.R.S., L.M.S., T.R.K., and M.D.C. approved final version of manuscript; R.C.-G., N.M., and M.D.C. prepared figures.
ACKNOWLEDGMENTS
Image collection and digital image analysis were conducted at the Microscopy Shared Resource Facility at the Icahn School of Medicine at Mount Sinai.
REFERENCES
- 1.Alessi DR, Zhang J, Khanna A, Hochdorfer T, Shang Y, Kahle KT. The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Sci Signal 7: 3, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science 253: 551–555, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Beck FX, Dorge A, Blumner E, Giebisch G, Thurau K. Cell rubidium uptake: a method for studying functional heterogeneity in the nephron. Kidney Int 33: 642–651, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Boim MA, Ho K, Shuck ME, Bienkowski MJ, Block JH, Slightom JL, Yang Y, Brenner BM, Hebert SC. ROMK inwardly rectifying ATP-sensitive K+ channel. II. Cloning and distribution of alternative forms. Am J Physiol Renal Fluid Electrolyte Physiol 268: F1132–F1140, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Valimaki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482: 98–102, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275: 6453–6461, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Candia S, Garcia ML, Latorre R. Mode of action of iberiotoxin, a potent blocker of the large conductance Ca2+-activated K+ channel. Biophys J 63: 583–590, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambrey R, Kurth I, Peti-Peterdi J, Houillier P, Purkerson JM, Leviel F, Hentschke M, Zdebik AA, Schwartz GJ, Hubner CA, Eladari D. Renal intercalated cells are rather energized by a proton than a sodium pump. Proc Natl Acad Sci USA 110: 7928–7933, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavez-Canales M, Zhang C, Soukaseum C, Moreno E, Pacheco-Alvarez D, Vidal-Petiot E, Castaneda-Bueno M, Vazquez N, Rojas-Vega L, Meermeier NP, Rogers S, Jeunemaitre X, Yang CL, Ellison DH, Gamba G, Hadchouel J. WNK-SPAK-NCC cascade revisited: WNK1 stimulates the activity of the Na-Cl cotransporter via SPAK, an effect antagonized by WNK4. Hypertension 64: 1047–1053, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Constantinescu A, Silver RB, Satlin LM. H-K-ATPase activity in PNA-binding intercalated cells of newborn rabbit cortical collecting duct. Am J Physiol Renal Physiol 272: F167–F177, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Cope G, Murthy M, Golbang AP, Hamad A, Liu CH, Cuthbert AW, O'Shaughnessy KM. WNK1 affects surface expression of the ROMK potassium channel independent of WNK4. J Am Soc Nephrol 17: 1867–1874, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Cornelius RJ, Wen D, Li H, Yuan Y, Wang-France J, Warner PC, Sansom SC. Low Na, high K diet and the role of aldosterone in BK-mediated K excretion. PLoS One 10: e0115515, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delaloy C, Lu J, Houot AM, Disse-Nicodeme S, Gasc JM, Corvol P, Jeunemaitre X. Multiple promoters in the WNK1 gene: one controls expression of a kidney-specific kinase-defective isoform. Mol Cell Biol 23: 9208–9221, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkins T, Ganetzky B, Wu CF. A Drosophila mutation that eliminates a calcium-dependent potassium current. Proc Natl Acad Sci USA 83: 8415–8419, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emmons C, Kurtz I. Functional characterization of three intercalated cell subtypes in the rabbit outer cortical collecting duct. J Clin Invest 93: 417–423, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engbretson BG, Stoner LC. Flow-dependent potassium secretion by rabbit cortical collecting tubule in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 253: F896–F903, 1987. [DOI] [PubMed] [Google Scholar]
- 17.Estilo G, Liu W, Pastor-Soler N, Mitchell P, Carattino MD, Kleyman TR, Satlin LM. Effect of aldosterone on BK channel expression in mammalian cortical collecting duct. Am J Physiol Renal Physiol 295: F780–F788, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farfel Z, Iaina A, Rosenthal T, Waks U, Shibolet S, Gafni J. Familial hyperpotassemia and hypertension accompanied by normal plasma aldosterone levels: possible hereditary cell membrane defect. Arch Intern Med 138: 1828–1832, 1978. [PubMed] [Google Scholar]
- 19.Frindt G, Palmer LG. Apical potassium channels in the rat connecting tubule. Am J Physiol Renal Physiol 287: F1030–F1037, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Frindt G, Palmer LG. Ca-activated K channels in apical membrane of mammalian CCT, and their role in K secretion. Am J Physiol Renal Fluid Electrolyte Physiol 252: F458–F467, 1987. [DOI] [PubMed] [Google Scholar]
- 21.Frindt G, Palmer LG. Low-conductance K channels in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 256: F143–F151, 1989. [DOI] [PubMed] [Google Scholar]
- 22.Giebisch G. Renal potassium transport: mechanisms and regulation. Am J Physiol Renal Physiol 274: F817–F833, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Good DW, Wright FS. Luminal influences on potassium secretion: sodium concentration and fluid flow rate. Am J Physiol Renal Fluid Electrolyte Physiol 236: F192–F205, 1979. [DOI] [PubMed] [Google Scholar]
- 24.Grantham JJ, Burg MB, Orloff J. The nature of transtubular Na and K transport in isolated rabbit renal collecting tubules. J Clin Invest 49: 1815–1826, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray DA, Frindt G, Palmer LG. Quantification of K+ secretion through apical low-conductance K channels in the CCD. Am J Physiol Renal Physiol 289: F117–F126, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Grimm PR, Foutz RM, Brenner R, Sansom SC. Identification and localization of BK β-subunits in the distal nephron of the mouse kidney. Am J Physiol Renal Physiol 293: F350–F359, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Grimm PR, Irsik DL, Liu L, Holtzclaw JD, Sansom SC. Role of BKb1 in Na+ reabsorption by cortical collecting ducts of Na+-deprived mice. Am J Physiol Renal Physiol 297: F420–F428, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He G, Wang HR, Huang SK, Huang CL. Intersectin links WNK kinases to endocytosis of ROMK1. J Clin Invest 117: 1078–1087, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heise CJ, Xu BE, Deaton SL, Cha SK, Cheng CJ, Earnest S, Sengupta S, Juang YC, Stippec S, Xu Y, Zhao Y, Huang CL, Cobb MH. Serum and glucocorticoid-induced kinase (SGK) 1 and the epithelial sodium channel are regulated by multiple with no lysine (WNK) family members. J Biol Chem 285: 25161–25167, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature 362: 31–38, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Holtzclaw JD, Grimm PR, Sansom SC. Intercalated cell BK-a/b4 channels modulate sodium and potassium handling during potassium adaptation. J Am Soc Nephrol 21: 634–645, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoorn EJ, Nelson JH, McCormick JA, Ellison DH. The WNK kinase network regulating sodium, potassium, and blood pressure. J Am Soc Nephrol 22: 605–614, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horrigan FT, Aldrich RW. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J Gen Physiol 120: 267–305, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter M, Lopes AG, Boulpaep EL, Giebisch GH. Single channel recordings of calcium-activated potassium channels in the apical membrane of rabbit cortical collecting tubules. Proc Natl Acad Sci USA 81: 4237–4239, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imai M, Nakamura R. Function of distal convoluted and connecting tubules studied by isolated nephron segments. Kidney Int 22: 465–472, 1982. [DOI] [PubMed] [Google Scholar]
- 36.Jin P, Weiger TM, Levitan IB. Reciprocal modulation between the alpha and beta 4 subunits of hSlo calcium-dependent potassium channels. J Biol Chem 277: 43724–43729, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Kahle KT, Wilson FH, Leng Q, Lalioti MD, O'Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 35: 372–376, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Khuri RN, Strieder WN, Giebisch G. Effects of flow rate and potassium intake on distal tubular potassium transfer. Am J Physiol 228: 1249–1261, 1975. [DOI] [PubMed] [Google Scholar]
- 39.Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM, Nielsen S. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol 283: F744–F754, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Knaus HG, Folander K, Garcia-Calvo M, Garcia ML, Kaczorowski GJ, Smith M, Swanson R. Primary sequence and immunological characterization of beta-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J Biol Chem 269: 17274–17278, 1994. [PubMed] [Google Scholar]
- 41.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38: 1124–1132, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Lazrak A, Liu Z, Huang CL. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci USA 103: 1615–1620, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li D, Wang Z, Sun P, Jin Y, Lin DH, Hebert SC, Giebisch G, Wang WH. Inhibition of MAPK stimulates the Ca2+ -dependent big-conductance K channels in cortical collecting duct. Proc Natl Acad Sci USA 103: 19569–19574, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu W, Morimoto T, Woda C, Kleyman TR, Satlin LM. Ca2+ dependence of flow-stimulated K secretion in the mammalian cortical collecting duct. Am J Physiol Renal Physiol 293: F227–F235, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Liu W, Schreck C, Coleman RA, Wade JB, Hernandez Y, Zavilowitz B, Warth R, Kleyman TR, Satlin LM. Role of NKCC in BK channel-mediated net K+ secretion in the CCD. Am J Physiol Renal Physiol 301: F1088–F1097, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu W, Wei Y, Sun P, Wang WH, Kleyman TR, Satlin LM. Mechanoregulation of BK channel activity in the mammalian cortical collecting duct (CCD): role of protein kinases A and C. Am J Physiol Renal Physiol 297: F904–F915, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Song X, Shi Y, Shi Z, Niu W, Feng X, Gu D, Bao HF, Ma HP, Eaton DC, Zhuang J, Cai H. WNK1 activates large-conductance Ca2+-activated K+ channels through modulation of ERK1/2 signaling. J Am Soc Nephrol 26: 844–854, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, Beaurain G, Bonnefond A, Sand O, Simian C., Vidal-Petiot E, Soukaseum C, Mandet C, Broux F, Chabre O, Delahousse M, Esnault V, Fiquet B, Houillier P, Bagnis CI, Koenig J, Konrad M, Landais P, Mourani C, Niaudet P, Probst V, Thauvin C, Unwin RJ, Soroka SD, Ehret G, Ossowski S, Caulfield M, International Consortium for Blood, Bruneval P, Estivill X, Froguel P, Hadchouel J, Schott JJ, Jeunemaitre X. KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet 44: 456–460, 2012. [DOI] [PubMed] [Google Scholar]
- 50.Lu R, Alioua A, Kumar Y, Eghbali M, Stefani E, Toro L. MaxiK channel partners: physiological impact. J Physiol 570: 65–72, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lynch IJ, Greenlee MM, Gumz ML, Rudin A, Xia SL, Wingo CS. Heterogeneity of H-K-ATPase-mediated acid secretion along the mouse collecting duct. Am J Physiol Renal Physiol 298: F408–F415, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madsen KM, Tisher CC. Structural-functional relationship along the distal nephron. Am J Physiol Renal Fluid Electrolyte Physiol 250: F1–F15, 1986. [DOI] [PubMed] [Google Scholar]
- 53.Malnic G, Berliner RW, Giebisch G. Flow dependence of K+ secretion in cortical distal tubules of the rat. Am J Physiol Renal Fluid Electrolyte Physiol 256: F932–F941, 1989. [DOI] [PubMed] [Google Scholar]
- 54.Malnic G, Klose RM, Giebisch G. Micropuncture study of distal tubular potassium and sodium transport in rat nephron. Am J Physiol 211: 529–547, 1966. [DOI] [PubMed] [Google Scholar]
- 55.McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev 91: 177–219, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCormick JA, Yang CL, Zhang C, Davidge B, Blankenstein KI, Terker AS, Yarbrough B, Meermeier NP, Park HJ, McCully B, West M, Borschewski A, Himmerkus N, Bleich M, Bachmann S, Mutig K, Argaiz ER, Gamba G, Singer JD, Ellison DH. Hyperkalemic hypertension-associated cullin 3 promotes WNK signaling by degrading KLHL3. J Clin Invest 124: 4723–4736, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meera P, Wallner M, Song M, Toro L. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0–S6), an extracellular N terminus, and an intracellular (S9–S10) C terminus. Proc Natl Acad Sci USA 94: 14066–14071, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Min X, Lee BH, Cobb MH, Goldsmith EJ. Crystal structure of the kinase domain of WNK1, a kinase that causes a hereditary form of hypertension. Structure 12: 1303–1311, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, Liu W, Kleyman TR, Satlin LM. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am J Physiol Renal Physiol 289: F922–F932, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Ohta A, Schumacher FR, Mehellou Y, Johnson C, Knebel A, Macartney TJ, Wood NT, Alessi DR, Kurz T. The CUL3-KLHL3 E3 ligase complex mutated in Gordon's hypertension syndrome interacts with and ubiquitylates WNK isoforms: disease-causing mutations in KLHL3 and WNK4 disrupt interaction. Biochem J 451: 111–122, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Reilly M, Marshall E, Speirs HJ, Brown RW. WNK1, a gene within a novel blood pressure control pathway, tissue-specifically generates radically different isoforms with and without a kinase domain. J Am Soc Nephrol 14: 2447–2456, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Pacha J, Frindt G, Sackin H, Palmer LG. Apical maxi K channels in intercalated cells of CCT. Am J Physiol Renal Fluid Electrolyte Physiol 261: F696–F705, 1991. [DOI] [PubMed] [Google Scholar]
- 63.Palmer LG, Frindt G. High-conductance K channels in intercalated cells of the rat distal nephron. Am J Physiol Renal Physiol 292: F966–F973, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Palmer LG, Frindt G. Regulation of apical K channels in rat cortical collecting tubule during changes in dietary K intake. Am J Physiol Renal Physiol 277: F805–F812, 1999. [DOI] [PubMed] [Google Scholar]
- 65.Pathare G, Hoenderop JG, Bindels RJ, San-Cristobal P. A molecular update on pseudohypoaldosteronism type II. Am J Physiol Renal Physiol 305: F1513–F1520, 2013. [DOI] [PubMed] [Google Scholar]
- 66.Pluznick JL, Sansom SC. BK channels in the kidney: role in K+ secretion and localization of molecular components. Am J Physiol Renal Physiol 291: F517–F529, 2006. [DOI] [PubMed] [Google Scholar]
- 67.Pluznick JL, Wei P, Carmines PK, Sansom SC. Renal fluid and electrolyte handling in BKCa-b1−/− mice. Am J Physiol Renal Physiol 284: F1274–F1279, 2003. [DOI] [PubMed] [Google Scholar]
- 68.Pluznick JL, Wei P, Grimm PR, Sansom SC. BK-b1 subunit: immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. Am J Physiol Renal Physiol 288: F846–F854, 2005. [DOI] [PubMed] [Google Scholar]
- 69.Ring AM, Cheng SX, Leng Q, Kahle KT, Rinehart J, Lalioti MD, Volkman HM, Wilson FH, Hebert SC, Lifton RP. WNK4 regulates activity of the epithelial Na+ channel in vitro and in vivo. Proc Natl Acad Sci USA 104: 4020–4024, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roy A, Goodman JH, Begum G, Donnelly BF, Pittman G, Weinman EJ, Sun D, Subramanya AR. Generation of WNK1 knockout cell lines by CRISPR/Cas-mediated genome editing. Am J Physiol Renal Physiol 308: F366–F376, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sabolic I, Herak-Kramberger CM, Breton S, Brown D. Na/K-ATPase in intercalated cells along the rat nephron revealed by antigen retrieval. J Am Soc Nephrol 10: 913–922, 1999. [DOI] [PubMed] [Google Scholar]
- 72.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci 7: 921–931, 2006. [DOI] [PubMed] [Google Scholar]
- 73.Satlin LM. Postnatal maturation of potassium transport in rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 266: F57–F65, 1994. [DOI] [PubMed] [Google Scholar]
- 74.Satlin LM, Carattino MD, Liu W, Kleyman TR. Regulation of cation transport in the distal nephron by mechanical forces. Am J Physiol Renal Physiol 291: F923–F931, 2006. [DOI] [PubMed] [Google Scholar]
- 75.Satlin LM, Matsumoto T, Schwartz GJ. Postnatal maturation of rabbit renal collecting duct. III. Peanut lectin-binding intercalated cells Am J Physiol Renal Fluid Electrolyte Physiol 262: F199–F208, 1992. [DOI] [PubMed] [Google Scholar]
- 76.Satlin LM, Palmer LG. Apical K+ conductance in maturing rabbit principal cell. Am J Physiol Renal Physiol 272: F397–F404, 1997. [DOI] [PubMed] [Google Scholar]
- 77.Satlin LM, Palmer LG. Apical Na+ conductance in maturing rabbit principal cell. Am J Physiol Renal Fluid Electrolyte Physiol 270: F391–F397, 1996. [DOI] [PubMed] [Google Scholar]
- 78.Sauer M, Flemmer A, Thurau K, Beck FX. Sodium entry routes in principal and intercalated cells of the isolated perfused cortical collecting duct. Pflugers Arch 416: 88–93, 1990. [DOI] [PubMed] [Google Scholar]
- 79.Schlatter E, Bleich M, Hirsch J, Markstahler U, Frobe U, Greger R. Cation specificity and pharmacological properties of the Ca2+-dependent K+ channel of rat cortical collecting ducts. Pflugers Arch 422: 481–491, 1993. [DOI] [PubMed] [Google Scholar]
- 80.Schuster VL, Bonsib SM, Jennings ML. Two types of collecting duct mitochondria-rich (intercalated) cells: lectin and band 3 cytochemistry. Am J Physiol Cell Physiol 251: C347–C355, 1986. [DOI] [PubMed] [Google Scholar]
- 81.Silver RB, Soleimani M. H+-K+-ATPases: regulation and role in pathophysiological states. Am J Physiol Renal Physiol 276: F799–F811, 1999. [DOI] [PubMed] [Google Scholar]
- 82.Stoner LC, Viggiano SC. Elevation of basolateral K+ induces K+ secretion by apical maxi K+ channels in Ambystoma collecting tubule. Am J Physiol Regul Integr Comp Physiol 276: R616–R621, 1999. [DOI] [PubMed] [Google Scholar]
- 83.Stoner LC, Viggiano SC. Environmental KCl causes an upregulation of apical membrane maxi K and ENaC channels in everted Ambystoma collecting tubule. J Membr Biol 162: 107–116, 1998. [DOI] [PubMed] [Google Scholar]
- 84.Subramanya AR, Liu J, Ellison DH, Wade JB, Welling PA. WNK4 diverts the thiazide-sensitive NaCl cotransporter to the lysosome and stimulates AP-3 interaction. J Biol Chem 284: 18471–18480, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Subramanya AR, Yang CL, Zhu X, Ellison DH. Dominant-negative regulation of WNK1 by its kidney-specific kinase-defective isoform. Am J Physiol Renal Physiol 290: F619–F624, 2006. [DOI] [PubMed] [Google Scholar]
- 86.Susa K, Sohara E, Rai T, Zeniya M, Mori Y, Mori T, Chiga M, Nomura N, Nishida H, Takahashi D, Isobe K, Inoue Y, Takeishi K, Takeda N, Sasaki S, Uchida S. Impaired degradation of WNK1 and WNK4 kinases causes PHAII in mutant KLHL3 knock-in mice. Hum Mol Genet 23: 5052–5060, 2014. [DOI] [PubMed] [Google Scholar]
- 87.Taniguchi J, Imai M. Flow-dependent activation of maxi K+ channels in apical membrane of rabbit connecting tubule. J Membr Biol 164: 35–45, 1998. [DOI] [PubMed] [Google Scholar]
- 88.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AH, Fenton RA, Zietse R, Hoorn EJ. K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl− cotransporter. Am J Physiol Renal Physiol 305: F1177–F1188, 2013. [DOI] [PubMed] [Google Scholar]
- 90.Vidal-Petiot E, Elvira-Matelot E, Mutig K, Soukaseum C, Baudrie V, Wu S, Cheval L, Huc E, Cambillau M, Bachmann S, Doucet A, Jeunemaitre X, Hadchouel J. WNK1-related familial hyperkalemic hypertension results from an increased expression of L-WNK1 specifically in the distal nephron. Proc Natl Acad Sci USA 110: 14366–14371, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wade JB, Fang L, Liu J, Li D, Yang CL, Subramanya AR, Maouyo D, Mason A, Ellison DH, Welling PA. WNK1 kinase isoform switch regulates renal potassium excretion. Proc Natl Acad Sci USA 103: 8558–8563, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wald H, Garty H, Palmer LG, Popovtzer MM. Differential regulation of ROMK expression in kidney cortex and medulla by aldosterone and potassium. Am J Physiol Renal Physiol 275: F239–F245, 1998. [DOI] [PubMed] [Google Scholar]
- 93.Wang B, Rothberg BS, Brenner R. Mechanism of beta4 subunit modulation of BK channels. J Gen Physiol 127: 449–465, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang WH, Schwab A, Giebisch G. Regulation of small-conductance K+ channel in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 259: F494–F502, 1990. [DOI] [PubMed] [Google Scholar]
- 95.Wang Z, Subramanya AR, Satlin LM, Pastor-Soler NM, Carattino MD, Kleyman TR. Regulation of large-conductance Ca2+-activated K+ channels by WNK4 kinase. Am J Physiol Cell Physiol 305: C846–C853, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wei A, Solaro C, Lingle C, Salkoff L. Calcium sensitivity of BK-type KCa channels determined by a separable domain. Neuron 13: 671–681, 1994. [DOI] [PubMed] [Google Scholar]
- 97.Wen D, Cornelius RJ, Yuan Y, Sansom SC. Regulation of BK-alpha expression in the distal nephron by aldosterone and urine pH. Am J Physiol Renal Physiol 305: F463–F476, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001. [DOI] [PubMed] [Google Scholar]
- 99.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001. [DOI] [PubMed] [Google Scholar]
- 100.Woda CB, Miyawaki N, Ramalakshmi S, Ramkumar M, Rojas R, Zavilowitz B, Kleyman TR, Satlin LM. Ontogeny of flow-stimulated potassium secretion in rabbit cortical collecting duct: functional and molecular aspects. Am J Physiol Renal Physiol 285: F629–F639, 2003. [DOI] [PubMed] [Google Scholar]
- 101.Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 275: 16795–16801, 2000. [DOI] [PubMed] [Google Scholar]
- 102.Xu BE, Stippec S, Chu PY, Lazrak A, Li XJ, Lee BH, English JM, Ortega B, Huang CL, Cobb MH. WNK1 activates SGK1 to regulate the epithelial sodium channel. Proc Natl Acad Sci USA 102: 10315–10320, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu JZ, Hall AE, Peterson LN, Bienkowski MJ, Eessalu TE, Hebert SC. Localization of the ROMK protein on apical membranes of rat kidney nephron segments. Am J Physiol Renal Physiol 273: F739–F748, 1997. [DOI] [PubMed] [Google Scholar]
- 104.Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039–1045, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yoo D, Kim BY, Campo C, Nance L, King A, Maouyo D, Welling PA. Cell surface expression of the ROMK (Kir 1.1) channel is regulated by the aldosterone-induced kinase, SGK-1, and protein kinase A. J Biol Chem 278: 23066–23075, 2003. [DOI] [PubMed] [Google Scholar]
- 106.Yue P, Zhang C, Lin DH, Sun P, Wang WH. WNK4 inhibits Ca-activated big-conductance potassium channels (BK) via mitogen-activated protein kinase-dependent pathway. Biochim Biophys Acta 1833: 2101–2110, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou H, Tate SS, Palmer LG. Primary structure and functional properties of an epithelial K channel. Am J Physiol Cell Physiol 266: C809–C824, 1994. [DOI] [PubMed] [Google Scholar]
- 108.Zhuang J, Zhang X, Wang D, Li J, Zhou B, Shi Z, Gu D, Denson DD, Eaton DC, Cai H. WNK4 kinase inhibits Maxi K channel activity by a kinase-dependent mechanism. Am J Physiol Renal Physiol 301: F410–F419, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]