Abstract
Patients with schizophrenia present with deficits in specific areas of cognition. These are quantifiable by neuropsychological testing and can be clinically observable as negative signs. Concomitantly, they self-administer nicotine in the form of cigarette smoking. Nicotine dependence is more prevalent in this patient population when compared to other psychiatric conditions or to non-mentally ill people. The target for nicotine is the neuronal nicotinic acetylcholine receptor (nAChR). There is ample evidence that these receptors are involved in normal cognitive operations within the brain. This review describes neuronal nAChR structure and function, focusing on both cholinergic agonist-induced nAChR desensitization and nAChR up-regulation. The several mechanisms proposed for the nAChR up-regulation are examined in detail. Desensitization and up-regulation of nAChRs may be relevant to the physiopathology of schizophrenia. The participation of several subtypes of neuronal nAChRs in the cognitive processing of non-mentally ill persons and schizophrenic patients is reviewed. The role of smoking is then examined as a possible cognitive remediator in this psychiatric condition. Finally, pharmacological strategies focused on neuronal nAChRs are discussed as possible therapeutic avenues that may ameliorate the cognitive deficits of schizophrenia.
Keywords: Schizophrenia, Nicotinic receptors
Introduction
Persons with schizophrenia present with alogia, avolition, apathy, anhedonia, asociality, flattening of affect, attentional deficits, decreased spontaneous movements, and clinically observable neurological signs. These signs, classically termed negative, may co-exist with classical positive symptoms such as delusions and hallucinations.
It is now recognized that these signs are directly correlated with profound cognitive deficits and that they represent the core feature of schizophrenia (Heinrichs and Zakzanis 1998; Elvevag and Goldberg 2000; Kuperberg and Heckers 2000; O’Leary et al. 2000; Friedman et al. 2001).
Several cognitive operations within the brain are a function of intact neuronal nicotinic acetylcholine receptors (neuronal nAChRs) localized in discrete neuroanatomical pathways (Levin and Simon 1998; Levin and Rezvani 2002; Levin et al. 2005, 2006). Dementia of the Alzheimer’s type offers a patent example of the correlation between profound cognitive impairment and impaired neuronal nAChR function (Perry et al. 2000). Hence, this review will focus on the evidence that links nAChR function and the cognitive deficits of schizophrenia.
In the first section the structure, localization, and function of neuronal nAChRs will be discussed. Then, the paradoxical phenomenon of nicotine-induced neuronal nAChR up-regulation will be described. This is particularly important since schizophrenic patients who smoke do not exhibit this phenomenon (Breese et al. 2000).
After establishing the basic science background, the possible participation of diverse neuronal nAChR subtypes in the pathophysiology of schizophrenia will be discussed. The emphasis will be on two receptor subtypes: the α7-nAChR subtype which is known to be implied in the pathophysiology of attentional deficits of schizophrenia (Freedman et al. 1996, 1997; Olincy et al. 2006) and in the α4β2-nAChR subtype. This is the most abundant subtype in the brain, and is preferentially activated, desensitized, and up-regulated by nicotine (Flores et al. 1992; Ochoa 1994; Vibat et al. 1995).
The importance of the α4β2-nAChR will be underscored by describing current therapeutic strategies that target this receptor subtype: varenicline, a α4β2-nAChR partial agonist recently approved by the FDA for smoking cessation (Coe et al. 2005a, b) and by allosteric potentiators of the nAChR such as galantamine (Albuquerque et al. 2001; Maelicke et al. 2001; Pereira et al. 2002; Santos et al. 2002). Galantamine has been already used to ameliorate alogia and the attentional and memory deficits of schizophrenic patients (Allen and McEvoy 2002; Rosse and Deutsch 2002; Ochoa and Clark 2004; Bora et al. 2005; Ochoa and Clark 2006; Schubert et al. 2006; Lee et al. 2007).
Nicotinic Acetylcholine Receptors
The nAChRs are ligand-gated cation channels that belong to a gene super family of homologous receptors that includes the NMDA receptor, the γ-aminobutyric acid (GABA) receptor, the glycine receptor, and the 5-HT3 serotonin receptor (Sargent 1993; Karlin and Akabas 1995). nAChRs are known as Cys-loop receptors due to the presence of a conserved sequence containing a pair of cysteines separated by 13 amino acid residues and linked by a disulfide bridge (Hogg et al. 2003). The nAChR has been proposed as a model of an allosteric protein in which effects initiated from the binding of a ligand to a site on the receptor can lead to structural changes in another part of the molecule. When an agonist molecule, such as ACh, binds to the nAChR it produces an allosteric transition that allows the channel to go from the closed to an open state, thus permitting the flow of sodium and potassium cations through the channel pore (Changeux and Edelstein 2005). Nicotinic AChRs are divided into two classes: muscle and neuronal receptors.
Cholinergic pathways have been implied as participating in the physiopathology of schizophrenia (for a recent review see Berman et al. 2007). Furthermore, neuronal nAChRs have been implicated in the pathogenesis of some deficits seen in schizophrenic patients (Freedman et al. 1994). Clinically, this concept is supported by the high prevalence of smoking among these patients (de Leon et al. 1995; DeLeon et al. 1995; Stassen et al. 2000) and by the amelioration effected by nicotine and its agonists of some of the neurophysiological deficits exhibited in this disorder (Levin and Rezvani 2002; Harris et al. 2004).
Neuronal nAChRs
Neuronal nAChRs are distributed throughout the central and peripheral nervous systems. In contrast with their muscle counterparts, they serve more of a modulatory function in synaptic transmission (Role and Berg 1996; Wonnacott 1997). Elucidation of the functional and structural characteristics of neuronal nAChRs started later due to their lower concentrations in more heterogeneous tissues (Lindstrom 1997). Many properties of the neuronal nAChRs, such as their ion selectivity and gating properties, are similar to their counterparts in skeletal muscle and Torpedo tissues.
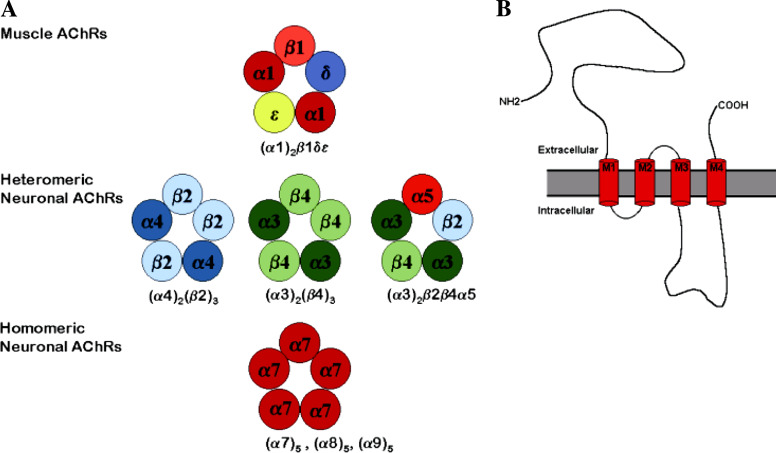
Although these receptors are distinguished by their great diversity (Role 1992; Sargent 1993), neuronal nAChR subunits share many of the structural hallmarks of their muscle relatives, including the prominent N-terminus, four transmembrane domains, a large cytoplasmic loop between M3 and M4 regions, and the short extracellular C-terminal domain (Fig. 1B).
Fig. 1.
(A) Putative subunit arrangements of some nAChR subtypes. The nAChRs have a pentameric structure consisting of five membrane-spanning subunits around a central ion channel. (B) Topology of nAChR subunit. All nAChR subunits share a similar hydrophobicity profile: a large hydrophilic N-terminal domain that faces the extracellular environment, four transmembrane segments (M1, M2, M3, and M4), a variable cytoplasmic domain between M3 and M4, and a short extracellular carboxylic domain
Overview of Neuronal Nicotinic Receptor Structure
Like nAChR from the neuromuscular endplate, neuronal nAChRs have a pentameric structure with five rod-like membrane spanning regions around a central ionic channel, but do not contain γ, δ, or ε subunits. Instead, most neuronal nAChRs are formed only by α and β subunits. Their functional properties result from the assembly of the α and β subunits within the receptor complex (Buisson and Bertrand 2001).
The α subunits are characterized by the presence of a cysteine pair homologous to position 192 and 193 of muscle α subunit, whereas β subunits lack this cysteine pair. Presently, nine subtypes of α subunitS (α2-α10) and three subtypes of β subunits (β2-β4) have been identified and cloned in vertebrate systems (Fig. 1A). The overall amino acid homology between the genes coding for the neuronal and muscle subunits genes from the same species is about 40–55% (Sargent 1993). Homology is higher (∼100%) in the membrane-spanning regions M1–M3 and in certain regions of the N-terminal domain (Fig. 1B), albeit the amino acid sequence of the cytoplasmic domain between M3 and M4 transmembrane segments is divergent (Sargent 1993).
Neuronal nAChRs also differ from their skeletal muscle counterparts in their biophysical properties. Two characteristics that distinguished neuronal nAChRs are: inward rectification and large calcium permeability (Bertrand et al. 1990; Vernino et al. 1992, 1994 Seguela et al. 1993). This inward rectification occurs independently of the current polarity and depends primarily upon the membrane potential and internal Mg2+ (Mathie et al. 1990; Neuhaus and Cachelin 1990; Sands and Barish 1992) reviewed by Sargent (1993). Different neuronal nAChRs have different permeabilities to Ca2+, but overall they are more permeable to this cation than muscle nAChRs. The pCa2+/pNa+ for neuronal nAChRs ranges from 15 to 0.5, whereas the muscle nAChR displays a pCa2+/pNa+ of ∼0.2 (Decker and Dani 1990; Sands and Barish 1991; Adams and Nutter 1992; Vernino et al. 1992; Changeux et al. 1998).
The passage of Ca2+ through nAChR channels could activate intracellular cascades or other ion channels and potentially induce changes in the phosphorylation states of specific nAChR subunits (Vijayaraghavan et al. 1990; Mulle et al. 1992; Vernino et al. 1992; Nakayama et al. 1993).
The neuronal nAChRs can be divided into two main groups: the heteromeric and the homomeric receptors (Fig. 1A). The heteromeric receptors are composed of α and β subunits in different stoichiometries. The homomeric receptors consist of one type of subunit only. When α2, α3, or α4 subunits are expressed in pair wise combinations with β2 or β4 subunits, functional receptors with different electrophysiological and pharmacological properties can be assembled (i.e., α2β2, α2β4, α3β2, α3β4, α4β2, α4β4) (Sargent 1993; McGehee and Role 1995; Gotti et al. 2006). Although α subunits contain the ACh binding sites, several studies have revealed that β subunits have a strong influence on the dissociation rate of agonists and antagonists from the receptor, as well as on the opening rate of an agonist-bound receptor (Papke and Heinemann 1991; Papke et al. 1993; Paterson and Nordberg 2000).
The assembly of three or more neuronal subunit types could also form functional heteromeric nAChRs (Role and Berg 1996; Wang et al. 1996). For example, although the α5 subunit cannot form functional receptors in combination with β2 or β4, when expressed with α3 and β2 or α3 and β4, it gives rise to functional nAChRs (Ramirez-Latorre et al. 1996; Wang et al. 1996). In this respect, a neuronal nAChR can be detected in ciliary ganglion neurons with a α3β2β4α5 subunit composition (Vernallis et al. 1993). Similarly, the β3 subunit, forms functional nicotinic receptors only when co-expressed with at least two other subunit types (Groot-Kormelink et al. 1998).
Neuronal nAChR Cellular Localization and Function
In contrast with muscle nAChRs that have a predominant postsynaptic localization, most neuronal nAChRs are located presynaptically at cholinergic and at noncholinergic terminals (i.e., they are heteroreceptors). At these locations, they regulate cholinergic, glutamatergic, dopaminergic, serotoninergic, adrenergic, and endogenous opiate neurotransmission (McGehee et al. 1995; McGehee and Role 1995; Wonnacott 1997).
Nicotine regulates ACh release from areas involved in cognition that are putatively defective in schizophrenia: cholinergic terminals from rat cortex and striatum (Rowell and Winkler 1984) (Rowell and Wonnacott 1990), (O’Shea and Ochoa 1993; Ochoa and O’Shea 1994) and from rat hippocampus (Wonnacott and Thorne 1990; Tandon and Ochoa 1992).
These sites are identified as functional presynaptic α3β2 and α4β2-nAChRs and modulate dopamine release from rat cortex and nigrostriatal terminals (Soliakov and Wonnacott 1996; Luo et al. 1998; Wonnacott et al. 2000; Hogg et al. 2003) (Sharples et al. 2000). There is also evidence to support the presynaptic locus of α7-nAChRs in the hippocampus, where they regulate glutamate release (Gray et al. 1996).
Presynaptic facilitation of neurotransmitter release by nAChRs implies their agonist-induced activation, which in turn induces depolarization of presynaptic membranes and Ca2+ entry into the presynaptic terminal (McGehee et al. 1995) through voltage activated Ca2+ channels (Hogg et al. 2003). Desensitization of these receptors may mediate inhibition of neurotransmitter release, providing a modulatory mechanism for this function.
Postsynaptic α9 nAChRs have been described in rat cochlear hair cells (Elgoyhen et al. 1994), where they mediate a long-lasting inhibitory response through Ca2+-activated potassium channels (Fuchs 1996). In the autonomic nervous system, α3 containing nAChRs also act primarily as postsynaptic receptors (Sargent 1993; Vernallis et al. 1993; Role and Berg 1996; Lindstrom 1997).
In addition to postsynaptic and presynaptic sites, nAChRs have been found at perisynaptic, extrasynaptic, and somatodendritic locations. Perisynaptic α7 nAChRs with trophic and neurotransmission functions have been detected in ciliary ganglia (Horch and Sargent 1995; Zhang et al. 1996; Ullian et al. 1997) and in hippocampal interneurons (Hurst et al. 2005). nAChRs located in the somatodendritic area are involved in catecholamine release (Rahman et al. 2003), whereas extrasynaptic nAChR localizations may provide for a novel mechanism of communication within the brain (Coggan et al. 2005).
Relevance of Presynaptic nAChRs in Schizophrenia
The most abundant nAChRs in the central nervous system are the α4β2-nAChRs, accounting for >90% of the high-affinity nicotine binding sites in the brain (Whiting and Lindstrom 1988; Flores et al. 1992; Brody et al. 2006).
This receptor subtype is the one preferentially activated, desensitized and up-regulated by nicotine (Flores et al. 1992; Ochoa 1994; Vibat et al. 1995) and it may be crucial to both the pathophysiology of nicotine dependence (Ochoa et al. 1990; Ochoa 1994) and schizophrenia (Freedman et al. 1997).
Dopaminergic neurons located at the mesencephalic ventral tegmental area (VTA) project to the dorsolateral prefrontal cortex (mesocortical dopaminergic pathway) and to several limbic areas of the brain such as the nucleus accumbens (mesolimbic dopaminergic pathway) (Di Chiara and Imperato 1988; Di Chiara 2000).
Nicotine stimulates dopamine release in the mesolimbic pathway (Corrigall et al. 1992) by way of nAChRs located in the VTA (Nisell et al. 1994, 1995) and the nucleus accumbens (Pontieri et al. 1996). This nicotine-induced release of dopamine has been correlated with the pleasurable effects of nicotine experienced by smokers (Benowitz 1992).
VTA neurons can be over stimulated in stressful conditions (Horger and Roth 1996) a fact particularly relevant to schizophrenia, where different types of stressors are known to precipitate a psychotic break (Lysaker et al. 2005).
Agonist-Induced Desensitization of nAChR Function
A general feature of nAChRs is that they are reversibly desensitized on chronic exposure to an agonist (Ochoa et al. 1989; Ochoa 1994; Dani and Heinemann 1996). Desensitization has been defined as a decrease or loss of the biological response after prolonged or repetitive stimulation (see review in Ochoa et al. 1989 and references therein).
The onset of desensitization depend on both time and agonist concentration (Katz and Thesleff 1957). These authors first demonstrated that prolonged exposure to sub-stimulating concentrations of nicotinic agonists reduced receptor function. They further proposed a cyclical model postulating inter-convertible high- and low-affinity agonist binding sites to explain the desensitization observed following an exposure to non-stimulating and stimulating concentration of agonists, respectively.
According to this model, the affinity of the nicotinic receptor for nicotine is higher in the desensitized state (D) than for the activable state (R) (k0 >> k1). Therefore, under prolonged nicotine exposure, receptors should stabilize in the agonist-bound desensitization state (AD).
Nicotine-Induced Up-Regulation of Neuronal nAChR
One of the most striking and puzzling effects of chronic nicotine exposure is the up-regulation of neuronal nAChRs, and in particular the α4β2 subtype in the CNS (Marks et al. 1983; Schwartz and Kellar 1985; Benwell et al. 1988a, b; Flores et al. 1992; Breese et al. 1997; Whiteaker et al. 1998). For a comprehensive review see Gentry and Lukas (2002).
Postmortem binding studies have revealed increased [3H]-nicotine and [3H]-ACh binding sites in the brains of smokers as compared to nonsmokers, with an increase in binding sites being dependent on nicotine dose (Benwell et al. 1988a, b; Breese et al. 1997). The nicotine-induced up-regulation of nAChR binding sites has been termed paradoxical (Wonnacott 1990), because chronic agonist treatment is expected to down-regulate receptor numbers.
Up-regulation of neuronal nAChR induced by chronic nicotine exposure has also been demonstrated in different in vivo systems, including rats (Schwartz and Kellar 1985; Collins 1990; Flores et al. 1992) and mice (Marks et al. 1983), and in vitro systems such as cell lines (Peng et al. 1994a, b; Gopalakrishnan et al. 1997; Whiteaker et al. 1998) and heterologous expression system (e.g., Xenopus oocytes) (Peng et al. 1994a, b; Fenster et al. 1999b; Lopez-Hernandez et al. 2004).
This phenomenon reflects an apparent increase in receptor number rather than an increase in their affinity for nicotine (Marks and Collins 1985; Schwartz and Kellar 1985; Sanderson et al. 1993; Peng et al. 1994a). It is not dependent on cell type, since α4β2-nAChRs expressed in fibroblasts or in oocytes also exhibit nicotine-induced up-regulation of these receptors (Peng et al. 1994a; Lopez-Hernandez et al. 2004). This notion, held for about 20 years, has been challenged recently (see below (Vallejo et al. 2005).
In humans, up-regulation is reversible (Breese et al. 1997). It is associated with tolerance to nicotine in rodents (Collins et al. 1988, 1990; Marks et al. 1993) and coincides with the time course of development of behavioral tolerance to nicotine (Marks et al. 1983; Hulihangiblin et al. 1990). Behavioral tolerance is related to desensitization of neuronal nAChR function (Marks and Collins 1993; Marks et al. 1993), but the relationship between up-regulation and dependence to nicotine is still obscure (Collins et al. 1990; McCallum et al. 1999, 2000).
Nicotine-induced up-regulation is not unique to the α4β2-nAChRs. Chronic intravenous infusion of mice with nicotine elicited an increase in brain [125I]-α-bungarotoxin ([125I]-αBgt) binding (Marks et al. 1985).
α7-nAChRs are the predominant αBgt-binding proteins in the brain. They have a higher affinity for nicotine than for ACh, but much lower affinity for nicotine than do α4β2-nAChRs (Anand et al. 1993). The extent and the duration of nicotine-induced up-regulation of [125I]-αBgt binding sites in rat brain were both less than the increase in [3H]-nicotine binding (Marks et al. 1985).
In vitro experiments showed that chronic nicotine exposure of hippocampus neurons elicits a 40% increase in the number of [125I]-αBgt binding sites (Barrantes et al. 1995). More recently, Molinari et al. reported that long-term exposure to nicotine increase the density of α7 nAChR stably expressed in HEK 293 cells (Molinari et al. 1998).
The half-maximal effective concentration to induce up-regulation of α4β2-nAChRs expressed in mouse fibroblasts (M10 cells) and Xenopus oocytes are 0.21 and 0.19 μM nicotine, respectively (Peng et al. 1994a, b). These values are very close to the typical, mean, steady state, serum concentration of nicotine in tobacco smokers (0.100-300 nM) (Benowitz 1990).
The affinity of nAChRs for nicotine and cholinergic ligands is unchanged (Marks et al. 1983; Schwartz and Kellar 1983, 1985; Collins and Marks 1987; Marks et al. 1992; Sanderson et al. 1993).
Up-regulation of α3 and α7 receptors, unlike α4β2-nAChRs, requires much higher-nicotine concentrations than those encountered in smokers. The upregulation of α3 receptors is more complex, for instance, α3β4 receptors do not upregulate well with nicotine, whereas α3β2 receptors do (Xiao and Kellar 2004). α3-containing and α7 nAChRs up-regulate at EC50 values of 100 and 65 μM nicotine, respectively, which are higher values than the nicotine concentration typical of smoker’s serum (Lindstrom 1997). Also, the extent of increase in cell surface nAChRs is less; and the mechanisms of up-regulation are different than for α4β2 nAChRs (Peng et al. 1997)
On the other hand, peripheral (i.e., skeletal muscle) nAChRs or ACh receptors of the muscarinic type are not up-regulated by nicotine (Marks et al. 1983; Collins and Marks 1987; Sanderson et al. 1993).
Up-Regulation of Neuronal nAChRs Induced by Agents other than Nicotine
Nicotine is not unique in eliciting up-regulation of α4β2 nAChRs following chronic treatment. Endogenous ACh can also up-regulate α4β2 receptors (Gopalakrishnan et al. 1997; Whiteaker et al. 1998). Studies in vivo have shown an increase in high-affinity agonist binding following chronic intraperitoneal injection with cytisine (Schwartz and Kellar 1985), chronic subcutaneous infusion of anatoxin-α (Rowell and Wannacott 1990), and chronic intraventicular injection of methylcarbachol to rats (Yang and Buccafusco 1994).
Also, chronic infusion of anabasine produced an increase of high-affinity nicotinic sites in mice (Bhat et al. 1991). Furthermore, studies in cells and cell lines have shown that some nicotinic agonists can induce up-regulation of α4β2-nAChRs (Gopalakrishnan et al. 1997; Whiteaker et al. 1998; Fenster et al. 1999b).
Repeated administration of the nicotinic agonist cytisine, also results in nicotinic receptor up-regulation (Schwartz and Kellar 1985). (+)-Anatoxin-α, a secondary amine, increased the density of nicotine binding sites in striatal synaptosomes (Rowell and Wonnacott 1990). Membrane impermeable quaternary amines, including the nicotinic agonist 1,1-dimethyl-4-phenylpiperazinium (DMPP) and carbamylcholine, also cause up-regulation of α4β2 nAChRs in vitro (Peng et al. 1994a). DMPP and carbamylcholine may be assumed to produce their effects from outside the cell on α4β2 nAChRs that are already in the surface membrane (Peng et al. 1994a).
Additionally, cholinergic ligands of human α4β2-nAChRs expressed in HEK293 cells also induce up-regulation (Gopalakrishnan et al. 1997). Treatment with (−)-cytisine, DMPP, (±)-epibatidine, ABT-418, and A-85380, for 168 hours increases [3H]-cytisine binding level in a concentration-dependent manner (Gopalakrishnan et al. 1997). The relative potencies were (±)-epibatidine >A-85380 > (−)-nicotine > (+)-nicotine > (−)-cytisine > ABT-418 > DMPP. The EC50 values for up-regulation correlated well with their binding affinities, indicating that receptor up-regulation may be related to the interaction of these ligands with the high-affinity desensitized state of nAChRs (Gopalakrishnan et al. 1997).
Other extensive studies of agonist-induced up-regulation of α4β2 nAChRs in M10 cells have covered a wide spectrum of nicotinic agonists, including (−)-nicotine, (±)-epibatidine, methylcarbamylcholine (MCC), (±)-Anatoxin-a, (−)-cytisine, ABT-418, and tetramethylammonium (TMA) (Whiteaker et al. 1998). The maximum up-regulation elicited by each of the agonists was similar, except forfs MCC and (±)-epibatidine, which elicited only 16% and 38% respectively, of the maximum up-regulation observed for (−)-nicotine. Thus, MCC and (±)-epibatidine were less efficacious as inducers of the up-regulation phenomenon.
Chronic treatment with neuronal-nAChR antagonists could elicit nAChR up-regulation, although data are contradictory for some ligands. For example, the noncompetitive antagonist mecamylamine causes up-regulation of the α4β2 nAChRs (Peng et al. 1994a) but a chronic injection effect has not been detected with this compound (Schwartz and Kellar 1985). In addition, no up-regulation of α4β2 nAChRs expressed in M10 cells for mecamylamine (Gopalakrishnan et al. 1997; Whiteaker et al. 1998).
Competitive antagonists such as d-tubocurarine, dihydro-β-erythroidine (DHβE), and methyllycaconitine increase [3H]-cytisine binding in HEK293 cells (Gopalakrishnan et al. 1997), leading to the proposal that nAChR functional stimulation is not required to induce up-regulation.
Since different expression systems and experimental conditions may be responsible for the aforementioned discrepancies, more work is needed to define the role of agonist, competitive and noncompetitive antagonists, competitive and noncompetitive blockers, allosteric activators and inhibitors in the up-regulation of nAChR number in these in vitro conditions
It has become very evident that the mechanisms involved in the up-regulation of nAChRs are diverse and structure-dependent. From a critical perspective, we suggest that nicotinic or other ligands acting at different sites (Fig. 2), and perhaps through diverse mechanisms, to regulate stabilization of nAChR in distinct conformations representing functionally active, functionally inactive, or up-regulated states.
Fig. 2.
Schematic cross section of the nAChR showing ion channel, ACh binding site and multiple type of ligands (NCA, CA, NCB, AA, AI, and CB) extracellular domains and ion-channel pathway of the receptor
In order to induce up-regulation, a given drug must induce a conformational state of the nAChR which is directly (or indirectly) linked to the trasmembrane signal that will trigger the incorporation of presynthesized receptors into the plasma membrane. This hypothesis departs from the traditional proposal that agonists lock nAChRs in a stable conformation, leading to their accumulation in the plasma membrane.
Functionality of Up-Regulated nAChRs
Chronic smokers typically have steady state plasma nicotine concentration of 10–50 ng/ml (100–300 nM nicotine; (Benowitz 1990)). However, nicotine levels in the mammalian brain are about three times higher (Ghosheh et al. 2001).
At this concentration of nicotine, the α4β2 nAChR will be activated preferentially over other subtypes. It has been suggested that in chronic smokers most α4β2-nAChRs (50%) are inactivated at average serum nicotine concentrations (Brody et al. 2006). The EC50 for this AChR subtype is 1–3 μM (Lopez-Hernandez et al. 2004).
Recent evidence using PET scans of human smokers with the newly developed radioligand 2-FA (specific for α4β2-nAChRs) showed that their α4β2-nAChRs are totally saturated in a 24 h period (Brody et al. 2006). Saturation should keep these receptors in the desensitized state (alleviating withdrawal symptoms), and induce up-regulation of nAChRs, as seen in chronic smokers (Benwell et al. 1988a, b).
Continued smoking, despite saturation of receptor sites, may occur in order to avoid having unoccupied receptors (responsible for craving). Alternatively, persistent smoking may produce positive reinforcement via nicotine-induced activation of other α4β2 receptors of unknown stoichiometry (which are not labeled by the radioligand) (Brody et al. 2006). This study is particularly relevant to elucidate the basic mechanisms of nicotine dependence in schizophrenic smokers.
In contrast, α3-nAChRs (EC50 30 μM), α7-nAChRs (EC50 10 μM) and muscle type nAChRs (EC50 100 μM) are more resistant to inactivation by chronic nicotine exposure This has been explained postulating that a significant fraction of the α3-nAChRs, and the α7-nAChRs subtypes are already in the desensitized state due to their higher affinity for nicotine (10nM and 1.0 μM) (Quick and Lester 2002; Giniatullin et al. 2005).
Thus, while α4β2-nAChRs are subject to desensitization and functional inactivation by low concentrations of nicotine, α3β2-nAChRs are not permanently inactivated by chronic exposure to nicotine (Hsu et al. 1996), and α7-nAChRs are subject to a more rapid desensitization than α4β2-nAChRs (Albuquerque et al. 2000).
Although nicotine-induced up-regulation of nAChRs have been demonstrated to occur in both in vivo and in vitro experiments, it is still unclear whether this increase in receptor number is accompanied by long-lasting inactivation or functional up-regulation.
It is commonly held that functional activity of neuronal nAChRs is lost as a result of a rapid and persistent desensitization induced by chronic nicotine exposure (Sharp and Beyer 1986; Lapchak et al. 1989; Lukas 1991; Marks and Collins 1993; Olale et al. 1997; Shioda et al. 1997; Ke et al. 1998).
Chronic nicotine treatments induce up-regulation, which produces a non-functional, desensitized receptor (Lapchak et al. 1989; Hulihangiblin et al. 1990; Marks et al. 1993). This can be caused by agonists (e.g., nicotine) or by any agent that promotes a nonfunctional state of the receptor [e.g., mecamylamine (Collins et al. 1994; Peng et al. 1994a) or chlorisondamine (El-Bizri and Clarke 1994). Behavioral desensitization, however, is specific to the agonist (Yang and Buccafusco 1994). Recently, Girod and Role (2001) reported that presynaptic nAChR function could be lost for >24 h following a 24–72 h treatment with low doses of nicotine.
In contrast with these traditional notions based on a series of experimental results, other studies using cell lines suggested that nAChRs are functionally more active with chronic nicotine exposure (Gopalakrishnan et al. 1997; Buisson and Bertrand 2001; Nelson et al. 2003; Vallejo et al. 2005).
The difference in the effects of nicotine detected in these studies could be explained by different incubation requirements, such as temperature or culture medium of the different expression systems. Furthermore, mammalian cell lines and Xenopus oocytes may differ in the protein kinase modulation of channels expressed or in the posttranslational modifications made inside the host cells [reviewed by (Hogg et al. 2003)].
Proposed Mechanisms of Neuronal nAChR Up-Regulation
Nicotine-Induced Desensitization of Neuronal nAChRs
Both acute and chronic specific desensitization of nAChR (Changeux and Revah 1987; Ochoa et al. 1989, 1990; Changeux 1990; Ochoa 1994) have been invoked to explain the effects of nicotine on the smoker’s brain. Specific acute desensitization (tachyphylaxis) to nicotine is reversible, has rapid rates of onset and recovery, and does not correlate with alterations in nAChR number. It is responsible for developing daily tolerance to several acute effects of nicotine that resensitize rapidly overnight (e.g., muscular relaxation or enhanced cognition).
Specific chronic desensitization occurs after prolonged exposure to nicotine is less reversible than the acute type and has slower rates of development and recovery, and may be responsible for tolerance to both the rewarding properties of nicotine and to aversive effects such as nausea or dizziness.
Chronic nicotine use may induce tolerance via repeated cycles of activation of reversibly desensitized receptors and a progressive shift of the total population of activatable receptors to the desensitized state (Ochoa et al. 1990). Chronic agonist treatment could lead to an incomplete recovery that results from a long-lasting accumulation of receptors in one or more desensitized states or a permanent loss of functional channels (Lukas 1991) reviewed by (Quick and Lester 2002).
Desensitization of neuronal nAChRs may be the driving force for the up-regulation (Marks et al. 1983; Schwartz and Kellar 1985; Fenster et al. 1999a), but acute administration of nicotine and rapid desensitization are not sufficient to produce up-regulation. Prolonged administration and chronic desensitization appear to be important (Schwartz and Kellar 1985).
Chronic agonist exposure may thus result in an initial rapid desensitization, leading to further chronic inactivation of function and cholinergic deficit which is then counteracted by an increase in receptor number (Schwartz and Kellar 1985). This increase in nAChR number could be responsible for nicotine tolerance and dependence.
When the concentration of nicotine is low or absent in the brain of chronic smokers, the excess nAChRs recover from desensitization, causing hyperexcitability at the cholinergic synapses (Dani and De Biasi 2001). The hyperexcitability of the nicotinic cholinergic system could explain the urge to continue smoking cigarettes throughout the day and the withdrawal symptoms experienced by smokers when they stop smoking.
Individual nAChR subtypes vary in sensitivity to desensitization and inactivation following agonist exposure. We have demonstrated that both the rate of desensitization resulting from prolonged exposure to an agonist and the rate of recovery from desensitization depends on the subunit composition of the receptors (Vibat et al. 1995).
Three rat neuronal nAChR subunit combinations were expressed in Xenopus laevis oocytes (α2β2, α3β2 and α4β2). In contrast with results obtained using the other two-subunit combinations, the α4β2-nAChR exhibited a depression of the maximum in the dose-response curves, a slower rate of nicotine desensitization, and a depression in the response to nicotine after repetitive application of the agonist.
Although the α4β2 subunit combination showed a slower-desensitization rate compared to its α2β2 and α3β2 counterparts, it remained longer in the desensitized state (Vibat et al. 1995). Precisely, the α4β2-nAChR is the one that is more abundant in the human brain and the one that is predominantly up-regulated after chronic nicotine exposure in the CNS (Flores et al. 1992; Whiteaker et al. 1998).
Later, Fenster et al. (1999b) presented four additional lines of evidence which suggest that nicotine-induced desensitization of neuronal nAChR initiates up-regulation: (1) the half-maximal nicotine concentration necessary to produce desensitization (9.7 nM) was the same as that needed to induced up-regulation (9.9 nM); (2) the concentration of [3H]-nicotine for half-maximal binding to surface nAChRs on intact oocytes (11 nM) was comparable with that for desensitization; (3) functional desensitization of α3β4 receptors required a 10-fold higher-nicotine concentrations that was mirrored by a 10-fold shift in concentrations necessary for up-regulation; and (4) mutant α4β2 receptors that do not recover fully from desensitization were up-regulated after acute (1 hour) application of nicotine.
Other lines of evidence support the desensitization hypothesis. For example: the concentrations of nicotine that induce half-maximal increases in nicotinic binding for α4β2, α3, and α7-containing nAChRs are qualitatively similar to the concentrations required for desensitization of rat receptors expressed in Xenopus oocytes (Peng et al. 1994a, 1997). In this cell, α4β2 receptors are 50-fold more sensitive to nicotine-induced desensitization than α3- or α7-containing subtypes (Fenster et al. 1997).
Protein Kinase Modulation of nAChR Desensitization
Phosphorylation of nAChR subunits regulates many aspects of receptor function, including desensitization [reviewed by (Quick and Lester 2002)]. It is possible that such intracellular mechanisms could confer long-lasting changes during abnormal conditions, as those that occur during and after chronic nicotine exposure (Peng et al. 1994a; Fenster et al. 1999b).
As previously discussed before, chronic nicotine exposure (at levels related to those found on active smokers) both activates and desensitizes α4β2-nAChRs (Vibat et al. 1995; Fenster et al. 1997), a phenomenon believed to be responsible for initiating nAChR up-regulation (Marks et al. 1983; Schwartz and Kellar 1985; Fenster et al. 1999b).
It seems conceivable that factors that regulate desensitization of α4β2-nAChRs may contribute to the long-term effects of nicotine on neuronal nAChR number and on their function (Fenster et al. 1999a).
Evidence for the role of phosphorylation in the desensitization process came from muscle nAChRs. Direct phosphorylation of γ and δ subunits at serine residues by protein kinase A (PKA) increased the rate of desensitization (Huganir et al. 1986). Subsequently, studies of neuronal nicotinic receptors in chick sympathetic ganglia (containing α3, α4, α5, α7, β2, β3 and β4 subunits) have indicated that cyclic AMP-dependent PKA and protein kinase C (PKC) can phosphorylate this receptors, and that phosphorylation may regulate agonist-induced desensitization (Downing and Role 1987; Vijayaraghavan et al. 1990).
As mentioned earlier, nAChRs display high Ca2+ permeability (Mulle et al. 1992; Vernino et al. 1992). Thus, Ca2+ entry through these channels could regulate nicotinic receptor desensitization either directly or indirectly via protein kinase activation (Quick and Lester 2002).
In addition to the effect of Ca2+ ions on desensitization of skeletal nAChRs (Miledi 1980), it was demonstrated that the recovery rate from desensitization was most influenced by Ca2+ (Khiroug et al. 1997; Fenster et al. 1999a). Nevertheless, the way in which this cation affects the rate of recovery from desensitization remains elusive.
Whereas increased levels in intracellular Ca2+ following nAChR stimulation inhibit recovery from desensitization (Khiroug et al. 1997), this process is largely eliminated after replacement of external Ca2+ with Ba2+(Fenster et al. 1999a). It has been proposed that Ca2+ facilitates recovery from desensitization through the activation of PKC (Fenster et al. 1999a).
Activation of protein kinases (such as PKA and PKC) has been demonstrated to promote recovery from desensitization (Khiroug et al. 1998; Fenster et al. 1999a). Moreover, several studies suggested that desensitization is mediated by an alteration in the balance between the functions of protein kinase and protein phosphatase: inhibition of a Ca2+-dependent phosphatase speeds recovery from desensitization and a decrease in cellular phosphorylation prolongs the time the receptor remains in the inactivated state (Quick and Lester 2002).
Since the role that phosphorylation of nAChR subunits play on the desensitization process is still unknown, it is important to evaluate what nAChR subunits are subjected to additions of phosphate moieties. The majority of the consensus sequences for phosphorylation are located in the cytoplasmic domain between M3 and M4 transmembrane regions (Swope et al. 1992).
For example, the α4 subunit contains at least nine serine phosphorylation consensus sites. Several in vitro studies have demonstrated that some of these sites are directly phosphorylated by PKA or PKC (Vijayaraghavan et al. 1990; Nakayama et al. 1993; Moss et al. 1996; Wecker et al. 2001). Biochemical studies on rat α4β2- nAChR either isolated from rat brain (Nakayama et al. 1993) or immunoprecipitated from Xenopus oocytes (Hsu et al. 1997) provided evidence that the α4 subunit is a substrate for phosphorylation by PKA.
There are also phosphorylation sites for cyclic AMP-dependent protein kinase (PKA) and protein kinase C in the amino acid sequence corresponding to the M3/M4 cytoplasmic domain of the α4 subunit expressed in Xenopus oocytes (Wecker et al. 2001; Guo and Wecker 2002; Wecker and Rogers 2003). Ser365, Ser472, and Ser491 are phosphorylated by PKA, suggesting that these positions represent posttranslational regulatory sites on the(α4β2 nAChR (Guo and Wecker 2002). S470, S493, S517, and S590 are not phosphorylated by PKC or PKA. Elimination of a PKC phosphorylation site in the α4 subunit, by the replacement of a serine at position 336 for an alanine residue, inhibited recovery from desensitization of α4β2 nAChRs expressed in Xenopus oocytes (Fenster et al. 1999a).
Since phosphorylation of nAChR subunits plays a pivotal role in nAChR desensitization, the study of factors that alter the balance of protein kinase/protein phosphatase function could be critical to fully understand α4β2-nAChR up-regulation.
Reduced Receptor Turnover
Although receptor desensitization may be important in the initial stages of receptor up-regulation, it is possible that other factors also mediate increases in receptor number. For example, chronic nicotine exposure of M10 cells expressing α4β2 receptors produced a decrease in the rate of receptor turnover (Peng et al. 1994a).
The decrease in the rate of receptor turnover could explain the nicotine-induced increase in nAChR number. It was unlikely that nicotine regulated nAChR expression by changing transcriptional activities, because the levels of messenger RNA for both the α4 and β2 protein subunits were not changed after chronic nicotine exposure (Peng et al. 1994a).
Surface Versus Internal Up-Regulation
A very elegant study by Darsow et al. appears to contradict the notion that increased turnover is a satisfactory explanation of receptor up-regulation (Darsow et al. 2005). Using HEK293 cells expressing the α4β2-nAChR and brefeldin A, a fungal metabolite that disrupts transport of secreted proteins from the endoplasmic reticulum to the Golgi, it was found that neither acute nor chronic administration of nicotine altered endocytic trafficking of α4β2 2nAChRs. Instead, chronic nicotine exposure increased α4β2-nAChR number transiting from the endoplasmic reticulum (ER) to the surface membrane. A similar result was obtained using the same HEK293 cells and human neuroblastoma cells transfected with the α4β2-nAChR (Sallette et al. 2005).
These results are consistent with previous studies on the muscle AChR, where the limiting step for surface expression appears to be at the ER (Ross et al. 1991; Wang et al. 2002). Based on these findings, nAChR up-regulation would occur prior to their insertion in the plasma membrane.
Influence of Receptor Subunit Composition in Up-Regulation of the α4β2-nAChR
Although the (α4)2(β2)3 was the subunit stoichiometry proposed for the α4β2- nAChR expressed in Xenopus oocytes (Anand et al. 1991; Cooper et al. 1991), several functional studies suggest that this is not the only stoichiometry present in cells that express this nAChR subtype.
Patch clamp recordings from oocytes expressing the α4β2 nAChR have demonstrated that single-channel conductance depends on the α:β ratio of the mRNA injected into the oocyte (Papke et al. 1989). When three different ratios of α4 and β2 were injected into the nucleus of Xenopus oocytes, different sensitivities to ACh and d-tubocurarine were obtained using voltage clamp recording (Zwart and Vijverberg 1998)suggesting that the subunit stoichiometry of functional heteromeric α4β2 nAChRs is not limited to (α4)2(β2)3.
Furthermore, using human embryonic kidney cells as a expression system, two functional types of α4β2 nAChRs have been reported (Nelson et al. 2003). The predominant subunit stoichiometry of α4β2 nAChRs expressed in human embryonic kidney cells was 3(α4):2(β2), but overnight nicotine exposure increased the proportion of nAChRs with a 2(α4):3(β2) stoichiometry.
The α4:β2 mRNA stoichiometry plays a critical role in nAChR up-regulation and functional loss induced by chronic nicotine exposure (Lopez-Hernandez et al. 2004). Oocytes microinjected with the 2(α4):3(β2) stoichiometry displayed an increase in membrane nAChR following chronic nicotine exposure. After chronic nicotine exposure, the 2(α4):3(β2) was the only stoichiometry that clearly up-regulated. In contrast, 4(α4):1(β2) which produced the largest amount of macroscopic current, apparently, showed down-regulation and 1(α4):4(β2) did not show a significant change. Since the stoichiometry with the highest proportion of the α4 subunit, 4(α4):1(β2), resulted in receptor down-regulation and drastic loss in function, our data suggest that the α4 subunit controls the inactivation and trafficking of the nAChR heteropentamer (Lopez-Hernandez et al. 2004).
The properties of agonist binding for α4β2 channel activation might also have distinct dynamics or perhaps structural requirements for ACh and nicotine.
In contrast to the nicotine-induced activation of α4β2-nAChR that appeared to be independent of the subunit ratio expressed on the oocyte surface, desensitization was remarkably affected by acute nicotine exposure. Activation and desensitization of α4β2-nAChR by nicotine could be triggered by two independent mechanisms, which in turn suggest the possibility of at least two distinct binding sites for nicotine.
Since nicotine-induced desensitization and up-regulation of α4β2 nAChR is regulated by subunit ratio (Lopez-Hernandez et al. 2004) and given that this receptor subtype is wide spread in brain presynaptic terminals, control of the subunit ratios of these heteropentamer receptors could regulate neurotransmitter release in the central nervous system and nicotine sensitivity in humans.
We suggest that abnormal up-regulation of receptor subtypes such as the one observed in vitro (Lopez-Hernandez et al. 2004) should be considered from an in vivo perspective, when considering the pathophysiology of schizophrenia, where neuronal nAChRs with different subunit types and stoichiometries could be responsible for defective up-regulation of nicotinic receptors (Breese et al. 2000) (see discussion below).
An indirect support for this argument could be found in a recent study by De Luca et al. (2006) where the polymorphisms in the CHRNA4 and the CHRNB2 genes (which control the expression of the neuronal α4 and β2 subunits respectively) were explored in 117 Canadian families having at least one schizophrenic patient.
The interactions between the CHRNA4 and CHRNB2 genes produced a significant risk effect for schizophrenia, but this was not the case for each gene acting separately. The effects of the interactions between other genes for other neuronal nAChRs are still unknown.
Another Mechanism Proposed for Up-Regulation
In contrast to the aforementioned studies, chronic nicotine treatment of K-177 cells expressing the human α4β2-nAChR resulted in functional receptor up-regulation (Buisson and Bertrand 2001). Using a heterologous expression system, the same authors reported an increase in the ACh-evoked response measured by whole cell voltage clamp, contradicting the notion of that these receptors are non functional after chronic exposure to nicotine (Vallejo et al. 2005).
These investigators hypothesized that chronic nicotine treatment produced a conversion from low-affinity nAChRs into high-affinity nAChRs (the phenomenon being termed “functional up-regulation”). The two distinct affinities observed for the ACh concentration–response curves were interpreted as a result of two distinct functional states of the α4β2 receptors. Subunit phosphorylation was suggested as a possible mechanism for the interconversion from low-affinity into high-affinity α4β2-nAChRs.
The up-regulated AChR displayed a larger single-channel conductance, suggesting a remarkable functional change of state with nicotine exposure. This functional change was probably not due to a change in subunit stoichiometry, since a change in receptor subunit stoichiometry was unlikely after receptor insertion into the plasma membrane.
Thus, nicotine may slowly transform α4β2-nAChRs into an up-regulated state that displays increased response and sensitivity to agonist (Vallejo et al. 2005).
Abnormal Nicotine-Induced Regulation of Receptor Number in Schizophrenia
The human brain exhibits the phenomenon of receptor up-regulation (Benwell et al. 1988a, b). Simmilarly, autopsy material from chronic smokers showed increased number of nAChRs depending on the number of cigarettes smoked daily (Breese et al. 2000). This was evidenced by 3H-epibatidine binding to postmortem material from hippocampus, cortex, caudate, and thalamus, suggesting that the up-regulation phenomenon had poor-region specificity.
The affinity for the ligand remained unchanged and the increase in receptor number was reversible (Breese et al. 1997, 2000). The same authors showed that administration of concomitant antipsychotic medication (in this case the typical antipsychotic haloperidol) did not influence the nicotine-induced receptor up-regulation (Breese et al. 2000).
Surprisingly, the study showed that schizophrenic patients have fewer high- affinity nAChRs than either chronic smokers or than chronic smokers who are mentally ill with comparable daily use of cigarettes (Breese et al. 2000). The subunit composition and the functionality of these receptors were unknown, although it is conceivable that they were α4β2-nAChRs due to their predominance in the brain over other receptor subtypes.
Overview of Neuronal nAChR Up-Regulation: Perspectives for Future Therapeutic Strategies Aimed at Cholinergic Deficit Remediation
A reduction in the number of nAChRs is associated with diseases such as Alzheimer’s disease (AD), dementia with Lewy bodies, and Parkinson’s disease (Nordberg 1994; Court et al. 1999; Perry et al. 2000). Furthermore, patients with schizophrenia who are chronic smokers do not up-regulate their nAChRs (Breese et al. 2000).
Since alterations in nicotine-induced nAChR number are associated to several pathologies, and due to the central role that these receptors have in cognitive operations within the brain (see below), there are several fundamental questions that deserve further experimental inquiry:
What is the functional state (active/inactive) of new neuronal nAChRs that are being exported from the EM (see Fig. 3)?
What is the mechanistic relationship (if any) between nAChR desensitization, inactivation, and up-regulation?
What is the relationship between allosteric states of the nAChR and their loss/activation of function? Does this relationship depend on the dose and on the length of nicotine exposure (see Fig. 3, R ↔ D ↔D*nic ↔ I nic)?
What is the effect of phosphorylation on nAChR function and up-regulation? What are the specific contributions of all the putative phosphorylation consensus sequences in the M3/M4 intracellular loop?
Is there any altered nAChR subunit stochiometry in a specific receptor subtype(s) in the schizophrenic brain that is responsible for a defective nicotine-induced nAChR up-regulation?
What is the potential chronic affect of medications that affect nAChRs (such as allosteric potentiators, see below) on their ability to increase the number of nAChRs. How does this affect the potential clinical benefits of these medications?
Fig. 3.
Overview of the proposed mechanisms for up-regulation of the α4β2 nAChR. A resting α4β2 nAChR is desensitized (D nic) after acute nicotine exposure. Recovery from D nic to the resting is relatively fast (5 min). After chronic exposure the D nic enters into a long-lived desensitized state from which recovery is very slow (hours) depending on the period of exposure). The long-lived desensitized state D*nic represents a different conformation than R and D nic. The majority of studies in heterologous and natural expression experimental systems have indicated that chronic nicotine induces a persistent inactivation (loss of functional responsiveness) and a numerical up-regulation of α4β2-nAChR. Mechanistically, the relation between numerical up-regulation and inactivation remains to be defined. Several posttranscriptional mechanisms have been demonstrated to contribute to the functional changes and numerical up-regulation (i.e. phosphorylation of the M3/M4 loop, increase in transport of the α4β2 nAChR from the ER to the plasma membrane and changes in α4/β2 subunit ratio). A subset of studies has suggested that chronic nicotine exposure does not produce a numerical up-regulation of the α4β2 nAChR. Rather than proposing numerical receptor up-regulation, these studies suggest that after chronic nicotine exposure the receptor increases its functional response and its sensitivity to the agonist
Understanding the basic mechanisms of nicotine-induced numerical regulation of nAChRs will facilitate the development of more effective therapeutic agents used in diseases where cognitive dysfunction is the prime target
Neuronal nAChRs are Involved in Normal Mammalian Cognitive Processess
Cholinergic receptors of both the nicotinic and the muscarinic type are involved in cognitive processes within the brain. The latter is substantiated by the acute confusional states induced in humans by antimuscarinic drugs (Tune and Egeli 1999; Tune 2001). The focus of this review, however, will remain on specific cognitive functions subserved by neuronal nAChRs.
Neuronal nAChRs participate in attentional and memory processes of humans and other animals (Levin 1992; Holscher 1999; Perry et al. 1999; Woolf 1999; Levin 2002; Levin et al. 2002, 2005). Administration of nicotine enhances cognition (Warburton 1992; Levin and Simon 1998; Grilly et al. 2000; Levin and Rezvani 2002), whereas nicotinic antagonists impair it (Levin et al. 2006).
As mentioned before, neuronal nAChRs are reduced in number in Dementia of the Alzheimer’s type and are responsible for the cognitive deficits exhibited by these patients (Coyle et al. 1983; Whitehouse and Kellar 1987; Nordberg et al. 1989; Maelicke and Albuquerque 2000; Perry et al. 2000).
Most of the information on the possible participation of specific subtypes of nAChRs in the pathophysiology of cognitive deficits in schizophrenia comes from the use of specific agonist/antagonist of receptor subtypes which are injected in key areas known to be affected in the disease (Levin et al. 2006).
The hippocampus is an important area of the brain involved in working memory. This can be demonstrated by injecting the nicotinic antagonist mecamylamine and by observing the subsequent impairment in this cognitive function (Ohno et al. 1993; Kim and Levin 1996). At least two subtypes of nAChRs are involved: α7-nAChRs and α4β2-nAChRs. Acute infusions of either MLA (an α7-nAChR antagonist) into the ventral hippocampus or of DHβE (an α4β2-nAChR antagonist) cause impairment in working memory (Levin et al. 2002). Administration of nicotine reverses only the DHβE effect, indicating that the nicotine enhancing effects on working memory fully depend on α7-nAChRs rather than on α4β2-nAChRs.
DHβE or MLA injections into the medial or the lateral-frontal cortex failed to impair working memory (Levin et al. 2004). As previously seen, these agents impair working memory when infused in the ventral hippocampus (Levin et al. 2002).
The thalamus is a site of great density of nicotinic receptors (Rubboli et al. 1994). When DHβE is infused in the mediodorsal thalamic nucleus (a nucleus that has reciprocal connections with the frontal cortex), working memory improves (Levin et al. 2004). The function of nicotinic receptors in the anterior thalamic nuclei (which receive projections from the limbic system) has not been characterized. Muscarinic receptors in this area may be important, since scopolamine infusion causes memory impairment (Mitchell et al. 2002).
Another important subcortical nucleus, the amygdala, is implied in working memory. Blocking α7 and α4β2-nAChRs in the basolateral amygdala causes deficits in this cognitive function (Levin and Rezvani 2002).
More caudally in the central nervous system, midbrain dopaminergic nuclei such as the VTA and the substantia nigra are also implied in working memory regulation. Mecamylamine (but not the muscarinic agent scopolamine) is able to impair working memory when injected into these nuclei (Levin et al. 1994).
Genetically engineered mice have provided another line of evidence regarding participation of neuronal nAChRs in cognition. The α4 (Tapper et al. 2004) and the β2 subunit (Picciotto et al. 1999; Maskos et al. 2005) are linked to both the reinforcing properties and to the cognitive augmenting effects of nicotine.
Cognitive Deficits in Schizophrenia: The Core Feature of the Disorder
Abnormalities in brain structure have been documented in certain patients with schizophrenia (Silbersweig et al. 1995; McCarley et al. 1999). About 15 to 30% of patients with schizophrenia have an enlargement of both the lateral and the third ventricles (Johnstone et al. 1976), a finding also confirmed in first episode patients (Lieberman et al. 1992). Abnormalities in the thalamus (Andreasen et al. 1994; Jones 1997; Gilbert et al. 2001) and in the left hemisphere have also been documented (Friston et al. 1992).
The cellular basis of these abnormalities appear to be a disruption in normal synaptic organization rather than neuronal loss (Selemon and Goldman-Rakic 1999) possibly due to aberrant programed cell death (Kozlovsky et al. 2002). Taken altogether, these abnormalities appear to be a sufficient anatomical substrate to explain cognitive deficits in patients with schizophrenia.
Clinically, these patients present as three distinct clusters of symptoms when factor analysis techniques are used (Liddle et al. 1989): the psychotic/reality distortion cluster (delusions and hallucinations), the disorganization cluster (disorganized behavior and thinking and inappropriate affect), and the psychomotor poverty cluster (decreased spontaneous movements, plus the classical negative signs identified as: attentional deficits, alogia, affective flattening, avolition/apathy, asociality, and anhedonia).
Negative signs and the disorganization cluster (but not the psychotic cluster) can be correlated with cognitive deficits using more elaborate neuropsychological testing (Elvevag and Goldberg 2000; Kuperberg and Heckers 2000; O’Leary et al. 2000).
Schizophrenic patients score one (or more) standard deviation below the mean of healthy controls across several neuropsychological tests (Saykin et al. 1991; Hoff et al. 1992; Mohamed et al. 1999). The NIMH-established program known as MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) (Green et al. 2004; Nuechterlein et al. 2004) has identified several key cognitive domains as impaired in schizophrenia (for a review see Green 2006).
These include deficits in speed of processing and visuomotor skills (Censits et al. 1997; Heinrichs and Zakzanis 1998) , selective attention, working memory, and executive function, and IQ values that go from normal to mental retardation values.
Verbal declarative memory and selective attention (vigilance, but not divided and sustained attention) are exquisitely impaired in schizophrenia. Language is also defective as demonstrated by simple tests that measure reasoning and verbal fluency. All these deficits are premorbid to the full blown syndrome of schizophrenia and predate antipsychotic treatment (Saykin et al. 1994; Torrey 2002).
Apart from the aforementioned negative signs, cognitive deficits manifest themselves in neurological signs: deficits in motor coordination, cerebellar signs as finger to nose or heel to shin, Romberg sign, tandem walking, and motor sequences that require an intact frontal cortex. Perceptual abnormalities are also demonstrated as poor stereognosis and graphestesia.
Negative symptoms and cognitive deficits can be confounded by depression, dyskinetic-inducing effects of antipsychotic medications, paranoia preventing social interaction, or the isolating effect of institutionalization. However, it is now recognized that cognitive impairment is a phenomenon unique and central to schizophrenia, since cognitive deficits predate the onset of psychosis (Saykin et al. 1994; Torrey 2002). Improvement in positive symptoms does not necessarily imply amelioration of cognitive impairment, and negative symptoms are stable over time once they appear (Hirsch and Weinberger 2003).
Cognitive deficits and the psychomotor poverty cluster (but not the psychotic/reality distortion cluster) are important as prognostic indicators of how well patients will be able to function more or less independently in the community (Andreasen 1982).
Due to the salient role of neuronal nAChRs in normal cognition, and given that schizophrenia is a disorder defined by specific cognitive deficits, a question that naturally follows is: what is the role of neuronal nAChRs in the pathophysiology of this brain disorder?
Nicotinic Receptors are Involved in Abnormal Human Cognitive Processess: Relevance to Schizophrenia
The α7-nAChR Subtype
When nonmentally ill subjects are exposed to paired auditory stimuli and the corresponding evoked potentials are recorded, the amplitude of the second auditory response (P50 component of the evoked response) is reduced, compared to the amplitude of the first response. The evoked potential change is cholinergically mediated (Luntz-Leybman et al. 1992) and reflects inhibitory mechanisms that reduce sensory overload in healthy subjects.
Indirect evidence from animal models suggests that activation of α7-nAChRs located in hippocampal interneurons is essential for normal attentional processes, although α4β2-nAChRs might be even more prominent in this respect as suggested by Albuquerque (Alkondon and Albuquerque 2001).
Interestingly, the impairment in sustained attention seen in schizophrenic patients can be correlated with neurophysiological deficits using the P50 auditory evoked potential (Freedman et al. 1997). These patients cannot filter, as normal controls do, the second of two paired auditory stimuli (Freedman et al. 1991; Waldo et al. 1991; Adler et al. 1992). Smoking (Adler et al. 1993) or administration of nicotine (Adler et al. 1992) reverses this P50 deficit.
This phenotypic trait in schizophrenia is transmitted as an autosomal dominant phenotype and has been linked to a dinucleotide repeat polymorphism located less than 120 kb from CHRNA7 on chromosome 15q14 (Freedman et al. 1997; Weiland et al. 2000).
Expression of the α7 subunit is decreased in postmortem hippocampus from schizophrenia patients when compared to controls (Freedman et al. 1995) and the frontal cortex of the schizophrenic brain shows decreased levels of this subunit (Guan et al. 1999). Antagonists of α7 induce sensory gating deficits analogous to those seen in schizophrenic patients (Stevens et al. 1996).
Another marked deficit exhibited by schizophrenic patients is reflected in smooth pursuit eye movement (Radant and Hommer 1992) a sensory defect also normalized by nicotine (Olincy et al. 1998).
The aforementioned body of evidence led to the hypothesis that defective activation of α7-nAChRs is implied in attentional deficits in schizophrenia and prompted the development of partial agonists of α7-nAChRs in a recent proof-of-concept trial of 12 patients with schizophrenia (Olincy et al. 2006).
Schizophrenic patients self-administer more nicotine than nonschizophrenics, because their smoking pattern is characterized by deep inhalation patterns, shown by elevated blood levels of cotinine when compared with nonmentally ill chronic smokers (Olincy et al. 1997). This behavior delivers more nicotine per puff and conceivably increases nicotine concentration in the vicinity of neurons (Olincy et al. 1997). It is not known whether such high concentrations of nicotine are capable of desensitizing and up-regulating α7 receptors.
Desensitization of α7-nAChRs has been invoked as the basis of sensory gating defects in schizophrenia (Griffith et al. 1998). At the concentrations seen in the plasma of smokers, nicotine activates nAChRs (Gray et al. 1996) and desensitizes nAChRs (Fenster et al. 1997), but it is unable to up-regulate α7-nAChRs (Marks et al. 1985; Collins et al. 1994). Only high concentrations of nicotine up-regulate α7-nAChRs (Marks et al. 1983).
Although the evidence on the α7-nAChR participation in schizophrenia is compelling, this nicotinic receptor subtype is only involved in selective attentional deficits (Olincy et al. 2006) leaving unanswered the question of what other nAChR sybtypes are involved in other deficits.
Given the diversity of nAChRs in the brain (see pertinent section of this review), it seems logical to conclude that nAChR subtypes other than the α7-nAChR might be involved in the pathophysiology of cognitive deficits seen in patients with schizophrenia.
Cigarette Smoking as a Possible Alleviation of Cognitive Deficits in Schizophrenia
Smoking is associated with significant morbidity and mortality in the general population (Piasecki and Newhouse 2000). The high prevalence of smoking in schizophrenia (O’Farrell et al. 1983; Masterson and O’Shea 1984; Hughes et al. 1986; Ziedonis et al. 1994; de Leon et al. 1995) may provide a clue for understanding the cognitive deficits that predate this neuropsychiatric disorder. Patients with schizophrenia smoke more than smokers who do not carry this psychiatric diagnosis (de Leon et al. 1995) or more than patients with other psychiatric disorders (Hughes et al. 1986; de Leon et al. 1995; Diwan et al. 1998).
The relationship between smoking and schizophrenia can be explained by two, not necessarily mutually exclusive, hypotheses (Dalack et al. 1998):
schizophrenic patients smoke to alleviate mood and cognitive symptoms induced by antipsychotic agents
schizophrenic patients smoke to alleviate mood and cognitive symptoms that predate the onset of the illness (Dalack and Meador-Woodruff 1996)
With regard to the first hypothesis, it has been shown that nicotine relieves the undesired side effects of the typical antipsychotic medications (Goff et al. 1992). Five milligrams of haloperidol given to either a non-schizophrenic smoker or to a schizophrenic patient who smokes, increases their consumption of cigarettes (Dawe et al. 1995; McEvoy et al. 1995a). It can be postulated that blockade of D2 receptors, concentrated in the so called “pleasure giving” dopaminergic pathways, reduces subjective levels of reward that can be compensated by nicotine.
Interestingly, clozapine decreases smoking in schizophrenic patients (McEvoy et al. 1995b, 1999), normalizes P50 gating at 500 msecs (Nagamoto et al. 1996), and induces c-fos expression in the prefrontal cortex (Deutch and Duman 1996) and the thalamic paraventricular nucleus (Deutch et al. 1995) by a yet to be identified mechanism. The effects of clozapine on nAChR function are still unknown.
There is a good evidence that cigarette smoking affects the blood concentration of antipsychotic medications or their metabolites. A significant reduction (20–40% lower) in plasma levels is documented for clozapine (Seppala et al. 1999), haloperidol (Shimoda et al. 1999) and olanzapine (Zyprexa, package insert).
With regard to the second hypothesis (smoking alleviates naturally occurring cognitive deficits), it has been proposed that the heavy smoking of schizophrenic patients may be an attempt to help them focus in a heavy-loaded sensory environment (Adler et al. 1992). In this regard, the cognitive deficits in schizophrenia can be partially ascribed to a defective prefrontal dopamine transmission in the dorsolateral prefrontal cortex (Laruelle 2003).
Amelioration of Cognitive Deficits in Schizophrenia: Focus on Neuronal nAChRs
Non-Pharmacological Strategies
Therapeutic approaches designed to reduce cognitive impairment in schizophrenia can be divided into non-pharmacological and pharmacological. Among the former, modalities such as Modularized Social Skills Training (Liberman 1998), Family Psychoeducation (Pitschel-Walz et al. 2006; Pollio et al. 2006), and Cognitive Behavioral Therapy (Chadwick and Lowe 1990, 1994) are all validated therapies.
Pharmacological Strategies
A variety of pharmacological treatments have been proposed, which include the cognitive enhancing effects of second generation antipsychotics (SGAs) (Purdon et al. 2000; Keefe et al. 2006) manipulations of the cholinergic nicotinic and muscarinic systems (Friedman 2004) or anabaseine derivatives (Olincy et al. 2006). Nicotine, of itself, improves attention but not other cognitive functions. Desensitization of nAChRs is the most probable explanation for this modest effect of nicotine on cognition (Harris et al. 2004).
Second-Generation Antipsychotics (SGA)
These agents have improved the natural course of schizophrenia but negative symptoms have proven to be somewhat refractory to pharmacotherapy (O’Leary et al. 2000). The cognitive enhancing properties of the so-called atypical antipsychotic drugs have been demonstrated at least for olanzapine and risperidone (Purdon et al. 2000; Keefe et al. 2006) although there is no evidence that they have direct effects on nAChRs. Interestingly, atypical antipsychotics such as olanzapine or risperidone reversibly and dose-dependently reduce the frequency of miniature endplate potentials for vertebrate muscle nAChRs (Nguyen et al. 2002). Although muscle nAChRs are structurally related to their brain counterparts, there is no conclusive evidence to date that interactions between atypical antipsychotics and neuronal nAChRs can be ascribed to clinically relevant antipsychotic or to cognitive-enhancing effects.
Galantamine
This drug is used clinically to enhance cholinergic transmission and has been approved by the FDA to treat the cognitive deficits associated with Dementia of the Alzheimer’s type (Tariot et al. 2000; Wilcock et al. 2000). Galantamine allosterically potentiates neuronal nAChRs (Albuquerque et al. 1997; Maelicke and Albuquerque 2000) and is also a cholinesterase inhibitor. The activity of cholinergic receptors of the muscarinic type is not affected by galantamine, indicating that its sensitizing effects on nAChRs is via true allosteric potentiation rather than by an enhancement in cholinergic transmission due to acetylcholinesterase inhibition (Samochocki et al. 2003).
Using mouse brain slices (Zhang et al. 2004) and in vivo microdyalisis experiments in rats (Schilstrom et al. 2006), it has been shown that galantamine increases dopamine release (Zhang et al. 2004) and the firing activity of dopaminergic cells in the ventral tegmental area via allosteric potentiation of presynaptic nAChRs (Schilstrom et al. 2006). The same mechanism was invoked to explain an increased release of dopamine in the medial prefrontal cortex (Schilstrom et al. 2006).
Preliminary evidence suggests that galantamine can improve negative symptoms in schizophrenia (Vovin et al. 1991, 1992; Rosse and Deutsch 2002; Arnold et al. 2004). It is thus reasonable to assume that enhancement of nAChRs function may improve cognitive deficits related to negative symptoms as well.
Galantamine differs from the pure AChE inhibitor donepezil which does not have effects on either positive or negative symptoms of schizophrenia (Buchanan et al. 2003), see also references in (Vovin et al. 1992). A randomized controlled trial using another cholinesterase inhibitor, rivastagmine, failed to demonstrate any beneficial effects on cognition in 40 patients with schizophrenia (Sharma et al. 2006).
The earliest report in the literature of cognitive benefits of galantamine was performed in Russia (Vovin et al. 1991, 1992) showing that 18 out of 30 patients clinically improved their attention in 3–4 weeks when galantamine (10–20 mg/d) was combined with the antipsychotic pimozide (unknown dose, unknown if patients were stable on the neuroleptic) and benactyzine, a muscarinic blocking agent (1–2 mg/d). The scales used were the BPRS (Rhoades and Overall 1988) and a scale used to assess negative deficits developed by the authors (Vovin et al. 1992). The smoking status of these patients was unknown.
In 2002, the case of two treatment-resistant patients with schizophrenia (smoking status unknown) was reported by Allen and McEvoy (2002). Risperidone (at unknown dose, unknown smoking status of patients) was combined with galantamine (8 mg/d). In both patients both positive and negative symptoms were assessed merely clinically. In one of the two patients, disorganized thinking cleared dramatically when the galantamine dose was escalated to 12 mg twice a day. The follow-up in this study was up to 2 months for one patient.
The case of a 42-year-old man with a 20-year history of schizophrenia who needed a complicated medication regimen (four neuroleptics) to maintain clinical stability (plus the use of nicotine gum) was reported by Rosse and Deutsch (2002). The patient was cotreated with galantamine (8 mg/d), which was increased to 24 mg/d for 2 months. There was an improvement in negative (but not positive) symptoms as measured with the total scores of the Scale for the assessment of Negative Symptoms (Scale for the Assessment of Negative Symptoms, SANS (Andreasen 1982)). This easily administered scale, is a reliable indicator of cognitive impairment (Andreasen 1982; O’Leary et al. 2000).
At day 55 of drug administration, galantamine was discontinued and the SANS scores dramatically increased (Rosse and Deutsch 2002). The same group also reported an adjuvant therapeutic effect of galantamine (8–24 mg/d) on apathy (using the Apathy Evaluation Scale; AES) in one smoking-free patient with schizophrenia treated stable on both (unknown doses) risperidone and olanzapine (Arnold et al. 2004).
All these reports support the notion of a galantamine-induced cognitive enhancement in schizophrenia, but do not indicate which cognitive domains are affected. We first reported cognitive changes using a psychometric measure of cognitive impairment in schizophrenia (Purdon 2005, Personal Communication) termed Scale for the Assessment of Cognitive Impairment in Psychosis (SCIP) and the SANS (Ochoa and Clark 2004). This was a rapid (15–20 days) improvement in negative symptoms of schizophrenia in thirteen in-patients who were smoke-free and treated with neuroleptics (mainly olanzapine, initiated at the time of admission) and adjuvant galantamine (8–12 mg/d). Clinical ratings of alogia and attention were specifically improved in most cases in 20–30 days (SANS scores). One patient also showed a marked improvement in both working memory and delayed recall with the SCIP (Ochoa and Clark 2006).
Furthermore, five patients (four were nonsmokers) with schizophrenia treated with 250–450 mg/d of clozapine (a drug that has potent anticholinergic effects) plus 16 mg/d galantamine after 8 weeks also showed improved sustained attention using a neurocognitive battery (Bora et al. 2005). The PANNS (Kay et al. 1987) scores of these patients were unchanged suggesting that cognitive amelioration was independent of clinical presentation.
The most reliable studies to date are two randomized controlled trials recently published. In the first study, 16 patients (all but one smokers) already stabilized on 4–6 mg/d risperidone were given supplemental galantamine (8–24 mg/d) (Schubert et al. 2006). Using the Repeatable Battery for the Assessment of Neuropsychological Status (R-BANS; (Randolph et al. 1998) these authors demonstrated improvements in total scores, and in the attention and delayed memory subscales. The improvement observed in these two subscales of the R-BANS coincided with the preliminary report using the SCIP, which suggested improvements in both delayed recall and attention (Ochoa and Clark 2006).
The second-randomized controlled trial is a 12-week study of 24 patients already stable on a typical or first generation antipsychotic (chlorpromazine dose equivalent to 1390 mg/d) that were randomized to either placebo or galantamine (8 mg/d). The dose of galantamine was increased to 12 mg/d after six weeks (Lee et al. 2007). The authors found no substantial differences between both groups except for the increased recognition in the recognition portion of the Rey Complex Figure Test (Loring et al. 1990).
Based on their findings the authors concluded that galantamine provided little if any cognitive improvement when added to first generation antipsychotics. Notwithstanding, two possible confounding factors in this trial are (1) patients had free access to nicotine during the trial, and (2) they were on first generation antipsychotics, medications that may have potential deleterious effects on cognition (Borison 1996; Gallhofer et al. 1996).
Overall, the evidence accumulated so far indicates that galantamine provides modest cognitive remediation in schizophrenia.
Interestingly, a recent in vitro study using rat brain, showed that galantamine up-regulates nAChRs on its own (Kume et al. 2005). This finding raises the following question: what is the in vivo relationship between neuronal nAChR up-regulation and the potential therapeutic benefits of this drug?
Anabaseine Derivatives
Benzylidene derivatives of a marine worm toxin, anabaseine, have high selectivity for the α7 receptor subtype. Substituting at the 2 and 4 positions of the benzylidene ring of anabaseine renders 3-anabaseine dihydrochloride (GTS-21 or DMXB-A) and 4OH-GTS-21. In particular, GTS-21 is a partial agonist at α7-nAChRs and an antagonist at α4β2-nAChRs (Briggs et al. 1997; Kem 2000).
Due to their α7 selectivity these two compounds may have potential for treating cognitive deficits of schizophrenia (Olincy et al. 2006). The following animal and human experiments support this contention.
Aged rats improve learning and memory with chronic administration of GTS-21 (Arendash et al. 1995). Also, GTS-21 improved passive avoidance behavior and spatial memory-related behaviors in rats with bilateral lesions of the nucleus basalis in a mecamylamine-sensitive manner. Xenopus oocyte-expressed α7-nAChRs were also activated by GTS-21 (Meyer et al. 1997).
GTS-21 ameliorates cognitive deficits (radial maze learning performance) caused by permanent occlusion of bilateral common carotid arteries in rats (Nanri et al. 1998) It also improves the auditory gating deficit seen in isolation-reared rats (O’Neill et al. 2003).
Certain inbred mouse strains (such as DBA mice) exhibit sensory deficits analogous to those in patients with schizophrenia. (diminished response of the hippocampal evoked potential to the second of closely paired auditory stimuli). The severity of the deficits has been correlated with reduced α7-nAChRs in the hippocampus. GTS-21 normalized inhibition of auditory. response in DBA mice (Stevens et al. 1998). Normalization of these deficits by GTS-21 is blocked by α-bungarotoxin but not mecamylamine, indicating that the response is mediated via α7-nAChRs (Simosky et al. 2001).
Nicotine and nicotinic agonists affect auditory gating as measured by a prepulse inhibition paradigm (PPI). In Sprague-Dawley rats, nicotine, epibatidine (a potent nAChR agonist) and A-85380 (a selective α4β2 agonist) all disrupted PPI. GTS-21 did not affect PPI in a consistent manner, in either rats or in a strain of mice that expresses a disrupted gating phenotype. In a strain that expresses a normal gating phenotype, nicotine, epibatidine, and A-85380 all augmented PPI. (Schreiber et al. 2002).
The data suggest that the effects of nAChR agonists on PPI are species-dependent and that stimulation of heteromeric nAChRs containing both α and β subunits (possibly α4β2) affect sensorimotor gating. Evidence supporting a role for α7-nAChRs in the control of PPI of the acoustic startle response was not obtained. (Schreiber et al. 2002).
The septo-hippocampal pathway is involved in eyeblink classical conditioning in the rabbit. GTS-21, facilitated acquisition of the reflex in older rabbits and ameliorated eyeblink classical conditioning deficits in older rabbits (Woodruff-Pak et al. 1994). It also facilitates acquisition, retention, and relearning in these animals (Woodruff-Pak et al. 2000) and reversed the mecamylamine impaired acquisition of conditioned responses (Woodruff-Pak, 2003).
With respect to human subjects, a randomized controlled trial on 18 healthy volunteers has shown that GTS-21 (between 75 and 150 mg) significantly enhanced three measures of cognitive function (attention, working memory, episodic secondary memory) compared to placebo (Kitagawa et al. 2003). More recently, a different group of investigators (Olincy et al. 2006) performed a randomized controlled crossover trial of two GTS-21 doses and one placebo arm in 12 schizophrenic patients who abstained from tobacco for at least 30 days. Cognitive improvement was assessed using both the R-BANS and paired auditory stimuli analyzing inhibition of the P50 auditory evoked potential. Improvement in the total scale score of the R-BANS (8–12 points) and improvement of P50 inhibition were obtained with lower (75 mg) doses of the drug.
Out of these 12 patients, 3 were on a typical neuroleptic (two on combined therapies and only one with fluphenazine decanoate as monotherapy), 8 were in monotherapy with second generation neuroleptics, and 4 on two second generation neuroleptics. When interpreting these data, the cognitive-enhancing effects of atypical neuroleptics have to be taken into consideration (Purdon et al. 2000; Keefe et al. 2006).
Varenicline
Among the latest strategies for smoking cessation (Fagerstrom and Balfour 2006) varenicline offers promising clinical results (Coe et al. 2005a, b; Obach et al. 2006). The molecule is derived from the structure of cytisine, a plant alkaloid selective for the α4β2 type of receptor that has weak partial agonist activity.
Varenicline displays partial agonist properties at α4β2-nAChRs in animal models (Coe et al. 2005a). These results have been extended to rat neuronal nAChRs expressed in Xenopus laevis oocytes: varenicline is a partial agonist at α4β2, α3β4, α3β2 and at receptors that contain the α6 subunit (Mihalak et al. 2006). Remarkably, varenicline is a full agonist at α7 homomeric receptors (Mihalak et al. 2006).
Clinically, varenicline has been tried in randomized controlled trials using non-mentally ill smokers, in a 12-week treatment period, with a 52-week period for follow-up. It was shown to be more efficacious than placebo (smoking abstinence rates of 44% vs. 17.7%) at all time-points of the study and than bupropion sustained release (Gonzales et al. 2006; Jorenby et al. 2006). A third study confirmed these results and established varenicline as a maintenance agent for smoking cessation (Tonstad et al. 2006).
Due to the effectiveness in smoking cessation and the good tolerability of varenicline (Gonzales et al. 2006; Jorenby et al. 2006; Tonstad et al. 2006) and due to its α7 full agonist properties, it is conceivable that the drug may be useful for treating both nicotine dependence in schizophrenic patients as well as acting as a cognitive remediator, a possibility that is under our current scrutiny.
Conclusions
Four major observations support the notion that neuronal nAChRs play a central role in the cognitive deficits found in schizophrenia:
(1) the high prevalence of smoking found in schizophrenic patients, (2) the abnormal up-regulation of high-affinity neuronal nAChR sites found in schizophrenic patients, (3) the fact that smoking alleviates cognitive deficits in schizophrenic patients, and (4) the decreased levels of α7 nAChR in the hippocampus and the frontal cortex of schizophrenic patients.
Furthermore, although the modest consistently demonstrated benefitial effects of adjuvant galantamine in schizophrenia reinforce the idea of a participation of neuronal nAChRs in the physiopathology of the disorder.
A central hypothesis that remains to be tested is that the overall result of chronic nicotine exposure in schizophrenic patients is to attenuate cholinergic hyperactivity in the brain. Several fundamental questions then need to be answered by experiment:
What is the mechanistic relationship if any, between agonist-induced desensitization, inactivation, and up-regulation of these neuronal nAChRs?
What is the functional state of the upregulated neuronal nAChR in vivo? Are they desensitized, inactivated, or functionally upregulated?
Does phosphorylation regulate the number and the function of nAChRs in vivo?
Do schizophrenic patients display alterations of subunit ratios in the heteromeric neuronal nAChRs?
The years to come will reveal more novel therapeutic techniques based on findings in the basic science of nAChRs and other receptor, giving researchers an impetus for what is now called Translational research: from bench to bedside and from bedside to the community (Carter 2006; Insel 2006).
Acknowledgments
The authors are grateful to Drs. Gretchen Lopez-Hernandez, and Javier Sanchez for insights and perspectives. Work in the author’s laboratory (JLD) is supported by the National Institutes of Health grants 2RO1GM56371–10, GM08102–27 and SNRP U54NS0430311. This work was supported in part by grants from the National Institutes of Health NIGMS 2RO1GM56371-10, GM08102-27, NINDS SNRP U54NS0430311 and UPR Insitutional Funds for Research.
Abbreviations
- ACh
Acetylcholine
- AES
Apathy Evaluation Scale
- αBgt
α-Bungarotoxin
- BPRS
Brief Psychiatric Rating Scale
- DHβE
Dihydro-β-erythroidine
- DMPP
1,1-dimethyl-4-phenylpiperazinium
- FDA
Food and Drug Administration
- GABA
Gamma aminobutyric acid
- 5-HT
5-Hydroxytryptamine
- MATRICS
Measurement and Treatment Research to Improve Cognition in Schizophrenia
- NIMH
National Institute of Mental Health
- MCC
Methylcarbamylcholine
- nAChR
Nicotinic acetylcholine receptor
- NMDA
N-methyl-d-aspartic acid
- OL
Open Label
- PANSS
Positive and Negative Symptom Scale for Schizophrenia
- PKA
Protein kinase A
- PKC
Protein kinase C
- RBANS
Repeatable Battery for the Assessment of Neuropsychological status
- RCFT
Rey Complex Figure Test
- RCT
Randomized Controlled Trial
- SANS
Scale for the Assessment of Negative Symptms in Schizoprenia
- SCIP
Scale for the Assessment of Cognitive Impairment in Psychosis
- SGA
Second generation antipsychotics
- SN
Substantia nigra
- TMA
Tetramethylammonium
- VTA
Ventral tegmental area
References
- Adams DJ, Nutter TJ (1992) Calcium permeability and modulation of nicotinic acetylcholine receptor-channels in rat parasympathetic neurons. J Physiol Paris 86:67–76 [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LD, Wiser A, Freedman R (1993) Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry 150:1856–1861 [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LJ, Griffith J, Waldo MC, Freedman R (1992) Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry 32:607–616 [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Alkondon M, Pereira EF, Castro NG, Schrattenholz A, Barbosa CT, Bonfante-Cabarcas R, Aracava Y, Eisenberg HM, Maelicke A (1997) Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther 280:1117–1136 [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Mike A, Eisenberg HM, Maelicke A, Alkondon M (2000) Neuronal nicotinic receptors in synaptic functions in humans and rats: physiological and clinical relevance. Behav Brain Res 113:131–141 [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Santos MD, Alkondon M, Pereira EF, Maelicke A (2001) Modulation of nicotinic receptor activity in the central nervous system: a novel approach to the treatment of Alzheimer disease. Alzheimer Dis Assoc Disord 15(Suppl. 1):S19–25 [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX (2001) Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol 86:3043–3055 [DOI] [PubMed] [Google Scholar]
- Allen TB, McEvoy JP (2002) Galantamine for treatment-resistant schizophrenia. Am J Psychiatry 159:1244–1245 [DOI] [PubMed] [Google Scholar]
- Anand R, Conroy WG, Schoepfer R, Whiting P, Lindstrom J (1991) Neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes have a pentameric quaternary structure. J Biol Chem 266:11192–11198 [PubMed] [Google Scholar]
- Anand R, Peng X, Lindstrom J (1993) Homomeric and native alpha 7 acetylcholine receptors exhibit remarkably similar but non-identical pharmacological properties, suggesting that the native receptor is a heteromeric protein complex. FEBS Lett 327:241–246 [DOI] [PubMed] [Google Scholar]
- Andreasen NC (1982) Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry 39:784–788 [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Arndt S, Swayze V II, Cizadlo T, Flaum M, O’Leary D, Ehrhardt JC, Yuh WT (1994) Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science 266:294–298 [DOI] [PubMed] [Google Scholar]
- Arendash GW, Sengstock GJ, Sanberg PR, Kem WR (1995) Improved learning and memory in aged rats with chronic administration of the nicotinic receptor agonist GTS-21. Brain Res 674:252–259 [DOI] [PubMed] [Google Scholar]
- Arnold DS, Rosse RB, Dickinson D, Benham R, Deutsch SI, Nelson MW (2004) Adjuvant Therapeutic effects of galantamine on apathy in a Schizophrenia patient. J Clin Psychiatry 65:1723–1724 [DOI] [PubMed] [Google Scholar]
- Barrantes GE, Rogers AT, Lindstrom J, Wonnacott S (1995) Alpha-Bungarotoxin Binding Sites in Rat Hippocampal and Cortical Cultures - Initial Characterisation, Colocalisation with Alpha-7 Subunits and Up-Regulation By Chronic Nicotine Treatment. Brain Res 672:228–236 [DOI] [PubMed] [Google Scholar]
- Benowitz N (1990) Pharmacokinetic considerations in understanding nicotine dependence. In The biology of nicotine dependence, CIBA foundation symposium, vol 152. Wiley, Chichester, pp 186–209 [DOI] [PubMed]
- Benowitz NL (1992) Cigarette smoking and nicotine addiction. Med Clin North Am 76:415–437 [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Anderson JM (1988a) Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J Neurochem 50:1243–1247 [DOI] [PubMed] [Google Scholar]
- Benwell MEM, Balfour DJK, Anderson JM (1988b) Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J Neurochem 50:1243–1247 [DOI] [PubMed] [Google Scholar]
- Berman JA, Talmage DA, Role LW (2007) Cholinergic circuits and signaling in the pathophysiology of schizophrenia. Int Rev Neurobiol 78:193–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Ballivet M, Rungger D (1990) Activation and blocking of neuronal nicotinic acetylcholine receptor reconstituted in Xenopus oocytes. Proc Natl Acad Sci USA 87:1993–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat RV, Turner SL, Selvaag SR, Marks MJ, Collins AC (1991) Regulation of brain nicotinic receptors by chronic agonist infusion. J Neurochem 56:1932–1939 [DOI] [PubMed] [Google Scholar]
- Bora E, Veznedaroglu B, Kayahan B (2005) The effect of galantamine added to clozapine on cognition of five patients with schizophrenia. Clin Neuropharmacol 28:139–141 [DOI] [PubMed] [Google Scholar]
- Borison RL (1996) The role of cognition in the risk–benefit and safety analysis of antipsychotic medication. Acta Psychiatr Scand Suppl 389:5–11 [DOI] [PubMed] [Google Scholar]
- Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, Marks MJ, Collins AC, Leonard S (2000) Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology 23:351–364 [DOI] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S (1997) Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther 282:7–13 [PubMed] [Google Scholar]
- Briggs CA, Anderson DJ, Brioni JD, Buccafusco JJ, Buckley MJ, Campbell JE, Decker MW, Donnelly-Roberts D, Elliott RL, Gopalakrishnan M, Holladay MW, Hui YH, Jackson WJ, Kim DJ, Marsh KC, O’Neill A, Prendergast MA, Ryther KB, Sullivan JP, Arneric SP (1997) Functional characterization of the novel neuronal nicotinic acetylcholine receptor ligand GTS-21 in vitro and in vivo. Pharmacol Biochem Behav 57:231–241 [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG (2006) Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry 63:907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Summerfelt A, Tek C, Gold J (2003) An open-labeled trial of adjunctive donepezil for cognitive impairments in patients with schizophrenia. Schizophr Res 59:29–33 [DOI] [PubMed] [Google Scholar]
- Buisson B, Bertrand D (2001) Chronic exposure to nicotine upregulates the human (alpha)4((beta)2 nicotinic acetylcholine receptor function. J Neurosci 21:1819–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS (2006) Re-conceptualizing schizophrenia as a disorder of cognitive and emotional processing: a shot in the arm for translational research. Biol Psychiatry 60:1169–1170 [DOI] [PubMed] [Google Scholar]
- Censits DM, Ragland JD, Gur RC, Gur RE (1997) Neuropsychological evidence supporting a neurodevelopmental model of schizophrenia: a longitudinal study. Schizophr Res 24:289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick PD, Lowe CF (1990) Measurement and modification of delusional beliefs. J Consult Clin Psychol 58:225–232 [DOI] [PubMed] [Google Scholar]
- Chadwick PD, Lowe CF (1994) A cognitive approach to measuring and modifying delusions. Behav Res Ther 32:355–367 [DOI] [PubMed] [Google Scholar]
- Changeux J-P(1990) Functional architecture and dynamics of the nicotinic acetylcholine receptor: an allosteric ligand-gated ion channel. In: Changeux J-P, Llinas RR, Purves D, Bloom FE (eds) Fidia research foundation neuroscience award lectures, Vol. 4. Raven Press, New York, pp 21–168
- Changeux JP, Bertrand D, Corringer PJ, Dehaene S, Edelstein S, Léna C, Le Novère N, Marubio L, Picciotto M, Zoli M (1998) Brain nicotinic receptors: structure and regulation, role in learning and reinforcement. Brain Res Brain Res Rev 26:198–216 [DOI] [PubMed] [Google Scholar]
- Changeux JP, Edelstein SJ (2005) Allosteric mechanisms of signal transduction. Science 308:1424–1428 [DOI] [PubMed] [Google Scholar]
- Changeux J-P, Revah F (1987) The Acetylcholine Receptor Molecule: Allosteric Sites and the Ion Channel. Trends in Neurosci 10:245–250 [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD III, O’Neill BT (2005a) Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 48:3474–3477 [DOI] [PubMed] [Google Scholar]
- Coe JW, Vetelino MG, Bashore CG, Wirtz MC, Brooks PR, Arnold EP, Lebel LA, Fox CB, Sands SB, Davis TI, Schulz DW, Rollema H, Tingley FD III, O’Neill BT (2005b) In pursuit of alpha4beta2 nicotinic receptor partial agonists for smoking cessation: carbon analogs of (−)-cytisine. Bioorg Med Chem Lett 15:2974–2979 [DOI] [PubMed] [Google Scholar]
- Coggan JS, Bartol TM, Esquenazi E, Stiles JR, Lamont S, Martone ME, Berg DK, Ellisman MH, Sejnowski TJ (2005) Evidence for ectopic neurotransmission at a neuronal synapse. Science 309:446–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AC (1990) An analysis of the addiction liability of nicotine. Adv Alcohol Subst Abuse 9:83–101 [DOI] [PubMed] [Google Scholar]
- Collins AC, Luo Y, Selvaag S, Marks MJ (1994) Sensitivity to nicotine and brain nicotinic receptors are altered by chronic nicotine and mecamylamine infusion. J Pharmacol Exp Ther 271:125–133 [PubMed] [Google Scholar]
- Collins AC, Marks MJ (1987) The effects of chronic nicotine administration on brain nicotinic receptor numbers. In: Martin WR, Van Loon GR, Iwamoto ET, Davis L (eds) Tobacco smoking and nicotine. A neurobiological approach. Plenum Press, New York-London, pp. 439–450
- Collins AC, Romm E, Wehner JM (1988) Nicotine tolerance: an analysis of the time course of its development and loss in the rat. Psychopharmacology (Berl) 96:7–14 [DOI] [PubMed] [Google Scholar]
- Collins AC, Romm E, Wehner JM (1990) Dissociation of the apparent relationship between nicotine tolerance and up-regulation of nicotinic receptors. Brain Res Bull 25:373–379 [DOI] [PubMed] [Google Scholar]
- Cooper E, Couturier S, Ballivet M (1991) Pentameric structure and subunit stoichiometry of a neuronal nicotinic acetylcholine receptor. Nature 350:235–238 [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB (1992) The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 107:285–289 [DOI] [PubMed] [Google Scholar]
- Court J, Spurden D, Lloyd S, McKeith I, Ballard C, Cairns N, Kerwin R, Perry R, Perry E (1999) Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha-bungarotoxin and nicotine binding in the thalamus [In Process Citation]. J Neurochem 73:1590–1597 [DOI] [PubMed] [Google Scholar]
- Coyle JT, Price DL, Delong MR (1983) Alzheimer’s disease: a disorder of cortical cholinergic innervation. Science (Washington DC) 219:1184–1190 [DOI] [PubMed] [Google Scholar]
- Dalack GW, Healy DJ, Meador-Woodruff JH (1998) Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Amer J Psych 155:1490–1501 [DOI] [PubMed] [Google Scholar]
- Dalack GW, Meador-Woodruff JH (1996) Smoking, smoking withdrawal and schizophrenia: case reports and a review of the literature. Schizophr Res 22:133–141 [DOI] [PubMed] [Google Scholar]
- Dani JA, De Biasi M (2001) Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav 70:439–446 [DOI] [PubMed] [Google Scholar]
- Dani JA, Heinemann S (1996) Molecular And Cellular Aspects Of Nicotine Abuse. Neuron 16:905–908 [DOI] [PubMed] [Google Scholar]
- Darsow T, Booker TK, Pina-Crespo JC, Heinemann SF (2005) Exocytic trafficking is required for nicotine-induced up-regulation of alpha 4 beta 2 nicotinic acetylcholine receptors. J Biol Chem 280:18311–18320 [DOI] [PubMed] [Google Scholar]
- Dawe S, Gerada C, Russell MA, Gray JA (1995) Nicotine intake in smokers increases following a single dose of haloperidol. Psychopharmacology 117:110–115 [DOI] [PubMed] [Google Scholar]
- de Leon J, Dadvand M, Canuso C, White AO, Stanilla JK, Simpson GM (1995) Schizophrenia and smoking: an epidemiological survey in a state hospital. Am J Psychiatry 152:453–455 [DOI] [PubMed] [Google Scholar]
- De Luca V, Voineskos S, Wong G, Kennedy JL (2006) Genetic interaction between alpha4 and beta2 subunits of high affinity nicotinic receptor: analysis in schizophrenia. Exp Brain Res 174:292–296 [DOI] [PubMed] [Google Scholar]
- Decker ER, Dani JA (1990) Calcium permeability of the nicotinic acetylcholine receptor: the single-channel calcium influx is significant. J Neurosci 10:3413–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeon J, Dadvand M, Canuso C, White AO, Stanilla JK, Simpson GM (1995) Schizophrenia and smoking—an epidemiological survey in a state hospital. Am J Psychiatry 152:453–455 [DOI] [PubMed] [Google Scholar]
- Deutch AY, Duman RS (1996) The effects of antipsychotic drugs on Fos protein expression in the prefrontal cortex: cellular localization and pharmacological characterization. Neuroscience 70:377–389 [DOI] [PubMed] [Google Scholar]
- Deutch AY, Ongür D, Duman RS (1995) Antipsychotic drugs induce Fos protein in the thalamic paraventricular nucleus: a novel locus of antipsychotic drug action. Neuroscience 66:337–346 [DOI] [PubMed] [Google Scholar]
- Di Chiara G (2000) Role of dopamine in the behavioral actions of nicotine related to addiction. Eur J Phamacol 393:295–314 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85:5274–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwan A, Castine M, Pomerleau CS, Meador-Woodruff JH, Dalack GW (1998) Differential prevalence of cigarette smoking in patients with schizophrenic vs mood disorders. Schizophrenia Res 33:113–118 [DOI] [PubMed] [Google Scholar]
- Downing JE, Role LW (1987) Activators of protein kinase C enhance acetylcholine receptor desensitization in sympathetic ganglion neurons. Proc Natl Acad Sci USA 84:7739–7743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bizri H, Clarke PBS (1994) Regulation of nicotinic receptors in rat brain following quasi-irreversible nicotinic blockade by chlorisondamine and chronic treatment with nicotine. Br J Pharmacol 113:917–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S (1994) Alpha-9—an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 79:705–715 [DOI] [PubMed] [Google Scholar]
- Elvevag B, Goldberg TE (2000) Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol 14:1–21 [PubMed] [Google Scholar]
- Fagerstrom K, Balfour DJ (2006) Neuropharmacology and potential efficacy of new treatments for tobacco dependence. Expert Opin Investig Drugs 15:107–116 [DOI] [PubMed] [Google Scholar]
- Fenster CP, Beckman ML, Parker JC, Sheffield EB, Whitworth TL, Quick MW, Lester RA (1999a) Regulation of alpha4beta2 nicotinic receptor desensitization by calcium and protein kinase C. Mol Pharmacol 55:432–443 [PubMed] [Google Scholar]
- Fenster CP, Rains MF, Noerager B, Quick MW, Lester RA (1997) Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J Neurosci 17:5747–5759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RA (1999b) Upregulation of surface alpha4beta2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J Neurosci 19:4804–4814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ (1992) A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol 41:31–37 [PubMed] [Google Scholar]
- Freedman R, Adler LE, Bickford P, Byerley W, Coon H, Cullum CM, Griffith JM, Harris JG, Leonard S, Miller C, Mylesworsley M, Nagamoto HT, Rose G, Waldo M (1994) Schizophrenia and nicotinic receptors. Harv Rev Psychiatry 2:179–192 [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Myles-Worsley M, Nagamoto HT, Miller C, Kisley M, McRae K, Cawthra E, Waldo M (1996) Inhibitory gating of an evoked response to repeated auditory stimuli in schizophrenic and normal subjects. Human recordings, computer simulation, and an animal model. Arch Gen Psychiatry 53:1114–1121 [DOI] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W (1997) Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA 94:587–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S (1995) Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry 38:22–33 [DOI] [PubMed] [Google Scholar]
- Freedman R, Waldo M, Bickford-Wimer P, Nagamoto H (1991) Elementary neuronal dysfunctions in schizophrenia. Schizophrenia Res 4:233–243 [DOI] [PubMed] [Google Scholar]
- Friedman JI (2004) Cholinergic targets for cognitive enhancement in schizophrenia: focus on cholinesterase inhibitors and muscarinic agonists. Psychopharmacology (Berl) 174:45–53 [DOI] [PubMed] [Google Scholar]
- Friedman JI, Harvey PD, Coleman T, Moriarty PJ, Bowie C, Parrella M, White L, Adler D, Davis KL (2001) Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: a comparison with Alzheimer’s disease and normal aging. Am J Psychiatry 158:1441–1448 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Liddle PF, Frith CD, Hirsch SR, Frackowiak RS (1992) The left medial temporal region and schizophrenia. A PET study Brain 115( Pt 2):367–382 [DOI] [PubMed] [Google Scholar]
- Fuchs PA (1996) Synaptic transmission at vertebrate hair cells. Curr Opin Neurobiol 6:514–519 [DOI] [PubMed] [Google Scholar]
- Gallhofer B, Bauer U, Lis S, Krieger S, Gruppe H (1996) Cognitive dysfunction in schizophrenia: comparison of treatment with atypical antipsychotic agents and conventional neuroleptic drugs. Eur Neuropsychopharmacol 6 Suppl 2:S13–20 [DOI] [PubMed] [Google Scholar]
- Gentry CL, Lukas RJ (2002) Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr Drug Targets CNS Neurol Disord 1:359–385 [DOI] [PubMed] [Google Scholar]
- Ghosheh OA, Dwoskin LP, Miller DK, Crooks PA (2001) Accumulation of nicotine and its metabolites in rat brain after intermittent or continuous peripheral administration of [2’-(14)C]nicotine. Drug Metab Dispos 29:645–651 [PubMed] [Google Scholar]
- Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS (2001) Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry 158:618–624 [DOI] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL (2005) Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci 28:371–378 [DOI] [PubMed] [Google Scholar]
- Girod R, Role LW (2001) Long-lasting enhancement of glutamatergic synaptic transmission by acetylcholine contrasts with response adaptation after exposure to low-level nicotine. J Neurosci 21:5182–5190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff DC, Henderson DC, Amico E (1992) Cigarette Smoking In Schizophrenia - Relationship To Psychopathology And Medication Side Effects. Am J Psychiatry 149:1189–1194 [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR (2006) Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 296:47–55 [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan M, Molinari EJ, Sullivan JP (1997) Regulation of human alpha4beta2 neuronal nicotinic acetylcholine receptors by cholinergic channel ligands and second messenger pathways. Mol Pharmacol 52:524–534 [PubMed] [Google Scholar]
- Gotti C, Riganti L, Vailati S, Clementi F (2006) Brain neuronal nicotinic receptors as new targets for drug discovery. Curr Pharm Des 12:407–428 [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA (1996) Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature 383:713–716 [DOI] [PubMed] [Google Scholar]
- Green MF (2006) Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry 67:3–8 [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, Fenton WS, Frese F, Goldberg TE, Heaton RK, Keefe RS, Kern RS, Kraemer H, Stover E, Weinberger DR, Zalcman S, Marder SR (2004) Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry 56:301–307 [DOI] [PubMed] [Google Scholar]
- Griffith JM, O’Neill JE, Petty F, Garver D, Young D, Freedman R (1998) Nicotinic receptor desensitization and sensory gating deficits in schizophrenia. Biol Psychiatry 44:98–106 [DOI] [PubMed] [Google Scholar]
- Grilly DM, Simon BB, Levin ED (2000) Nicotine enhances stimulus detection performance of middle- and old-aged rats: a longitudinal study. Pharmacol Biochem Behav 65:665–670 [DOI] [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Luyten WH, Colquhoun D, Sivilotti LG (1998) A reporter mutation approach shows incorporation of the “orphan” subunit beta3 into a functional nicotinic receptor. J Biol Chem 273:15317–15320 [DOI] [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Blennow K, Nordberg A (1999) Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport 10:1779–1782 [DOI] [PubMed] [Google Scholar]
- Guo X, Wecker L (2002) Identification of three cAMP-dependent protein kinase (PKA) phosphorylation sites within the major intracellular domain of neuronal nicotinic receptor alpha4 subunits. J Neurochem 82:439–447 [DOI] [PubMed] [Google Scholar]
- Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, Zerbe G, Freedman R (2004) Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology 29:1378–1385 [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK (1998) Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 12:426–445 [DOI] [PubMed] [Google Scholar]
- Hirsch SR, Weinberger D (2003) Schizophrenia. Blackwell, Massachussetts [Google Scholar]
- Hoff AL, Riordan H, O’Donnell DW, Morris L, DeLisi LE (1992) Neuropsychological functioning of first-episode schizophreniform patients. Am J Psychiatry 149:898–903 [DOI] [PubMed] [Google Scholar]
- Hogg RC, Raggenbass M, Bertrand D (2003) Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol 147:1–46 [DOI] [PubMed] [Google Scholar]
- Holscher C (1999) Consciousness in mind: a correlate for ACh? Trends in Neurosciences 22:541–542 [DOI] [PubMed] [Google Scholar]
- Horch HL, Sargent PB (1995) Perisynaptic surface distribution of multiple classes of nicotinic acetylcholine receptors on neurons in the chicken ciliary ganglion. J Neurosci 15:7778–7795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horger BA, Roth RH (1996) The role of mesoprefrontal dopamine neurons in stress. Crit Rev Neurobiol 10:395–418 [DOI] [PubMed] [Google Scholar]
- Hsu YN, Amin J, Weiss DS, Wecker L (1996) Sustained nicotine exposure differentially affects alpha 3 beta 2 and alpha 4 beta 2 neuronal nicotinic receptors expressed in Xenopus oocytes. J Neurochem 66:667–675 [DOI] [PubMed] [Google Scholar]
- Hsu YN, Edwards SC, Wecker L (1997) Nicotine enhances the cyclic AMP-dependent protein kinase-mediated phosphorylation of alpha4 subunits of neuronal nicotinic receptors. J Neurochem 69:2427–2431 [DOI] [PubMed] [Google Scholar]
- Huganir RL, Delcour AH, Greengard P, Hess GP (1986) Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature 321:774–776 [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgreen LA (1986) Prevalence of smoking among psychiatric outpatients. Amer J Psych 143:993–997 [DOI] [PubMed] [Google Scholar]
- Hulihangiblin BA, Lumpkin MD, Kellar KJ (1990) Effects of chronic administration of nicotine on prolactin release in the rat—inactivation of prolactin response by repeated injections of nicotine. J Pharmacol Exp Ther 252:21–25 [PubMed] [Google Scholar]
- Hurst RS, Hajos M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, Groppi VE, Allaman G, Ogier R, Bertrand S, Bertrand D, Arneric SP (2005) A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci 25:4396–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR (2006) Translational research in the decade of discovery. Horm Behav 50:504–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone EC, Crow TJ, Frith CD, Husband J, Kreel L (1976) Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet 2:924–926 [DOI] [PubMed] [Google Scholar]
- Jones EG (1997) Cortical development and thalamic pathology in schizophrenia. Schizophr Bull 23:483–501 [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR (2006) Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 296:56–63 [DOI] [PubMed] [Google Scholar]
- Karlin A, Akabas MH (1995) Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron 15:1231–1244 [DOI] [PubMed] [Google Scholar]
- Katz B, Thesleff S (1957) A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol 138:63–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276 [DOI] [PubMed] [Google Scholar]
- Ke L, Eisenhour CM, Bencherif M, Lukas RJ (1998) Effects of chronic nicotine treatment on expression of diverse nicotinic acetylcholine receptor subtypes. I. Dose- and time-dependent effects of nicotine treatment. J Pharmacol Exp Ther 286:825–840 [PubMed] [Google Scholar]
- Keefe RS, Young CA, Rock SL, Purdon SE, Gold JM, Breier A (2006) One-year double-blind study of the neurocognitive efficacy of olanzapine, risperidone, and haloperidol in schizophrenia. Schizophr Res 81:1–15 [DOI] [PubMed] [Google Scholar]
- Kem WR (2000) The brain alpha7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimer’s disease: studies with DMXBA (GTS-21) Behav Brain Res 113:169–181 [DOI] [PubMed] [Google Scholar]
- Khiroug L, Giniatullin R, Sokolova E, Talantova M, Nistri A (1997) Imaging of intracellular calcium during desensitization of nicotinic acetylcholine receptors of rat chromaffin cells. Br J Pharmacol 122:1323–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug L, Sokolova E, Giniatullin R, Afzalov R, Nistri A (1998) Recovery from desensitization of neuronal nicotinic acetylcholine receptors of rat chromaffin cells is modulated by intracellular calcium through distinct second messengers. J Neurosci 18:2458–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Levin ED (1996) Nicotinic, muscarinic and dopaminergic actions in the ventral hippocampus and the nucleus accumbens: effects on spatial working memory in rats. Brain Res 725:231–240 [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Takenouchi T, Azuma R, Wesnes KA, Kramer WG, Clody DE, Burnett AL (2003) Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology 28:542–551 [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Belmaker RH, Agam G (2002) GSK-3 and the neurodevelopmental hypothesis of schizophrenia. Eur Neuropsychopharmacol 12:13–25 [DOI] [PubMed] [Google Scholar]
- Kume T, Sugimoto M, Takada Y, Yamaguchi T, Yonezawa A, Katsuki H, Sugimoto H, Akaike A (2005) Up-regulation of nicotinic acetylcholine receptors by central-type acetylcholinesterase inhibitors in rat cortical neurons. Eur J Pharmacol 527:77–85 [DOI] [PubMed] [Google Scholar]
- Kuperberg G, Heckers S (2000) Schizophrenia and cognitive function. Curr Opin Neurobiol 10:205–210 [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Quirion R, Collier B (1989) Effect of chronic nicotine treatment on nicotinic autoreceptor function and N-[3H] methylcarbamylcholine binding sites in the rat brain. J Neurochem 52:483–491 [DOI] [PubMed] [Google Scholar]
- Laruelle M (2003) Dopamine transmission in the schizophrenic brain. In: Hirsch SR, Weinberger D (eds), Schizophrenia, Blackwell, Massachussetts, pp. 365–387
- Lee SW, Lee JG, Lee BJ, Kim YH (2007) A 12-week, double-blind, placebo-controlled trial of galantamine adjunctive treatment to conventional antipsychotics for the cognitive impairments in chronic schizophrenia. Int Clin Psychopharmacol 22:63–68 [DOI] [PubMed] [Google Scholar]
- Levin ED (1992) Nicotinic systems and cognitive function. Psychopharmacology (Berl) 108:417–431 [DOI] [PubMed] [Google Scholar]
- Levin ED (2002) Nicotinic receptor subtypes and cognitive function. J Neurobiol 53:633–640 [DOI] [PubMed] [Google Scholar]
- Levin ED, Blackwelder WP, Lau E, Brotherton J (2004) Nicotinic alpha4-beta2 and alpha7 nicotinic antagonistic effects in the mediodorsal thalamic nucleus and frontal cortex on memory function. Society for Neuroscience Abstracts San Diego, CA
- Levin ED, Bradley A, Addy N, Sigurani N (2002) Hippocampal alpha 7 and alpha 4 beta 2 nicotinic receptors and working memory. Neuroscience 109:757–765 [DOI] [PubMed] [Google Scholar]
- Levin ED, Briggs SJ, Christopher NC, Auman JT (1994) Working memory performance and cholinergic effects in the ventral tegmental area and substantia nigra. Brain Res 657:165–170 [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH (2005) Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl):1–17 [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH (2006) Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 184:1–17 [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH (2002) Nicotinic treatment for cognitive dysfunction. Curr Drug Target CNS Neurol Disord 1:423–431 [DOI] [PubMed] [Google Scholar]
- Levin ED, Simon BB (1998) Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology 138:217–230 [DOI] [PubMed] [Google Scholar]
- Liberman RP (1998) International perspectives on skill training for the mentally disabled. Int Rev Psychiatry 10:5–8 [Google Scholar]
- Liddle PF, Barnes TR, Morris D, Haque S (1989) Three syndromes in chronic schizophrenia. Br J Psychiatry Suppl:119–122 [PubMed]
- Lieberman J, Bogerts B, Degreef G, Ashtari M, Lantos G, Alvir J (1992) Qualitative assessment of brain morphology in acute and chronic schizophrenia. Am J Psychiatry 149:784–794 [DOI] [PubMed] [Google Scholar]
- Lindstrom J (1997) Nicotinic acetylcholine receptors in health and disease. Mol Neurobiol 15:193–222 [DOI] [PubMed] [Google Scholar]
- Lopez-Hernandez GY, Sanchez-Padilla J, Ortiz-Acevedo A, Lizardi-Ortiz J, Salas-Vincenty J, Rojas LV, Lasalde-Dominicci JA (2004) Nicotine-induced up-regulation and desensitization of alpha4beta2 neuronal nicotinic receptors depend on subunit ratio. J Biol Chem 279:38007–38015 [DOI] [PubMed] [Google Scholar]
- Loring DW, Martin RC, Meador KJ, Lee GP (1990) Psychometric construction of the Rey-Osterrieth Complex Figure: methodological considerations and interrater reliability. Arch Clin Neuropsychol 5:1–14 [PubMed] [Google Scholar]
- Lukas RJ (1991) Effects of chronic nicotinic ligand exposure on functional activity of nicotinic acetylcholine receptors expressed by cells of the PC12 rat pheochromocytoma or the TE671/RD human clonal line. J Neurochem 56:1134–1145 [DOI] [PubMed] [Google Scholar]
- Luntz-Leybman V, Bickford PC, Freedman R (1992) Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res 587:130–136 [DOI] [PubMed] [Google Scholar]
- Luo S, Kulak JM, Cartier GE, Jacobsen RB, Yoshikami D, Olivera BM, McIntosh JM (1998) alpha-conotoxin AuIB selectively blocks alpha3 beta4 nicotinic acetylcholine receptors and nicotine-evoked norepinephrine release. J Neurosci 18:8571–8579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysaker PH, Davis LW, Lightfoot J, Hunter N, Stasburger A (2005) Association of neurocognition, anxiety, positive and negative symptoms with coping preference in schizophrenia spectrum disorders. Schizophr Res 80:163–171 [DOI] [PubMed] [Google Scholar]
- Maelicke A, Albuquerque EX (2000) Allosteric modulation of nicotinic acetylcholine receptors as a treatment strategy for Alzheimer’s disease. Eur J Pharmacol 393:165–170 [DOI] [PubMed] [Google Scholar]
- Maelicke A, Samochocki M, Jostock R, Fehrenbacher A, Ludwig J, Albuquerque EX, Zerlin M (2001) Allosteric sensitization of nicotinic receptors by galantamine, a new treatment strategy for Alzheimer’s disease. Biol Psychiatry 49:279–288 [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burchs JB, Collins AC (1983) Effects of chronic nicotine infusion on tolerance development and cholinergic receptors. J Pharmacol Exp Ther 226:817–825 [PubMed] [Google Scholar]
- Marks MJ, Collins AC (1985) Tolerance, cross tolerance, and receptors after chronic nicotine or oxotremorine. Pharmacol Biochem Behav 22:283–291 [DOI] [PubMed] [Google Scholar]
- Marks MJ, Collins AC (1993) Desensitization of nicotine-induced 86Rb+ efflux. Soc Neurosci Abstr 19:289 [Google Scholar]
- Marks MJ, Grady SR, Collins AC (1993) Downregulation of nicotinic receptor function after chronic nicotine infusion. J Pharmacol Exp Ther 266:1268–1276 [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC (1992) Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci 12:2765–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC (1985) Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. J Pharmacol Exp Ther 235:619–628 [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloez-Tayarani I, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux JP (2005) Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436:103–107 [DOI] [PubMed] [Google Scholar]
- Masterson E, O’Shea B (1984) Smoking and malignancy in schizophrenia. Br J Psychiatry 145:429–432 [DOI] [PubMed] [Google Scholar]
- Mathie A, Colquhoun D, Cull-Candy SG (1990) Rectification of currents activated by nicotinic acetylcholine receptors in rat sympathetic ganglion neurones. J Physiol 427:625–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum SE, Caggiula AR, Booth S, Breese CR, Lee MJ, Donny EC, Leonard S, Sved AF (2000) Mecamylamine prevents tolerance but enhances whole brain [3H]epibatidine binding in response to repeated nicotine administration in rats. Psychopharmacology (Berl) 150:1–8 [DOI] [PubMed] [Google Scholar]
- McCallum SE, Caggiula AR, Epstein LH, Saylor S, Ploskina T, Sved AF (1999) Mecamylamine blocks the development of tolerance to nicotine in rats: implications for the mechanisms of tolerance. Psychopharmacology (Berl) 141:332–338 [DOI] [PubMed] [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME (1999) MRI anatomy of schizophrenia. Biol Psychiatry 45:1099–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy J, Freudenreich O, Mcgee M, Vanderzwaag C, Levin E, Rose J (1995a) Clozapine Decreases Smoking In Patients With Chronic Schizophrenia. Biol Psychiatry 37:550–552 [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Freudenreich O, Levin ED, Rose JE (1995b) Haloperidol Increases Smoking In Patients With Schizophrenia. Psychopharmacology 119:124–126 [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Freudenreich O, Wilson WH (1999) Smoking and therapeutic response to clozapine in patients with schizophrenia. Biol Psych 46:125–129 [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJS, Gelber S, Devay P, Role LW (1995) Nicotine Enhancement Of Fast Excitatory Synaptic Transmission In Cns By Presynaptic Receptors. Science 269:1692–1696 [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW (1995) Physiological Diversity Of Nicotinic Acetylcholine Receptors Expressed By Vertebrate Neurons. Annu Rev Physiol 57:521–546 [DOI] [PubMed] [Google Scholar]
- Meyer EM, Tay ET, Papke RL, Meyers C, Huang GL, de Fiebre CM (1997) 3-[2,4-Dimethoxybenzylidene]anabaseine (DMXB) selectively activates rat alpha7 receptors and improves memory-related behaviors in a mecamylamine-sensitive manner. Brain Res 768:49–56 [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW (2006) Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol 70:801–805 [DOI] [PubMed] [Google Scholar]
- Miledi R (1980) Intracellular Calcium and Desensitization of Acetylcholine Receptors. Proc R Soc London (Biol) 209:447–452 [DOI] [PubMed] [Google Scholar]
- Mitchell AS, Dalrymple-Alford JC, Christie MA (2002) Spatial working memory and the brainstem cholinergic innervation to the anterior thalamus. J Neurosci 22:1922–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed S, Paulsen JS, O’Leary D, Arndt S, Andreasen N (1999) Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Arch Gen Psychiatry 56:749–754 [DOI] [PubMed] [Google Scholar]
- Molinari EJ, Delbono O, Messi ML, Renganathan M, Arneric SP, Sullivan JP, Gopalakrishnan M (1998) Up-regulation of human alpha7 nicotinic receptors by chronic treatment with activator and antagonist ligands. Eur J Pharmacol 347:131–139 [DOI] [PubMed] [Google Scholar]
- Moss SJ, McDonald BJ, Rudhard Y, Schoepfer R (1996) Phosphorylation of the predicted major intracellular domains of the rat and chick neuronal nicotinic acetylcholine receptor alpha 7 subunit by cAMP-dependent protein kinase. Neuropharmacology 35:1023–1028 [DOI] [PubMed] [Google Scholar]
- Mulle C, Choquet D, Korn H, Changeux JP (1992) Calcium influx through nicotinic receptor in rat central neurons: its relevance to cellular regulation. Neuron 8:135–143 [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Hea RA, Griffith JM, McRae KA, Freedman R (1996) Gating of auditory P50 in schizophrenics: unique effects of clozapine. Biological Psychiatry 40:181–188 [DOI] [PubMed] [Google Scholar]
- Nakayama H, Okuda H, Nakashima T (1993) Phosphorylation of rat brain nicotinic acetylcholine receptor by camp-dependent protein kinase invitro. Mol Brain Res 20:171–177 [DOI] [PubMed] [Google Scholar]
- Nanri M, Miyake H, Murakami Y, Matsumoto K, Watanabe H (1998) GTS-21, a nicotinic agonist, attenuates multiple infarctions and cognitive deficit caused by permanent occlusion of bilateral common carotid arteries in rats. Jpn J Pharmacol 78:463–469 [DOI] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J (2003) Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol 63:332–341 [DOI] [PubMed] [Google Scholar]
- Neuhaus R, Cachelin AB (1990) Changes in the conductance of the neuronal nicotinic acetylcholine receptor channel induced by magnesium. Proc Biol Sci 241:78–84 [DOI] [PubMed] [Google Scholar]
- Nguyen QT, Yang J, Miledi R (2002) Effects of atypical antipsychotics on vertebrate neuromuscular transmission. Neuropharmacology 42:670–676 [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH (1994) Systemic nicotine-induced dopamine dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse 16:36–44 [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH (1995) Nicotine Dependence, Midbrain Dopamine Systems And Psychiatric Disorder. Pharm Tox 76:157–162 [DOI] [PubMed] [Google Scholar]
- Nordberg A (1994) Human nicotinic receptors: their role in aging and dementia. Neurochem Int 25:93–97 [DOI] [PubMed] [Google Scholar]
- Nordberg A, Nilsson-Hakanson L, Adem A, Hardy S, Alafuzoff I, Lai Z, Herrera-Marschitz M, Winblad B (1989) The role of nicotinic receptors in the pathophysiology of Alzheimer’s disease. Prog Brain Res 79:353–362 [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK (2004) Identification of separable cognitive factors in schizophrenia. Schizophr Res 72:29–39 [DOI] [PubMed] [Google Scholar]
- Obach RS, Reed-Hagen AE, Krueger SS, Obach BJ, O’Connell TN, Zandi KS, Miller S, Coe JW (2006) Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab Dispos 34:121–130 [DOI] [PubMed] [Google Scholar]
- Ochoa EL, Li L, McNamee MG (1990) Desensitization of central cholinergic mechanisms and neuroadaptation to nicotine. Mol Neurobiol 4:251–287 [DOI] [PubMed] [Google Scholar]
- Ochoa ELM (1994) Nicotine-related brain disorders: the neurobiological basis of nicotine dependence. Cell Mol Neurobiol 14:195–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa ELM, Chattopadhyay A, McNamee MG (1989) Desensitization of the nicotinic acetylcholine receptor: molecular mechanisms and effect of modulators. Cell Mol Neurobiol 9:141–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa ELM, Clark E (2006) Galantamine may improve Attention and Speech in Schizophrenia. Human Psycopharmacol 21:127–128 [DOI] [PubMed] [Google Scholar]
- Ochoa ELM, Clark E (2004) Online (2004) Galantamine as an adjuvant treatment for negative symptoms in schizophrenia: a pilot study. Program No. 351.8. 2004 Abstract Viewer/Itinerary Planner Society for Neuroscience, Washington, DC
- Ochoa ELM, O’Shea SM (1994) Concomitant protein phosphorylation and endogenous acetylcholine release induced by nicotine: dependency on neuronal nicotinic receptors and desensitization. Cell Mol Neurobiol 14:315–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Farrell TJ, Connors GJ, Upper D (1983) Addictive behaviors among hospitalized psychiatric patients. Addict Behav 18:329–333 [DOI] [PubMed] [Google Scholar]
- Ohno M, Yamamoto T, Watanabe S (1993) Blockade of hippocampal nicotinic receptors impairs working memory but not reference memory in rats. Pharmacol Biochem Behav 45:89–93 [DOI] [PubMed] [Google Scholar]
- Olale F, Gerzanich V, Kuryatov A, Wang F, Lindstrom J (1997) Chronic nicotine exposure differentially affects the function of human alpha3, alpha4, and alpha7 neuronal nicotinic receptor subtypes. J Pharmacol Exp Ther 283:675–683 [PubMed] [Google Scholar]
- O’Leary DS, Flaum M, Kesler ML, Flashman LA, Arndt S, Andreasen NC (2000) Cognitive correlates of the negative, disorganized, and psychotic symptom dimensions of schizophrenia. J Neuropsychiatry Clin Neurosci 12:4–15 [DOI] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, Ellis J, Zerbe GO, Leonard S, Stevens KE, Stevens JO, Martin L, Adler LE, Soti F, Kem WR, Freedman R (2006) Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry 63:630–638 [DOI] [PubMed] [Google Scholar]
- Olincy A, Ross RG, Young DA, Roath M, Freedman R (1998) Improvement in smooth pursuit eye movements after cigarette smoking in schizophrenic patients. Neuropsychopharmacology 18:175–185 [DOI] [PubMed] [Google Scholar]
- Olincy A, Young DA, Freedman R (1997) Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry 42:1–5 [DOI] [PubMed] [Google Scholar]
- O’Neill HC, Rieger K, Kem WR, Stevens KE (2003) DMXB, an alpha7 nicotinic agonist, normalizes auditory gating in isolation-reared rats. Psychopharmacology (Berl) 169:332–339 [DOI] [PubMed] [Google Scholar]
- O’Shea SM, Ochoa ELM (1993) Nicotine-induced synapsin I phosphorylation and endogenous acetylcholine release in cholinergic nerve endings. Soc Neurosci Abstr 19:902 [Google Scholar]
- Papke RL, Boulter J, Patrick J, Heinemann S (1989) Single-channel currents of rat neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Neuron 3:589–596 [DOI] [PubMed] [Google Scholar]
- Papke RL, Duvoisin RM, Heinemann SF (1993) The amino terminal half of the nicotinic beta-subunit extracellular domain regulates the kinetics of inhibition by neuronal bungarotoxin. Proc Biol Sci 252:141–148 [DOI] [PubMed] [Google Scholar]
- Papke RL, Heinemann SF (1991) The role of the beta 4-subunit in determining the kinetic properties of rat neuronal nicotinic acetylcholine alpha 3-receptors. J Physiol 440:95–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson D, Nordberg A (2000) Neuronal nicotinic receptors in the human brain. Prog Neurobiol 61:75–111 [DOI] [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Wang F, Lindstrom J (1997) Chronic nicotine treatment up-regulates alpha3 and alpha7 acetylcholine receptor subtypes expressed by the human neuroblastoma cell line SH-SY5Y. Mol Pharmacol 51:776–784 [DOI] [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J (1994a) Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Mol Pharmacol 46:523–530 [PubMed] [Google Scholar]
- Peng X, Katz M, Gerzanich V, Anand R, Lindstrom J (1994b) Human Alpha-7-Acetylcholine Receptor—Cloning of the Alpha-7-Subunit From The Sh-Sy5Y cell line and determination of pharmacological properties of native receptors and functional Alpha-7-homomers expressed in Xenopus-oocytes. Molecular Pharmacology 45:546–554 [PubMed] [Google Scholar]
- Pereira EF, Hilmas C, Santos MD, Alkondon M, Maelicke A, Albuquerque EX (2002) Unconventional ligands and modulators of nicotinic receptors. J Neurobiol 53:479–500 [DOI] [PubMed] [Google Scholar]
- Perry E, Martin-Ruiz C, Lee M, Griffiths M, Johnson M, Piggott M, Haroutunian V, Buxbaum JD, Nãsland J, Davis K, Gotti C, Clementi F, Tzartos S, Cohen O, Soreq H, Jaros E, Perry R, Ballard C, McKeith I, Court J (2000) Nicotinic receptor subtypes in human brain ageing, Alzheimer and Lewy body diseases. Eur J Pharmacol 393:215–222 [DOI] [PubMed] [Google Scholar]
- Perry E, Walker M, Grace J, Perry R (1999) Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci 22:273–280 [DOI] [PubMed] [Google Scholar]
- Piasecki M, Newhouse P (2000) Nicotine in psychiatry: psychopathology and emerging therapeutics. American Psychiatric Press, Washington, DC [Google Scholar]
- Picciotto MR, Zoli M, Changeux JP (1999) Use of knock-out mice to determine the molecular basis for the actions of nicotine. Nicotine Tob Res 1(Suppl 2):S121–125; discussion S139–140 [DOI] [PubMed] [Google Scholar]
- Pitschel-Walz G, Bauml J, Bender W, Engel RR, Wagner M, Kissling W (2006) Psychoeducation and compliance in the treatment of schizophrenia: results of the Munich Psychosis Information Project Study. J Clin Psychiatry 67:443–452 [DOI] [PubMed] [Google Scholar]
- Pollio DE, North CS, Reid DL, Miletic MM, McClendon JR (2006) Living with severe mental illness–what families and friends must know: evaluation of a one-day psychoeducation workshop. Soc Work 51:31–38 [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G (1996) Effects of nicotine on the nucleus accumbens and similarity to those of the addictive drugs. Nature 382:255–257 [DOI] [PubMed] [Google Scholar]
- Purdon SE, Jones BD, Stip E, Labelle A, Addington D, David SR, Breier A, Tollefson GD (2000) Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidol. The Canadian Collaborative Group for research in schizophrenia. Arch Gen Psychiatry 57:249–258 [DOI] [PubMed] [Google Scholar]
- Quick MW, Lester RA (2002) Desensitization of neuronal nicotinic receptors. J Neurobiol 53:457–478 [DOI] [PubMed] [Google Scholar]
- Radant AD, Hommer D W (1992) A quantitative analysis of saccades and smooth pursuit during visual pursuit tracking. A comparison of schizophrenics with normals and substance abusing controls. Schizophrenia Res 6:225–235 [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Corrigall W A (2003) Effects of acute and chronic nicotine on somatodendritic dopamine release of the rat ventral tegmental area: in vivo microdialysis study. Neurosci Lett 348:61–64 [DOI] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu C R, Qu X, Perin F, Karlin A, Role L (1996) Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature 380:347–351 [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney M C, Mohr E, Chase T N (1998) The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 20:310–319 [DOI] [PubMed] [Google Scholar]
- Rhoades HM, Overall J E (1988) The semistructured BPRS interview and rating guide. Psychopharmacol Bull 24:101–104 [PubMed] [Google Scholar]
- Role LW (1992) Diversity in primary structure and function of neuronal nicotinic acetylcholine receptor channels. Curr Opin Neurobiol 2:254–262 [DOI] [PubMed] [Google Scholar]
- Role LW, Berg DK (1996) Nicotinic receptors in the development and modulation of CNS synapses. Neuron 16:1077–1085 [DOI] [PubMed] [Google Scholar]
- Ross AF, Green WN, Hartman DS, Claudio T (1991) Efficiency of acetylcholine receptor subunit assembly and its regulation by cAMP. J Cell Biol 113:623–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse RB, Deutsch SI (2002) Adjuvant galantamine administration improves negative symptoms in a patient with treatment-refractory schizophrenia. Clin Neuropharmacol 25:272–275 [DOI] [PubMed] [Google Scholar]
- Rowell PP, Winkler DL (1984) Nicotinic stimulation of [3H]acetylcholine release from mouse cerebral cortical synaptosomes. J Neurochem 43:1593–1598 [DOI] [PubMed] [Google Scholar]
- Rowell PP, Wonnacott S (1990) Evidence for functional activity of up-regulated nicotine binding sites in rat striatal synaptosomes. J Neurochem 55:2105–2110 [DOI] [PubMed] [Google Scholar]
- Rubboli F, Court JA, Sala C, Morris C, Perry E, Clementi F (1994) Distribution of neuronal nicotinic receptor subunits in human brain. Neurochem Int 25:69–71 [DOI] [PubMed] [Google Scholar]
- Sallette J, Pons S, Devillers-Thiery A, Soudant M, Prado de Carvalho L, Changeux JP, Corringer PJ (2005) Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron 46:595–607 [DOI] [PubMed] [Google Scholar]
- Samochocki M, Hoffle A, Fehrenbacher A, Jostock R, Ludwig J, Christner C, Radina M, Zerlin M, Ullmer C, Pereira EF, Lubbert H, Albuquerque EX, Maelicke A (2003) Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther 20:20 [DOI] [PubMed] [Google Scholar]
- Sanderson EM, Drasdo AL, McCrea K, Wonnacott S (1993) Upregulation of nicotinic receptors following continuous infusion of nicotine is brain-region-specific. Brain Res 617:349–352 [DOI] [PubMed] [Google Scholar]
- Sands SB, Barish ME (1991) Calcium permeability of neuronal nicotinic acetylcholine receptor channels in PC12 cells. Brain Res 560:38–42 [DOI] [PubMed] [Google Scholar]
- Sands SB, Barish ME (1992) Neuronal nicotinic acetylcholine receptor currents in phaeochromocytoma (PC12) cells: dual mechanisms of rectification. J Physiol (Lond) 447:467–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos MD, Alkondon M, Pereira EF, Aracava Y, Eisenberg HM, Maelicke A, Albuquerque EX (2002) The nicotinic allosteric potentiating ligand galantamine facilitates synaptic transmission in the mammalian central nervous system. Mol Pharmacol 61:1222–1234 [DOI] [PubMed] [Google Scholar]
- Sargent PB (1993) The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci 16:403–443 [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P (1991) Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry 48:618–624 [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC (1994) Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry 51:124–131 [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Ivanov VB, Wiker C, Svensson TH (2006) Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology [DOI] [PubMed]
- Schreiber R, Dalmus M, De Vry J (2002) Effects of alpha 4/beta 2- and alpha 7-nicotine acetylcholine receptor agonists on prepulse inhibition of the acoustic startle response in rats and mice. Psychopharmacology (Berl) 159:248–257 [DOI] [PubMed] [Google Scholar]
- Schubert MH, Young KA, Hicks PB (2006) Galantamine improves cognition in schizophrenic patients stabilized on risperidone. Biol Psychiatry (Jun 23 On Line Publication) [DOI] [PubMed]
- Schwartz RD, Kellar KJ (1983) Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science 220:214–216 [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ (1985) In vivo regulation of [3H] acetylcholine recognition sites in brain by nicotinic cholinergic drugs. J Neurochem 45:427–433 [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley MK, Dani JA, Patrick JW (1993) Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci 13:596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS (1999) The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 45:17–25 [DOI] [PubMed] [Google Scholar]
- Seppala NH, Leinonen EV, Lehtonen ML, Kivisto KT (1999) Clozapine serum concentrations are lower in smoking than in non-smoking schizophrenic patients. Pharmacol Toxicol 85:244–246 [DOI] [PubMed] [Google Scholar]
- Sharma T, Reed C, Aasen I, Kumari V (2006) Cognitive effects of adjunctive 24-weeks Rivastigmine treatment to antipsychotics in schizophrenia: a randomized, placebo-controlled, double-blind investigation. Schizophr Res 85:73–83 [DOI] [PubMed] [Google Scholar]
- Sharp BM, Beyer HS (1986) Rapid desensitization of the acute stimulatory effects of nicotine on rat plasma adrenocorticotropin and prolactin. J Pharmacol Exp Ther 238:486–491 [PubMed] [Google Scholar]
- Sharples CG, Kaiser S, Soliakov L, Marks MJ, Collins AC, Washburn M, Wright E, Spencer JA, Gallagher T, Whiteaker P, Wonnacott S (2000) UB-165: a novel nicotinic agonist with subtype selectivity implicates the alpha4beta2* subtype in the modulation of dopamine release from rat striatal synaptosomes. J Neurosci 20:2783–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda K, Someya T, Morita S, Hirokane G, Noguchi T, Yokono A, Shibasaki M, Takahashi S (1999) Lower plasma levels of haloperidol in smoking than in nonsmoking schizophrenic patients. Ther Drug Monit 21:293–296 [DOI] [PubMed] [Google Scholar]
- Shioda S, Nakajo S, Hirabayashi T, Nakayama H, Nakaya K, Matsuda K, Nakai Y (1997) Neuronal nicotinic acetylcholine receptor in the hypothalamus: morphological diversity and neuroendocrine regulations. Brain Res Mol Brain Res 49:45–54 [DOI] [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, Seaward J, McKenna P, Chua SE, Schnorr L, et, a. l (1995) A functional neuroanatomy of hallucinations in schizophrenia. Nature 378:176–179 [DOI] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Kem WR, Freedman R (2001) Intragastric DMXB-A, an alpha7 nicotinic agonist, improves deficient sensory inhibition in DBA/2 mice. Biol Psychiatry 50:493–500 [DOI] [PubMed] [Google Scholar]
- Soliakov L, Wonnacott S (1996) Voltage-sensitive Ca2+ channels involved in nicotinic receptor-mediated [3H]dopamine release from rat striatal synaptosomes. J Neurochem 67:163–170 [DOI] [PubMed] [Google Scholar]
- Stassen HH, Bridler R, Hägele S, Hergersberg M, Mehmann B, Schinzel A, Weisbrod M, Scharfetter C (2000) Schizophrenia and smoking: evidence for a common neurobiological basis? Am. J Med Genet 96:173–177 [PubMed] [Google Scholar]
- Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks JM, Rose GM (1996) Genetic Correlation Of Inhibitory Gating Of Hippocampal Auditory Evoked Response And Alpha-Bungarotoxin-Binding Nicotinic Cholinergic Receptors In Inbred Mouse Strains. Neuropsychopharmacology 15:152–162 [DOI] [PubMed] [Google Scholar]
- Stevens KE, Kem WR, Mahnir VM, Freedman R (1998) Selective alpha7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology (Berl) 136:320–327 [DOI] [PubMed] [Google Scholar]
- Swope SL, Moss SJ, Blackstone CD, Huganir RL (1992) Phosphorylation of ligand-gated ion channels: a possible mode of synaptic plasticity. Faseb J 6:2514–2523 [PubMed] [Google Scholar]
- Tandon T, Ochoa ELM (1992) Calcium and nicotine induced desensitization of endogenous acetylcholine release from mammalian brain cholinergic nerve endings. Soc Neurosci Abs 18:634 [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA (2004) Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science 306:1029–1032 [DOI] [PubMed] [Google Scholar]
- Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C (2000) A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology 54:2269–2276 [DOI] [PubMed] [Google Scholar]
- Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR (2006) Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 296:64–71 [DOI] [PubMed] [Google Scholar]
- Torrey EF (2002) Studies of individuals with schizophrenia never treated with antipsychotic medications: a review. Schizophr Res 58:101–115 [DOI] [PubMed] [Google Scholar]
- Tune LE (2001) Anticholinergic effects of medication in elderly patients. J Clin Psychiatry 62:(Suppl 21):11–14 [PubMed] [Google Scholar]
- Tune LE, Egeli S (1999) Acetylcholine and delirium. Dement Geriatr Cogn Disord 10:342–344 [DOI] [PubMed] [Google Scholar]
- Ullian EM, McIntosh JM, Sargent PB (1997) Rapid synaptic transmission in the avian ciliary ganglion is mediated by two distinct classes of nicotinic receptors. J Neurosci 17:7210–7219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo YF, Buisson B, Bertrand D, Green WN (2005) Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J Neurosci 25:5563–5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernallis AB, Conroy WG, Berg DK (1993) Neurons assemble acetylcholine receptors with as many as three kinds of subunits while maintaining subunit segregation among receptor subtypes. Neuron 10:451–464 [DOI] [PubMed] [Google Scholar]
- Vernino S, Amador M, Luetje CW, Patrick J, Dani JA (1992) Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron 8:127–134 [DOI] [PubMed] [Google Scholar]
- Vernino S, Rogers M, Radcliffe KA, Dani JA (1994) Quantitative Measurement Of Calcium Flux Through Muscle And Neuronal Nicotinic Acetylcholine Receptors. J Neurosci 14:5514–5524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibat CR, Lasalde JA, McNamee MG, Ochoa EL (1995) Differential desensitization properties of rat neuronal nicotinic acetylcholine receptor subunit combinations expressed in Xenopus laevis oocytes. Cell Mol Neurobiol 15:411–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraghavan S, Schmid HA, Halvorsen SW, Berg DK (1990) Cyclic AMP-dependent phosphorylation of a neuronal acetylcholine receptor alpha-type subunit. J Neurosci 10:3255–3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vovin R, Fakturovich A, Golenkov AV, Lukin VO (1991) Correction of apathetic-abulic manifestations of schizophrenia with cholinotropic drugs. Zh. Nevropatol. Psikhiatr Im S S Korsakova 91:111–115 [PubMed] [Google Scholar]
- Vovin R, Fakturovich A, Golenkov AV, Lukin VO (1992) Correction of apathic-abulic manifestations of the processual defect by cholinotropic preparations. Neurosci Behav Physiol 22:241–245 [DOI] [PubMed] [Google Scholar]
- Waldo MC, Carey G, Myles-Worsley M, Cawthra E, Adler LE, Nagamoto HT, Wender P, Byerley W, Plaetke R, Freedman R (1991) Codistribution of a sensory gating deficit and schizophrenia in multi-affected families. Psychiatry Res 39:257–268 [DOI] [PubMed] [Google Scholar]
- Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, Lindstrom J (1996) Assembly of human neuronal nicotinic receptor alpha5 subunits with alpha3, beta2, and beta4 subunits. J Biol Chem 271:17656–17665 [DOI] [PubMed] [Google Scholar]
- Wang JM, Zhang L, Yao Y, Viroonchatapan N, Rothe E, Wang ZZ (2002) A transmembrane motif governs the surface trafficking of nicotinic acetylcholine receptors. Nat Neurosci 5:963–970 [DOI] [PubMed] [Google Scholar]
- Warburton DM (1992) Nicotine as a cognitive enhancer. Prog Neuropsychopharmacol Biol Psychiatry 16:181–191 [DOI] [PubMed] [Google Scholar]
- Wecker L, Guo X, Rycerz AM, Edwards SC (2001) Cyclic AMP-dependent protein kinase (PKA) and protein kinase C phosphorylate sites in the amino acid sequence corresponding to the M3/M4 cytoplasmic domain of alpha4 neuronal nicotinic receptor subunits. J Neurochem 76:711–720 [DOI] [PubMed] [Google Scholar]
- Wecker L, Rogers CQ (2003) Phosphorylation sites within alpha4 subunits of alpha4beta2 neuronal nicotinic receptors: a comparison of substrate specificities for cAMP-dependent protein kinase (PKA) and protein kinase C (PKC) Neurochem Res 28:431–436 [DOI] [PubMed] [Google Scholar]
- Weiland S, Bertrand D, Leonard S (2000) Neuronal nicotinic acetylcholine receptors: from the gene to the disease. Behav Brain Res 113:43–56 [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Sharples CG, Wonnacott S (1998) Agonist-induced up-regulation of alpha4beta2 nicotinic acetylcholine receptors in M10 cells: pharmacological and spatial definition. Mol Pharmacol 53:950–962 [PubMed] [Google Scholar]
- Whitehouse PJ, Kellar KJ (1987) Nicotinic and muscarinic cholinergic receptors in Alzheimers’s disease and related disorders. J Neural Transm (suppl) 24:175–182 [PubMed] [Google Scholar]
- Whiting PJ, Lindstrom JM (1988) Characterization of bovine and human neuronal nicotinic acetylcholine receptors using monoclonal antibodies. J Neurosci 8:3395–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock GK, Lilienfeld S, Gaens E (2000) Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicentre randomised controlled trial. Galantamine International-1 Study Group. Brit Med J 321:1445–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S (1990) The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharm Sci 11:216–219 [DOI] [PubMed] [Google Scholar]
- Wonnacott S (1997) Presynaptic nicotinic ACh receptors. Trends Neurosci 20:92–98 [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Kaiser S, Mogg A, Soliakov L, Jones IW (2000) Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur J Pharmacol 393:51–58 [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Thorne B (1990) Separation of pre- and post-synaptic receptors on Percoll gradients. Biochem Soc Trans 18:885–886 [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS (2003) Mecamylamine reversal by nicotine and by a partial alpha7 nicotinic acetylcholine receptor agonist (GTS-21) in rabbits tested with delay eyeblink classical conditioning. Behav Brain Res 143:159–167 [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Green JT, Coleman-Valencia C, Pak JT (2000) A nicotinic cholinergic agonist (GTS-21) and eyeblink classical conditioning: acquisition, retention, and relearning in older rabbits. Exp Aging Res 26:323–336 [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Li YT, Kem WR (1994) A nicotinic agonist (GTS-21), eyeblink classical conditioning, and nicotinic receptor binding in rabbit brain. Brain Res 645:309–317 [DOI] [PubMed] [Google Scholar]
- Woolf NJ (1999) Cholinergic correlates of consciousness: from mind to molecules. Trend Neurosci 22:540–541 [DOI] [PubMed] [Google Scholar]
- Xiao Y, Kellar KJ (2004) The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sites stably expressed in transfected mammalian cells. J Pharmacol Exp Ther 310:98–107 [DOI] [PubMed] [Google Scholar]
- Yang XH, Buccafusco JJ (1994) Effect of chronic central treatment with the acetylcholine analog methylcarbamylcholine on cortical nicotinic receptors—correlation between receptor changes and behavioral function. J Pharmacol Exp Ther 271:651–659 [PubMed] [Google Scholar]
- Zhang L, Zhou FM, Dani JA (2004) Cholinergic drugs for Alzheimer’s disease enhance in vitro dopamine release. Mol Pharmacol 66:538–544 [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Coggan JS, Berg DK (1996) Synaptic currents generated by neuronal acetylcholine receptors sensitive to alpha-bungarotoxin. Neuron 17:1231–1240 [DOI] [PubMed] [Google Scholar]
- Ziedonis DM, Kosten TR, Glazer WM, Frances RJ (1994) Nicotine dependence and schizophrenia. Hosp Community Psychiatry 45:204–206 [DOI] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HP (1998) Four pharmacologically distinct subtypes of alpha4beta2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol Pharmacol 54:1124–1131 [PubMed] [Google Scholar]