Abstract
This article reviews ocular adverse events (AEs) reported in association with administration of antibody–drug conjugates (ADCs) in human clinical trials. References reporting ocular toxicity or AEs associated with ADCs were collected using online publication searches. Articles, abstracts, or citations were included if they cited ocular toxicities or vision-impairing AEs with a confirmed or suspected association with ADC administration. Twenty-two references were found citing ocular or vision-impairing AEs in association with ADC administration. All references reported use of ADCs in human clinical trials for treatment of various malignancies. The molecular target and cytotoxic agent varied depending on the ADC used. Ocular AEs affected a diversity of ocular tissues. The most commonly reported AEs involved the ocular surface and included blurred vision, dry eye, and corneal abnormalities (including microcystic corneal disease). Most ocular AEs were not severe (≤ grade 2) or dose limiting. Clinical outcomes were not consistently reported, but when specified, most AEs improved or resolved with cessation of treatment or with ameliorative therapy. A diverse range of ocular AEs are reported in association with administration of ADCs for the treatment of cancer. The toxicologic mechanism(s) and pathogenesis of such events are not well understood, but most are mild in severity and reversible. Drug development and medical professionals should be aware of the clinical features of these events to facilitate early recognition and intervention in the assessment of preclinical development programs and in human clinical trials.
Introduction
While conventional chemotherapeutic agents serve as the foundations of most cancer treatment protocols, drug toxicities commonly result in dose-limiting adverse events (AEs). Targeted agents such as monoclonal antibodies (mAbs), however, aim to reduce toxicity and demonstrate encouraging potential in the clinical setting.1,2
As of February 2015, over 35 mAbs have been approved by the Food and Drug Administration (FDA), and at least 15 mAbs were first approved for the treatment of cancer.3 Despite proven activity against malignancies, however, most mAbs are prescribed only as adjuncts to conventional chemotherapy protocols due to limited efficacy as single-agent therapies.4 Putatively contributing to these limitations are factors such as target heterogeneity or loss of targets on tumor cells, as well as insufficiency of the desired antitumor immune response.4 Furthermore, the presence of similar targets in healthy tissues has contributed to a variety of drug-related toxicities, including ocular toxicities.5–7
The eye may be susceptible to toxicity due to several factors, including its inherently robust blood supply, presence of subpopulations of rapidly dividing cells, and an abundance and variety of cell surface receptors. In turn, the ocular AEs associated with targeted agents such as mAbs are diverse, affecting a variety of structures. Severities of mAb-associated ocular toxicities are also variable, ranging from minor ocular irritation to severe vision-threatening events.5–7
The newest generation of targeted cancer therapies, the antibody–drug conjugates (ADCs), capitalize on molecular binding of an mAb and cytotoxin through a chemical linker.8 Once directed to a tumor cell by its mAb, the conjugate is internalized and undergoes lysosomal degradation, liberating its cytotoxic payload to act on its intracellular target.8 Most ADCs employ powerful tubulin-inhibiting cytotoxins (maytansinoids, auristatins) or other potent agents that target and disrupt DNA (calicheamicin, duocarmycin).9,10 Preclinical and clinical investigations of ADCs have demonstrated considerable antitumor efficacy and therefore great potential to function as single-agent therapies for certain cancers.9–12
Despite their promise, the design of ADCs and refinement of their pharmacologic properties are challenging. Limitations related to linker stability, target specificity, and payload delivery have been encountered, influencing efficacy and margin of safety.9 Despite a paucity of published evidence regarding ocular toxicity of ADCs in the preclinical literature, ocular AEs have been reported in clinical investigations. The following is a review of the clinical literature reporting those ocular toxicities and AEs associated with ADCs.
Methods
Data regarding ocular AEs associated with ADCs were collected using online publication searches, including PubMed, Medline, GoogleScholar™, and Scopus™, as well as the FDA Adverse Event Reporting System database, and the website of the US Patent and Trademark Office. Keywords or terms searched included “antibody-drug conjugate” (“ADC”), “eye,” “ocular,” “ocular toxicity,” “ophthalmologic,” “vision,” “keratitis,” “cornea,” “corneal microcyst,” “corneal inclusions,” “conjunctivitis,” “dry eye,” “uveitis,” “cataract,” “neuropathy,” “retina,” and “blindness.” Articles or abstracts were included in the review if they cited ocular toxicity or vision-impairing ocular AE(s) in association with administration of an ADC. When available, descriptions of AEs and data reporting incidence, severity, and reversibility were compiled; the features of associated ADCs were compared with those without reported association with ocular AEs.
Results
Twenty-two references were found citing ocular or vision-impairing AEs associated with 13 different ADCs, summarized in Table 1. All references cited phase I or II clinical trials determining the safety, tolerability, activity, pharmacokinetics, and/or maximum tolerated dose (MTD) of ADCs. The indication for ADC administration in all references was treatment of cancer (solid tumors in 14 references and hematopoietic/lymphoid neoplasia in 10 references). In almost all references, patients had refractory or recurrent malignant neoplasms and had undergone prior chemotherapeutic treatment. In 1 study,13 some patients naïve to chemotherapeutic treatment were enrolled. Only 1 ADC was administered to a patient in any trial.
Table 1.
Summary of Features of Ocular and/or Vision-Impairing AEs Associated with Administration of Antibody–Drug Conjugates
| ADC | Indication (neoplasm) | Target antigen/cytotoxin/linker | Toxicity/symptom | Incidence | Gradea | Outcome | Reference |
|---|---|---|---|---|---|---|---|
| IMGN242 (huC242-DM4) | CanAg-expressing solid tumors | CanAg/DM4/SPDB | Decreased visual acuity, corneal deposits, keratitis | 2/30 (during second treatment cycle, at 223 mg/m2) | Not specified | Resolved in 1 patient; significantly improved in the other | Mita et al.18 |
| IMGN242 (huC242-DM4) | CanAg-positive gastric or gastroesophageal junction tumors | CanAg/DM4/SPDB | Not specified | 3/6 (receiving 168 mg/m2) | Not specified | Not specified | Goff et al.19 |
| Trastuzumab emtansine (T-DM1) | Advanced HER2+ breast cancer | HER2/DM1/SMCC | Conjunctivitis, photophobia, swollen tear duct | 1/24b | Grade 1–2 | Not specified | Krop et al.26 |
| Trastuzumab emtansine (T-DM1) | HER2+ breast cancer | HER2/DM1/SMCC | Dry eye, increased lacrimation, vision blurred/vision impairment, conjunctivitisc | 35/112 | 1–2c | Not specified | Burris et al.27 |
| Trastuzumab emtansine (T-DM1) | Advanced HER2+ breast cancer | HER2/DM1/SMCC | Cataract, ocular surface disease, punctuate keratitis, dry eye | 13/28 | 3 (2 patients); others not specified | Not specified | Beeram et al.28 |
| SAR3419 (huB4-DM4) | Relapsed/refractory B-cell lymphoma | CD19/DM4/SPDB | Severe blurred vision, microcystic corneal epithelial changesd | 17/39 | 2 (7 patients); 3 (5 patients); 4 (1 patient) | Reversibled | Younes et al.35 |
| SAR3419 (huB4-DM4) | Relapsed/refractory diffuse large B-cell lymphoma (CD19+ and CD20+) | CD19/DM4/SPDB | Blurred vision, dry eye, conjunctivitis, diplopia, eye irritation, corneal deposits, keratitis, keratoconjunctivitis, scotoma, optic neuropathy | Blurred vision (5 patients); dry eye (3 patients); diplopia (2 patients); all others (1 patient each) | 1–2 (except optic neuropathy associated with grade 3–4 blurred vision and eye irritation in a single patient) | Not specified | Coiffier et al.36 |
| SAR3419 (huB4-DM4) | Relapsed/refractory B-cell NHL | CD19/DM4/SPDB | Weekly: blurred vision, optic neuropathy, corneal deposit/microcysts.e | Weekly: 10/44 (blurred vision and corneal microcysts)e | 2 (corneal deposit) and 3 (optic neuropathy) | Reversible corneal toxicity (outcome of bilateral uveitis unknown due to patient death) | Ribrag et al.37 |
| Optimized schedule: blurred vision, bilateral uveitis w/decreased visual acuity; optic neuropathy (with blurred vision), diplopia, eye irritation | Optimized schedule: 4/25 | ||||||
| SGN-CD19A | Relapsed or refractory B-cell NHL, mantle cell lymphoma, or grade 3 follicular lymphoma | CD19/MMAF (auristatin)/mc | Blurred vision, dry eye, keratopathy, microcystic keratopathy | Blurred vision (59%), dry eye (39%), keratopathy (23%), microcystic keratopathy (57%) | Grade 3 or 4 in 4 patients receiving higher doses; otherwise, mostly grade 1–2 | Resolved or improved following treatment with steroid eye drops | Moskowitz et al.38 |
| SGN-CD19A | Relapsed or refractory B-lineage acute leukemia and highly aggressive lymphoma | CD19/MMAF (auristatin)/mc | Blurred vision, dry eye, superficial microcystic keratopathy | Superficial microcystic keratopathy in 13 adult patients (34%) and 1 pediatric patient (9%) | Grade 3/4 corneal AEs in 4 adult patients | Majority resolved or improved to grade 1–2 with steroid eye drops | Fathi et al.39 |
| AVE9633 | Relapsed/refractory acute myeloid leukemia | CD33/DM4/SPDB | Keratitis (dose-limiting) | 1/52 | 3 | Reversible | Lapusan et al.43 |
| SGN-75 | CD70+ metastatic RCC or relapsed/refractory NHL | CD70/MMAF (auristatin)/mc | Iridocyclitis | Not specified | 2 | Not specified | Thompson et al.48 |
| SGN-75 | CD70-positive relapsed/refractory NHL or metastatic RCC | CD70/MMAF (auristatin)/mc | Corneal epitheliopathy, dry eye, blurred vision, keratitis | Q3week dosing (57%): Corneal epitheliopathy (15%), dry eye (30%), blurred vision (11%), keratitis (9%) | Q3week dosing: ≥ Grade 3 in 23% | Generally reversible (resolved or resolving) with artificial tears and steroid eye drops | Tannir et al.47 |
| Weekly dosing (36%): | Weekly dosing: | ||||||
| Dry eye (27%), blurred vision (18%) | Grade 3: 1 patient with dry eye | ||||||
| SAR566658 | CA6+ advanced solid tumors | DS6/DM4/SPDB | Keratitis | 11/34 | 3 (2 patients); others not specified | Reversible | Boni et al.50 |
| Gemtuzumab ozogamicin | Acute myeloid leukemia | CD33/calicheamicin/hydrazone | Ocular bleeding (anatomic location not specified) | 1/9 | 4 | Not specified | Piccaluga et al.13 |
| MEDI-547 | Relapsed or refractory solid tumors | Ephrin type A receptor 2 (EphA2)/mcMMAF (auristatin)/mc | Eye pain, conjunctival hemorrhage | 1/6 (each for eye pain and hemorrhage) | Not specified (but classified as “serious”) | Not specified | Annunziata et al.58 |
| Lorvotuzumab mertansine (IMGN901) | CD56+ solid tumors | CD56/DM1/SPP | Eye redness | Total 3/64; 1/29 (4–48 mg/m2/day); 1/22 (75 mg/m2/day; 1/2 (94 mg/m2/day) | 1–2 | Not specified | Woll et al.63 |
| Lorvotuzumab mertansine (IMGN901) | Merkel cell carcinoma | CD56/DM1/SPP | Cortical blindness (consistent with reversible posterior leukoencephalopathy syndrome) | Single patient (case report) | Not specified | Slow progressive improvement with persistent visual field deficit | Wilson et al.64 |
| BT062 | Relapsed/refractory multiple myeloma | CD138/DM4/SPDB | Dry eyes, blurred vision, epithelial corneal damage, crystal inclusions | One patient with dry eyes/blurred vision; 1 patient with corneal epithelial disease/inclusions | 2 | Not specified | Chanan-Khan et al.67 |
| IMGN853 | FRα+ solid tumors | FRα/DM4/SPDB | TBW dosing: punctate keratitis, blurred vision, unspecified other ocular toxicity | TBW dosing: 4/11 at 5.0 mg/kg; 5/5 at 7.0 mg/kg | TBW dosing: 1–3 AIBW dosing: 1–2 for all AEs | Reversible | Moore et al.68 |
| AIBW dosing: punctate keratitis, blurred vision, retinopathy, floaters | AIBW dosing: 1/7 at 5.0 mg/kg; 3/7 at 6.0 mg/kg | ||||||
| IMGN853 | Platinum-resistant epithelial ovarian cancer | FRα/DM4/SPDB | Blurred vision, keratitis, punctate keratitis, corneal epithelial microcysts, retinal vein occlusion/vision impairment, corneal cyst, eye pain | Blurred vision (∼50%), keratitis or punctate keratitis (<20% each), corneal epithelial microcysts (1 patient), retinal vein occlusion/vision impairment (2 patients), corneal cyst (2 patients), eye pain (1 patient) | All ≤ grade 3 | Manageable with dose modifications | Moore et al.70 |
| AGS-16M8F-MMAF and AGS-16C3F-MMAF | Refractory RCCs (clear cell and papillary) | ENPP3/MMAF (auristatin)/mc | Most commonly reversible keratopathy, but otherwise not specified | AGS-16M8F: 8/26 | AGS-16M8F: 1 ≥ grade 3 | Reversible | Thompson et al.72 |
| AGS-16C3F: 29/34 | AGS-16C3F (any dose): 12 ≥ grade 3 | ||||||
| AGS-16C3F (1.8 mg/kg): 12/13 |
US Department of Health and Human Services. “Common terminology criteria for adverse events (CTCAE) version 4.0.” National Institutes of Health, National Cancer Institute (2009).
Incidence of conjunctivitis, photophobia, and swollen tear duct was reported as 1/24 for each, respectively. Whether these AEs were observed in separate patients was not specified.
One patient with a history of glaucoma developed grade 3 glaucoma and transient grade 4 reduced visual acuity, reported as unrelated to T-DM1. The patient remained on therapy without recurrence.
Most common finding was bilateral microcystic corneal epitheliopathy, typically starting at corneal periphery in a ring-like manner, migrating toward the papillary axis with occasional whitish clumping at the epithelial level. Corneal changes were reversible in all patients, and the single patient with grade 4 corneal changes experienced resolution to baseline within 3 days of discontinuation. When additional doses were administered following a previous ocular AE, corneal toxicity was recurrent with subsequent cycles, but again reversible in all patients.
Most common finding at slit-lamp examination was bilateral corneal epitheliopathy with microcystic appearance typically starting at the corneal periphery in a ring-like manner, migrating toward the axis with occasional whitish clumping at the epithelial level. Patients tear function and corneal thickness were largely not affected and the rest of the examination was unremarkable.
ADCs, antibody-drug conjugates; AIBW, adjusted ideal body weight; ENPP3, ectonucleotide pyrophosphatase/phosphodiesterase 3; FRα; folate receptor α; GO, gemtuzumab ozogamicin; HER2, human epidermal growth factor receptor 2; MMAF, monomethyl auristatin F; NHL, non-Hodgkin lymphoma; RCC, renal cell carcinoma; TBW, total body weight.
Among all 13 ADCs, 11 distinct molecules were targeted, the mAb component of each varying depending on the tumor being treated. Table 2 presents a list of cells, tissues, and neoplasms expressing the targets cited in this review. Most ADCs (12/13) employed tubulin-inhibiting cytotoxins (8 with maytansinoids and 4 with auristatins). Only 1 employed a DNA-targeting cytotoxin (calicheamicin).
Table 2.
Antigens Targeted by ADCs Reported to Cause Ocular AEs; the Cells, Tissues, and/or Neoplasms Expressing Each Antigen
| Target antigen | Cells/tissues/neoplasms expressing antigen | Reference(s) |
|---|---|---|
| CanAg | Gastrointestinal and pancreatic carcinomas, some nonsmall cell lung tumors | 16 |
| HER2 | Breast tumors (∼10%–30%) | 20,21 |
| CD19 | B lymphocytes (precursor and differentiated with the exception of differentiated plasma cells) and neoplastic B lymphocytes | 8,29,30 |
| CD33 | Acute myeloid leukemia, myeloid precursor cells, mature monocytes, and macrophages | 40,41,43 |
| CD70 | Activated T and B lymphocytes, mature dendritic cells, lymphoma, RCC, glioblastoma | 44,45 |
| CA6 | Solid tumors (pancreas, ovary, breast, bladder) | 9 |
| Ephrin type A receptor 2 (EphA2) | Overexpressed in some carcinomas, melanomas, and gliomas; normal epithelial tissues (skin, lung, colon, ovary, bladder) | 54 |
| CD56 | Neurons, astrocytes, Schwann cells, natural killer cells, and some activated T lymphocytes | 44,59 |
| CD138 | Mature epithelial cells, overexpression has been documented on B-cell and plasma cell precursors, as well as mature plasma cells, including those associated with multiple myeloma | 65,66 |
| FRα | Various solid tumors (ovarian, endometrial, and some lung tumors) | 68,69 |
| ENPP3 | Renal carcinomas (clear cell and papillary) | 71,72 |
AEs, adverse events.
A summary of ocular AE incidence across all 22 references is presented in Table 3. AEs commonly involved the ocular surface, including keratitis (8/22), dry eye (7/22), corneal microcysts (5/22), corneal deposits/inclusions (4/22), conjunctivitis/keratoconjunctivitis (3/22), and unspecified keratopathy (2/22). Corneal epithelial defect/damage, swollen tear duct, increased lacrimation, conjunctival hemorrhage, and eye redness were cited in 1 reference each. The most common symptom reported by affected patients was blurred vision (10/22). Decreased visual acuity and diplopia were each reported in 2/22. Intraocular AEs were uncommon, reported sporadically in individual patients. Neuro-ophthalmic or neurologic AEs included optic neuropathy (2/22) and cortical blindness (1/22), presumed in the latter to be reversible posterior leukoencephalopathy syndrome (RPLS). When specified, 13/22 references cited ocular toxicities or AEs equaling or exceeding grade 3 (according to the Common Terminology Criteria for Adverse Events, US Department of Health and Human Services, National Institutes of Health, National Cancer Institute).14
Table 3.
Summary of the Incidence of Ocular AEs Across All References
| Adverse event | Number of references (of 22) citing AE |
|---|---|
| Blurred vision | 10 |
| Keratitis (including punctate) | 8 |
| Dry eye | 7 |
| Corneal microcysts/microcystic epithelial changes | 5 |
| Corneal deposits/inclusions | 4 |
| Conjunctivitis/keratoconjunctivitis | 3 |
| Decreased visual acuity | 2 |
| Unspecified keratopathy | 2 |
| Optic neuropathy | 2 |
| Eye irritation | 2 |
| Diplopia | 2 |
| Corneal epithelial defect/damage | 1 |
| Unspecified ocular toxicity | 1 |
| Ocular surface disease | 1 |
| Swollen tear duct | 1 |
| Increased lacrimation | 1 |
| Conjunctival hemorrhage | 1 |
| Eye redness | 1 |
| Ocular bleeding (location unspecified) | 1 |
| Uveitis (bilateral) | 1 |
| Iridocyclitis | 1 |
| Photophobia | 1 |
| Cataract | 1 |
| Cortical blindness | 1 |
| Scotoma | 1 |
| Nonspecific retinopathy | 1 |
| Eye pain | 1 |
| Floaters | 1 |
| Retinal vein occlusion | 1 |
For comparison, the structural components of ADCs associated with and unassociated with ocular AEs are compared in Table 4.
Table 4.
Structural Components of ADCS Associated with and Unassociated with Ocular Adverse Events
| ADC | MAb mode | Target | Cytotoxin | Linker | |
|---|---|---|---|---|---|
| ADCs associated with ocular AEs | Trastuzumab emtansine (Kadcyla®, T-DM1) | Humanized | Her-2 | Maytansine (DM1) | SMCC |
| Lorvotuzumab mertansine (IMGN-901) | Humanized | CD56 | Maytansine (DM1) | SPP | |
| SAR3419 (HuB4-DM4) | Humanized | CD19 | Maytansine (DM4) | SPDB | |
| BT-062 | Chimerized | CD138 Syndecan1) | Maytansine (DM4) | SPDB | |
| SAR566658 (HuDS6-DM4) | Humanized | Muc1 (CA6) | Maytansine (DM4) | SPDB | |
| SGN-75 | Humanized | CD70 | Auristatin (MMAF) | Maleimidocaproyl (mc) | |
| IMGN242 (huc242-DM4) | Humanized | CanAg | Maytansine (DM4) | SPDB | |
| SGN-CD19A | Humanized | CD19 | Auristatin (MMAF) | Maleimidocaproyl (mc) | |
| AVE9633 | Humanized | CD33 | Maytansine (DM4) | SPDB | |
| GO | Humanized | CD33 | Calicheamicin | Hydrazone | |
| MEDI-547 | Human | EphA2 | Auristatin (MMAF) | Maleimidocaproyl (mc) | |
| IMGN 853 | Humanized | FRa | Maytansine (DM4) | SPDB | |
| AGS-16M8F (AGS-6MF) | Fully human | ENPP3 | Auristatin (MMAF) | Maleimidocaproyl (mc) | |
| ADCs unassociated with ocular AEsa | Brentuximab vedotin (Adcetris®, SGN-35) | Chimeric | CD30 | Auristatin (MMAE) | vc |
| Inotuzumab ozogamicin (CMC-544) | Humanized | CD22 | Calicheamicin | Hydrazone/AcBut | |
| Glembatumumab vedotin (CDX-011, CR-011-vcMMAE) | Fully human | GPNMB | Auristatin (MMAE) | vc | |
| Milatuzumab–doxorubicin (IMMU-110) | Humanized | CD74 | Doxorubicin | Hydrazone | |
| AGS-22M6E (ASG-22ME) | Fully human | Nectin-4 | Auristatin (MMAE) | vc | |
| AMG-172 | ND | ND | ND | ND | |
| AMG-595 | Fully human | EGFRvIII | Maytansinoid | Noncleavable | |
| ASG-5ME (AGS-5M2E) | Fully human | SLC44A4 | Auristatin (MMAE) | vc | |
| DEDN-6526A | Humanized | ET8R (endothelin B) | Auristatin (MMAE) | vc | |
| IMGN 529 (K7153A-SMCC-DM1) | Humanized | CD37 | Maytansine (DM1) | SMCC | |
| IMMU-130 (hMN14-SN38) | Humanized | CEACAM5 | SN-38 | CL2 | |
| MDX-1203 | Fully human | CD70 | MGBA | vc | |
| PSMA-ADC (PSMA-ADC-1301) | Fully human | PSMA | Auristatin (MMAE) | vc | |
| RG-7450 (DSTP-3086S) | ND | ND | Auristatin | ND | |
| RG-7458 | ND | MUC16 (CA125) | Auristatin (MMAE) | ND | |
| RG-7593 (pinatuzumab vedotin, DCDT-2980S) | Humanized | CD22 | Auristatin (MMAE) | vc | |
| RG-7596 (DCDS-4501A) | Humanized | CD79b | Auristatin | ND | |
| RG-7598 | ND | ND | Auristatin | ND | |
| RG-7599 | ND | MUC16 (CA125) | Auristatin | ND | |
| RG-7600 | ND | ND | Auristatin | ND | |
| BAY 94–9343 | Fully human | Mesothelin | Maytansine (DM4) | SPDB |
Modified from Sassoon and Blanc.112
EGFR, epidermal growth factor receptor; MMAE, monomethyl auristatin E; ND, nondisclosed.
IMGN242 (huC242-DM4)
IMGN242 (huC242-DM4) is a conjugate of a humanized antibody (huC242) and DM4 (a maytansinoid). huC242 demonstrates affinity for a tumor-associated epitope of CanAg, a glycoform molecule of MUC1.15 CanAg is a desirable target expressed by gastrointestinal and pancreatic carcinomas and nonsmall cell lung tumors.16 A preclinical abstract reported superior activity of IMGN242 against CanAg-expressing solid tumors in a mouse xenograft model.17
In phase I/II clinical trials, ocular AEs in some patients prompted dose alteration and ameliorative therapy. In a phase I study, patients with refractory and/or inoperable CanAg-expressing solid tumors were treated with single intravenous (IV) infusions of IMGN242 (18–297 mg/m2) once every 3 weeks.18 Dose-limiting toxicities, including decreased visual acuity, corneal deposits, and keratitis, were reported in 2 patients receiving 223 mg/m2 during the second treatment cycle. Ameliorative lubricating eye drops were prescribed. Ocular AEs were reversible, with 1 patient experiencing marked improvement and the other returning to baseline. Grades of severity were not specified.
In a phase II study, patients with CanAg-positive metastatic or locally advanced gastric/gastroesophageal tumors were eligible if treated with at least 1 chemotherapeutic regimen before enrollment.19 Six patients received a single IV infusion at the MTD (168 mg/m2) every 3 weeks. Three patients developed unspecified ocular AEs, but additional details were not reported.
Trastuzumab emtansine (Kadcyla® (formerly T-DM1), Genentech©)
Trastuzumab emtansine (Kadcyla (formerly T-DM1), Genentech) is a conjugate of the human epidermal growth factor receptor 2 (HER2)-binding antibody trastuzumab (Herceptin®, Genentech) and DM1 (a maytansinoid). HER2 is abundantly expressed in 10%–30% of primary human breast tumors20,21; and tumor cells overexpressing HER2 often demonstrate aggressive growth associated with poorer clinical outcomes.20,22 Despite these prognostic implications, HER2 is an attractive therapeutic target. When combined with chemotherapy, unconjugated trastuzumab has prolonged survival in patients with HER2+ breast cancer.23 To date, T-DM1 is considered one of the most successful examples of ADC development into the clinic.16 Correspondingly, preclinical evaluation has demonstrated excellent stability, safety, and efficacy in HER2-expressing tumor models.24,25
In a phase I, dose-escalation clinical trial, patients with metastatic HER2-positive breast cancer received T-DM1 IV every 3 weeks (0.3–4.8 mg/kg).26 Patients received a mean of 4 chemotherapeutic treatments before enrollment. While the majority of AEs were nonocular, conjunctivitis, photophobia, and swollen tear duct (all grade 1–2) were infrequently reported. Further details concerning management or follow-up were not specified.
In a phase II study, patients with tumor progression despite treatment with HER2-directed therapy and prior chemotherapy, received T-DM1 IV every 3 weeks at the MTD (3.6 mg/kg).27 Ocular AEs (mostly grade 1–2) were reported in 31.3% of patients, including dry eye, increased lacrimation, blurring of vision/vision impairment, and conjunctivitis. Follow-up information was not specified. In this study, a single patient with historic glaucoma developed grade 3 glaucoma with transient grade 4 reduced visual acuity, but this was not considered ADC related. That patient continued treatment without recurrence.
In another phase I dose-escalation study of patients with advanced HER2-positive breast cancer, T-DM1 was administered weekly or every 3 weeks.28 Patients had previously received trastuzumab. Ocular AEs were reported in 13/28 patients, including cataract, ocular surface disease, punctuate keratitis, and dry eye. In 2 patients, AEs were classified as grade 3.
SAR3419 (CD19-DM4)
SAR3419 (CD19-DM4) is a conjugate of humanized IgG1 anti-CD19 monoclonal antibody and DM4. CD19 is a glycoprotein member of the immunoglobulin superfamily, expressed on normal and cancerous B lymphocytes.8 It is more abundant early in cellular development from pre-B-cell differentiation through plasma cell differentiation.29,30 It is believed that CD19 targeting can eliminate potentially malignant immature B cells that otherwise escape targeting by other B-cell antigens such as CD20.29 SAR3419 has been associated with minimal toxicity and encouraging efficacy in animal models for lymphoma, demonstrating superior antitumor activity when compared with unconjugated maytansinoid or antibody alone.31–34
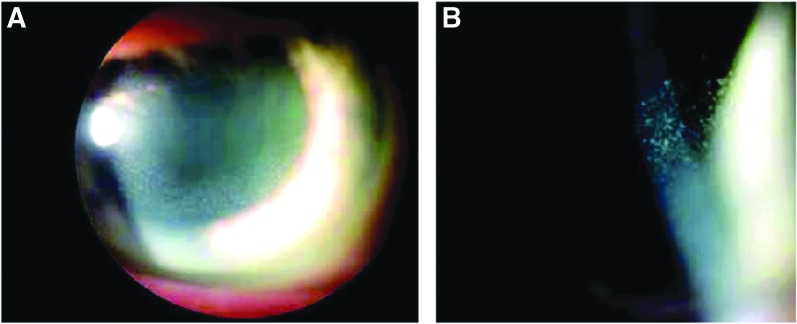
In a phase I multidose-escalation study, SAR3419 was administered to patients with relapsed or refractory B-cell lymphoma.35 Patients had undergone a median of 4 prior treatment regimens, some receiving stem cell transplantation and all receiving rituximab. Escalating IV doses of SAR3419 (10–270 mg/m2) were administered every 3 weeks. Dose-limiting ocular AEs were observed in 17 patients (44%), primarily at higher doses (≥160 mg/m2) after the second administration. Blurring of vision was reported in 16 patients, typically after the second dose. Microcystic corneal epitheliopathy was commonly observed on ophthalmic examination, typically distributed at the corneal periphery in a ring-like pattern, migrating toward the central cornea (Fig. 1). Clumping of whitish intraepithelial material was also occasionally observed. Seven patients had grade 2 AEs, 5 had grade 3, and 1 had grade 4. Based on these findings, the MTD was 160 mg/m2. Corneal AEs were reversible in all affected patients, but recovery to baseline vision required a dose delay of 1–2 weeks. Even following dose delay, reversible corneal toxicity was again observed.
FIG. 1.
Clinical images of microcystic corneal epithelial changes associated with SAR3419 (huB4-DM4; Younes et al.35). (A) Slit lamp photo of corneal toxicity, epithelial haze in the mid-periphery of the cornea. (B) High-power photo showing micro-cystic appearance within the corneal epithelium. Color images available online at www.liebertpub.com/jop
In another phase I/II dose-escalation study, SAR3419 was dosed weekly for a total of 8 doses.36 Dose-dependent ocular AEs were observed, including blurred vision, dry eye, conjunctivitis, diplopia, eye irritation, corneal deposits, keratitis, keratoconjunctivitis, scotoma, and optic neuropathy. The only ocular AE exceeding grade 2 occurred in a patient with optic neuropathy associated with grade 3–4 blurred vision and eye irritation.
A subsequent phase I study evaluated a weekly IV dose and an optimized IV dosing schedule (4 weekly infusions, followed by 4 biweekly infusions) at 55 mg/m2. The optimized schedule yielded an improved safety profile.37 Of 44 patients receiving weekly treatment, 10 (23%) reported blurring of vision, and bilateral microcystic corneal changes with whitish epithelial clumps, similar to those reported by Younes et al., were described on slit-lamp examination.35 With the optimized schedule, only 4/25 patients (16%) developed ocular AEs, including blurred vision, bilateral uveitis with decreased visual acuity (1 patient), and optic neuropathy with diplopia and eye irritation (1 patient). The only AE exceeding grade 2 was optic neuropathy (grade 3). All corneal toxicities were reversible. Visual acuity returned to 90% in the patient with uveitis within 6 weeks, but further follow-up was not available due to death of the patient.
SGN-CD19A
SGN-CD19A is a conjugate of a humanized anti-CD19 antibody and an auristatin drug, monomethyl auristatin F (MMAF). CD19 is commonly expressed in patients with B-cell non-Hodgkin lymphoma (NHL).30
In a phase I dose-escalation study evaluating SGN-CD19A in patients with relapsed or refractory lymphomas, SGN-CD19A was administered IV (0.5–6.0 mg/kg) at 21-day intervals.38 Patients were enrolled if they had received at least 1 systemic treatment regimen for their malignancy. Ocular AEs were reported in ≥20% of patients, including blurred vision (59%), dry eye (39%), and keratopathy (23%). In 25 patients (57%), corneal examination confirmed superficial microcystic keratopathy (grade 1–2). In 4 patients receiving higher doses, grade 3–4 corneal AEs were observed.
In another phase I dose-escalation study, SGN-CD19A was administered to adult and pediatric patients with relapsed/refractory B-cell leukemia or highly aggressive lymphomas.39 Patients were enrolled if they had received at least 1 treatment regimen for their malignancy. SGN-CD19A was administered according to 2 IV dosing schedules: weekly on days 1 and 8 of 21-day cycles (0.3–4.5 mg/kg) or every 3 weeks (0.5–6 mg/kg). Blurred vision and dry eye were among the most frequently reported AEs. Superficial microcystic keratopathy was observed in 13 adult patients (34%) and 1 pediatric patient (9%). Grade 3–4 corneal AEs were observed in 4 adult patients.
In both studies, patients experiencing ocular AEs were prescribed ophthalmic steroids and most experienced resolution or improvement to grade 1–2 by last follow-up. This observation prompted topical steroid prophylaxis in both trials, administered before each ADC dose. At interim analysis, the authors of the latter study reported that this prophylaxis had reduced the incidence of grade 3–4 events.39
AVE9633
AVE9633 is a conjugate of an anti-CD33 antibody and DM4. CD33 is expressed on myeloid precursor cells and mature monocytes and in up to 90% of patients with acute myeloid leukemia (AML), making it an attractive therapeutic target.40,41 In preclinical evaluation, AVE9633 demonstrated encouraging antitumor activity in an animal model of AML.42
Data from 3 phase I single-agent trials have been reported, evaluating AVE9633 in patients with relapsed or refractory AML.43 AVE9633 was administered at escalating IV doses at 3 different schedules; day 1 of a 21-day cycle, (15–260 mg/m2), days 1 and 8 of a 28-day cycle (30–150 mg/m2), and days 1, 4, and 7 of a 28-day cycle (30–90 mg/m2). One patient receiving the day 1/8 schedule developed dose-limiting, reversible grade 3 keratitis during the second cycle at 150 mg/m2.
SGN-75 (CD70-MMAF)
SGN-75 (CD70-MMAF) is a conjugate of a humanized anti-CD70 antibody and MMAF. CD70 is a member of the tumor necrosis factor family, expressed on the surfaces of activated T and B lymphocytes, natural killer cells, and mature dendritic cells.44 It is also expressed in some malignancies, including lymphoma, renal cell carcinoma (RCC), and glioblastoma.44,45 In a preclinical study, SGN-75 demonstrated antitumor activity in a mouse model of human RCC.45,46
SGN-75 was evaluated in phase I clinical trials for treatment of patients with RCC and/or NHL.47,48 In a phase I dose-escalation study, SGN-75 was administered IV (0.6–3 mg/kg) weekly or every 3 weeks.48 The only ocular AE reported was grade 2 iridocyclitis, but details regarding incidence and follow-up were not specified.
In the other study, SGN-75 was administered IV every 3 weeks (0.3–4.5 mg/kg) or on days 1, 8, and 15 of 28-day cycles (0.3–0.6 mg/kg).47 Dose-dependent ocular AEs (some dose limiting) were reported in 57% of patients dosed every 3 weeks, typically observed following multiple doses. These AEs included dry eye (30%), corneal epitheliopathy (15%), blurred vision (11%), and keratitis (9%). In 23% of those patients, ocular AEs were ≥ grade 3. Ocular AEs were reported in 36% of patients dosed weekly, including dry eye (27%) and blurred vision (18%), but corneal epitheliopathy was not observed. Only 1 patient experienced an AE (dry eye) ≥ grade 3. Ocular AEs were reversible, with even grade 3 events reported to improve or resolve following discontinuation of treatment. Observation of ocular AEs prompted a subsequent amendment, requiring clinical assessment of affected patients by a corneal specialist. Examination identified a ring pattern of microcystic-appearing corneal epithelial changes, starting in the periphery and migrating centrally. This finding was also associated with astigmatism and refractive error. Artificial tears and steroid eye drops were prescribed, mitigating the duration and severity of ocular symptoms. Thereafter, steroid eye drops were recommended for all patients with evidence of microcystic corneal epitheliopathy, even if asymptomatic.
SAR566658 (DS6-DM4)
SAR566658 (DS6-DM4) is a conjugate of a CA6-targeting antibody (huDS6) and DM4. CA6 is a glycol-epitope of MUC1, overexpressed in some solid tumors, including those of the pancreas, ovary, breast, and bladder.9 Preclinical evaluation has been performed in mouse models of solid human tumors.49
In a phase I, dose-escalation clinical trial, SAR566658 (SAR) was evaluated in patients with CA6-expressing solid tumors. Patients received single-agent therapy with escalating IV doses (10–240 mg/m2) every 3 weeks.50 Keratitis was reported in 11/34 patients, observed in those receiving 150 mg/m2 late in the study. In 2 patients, the keratitis was classified as grade 3. Keratitis was reportedly reversible, but additional information was not specified.
Gemtuzumab ozogamicin (Mylotarg™, Pfizer©)
Gemtuzumab ozogamicin (GO; Mylotarg, Pfizer) was a conjugate of a CD33-binding antibody and a calicheamicin cytotoxin. CD33 is associated with myeloid differentiation and highly expressed on myeloid precursor cells and circulating monocytes.40,43 Expression is lower on granulocytes and macrophages and constitutive on dendritic cells.40 GO demonstrated potent antitumor activity in a preclinical study,51 receiving FDA approval in 2000 for treatment of patients with CD33-positive AML.11 Despite early promise in phase II trials, Mylotarg was withdrawn from the market in 2010 due to concerns about relative efficacy.52,53
In a clinical study, 9 elderly patients with AML (untreated or relapsed) were treated with GO (6 mg/m2 on day 1 and 4 mg/m2 on day 8) in combination with cytarabine (100 mg/m2 daily for days 1–7). One patient experienced grade 4 ocular bleeding.13 Additional details were not reported, but it was presumed to be associated with drug-induced thrombocytopenia following myelosuppression.
MEDI-547
MEDI-547 is a conjugate of a monoclonal antibody to EphA2 and MMAF. EphA2 is a member of the tyrosine kinase family and is expressed at relatively low levels in most normal adult tissues, with higher expression confined mainly to epithelial tissues (skin, lung, colon, ovary, and bladder).54 It is also abundantly expressed in some carcinomas, melanomas, and gliomas. Overexpression of EphA2 in patients with some malignancies has been associated with a poorer clinical outcome.54 Potent antitumor effects of MEDI-547 have been demonstrated in preclinical animal models.55–57
In a phase I study, MEDI-547 was evaluated in patients with relapsed or refractory solid tumors.58 MEDI-547 was initially administered IV (0.08 mg/kg) every 3 weeks. All patients in the study, however, discontinued treatment and dose escalation was never pursued, primarily due to treatment-related hemorrhage or coagulation events. Eye pain was also reported in 1/6 patients, prompting discontinuation of treatment. Conjunctival hemorrhage was observed in 1/6 patients.
Lorvotuzumab mertansine (IMGN901)
Lorvotuzumab mertansine (IMGN901) is a conjugate of a CD56-targeting antibody and DM1. CD56 [neural cell adhesion molecule (NCAM)] is a glycoprotein expressed on neurons, astrocytes, Schwann cells, skeletal muscle, natural killer cells, and some activated T lymphocytes. It is also expressed on many multiple myeloma cells and some solid tumor cells.44,59 IMGN901 demonstrated potent antitumor activity in preclinical models of small cell lung and ovarian cancers and multiple myeloma.60–62
A phase I, dose-escalation clinical study evaluated IMGN901 in patients with CD56-positive solid tumors.63 Patients were enrolled if they had received 1–6 prior chemotherapeutic regimens. IMGN901 was administered IV, once daily for 3 consecutive days, every 3 weeks. Ocular AEs were uncommon, but eye redness (≤ grade 1–2) was reported in 3 patients at 3 different dose levels (4–48, 75, and 94 mg/m2/day). Details regarding duration of the AE and/or reversibility were not specified.
A case report described cortical blindness in a single patient with CD56-positive Merkel cell carcinoma (MCC) receiving IMGN901 in a phase I study.64 On day 16 of the study, the patient developed nausea, vomiting, headache, and confusion and disorientation. Brain magnetic resonance imaging (MRI) revealed bilaterally symmetrical cortical and subcortical lesions involving the parietal and occipital white matter characteristic of RPLS. Although the etiology of RPLS is unclear and a causative relationship with IMGN901 is not verifiable, the onset of clinical signs and characteristic MRI findings were highly suggestive. Despite improvement with general supportive care and monitoring, vision recovery was slow and a visual field deficit persisted.
BT-062
BT-062 is a conjugate of an anti-CD138 antibody and DM4. CD138 is a cell surface proteoglycan molecule involved with cell adhesion. CD138 is primarily expressed on the surfaces of mature epithelial cells. Overexpression has been demonstrated on B-cell and plasma cell precursors, including those associated with multiple myeloma.65,66 In vivo preclinical investigations have demonstrated antitumor activity.65
BT-062 was evaluated in a phase I dose-escalation study of patients with relapsed or refractory multiple myeloma.67 BT-062 was administered IV every 3 weeks (10–200 mg/m2). Four days following the fourth cycle of treatment (at 160 mg/m2), 1 female patient experienced dry eyes and blurry vision. Three days after the third cycle of treatment, at the same dose, a male patient developed corneal epithelial damage and crystal corneal inclusions. In both patients, these AEs were designated as grade 2 and medically significant. Further details regarding reversibility and/or follow-up were not reported.
IMGN853
IMGN853 is a conjugate of a folate receptor α (FRα)-binding antibody and DM4. FRα is abundantly expressed on several solid tumors, including those of the ovary, endometrium, and lung.68,69
In a phase I dose-escalation study of patients with refractory FRα+ solid tumors, doses of IMGN853 [based on total body weight (TBW)] were administered IV at 21-day intervals.68 Ocular AEs were observed in 4/11 patients at 5.0 mg/kg and 5/5 patients at 7.0 mg/kg. The nature of all AEs was not specified, but severity ranged from grade 1 to 3 at both dose levels, including dose-limiting punctate keratitis and blurred vision in 2 patients, respectively, at 7.0 mg/kg. Pharmacokinetic analysis determined an association between ocular AEs and earlier exposure to higher plasma levels of IMGN853 and poor correlation between patient plasma volume and TBW. To mitigate this discrepancy, patients' adjusted ideal body weights (AIBWs) were calculated and thereafter used to determine the administered dose, aiming to decrease variability in early exposure levels. The use of AIBWs yielded visual disturbance in only 1/7 patients at 5.0 mg/kg (grade 1–2). At 6.0 mg/kg, however, 3/7 patients developed grade 1–2 ocular AEs, including blurred vision with punctate keratitis, blurred vision with floaters, and unspecified retinopathy in 3 patients, respectively. All ocular AEs were reversible. No patients receiving IMGN853 at 5.0 or 6.0 mg/kg using AIBWs developed a ≥ grade 3 ocular AE.
Another study evaluated IMGN853 in patients with FRα-positive platinum-resistant epithelial ovarian cancer (EOC). Patients were enrolled if they had platinum-resistant histologically confirmed EOC, primary peritoneal cancer, or fallopian tube cancer and had received no more than 5 prior systemic treatment regimens.70 Ocular AEs included blurred vision (∼50%) and keratitis or punctate keratitis (<20% each). Corneal epithelial microcysts, corneal cysts, and eye pain were also infrequently reported and, in some cases, warranted dose delay, reduction, or continuation. When specified, all ocular AEs were grade 1 or 2. Follow-up was not provided, but all ocular AEs were reportedly manageable with dose modifications.
AGS-16M8F-MMAF and AGS-16C3F-MMAF
AGS-16M8F-MMAF and AGS-16C3F-MMAF are conjugates of an anti-ectonucleotide pyrophosphatase/phosphodiesterase 3 (ENPP3) antibody and MMAF. Expression of the ENPP3 receptor is relatively restricted in normal tissues, but abundant in RCC cells (90% of clear cell RC and 69% of papillary RC).71 In preclinical studies using xenograft models, AGS-16M8F-MMAF demonstrated tumor inhibition and was well tolerated in nonhuman primates.71
Phase I clinical studies evaluated AGS-16M8F and AGS-16C3F in patients with refractory RCC.72 Both were administered every 3 weeks at dose ranges of 0.6–4.8 and 1.8–4.8 mg/kg, respectively. In patients receiving AGS-16M8F, 8/26 patients experienced ocular AEs (only 1 patient ≥ grade 3), most commonly a reversible keratopathy occurring at higher doses, prompting treatment discontinuation in 3 patients. Ocular AEs were more prominent in patients receiving AGS-16C3F, affecting 29/34 patients (≥ grade 3 in 10 patients). When the dose was de-escalated to 1.8 mg/kg, however, tolerability improved, but incidence of ocular AEs remained at 12/13 (≥ grade 3 in 2 patients).
Discussion
The available literature demonstrates a diversity of ocular or vision-related AEs associated with ADCs. Compared with nonocular toxicities, AEs affecting the eye were infrequent, rarely exceeding 50% of enrolled patients in any study. Conclusions regarding overall incidence of ocular AEs, however, are difficult to draw due to the relatively small number of references, varied study designs, and presumed variability within and between study populations. Furthermore, a range of individual patient- and treatment-related factors affecting pharmacokinetics and clearance in cancer patients, particularly those with advanced disease, may influence the incidence of AEs.73
The clinical methods used to assess affected patients were rarely reported. Slit-lamp biomicroscopic examination was specifically cited in only 2 studies,37,47 and information regarding other assessment or diagnostic techniques was scarce. Consultation with a corneal specialist was only reported in 1 study.47 Lack of detailed clinical description, particularly of ocular surface findings, may underrepresent distinguishing features of certain toxicities.
Clinical outcomes in affected patients were only mentioned in 10/22 references. When specified, however, most either improved or resolved with ameliorative therapy and/or dose alteration/discontinuation. Furthermore, 3 references38,39,47 cited treatment with a topical steroid, reporting benefit in patients with corneal AEs. In 2 studies evaluating SGN-CD19A,38,39 prophylactic treatment with a topical steroid was employed following identification of corneal AEs, and in one,39 it was associated with a reduction in incidence. Response to therapy in these studies suggests an inflammatory component to ocular toxicity, but causality or reversibility by other mechanisms (including self-limiting resolution) cannot be ruled out.
Determining the toxic mechanism(s) of ocular AEs is difficult due to the diversity among AE descriptions as well as the complexities of ADC structure and pharmacology. Hypothetically, any component of an ADC could contribute to ocular toxicity whether through on-target (antibody-mediated delivery) or off-target (delivery of an unconjugated cytotoxin) mechanisms.74 Regardless of the mode of delivery, the weight of the evidence presented here suggests a strong association between the cytotoxin and ocular AEs. Of 13 ADCs associated with ocular AEs, 12 employed potent maytansinoids. Comparatively, of ADCs without reported association to ocular AEs (Table 4), only 3/21 employed maytansinoids, but 13/21 employed auristatins, and when disclosed, the only auristatin cited was monomethyl auristatin E (MMAE).
Corneal toxicity was reported in all references citing ADCs with DM4 as their cytotoxic payload. Ocular surface AEs, including reversible superficial keratopathy, have been reported in association with other unconjugated tubulin-binding drugs (i.e., docetaxel and paclitaxel), although the exact toxic mechanism(s) are undetermined.75–79 It is plausible that similarly ocular AEs associated with ADCs result from off-target delivery of naked, but active, cytotoxin released from an unstable conjugate. Optimization of conjugate stability, and linker stability in particular, remains a principle challenge in development and refinement of ADCs. The ideal linker mitigates toxicity by remaining stable in the extracellular environment, resisting dissociation in circulation or at off-target sites, and efficiently releasing cytotoxin within the target cell. Achieving this profile in vivo, however, is complicated by factors that promote premature deconjugation and off-target delivery such as degradative proteases or other enzymes in blood or off-target tissues and variations in extracellular and intracellular conditions (i.e., pH).80
While off-target delivery of an unconjugated cytotoxin presents a simple model for ocular toxicity, other features of ADC metabolism suggest more complex mechanisms. Linker–cytotoxin chemistry, for example, considerably influences the active metabolite(s) delivered to the intracellular environment after successful targeted delivery and may contribute to on-target toxicity. In this review, both noncleavable and cleavable linkers were employed in studies evaluating a diversity of ADCs whether associated or unassociated with ocular AEs (Table 4). The majority of AE-associated ADCs, however, employed linker–cytotoxin combinations of SPDB (a cleavable disulfide linker) and DM4 (6/13) or maleimidocaproyl (a noncleavable linker) and MMAF (4/13). Conversely, these same combinations were only rarely reported (when disclosed) in ADCs unassociated with ocular AEs (Table 4).
In comparative in vitro and in vivo studies, intracellular cleavage of a disulfide-linked maytansinoid ADC (SPDB-DM4) yielded a noncharged, membrane-permeable active metabolite.81 Correspondingly, these effects of this highly toxic metabolite extended to adjacent bystander cells through diffusion across cell membranes. Despite presenting an advantage in the treatment of some heterogeneous tumors, it also risks greater distribution of toxicity.82 Conversely, intracellular degradation of a similar ADC with a noncleavable linker (SMCC-DM1) yielded a charged metabolite incapable of membrane diffusion unless assisted by active transport. Although intracellular accumulation of an active metabolite may also contribute to toxicity (and some ocular AEs were seen with SMCC-DM1 in this review), the wider distribution of a cell-permeable toxin may provide at least a partial explanation for an association with higher incidence of ocular toxicity.
Similar to maytansinoids, there is disparity in the behavior of the active metabolites of auristatins (i.e., MMAE and MMAF). Cleavage of vcMMAE [a common cleavable linker–cytotoxin pair in ADCs not associated with ocular AEs (Table 4)] yields a noncharged membrane-permeable drug capable of bystander toxicity (such as SPDB-DM4). Cleavage of mcMMAF, however, produces a charged metabolite incapable of diffusing across cellular membranes (such as SMCC-DM1).83 Dissimilar to the mechanism suggested by noncharged metabolites of the maytansinoids, toxicity associated with MMAE may be more likely related to intracellular accumulation.
To illustrate this disparity, consider the microcystic keratopathies described nearly identically in 2 unrelated clinical trials35,47 (Fig. 1). In 1 study, the administered linker–cytotoxin was SPDB-DM4, and in the other, mcMMAF. While only representing 2 references, this reinforces that (while the trends presented here may provide insights) multiple mechanisms of on-target toxicity must be considered. Furthermore, clinical descriptions of corneal toxicities associated with ADCs often lacked detail. Therefore, side-by-side comparison of corneal toxicities between different studies is challenging, further complicating determination of a toxic mechanism.
Corneal epithelial microcysts have been reported as a nonspecific consequence of generalized corneal disease and corneal dystrophies and in association with cytosine arabinoside (Ara-C), a common chemotherapeutic agent.84–88 Recent confocal microscopic investigation, however, failed to demonstrate true cysts within or under the affected corneal epithelium.89 Instead, necrotic epithelial foci were observed within the basal layer early, thereafter progressing to involve the apical layers as disease progressed. The self-limiting nature of the toxicity is presumed to result from eventual desquamation of the necrotic epithelial cells. It is noteworthy that one of the referenced clinical trials13 described concurrent administration of an ADC (GO) and Ara-C. No corneal AEs, however, were observed.
Although this keratopathy's features may bear resemblance to the microcystic lesions observed in association with SAR3419, lesion distribution is different in the latter, concentrated primarily at the periphery. Therefore, the mechanism of toxicity for an ADC such as SAR3419 may differ. Assuming that the microcystic corneal lesions associated with ADCs are truly cysts and not necrotic foci, other mechanisms could include focal separation between the epithelium and basement membrane or toxicity to limbal stem cells, resulting in altered centripetal differentiation. Further characterization of these lesions with advanced imaging (i.e., optical coherence tomography and/or confocal microscopy) is indicated in any study to investigate the pathogenesis.
Given the diversity of cellular targets presented here, ocular AEs secondary to on-target toxicity of the antibody component itself are less plausible, but may warrant consideration. Epidermal growth factor receptor (EGFR) is abundant in epithelial tissues of the adnexa and ocular surface, with ocular AEs reported in up to 15% of patients receiving targeted agents such as cetuximab or panitumumab.5,90–95 It is important to note, however, while trastuzumab-targeted HER2 is within the EGFR family, its structure and expression are distinct and it is only readily expressed on breast cancer cells.96 Despite some homology of the 2 receptors, the study has failed to demonstrate a consistent cross-reactivity between anti-HER2 antibodies and HER1 (EGFR).97
On-target ocular toxicity of mAbs targeting leukocytic cluster of differentiation (CD) molecules is not easily explained. CD19-expressing B cells and CD33-expressing mononuclear cells are not present within the healthy cornea, but may be delivered from the limbus and/or conjunctiva to the corneal epithelium through centripetal migration.98–100 Others such as CD70 and CD138 may be present in corneal dendritic cells or corneal epithelial cells, respectively, but not in abundance.67,98,101 Furthermore, ephrin type A is only found at low levels and in many tissues, and DS6 is only reported on the surfaces of solid tumors.58 The relationship between targeting of CD56 (NCAM) and the RPLS reported in association with IMGN90164 may be more directly explained due to toxicity of the mAb or DM1. The exact pathophysiology of RPLS, however, is poorly understood, and furthermore, this single report indicated that the late onset of signs following dosing argues against direct toxicity.
As with other pharmaceuticals, exposure is largely dictated by the pharmacokinetic properties of a drug and could provide insights into mechanisms of toxicity. Even though the antibody component of an ADC contributes the most to its pharmacokinetic profile, some attributes of ADCs may yield more unpredictable pharmacokinetics.102 Some ADCs, including those approved for clinical trials, possess considerable variation in the number of drug molecules bound to each antibody and heterogeneity in molecular binding sites.103 In 1 study evaluating an auristatin ADC, higher cytotoxic load per antibody actually decreased the conjugate's therapeutic index in vivo, at least partially, due to its faster clearance.104 Studies determining the impact of optimal drug load on conjugate stability remain an active area of investigation.105,106
Patient-related factors that influence ADC pharmacokinetics and increase individual drug exposure may also play important roles in ocular toxicity. Ocular AEs associated with IMGN242 prompted a pharmacokinetic and pharmacodynamic investigation of the effect of a soluble plasma form of the CanAg antigen in patients with CanAg-expressing tumors.107 This study demonstrated that patients with low plasma CanAg levels (<1,000 U/mL) more consistently developed ocular AEs than those with higher levels, putatively due to comparatively lower clearance. Binding of the conjugate in circulation and, subsequently, increased rate of clearance likely contributed to this difference, which was 3- to 5-fold higher in patients with CanAg levels exceeding 1,000 U/mL. A corresponding correlation between circulating CanAg levels and tumor CanAg expression, however, was not observed. Based on these findings, the dosing regimen reported in the phase II trial was amended,19 guided instead by an individual patient's plasma CanAg levels. In the 3 patients treated with the amended protocol, no ocular AEs were reported.
To these authors' knowledge, ocular AEs have not been reported in published preclinical evaluations of ADCs. In light of the unknown mechanism(s) of toxicity, AEs in clinical trials have led to changes in the approaches to preclinical evaluation of these agents.108 Areas of focus include incorporation of ophthalmic examinations into preclinical studies, improvement in tumor modeling,109,110 and greater attention to pharmacokinetics and pharmacodynamics.111 A more exhaustive preclinical assessment in multiple species will contribute more translatable data, with greater relevance to human toxicity.
Administration of ADCs is associated with a diversity of ocular AEs in human clinical trials, but the exact mechanisms of toxicity are undetermined. While trends were observed (i.e., higher comparative incidence of ocular AEs in association with maytansinoid- and MMAF-containing ADCs), the data presented here indicate that multiple on- and off-target mechanisms of toxicity must be considered. The majority of ocular AEs were mild and reversible, some manageable with dose alteration and/or palliative therapy. Drug development and medical professionals alike should be aware of the clinical features of these effects to facilitate early recognition and intervention in both preclinical and clinical investigations.
Acknowledgment
The authors would like to thank Dr. Anas Younes for permission to use clinical images from Younes et al.35
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Scott A.M., Wolchok J.D., and Old L.J. Antibody therapy of cancer. Nat. Rev. Cancer. 12:278–287, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Oldham R.K., and Dillman R.O. Monoclonal antibodies in cancer therapy: 25 years of progress. J. Clin. Oncol. 26:1774–1777, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Reichert J. Therapeutic monoclonal antibodies approved or in review in the European Union or United States. http://www.antibodysociety.org/news/approved_mabs.php; 2015. Last accessed August20, 2015
- 4.Zigler M., Shir A., and Levitzki A. Targeted cancer immunotherapy. Curr. Opin. Pharmacol. 13:504–510, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Kheir W.J., Sniegowski M.C., El-Sawy T., et al. Ophthalmic complications of targeted cancer therapy and recently recognized ophthalmic complications of traditional chemotherapy. Surv. Ophthalmol. 59:493–502, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Renouf D.J., Velazquez-Martin J.P., Simpson R., et al. Ocular toxicity of targeted therapies. J. Clin. Oncol. 30:3277–3286, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Jusufbegovic D., Triozzi P.L., and Singh A.D. Targeted therapy and their ocular complications. In: Singh A.D. and Damato B., eds. Clinical Ophthalmic Oncology. Berlin: Springer; 2014; p. 123–132 [Google Scholar]
- 8.Trail P.A. Antibody directed delivery for treatment of cancer: antibody drug conjugates and immunotoxins. In: Phillips G.L., ed. Antibody-Drug Conjugates and Immunotoxins. New York: Springer; 2013; p. 3–22 [Google Scholar]
- 9.Lambert J.M. Drug-conjugated antibodies for the treatment of cancer. Br. J. Clin. Pharmacol. 76:248–262, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stack G.D., and Walsh J.J. Optimising the delivery of tubulin targeting agents through antibody conjugation. Pharm. Res. 29:2972–2984, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Perez H.L., Cardarelli P.M., Deshpande S., et al. Antibody-drug conjugates: current status and future directions. Drug Discov. Today. 19:869–881, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Dosio F., Stella B., Cerioni S., et al. Advances in anticancer antibody-drug conjugates and immunotoxins. Recent Pat. Anticancer Drug Discov. 9:35–65, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Piccaluga P.P., Martinelli G., Rondoni M., et al. First experience with gemtuzumab ozogamicin plus cytarabine as continuous infusion for elderly acute myeloid leukaemia patients. Leuk. Res. 28:987–990, 2004 [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services. Common teminology criteria for adverse events (CTCAE), Version 4.0 Bethesda: National Institutes of Health, National Cancer Institute, 2009. evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf Last accessed August20, 2015
- 15.Tolcher A.W., Ochoa L., Hammond L.A., et al. Cantuzumab mertansine, a maytansinoid immunoconjugate directed to the CanAg antigen: a phase I, pharmacokinetic, and biologic correlative study. J. Clin. Oncol. 21:211–222, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Ricart A.D. Immunoconjugates against solid tumors: mind the gap. Clin. Pharmacol. Ther. 89:513–523, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Lutz R.J., Xie H., Widdison W.C., et al. HuC242-DM4, an antibody-maytansinoid conjugate with superior preclinical activity in human CanAg-positive tumor xenograft models in SCID mice. AACR Meeting Abstracts. 334, 2005 [Google Scholar]
- 18.Mita M.M., Ricart A.D., Mita A.C., et al. A phase I study of a CanAg-targeted immunoconjugate, huC242-DM4, in patients with Can Ag-expressing solid tumors. J. Clin. Oncol. 25: 3062, 2007 [Google Scholar]
- 19.Goff L.W., Papadapoulos K., Posey J.A., et al. Abstract: a phase II study of IMGN242 (huC242-DM4) in patients with CanAg-positive gastric or gastroesophageal (GE) junction cancer. J. Clin. Oncol. 27:e15625, 2009 [Google Scholar]
- 20.Pegram M., and Slamon D. Biological rationale for HER2/neu (c-erbB2) as a target for monoclonal antibody therapy. Semin. Oncol. 27:13–19, 2000 [PubMed] [Google Scholar]
- 21.Mitri Z., Constantine T., and O'Regan R. The HER2 receptor in breast cancer: pathophysiology, clinical use, and new advances in therapy. Chemother. Res. Pract. 2012:743193, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slamon D.J., Clark G.M., Wong S.G., et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 235:177–182, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Rugo H.S., Krop I.E., and Chu Y.-W. Trastuzumab emtansine (T-DM1) for the treatment of HER2-positive cancer with a focus on breast cancer. In: Phillips G.L., ed. Antibody-Drug Conjugates and Immunotoxins. New York: Springer; 2013; p. 179–210 [Google Scholar]
- 24.Hudis C.A. Trastuzumab—mechanism of action and use in clinical practice. N. Engl. J. Med. 357:39–51, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Phillips G.D.L., Li G., Dugger D.L., et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Cancer Res. 68:9280–9290, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Krop I.E., Beeram M., Modi S., et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J. Clin. Oncol. 28:2698–2704, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Burris H.A., Rugo H.S., Vukelja S.J., et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)—positive breast cancer after prior HER2-directed therapy. J. Clin. Oncol. 29:398–405, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Beeram M., Krop I.E., Burris H.A., et al. A phase 1 study of weekly dosing of trastuzumab emtansine (T-DM1) in patients with advanced human epidermal growth factor 2—positive breast cancer. Cancer. 118:5733–5740, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Lambert J.M., Blanc V., Le Bail N., et al. Targeting CD19 with SAR3419, an anti-CD19-maytansinoid conjugate for the treatment of B cell malignancies. In: Phillips G.L., ed. Antibody-Drug Conjugates and Immunotoxins. New York: Springer; 2013; p. 149–160 [Google Scholar]
- 30.Scheuermann R., and Racila E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk. Lymphoma. 18:385–397, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Lutz R.J., Zuany-Amorim C., Vrignaud P., et al. Preclinical evaluation of SAR3419 (huB4-DM4), an anti-CD19-maytansinoid immunoconjugate, for the treatment of B cell lymphomas. AACR Meeting Abstracts. 47:877, 2006 [Google Scholar]
- 32.Al-Katib A.M., Aboukameel A., Mohammad R., et al. Superior antitumor activity of SAR3419 to rituximab in xenograft models for non-Hodgkin's lymphoma. Clin. Cancer. Res. 15:4038–4045, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Carol H., Szymanska B., Evans K., et al. The anti-CD19 antibody–drug conjugate SAR3419 prevents hematolymphoid relapse postinduction therapy in preclinical models of pediatric acute lymphoblastic leukemia. Clin. Cancer Res. 19:1795–1805, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanc V., Bousseau A., Caron A., et al. SAR3419: an anti-CD19-maytansinoid immunoconjugate for the treatment of B-cell malignancies. Clin. Cancer Res. 17:6448–6458, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Younes A., Kim S., Romaguera J., et al. Phase I multidose-escalation study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered by intravenous infusion every 3 weeks to patients with relapsed/refractory B-cell lymphoma. J. Clin. Oncol. 30:2776–2782, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coiffier B., Ribrag V., Dupuis J., et al. Phase I/II study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered weekly to patients with relapsed/refractory B-cell non-Hodgkins lymphoma (NHL). J. Clin. Oncol. 29, 2011. Abstract 8017 [Google Scholar]
- 37.Ribrag V., Dupuis J., Tilly H., et al. A dose-escalation study of SAR3419, an anti-CD19 antibody maytansinoid conjugate, administered by intravenous infusion once weekly in patients with relapsed/refractory B-cell non-Hodgkin lymphoma. Clin. Cancer Res. 20:213–220, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Moskowitz C.H., Forero-Torres A., Shah B.D., et al. Interim analysis of a phase 1 study of the antibody-drug conjugate sgn-cd19a in relapsed or refractory b-lineage non-Hodgkin lymphoma. Blood. 124:1741, 2014 [Google Scholar]
- 39.Fathi A.T., Chen R., Trippett T.M., et al. Interim analysis of a phase 1 study of the antibody-drug conjugate SGN-CD19A in relapsed or refractory B-lineage acute leukemia and highly aggressive lymphoma. Blood. 124:963–963, 2014. 24833353 [Google Scholar]
- 40.Hernández-Caselles T., Martínez-Esparza M., Pérez-Oliva A.B., et al. A study of CD33 (SIGLEC-3) antigen expression and function on activated human T and NK cells: two isoforms of CD33 are generated by alternative splicing. J. Leukoc. Biol. 79:46–58, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Larson R., Boogaerts M., Estey E., et al. Antibody-targeted chemotherapy of older patients with acute myeloid leukemia in first relapse using Mylotarg (gemtuzumab ozogamicin). Leukemia. 16:1627–1636, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Chari R.J.V., Xie H., Leece B.L., et al. Preclinical evaluation of an anti-CD33-maytansinoid immunoconjugate, huMy9-6-DM4 (AVE9633) for the targeted therapy of acute myeloid leukemia. Am. Assoc. Cancer Res. Abstract-LB–287, 2005 [Google Scholar]
- 43.Lapusan S., Vidriales M.B., Thomas X., et al. Phase I studies of AVE9633, an anti-CD33 antibody-maytansinoid conjugate, in adult patients with relapsed/refractory acute myeloid leukemia. Invest. New Drugs. 30:1121–1131, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Polson A.G., Ho W.Y., and Ramakrishnan V. Investigational antibody-drug conjugates for hematological malignancies. Expert Opin. Investig. Drugs. 20:75–85, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Oflazoglu E., Stone I.J., Gordon K., et al. Potent anticarcinoma activity of the humanized anti-CD70 antibody h1F6 conjugated to the tubulin inhibitor auristatin via an uncleavable linker. Clin. Cancer Res. 14:6171–6180, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Alley S.C., Zhang X., Okeley N.M., et al. The pharmacologic basis for antibody-auristatin conjugate activity. J. Pharmacol. Exp. Ther. 330:932–938, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Tannir N.M., Forero-Torres A., Ramchandren R., et al. Phase I dose-escalation study of SGN-75 in patients with CD70-positive relapsed/refractory non-Hodgkin lymphoma or metastatic renal cell carcinoma. Invest. New Drugs. 32:1246–1257, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Thompson J., Forero-Torres A., Heath E., et al. The effect of SGN-75, a novel antibody-drug conjugate (ADC), in treatment of patients with renal cell carcinoma (RCC) or non-Hodgkin lymphoma (NHL): a phase I study. ASCO Annual Meeting Proceedings. 29:3071, 2011 [Google Scholar]
- 49.Carrigan C., Zuany-Amorim C., Mayo M., et al. 525 POSTER preclinical evaluation of SAR566658 (huDS6-DM4) in mice bearing human tumor xenografts of breast, ovarian, lung, cervical and pancreatic cancer. Eur. J. Cancer Suppl. 6:166, 2008 [Google Scholar]
- 50.Boni V., Rixe O., Rasco D., et al. Abstract A73: a phase I first-in-human (FIH) study of SAR566658, an anti CA6-antibody drug conjugate (ADC), in patients (Pts) with CA6-positive advanced solid tumors (STs) (NCT01156870). Mol. Cancer Ther. 12:A73, 2013 [Google Scholar]
- 51.Hamann P.R., Hinman L.M., Beyer C.F., et al. An anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Choice of linker. Bioconjug. Chem. 13:40–46, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Ravandi F. Gemtuzumab ozogamicin: one size does not fit all—the case for personalized therapy. J. Clin. Oncol. 29:349–351, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Petersdorf S., Kopecky K., Stuart R.K., et al. Preliminary results of Southwest Oncology Group Study S0106: an international intergroup Phase 3 randomized trial comparing the addition of gemtuzumab ozogamicin to standard induction therapy versus standard induction therapy followed by a second randomization to post-consolidation gemtuzumab ozogamicin versus no additional therapy for previously untreated acute myeloid leukemia. Blood. 114:790, 2009 [Google Scholar]
- 54.Xiao Z., Jackson D., and Tice D.A. EphA2 immunoconjugate. In: Phillips G.L., ed. Antibody-Drug Conjugates and Immunotoxins. New York: Springer; 2013; p. 241–253 [Google Scholar]
- 55.Jackson D., Gooya J., Mao S., et al. A human antibody-drug conjugate targeting EphA2 inhibits tumor growth in vivo. Cancer Res. 68:9367–9374, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Lee J.-W., Han H.D., Shahzad M.M., et al. EphA2 immunoconjugate as molecularly targeted chemotherapy for ovarian carcinoma. J. Natl. Cancer Inst. 101:1193–1205, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee J.-W., Stone R.L., Lee S.J., et al. EphA2 targeted chemotherapy using an antibody drug conjugate in endometrial carcinoma. Clin. Cancer Res. 16:2562–2570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Annunziata C.M., Kohn E.C., LoRusso P., et al. Phase 1, open-label study of MEDI-547 in patients with relapsed or refractory solid tumors. Invest. New Drugs. 31:77–84, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lambert J.M., O'Leary J., Whiteman K.R., et al. Targeting CD56 (NCAM)-expressing neoplasms with lorvotuzumab mertansine. In: Phillips G.L., ed. Antibody-Drug Conjugates and Immunotoxins. New York: Springer; 2013; p. 273–293 [Google Scholar]
- 60.Tassone P., Goldmacher V.S., Neri P., et al. Cytotoxic activity of the maytansinoid immunoconjugate B-B4–DM1 against CD138+ multiple myeloma cells. Blood. 104:3688–3696, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Tassone P., Gozzini A., Goldmacher V., et al. In vitro and in vivo activity of the maytansinoid immunoconjugate huN901-N2′-deacetyl-N2′-(3-mercapto-1-oxopropyl)-maytansine against CD56+ multiple myeloma cells. Cancer Res. 64:4629–4636, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Lutz R.J., and Whiteman K.R. Antibody-maytansinoid conjugates for the treatment of myeloma. MAbs. 1:548–551, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woll P.J., O'Brien M., Fossella F., et al. Phase I study of lorvotuzumab mertansine (IMGN901) in patients with CD56-positive solid tumors. Ann. Oncol. 21, 2010 [Google Scholar]
- 64.Wilson C., Leaning D., Shankland K., et al. Cortical blindness as an unusual adverse drug reaction. J. Med. Cases. 1:47–50, 2010 [Google Scholar]
- 65.Ikeda H., Hideshima T., Fulciniti M., et al. The monoclonal antibody nBT062 conjugated to cytotoxic Maytansinoids has selective cytotoxicity against CD138-positive multiple myeloma cells in vitro and in vivo. Clin. Cancer Res. 15:4028–4037, 2009 [DOI] [PubMed] [Google Scholar]
- 66.O'Connell F.P., Pinkus J.L., and Pinkus G.S. CD138 (Syndecan-1), a plasma cell marker immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. Am. J. Clin. Pathol. 121:254–263, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Chanan-Khan A., Jagannath S., Heffner T., et al. Phase I study of BT062 given as repeated single dose once every 3 weeks in patients with relapsed or relapsed/refractory multiple myeloma. Blood. 114:69–73, 2009 [Google Scholar]
- 68.Moore K., Ponte J., LoRusso P., et al. Relationship of pharmacokinetics (PK), toxicity and initial evidence of clinical activity with IMGN853, a folate receptor alpha (FRα)-targeting antibody drug conjugate in patients with epithelial ovarian cancer and other FRα-positive solid tumors. J. Clin. Oncol. 32:5571, 2014 [Google Scholar]
- 69.Timotheadou E. Emerging targeted agents in endometrial cancer treatment. OA Cancer. 1:9, 2013 [Google Scholar]
- 70.Moore K., Martin L., Seward S., et al. Preliminary single agent activity of IMGN853, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in platinum-resistant epithelial ovarian cancer (EOC) patients (pts): Phase I trial. J. Clin. Oncol. 33:5518, 2015 [Google Scholar]
- 71.Beck A., Lambert J., Sun M., et al. Fourth world antibody-drug conjugate summit: February 29–March 1, 2012, Frankfurt, Germany. MAbs. 4:637–647, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson J.A., Motzer R., Molina A.M., et al. Phase I studies of anti-ENPP3 antibody drug conjugates (ADCs) in advanced refractory renal cell carcinomas (RRCC). J. Clin. Oncol. 33:2503, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ratain M.J., and Plunkett W.K. Principles of pharmacodynamics. In: Kufe D.W., Pollock R.E., Weichselbaum R.R., et al. eds. Holland-Frei Cancer Medicine. Hamilton, ON: BC Decker; 2003. www.ncbi.nlm.nih.gov/books/NBK13774/ Last accessed August15, 2015 [Google Scholar]
- 74.Rudmann D.G. On-target and off-target-based toxicologic effects. Toxicol. Pathol. 41:310–314, 2013 [DOI] [PubMed] [Google Scholar]
- 75.Burstein H.J., Manola J., Younger J., et al. Docetaxel administered on a weekly basis for metastatic breast cancer. J. Clin. Oncol. 18:1212–1219, 2000 [DOI] [PubMed] [Google Scholar]
- 76.Al-Tweigeri T., Nabholtz J.-M., and Mackey J.R. Ocular toxicity and cancer chemotherapy: a review. Cancer. 78:1359–1373, 1996 [DOI] [PubMed] [Google Scholar]
- 77.Ibrahim N.K., Desai N., Legha S., et al. Phase I and pharmacokinetic study of ABI-007, a cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin. Cancer Res. 8:1038–1044, 2002 [PubMed] [Google Scholar]
- 78.Esmaeli B., Ahmadi M.A., Rivera E., et al. Docetaxel secretion in tears: association with lacrimal drainage obstruction. Arch. Ophthal. 120:1180–1182, 2002 [PubMed] [Google Scholar]
- 79.Esmaeli B., Hortobagyi G., Esteva F., et al. Canalicular stenosis secondary to weekly docetaxel: a potentially preventable side effect. Ann. Oncol. 13:218–221, 2002 [DOI] [PubMed] [Google Scholar]
- 80.Goldmacher V.S., Singh R., Chittenden T., et al. Linker technology and impact of linker design on ADC properties. In: Phillips G.L., ed. Antibody-Drug Conjugates and Immunotoxins. New York: Springer; 2013; p. 117–135 [Google Scholar]
- 81.Erickson H., Wilhelm S., Widdison W., et al. Evaluation of the cytotoxic potencies of the major maytansinoid metabolites of antibody maytansinoid conjugates detected in vitro and in preclinical mouse models. AACR Meeting Abstracts. 2008:2150, 2008 [Google Scholar]
- 82.Kovtun Y.V., Audette C.A., Ye Y., et al. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 66:3214–3221, 2006 [DOI] [PubMed] [Google Scholar]
- 83.Widdison W.C., and Chari R.V. Factors involved in the design of cytotoxic payloads for antibody–drug conjugates. In: Phillips G.L., ed. Antibody-Drug Conjugates and Immunotoxins. New York: Springer; 2013; p. 93–115 [Google Scholar]
- 84.Fracht H.U., Harvey T.J., and Bennett T.J. Transient corneal microcysts associated with interferon therapy. Cornea. 24:480–481, 2005 [DOI] [PubMed] [Google Scholar]
- 85.Dhillon V.K., Faraj L.A., Elalfy M.S., et al. Corneal microcysts. Br. J. Ophthalmol. 98:138–140, 2014 [DOI] [PubMed] [Google Scholar]
- 86.Hopen G., Mondino B.J., Johnson B.L., et al. Corneal toxicity with systemic cytarabine. Am. J. Ophthalmol. 91:500–504, 1981 [DOI] [PubMed] [Google Scholar]
- 87.Herzig R.H., Wolff S.N., Lazarus H.M., et al. High-dose cytosine arabinoside therapy for refractory leukemia. Blood. 62:361–369, 1983 [PubMed] [Google Scholar]
- 88.Gressel M., and Tomsak R. Keratitis from high dose intravenous cytarabine. Lancet. 320:273, 1982 [DOI] [PubMed] [Google Scholar]
- 89.Guthoff T., Tietze B., Meinhardt B., et al. Cytosine-arabinoside-induced keratopathy: a model of corneal proliferation kinetics. Ophthalmologica. 224:308–311, 2009 [DOI] [PubMed] [Google Scholar]
- 90.Vaccaro M., Pollicino A., Barbuzza O., et al. Trichomegaly of the eyelashes following treatment with cetuximab. Clin. Exp. Dermatol. 34:402–403, 2009 [DOI] [PubMed] [Google Scholar]
- 91.Fraunfelder F.T., and Fraunfelder F.W. Trichomegaly and other external eye side effects associated with epidermal growth factor. Cutan. Ocul. Toxicol. 31:195–197, 2012 [DOI] [PubMed] [Google Scholar]
- 92.Garibaldi D.C., and Adler R.A. Cicatricial ectropion associated with treatment of metastatic colorectal cancer with cetuximab. Ophthal. Plast. Reconstr. Surg. 23:62–63, 2007 [DOI] [PubMed] [Google Scholar]
- 93.Foerster C.G., Cursiefen C., and Kruse F.E. Persisting corneal erosion under cetuximab (Erbitux) treatment (epidermal growth factor receptor antibody). Cornea. 27:612–614, 2008 [DOI] [PubMed] [Google Scholar]
- 94.Basti S. Ocular toxicities of epidermal growth factor receptor inhibitors and their management. Cancer Nurs. 30:S10–S16, 2007 [DOI] [PubMed] [Google Scholar]
- 95.Dranko S., Kinney C., and Ramanathan R.K. Ocular toxicity related to cetuximab monotherapy in patients with colorectal cancer. Clin. Colorectal Cancer. 6:224–225, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Mahipal A., Kothari N., and Gupta S. Epidermal growth factor receptor inhibitors: coming of age. Cancer Control. 21:74–79, 2014 [DOI] [PubMed] [Google Scholar]
- 97.Schrohl A.S., Pedersen H.C., Jensen S.S., et al. Human epidermal growth factor receptor 2 (HER2) immunoreactivity: specificity of three pharmacodiagnostic antibodies. Histopathology. 59:975–983, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Knop E., and Knop N. Anatomy and immunology of the ocular surface. In: Niederkorn J.Y. and Kaplan H.J., eds. Chemical Immunology and Allergy. Basel: Karger; 2007; p. 36–49 [DOI] [PubMed] [Google Scholar]
- 99.Smolin G. Cellular response to inflammation at the limbus. Eye. 3:167–171, 1989 [DOI] [PubMed] [Google Scholar]
- 100.Hoyer J.D., Grogg K.L., Hanson C.A., et al. CD33 detection by immunohistochemistry in paraffin-embedded tissues: a new antibody shows excellent specificity and sensitivity for cells of myelomonocytic lineage. Am. J. Clin. Pathol. 129:316–323, 2008 [DOI] [PubMed] [Google Scholar]
- 101.Hamrah P., and Dana M.R. Corneal antigen-presenting cells. In: Niederkorn J.Y. and Kaplan H.J., eds. Chemical Immunology and Allergy. Basel: Karger; 2007; p. 58–70 [DOI] [PubMed] [Google Scholar]
- 102.Han T.H., and Zhao B. Absorption, distribution, metabolism, and excretion considerations for the development of antibody-drug conjugates. Drug Metab. Dispos. 42:1914–1920, 2014 [DOI] [PubMed] [Google Scholar]
- 103.Wang L., Amphlett G., Blättler W.A., et al. Structural characterization of the maytansinoid–monoclonal antibody immunoconjugate, huN901–DM1, by mass spectrometry. Prot. Sci. 14:2436–2446, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hamblett K.J., Senter P.D., Chace D.F., et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin. Cancer Res. 10:7063–7070, 2004 [DOI] [PubMed] [Google Scholar]
- 105.Strop P., Liu S.-H., Dorywalska M., et al. Location matters: site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem. Biol. 20:161–167, 2013 [DOI] [PubMed] [Google Scholar]
- 106.Behrens C.R., and Liu B. Methods for site-specific drug conjugation to antibodies. MAbs. 6:46–53, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qin A., Watermill J., Mastico R.A., et al. The pharmacokinetics and pharmacodynamics of IMGN242 (huC242-DM4) in patients with CanAg-expressing solid tumors. J. Clin. Oncol. 26, 2008 [Google Scholar]
- 108.Jackson D., and Stover D. Using the lessons learned from the clinic to improve the preclinical development of antibody drug conjugates. Pharm. Res. 1–12, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Siolas D., and Hannon G.J. Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer Res. 73:5315–5319, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sausville E.A., and Burger A.M. Contributions of human tumor xenografts to anticancer drug development. Cancer Res. 66:3351–3354, 2006 [DOI] [PubMed] [Google Scholar]
- 111.Haddish-Berhane N., Shah D.K., Ma D., et al. On translation of antibody drug conjugates efficacy from mouse experimental tumors to the clinic: a PK/PD approach. J. Pharmacokinet. Pharmacodyn. 40:557–571, 2013 [DOI] [PubMed] [Google Scholar]
- 112.Sassoon I., and Blanc V. Antibody-drug conjugate (ADC) clinical pipeline: a review.” Methods Mol. Biol. 1045:1–27, 2013 [DOI] [PubMed] [Google Scholar]