Abstract
The purpose of this review is to survey the use of experimental animal models for studying the chronic histopathological and behavioral consequences of traumatic brain injury (TBI). The strategies employed to study the long-term consequences of TBI are described, along with a summary of the evidence available to date from common experimental TBI models: fluid percussion injury; controlled cortical impact; blast TBI; and closed-head injury. For each model, evidence is organized according to outcome. Histopathological outcomes included are gross changes in morphology/histology, ventricular enlargement, gray/white matter shrinkage, axonal injury, cerebrovascular histopathology, inflammation, and neurogenesis. Behavioral outcomes included are overall neurological function, motor function, cognitive function, frontal lobe function, and stress-related outcomes. A brief discussion is provided comparing the most common experimental models of TBI and highlighting the utility of each model in understanding specific aspects of TBI pathology. The majority of experimental TBI studies collect data in the acute postinjury period, but few continue into the chronic period. Available evidence from long-term studies suggests that many of the experimental TBI models can lead to progressive changes in histopathology and behavior. The studies described in this review contribute to our understanding of chronic TBI pathology.
Key words: : behavior, chronic, function, histopathology, TBI
Introduction
Overview and purpose
It is well-established that traumatic brain injury (TBI) leads to diverse histopathological and behavioral consequences that begin in the acute period (hours to days) and persist chronically (weeks, months, and years after injury). Chronic symptoms negatively affect survivors' quality of life and hinder their independence and ability to return to preinjury responsibilities.1–6 Even with modern medical care, an estimated 3.2–5.3 million Americans are living with one or more residual problem attributed to TBI.2,7 There is an impetus to better understand the long-term consequences of TBI, given that chronic symptoms are distressing for both TBI survivors and their families and are coupled with significant health service utilization and cost.
Animal models have been a mainstay of TBI research for over a century.8–11 Several types of experimental TBI models have been developed to model the consequences of TBI, and four of the most commonly used options are discussed in this article: fluid percussion injury (FPI); controlled cortical impact (CCI); blast TBI (bTBI); and closed-head injury (CHI). In this review, separate headings are used for different injury induction methods with the test species, location, or injury severity denoted in the body of the text when relevant. It is important that additional long-term studies of experimental TBI are conducted so that the persistence and progression of TBI pathology may be better understood, given that this information can be applied to guide the development of therapeutic interventions that reduce chronic disability. To date, the chronic consequences of experimental TBI remain less characterized than the acute pathology. This was confirmed by Gold and colleagues, who reviewed 314 data-based publications from experimental TBI studies, which met the following criteria: used rodent models, compared TBI-exposed cases to controls, and included functional outcome assessments.12 The researchers found that only 32% of all experimental TBI publications meeting the above-mentioned criteria included outcomes beyond 1 month postinjury, and a mere 8.6% of studies assessed outcomes beyond 2 months postinjury.12 Other TBI researchers have acknowledged that only limited attention has been given to understanding the chronic effects of experimental TBI.13 Only a handful of studies have included postinjury outcomes out to 1 year or beyond.14–18 Despite the limited quantity of evidence, the literature that is available from experimental models of TBI suggests that histopathological and functional outcome deficits persist into the long term, as observed in TBI survivors.19–21
The purpose of this review is to summarize the key evidence available from long-term studies of FPI, CCI, bTBI, and CHI. Less commonly used methods for inducing experimental TBI, such as cold probe injury,22 penetrating ballistic-like brain injury,23–26 and combinatorial models (e.g., blast+stress), are not included. In this review, long-term outcomes, defined as evidence collected at or beyond 2 weeks after injury, are emphasized. Our rationale for using a 2-week cutoff for chronic outcomes in this review is based on a desire to provide the most comprehensive review possible, keeping in mind the relative dearth of long-term outcome assessments highlighted in the Gold and colleagues review. Notably, there is no consensus regarding how the time course of recovery observed in humans compares to those observed in the various experimental TBI models.
The resources at a large university library system and online search engines (e.g., PubMed, PubMed Central, Google Scholar, and Web of Science) were used to identify relevant literature. In total, 193 articles were identified, as summarized in Table 1. Assessment time points are not always reported and when they are the units of time varied (e.g., days, weeks, months, or years). To facilitate ease of comparison in this review, all data collection time points were converted to approximate number of days based off a 30-day month and a 365-day year. Study length in days was used to create ordinal groups: 14–29, 30–59, 60–89, 90–119, 120–149, 150–179, 180–209, 210–239, 240–269, 270–299, 300–329, 330–365, and 366+days postinjury. Group frequencies were generated, which revealed that, of the long-term studies identified, only one quarter (26.4%) collected outcomes at or beyond 2 months (60 days) postinjury. A mere nine studies (4.6%) collected outcomes at 1 year (365 days) postinjury,14–18,27–30 with one extending beyond 1 year (0.5%).26,31
Table 1.
Summary of the 193 Long-Term Studies Identified
| Characteristic | Frequency (%) |
|---|---|
| Type test animal | |
| Rat | 126 (65.3) |
| Mouse | 60 (31.1) |
| Pig | 4 (2.1) |
| Nonhuman primate | 2 (1.0) |
| Rabbit | 1 (0.5) |
| Sex | |
| Male | 161 (83.4) |
| Female | 8 (4.1) |
| Both male and female | 7 (3.6) |
| Unspecified | 17 (8.8) |
| Study duration, days | |
| 14–29 | 73 (37.8) |
| 30–59 | 69 (35.8) |
| 60–89 | 15 (7.8) |
| 90–119 | 12 (6.2) |
| 120–149 | 2 (1.0) |
| 150–179 | 4 (2.1) |
| 180–209 | 5 (2.6) |
| 210–239 | 1 (0.5) |
| 240–269 | 0 (0) |
| 270–299 | 1 (0.5) |
| 300–329 | 1 (0.5) |
| 330–365 | 9 (4.7) |
| 366+ | 1 (0.5) |
Type of animal used, animal sex, and study duration are noted.
Of the 193 articles identified, the vast majority (96.4%) used rodent models, with 65.3% of studies being rat studies and 31.1% mouse studies. The remaining 3.6% of studies used pigs, nonhuman primates, and rabbits (Table 1). No exclusions were made based on the species used, but efforts were made to denote the species with the evidence, especially for nonrodent models.
Notably, the vast majority of long-term studies identified used male animals exclusively (83.4%), whereas only a handful of studies used females exclusively (4.1%) or both males and females (3.6%). The remaining 8.8% did not specify the sex of the animal used (Table 1). In this review, the long-term evidence highlighted will be limited to studies exclusively and explicitly using male animals. The rationale for this decision is 2-fold: 1) outcomes of TBI may be moderated by sex and/or the effect of sex hormones32–35 and 2) human males in all age groups are more likely to experience TBI than females.36 Whereas long-term evidence will be limited to males, studies using female animals or not specifying animal sex may be cited in supporting text (e.g., introducing/defining concepts, describing models, in the Discussion section).
Age of the test animals is another study variable that is often not reported by researchers. Indeed, nearly one third (31.6%) of studies did not specify the age, with many of these also not indicating weight, which could be used to approximate age of the test animals. Another one quarter of the studies (25.9%) vaguely listed the age as adult. A mere 12 studies (6.2%) explicitly described the sample as consisting of immature (i.e., juvenile, pediatric, and adolescent) animals or provided animal ages that correspond to an immature animal of that type/strain. These 12 studies were excluded from the evidence highlighted in this review. However, owing to the large percentage of studies that did not specify animal age, studies were not excluded from this review if age was unspecified, as was true for studies that did not specify sex.
For each of the five TBI models included, a brief historical overview is provided with commonly used species noted. A summary of key evidence from studies using each model is divided into histopathological and functional outcomes. The histopathological consequences of interest in this review are gross morphological changes, ventricular enlargement, gray/white matter (GM/WM) shrinkage, cell death, axonal injury, cerebrovascular histopathology, inflammation, and neurogenesis. With respect to functional outcomes, this review includes overall neurological function, motor function, frontal lobe function, and stress-related outcomes.
In the summary of the evidence provided, injury severity is sometimes included to provide additional details that may be of interest to readers. However, it is important to note that injury severities included in this review are based off the original description of injury level from the primary source; these severity levels may not be consistent across research groups complicating comparisons across studies. Moreover, these injury severity levels may or may not correlate with clinical TBI severity.
In summarizing the evidence pertaining to each outcome of interest for the five models, the emphasis is on evidence gathered over the longest study period from the publications identified during the literature review. After the model-specific discussion of the long-term histopathological and behavioral consequences of experimental TBI, a brief description of the comparisons and contrasts between the models is provided, including a table to summarize key comparisons. The primary aims of this review are to highlight the literature detailing the chronic histopathological and behavioral outcomes of TBI, and leave the reader with an understanding of the techniques available for the study of chronic TBI in animals. Careful study planning is important to select the appropriate model characteristics to best address the research question(s).
Outcomes of interest
A short definition/description of each histopathological and behavioral outcome is provided, and the clinical relevance of the outcomes of interest is noted. Gross morphological and histopathological changes are apparent with the use of simple staining and microscopy. Examples include gross contusion, lesion, or volume loss of particular brain structures. These overt changes in brain morphology have been observed after clinical TBI37 and replicated in experimental models.38 The manifestation of post-traumatic functional impairments can exist in the absence of overt morphological changes,39 and thus conclusions from studies are strengthened by the inclusion of both histopathological and functional deficits.
Three related changes are discussed under a single heading: ventricular enlargement, shrinkage of GM, and shrinkage of WM. Ventricular enlargement has been observed after clinical TBI where it correlated with deficits in cognition and memory.40–43 Similarly, GM shrinkage has been observed after clinical TBI.44,45 Gale and colleagues used voxel-based morphometry in TBI survivors approximately 1 year postinjury and reported that GM concentration was significantly lower than in matched controls.45 WM atrophy contributes to tissue shrinkage observed after clinical TBI42,46 and can be studied using animal models.28,47 Changes in GM/WM concentration28,29,48,49 and ventricular enlargement have also been reported after experimental TBI.14,27
A factor contributing to the aforementioned changes to GM and WM is cell death. Cell death occurs in multiple types of central nervous system (CNS) cells after TBI through multiple mechanisms: apoptosis, necrosis, and autophagy.50,51 Depending on the types of cell death (or subset of a particular cell death pathway) that are of interest, the researcher can choose between available models (e.g. FPI, CCI, bTBI, and CHI), which differ in their histopathological and behavioral consequences, as described in this review. Discussion of cell death will be broken down under three headings pertaining to apoptosis, necrosis, and autophagy; when relevant, a discussion of the patterns of cell death will be included.
Early evidence of axonal injury, a common TBI pathology, was reported nearly 70 years ago by Rand and Courville, who observed histopathological changes throughout many brain regions post-TBI.52 Additional evidence accrued regarding axonal pathology at early post-TBI time points in varying injury severities.53–55 More in-depth categorization of these changes occurred during the 1980s, leading to the now widely use concept of diffuse axonal injury (DAI).56,57 DAI is characterized by early axonal changes and swelling during the first 1–2 days post-TBI with the progression to more extensive secondary damage and ultimate axonal disconnection.58 Axonal injury has been reported in numerous experimental TBI models.57,59,60
Cerebrovascular histopathology includes disruption of the blood–brain barrier (BBB), endothelial cell damage, and dysregulation in cerebrovascular blood flow. These changes to the cerebrovascular system have been observed in the clinical setting post-TBI.61–64 Evidence of cerebrovascular histopathology has also been reported in experimental models of TBI.65–67
The study of inflammation began in antiquity, with Celsus.68 More recently, scientific understanding of inflammation has broadened to include both the multiple influences and outcomes when defined as a “multi-mediated phenomenon, of a pattern type in which all mediators would come and go at the appropriate moment…increasing vascular permeability, attracting leucocytes, producing pain, local edema and necrosis.”69 Historically, the brain was considered immune privileged; however, CNS inflammation has since been discovered, as evidenced by major histocompatibility complex expression, glial activation, acute phase response, complement activation, expression of adhesion molecules, inflammatory mediator synthesis, edema, and immune cell invasion.70 In the context of clinical TBI, inflammation represents a key pathophysiological process during both the acute and chronic periods46,71–73 and is replicated by experimental TBI.49,74,75 Whereas microglial and astrocytic responses are also part of the inflammatory cascade, they are not emphasized in this review; rather, the focus is primarily on cytokines because these are better categorized in the long term postinjury.
Neurogenesis can be defined as the process in which new neurons are generated through differentiation and maturation of neural stem cells and neuronal precursor cells.76–79 Following the observation that ongoing neurogenesis occurs in the adult human brain,80,81 this physiological process has been explored in animal models as a mechanism by which new neurons could be generated to compensate for cell loss after TBI.82–86 Whereas adult neurogenesis occurs in both the subgranular zone of the hippocampus and the subventricular zone of the lateral ventricle, this review focuses on hippocampal neurogenesis because the hippocampus is an important contributor to specific cognitive tasks.87–90 Studies using many of the aforementioned animal models of TBI demonstrate that therapeutic interventions designed to enhanced neurogenesis may contribute to improved neurobehavioral function post-TBI.84,91–93
There are six main behavioral outcomes of interest in this review; each outcome is studied using a variety of experimental methodologies. Overall neurological function is negatively impacted by clinical TBI and can be evaluated in TBI patients using tools such as the Glasgow Outcomes Scale (GOS), Glasgow Outcomes Scale Extended, and Neurobehavioral Rating Scale.94–98 In experimental models of TBI, overall neurological function is assessed using various composite tools.99–102
Motor deficits are common after clinical and experimental TBI. In pre-clinical studies, motor deficits are assessed using the beam balance task (BBT), beam walking task (BWT), inclined plane task, cylinder test, foot fault test, bilateral tactile adhesive removal task, rotarod, and rotating pole task. Impairments in acute and chronic motor function have been observed on these assessments when applied to studies using varied experimental TBI models. A composite neuroscore, which combines several motor assessments into a single score,99,103 can be used by researchers to evaluate gross and/or fine motor function, depending on the task(s) utilized and study time frame.
Cognitive dysfunction is cited by TBI survivors as one of the greatest contributors to reduced quality of life.104 In animal models, the components of cognitive function commonly studied include spatial memory/learning, nonspatial learning, and reference memory. Spatial memory is most commonly assessed using the Morris water maze (MWM) hidden platform task, reference memory with the MWM probe trial, and nonspatial learning using the passive avoidance task.105 Passive learning is assessed using cued and contextual fear conditioning.106
Frontal lobe function encompasses executive function, memory, language, aggression, and reasoning.107,108 TBI in humans is known to affect the frontal lobe and cause dysfunction in the cognitive processes controlled by this brain region.109–111 However, frontal lobe dysfunction remains understudied in animals because it is challenging to propose experimental analogs of the complex frontal lobe functions observed in humans (e.g., executive function, impulse control, and language). To date, frontal lobe function after experimental TBI has been most studied in the context of CCI.
Several stress-related outcomes have been associated with TBI, most notably post-traumatic stress disorder (PTSD).112–117 Fear and stress can lead to emotional and physical consequences, including outcomes associated with sympathetic nervous system actions.118,119 In experimental models of TBI, heightened stress and fear have been reported.120,121 Often, bTBI studies include stress-, fear-, and anxiety-related outcome measurements, such as the elevated plus maze, zero maze, and responses to predator stress exposure.122,123
Fluid Percussion Injury: Model Overview and History
FPI is one of the earliest standardized models of TBI described and remains relevant and widely used to this day (Fig. 1). FPI was developed for use in rabbits by Lindgren and Rinder.11 More than a decade later, the model was applied by Sullivan and colleagues to cats.124 Subsequently, Dixon and colleagues applied FPI to rats,125 and recently, the model has been applied to mice126 and swine.127,128
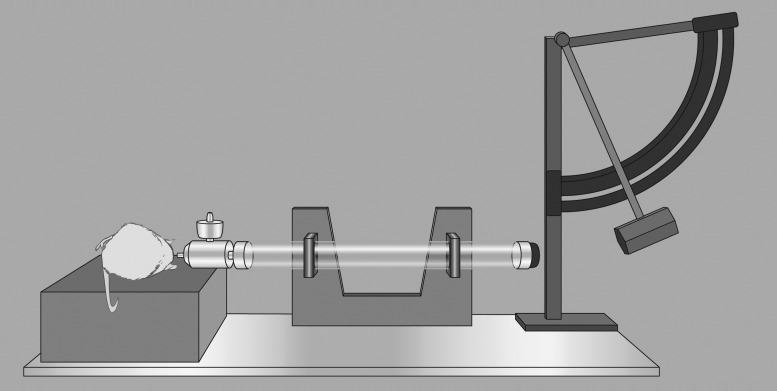
FIG. 1.
Fluid percussion injury diagram. A saline-filled tube with a flexible end cap is struck by a pendulum; this rapidly injects a saline bolus into the closed cranium to produce traumatic brain injury. A protractor on the pendulum allows for precise and repeatable forces.
FPI uses a bolus of saline that is injected at a rapid rate into the closed cranium to produce a brain injury. Preceding the FPI, a craniotomy is performed, typically in one of two locations: 1) at midline in the location of the sagittal sinus125 (i.e., medial FPI, midline FPI, or central FPI), or 2) lateral in the location of the parietal cortex103,129,130 (i.e., lateral FPI or parasagittal). Cemented over the craniotomy site is a plastic cap coupled with a female Leur-Loc connector. The Leur-Loc is temporarily attached to a Plexiglas or metal tube connected to a saline-filled reservoir. The release of a swinging pendulum and the resulting contact with the plunger causes a fluid pulse, measured in atmospheres (atm) that contacts the dura and enters the epidural space. The percussion produces brief displacement and deformation of neural tissue.131,132 Injury severity is modified by adjusting the height of the pendulum to alter the magnitude of fluid pulse pressure. More recently, Kabadi enhanced the reproducibility of FPI by adapting the instrument to be pneumatically driven and controlled by a microprocessing unit.131
Since its development, FPI has become recognized as a clinically relevant model of brain injury that is particularly useful for studying specific characteristic features of TBI. Lateral FPI is frequently utilized to study alterations in blood flow within the cerebrovasculature,133 WM damage,134 hippocampal cell death,103 and parieto-occipital cortex.135 Midline FPI is commonly chosen to model concussive injuries and DAI.125,136
Fluid Percussion Injury: Chronic Histopathological Outcomes
Shrinkage of gray/white matter and enlargement of ventricles
Carbonell and Grady reported that parasagittal FPI in mice led to WM degeneration in multiple brain regions, which was evident by 4 days postinjury and progressed at least 35 days postinjury. The researchers concluded that the spatial pattern of damage was similar in mice and rats post-FPI, but did note one key difference: Mice exhibited a shorter period of damage, particularly within the thalamus and hippocampus.137 In rats, enlargement of the lateral ventricles has been reported 8 weeks post-FPI.138 Long-term ventricular enlargement post-FPI has also been reported by Nonaka and colleagues, who found progressive ventricular expansion up to 1 year after parasagittal FPI in rats.16
Cell death
Patterns
The existing body of literature suggests that cell death begins rapidly post-TBI and persists into the long term. Early pre-clinical work by Bramlett and colleagues suggests that significant hippocampal cell loss within the ipsilateral dentate hilar region was found after moderate FPI in rats.138 Patterns of cell death post-FPI have been reported in the literature, contributing substantially to our understanding of the chronic effects of TBI. Bramlett and colleagues conducted a 1-year study in rats and reported that the penumbral tissue exhibited ongoing cell death that resulted in an enlarged cavity lined with glia.28 Smith and colleagues described progressive tissue loss starting within 1 h of initial injury and continuing into the subsequent weeks, months, and out to 1 year after parasagittal FPI in rats.14 This study used multiple assessment techniques, including hematoxylin-eosin (H&E) staining, photomicrographs of glial fibrillary acid protein (GFAP), and quantification of the percent of cortical tissue lost; continued ventricular expansion and progression of the cortical lesion at 6 months postinjury was a key finding of this study.14 H&E staining at 6 and 12 months postinjury further revealed long-term hippocampal changes, including progressive shrinkage of the layer of pyramidal cells and sustained loss of neurons in the dentate hilus at 12 months.14 After lateral FPI, chronic neuron loss in the ipsilateral cortex and hippocampus were observed at 1–4 weeks postinjury.135 Functional deficits can be present in the absence of overt histopathology as shown in a study of mild, moderate, and severe FPI, which failed to find evidence of significant cell death in the hippocampal CA3 region and cerebral cortex, despite MWM deficits, when assessed 2 weeks postinjury.139
Apoptosis
The term apoptosis was first coined by Kerr and colleagues to describe cells exhibiting certain morphological characteristics of cell death, including, but not limited to, chromatin condensation, karyorrhexis (i.e., nuclear fragmentation), pyknosis (reduced cell volume), and phagocytic engulfment.140 Apoptosis has been observed post-FPI, though chronic evidence is limited and only a small number of studies have reported delayed apoptosis post-FPI. Conti and colleagues studied apoptosis 2 weeks after lateral FPI in rats using double-labeled immunocytochemistry to identify terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive neurons. The researchers observed apoptosis immediately postinjury and again starting at 1 week postinjury, peaking at 2 weeks postinjury, and returning to preinjury levels by the 2-month assessment time point.141 In this study, apoptosis was found to affect the injured cortex, thalamus, and, to a lesser extent, the hippocampus.
Autophagy
As with apoptosis, autophagy is defined according to its characteristic features, namely, degeneration and clearing of cytoplasmic material using autophagosomes.142,143 Post-FPI in rats, immunoblot analysis of tissue from the neocortical regions showed markedly redistributed autophagy-related gene (ATG)12-ATG5 conjugates on day 15 postinjury.144 Longer-term evidence of autophagy post-FPI was not identified.
Necrosis
Necrosis is associated with organelle swelling, oncosis (increased cell volume), rupture of the plasma membrane, and the resulting release of cellular contents.145 Unlike apoptosis, necrosis lacks chromatin fragmentation. Necrosis is frequently observed acutely after TBI, but relatively few studies have examined it chronically. Bramlett and colleagues evaluated rat brain tissue 8 weeks after moderate FPI, and reported focal necrosis within a subset of thalamic cells.138
Axonal injury
The FPI model has been used to characterize the progression of axonal injury.59,146 However, only a handful of studies have evaluated chronic axonal injury. A study by Pierce and colleagues was among the first to include both histopathological and behavioral outcomes out to 1 year after severe lateral FPI.27 Notable findings from this study were year-long functional deficits (e.g., learning, motor), which co-occurred with axonal degeneration in the injured cortex, corpus callosum (CC), and striatum. Smith and colleagues published several experimental TBI studies that contributed to the understanding of the prolonged time course of traumatic axonal damage.147–150 Axon swelling throughout the brain has been observed out to 6 months postinjury, and time-dependent reductions in the number of myelinated axons in the thalamus and cortex were reported up to 1 year postinjury.29 In a study of parasagittal FPI, Dietrich and colleagues found a spatial relationship between areas where axonal damage was found and those where long-term GFAP messenger RNA was observed.151
Using an FPI model to produce a mild TBI (mTBI), Spain and colleagues found evidence of spatially progressive axonal degeneration within the external capsule and the dorsal thalamus up to 6 weeks after insult. At the 4-week time point, elevated levels of axonal bulbs within the external capsule were evident in injured animals, whereas no major differences were present in other areas of the brain. In addition, myelin basic protein immunostaining showed no damage to myelin during any time point within the study.152
Cerebrovascular histopathology
Cerebrovascular dysfunction has not been well studied during the chronic period post-FPI. One study by Guo and colleagues documented evidence of ongoing angiogenesis out to 2 weeks postinjury. In this study, CD34-positive cells were reported starting 72 h after injury and persisting for 14 days post-TBI.153 Existing evidence suggests that CD34-positive cells are directly incorporated into expanding vascular cells.154 CD34-positive cells have also been found to impact secretion of angiogenic growth factors, such as vascular endothelial growth factor (VEGF).155 Thus, CD34-positive cells are believed to promote angiogenesis both directly and indirectly. However, the exact mechanisms by which CD34-positive cells contribute to angiogenesis remains unknown.156
Neurogenesis
A recent experimental study observed neurons expressing neural progenitor and immature neuron markers within perilesional regions, including the cortex, WM, and hippocampus, in resected tissue from adults humans post-TBI.157 After an FPI, the hippocampus exhibits a robust increase in cellular proliferation of neural stem cells in the subgranular zone of the dentate gyrus within the first week of brain injury86,158,159; however, the transient elevation in the rate of cellular proliferation returns to baseline by 2 weeks postinjury.86 Cellular proliferation is identified by the incorporation of bromodeoxyuridine or other thymidine analog into the DNA during cellular replication. As an alternative, researchers can assess cell proliferation using Ki67 immunoreactivity; Ki67 is a protein expressed during several phases of the cell cycle (e.g., late G1, S, G2, and mitosis).160,161 Many of the newly proliferated cells will adopt an astrocytic or GFAP-positive lineage, but a subpopulation will differentiate and express neuronal markers in the weeks postinjury.162 In the context of FPI, two studies demonstrate that newly generated neurons extend axonal projections that integrate into CA3 at 2159 and 8 weeks162 postinjury. These studies demonstrate that FPI stimulates a post-traumatic neurogenic response that results in an elevation in the number of new neurons that can functionally incorporate into the hippocampus in the weeks after injury.
Fluid Percussion Injury: Chronic Behavioral Outcomes
Overall neurological function
Kabadi and colleagues found distinct injury-induced neurological score impairment after sham, mild FPI, and moderate FPI out to 3 weeks postinjury,131 with scoring as previously described.163–165 Briefly, neurological score was calculated based on four motor assessments: 1) right (R) and left (L) forelimb contraflexion when rats were suspended by the tail; 2) R&L hind limb flexion when rats were suspended by the tail; 3) resistance to R&L lateral pulsion; and 4) ability to stand on an inclined angle board.131 Another study found that severe lateral FPI worsened composite neuroscore performance out to 2 months postinjury.27 McIntosh and colleagues evaluated composite neuroscore score using five motor assessments: 1) forelimb flexion during tail suspension; 2) circling behavior during spontaneous ambulation; 3) decreased resistance to lateral pulsion; 4) ability to balance on a narrow (2 cm wide) wooden beam; and 5) ability to stand on an angled board with the maximal angle the animal could stand on for 5 sec recorded.99 This 5-point neurological assessment results in scores ranging from 0 to 20, interpreted as follows: 20=normal, 15=slight impairment, 10=moderate impairment, 5=severe impairment, and 0=afunctional. Whereas McIntosh and colleagues found no effects of mild injury, moderate FPI led to deficits up to 4 weeks postinjury in this study.103 Other researchers have reported neuroscore deficits post-FPI, as described in the following section.
Motor function
Deficits on the BBT have been observed up to 4 months post- FPI.166,167 Impaired rotarod performance beyond 4 weeks postinjury has been observed after lateral FPI.168 A number of motor behaviors assessed by Smith and colleagues, including BWT, skilled forelimb reaching, and locomotor placing, were assessed after lateral FPI.169 The BWT is commonly used by researchers to study the consequences of experimental TBI on fine motor performance. In this assessment, animals are motivated, using negative reinforcement, to traverse a narrow beam. Deficits on the BWT have been observed after a single head trauma modeled using lateral FPI,167,170–172 as well as repeated head trauma modeled using lateral FPI. The bilateral adhesive removal task is another fine motor task that has shown deficits out to 1 month after lateral FPI.173 Other less-common assessments that have shown long-term deficits in FPI studies include the foot fault test of locomotor function,130 the rotarod test of balance,173 the cylinder test of dorsal raphe nuclei and median raphe function,174 and the rotating pole test of coordination,166,168,173 all of which have shown deficits 1 month postinjury.
Other common assessments of motor function are the composite neuroscore and MWM. Early use of the composite neuroscore by McIntosh and colleagues combined five motor assessments into a single test, as described above.99,103 Motor deficits assessed using the composite neuroscore have been reported at 1 month postinjury in several studies.27,166,168,175–177 One of the studies found that deficits persisted out to 2 months postinjury.27 The MWM, discussed below in relation to its use for assessment of cognitive function, such as spatial learning/memory and reference memory, also collects swim speed data that provides information about overall motor function. Long-term deficits on swim speed have been observed after both isolated lateral FPI178 and repeated lateral FPI.179
Cognitive function
Learning deficits assessed using the MWM were evident out to 2 weeks in a rat model of severe FPI.180 MWM deficits have been observed to persist for up to 4 weeks post-FPI in some studies179,181,182 and 8 weeks postinjury in others.167,183,184 When the severity of injury was increased, MWM deficits were observed out to 1 year postinjury.27 An alternative assessment of learning, the reversal learning test, also showed impairments 8 weeks after a single lateral FPI186 or repeated lateral FPI.179,181
Reference memory is also impaired after FPI. Injury effects on the MWM have been reported out to 2 weeks post-FPI.185 Other studies in rodents have reported reference memory deficits out to 1 month after both lateral FPI,130,175,176,184,186,187 as well as medial FPI.188,189 Sun and colleagues observed cognitive deficits assessed by the MWM on days 11–15 and 26–30 postinjury; in this study, deficits were resolved by days 56–60 postinjury.162
Radial arm maze task for assessment of spatial learning and memory was found to be sensitive to FPI out to 7 weeks postinjury.167 As an alternative, researchers can assess spatial learning/memory using the Barnes Maze, where rodents are placed on a well-lit circular platform with 40 holes and trained to locate a specific hole under which a goal box is found.190 Injured animals assessed at 1 and 3 months postinjury impaired Barnes Maze performance.191 Impaired spatial learning was observed 3 weeks post-FPI with concomitant localized axonal damage within the external capsule and dorsal thalamus, despite unaltered myelin.152
Stress-related outcomes
Overall, stress-related outcomes are rarely assessed into the long term post-FPI. Anxiety, assessed using the elevated plus maze, has been reported out to 1 month after a single lateral FPI,172 as well as after repeated lateral FPI.179,181 Depression-like behavior in rodents are observed 1 month after lateral FPI in the forced swim test.179
Controlled Cortical Impact: Model Overview and History
CCI is a widely used, well-characterized model of injury, which is valued for its quantifiable, replicable, and programmable nature. CCI was first developed for use in ferrets192 and within a few years was translated to rats60 and mice.48,193 CCI experiments with primates have also been reported in the literature.194 Briefly, CCI produces a TBI by transferring mechanical energy onto the exposed dura matter. Most commonly, the impact in CCI is delivered by a pneumatic cylinder (Fig. 2); however, an electromechanical alternative is also available. CCI is well regarded for the precise control it affords over injury parameters; the velocity at which the impactor tip moves, depth of depression of neural tissue, dwell time, and tip geometry are all precisely controlled, which ultimately enhances the reproducibility of the injury and consistency across test animals. CCI is considered to be clinically relevant and replicates many important secondary injury features seen in TBI patients. CCI has been used in a handful of studies evaluating injury progression up to 1 year postinjury. One study found that long-term histopathological changes, including enlarged contusion volume, progressive tissue loss, and ventricular expansion, occur out to 1 year postinjury.15
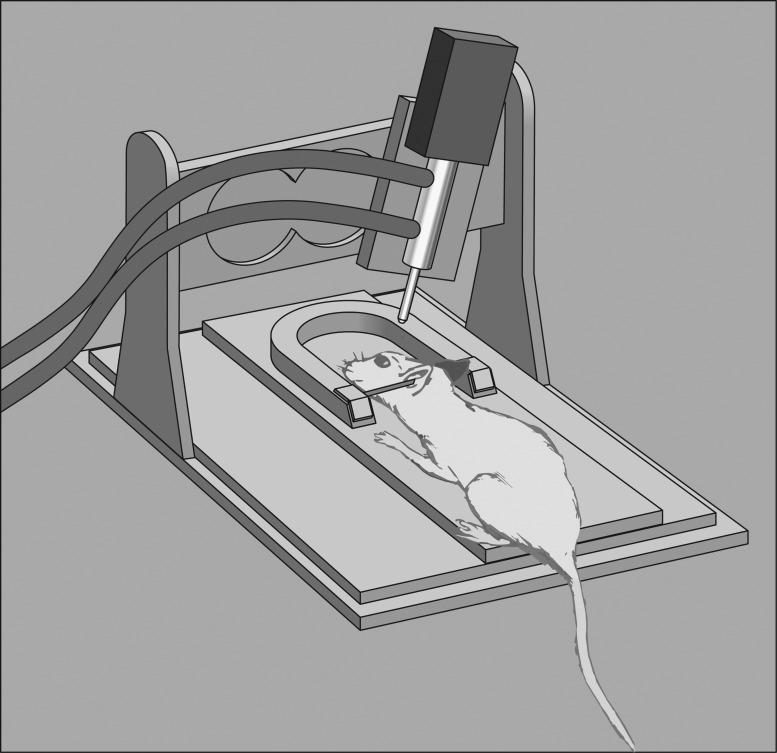
FIG. 2.
Controlled Cortical impact diagram. Test animals are held in a stereotactic frame using ear bars and an incisor bar. A double-acting pneumatic piston drives an impactor tip into the test animal's intact dura overlying the brain or, less commonly, onto the intact skull. The piston can be precisely moved to control the depth of impact and the velocity is determined by controlling the pressure applied to the piston. There is an electromagnetically driven alternative to a pneumatically actuated piston (not pictured).
Controlled Cortical Impact: Chronic Histopathological Outcomes
Gross morphological and histological changes
One study compared animals exposed to CCI and sham and found that CCI-exposed animals had vastly larger contusion volumes at 3 weeks postinjury.15 Another notable finding of this study was that the size of the contusion nearly doubled by 1 year post-CCI.15 Though progressive gross changes have been reported, evidence of long-term morphological changes remains sparse.
Enlargement of ventricles and shrinkage of gray/white matter
Post-CCI in rats, volume of ipsilateral ventricles increased approximately 3-fold between 3 weeks and 1 year postinjury, suggesting chronic loss of GM and WM.15 A CCI model of repetitive mTBI found that bilateral contusion injuries spaced 1 week apart resulted in continued damage to the myelin within the CC that was not present in animals with only a single injury at 60 days postinjury.195
Cell death
Patterns
Fox and colleagues used TUNEL, Hoechst staining, as well as anti-NeuN antibodies and found that apoptosis was frequent and abundant.196 More recently, Zhou and colleagues used Fluor-Jade B at several acute time points and one chronic time point at 14 days post-CCI. This study identified that most neurons degenerate within the first 24 h of initial injury, but cell death was ongoing up to 14 days postinjury.197
Apoptosis
Apoptosis, defined previously in the FPI section, was first reported after experimental TBI in a CCI study by Colicos and colleagues.198 Delayed apoptotic cell death was observed in the hippocampal CA1 and CA3 out to 2 weeks post-CCI.198 In this study, characteristic apoptotic features were evaluated by silver impregnation and cresyl violet staining.198 Another study observed increased levels of the apoptotic executioner protein, cleaved caspase 3, at 2 weeks postinjury.199 Notably, outside of a handful of studies that assessed apoptotic outcomes at or beyond 2 weeks, the majority of studies assessing apoptosis do so in the acute period.
Necrosis
Characteristics of necrotic death have been reported in the short term post-CCI, including cellular swelling, blebbing, and lysis.200 Evidence of necrosis in the chronic postinjury period is sparse. Necrosis has been observed to begin rapidly post-CCI and persist for at least 2 weeks, albeit at a low level.197 Necrosis of subcortical WM and concomitant working memory deficits in the absence of substantial somatosensory damage has been reported 21–23 days after mild CCI.196
Axonal injury
Axonal injury has traditionally been included as a short-term outcome post-CCI.201,202 Myelin loss is another change to axons that has been reported in the long term post-CCI. Glushakova and colleagues used Luxol fast blue to detect loss of myelin out to 3 months postinjury in conjunction with microbleeds.203 The vascular consequences observed in this study are described in additional detail in the next section.203
Cerebrovascular histopathology
In the aforementioned Glushkova and colleagues study, microbleeds were associated with myelin loss and glial scarring. Taken together, the findings suggest that vascular damage is associated with the combination of breakdown of the BBB and inflammatory processes.203 Moreover, the researchers assert that enhanced understanding of delayed microvascular changes could lead to a better understanding of long-term TBI pathology.
Inflammation
Overall, cyclooxygenase 2 (COX-2) activity produces benefits in the context of TBI; however, ongoing production of COX-2 may be associated with secondary damage from oxidative stress, deficits in cellular metabolism, vascular changes, and associated symptoms.204 Evidence of long-term inflammation was reported in a study by Briones and colleagues, in which rats sacrificed one month post-CCI exhibited elevated interleukin (IL)-1β and tumor necrosis factor alpha in both the cortex and hippocampus.205 The anti-inflammatory cytokine, IL-10, was elevated in both the cortex and hippocampus when assessed one month post-CCI.205
Neurogenesis
In addition to mature granular neuron loss, immature hippocampal neurons are particularly vulnerable to TBI, resulting in a reduction in immature neuron density up to 2 weeks post-CCI.82,83,206,207 Similar to FPI, CCI stimulates an increase in cellular proliferation and neural stem cell expansion during the week postinjury,82,206,208,209 resulting in the generation of newborn neurons in the granular layer.82,206–208,210 However, the majority of newly proliferated cells adopt a GFAP-positive lineage, which could include glial cells and neural progenitor pool repopulation in the subgranular zone at 1 month post-CCI.82
Controlled Cortical Impact: Chronic Behavioral Outcomes
Overall neurological function
Several studies have found that CCI leads to global neurological deficits at 1 month postinjury.211–214 Although there has been some debate about the reliability, validity, and bias of composite scores,12 assessments of overall neurological function have been widely applied in CCI and other experimental models of TBI.
Motor function
Motor function deficits have been reported using the BBT out to 1 month postinjury.215 Fine motor deficits have also been observed 1 month after CCI using the BWT.196,212,216,217 Two experimental CCI studies demonstrated motor function deficits with vermicelli test of manual dexterity up to 8 weeks postinjury.218,219 Animals assessed with the cylinder test exhibited paw placement preference out to1 month post-CCI.220 Balance deficits have been observed on the rotarod test 1 month post-CCI,221 as well as out to 11 weeks after moderate CCI.222 One study found that grid walk performance was impaired at 4 weeks after CCI in mice, and spontaneous forelimb elevation was reduced at 5 months postinjury.223 Reduced forelimb function after moderate bilateral CCI has been reported up to 18 days postinjury.224
Cognitive function
Dixon and colleagues reported that moderate CCI produced persistent spatial memory deficits on the MWM up to 1 year postinjury.15 More recently MWM deficits have been observed as early as 2 weeks and up to 1 month post-CCI.47,211,212,214–216,225–232 Chronic deficits have been observed out to 1 month post-CCI, when reference memory is assessed using the MWM probe trial.15,227,232,233 In mice, evaluation of learning using the MWM has revealed deficits out to 24 days postinjury234,235 and working memory deficits out to 4 weeks post-CCI.196 Teresita Briones and her colleagues found that the CCI significantly increased latency on the MWM.205
Fox and colleagues showed impaired performance on the Barnes Maze task up to 24 days postinjury in mice subjected to CCI.217,234 Deficits in working memory function have been reported out to 1 month post-CCI versus controls.233 Nonspatial learning shows an injury effect out to 1 month post-CCI when assessed using the passive avoidance task.236 Attenuation of CCI-induced cognitive deficits was observed at 3 months postinjury in mice that exercised beginning 5 weeks after trauma.237
Frontal lobe function
The attentional set-shifting test (AST) is a test of frontal lobe function in which animals complete a series of perceptual discriminations of increasing difficulties with food as a reward. Animals are required to both form and maintain an attentional set and then shift from stimulus to stimulus. The food rewards are hidden within terra cotta pots filled with distinctive digging material; each pot has a distinctive odor around the rim that animals use to learn the location of the reward.238–240 Previous applications of the AST include rodent models of attention deficit hyperactivity disorder,241 Parkinson's disease,242 aging,243 and stress.238,244,245 Bondi and colleagues first applied the AST to an experimental TBI study in rats and found deficits at 4 weeks post-CCI. In this study, a series of discriminative tasks were used, with difficulty of discrimination increasing progressively and included simple discriminations, compound discriminations, stimulus reversals, as well as intra- and extradimensional shifts. TBI was found to result in AST deficits, with extradimensional set-shifting and stimulus reversal performance worsening with increasing injury severity.246
Blast Injury: Model Overview and History
bTBI is a common mechanism of head injury for combat military personnel. In a study from one combat site in Operation Iraqi Freedom, a staggering 78% of all injuries were attributed to explosions.247 Another research team found that among individuals who experienced TBI severe enough to result in loss of consciousness (LOC), the majority (79%) of cases were related to a blast exposure.248 When individuals are in close proximity to the location of the explosion, injury tends to be severe, but the majority of exposures occur at a distance and result in mTBI; notably, even mild bTBI can produce long-term or delayed cognitive and neuropsychiatric symptoms.249 Thus, there is an impetus to better understand bTBI to reduce morbidity and mortality among military members. For this reason, many experimental bTBI labs are being utilized to simulate blast waves similar to those produced by detonation of an explosive. Briefly, animals are placed in a large tube and exposed to pressure waves caused by air pressure or an explosion (Fig. 3).250,251 There have been several attempts to design clinically relevant, reliable, and valid models of bTBI.252–254 Recently, efforts have been made to scale the blast wave used to replicate exposures observed in human bTBI by scaling the thickness of the membrane to control peak overpressures.255 Models designed to replicate explosive blasts affecting high-mobility multipurpose wheeled vehicles and within walled areas have been developed for use with pigs.254 As an alternative to using animals, three-dimensional models of bTBI have been developed, such as a physical head model constructed using a polycarbonate shell and filled with gel.256 In one study by Zhang and colleagues, the physical head model was subjected to six blast tests with varying orientations (e.g., frontal, side, 45-degree oblique) and recorded a wide range of blast overpressures (129.5–769.3 kPa). This study also reported on two distinct stages of the bTBI response: an overpressure stage followed by a blast wind stage.256
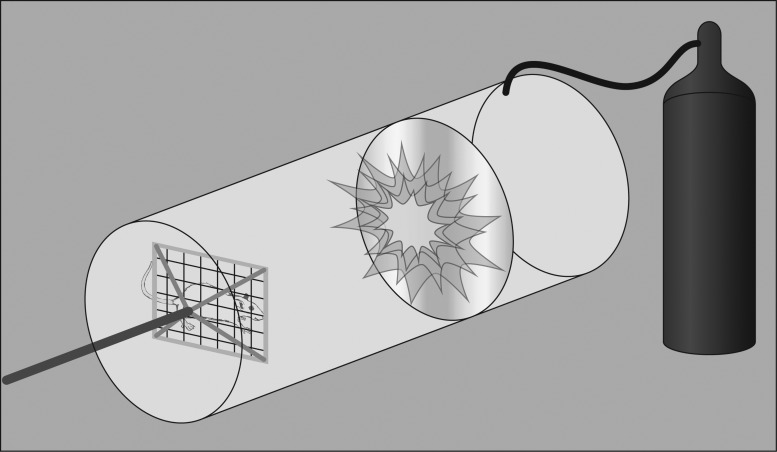
FIG. 3.
Blast injury diagram. Nonexplosive shock tubes are commonly used to study blast traumatic brain injury and are typically comprised of a driver section separated by a longer driven section by a diaphragm. The driver section of the tube is pressurized, and when the pressure is sufficient, the diaphragm bursts and rapid expansion of the gas into the driven section produces a pressure waveform. By varying the diaphragm's thickness, the bursting pressure can be controlled. Depending on the goals of the study, the test animal can be placed in varying positions with respect to the tube. Body armor can be used to minimize noncerebral damage, if desired.
In a review by Risling and Davidsson, they describe the blast mechanism as consisting of four components to blast injury.257 The primary blast wave consists of the initial wave of energy caused by the blast, and secondary effects include injury caused by the impact of objects displaced by the initial blast. Advancements in helmet technology have helped to curb some of the effects of secondary impacts; however, without the use of helmet sensors, there are challenges to scaling experimental models to replicate battlefield blasts.258 The third component of blast injury is the acceleration and rotation of the brain within the skull; this aspect of blast injury is typically associated with DAI.257 The final component of bTBI is the resultant chemical emissions and thermal energy associated with typical battlefield blasts. Several different models have been developed in an attempt to replicate either a single or multiple components of blast injury. These include the aforementioned blast tube fragmentation models that seek to replicate the secondary effects of blasts, which often accompany severe TBI, and penetrating ballistic models that replicate the path and cavitation caused by bullets. Currently, pneumatically driven shock tubes are the most frequently used model to simulate pressure-related features of bTBI. Various models are used to replicate acceleration and deceleration injuries associated with blast. Few models exist to test the quaternary effects of blasts, and therefore limited data have been generated on the quaternary effects of bTBI.257
Blast Injury: Chronic Histopathological Outcomes
Enlargement of ventricles and shrinkage of gray/white matter
As is true for other models of injury, WM damage is a common pathology of bTBI. WM damage has been observed in both pre-clinical and clinical work and is commonly reported in the CC, brainstem, and parasagittal WM.259–261 Notably, in a rat model of bTBI induced using a shock tube with body shielding, the most prominent histopathological consequence observed 2 weeks postinjury was degeneration of WM within the fiber track of the cerebellum using silver staining.262
Cell death
Apoptosis
Povlishock and Katz posited that cell death mechanisms likely underlie the destruction of WM commonly reported post-bTBI. Specifically, they hypothesize that apoptosis and necrosis contribute to this matter pathology, which continue into the long term subsequent to the initial blast exposure.261 Empirical evidence of ongoing apoptosis has been reported 2 months postinjury using TUNEL staining. No other evidence of long-term apoptosis post-bTBI was identified.
Axonal injury
Among rat models of TBI, axonal injury has been reported post-bTBI.262 Garman and colleagues evaluated axonal degeneration using amino silver staining and found multi-focal axonal degeneration at all data collection time points after the initial blast-induced TBI, including up to 2 weeks postinjury.262 The researchers suggest that silver staining is a valuable, yet underutilized, method of analyzing structural outcomes of experimental TBI, such as axonal injury.262 However, they also acknowledge that because of the limited sensitivity of the silver staining, it is best to confirm axonal injury using morphological staining patterns.262
Axonal injury has also been reported in swine up to 2 weeks post-bTBI; specifically, elevated amyloid precursor protein was found, compared to naïve and sham controls, in a study by de Lanerolle and colleagues.263 In a study of blast injury with body shielding in rats, scattered axonal spheroids were observed in the deep cerebellar WM and brainstem at 2 weeks postinjury, suggesting DAI.262 Axonal density changes have also been reported in the cingulum bundle out to 14 days after laser-induced shock-wave exposure (0.5–1.5 J/cm2) in rats.264
Aside from axonal injury, changes surrounding myelin have been observed post-bTBI. One month after a single high-intensity (200 kPa) blast in macaques, researchers identified axons completely devoid of myelin staining in both transverse and longitudinal sections at 1 month postinjury, suggesting long-term evidence of axonal pathology post-bTBI.265
Cerebrovascular histopathology
Available evidence suggests that bTBI leads to impairments in cerebrovascular compensatory responses. This can contribute to secondary insults and development of chronic deficits. However, there remains a gap in the knowledge regarding the effects of blast injury on acute and chronic cerebral vascular reactivity.266 One bTBI study in rats observed vessel tortuosity within the primary visual cortex along with evidence of collagenous remnants at 10 months after three blast injuries induced on 3 consecutive days.267 H&E staining after 3 consecutive 74.5-kPa exposures revealed hemorrhage in the region of the fimbria, third ventricle, and adjacent to the periventricular nucleus at 10 months after the final bTBI.267 In a rat model of bTBI induced using a compression-driven shock tube (20.6 psi), Kovesdi and colleagues observed vasculogenesis, as evidenced by elevated VEGF268 One month post-bTBI induced using trinitrotoluene, increased BBB permeability was observed.269 Another study found that early elevation of VEGF remained significantly elevated when assessed 2 weeks after overpressure blast injury in pigs wearing body armor.270
Inflammation
Long-term evidence of inflammation was reported by Cernak and colleagues. In this study, mild (rupture pressure=183±14 kPa) and moderate (213±17 kPa) bTBI led to elevation in macrophage-inhibiting factor related protein 8, a macrophage-related protein. These changes were reported within the brainstem and hippocampus at 14 and 30 days postinjury.271
Blast Injury: Chronic Behavioral Outcomes
Cognitive function
Long-term memory deficits have been reported post-bTBI induced using a compression-driven shock tube (20.6 psi) and was ameliorated by environmental enrichment, when compared to standard housing.268 Spatial memory deficits have been reported out to 1 month with the Y maze task and Novel Object Recognition (NOR) task post-bTBI induced using 500 g of detonated trinitrotoluene.269 Similarly, blast overpressure exposure in mice has been associated with memory deficits on the NOR task.272
In this study by Cernak and colleagues, the active avoidance response task was used to evaluated cognitive function; mild injury was found to result in cognitive deficits out to 21 days postinjury, but these deficits were attenuated by 30 days postinjury. Conversely, moderate bTBI resulted in persistent cognitive deficits out to 30 days postinjury.271
Stress-related outcomes
A review by Graner and colleagues summarizing the applications of functional magnetic resonance imaging (MRI) suggested that bTBI survivors may experience symptoms related to stress and emotional disturbance related to underlying brain change (e.g., activation of the amygdala).273 Clinically, there is a challenge in separating out these problems from more-severe psychological consequences, such as PTSD and major depressive disorder, both of which are common in military personnel.274,275
In the context of animal models, bTBI led to prolonged psychological dysfunction for months, including enhanced contextual fear conditioning.123 Elder and colleagues found that post-TBI stress-related traits persisted for many months after injury, including contextual fear conditioning, increased anxiety, and an altered response in a predator scent assay. Another study reported that rats exposed to blast injury exhibited increased levels of anxiety in the elevated plus maze (EPM) at 1 month; however, at 2 months postinjury, there was no difference between injured and sham animals.122 Kovesdi and colleagues found that injured animals displayed increased anxiety on the EPM at 45 days post-bTBI induced using a shock tube (20.6 psi) 276
Other researchers have evaluated potential strategies to target anxiety post-bTBI. Kovesdi and colleagues observed elevated anxiety peaking at 6 weeks post-bTBI induced within a compression-driven shock tube (20.6 psi), which returned to normal by 2 months postinjury.268 Long-term evidence of depression has also been reported after blast-induced TBI in mice. Specifically, Cernak and colleagues used two- and three-dimensional mapping of exploratory activity, which revealed hypoactivity, preference for corners, and freezing activity consistent with depressive behaviors at 14 and 30 days postinjury.271
Closed Head Injury: Model Overview and History
Marmarou's weight drop impact-acceleration model was initially developed in the mid-1990s.12,38,277 Preceding injury, the rodent has a metal disk fastened to its skull; the weight is dropped onto this disk from a desired height to produce the TBI. A key feature of Marmarou's model is that animals are placed on a foam pad during injury to limit linear acceleration (Fig. 4). Feeney and colleagues proposed an alternative weight drop model, in which a weight is dropped onto the intact dura after a craniotomy.38,278,279 Another method of inducing closed-head trauma uses a weight propelled by a spring down a tube onto a helmet.280 The projective concussive impact model, proposed by Leung and colleagues, induces concussion with a blunt impact.281 CHI is frequently used to study the effects of repeated TBI.
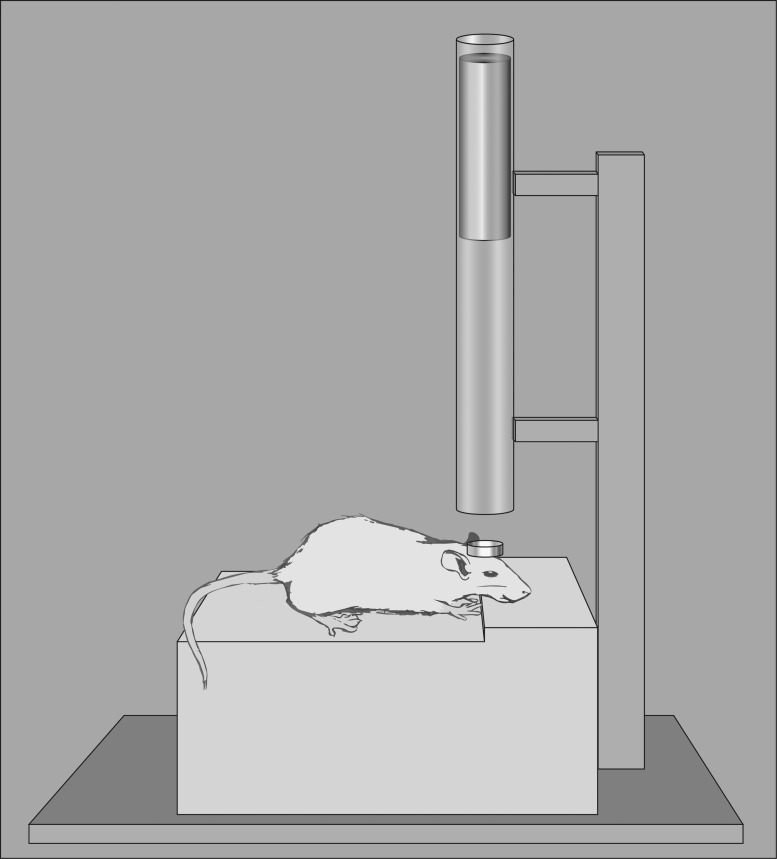
FIG. 4.
Closed head injury diagram. This model depicts the most commonly used version of closed head injury: the Marmarou model, in which the animal's head is placed on a foam pad to limit linear acceleration. Before injury induction, a metal disk is affixed to the skull and the weight is dropped onto the disk. Both the mass of the weight used and the height from which it is dropped can be controlled by researchers.
Closed-Head Injury: Chronic Histopathological Outcomes
Gross morphological and histological changes
In a repeated mTBI weight drop model, Mannix and colleagues subjected mice to repeated CHI over a 9-day period, in an attempt to replicate repeated human concussion in the absence of convulsion or LOC. No gross morphological changes were observed using MRI out to 6 months after final injury.282 Further, none of the histopathological testing in this study suggested major WM tract disruption.282
Axonal injury
Weight drop impact acceleration is well known for resulting in widespread damage to axons and thus may be chosen by researchers interested in axonal injury as an outcome variable. When silver staining was used after repetitive closed-skull TBI in mice, the silver staining intensity was significantly higher at 1, 2, and 3 weeks postinjury than it was at baseline.283 In a weight drop model of repeated CHI (7 injuries over 9 days), no axonal pathology was present when assessed 6 months after final injury.282
Neurogenesis
In the context of weight drop impact acceleration, little is known about the vulnerability of immature neurons in the hippocampus after injury. Many of the studies evaluating long-term post-traumatic neurogenesis after weight drop impact acceleration do not assess the hippocampus, but instead the subventricular zone,84,284 and were not included in this discussion. Bye and colleagues demonstrated that, similar to FPI and CCI, weight drop impact acceleration increases the rate of cellular proliferation during the week postinjury and leads to an increased number of newly generated neurons in the granular layer at 8 weeks postinjury.85
Closed Head Injury: Chronic Behavioral Outcomes
Overall neurological function
Notably, an early pre-clinical assessment of global neurological function was first applied to a rat model of CHI by Shapira and colleagues.285 This composite score was based off the following five assessments: 1) righting reflex; 2) presence of hemiplegia; 3) presence of hemiparesis; 4) seeking behavior; and 5) ability to exit a large ring (50 cm diameter).285 Since the original publication of the Neurological Severity Score (NSS), it has been modified to assess specific aspects of motor function that may be impaired post-TBI. For example, additional assessments of reflexes were added by Shohami and colleagues.286 More recently, the scoring of the NSS was further expanded to a 14-point scale287,288 based off assessment of reflexes, along with motor and sensory functions, which was further adapted into the modified NSS (mNSS).289 The mNSS is also measured on a 14-point scale and includes assessments of reflexes, motor function, balance, and sensory function.287,288,290
Motor function
Coordination deficits assessed by rotating pole test have been reported out to 1 month after repeated CHI. This study also observed deficits in balance assessed by the rotarod task.291 Notably, no additional long-term evidence of motor deficit post-CHI was found by us during the literature search.
Cognitive function
MWM deficits have been observed out to 1 month after a single CHI.292 Another study reported working memory function deficits post-CHI has been reported out to 1 month postinjury.293 No other long-term evidence of cognitive deficits post-CHI was identified.
Stress-related outcomes
Depression- and anxiety-like behaviors have been observed after impact accelerated TBI induced using a modified weight drop protocol.294 In this study, depression-like behavior was observed post-TBI using the open-field exploration task, the EPM task, along with observations of changes in social behavior and hypermotionality. Anxiety-like behavior was assessed using observations of social interaction and the marble-burying tests.294
Discussion and Conclusion
Overall, this review of the literature demonstrates that several experimental TBI models are being used to study chronic TBI pathology. Both histopathological and behavioral outcomes of TBI are reported into the long term postinjury. Notably, compared to the acute effects of experimental TBI, the chronic effects remain poorly characterized. Of the long-term evidence published to date, studies exclusively using male test animals are, by far, the most common; additional characterization of long-term outcomes of TBI in female animals is warranted. Both adult and pediatric studies have been conducted into the long term, but a number of studies fail to report the age of the animal or provide a vague characterization of adult or young adult. Under-reporting of sex, age, and other important study characteristics may affect interpretation of findings and the ability of other researchers to replicate results. This under-reporting also highlights the need for common data elements (CDEs).
The need for CDEs has also been recognized by the National Institute for Neurological Diseases and Stroke (NINDS), which is establishing a set of CDEs in an effort to enhance the extent to which comparisons can be made across studies. These developing CDEs span several categories, including characteristics of the animals used (e.g., species, sex, and supplier), age, weight, and the outcome assessment(s) used (e.g., timing of assessment and measures). The NINDS has also released guidelines regarding optimizing experimental design in pre-clinical TBI studies to reduce bias and promote best practices in data interpretation and reporting.295 These guidelines were designed to promote clinical relevance of TBI research and ultimately promote translation to clinical trials and care. The reader is encouraged to stay up to date with these guidelines and incorporate them into their publications. The following is a brief discussion regarding how the various models of TBI compare and contrast. Notably, not all histopathological and functional outcomes of interest in this review have been observed in the chronic period postinjury using each of the included models. A summary of long-term histopathological and functional outcomes by model type is shown in Tables 2 and 3.
Table 2.
Summary of Long-Term Histopathological Deficits by Model
| FPI | Controlled cortical impact | Blast TBI | Closed head injury | |
|---|---|---|---|---|
| Gross changes in morphology/histology | X | X | ||
| Ventricular enlargement and gray/white matter shrinkage | X | X | X | |
| Cell death patterns | X | X | ||
| Apoptosis | X | X | X | |
| Autophagy | X | |||
| Necrosis | X | X | ||
| Axonal injury | X | X | X | X |
| Cerebrovascular histopathology | X | X | X | |
| Inflammation | X | X | ||
| Neurogenesis | X | X | X |
This table states whether or not literature was identified that reported long-term effects of TBI on the histopathological outcomes of interest in this review; however, it does not compare model subtypes (e.g., medial FPI vs. lateral FPI) nor does it provide information regarding extent or severity of each outcome.
TBI, traumatic brain injury; FPI, fluid percussion injury.
Table 3.
Summary of Long-Term Behavioral Deficits by Model
| FPI | Controlled cortical impact | Blast TBI | Closed head injury | |
|---|---|---|---|---|
| Overall neurological function | X | X | X | |
| Motor function | X | X | X | |
| Cognitive function | X | X | X | X |
| Frontal lobe function | X | |||
| Stress-related outcomes | X | X | X |
This table states whether or not literature was identified that reported long-term effects of TBI on the behavioral outcomes of interest in this review; however, it does not compare model subtypes (e.g., medial FPI vs. lateral FPI) nor does it provide information regarding extent or severity of each outcome.
TBI, traumatic brain injury; FPI, fluid percussion injury.
FPI was the first widely used injury induction method for experimental brain injury. Since FPI was developed, this model has been widely applied to study chronic outcomes of TBI. Similarly, the CCI model of TBI, also one of the earlier developed models, is widely used today. Overall, the chronic histopathological and behavioral consequences of CCI are well established.
FPI and CCI are similar, in that both lead to numerous histopathological deficits and behavioral dysfunction. Post-CCI, deficits are common on the MWM (compared to control animals),211,212,214–216,225–233 especially when compared to the rare long-term deficits observed after lateral FPI179,181 and medial FPI.182 Moreover, several studies suggest that CCI leads to chronic foot fault test deficits,92,213,214,296,297 whereas only one study showing long-term foot fault deficits after FPI was identified.130
Conversely, in preparing this review, no evidence of long-term reversal learning task impairment post-CCI was identified; however, injury effects have been observed on this task after a single lateral FPI186 as well as repeated lateral FPI.179,181 Moreover, no references were identified where this test was applied and deficits found post-CCI or other models included in this review.
Some models that were less commonly used historically are gaining popularity. For example, bTBI has been a rapidly developing area of TBI research. This increasing interest in bTBI is owing, in part, to the fact that this represents a major injury mechanism affecting combat military personnel. CHI is less studied. Still, valuable evidence has been gleaned from these models. Moreover, each of the models of TBI have merit and may be useful for studying some particular pathoanatomical or behavioral feature of TBI.
All the experimental models are characterized by postinjury survival and the initiation of numerous pathologic and protective processes. Notably, some models have reported mortality, which may or may not correlate with injury severity.298 Overall, the evidence suggests that TBI is a diverse process that begins in the acute period and continues chronically. Some models of TBI may be better suited than others for studying certain features of TBI. For instance, FPI and CCI have been used to study apoptosis, necrosis, and autophagy, whereas bTBI has been used to study apoptosis.
It is also worth briefly noting that some outcomes of TBI remain understudied across all TBI models. Social dysfunction is a common, debilitating outcome of clinical brain injury, only recently studied using animal models of TBI and especially understudied in the long term. For example, Bridgette Semple and her colleagues have evaluated the long-term effects of TBI on social functioning in pediatric and adolescent animals.299,300 Additional research exploring sociosexual outcomes of experimental TBI is warranted, along with expansion of this work to include adult animals, with the goal of reducing sociosexual symptoms known to affect TBI survivors.301–304 Evidence of long-term frontal lobe dysfunction has only been reported in the context of CCI. Extending underutilized assessments in less-characterized models of TBI represents an opportunity for researchers.
Strengths
Overall, the available evidence suggests that animal models of TBI replicate most histopathological and functional outcomes observed clinically. That said, as discussed previously, some models replicate certain aspects of TBI better than others. In addition to the clinical relevance and advantages over in vitro models, in some applications, experimental studies have advantages over clinical trials. For example, pre-clinical studies of TBI are useful for building a preliminary evidence base and controlling confounding variables that could affect study findings. For example, in animal models, diet and environment can be precisely and acurately controlled.215,305
An additional strength of experimental models of TBI is that whereas many of the same histopathological outcomes can be assessed in humans, experimental TBI studies facilitate specifically timed data collection in ways that would be neither feasible nor ethical in humans. Experimental TBI studies allow researchers to precisely and accurately control timing of animal sacrifice and collect fresh postmortem tissue for analysis.
Trials of potential TBI therapeutics represent one of the most important applications of experimental TBI models. It is critical that safety and efficacy of a potential therapeutic be established in animals before translating the agent into clinical trials and, ultimately, patient care. Experimental TBI drug studies also facilitate identification of potential adverse treatment effects.
Weaknesses
The published evidence surrounding the long-term histopathological and functional outcomes of experimental TBI is limited in scope and volume. Few studies include outcome assessments beyond 1 month, with even fewer extending out to 3, 6, or 12 months (Table 1). This narrow timeframe of data collection may obscure our understanding of long-term consequences. Additional research assessing outcomes of TBI into the long term is needed. Arguably, the most significant barrier to conducting long-term experimental TBI studies is the high cost associated with animals, housing, veterinary care, food, and the need for specialized facilities. Concern for the ethical treatment of laboratory animals may further contribute to the preference for shorter-term studies.
Although animal research facilitates control of important variables, including genetics, environment, and diet, there are some unique considerations for researchers to take into account when conducting this type of work. Notably, the clinical population is not as homogenous as those observed in experimental studies, with respect to age, genes, and environment. Thus, additional research efforts are needed to promote generalizability of study findings and/or facilitate clinical translation.
A final limitation of using animal models to study the long-term histopathological and functional outcomes of TBI is that the relationship between structural and functional deficits is not one to one. For example, it is possible to have functional deficits with or without the development of a lesion. Morphological changes can be sufficient to produce functional deficits, but are not necessary. The opposite is also true; pathophysiological changes can occur in the absence of functional deficits. For instance, in a weight drop model of focal brain injury in rats, there was inflammation in the absence of functional deficits.13 That said, limitations in the available research tools could prevent consequences of TBI from being detected.
Closing remarks
Animal models of TBI have been widely used to understand the histopathological and behavioral consequences of brain trauma in humans. Animal models of head trauma remain relevant today. Researchers using animal models to study TBI have a number of critical decisions regarding experimental parameters to consider, including, but not limited to, the species to use, injury method, severity, injury location, and outcome measures. As part of the effort to promote rigorous TBI studies, researchers are advised to follow the aforementioned criteria proposed by the NINDS.295 Researchers considering the use of an experimental TBI model should give careful consideration and attention to ensure that the most appropriate model is chosen to address the research question(s). Researchers can control for injury severity and target damage to different brain regions by selection of appropriate models.
Careful study planning and thoughtful consideration are necessary to promote clinical relevance of animal studies of TBI. Specifically, attention should be given to selecting clinically relevant outcome variables that are comparable to what is observed in human patients. There are obvious challenges in assessing cognition in animals, compared to humans; for example, verbal assessments are not possible. Moreover, in deciding the types of long-term outcomes to evaluate in pre-clinical studies, researchers must have an understanding of the types of functional deficits that are commonly experienced by, and particularly distressing for, TBI survivors. Notably, some commonly reported problems, such as difficulties in devoting attention to multiple competing stimuli, cannot be easily studied in animals306; however, Corina Bondi and her colleagues have proposed and tested one such assessment.246
Assessment of functional outcomes in animal models requires inference in line with classic Skinnerian thought. As mentioned above, there are some functional outcomes of TBI in humans that have no clear analog in animals. Additional efforts are needed to map human to animal domains and develop additional reliable, valid assessments of functionality for use in animals. Moreover, it is worth noting that functional tests can be completed within hours in humans, whereas assessment of neurobehavioral function in animals can take much longer.
There are some relevant considerations for researchers regarding the species to use. Not all outcome variables can be assessed in all species. For example, if behavioral tests are of interest, a rodent model may be preferred given that functional outcome tests are well characterized in this context, especially when compared to large animals.307 Rodent models afford unique opportunities to assess histopathological and behavioral consequences of TBI under controlled conditions and at precisely timed intervals. However, these small animal models are associated with their own set of pitfalls, such as limited generalizability, the potential for test strains to exhibit deficits that would confound study results, and the need for substantial research efforts before translation.308,309 Most behavioral tests developed for use in rats have been scaled for translation to mice; however, the tests may not yield similar injury effects across species.
Likewise, selection of assessment time point(s) can affect results. If assessed too early, the effects of anesthesia may confound findings, whereas, if the assessment occurs too late, the injury effect may have subsided. Thus, Fujimoto and colleagues suggest that researchers should assess the functional outcomes of interest at multiple time points. Previously, experimental TBI researchers have suggested that additional long-term studies are needed.310
Overall, experimental models have contributed substantially to our understanding of acute and chronic TBI outcomes. Despite available research, chronic changes remain relatively understudied, though available evidence suggests that chronic outcomes of experimental TBI are diverse and include histopathological and behavioral changes. In this review, effort was given to include as much evidence as possible; however, this review is not intended to be fully comprehensive. Readers of this review will take away a familiarization of key evidence from four common experimental TBI models (FPI, CCI, bTBI, and CHI), along with the key assessments used to evaluate the long-term histopathological and behavioral consequences of these models.
Acknowledgments
This work was supported by three federally sponsored grants: National Institutes of Health (NIH)-NINDS grants R01-NS079061 and 5T32HD040686-14, Department of Veterans Affairs grant RR&D B1127-I, and NIH-NINR grants 1F31NR014957-01 and T32NR009759. Additional support for this work was provided by grants from the following foundations and professional societies: The Pittsburgh Foundation, Sigma Theta Tau International Eta Chapter, International Society for Nurses in Genetics, and the American Association of Neuroscience Nursing/Neuroscience Nursing Foundation. The authors thank Michael Farmer for his time and assistance with the figures, Marilyn Farmer for her editorial support, as well as Lori Beck, Emad Madha, Lan Pham, and Amanda Savarese for their assistance in identifying and managing the long-term publications cited in this review.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Tate R.L., and Broe G.A. (1999). Psychosocial adjustment after traumatic brain injury: what are the important variables? Psychol. Med. 29, 713–725 [DOI] [PubMed] [Google Scholar]
- 2.Corrigan J.D., Selassie A.W., and Orman J.A. (2010). The epidemiology of traumatic brain injury. J. Head Trauma Rehabil. 25, 72–80 [DOI] [PubMed] [Google Scholar]
- 3.McKinlay W.W., Brooks D.N., Bond M.R., Martinage D.P., and Marshall M.M. (1981). The short-term outcome of severe blunt head injury as reported by relatives of the injured persons. J. Neurol. Neurosurg. Psychiatry 44, 527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schalen W., Hansson L., Nordstrom G., and Nordstrom C.H. (1994). Psychosocial outcome 5–8 years after severe traumatic brain lesions and the impact of rehabilitation services. Brain Inj. 8, 49–64 [DOI] [PubMed] [Google Scholar]
- 5.Ergh T.C., Rapport L.J., Coleman R.D., and Hanks R.A. (2002). Predictors of caregiver and family functioning following traumatic brain injury: social support moderates caregiver distress. J. Head Trauma Rehabil. 17, 155–174 [DOI] [PubMed] [Google Scholar]
- 6.Anderson M.I., Parmenter T.R., and Mok M. (2002). The relationship between neurobehavioural problems of severe traumatic brain injury (TBI), family functioning and the psychological well-being of the spouse/caregiver: path model analysis. Brain Inj. 16, 743–757 [DOI] [PubMed] [Google Scholar]
- 7.Thurman D.J., Alverson C., Dunn K.A., Guerrero J., and Sniezek J.E. (1999). Traumatic brain injury in the United States: a public health perspective. J. Head Trauma Rehabil 14, 602–615 [DOI] [PubMed] [Google Scholar]
- 8.Kramer S.P. (1896). A contribution to the theory of cerebral concussion. Anim. Surg. 23, 163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinder L., and Olsson Y. (1968). Studies on vascular permeability changes in experimental brain concussion. I. Distribution of circulating fluorescent indicators in brain and cervical cord after sudden mechanical loading of the brain. Acta Neuropathol. 11, 183–200 [DOI] [PubMed] [Google Scholar]
- 10.Denny-Brown D., and Russell W. (1941). Experimental concussion. Brain 64, 93–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindgren S., and Rinder L. (1965). Experimental studies in head injury. I. Some factors influencing results of model experiments. Biophysik 2, 320–329 [PubMed] [Google Scholar]
- 12.Gold E.M., Su D., López-Velázquez L., Haus D.L., Perez H., Lacuesta G.A., Anderson A.J., and Cummings B.J. (2013). Functional assessment of long-term deficits in rodent models of traumatic brain injury. Regen. Med. 8, 483–516 [DOI] [PubMed] [Google Scholar]
- 13.Holmin S., and Mathiesen T. (1999). Long-term intracerebral inflammatory response after experimental focal brain injury in rat. Neuroreport 10, 1889–1891 [DOI] [PubMed] [Google Scholar]
- 14.Smith D.H., Chen X.-H., Pierce J.E., Wolf J.A., Trojanowski J.Q., Graham D.I., and McIntosh T.K. (1997). Progressive atrophy and neuron death for one year following brain trauma in the rat. J. Neurotrauma 14, 715–727 [DOI] [PubMed] [Google Scholar]
- 15.Dixon C., Kochanek P., Yan H., Schiding J., Griffith R., Baum E., Marion D., and DeKosky S. (1999). One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J. Neurotrauma 16, 109–122 [DOI] [PubMed] [Google Scholar]
- 16.Nonaka M., Chen X.H., Pierce J.E., Leoni M.J., McIntosh T.K., Wolf J.A., and Smith D.H. (1999). Prolonged activation of NF-kappaB following traumatic brain injury in rats. J. Neurotrauma 16, 1023–1034 [DOI] [PubMed] [Google Scholar]
- 17.Loane D.J., Kumar A., Stoica B.A., Cabatbat R., and Faden A.I. (2014). Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 73, 14–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannix R.C., Zhang J., Park J., Zhang X., Bilal K., Walker K., Tanzi R.E., Tesco G., and Whalen M.J. (2011). Age-dependent effect of apolipoprotein E4 on functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 31, 351–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halldorsson J.G., Arnkelsson G.B., Tomasson K., Flekkoy K.M., Magnadottir H.B., and Arnarson E.O. (2013). Long-term outcome of medically confirmed and self-reported early traumatic brain injury in two nationwide samples. Brain Inj. 27, 1106–1118 [DOI] [PubMed] [Google Scholar]
- 20.Godbolt A.K., Cancelliere C., Hincapié C.A., Marras C., Boyle E., Kristman V.L., Coronado V.G., and Cassidy J.D. (2014). Systematic review of the risk of dementia and chronic cognitive impairment after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil. 95, S245–S256 [DOI] [PubMed] [Google Scholar]
- 21.Andruszkow H., Deniz E., Urner J., Probst C., Grün O., Lohse R., Frink M., Krettek C., Zeckey C., and Hildebrand F. (2014). Physical and psychological long-term outcome after traumatic brain injury in children and adult patients. Health Qual. Life Outcomes 12, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barth M., Schilling L., and Schmiedek P. (2000). Time course of apoptotic cell death after experimental neurotrauma. Acta Neurochir. Suppl. 76, 121–124 [DOI] [PubMed] [Google Scholar]
- 23.Williams A.J., Hartings J.A., Lu X.-C.M., Rolli M.L., and Tortella F.C. (2006). Penetrating ballistic-like brain injury in the rat: differential time courses of hemorrhage, cell death, inflammation, and remote degeneration. J. Neurotrauma 23, 1828–1846 [DOI] [PubMed] [Google Scholar]
- 24.Williams A.J., Hartings J.A., Lu X.-C.M., Rolli M.L., Dave J.R., and Tortella F.C. (2005). Characterization of a new rat model of penetrating ballistic brain injury. J. Neurotrauma 22, 313–331 [DOI] [PubMed] [Google Scholar]
- 25.Allen I. V, Scott R., and Tanner J.A. (1982). Experimental high-velocity missile head injury. Injury 14, 183–193 [DOI] [PubMed] [Google Scholar]
- 26.Carey M.E., Sarna G.S., Farrell J.B., and Happel L.T. (1989). Experimental missile wound to the brain. J. Neurosurg. 71, 754–764 [DOI] [PubMed] [Google Scholar]
- 27.Pierce J.E., Smith D.H., Trojanowski J.Q., and McIntosh T.K. (1998). Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience 87, 359–369 [DOI] [PubMed] [Google Scholar]
- 28.Bramlett H.M., and Dietrich W.D. (2002). Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol. 103, 607–614 [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Paez A.C., Brunschwig J.P., and Bramlett H.M. (2005). Light and electron microscopic assessment of progressive atrophy following moderate traumatic brain injury in the rat. Acta Neuropathol. 109, 603–616 [DOI] [PubMed] [Google Scholar]
- 30.Nagamoto-Combs K., McNeal D.W., Morecraft R.J., and Combs C.K. (2007). Prolonged microgliosis in the rhesus monkey central nervous system after traumatic brain injury. J. Neurotrauma 24, 1719–1742 [DOI] [PubMed] [Google Scholar]
- 31.Kagan V.E., Tyurin V.A., Jiang J., Tyurina Y.Y., Ritov V.B., Amoscato A.A., Osipov A.N., Belikova N.A., Kapralov A.A., Kini V., Vlasova II, Zhao Q., Zou M., Di P., Svistunenko D.A., Kurnikov I.V., and Borisenko G.G. (2005). Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 1, 223–232 [DOI] [PubMed] [Google Scholar]
- 32.O'Connor C.A., Cernak I., Johnson F., and Vink R. (2007). Effects of progesterone on neurologic and morphologic outcome following diffuse traumatic brain injury in rats. Exp. Neurol. 205, 145–153 [DOI] [PubMed] [Google Scholar]
- 33.Ratcliff J.J., Greenspan A.I., Goldstein F.C., Stringer A.Y., Bushnik T., Hammond F.M., Novack T.A., Whyte J., and Wright D.W. (2007). Gender and traumatic brain injury: do the sexes fare differently? Brain Inj. 21, 1023–1030 [DOI] [PubMed] [Google Scholar]
- 34.Slewa-Younan S., Green A.M., Baguley I.J., Gurka J.A., and Marosszeky J.E. (2004). Sex differences in injury severity and outcome measures after traumatic brain injury. Arch. Phys. Med. Rehabil. 85, 376–379 [DOI] [PubMed] [Google Scholar]
- 35.Wagner A.K., Willard L.A., Kline A.E., Wenger M.K., Bolinger B.D., Ren D., Zafonte R.D., and Dixon C.E. (2004). Evaluation of estrous cycle stage and gender on behavioral outcome after experimental traumatic brain injury. Brain Res. 998, 113–121 [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. (2010). Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths 2002–2006. http://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf Accessed April8, 2015
- 37.Tomaiuolo F., Carlesimo G.A., Di Paola M., Petrides M., Fera F., Bonanni R., Formisano R., Pasqualetti P., and Caltagirone C. (2004). Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: a T1 weighted MRI study. J. Neurol. Neurosurg. Psychiatry 75, 1314–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foda M.A., and Marmarou A. (1994). A new model of diffuse brain injury in rats. Part II: Morphological characterization. J. Neurosurg. 80, 301–313 [DOI] [PubMed] [Google Scholar]
- 39.Lyeth B.G., Jenkins L.W., Hamm R.J., Dixon C.E., Phillips L.L., Clifton G.L., Young H.F., and Hayes R.L. (1990). Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 526, 249–258 [DOI] [PubMed] [Google Scholar]
- 40.Levin H.S., Meyers C.A., Grossman R.G., and Sarwar M. (1981). Ventricular enlargement after closed head injury. Arch. Neurol. 38, 623–629 [DOI] [PubMed] [Google Scholar]
- 41.Meyers C.A., Levin H.S., Eisenberg H.M., and Guinto F.C. (1983). Early versus late lateral ventricular enlargement following closed head injury. J. Neurol. Neurosurg. Psychiatry 46, 1092–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson S.C., Bigler E.D., Burr R.B., and Blatter D.D. (1994). White matter atrophy, ventricular dilation, and intellectual functioning following traumatic brain injury. Neuropsychology 8, 307–315 [Google Scholar]
- 43.Poca M.A., Sahuquillo J., Mataró M., Benejam B., Arikan F., and Báguena M. (2005). Ventricular enlargement after moderate or severe head injury: a frequent and neglected problem. J. Neurotrauma 22, 1303–1310 [DOI] [PubMed] [Google Scholar]
- 44.Jorge R.E., Robinson R.G., Moser D., Tateno A., Crespo-Facorro B., and Arndt S. (2004). Major depression following traumatic brain injury. Arch. Gen. Psychiatry 61, 42–50 [DOI] [PubMed] [Google Scholar]
- 45.Gale S.D., Baxter L., Roundy N., and Johnson S.C. (2005). Traumatic brain injury and grey matter concentration: a preliminary voxel based morphometry study. J. Neurol. Neurosurg. Psychiatry 76, 984–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson V.E., Stewart J.E., Begbie F.D., Trojanowski J.Q., Smith D.H., and Stewart W. (2013). Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136, 28–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flygt J., Djupsjö A., Lenne F., and Marklund N. (2013). Myelin loss and oligodendrocyte pathology in white matter tracts following traumatic brain injury in the rat. Eur. J. Neurosci. 38, 2153–2165 [DOI] [PubMed] [Google Scholar]
- 48.Smith D.H., Soares H.D., Pierce J.S., Perlman K.G., Saatman K.E., Meaney D.F., Dixon C.E., and McIntosh T.K. (1995). A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J. Neurotrauma 12, 169–178 [DOI] [PubMed] [Google Scholar]
- 49.Sauerbeck A., Gao J., Readnower R., Liu M., Pauly J.R., Bing G., and Sullivan P.G. (2011). Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp. Neurol. 227, 128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kochanek P.M., Clark R.S., Ruppel R.A., Adelson P.D., Bell M.J., Whalen M.J., Robertson C.L., Satchell M.A., Seidberg N.A., Marion D.W., and Jenkins L.W. (2000). Biochemical, cellular, and molecular mechanisms in the evolution of secondary damage after severe traumatic brain injury in infants and children: lessons learned from the bedside. Pediatr. Crit. Care Med. 1, 4–19 [DOI] [PubMed] [Google Scholar]
- 51.Raghupathi R. (2004). Cell death mechanisms following traumatic brain injury. Brain Pathol. 14, 215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rand C.W., and Courville C.B. (1946). Histologic changes in the brain in cases of fatal injury to the head; alterations in nerve cells. Arch. Neurol. Psychiatry 55, 79–110 [DOI] [PubMed] [Google Scholar]
- 53.Nevin N.C. (1967). Neuropathological changes in the white matter following head injury. J. Neuropathol. Exp. Neurol. 26, 77–84 [DOI] [PubMed] [Google Scholar]
- 54.Oppenheimer D.R. (1968). Microscopic lesions in the brain following head injury. J. Neurol. Neurosurg. Psychiatry 31, 299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peerless S.J., and Rewcastle N.B. (1967). Shear injuries of the brain. Can. Med. Assoc. J. 96, 577–582 [PMC free article] [PubMed] [Google Scholar]
- 56.Adams J.H., Graham D.I., Murray L.S., and Scott G. (1982). Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann. Neurol. 12, 557–563 [DOI] [PubMed] [Google Scholar]
- 57.Gennarelli T.A., Thibault L.E., Adams J.H., Graham D.I., Thompson C.J., and Marcincin R.P. (1982). Diffuse axonal injury and traumatic coma in the primate. Ann. Neurol. 12, 564–574 [DOI] [PubMed] [Google Scholar]
- 58.Smith D.H., Hicks R., and Povlishock J.T. (2013). Therapy development for diffuse axonal injury. J. Neurotrauma 30, 307–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erb D.E., and Povlishock J.T. (1988). Axonal damage in severe traumatic brain injury: an experimental study in cat. Acta Neuropathol. 76, 347–358 [DOI] [PubMed] [Google Scholar]
- 60.Dixon C., Clifton G., Lighthall J., Yaghmai A., and Hayes R. (1991). A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 39, 253–262 [DOI] [PubMed] [Google Scholar]
- 61.Muradashvili N., and Lominadze D. (2013). Role of fibrinogen in cerebrovascular dysfunction after traumatic brain injury. Brain Inj. 27, 1508–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dias C., Silva M.J., Pereira E., Silva S., Cerejo A., Smieleweski P., Rocha A.P., Gaio A.R., Paiva J.-A., and Czosnyka M. (2014). Post-traumatic multimodal brain monitoring: response to hypertonic saline. J. Neurotrauma 31, 1872–1880 [DOI] [PubMed] [Google Scholar]
- 63.Varsos G.V., Budohoski K.P., Kolias A.G., Liu X., Smielewski P., Varsos V.G., Hutchinson P.J., Pickard J.D., and Czosnyka M. (2014). Relationship of vascular wall tension and autoregulation following traumatic brain injury. Neurocrit. Care 21, 266–274 [DOI] [PubMed] [Google Scholar]
- 64.Alves J.L. (2014). Blood-brain barrier and traumatic brain injury. J. Neurosci. Res. 92, 141–147 [DOI] [PubMed] [Google Scholar]
- 65.Pascual J.L., Murcy M.A., Li S., Gong W., Eisenstadt R., Kumasaka K., Sims C., Smith D.H., Browne K., Allen S., and Baren J. (2013). Neuroprotective effects of progesterone in traumatic brain injury: blunted in vivo neutrophil activation at the blood-brain barrier. Am. J. Surg. 206, 840–845; discussion, 845–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyamoto K., Ohtaki H., Dohi K., Tsumuraya T., Nakano H., Kiriyama K., Song D., Aruga T., and Shioda S. (2013). Edaravone increases regional cerebral blood flow after traumatic brain injury in mice. Acta Neurochir. Suppl. 118, 103–109 [DOI] [PubMed] [Google Scholar]
- 67.Abdul-Muneer P.M., Schuetz H., Wang F., Skotak M., Jones J., Gorantla S., Zimmerman M.C., Chandra N., and Haorah J. (2013). Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic. Biol. Med. 60, 282–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scott A. (2004). What is “inflammation”? Are we ready to move beyond Celsus? Br. J. Sports Med. 38, 248–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rocha e Silva M. (1994). A brief survey of the history of inflammation. 1978. Agents Actions 43, 86–90 [DOI] [PubMed] [Google Scholar]
- 70.Lucas S.-M., Rothwell N.J., and Gibson R.M. (2006). The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 147, Suppl. 1, S232–S2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cederberg D., and Siesjö P. (2010). What has inflammation to do with traumatic brain injury? Childs. Nerv. Syst. 26, 221–226 [DOI] [PubMed] [Google Scholar]
- 72.Ramlackhansingh A.F., Brooks D.J., Greenwood R.J., Bose S.K., Turkheimer F.E., Kinnunen K.M., Gentleman S., Heckemann R.A., Gunanayagam K., Gelosa G., and Sharp D.J. (2011). Inflammation after trauma: microglial activation and traumatic brain injury. Ann. Neurol. 70, 374–383 [DOI] [PubMed] [Google Scholar]
- 73.Nekludov M., Antovic J., Bredbacka S., and Blombäck M. (2007). Coagulation abnormalities associated with severe isolated traumatic brain injury: cerebral arterio-venous differences in coagulation and inflammatory markers. J. Neurotrauma 24, 174–180 [DOI] [PubMed] [Google Scholar]
- 74.Whalen M., Carlos T., Clark R., Marion D., DeKosky S., Heineman S., Schiding J., Memarzadeh F., and Kochanek P. (1997). The effect of brain temperature on acute inflammation after traumatic brain injury in rats. J. Neurotrauma 14, 561–572 [DOI] [PubMed] [Google Scholar]
- 75.Chen T., Liu W., Chao X., Zhang L., Qu Y., Huo J., and Fei Z. (2011). Salvianolic acid B attenuates brain damage and inflammation after traumatic brain injury in mice. Brain Res. Bull. 84, 163–168 [DOI] [PubMed] [Google Scholar]
- 76.Ming G.-L., and Song H. (2011). Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deng W., Aimone J.B., and Gage F.H. (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao C., Deng W., and Gage F.H. (2008). Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660 [DOI] [PubMed] [Google Scholar]
- 79.Zhao C., Teng E.M., Summers R.G., Ming G.-L., and Gage F.H. (2006). Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 26, 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eriksson P.S., Perfilieva E., Björk-Eriksson T., Alborn A.M., Nordborg C., Peterson D.A., and Gage F.H. (1998). Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317 [DOI] [PubMed] [Google Scholar]
- 81.Sanai N., Tramontin A.D., Quiñones-Hinojosa A., Barbaro N.M., Gupta N., Kunwar S., Lawton M.T., McDermott M.W., Parsa A.T., Manuel-García Verdugo J., Berger M.S., and Alvarez-Buylla A. (2004). Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427, 740–744 [DOI] [PubMed] [Google Scholar]
- 82.Rola R., Mizumatsu S., Otsuka S., Morhardt D.R., Noble-Haeusslein L.J., Fishman K., Potts M.B., and Fike J.R. (2006). Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp. Neurol. 202, 189–199 [DOI] [PubMed] [Google Scholar]
- 83.Gao X., Deng-Bryant Y., Cho W., Carrico K.M., Hall E.D., and Chen J. (2008). Selective death of newborn neurons in hippocampal dentate gyrus following moderate experimental traumatic brain injury. J. Neurosci. Res. 86, 2258–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thau-Zuchman O., Shohami E., Alexandrovich A.G., and Leker R.R. (2010). Vascular endothelial growth factor increases neurogenesis after traumatic brain injury. J. Cereb. Blood Flow Metab. 30, 1008–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bye N., Carron S., Han X., Agyapomaa D., Ng S.Y., Yan E., Rosenfeld J. V, and Morganti-Kossmann M.C. (2011). Neurogenesis and glial proliferation are stimulated following diffuse traumatic brain injury in adult rats. J. Neurosci. Res. 89, 986–1000 [DOI] [PubMed] [Google Scholar]
- 86.Urrea C., Castellanos D.A., Sagen J., Tsoulfas P., Bramlett H.M., and Dietrich W.D. (2007). Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat. Restor. Neurol. Neurosci. 25, 65–76 [PubMed] [Google Scholar]
- 87.Clelland C.D., Choi M., Romberg C., Clemenson G.D., Fragniere A., Tyers P., Jessberger S., Saksida L.M., Barker R.A., Gage F.H., and Bussey T.J. (2009). A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jessberger S., Clark R.E., Broadbent N.J., Clemenson G.D., Consiglio A., Lie D.C., Squire L.R., and Gage F.H. (2009). Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 16, 147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sahay A., Scobie K.N., Hill A.S., O'Carroll C.M., Kheirbek M.A., Burghardt N.S., Fenton A.A., Dranovsky A., and Hen R. (2011). Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blaiss C.A., Yu T.-S., Zhang G., Chen J., Dimchev G., Parada L.F., Powell C.M., and Kernie S.G. (2011). Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J. Neurosci. 31, 4906–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kleindienst A., McGinn M.J., Harvey H.B., Colello R.J., Hamm R.J., and Bullock M.R. (2005). Enhanced hippocampal neurogenesis by intraventricular S100B infusion is associated with improved cognitive recovery after traumatic brain injury. J. Neurotrauma 22, 645–655 [DOI] [PubMed] [Google Scholar]
- 92.Xiong Y., Mahmood A., Meng Y., Zhang Y., Qu C., Schallert T., and Chopp M. (2010). Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J. Neurosurg. 113, 598–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun D., Bullock M.R., McGinn M.J., Zhou Z., Altememi N., Hagood S., Hamm R., and Colello R.J. (2009). Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp. Neurol. 216, 56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Levin H.S., High W.M., Goethe K.E., Sisson R.A., Overall J.E., Rhoades H.M., Eisenberg H.M., Kalisky Z., and Gary H.E. (1987). The neurobehavioural rating scale: assessment of the behavioural sequelae of head injury by the clinician. J. Neurol. Neurosurg. Psychiatry 50, 183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barlow K.M., Thomson E., Johnson D., and Minns R.A. (2005). Late neurologic and cognitive sequelae of inflicted traumatic brain injury in infancy. Pediatrics 116, e174–e185 [DOI] [PubMed] [Google Scholar]
- 96.Jennett B., Snoek J., Bond M.R., and Brooks N. (1981). Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J. Neurol. Neurosurg. Psychiatry 44, 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Becker D.P., Miller J.D., Ward J.D., Greenberg R.P., Young H.F., and Sakalas R. (1977). The outcome from severe head injury with early diagnosis and intensive management. J. Neurosurg. 47, 491–502 [DOI] [PubMed] [Google Scholar]
- 98.Cooper D.J., Myles P.S., McDermott F.T., Murray L.J., Laidlaw J., Cooper G., Tremayne A.B., Bernard S.S., and Ponsford J. (2004). Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. JAMA 291, 1350–1357 [DOI] [PubMed] [Google Scholar]
- 99.McIntosh T.K., Noble L., Andrews B., and Faden A.I. (1987). Traumatic brain injury in the rat: characterization of a midline fluid-percussion model. Cent. Nerv. Syst. Trauma 4, 119–134 [DOI] [PubMed] [Google Scholar]
- 100.Friess S.H., Ichord R.N., Ralston J., Ryall K., Helfaer M.A., Smith C., and Margulies S.S. (2009). Repeated traumatic brain injury affects composite cognitive function in piglets. J. Neurotrauma 26, 1111–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sullivan S., Friess S.H., Ralston J., Smith C., Propert K.J., Rapp P.E., and Margulies S.S. (2013). Behavioral deficits and axonal injury persistence after rotational head injury are direction dependent. J. Neurotrauma 30, 538–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raghupathi R., Fernandez S.C., Murai H., Trusko S.P., Scott R.W., Nishioka W.K., and McIntosh T.K. (1998). BCL-2 overexpression attenuates cortical cell loss after traumatic brain injury in transgenic mice. J. Cereb. Blood Flow Metab. 18, 1259–1269 [DOI] [PubMed] [Google Scholar]
- 103.McIntosh T.K., Vink R., Noble L., Yamakami I., Fernyak S., Soares H., and Faden A.L. (1989). Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28, 233–244 [DOI] [PubMed] [Google Scholar]
- 104.Truelle J.-L., Koskinen S., Hawthorne G., Sarajuuri J., Formisano R., Von Wild K., Neugebauer E., Wilson L., Gibbons H., Powell J., Bullinger M., Höfer S., Maas A., Zitnay G., and Von Steinbuechel N. (2010). Quality of life after traumatic brain injury: the clinical use of the QOLIBRI, a novel disease-specific instrument. Brain Inj. 24, 1272–1291 [DOI] [PubMed] [Google Scholar]
- 105.Hamm R.J., Lyeth B.G., Jenkins L.W., O'Dell D.M., and Pike B.R. (1993). Selective cognitive impairment following traumatic brain injury in rats. Behav. Brain Res. 59, 169–173 [DOI] [PubMed] [Google Scholar]
- 106.Curzon P., Rustay N.R., and Browman K.E. (2009). Cued and Contextual Fear Conditioning for Rodents, in: Methods of Behavior Analysis in Neuroscience, 2nd ed. Buccafusco J.J. (ed). CRC: Boca Raton, FL: [PubMed] [Google Scholar]
- 107.Séguin J.R. (2009). The frontal lobe and aggression. Eur. J. Dev. Psychol. 6, 100–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chayer C., and Freedman M. (2001). Frontal lobe functions. Curr. Neurol. Neurosci. Rep. 1, 547–552 [DOI] [PubMed] [Google Scholar]
- 109.Hoover S., Zottoli T.M., and Grose-Fifer J. (2014). ERP correlates of malingered executive dysfunction. Int. J. Psychophysiol. 91, 139–146 [DOI] [PubMed] [Google Scholar]
- 110.Krpan K.M., Anderson N.D., and Stuss D.T. (2013). Obstacles to remediating coping following traumatic brain injury. NeuroRehabilitation 32, 721–728 [DOI] [PubMed] [Google Scholar]
- 111.Cook L.G., Chapman S.B., Elliott A.C., Evenson N.N., and Vinton K. (2014). Cognitive gains from gist reasoning training in adolescents with chronic-stage traumatic brain injury. Front. Neurol. 5, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bolzenius J.D., Roskos P.T., Salminen L.E., Paul R.H., and Bucholz R.D. (2014). Cognitive and self-reported psychological outcomes of blast-induced mild traumatic brain injury in veterans: a preliminary study. Appl. Neuropsychol. Adult 22, 79–87 [DOI] [PubMed] [Google Scholar]
- 113.Klein E., Caspi Y., and Gil S. (2003). The relation between memory of the traumatic event and PTSD: evidence from studies of traumatic brain injury. Can. J. Psychiatry 48, 28–33 [DOI] [PubMed] [Google Scholar]
- 114.King N.S. (2008). PTSD and traumatic brain injury: folklore and fact? Brain Inj. 22, 1–5 [DOI] [PubMed] [Google Scholar]
- 115.Ohry A., Rattok J., and Solomon Z. (1996). Post-traumatic stress disorder in brain injury patients. Brain Inj. 10, 687–696 [DOI] [PubMed] [Google Scholar]
- 116.Williams W.H., Evans J.J., Wilson B.A., and Needham P. (2002). Brief report: prevalence of post-traumatic stress disorder symptoms after severe traumatic brain injury in a representative community sample. Brain Inj. 16, 673–679 [DOI] [PubMed] [Google Scholar]
- 117.Glaesser J., Neuner F., Lütgehetmann R., Schmidt R., and Elbert T. (2004). Posttraumatic Stress Disorder in patients with traumatic brain injury. BMC Psychiatry 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Esler M., and Kaye D. (2000). Sympathetic nervous system activation in essential hypertension, cardiac failure and psychosomatic heart disease. J. Cardiovasc. Pharmacol. 35, S1–S7 [DOI] [PubMed] [Google Scholar]
- 119.Hoehn-Saric R., and McLeod D.R. (1988). The peripheral sympathetic nervous system. Its role in normal and pathologic anxiety. Psychiatr. Clin. North Am. 11, 375–386 [PubMed] [Google Scholar]
- 120.Genovese R.F., Simmons L.P., Ahlers S.T., Maudlin-Jeronimo E., Dave J.R., and Boutte A.M. (2013). Effects of mild TBI from repeated blast overpressure on the expression and extinction of conditioned fear in rats. Neuroscience 254, 120–129 [DOI] [PubMed] [Google Scholar]
- 121.Reger M.L., Poulos A.M., Buen F., Giza C.C., Hovda D.A., and Fanselow M.S. (2012). Concussive brain injury enhances fear learning and excitatory processes in the amygdala. Biol. Psychiatry 71, 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kwon S.-K., Kovesdi E., Gyorgy A.B., Wingo D., Kamnaksh A., Walker J., Long J.B., and Agoston D. V. (2011). Stress and traumatic brain injury: a behavioral, proteomics, and histological study. Front. Neurol. 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Elder G.A., Dorr N.P., De Gasperi R., Gama Sosa M.A., Shaughness M.C., Maudlin-Jeronimo E., Hall A.A., McCarron R.M., and Ahlers S.T. (2012). Blast exposure induces post-traumatic stress disorder-related traits in a rat model of mild traumatic brain injury. J. Neurotrauma 29, 2564–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sullivan H.G., Martinez J., Becker D.P., Miller J.D., Griffith R., and Wist A.O. (1976). Fluid-percussion model of mechanical brain injury in the cat. J. Neurosurg. 45, 521–534 [PubMed] [Google Scholar]
- 125.Dixon C., Lyeth B., Povlishock J., Findling R., Hamm R., Marmarou A., Young H., and Hayes R. (1987). A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 67, 110–119 [DOI] [PubMed] [Google Scholar]
- 126.Carbonell W.S., Maris D.O., McCall T., and Grady M.S. (1998). Adaptation of the fluid percussion injury model to the mouse. J. Neurotrauma 15, 217–229 [DOI] [PubMed] [Google Scholar]
- 127.Zink B.J., Walsh R.F., and Feustel P.J. (1993). Effects of ethanol in traumatic brain injury. J. Neurotrauma 10, 275–286 [DOI] [PubMed] [Google Scholar]
- 128.Stern S.A., Zink B.J., Mertz M., Wang X., and Dronen S.C. (2000). Effect of initially limited resuscitation in a combined model of fluid-percussion brain injury and severe uncontrolled hemorrhagic shock. J. Neurosurg. 93, 305–314 [DOI] [PubMed] [Google Scholar]
- 129.Muir K.W. (2006). Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr. Opin. Phamacol. 6, 53–60 [DOI] [PubMed] [Google Scholar]
- 130.Rau T.F., Kothiwal A.S., Rova A.R., Brooks D.M., and Poulsen D.J. (2012). Treatment with low-dose methamphetamine improves behavioral and cognitive function after severe traumatic brain injury. J. Trauma Acute Care Surg. 73, S165–S172 [DOI] [PubMed] [Google Scholar]
- 131.Kabadi S.V., Hilton G.D., Stoica B.A., Zapple D.N., and Faden A.I. (2010). Fluid-percussion-induced traumatic brain injury model in rats. Nat. Protoc. 5, 1552–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cernak I. (2005). Animal models of head trauma. NeuroRx 2, 410–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Long J.B., Gordon J., Bettencourt J.A., and Bolt S.L. (1996). Laser-Doppler flowmetry measurements of subcortical blood flow changes after fluid percussion brain injury in rats. J. Neurotrauma 13, 149–162 [DOI] [PubMed] [Google Scholar]
- 134.Graham D.I., Raghupathi R., Saatman K.E., Meaney D., and McIntosh T.K. (2000). Tissue tears in the white matter after lateral fluid percussion brain injury in the rat: relevance to human brain injury. Acta Neuropathol. 99, 117–124 [DOI] [PubMed] [Google Scholar]
- 135.Cortez S.C., McIntosh T.K., and Noble L.J. (1989). Experimental fluid percussion brain injury: vascular disruption and neuronal and glial alterations. Brain Res. 482, 271–282 [DOI] [PubMed] [Google Scholar]
- 136.Hayes R.L., Stalhammar D., Povlishock J.T., Allen A.M., Galinat B.J., Becker D.P., and Stonnington H.H. (1994). A new model of concussive brain injury in the cat produced by extradural fluid volume loading: II. Physiological and neuropathological observations. Brain Inj. 1, 93–112 [DOI] [PubMed] [Google Scholar]
- 137.Carbonell W.S., and Grady M.S. (1999). Regional and temporal characterization of neuronal, glial, and axonal response after traumatic brain injury in the mouse. Acta Neuropathol. 98, 396–406 [DOI] [PubMed] [Google Scholar]
- 138.Bramlett H.M., Dietrich W.D., Green E.J., and Busto R. (1997). Chronic histopathological consequences of fluid-percussion brain injury in rats: effects of post-traumatic hypothermia. Acta Neuropathol. 93, 190–199 [DOI] [PubMed] [Google Scholar]
- 139.Gurkoff G.G., Giza C.C., and Hovda D.A. (2006). Lateral fluid percussion injury in the developing rat causes an acute, mild behavioral dysfunction in the absence of significant cell death. Brain Res. 1077, 24–36 [DOI] [PubMed] [Google Scholar]
- 140.Kerr J.F., Wyllie A.H., and Currie A.R. (1972). Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Conti A.C., Raghupathi R., Trojanowski J.Q., and McIntosh T.K. (1998). Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period. J. Neurosci. 18, 5663–5672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Levine B., and Klionsky D.J. (2004). Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell 6, 463–477 [DOI] [PubMed] [Google Scholar]
- 143.Levine B., and Kroemer G. (2008). Autophagy in the pathogenesis of disease. Cell 132, 27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu C.L., Chen S., Dietrich D., and Hu B.R. (2008). Changes in autophagy after traumatic brain injury. J. Cereb. Blood Flow Metab. 28, 674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kroemer G., Galluzzi L., Vandenabeele P., Abrams J., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V., El-Deiry W.S., Golstein P., Green D.R., Hengartner M., Knight R.A., Kumar S., Lipton S.A., Malorni W., Nuñez G., Peter M.E., Tschopp J., Yuan J., Piacentini M., Zhivotovsky B., and Melino G. (2009). Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 16, 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Povlishock J.T., Becker D.P., Cheng C.L., and Vaughan G.W. (1983). Axonal change in minor head injury. J. Neuropathol. Exp. Neurol. 42, 225–242 [DOI] [PubMed] [Google Scholar]
- 147.Greer J.E., McGinn M.J., and Povlishock J.T. (2011). Diffuse traumatic axonal injury in the mouse induces atrophy, c-Jun activation, and axonal outgrowth in the axotomized neuronal population. J. Neurosci. 31, 5089–5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lifshitz J., Kelley B.J., and Povlishock J.T. (2007). Perisomatic thalamic axotomy after diffuse traumatic brain injury is associated with atrophy rather than cell death. J. Neuropathol. Exp. Neurol. 66, 218–229 [DOI] [PubMed] [Google Scholar]
- 149.Fujita M., Wei E.P., and Povlishock J.T. (2012). Intensity- and interval-specific repetitive traumatic brain injury can evoke both axonal and microvascular damage. J. Neurotrauma 29, 2172–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bailes J.E., and Mills J.D. (2010). Docosahexaenoic acid reduces traumatic axonal injury in a rodent head injury model. J. Neurotrauma 27, 1617–1624 [DOI] [PubMed] [Google Scholar]
- 151.Dietrich W.D., Truettner J., Zhao W., Alonso O.F., Busto R., and Ginsberg M.D. (1999). Sequential changes in glial fibrillary acidic protein and gene expression following parasagittal fluid-percussion brain injury in rats. J. Neurotrauma 16, 567–581 [DOI] [PubMed] [Google Scholar]
- 152.Spain A., Daumas S., Lifshitz J., Rhodes J., Andrews P.J., Horsburgh K., and Fowler J.H. (2010). Mild fluid percussion injury in mice produces evolving selective axonal pathology and cognitive deficits relevant to human brain injury. J. Neurotrauma 27, 1429–1438 [DOI] [PubMed] [Google Scholar]
- 153.Guo X., Liu L., Zhang M., Bergeron A., Cui Z., Dong J.-F., and Zhang J. (2009). Correlation of CD34+ cells with tissue angiogenesis after traumatic brain injury in a rat model. J. Neurotrauma 26, 1337–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Majka M., Janowska-Wieczorek A., Ratajczak J., Ehrenman K., Pietrzkowski Z., Kowalska M.A., Gewirtz A.M., Emerson S.G., and Ratajczak M.Z. (2001). Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood 97, 3075–3085 [DOI] [PubMed] [Google Scholar]
- 155.Yoshioka T., Ageyama N., Shibata H., Yasu T., Misawa Y., Takeuchi K., Matsui K., Yamamoto K., Terao K., Shimada K., Ikeda U., Ozawa K., and Hanazono Y. (2005). Repair of infarcted myocardium mediated by transplanted bone marrow-derived CD34+ stem cells in a nonhuman primate model. Stem Cells 23, 355–364 [DOI] [PubMed] [Google Scholar]
- 156.Mackie A.R., and Losordo D.W. (2011). CD34-positive stem cells: in the treatment of heart and vascular disease in human beings. Tex. Heart Inst. J. 38, 474–485 [PMC free article] [PubMed] [Google Scholar]
- 157.Zheng W., ZhuGe Q., Zhong M., Chen G., Shao B., Wang H., Mao X., Xie L., and Jin K. (2013). Neurogenesis in adult human brain after traumatic brain injury. J. Neurotrauma 30, 1872–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hoane M.R. (2005). Treatment with magnesium improves reference memory but not working memory while reducing GFAP expression following traumatic brain injury. Restor. Neurol. Neurosci. 23, 67–77 [PubMed] [Google Scholar]
- 159.Emery D.L., Fulp C.T., Saatman K.E., Schütz C., Neugebauer E., and McIntosh T.K. (2005). Newly born granule cells in the dentate gyrus rapidly extend axons into the hippocampal CA3 region following experimental brain injury. J. Neurotrauma 22, 978–988 [DOI] [PubMed] [Google Scholar]
- 160.Taupin P. (2007). BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res. Rev. 53, 198–214 [DOI] [PubMed] [Google Scholar]
- 161.Kee N., Sivalingam S., Boonstra R., and Wojtowicz J.M. (2002). The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J. Neurosci. Methods 115, 97–105 [DOI] [PubMed] [Google Scholar]
- 162.Sun D., McGinn M.J., Zhou Z., Harvey H.B., Bullock M.R., and Colello R.J. (2007). Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp. Neurol. 204, 264–272 [DOI] [PubMed] [Google Scholar]
- 163.Cernak I., Vink R., Zapple D.N., Cruz M.I., Ahmed F., Chang T., Fricke S.T., and Faden A.I. (2004). The pathobiology of moderate diffuse traumatic brain injury as identified using a new experimental model of injury in rats. Neurobiol. Dis. 17, 29–43 [DOI] [PubMed] [Google Scholar]
- 164.Hilton G.D., Stoica B.A., Byrnes K.R., and Faden A.I. (2008). Roscovitine reduces neuronal loss, glial activation, and neurologic deficits after brain trauma. J. Cereb. Blood Flow Metab. 28, 1845–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Thompson H.J., Lifshitz J., Marklund N., Grady M.S., Graham D.I., Hovda D.A., and McIntosh T.K. (2005). Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma 22, 42–75 [DOI] [PubMed] [Google Scholar]
- 166.Keck C.A., Thompson H.J., Pitkänen A., LeBold D.G., Morales D.M., Plevy J.B., Puri R., Zhao B., Dichter M., and McIntosh T.K. (2007). The novel antiepileptic agent RWJ-333369-A, but not its analog RWJ-333369, reduces regional cerebral edema without affecting neurobehavioral outcome or cell death following experimental traumatic brain injury. Restor. Neurol. Neurosci. 25, 77–90 [PMC free article] [PubMed] [Google Scholar]
- 167.Lyeth B.G., Gong Q.Z., Shields S., Muizelaar J.P., and Berman R.F. (2001). Group I metabotropic glutamate antagonist reduces acute neuronal degeneration and behavioral deficits after traumatic brain injury in rats. Exp. Neurol. 169, 191–199 [DOI] [PubMed] [Google Scholar]
- 168.Hoover R.C., Motta M., Davis J., Saatman K.E., Fujimoto S.T., Thompson H.J., Stover J.F., Dichter M.A., Twyman R., White H.S., and McIntosh T.K. (2004). Differential effects of the anticonvulsant topiramate on neurobehavioral and histological outcomes following traumatic brain injury in rats. J. Neurotrauma 21, 501–512 [DOI] [PubMed] [Google Scholar]
- 169.Smith D.C., Modglin A.A., Roosevelt R.W., Neese S.L., Jensen R.A., Browning R.A., and Clough R.W. (2005). Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J. Neurotrauma 22, 1485–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hallam T.M., Floyd C.L., Folkerts M.M., Lee L.L., Gong Q.-Z., Lyeth B.G., Muizelaar J.P., and Berman R.F. (2004). Comparison of behavioral deficits and acute neuronal degeneration in rat lateral fluid percussion and weight-drop brain injury models. J. Neurotrauma 21, 521–539 [DOI] [PubMed] [Google Scholar]
- 171.Lee L.L., Galo E., Lyeth B.G., Muizelaar J.P., and Berman R.F. (2004). Neuroprotection in the rat lateral fluid percussion model of traumatic brain injury by SNX-185, an N-type voltage-gated calcium channel blocker. Exp. Neurol. 190, 70–78 [DOI] [PubMed] [Google Scholar]
- 172.Bao F., Shultz S.R., Hepburn J.D., Omana V., Weaver L.C., Cain D.P., and Brown A. (2012). A CD11d monoclonal antibody treatment reduces tissue injury and improves neurological outcome after fluid percussion brain injury in rats. J. Neurotrauma 29, 2375–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Riess P., Bareyre F.M., Saatman K.E., Cheney J.A., Lifshitz J., Raghupathi R., Grady M.S., Neugebauer E., and McIntosh T.K. (2001). Effects of chronic, post-injury Cyclosporin A administration on motor and sensorimotor function following severe, experimental traumatic brain injury. Restor. Neurol. Neurosci. 18, 1–8 [PubMed] [Google Scholar]
- 174.Carballosa Gonzalez M.M., Blaya M.O., Alonso O.F., Bramlett H.M., and Hentall I.D. (2013). Midbrain raphe stimulation improves behavioral and anatomical recovery from fluid-percussion brain injury. J. Neurotrauma 30, 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hayward N.M.E.A., Immonen R., Tuunanen P.I., Ndode-Ekane X.E., Gröhn O., and Pitkänen A. (2010). Association of chronic vascular changes with functional outcome after traumatic brain injury in rats. J. Neurotrauma 27, 2203–2219 [DOI] [PubMed] [Google Scholar]
- 176.Marklund N., Bareyre F.M., Royo N.C., Thompson H.J., Mir A.K., Grady M.S., Schwab M.E., and McIntosh T.K. (2007). Cognitive outcome following brain injury and treatment with an inhibitor of Nogo-A in association with an attenuated downregulation of hippocampal growth-associated protein-43 expression. J. Neurosurg. 107, 844–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schütz C., Stover J.F., Thompson H.J., Hoover R.C., Morales D.M., Schouten J.W., McMillan A., Soltesz K., Motta M., Spangler Z., Neugebauer E., and McIntosh T.K. (2006). Acute, transient hemorrhagic hypotension does not aggravate structural damage or neurologic motor deficits but delays the long-term cognitive recovery following mild to moderate traumatic brain injury. Crit. Care Med. 34, 492–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lenzlinger P.M., Shimizu S., Marklund N., Thompson H.J., Schwab M.E., Saatman K.E., Hoover R.C., Bareyre F.M., Motta M., Luginbuhl A., Pape R., Clouse A.K., Morganti-Kossmann C., and McIntosh T.K. (2005). Delayed inhibition of Nogo-A does not alter injury-induced axonal sprouting but enhances recovery of cognitive function following experimental traumatic brain injury in rats. Neuroscience 134, 1047–1056 [DOI] [PubMed] [Google Scholar]
- 179.Shultz S.R., Bao F., Omana V., Chiu C., Brown A., and Cain D.P. (2012). Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. J. Neurotrauma 29, 281–294 [DOI] [PubMed] [Google Scholar]
- 180.Passineau M.J., Green E.J., and Dietrich W.D. (2001). Therapeutic effects of environmental enrichment on cognitive function and tissue integrity following severe traumatic brain injury in rats. Exp. Neurol. 168, 373–384 [DOI] [PubMed] [Google Scholar]
- 181.Shultz S.R., Bao F., Weaver L.C., Cain D.P., and Brown A. (2013). Treatment with an anti-CD11d integrin antibody reduces neuroinflammation and improves outcome in a rat model of repeated concussion. J. Neuroinflammation 10, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hamm R.J., Pike B.R., Temple M.D., O'Dell D.M., and Lyeth B.G. (1995). The effect of postinjury kindled seizures on cognitive performance of traumatically brain-injured rats. Exp. Neurol. 136, 143–148 [DOI] [PubMed] [Google Scholar]
- 183.Sanders M.J., Dietrich W.D., and Green E.J. (1999). Cognitive function following traumatic brain injury: effects of injury severity and recovery period in a parasagittal fluid-percussive injury model. J. Neurotrauma 16, 915–925 [DOI] [PubMed] [Google Scholar]
- 184.Schmidt R.H., Scholten K.J., and Maughan P.H. (1999). Time course for recovery of water maze performance and central cholinergic innervation after fluid percussion injury. J. Neurotrauma 16, 1139–1147 [DOI] [PubMed] [Google Scholar]
- 185.Sinson G., Perri B.R., Trojanowski J.Q., Flamm E.S., and McIntosh T.K. (1997). Improvement of cognitive deficits and decreased cholinergic neuronal cell loss and apoptotic cell death following neurotrophin infusion after experimental traumatic brain injury. J. Neurosurg. 86, 511–518 [DOI] [PubMed] [Google Scholar]
- 186.Thompson H.J., LeBold D.G., Marklund N., Morales D.M., Hagner A.P., and McIntosh T.K. (2006). Cognitive evaluation of traumatically brain-injured rats using serial testing in the Morris water maze. Restor. Neurol. Neurosci. 24, 109–114 [PMC free article] [PubMed] [Google Scholar]
- 187.Immonen R.J., Kharatishvili I., Gröhn H., Pitkänen A., and Gröhn O.H.J. (2009). Quantitative MRI predicts long-term structural and functional outcome after experimental traumatic brain injury. Neuroimage 45, 1–9 [DOI] [PubMed] [Google Scholar]
- 188.Yamaki T., Murakami N., Iwamoto Y., Sakakibara T., Kobori N., Ueda S., Kikuchi T., and Uwahodo Y. (1997). Evaluation of learning and memory dysfunction and histological findings in rats with chronic stage contusion and diffuse axonal injury. Brain Res. 752, 151–160 [DOI] [PubMed] [Google Scholar]
- 189.Yamaki T., Murakami N., Iwamoto Y., Sakakibara T., Kobori N., Ueda S., Uwahodo Y., and Kikuchi T. (1998). Cognitive dysfunction and histological findings in rats with chronic-stage contusion and diffuse axonal injury. Brain Res. Brain Res. Protoc. 3, 100–106 [DOI] [PubMed] [Google Scholar]
- 190.Barnes C.A. (1979). Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 93, 74–104 [DOI] [PubMed] [Google Scholar]
- 191.Lima F.D., Souza M.A., Furian A.F., Rambo L.M., Ribeiro L.R., Martignoni F.V., Hoffmann M.S., Fighera M.R., Royes L.F., Oliveira M.S., and de Mello C.F. (2008). Na+,K+-ATPase activity impairment after experimental traumatic brain injury: relationship to spatial learning deficits and oxidative stress. Behav. Brain Res. 193, 306–310 [DOI] [PubMed] [Google Scholar]
- 192.Lighthall J.W. (1988). Controlled cortical impact: a new experimental brain injury model. J. Neurotrauma 5, 1–15 [DOI] [PubMed] [Google Scholar]
- 193.Hannay H.J., Feldman Z., Phan P., Keyani A., Panwar N., Goodman J.C., and Robertson C.S. (1999). Validation of a controlled cortical impact model of head injury in mice. J. Neurotrauma 16, 1103–1114 [DOI] [PubMed] [Google Scholar]
- 194.Xiong Y., Mahmood A., and Chopp M. (2013). Animal models of traumatic brain injury. Nat. Rev. Neurosci. 14, 128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Donovan V., Kim C., Anugerah A.K., Coats J.S., Oyoyo U., Pardo A.C., and Obenaus A. (2014). Repeated mild traumatic brain injury results in long-term white-matter disruption. J. Cereb. Blood Flow Metab. 34, 715–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fox G.B., Fan L., LeVasseur R.A., and Faden A.I. (1998). Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J. Neurotrauma 15, 599–614 [DOI] [PubMed] [Google Scholar]
- 197.Zhou H., Chen L., Gao X., Luo B., and Chen J. (2012). Moderate traumatic brain injury triggers rapid necrotic death of immature neurons in the hippocampus. J. Neuropathol. Exp. Neurol. 71, 348–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Colicos M.A., Dixon C.E., and Dash P.K. (1996). Delayed, selective neuronal death following experimental cortical impact injury in rats: possible role in memory deficits. Brain Res. 739, 111–119 [DOI] [PubMed] [Google Scholar]
- 199.Patel A.D., Gerzanich V., Geng Z., and Simard J.M. (2010). Glibenclamide reduces hippocampal injury and preserves rapid spatial learning in a model of traumatic brain injury. J. Neuropathol. Exp. Neurol. 69, 1177–1190 [DOI] [PubMed] [Google Scholar]
- 200.Knoblach S.M., Alroy D.A., Nikolaeva M., Cernak I., Stoica B.A., and Faden A.I. (2004). Caspase inhibitor z-DEVD-fmk attenuates calpain and necrotic cell death in vitro and after traumatic brain injury. J. Cereb. Blood Flow Metab. 24, 1119–1132 [DOI] [PubMed] [Google Scholar]
- 201.Chen S.-F., Richards H.K., Smielewski P., Johnström P., Salvador R., Pickard J.D., and Harris N.G. (2004). Relationship between flow-metabolism uncoupling and evolving axonal injury after experimental traumatic brain injury. J. Cereb. Blood Flow Metab. 24, 1025–1036 [DOI] [PubMed] [Google Scholar]
- 202.Hall E.D., Sullivan P.G., Gibson T.R., Pavel K.M., Thompson B.M., and Scheff S.W. (2005). Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J. Neurotrauma 22, 252–265 [DOI] [PubMed] [Google Scholar]
- 203.Glushakova O.Y., Johnson D., and Hayes R.L. (2014). Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood-brain barrier disruption, and progressive white matter damage. J. Neurotrauma 31, 1180–1193 [DOI] [PubMed] [Google Scholar]
- 204.Strauss K.I., Barbe M.F., Marshall R.M., Raghupathi R., Mehta S., and Narayan R.K. (2000). Prolonged cyclooxygenase-2 induction in neurons and glia following traumatic brain injury in the rat. J. Neurotrauma 17, 695–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Briones T.L., Woods J., and Rogozinska M. (2013). Decreased neuroinflammation and increased brain energy homeostasis following environmental enrichment after mild traumatic brain injury is associated with improvement in cognitive function. Acta Neuropathol. Commun. 1, 57. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 206.Carlson S.W., Madathil S.K., Sama D.M., Gao X., Chen J., and Saatman K.E. (2014). Conditional overexpression of insulin-like growth factor-1 enhances hippocampal neurogenesis and restores immature neuron dendritic processes after traumatic brain injury. J. Neuropathol. Exp. Neurol. 73, 734–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yu T.-S., Zhang G., Liebl D.J., and Kernie S.G. (2008). Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J. Neurosci. 28, 12901–12912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dash P.K., Mach S.A., and Moore A.N. (2001). Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J. Neurosci. Res. 63, 313–319 [DOI] [PubMed] [Google Scholar]
- 209.Gao X., Enikolopov G., and Chen J. (2009). Moderate traumatic brain injury promotes proliferation of quiescent neural progenitors in the adult hippocampus. Exp. Neurol. 219, 516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cai W., Carlson S.W., Brelsfoard J.M., Mannon C.E., Moncman C.L., Saatman K.E., and Andres D.A. (2012). Rit GTPase signaling promotes immature hippocampal neuronal survival. J. Neurosci. 32, 9887–9897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Longhi L., Watson D.J., Saatman K.E., Thompson H.J., Zhang C., Fujimoto S., Royo N., Castelbuono D., Raghupathi R., Trojanowski J.Q., Lee V.M., Wolfe J.H., Stocchetti N., and McIntosh T.K. (2004). Ex vivo gene therapy using targeted engraftment of NGF-expressing human NT2N neurons attenuates cognitive deficits following traumatic brain injury in mice. J. Neurotrauma 21, 1723–1736 [DOI] [PubMed] [Google Scholar]
- 212.Longhi L., Gesuete R., Perego C., Ortolano F., Sacchi N., Villa P., Stocchetti N., and De Simoni M.-G. (2011). Long-lasting protection in brain trauma by endotoxin preconditioning. J. Cereb. Blood Flow Metab. 31, 1919–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Xiong Y., Mahmood A., Zhang Y., Meng Y., Zhang Z.G., Qu C., Sager T.N., and Chopp M. (2011). Effects of posttraumatic carbamylated erythropoietin therapy on reducing lesion volume and hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome in rats following traumatic brain injury. J. Neurosurg. 114, 549–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Meng Y., Xiong Y., Mahmood A., Zhang Y., Qu C., and Chopp M. (2011). Dose-dependent neurorestorative effects of delayed treatment of traumatic brain injury with recombinant human erythropoietin in rats. J. Neurosurg. 115, 550–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cheng J.P., Shaw K.E., Monaco C.M., Hoffman A.N., Sozda C.N., Olsen A.S., and Kline A.E. (2012). A relatively brief exposure to environmental enrichment after experimental traumatic brain injury confers long-term cognitive benefits. J. Neurotrauma 29, 2684–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fox G.B., and Faden A.I. (1998). Traumatic brain injury causes delayed motor and cognitive impairment in a mutant mouse strain known to exhibit delayed Wallerian degeneration. J. Neurosci. Res. 53, 718–727 [DOI] [PubMed] [Google Scholar]
- 217.Fox G.B., Fan L., LeVasseur R.A., and Faden A.I. (1998). Effect of traumatic brain injury on mouse spatial and nonspatial learning in the Barnes circular maze. J. Neurotrauma 15, 1037–1046 [DOI] [PubMed] [Google Scholar]
- 218.Lam T.I., Bingham D., Chang T.J., Lee C.C., Shi J., Wang D., Massa S., Swanson R.A., and Liu J. (2013). Beneficial effects of minocycline and botulinum toxin-induced constraint physical therapy following experimental traumatic brain injury. Neurorehabil. Neural Repair 27, 889–899 [DOI] [PubMed] [Google Scholar]
- 219.d'Avila J.C., Lam T.I., Bingham D., Shi J., Won S.J., Kauppinen T.M., Massa S., Liu J., and Swanson R.A. (2012). Microglial activation induced by brain trauma is suppressed by post-injury treatment with a PARP inhibitor. J. Neuroinflammation 9, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hånell A., Clausen F., Björk M., Jansson K., Philipson O., Nilsson L.N.G., Hillered L., Weinreb P.H., Lee D., McIntosh T.K., Gimbel D.A., Strittmatter S.M., and Marklund N. (2010). Genetic deletion and pharmacological inhibition of Nogo-66 receptor impairs cognitive outcome after traumatic brain injury in mice. J. Neurotrauma 27, 1297–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shear D.A., Tate C.C., Tate M.C., Archer D.R., LaPlaca M.C., Stein D.G., and Dunbar G.L. (2011). Stem cell survival and functional outcome after traumatic brain injury is dependent on transplant timing and location. Restor. Neurol. Neurosci. 29, 215–225 [DOI] [PubMed] [Google Scholar]
- 222.Lindner M.D., Plone M.A., Cain C.K., Frydel B., Francis J.M., Emerich D.F., and Sutton R.L. (1998). Dissociable long-term cognitive deficits after frontal versus sensorimotor cortical contusions. J. Neurotrauma 15, 199–216 [DOI] [PubMed] [Google Scholar]
- 223.Baskin Y.K., Dietrich W.D., and Green E.J. (2003). Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J. Neurosci. Methods 129, 87–93 [DOI] [PubMed] [Google Scholar]
- 224.Hoffman S.W., Fülöp Z., and Stein D.G. (1994). Bilateral frontal cortical contusion in rats: behavioral and anatomic consequences. J. Neurotrauma 11, 417–431 [DOI] [PubMed] [Google Scholar]
- 225.Xiong Y., Zhang Y., Mahmood A., Meng Y., Zhang Z.G., Morris D.C., and Chopp M. (2012). Neuroprotective and neurorestorative effects of thymosin β4 treatment initiated 6 hours after traumatic brain injury in rats. J. Neurosurg. 116, 1081–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dixon C.E., Hamm R.J., Taft W.C., and Hayes R.L. (1994). Increased anticholinergic sensitivity following closed skull impact and controlled cortical impact traumatic brain injury in the rat. J. Neurotrauma 11, 275–287 [DOI] [PubMed] [Google Scholar]
- 227.Chauhan N.B., and Gatto R. (2011). Restoration of cognitive deficits after statin feeding in TBI. Restor. Neurol. Neurosci. 29, 23–34 [DOI] [PubMed] [Google Scholar]
- 228.Byrnes K.R., Loane D.J., Stoica B.A., Zhang J., and Faden A.I. (2012). Delayed mGluR5 activation limits neuroinflammation and neurodegeneration after traumatic brain injury. J. Neuroinflammation 9, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhang Y., Chopp M., Mahmood A., Meng Y., Qu C., and Xiong Y. (2012). Impact of inhibition of erythropoietin treatment-mediated neurogenesis in the dentate gyrus of the hippocampus on restoration of spatial learning after traumatic brain injury. Exp. Neurol. 235, 336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tomasevic G., Laurer H.L., Mattiasson G., van Steeg H., Wieloch T., and McIntosh T.K. (2012). Delayed neuromotor recovery and increased memory acquisition dysfunction following experimental brain trauma in mice lacking the DNA repair gene XPA. J. Neurosurg. 116, 1368–1378 [DOI] [PubMed] [Google Scholar]
- 231.Xiong Y., Zhang Y., Mahmood A., Meng Y., Qu C., and Chopp M. (2011). Erythropoietin mediates neurobehavioral recovery and neurovascular remodeling following traumatic brain injury in rats by increasing expression of vascular endothelial growth factor. Transl. Stroke Res. 2, 619–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Han R.-Z., Hu J.-J., Weng Y.-C., Li D.-F., and Huang Y. (2009). NMDA receptor antagonist MK-801 reduces neuronal damage and preserves learning and memory in a rat model of traumatic brain injury. Neurosci. Bull. 25, 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chauhan N.B., and Gatto R. (2010). Synergistic benefits of erythropoietin and simvastatin after traumatic brain injury. Brain Res. 1360, 177–192 [DOI] [PubMed] [Google Scholar]
- 234.Fox G.B., LeVasseur R.A., and Faden A.I. (1999). Behavioral responses of C57BL/6, FVB/N, and 129/SvEMS mouse strains to traumatic brain injury: implications for gene targeting approaches to neurotrauma. J. Neurotrauma 16, 377–389 [DOI] [PubMed] [Google Scholar]
- 235.Sinz E.H., Kochanek P.M., Dixon C.E., Clark R.S., Carcillo J.A., Schiding J.K., Chen M., Wisniewski S.R., Carlos T.M., Williams D., DeKosky S.T., Watkins S.C., Marion D.W., and Billiar T.R. (1999). Inducible nitric oxide synthase is an endogenous neuroprotectant after traumatic brain injury in rats and mice. J. Clin. Invest. 104, 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhao Z., Loane D.J., Murray M.G., Stoica B.A., and Faden A.I. (2012). Comparing the predictive value of multiple cognitive, affective, and motor tasks after rodent traumatic brain injury. J. Neurotrauma 29, 2475–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Piao C.-S., Stoica B.A., Wu J., Sabirzhanov B., Zhao Z., Cabatbat R., Loane D.J., and Faden A.I. (2013). Late exercise reduces neuroinflammation and cognitive dysfunction after traumatic brain injury. Neurobiol. Dis. 54, 252–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Bondi C.O., Rodriguez G., Gould G.G., Frazer A., and Morilak D.A. (2008). Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 33, 320–331 [DOI] [PubMed] [Google Scholar]
- 239.Birrell J.M., and Brown V.J. (2000). Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 20, 4320–4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lapiz M.D., and Morilak D.A. (2006). Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience 137, 1039–1049 [DOI] [PubMed] [Google Scholar]
- 241.Cao A., Yu L., Wang Y., Wang J., Yang L., and Lei G.-F. (2012). Effects of methylphenidate on attentional set-shifting in a genetic model of attention-deficit/hyperactivity disorder. Behav. Brain Funct. 8, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tait D.S., Brown V.J., Farovik A., Theobald D.E., Dalley J.W., and Robbins T.W. (2007). Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur. J. Neurosci. 25, 3719–24 [DOI] [PubMed] [Google Scholar]
- 243.Young J.W., Powell S.B., Geyer M.A., Jeste D. V, and Risbrough V.B. (2010). The mouse attentional-set-shifting task: a method for assaying successful cognitive aging? Cogn. Affect. Behav. Neurosci. 10, 243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bondi C.O., Jett J.D., and Morilak D.A. (2010). Beneficial effects of desipramine on cognitive function of chronically stressed rats are mediated by alpha1-adrenergic receptors in medial prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 913–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Liston C., Miller M.M., Goldwater D.S., Radley J.J., Rocher A.B., Hof P.R., Morrison J.H., and McEwen B.S. (2006). Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 26, 7870–7874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bondi C.O., Cheng J.P., Tennant H.M., Monaco C.M., and Kline A.E. (2014). Old dog, new tricks: the attentional set-shifting test as a novel cognitive behavioral task after controlled cortical impact injury. J. Neurotrauma 31, 926–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Murray C.K., Reynolds J.C., Schroeder J.M., Harrison M.B., Evans O.M., and Hospenthal D.R. (2005). Spectrum of care provided at an echelon II Medical Unit during Operation Iraqi Freedom. Mil. Med. 170, 516–520 [DOI] [PubMed] [Google Scholar]
- 248.Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., and Castro C.A. (2008). Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463 [DOI] [PubMed] [Google Scholar]
- 249.Kobeissy F., Mondello S., Tümer N., Toklu H.Z., Whidden M.A., Kirichenko N., Zhang Z., Prima V., Yassin W., Anagli J., Chandra N., Svetlov S., and Wang K.K. (2013). Assessing neuro-systemic & behavioral components in the pathophysiology of blast-related brain injury. Front. Neurol. 4, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Cernak I., Wang Z., Jiang J., Bian X., and Savic J. (2001). Ultrastructural and functional characteristics of blast injury-induced neurotrauma. J. Trauma 50, 695–706 [DOI] [PubMed] [Google Scholar]
- 251.Säljö A., Bao F., Haglid K.G., and Hansson H.A. (2000). Blast exposure causes redistribution of phosphorylated neurofilament subunits in neurons of the adult rat brain. J. Neurotrauma 17, 719–726 [DOI] [PubMed] [Google Scholar]
- 252.Rafaels K., Bass C.R., Salzar R.S., Panzer M.B., Woods W., Feldman S., Cummings T., and Capehart B. (2011). Survival risk assessment for primary blast exposures to the head. J. Neurotrauma 28, 2319–2328 [DOI] [PubMed] [Google Scholar]
- 253.Rafaels K.A., Bass C.R., Panzer M.B., Salzar R.S., Woods W.A., Feldman S.H., Walilko T., Kent R.W., Capehart B.P., Foster J.B., Derkunt B., and Toman A. (2012). Brain injury risk from primary blast. J. Trauma Acute Care Surg. 73, 895–901 [DOI] [PubMed] [Google Scholar]
- 254.Bauman R.A., Ling G., Tong L., Januszkiewicz A., Agoston D.D., Delanerolle N., Kim Y., Ritzel D., Bell R., Ecklund J., Armonda R., Bandak F., and Parks S. (2009). An introductory characterization of a combat-casualty-care relevant swine model of closed head injury resulting from exposure to explosive blast. J. Neurotrauma 26, 841–860 [DOI] [PubMed] [Google Scholar]
- 255.Turner R.C., Naser Z.J., Logsdon A.F., DiPasquale K.H., Jackson G.J., Robson M.J., Gettens R.T., Matsumoto R.R., Huber J.D., and Rosen C.L. (2013). Modeling clinically relevant blast parameters based on scaling principles produces functional & histological deficits in rats. Exp. Neurol. 248, 520–529 [DOI] [PubMed] [Google Scholar]
- 256.Zhang J., Pintar F.A., Yoganandan N., Gennarelli T.A., and Son S.F. (2009). Experimental study of blast-induced traumatic brain injury using a physical head model. Stapp Car Crash J. 53, 215–227 [DOI] [PubMed] [Google Scholar]
- 257.Risling M., and Davidsson J. (2012). Experimental animal models for studies on the mechanisms of blast-induced neurotrauma. Front. Neurol. 3, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Rigby P., Wong J., Juhas B., Eslami P., Rapo M., and Baumer T. (2011). Using helmet sensors in predicting head kinematics, in: A Survey of Blast Injury across the Full Landscape of Military Science. NATO HFM-207: Halifax, Nova Scotia, Canada [Google Scholar]
- 259.Cernak I., and Noble-Haeusslein L.J. (2010). Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J. Cereb. Blood Flow Metab. 30, 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Meythaler J.M., Peduzzi J.D., Eleftheriou E., and Novack T.A. (2001). Current concepts: diffuse axonal injury–associated traumatic brain injury. Arch. Phys. Med. Rehabil. 82, 1461–1471 [DOI] [PubMed] [Google Scholar]
- 261.Povlishock J.T., and Katz D.I. (2005). Update of neuropathology and neurological recovery after traumatic brain injury. J. Head Trauma Rehabil. 20, 76–94 [DOI] [PubMed] [Google Scholar]
- 262.Garman R.H., Jenkins L.W., Switzer R.C., Bauman R.A., Tong L.C., Swauger P. V, Parks S.A., Ritzel D. V, Dixon C.E., Clark R.S.B., Bayir H., Kagan V., Jackson E.K., and Kochanek P.M. (2011). Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. J. Neurotrauma 28, 947–959 [DOI] [PubMed] [Google Scholar]
- 263.De Lanerolle N.C., Bandak F., Kang D., Li A.Y., Du F., Swauger P., Parks S., Ling G., and Kim J.H. (2011). Characteristics of an explosive blast-induced brain injury in an experimental model. J. Neuropathol. Exp. Neurol. 70, 1046–1057 [DOI] [PubMed] [Google Scholar]
- 264.Takeuchi S., Nawashiro H., Sato S., Kawauchi S., Nagatani K., Kobayashi H., Otani N., Osada H., Wada K., and Shima K. (2013). A better mild traumatic brain injury model in the rat. Acta Neurochir. Suppl. 118, 99–101 [DOI] [PubMed] [Google Scholar]
- 265.Lu J., Ng K.C., Ling G., Wu J., Poon D.J., Kan E.M., Tan M.H., Wu Y.J., Li P., Moochhala S., Yap E., Lee L.K., Teo M., Yeh I.B., Sergio D.M., Chua F., Kumar S.D., and Ling E.-A. (2012). Effect of blast exposure on the brain structure and cognition in Macaca fascicularis. J. Neurotrauma 29, 1434–1454 [DOI] [PubMed] [Google Scholar]
- 266.DeWitt D.S., and Prough D.S. (2009). Blast-induced brain injury and posttraumatic hypotension and hypoxemia. J Neurotrauma 26, 877–887 [DOI] [PubMed] [Google Scholar]
- 267.Gama Sosa M.A., De Gasperi R., Janssen P.L., Yuk F.J., Anazodo P.C., Pricop P.E., Paulino A.J., Wicinski B., Shaughness M.C., Maudlin-Jeronimo E., Hall A.A., Dickstein D.L., McCarron R.M., Chavko M., Hof P.R., Ahlers S.T., and Elder G.A. (2014). Selective vulnerability of the cerebral vasculature to blast injury in a rat model of mild traumatic brain injury. Acta Neuropathol. Commun. 2, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kovesdi E., Gyorgy A.B., Kwon S.-K., Wingo D.L., Kamnaksh A., Long J.B., Kasper C.E., and Agoston D.V. (2011). The effect of enriched environment on the outcome of traumatic brain injury; a behavioral, proteomics, and histological study. Front. Neurosci. 5, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Rubovitch V., Ten-Bosch M., Zohar O., Harrison C.R., Tempel-Brami C., Stein E., Hoffer B.J., Balaban C.D., Schreiber S., Chiu W.-T., and Pick C.G. (2011). A mouse model of blast-induced mild traumatic brain injury. Exp. Neurol. 232, 280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Ahmed F., Gyorgy A., Kamnaksh A., Ling G., Tong L., Parks S., and Agoston D. (2012). Time-dependent changes of protein biomarker levels in the cerebrospinal fluid after blast traumatic brain injury. Electrophoresis 33, 3705–3711 [DOI] [PubMed] [Google Scholar]
- 271.Cernak I., Merkle A.C., Koliatsos V.E., Bilik J.M., Luong Q.T., Mahota T.M., Xu L., Slack N., Windle D., and Ahmed F.A. (2011). The pathobiology of blast injuries and blast-induced neurotrauma as identified using a new experimental model of injury in mice. Neurobiol. Dis. 41, 538–551 [DOI] [PubMed] [Google Scholar]
- 272.Cho H.J., Sajja V.S., Vandevord P.J., and Lee Y.W. (2013). Blast induces oxidative stress, inflammation, neuronal loss and subsequent short-term memory impairment in rats. Neuroscience 253, 9–20 [DOI] [PubMed] [Google Scholar]
- 273.Graner J., Oakes T.R., French L.M., and Riedy G. (2013). Functional MRI in the investigation of blast-related traumatic brain injury. Front. Neurol. 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Tanielian T., and Jaycox L.H. (eds). (2008). Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. RAND Corporation: Santa Monica, CA [Google Scholar]
- 275.Elder G.A., Mitsis E.M., Ahlers S.T., and Cristian A. (2010). Blast-induced mild traumatic brain injury. Psychiatr. Clin. North Am. 33, 757–781 [DOI] [PubMed] [Google Scholar]
- 276.Kovesdi E., Kamnaksh A., Wingo D., Ahmed F., Grunberg N.E., Long J.B., Kasper C.E., and Agoston D.V. (2012). Acute minocycline treatment mitigates the symptoms of mild blast-induced traumatic brain injury. Front. Neurol. 3, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Marmarou A., Foda M.A., van den Brink W., Campbell J., Kita H., and Demetriadou K. (1994). A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J. Neurosurg. 80, 291–300 [DOI] [PubMed] [Google Scholar]
- 278.Xiong Y., Mahmood A., and Chopp M. (2013). Animal models of traumatic brain injury. Nat. Rev. Neurosci. 14, 128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Feeney D.M., Boyeson M.G., Linn R.T., Murray H.M., and Dail W.G. (1981). Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 211, 67–77 [DOI] [PubMed] [Google Scholar]
- 280.Ellingson B.M., Fijalkowski R.J., Pintar F.A., Yoganandan N., and Gennarelli T.A. (2005). New mechanism for inducing closed head injury in the rat. Biomed. Sci. Instrum. 41, 86–91 [PubMed] [Google Scholar]
- 281.Leung L.Y., Larimore Z., Holmes L., Cartagena C., Mountney A., Deng-Bryant Y., Schmid K., Shear D., and Tortella F. (2014). The WRAIR projectile concussive impact model of mild traumatic brain injury: re-design, testing and preclinical validation. Ann. Biomed. Eng. 42, 1618–1630 [DOI] [PubMed] [Google Scholar]
- 282.Mannix R., Meehan W.P., Mandeville J., Grant P.E., Gray T., Berglass J., Zhang J., Bryant J., Rezaie S., Chung J.Y., Peters N.V., Lee C., Tien L.W., Kaplan D.L., Feany M., and Whalen M. (2013). Clinical correlates in an experimental model of repetitive mild brain injury. Ann. Neurol. 74, 65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Shitaka Y., Tran H.T., Bennett R.E., Sanchez L., Levy M.A., Dikranian K., and Brody D.L. (2011). Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J. Neuropathol. Exp. Neurol. 70, 551–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Thau-Zuchman O., Shohami E., Alexandrovich A.G., and Leker R.R. (2012). Combination of vascular endothelial and fibroblast growth factor 2 for induction of neurogenesis and angiogenesis after traumatic brain injury. J. Mol. Neurosci. 47, 166–72 [DOI] [PubMed] [Google Scholar]
- 285.Shapira Y., Shohami E., Sidi A., Soffer D., Freeman S., and Cotev S. (1988). Experimental closed head injury in rats: mechanical, pathophysiologic, and neurologic properties. Crit. Care Med. 16, 258–265 [DOI] [PubMed] [Google Scholar]
- 286.Shohami E., Novikov M., and Bass R. (1995). Long-term effect of HU-211, a novel non-competitive NMDA antagonist, on motor and memory functions after closed head injury in the rat. Brain Res. 674, 55–62 [DOI] [PubMed] [Google Scholar]
- 287.Lu D., Mahmood A., Wang L., Li Y., Lu M., and Chopp M. (2001). Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport 12, 559–563 [DOI] [PubMed] [Google Scholar]
- 288.Lu D., Mahmood A., Qu C., Hong X., Kaplan D., and Chopp M. (2007). Collagen scaffolds populated with human marrow stromal cells reduce lesion volume and improve functional outcome after traumatic brain injury. Neurosurgery 61, 596–602; discussion, 602–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Li Y., Chopp M., Chen J., Wang L., Gautam S.C., Xu Y.X., and Zhang Z. (2000). Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J. Cereb. Blood Flow Metab. 20, 1311–1319 [DOI] [PubMed] [Google Scholar]
- 290.Chen J., Li Y., Wang L., Zhang Z., Lu D., Lu M., and Chopp M. (2001). Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 32, 1005–1011 [DOI] [PubMed] [Google Scholar]
- 291.Laurer H.L., Bareyre F.M., Lee V.M., Trojanowski J.Q., Longhi L., Hoover R., Saatman K.E., Raghupathi R., Hoshino S., Grady M.S., and McIntosh T.K. (2001). Mild head injury increasing the brain's vulnerability to a second concussive impact. J. Neurosurg. 95, 859–870 [DOI] [PubMed] [Google Scholar]
- 292.Maruichi K., Kuroda S., Chiba Y., Hokari M., Shichinohe H., Hida K., and Iwasaki Y. (2009). Transplanted bone marrow stromal cells improves cognitive dysfunction due to diffuse axonal injury in rats. Neuropathology 29, 422–432 [DOI] [PubMed] [Google Scholar]
- 293.Baratz R., Rubovitch V., Frenk H., and Pick C.G. (2010). The influence of alcohol on behavioral recovery after mTBI in mice. J. Neurotrauma 27, 555–563 [DOI] [PubMed] [Google Scholar]
- 294.Pandey D.K., Yadav S.K., Mahesh R., and Rajkumar R. (2009). Depression-like and anxiety-like behavioural aftermaths of impact accelerated traumatic brain injury in rats: a model of comorbid depression and anxiety? Behav. Brain Res. 205, 436–442 [DOI] [PubMed] [Google Scholar]
- 295.RIGOR. ([date unknown]). Improving the quality of NINDS-supported pre-clinical and clinical research through rigorous study design and transparent reporting. www.ninds.nih.gov/funding/transparency_in_reporting_guidance.pdf Accessed April8, 2015
- 296.Zhang Y., Xiong Y., Mahmood A., Meng Y., Qu C., Schallert T., and Chopp M. (2009). Therapeutic effects of erythropoietin on histological and functional outcomes following traumatic brain injury in rats are independent of hematocrit. Brain Res. 1294, 153–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Xiong Y., Mahmood A., Meng Y., Zhang Y., Zhang Z.G., Morris D.C., and Chopp M. (2011). Treatment of traumatic brain injury with thymosin β4 in rats. J. Neurosurg. 114, 102–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hines-Beard J., Marchetta J., Gordon S., Chaum E., Geisert E.E., and Rex T.S. (2012). A mouse model of ocular blast injury that induces closed globe anterior and posterior pole damage. Exp. Eye Res. 99, 63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Semple B.D., Noble-Haeusslain L.J., Kwon Y.J., Sam P.N., Gibson A.M., Grissom S., Brown S., Adahman Z., Hollingsworth C.A., Kwakye A., Gimlin K., Wilde E.A., Hanten G., Levin H.S., and Schenk A.K. (2014). Sociosexual and communication deficits after traumatic injury to the developing murine brain. PLoS One 9, e103386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Semple B.D., Canchola S.A., and Noble-Haeusslein L.J. (2012). Deficits in social behavior emerge during development after pediatric traumatic brain injury in mice. J. Neurotrauma 29, 2672–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Nochi M. (1998). Struggling with the labeled self: people with traumatic brain injuries in social settings. Qual. Heal. Res. 8, 665–681 [DOI] [PubMed] [Google Scholar]
- 302.Oddy M., Coughlan T., Tyerman A., and Jenkins D. (1985). Social adjustment after closed head injury: a further follow-up seven years after injury. J. Neurol. Neurosurg. Psychiatry 48, 564–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Kreuter M., Dahllöf A.-G., Gudjonsson G., Sullivan M., and Siösteen A. (1998). Sexual adjustment and its predictors after traumatic brain injury. Brain Inj. 12, 349–368 [DOI] [PubMed] [Google Scholar]
- 304.Ponsford J. (2003). Sexual changes associated with traumatic brain injury. Neuropsychol. Rehabil. 13, 275–289 [DOI] [PubMed] [Google Scholar]
- 305.Johnson E.M., Traver K.L., Hoffman S.W., Harrison C.R., and Herman J.P. (2013). Environmental enrichment protects against functional deficits caused by traumatic brain injury. Front. Behav. Neurosci. 7, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Arciniegas D., Adler L., Topkoff J., Cawthra E., Filley C.M., and Reite M. (1999). Attention and memory dysfunction after traumatic brain injury: cholinergic mechanisms, sensory gating, and a hypothesis for further investigation. Brain Inj. 13, 1–13 [DOI] [PubMed] [Google Scholar]
- 307.Fujimoto S.T., Longhi L., Saatman K.E., Conte V., Stocchetti N., and McIntosh T.K. (2004). Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci. Biobehav. Rev. 28, 365–378 [DOI] [PubMed] [Google Scholar]
- 308.Steward O., Schauwecker P.E., Guth L., Zhang Z., Fujiki M., Inman D., Wrathall J., Kempermann G., Gage F.H., Saatman K.E., Raghupathi R., and McIntosh T. (1999). Genetic approaches to neurotrauma research: opportunities and potential pitfalls of murine models. Exp. Neurol. 157, 19–42 [DOI] [PubMed] [Google Scholar]
- 309.Võikar V., Kõks S., Vasar E., and Rauvala H. (2001). Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol. Behav. 72, 271–281 [DOI] [PubMed] [Google Scholar]
- 310.Risling M., Plantman S., Angeria M., Rostami E., Bellander B.-M.M., Kirkegaard M., Arborelius U., and Davidsson J. (2011). Mechanisms of blast induced brain injuries, experimental studies in rats. Neuroimage 54 Suppl 1, S89–S97 [DOI] [PubMed] [Google Scholar]