Abstract
Background
Medulloblastomas in children can be categorized into 4 molecular subgroups with differing clinical characteristics, such that subgroup determination aids in prognostication and risk-adaptive treatment strategies. Magnetic resonance spectroscopy (MRS) is a widely available, noninvasive tool that is used to determine the metabolic characteristics of tumors and provide diagnostic information without the need for tumor tissue. In this study, we investigated the hypothesis that metabolite concentrations measured by MRS would differ between molecular subgroups of medulloblastoma and allow accurate subgroup determination.
Methods
MRS was used to measure metabolites in medulloblastomas across molecular subgroups (SHH = 12, Groups 3/4 = 17, WNT = 1). Levels of 14 metabolites were analyzed to determine those that were the most discriminant for medulloblastoma subgroups in order to construct a multivariable classifier for distinguishing between combined Group 3/4 and SHH tumors.
Results
Medulloblastomas across molecular subgroups revealed distinct spectral features. Group 3 and Group 4 tumors demonstrated metabolic profiles with readily detectable taurine, lower levels of lipids, and high levels of creatine. SHH tumors showed prominent choline and lipid with low levels of creatine and little or no evidence of taurine. A 5-metabolite subgroup classifier inclusive of creatine, myo-inositol, taurine, aspartate, and lipid 13a was developed that could discriminate between Group 3/4 and SHH medulloblastomas with excellent accuracy (cross-validated area under the curve [AUC] = 0.88).
Conclusions
The data show that medulloblastomas of Group 3/4 differ metabolically as measured using MRS when compared with SHH molecular subgroups. MRS is a useful and accurate tool to determine medulloblastoma molecular subgroups.
Keywords: medulloblastoma, molecular subtypes, MR spectroscopy
Medulloblastoma is the most common malignant brain tumor in children. Current treatment regimens are multimodal and include surgical resection, intensive chemotherapy, and irradiation.1–4 Recent discoveries have demonstrated 4 molecular subgroups of medulloblastoma: WNT, SHH, Group 3, and Group 4.5–8 Clinical outcomes of these subgroups vary significantly.9–11 Treatment strategies in the future will likely be subgroup specific in order to incorporate targeted therapies, avoid/minimize irradiation, or intensify therapy when appropriate, with the goal of improving outcomes for Group 3 and Group 4 medulloblastomas and reduce the late effects of toxic therapy in children with WNT and a subset of SHH tumors.
A standard method of classification has not yet been established, with published methods relying mostly on gene expression and methylation analyses.12–16 Given the importance of subgroup determination, researchers continue to search for fast, reliable, and accessible methods to assign molecular subgroup.
Magnetic resonance spectroscopy (MRS) is a widely available tool, readily integrated with magnetic resonance imaging, which provides useful information on the metabolism of tumors. For example, elevated taurine and choline levels in posterior fossa pediatric brain tumors distinguish medulloblastomas from other tumor types.17,18 Despite general differences between different tumor types, a significant metabolic heterogeneity within tumors of the same type has been observed.19
We hypothesized that SHH and WNT tumors have different metabolic profiles as compared with Group 3/4 tumors, due to the frequent MYC or MYCN amplification in the latter subgroups. These oncogenes may be associated with distinct metabolic profiles20 that may be detectable by MRS, thereby allowing in vivo identification of medulloblastoma subgroups. In the current study, we analyzed MRS data obtained prior to surgical tumor resection from patients whose medulloblastoma subgroup was determined postoperatively using a medulloblastoma specific 31-gene (MB31) signature.21 Our findings provide new insights about differences in the metabolic profile of medulloblastoma subgroups and provide the basis for constructing a 5-metabolite model that distinguishes Groups 3/4 tumors from SHH and WNT tumors.
Materials and Methods
Patient Characteristics
Frozen tumor tissues from 30 patients diagnosed with medulloblastoma at Children's Hospital Los Angeles between 2001 and 2012 were studied for molecular subgroup determination. All patients also had available MRSs, which had been performed at the time of diagnosis. Patient characteristics are presented in Table 1. Cerebellar MRSs were obtained from 21 children without brain tumors to serve as controls. Informed consents were obtained in accordance with institutional review board policies.
Table 1.
Patient characteristics
| No. of patients | % | |
|---|---|---|
| Total patients | 30 | |
| Molecular subgroup | ||
| WNT | 1 | 3 |
| SHH | 12 | 40 |
| Group 3 | 6 | 20 |
| Group 4 | 11 | 37 |
| Age group, years | ||
| ≤3 | 9 | 30 |
| 3–6 | 6 | 20 |
| 6–10 | 8 | 27 |
| ≥10 | 7 | 23 |
| Sex | ||
| Male | 18 | 60 |
| Female | 12 | 40 |
| M Stage | ||
| M0 | 23 | 77 |
| M1 | 1 | 3 |
| M2 | 0 | 0 |
| M3 | 6 | 20 |
| Histology | ||
| Desmoplastic | 6 | 20 |
| Classic | 21 | 70 |
| Anaplastic | 3 | 10 |
| No. of events | 10 | |
| No. of deaths | 7 | |
Magnetic Resonance Imaging and Spectroscopy Protocol
All patient MRS studies were performed on a 1.5 T MR system (Signa LX, GE Healthcare). Patients aged 5 years and younger were anesthetized with 100–200 μg/min/kg propofol throughout the MRS study. Single-voxel point-resolved spectroscopy (PRESS) with an echo time (TE) of 35 milliseconds, a repetition time (TR) of 1.5 seconds, and 128 signal averages was used for all acquisitions, resulting in a total acquisition time of <5 minutes including scanner-adjustment minutes. All MRS data were acquired prior to contrast injection (Magnevist [gadopentetate dimeglumine]). T2-weighted fast spin-echo, fluid-attenuated inversion recovery (FLAIR), and T1-weighted FLAIR images were acquired in all cases prior to MRS and reviewed to determine the extent of tumor lesions. The regions of interest (ROIs) for MRS were identified based on MRI images from all 3 axes and centered in the solid parts of the tumors, excluding any surrounding normal brain tissue or edema and in a manner to minimize inclusion of cystic or necrotic areas (Supplementary material, Fig. S1). An attending radiologist reviewed and approved ROI for each patient and confirmed that the MRS profile was representative for tumor tissue only. Sizes and shapes of the ROIs were adjusted to lesion size and typically varied between 5–10 cm3.
Spectra were processed using fully automated LCModel version 6.1–4Fsoftware (Stephen Provencher Inc), and absolute metabolite concentrations (in mmol/kg tissue) were obtained for 27 metabolites and lipid signals.17,18 The LCModel software also provided the Cramer-Rao lower bounds (CRLB) for each of the measured metabolites and lipids. CRLB are objective indicators of the reliability of an MRS measurement that take into account the quality of an acquisition such as the signal-to-noise ratio. A priori, it was decided to include only metabolites and lipids that were typically measured with CRLB <50% (ie, the model deemed the reported concentration to be accurate within ±50%). Fourteen metabolites and lipid resonances fulfilled this quality criterion and were retained for further analysis.
Statistical Analysis
The objectives of the statistical analysis were to determine the most discriminating MRS metabolites for medulloblastoma subgroups, with the primary focus being to differentiate combined Group 3 and Group 4 medulloblastomas (denoted Group 3/4) from SHH medulloblastomas and to construct a multivariable classifier to distinguish between combined Group 3/4 and SHH tumors and determine its accuracy.
In all analyses, metabolite values were transformed using the Box-Cox transformation——with λ = 0.5, in order to approximate normality (Supplementary material, Fig. S2). Unsupervised principal components analysis was used to visualize the separation between molecular risk groups due to differences in metabolite levels. A linear discriminant analysis (LDA) was developed to distinguish Group 3/4 from SHH tumors.22 The prior probabilities of group membership were estimated from the data. Individual metabolites were screened for their ability to discriminate between Group 3/4 and SHH using the Wilcoxon rank-sum test. This test is equivalent to the test that the AUC of the nonparametric receiver operating characteristic (ROC) curve is >0.5.21 The screening criterion of P < .05 was used to include individual metabolites in the LDA. Error rates were estimated via leave-one-out cross validation (LOOCV),23 wherein each patient was excluded from analysis in turn, and the complete cycle of univariate metabolite selection and LDA development was repeated, with the left-out case being classified by the resulting classifier. Statistical computations were performed with Stata 11 (Stata Statistical Software).
Results
Distinct Patterns of Magnetic Resonance Spectroscopy Among Medulloblastoma Subgroups
MRS studies selected for analysis were obtained from children whose tumors were later confirmed histologically as medulloblastoma (n = 30) and from whom frozen tissue was available for molecular subgroup classification. The molecular subgroup determination was made using a 31-gene TaqMan Low Density Array (TLDA) signature.24 The distribution of medulloblastoma tumors (3% WNT, 40% SHH, 20% Group 3, 37% Group 4) and clinical characteristics across molecular subgroups was consistent with previously published data (Table 1). Averaged spectra of the molecular subgroups revealed distinct metabolic features. Group 3 and Group 4 tumors demonstrated metabolic profiles with readily detectable taurine and creatine levels. SHH tumors showed prominent choline and lipids, notably low creatine levels, and little or no evidence of taurine (Fig. 1A).
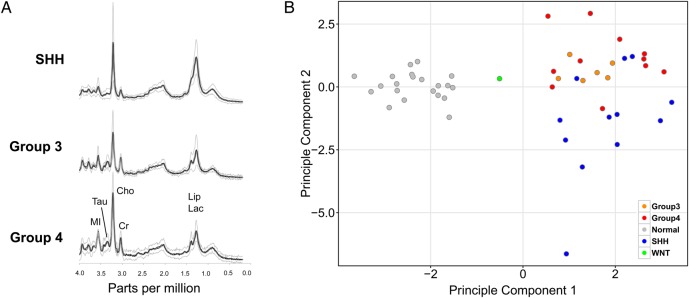
Fig. 1.
MR spectra patterns in medulloblastoma subgroups (A) Average and standard deviation of MR spectra of Group 4 (n = 11), Group 3 (n = 6), and SHH (n = 12) medulloblastomas scaled to the measured absolute concentrations to allow direct comparison. (B) Unsupervised principal component analysis of MRS metabolites (n = 14) demonstrating separation of medulloblastoma (n = 30) and normal cerebella (n = 21) samples (grey=normal cerebella, medulloblastomas; orange=Group 3; red=Group 4; blue=SHH; green=WNT).
An unsupervised principal component analysis using all available metabolite concentration levels showed distinct clusters of medulloblastoma tumors compared with normal cerebella, as expected. The analysis also demonstrated clustering of samples based on medulloblastoma molecular subgroups, with separation noted between the SHH and the Group 3/4 tumors (Fig. 1B). Our sample cohort contained only one WNT sample, which appeared to be distinct from the other groups.
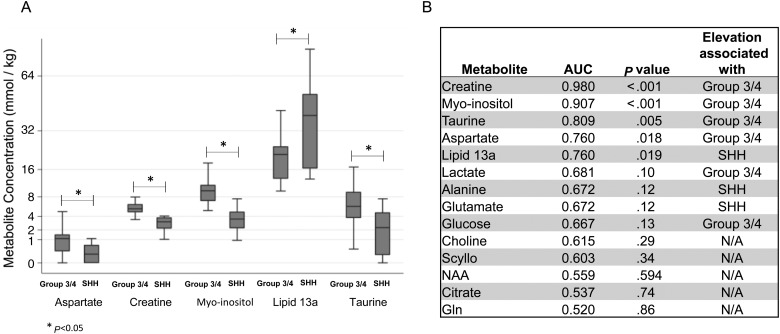
Five metabolites—creatine, myo-inositol (which may include signal from underlying glycine), taurine, aspartate, and lipid 13a—showed statistically significant difference (P < .05) in concentrations between the SHH and the Group 3/4 tumors (Fig. 2). SHH tumors were associated with lower levels of aspartate, creatine, myo-inositol, and taurine and higher levels of lipid 13a. A principal component analysis using only the concentration levels of these top 5 metabolites in medulloblastoma tumors also delineated SHH and Group 3/4 tumors, again with the one WNT sample that could not be clustered with the other tumors (Fig. 3B).
Fig. 2.
Top discriminating metabolites among medulloblastoma subgroups. (A) Concentration levels of the top 5 discriminating metabolites comparing SHH tumors with Group 3/4 tumors. (B) List of the top 14 discriminating metabolites along with their individual area under the curve (AUC) values and significance of metabolite concentration comparisons between SHH and Group 3/4 medulloblastomas. Significance values are 2-sided P values from Wilcoxon rank-sum test.
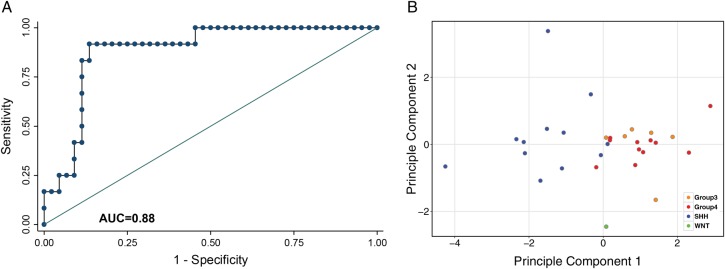
Fig. 3.
Accuracy of the 5-metabolite model in predicting medulloblastoma subgroups (A) The accuracy of the 5-metabolite model determined using receiver-operating-characteristic curve (ROC) based on true-positive rate (sensitivity) and false-positive rate (1-specificity). The area under the curve (AUC) value of the ROC curve was obtained using leave-one-out cross-validation of the logistic probability and demonstrated high accuracy (AUC = 0.88) for this model. (B) Principle component analysis of the top 5 discriminating metabolites provides evidence of 2 primary clusters of samples (SHH and Group 3/4) and a WNT sample that occupies a distinct area.
A 5-Metabolite Model Predicts Medulloblastoma Subgroups
The 5 metabolites that satisfied our screening criteria (see Materials and Methods) were used to construct an LDA classifier. The canonical loadings of the individual metabolites (ie, a measure of the importance of the metabolite in the classifier) revealed creatine, myo-inositol, and lipid 13a as the most influential metabolites in this classifier (Supplementary material, Table S2). The accuracy of the model for predicting medulloblastoma subgroups (SHH vs Group 3/4) using LOOCV AUC estimates was 0.88 (Fig. 3A). These data suggest that tumor-specific metabolites can be used to identify medulloblastoma subgroups.
Discussion
The molecular classification of medulloblastomas based on ex vivo analyses of tumor tissue by nucleic acid derivatives or protein expression has been well established.5–8,14,15 Our study demonstrates for the first time that in vivo analysis of tumor metabolites by MRS can also identify medulloblastoma molecular subgroups. A 5-metabolite model can distinguish SHH from Group 3/4 tumors with a high degree of accuracy. The relative ease of obtaining MRS in clinical settings should allow incorporation of this technique into future clinical trials aimed at validation of these findings and improving diagnostic classification of children with medulloblastoma.
MRS is noninvasive, widely available in the clinical setting, requires minimal time for analysis, and has been shown to successfully distinguish medulloblastoma from other tumors in the posterior fossa.17,18 Metabolic profiling of intact biological samples ex vivo using high-resolution MRS has also been shown to enable measurement of multiple cellular metabolites simultaneously. Profiling gene expression and metabolite content of the same breast carcinoma samples enabled comparisons of molecular and metabolite findings, revealing high correlation in identifying breast cancer subtypes.25
The significance of molecular subgroups of medulloblastoma has been demonstrated by their distinct biologic, molecular, and tumor microenvironment characteristics.9,11,24,26 Survival outcomes also differ based on molecular subgroup, with higher survival observed in children with WNT tumors and infants with SHH tumors.10,11,27 The current risk stratification is based on clinical variables, with age, evidence of metastasis at presentation, and/or an incomplete resection used to stratify patients into standard and high-risk subsets. Clinical trials are currently being developed to incorporate molecular subgroups into the risk and treatment stratifications. Several methods are available for molecular subgroup determination, with many currently being established as laboratory-developed tests. These techniques all rely on tumor tissues obtained at the time of diagnosis. Our in vivo MRS method for medulloblastoma classification could provide the health care team with additional information for establishing accurate risk stratification at diagnosis. Current standard of care dictates aggressive surgical approaches aimed at total or near total resection, given the improved survival for patients in whom a gross total resection is achieved. However, gross total resection also carries significant risks of morbidity including cerebellar mutism.28,29 With further research and validation of MRS-based stratification, we envision the possibility of clinical research questions that would utilize MRS with limited biopsies for molecular profiling and neo-adjuvant therapies.
The limitations of the MRS-based stratification method are centered around access to the technology and skilled personnel to interpret the data. The software and analytical tools used in this analysis are commercially available and can be used to conduct multicenter clinical research. While institutions are increasingly employing advanced imaging modalities as adjunctive methods of diagnosis, these practices are not universal. As the field of MRS continues to advance, clinical investigations incorporating this technology will become increasingly prevalent. The accuracy and feasibility of MRS for predicting molecular subgroups suggests that MRS may be a useful addition to the other strategies used for subgroup determination and treatment stratification in children with medulloblastoma.
Supplementary Material
Funding
This work was supported by a grant to S.A. from the American Cancer Society, Alex′s Lemonade Stand Foundation, and Brad Kamisnky Foundation. This work was also supported by a training grant from the National Institute of Health T32 CA009659-18 (trainee: AM). Support was also provided by the Rudi Schulte Research Institute (S.B.) and by the Ian′s Friends Foundation (S.B.).
Author Contributions
S.A. and S.B. conceived and led the project. A.M. performed the 31-gene signature assay for gene expression-based subgrouping along with support from R.K. S.B. obtained and processed the MR spectra. M.N. supervised and performed quality control for the MR spectra. N.R., M.V., L.H., S.M., J.F., A.E., F.G., A.J., M.K., and G.D. acquired the data (acquired and managed patients, selected and characterized samples, provided disease-specific histopathological analysis). S.B., A.M., and S.A. co-wrote the manuscript with input from all co-authors.
Conflict of interest statement. The authors have no conflicts of interest to report.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Ms. Karen Miller and the CHLA's Department of Pathology Biospecimens Core for their technical assistance in obtaining tumor samples.
References
- 1.Packer RJ, Gajjar A, Vezina G et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4208. [DOI] [PubMed] [Google Scholar]
- 2.Gajjar A, Chintagumpala M, Ashley D et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective multicentre trial. Lancet Oncol. 2006;7(10):813–820. [DOI] [PubMed] [Google Scholar]
- 3.Dhall G, Grodman H, Ji L et al. Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “Head Start” I and II protocols. Pediatr Blood Cancer. 2008;50(6):1169–1175. [DOI] [PubMed] [Google Scholar]
- 4.Jakacki RI, Burger PC, Zhou T et al. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children's Oncology Group Phase I/II study. J Clin Oncol. 2012;30(21):2648–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson MC, Fuller C, Hogg TL et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24(12):1924–1931. [DOI] [PubMed] [Google Scholar]
- 6.Kool M, Koster J, Bunt J et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS ONE 2008;3(8):e3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Northcott PA, Korshunov A, Witt H et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29(11):1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho YJ, Tsherniak A, Tamayo P et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29(11):1424–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellison DW, Kocak M, Dalton J et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011;29(11):1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kool M, Korshunov A, Remke M et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123(4):473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor MD, Northcott PA, Korshunov A et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwalbe EC, Lindsey JC, Straughton D et al. Rapid diagnosis of medulloblastoma molecular subgroups. Clin Cancer Res. 2011;17(7):1883–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons DW, Li M, Zhang X et al. The genetic landscape of the childhood cancer medulloblastoma. Science. 2011;331(6016):435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunder R, Jalali R, Sridhar E et al. Real-time PCR assay based on the differential expression of microRNAs and protein-coding genes for molecular classification of formalin-fixed paraffin embedded medulloblastomas. Neuro Oncol. 2013;15(12):1644–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwalbe EC, Williamson D, Lindsey JC et al. DNA methylation profiling of medulloblastoma allows robust subclassification and improved outcome prediction using formalin-fixed biopsies. Acta Neuropathol. 2013;125(3):359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Northcott PA, Shih DJH, Remke M et al. Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol. 2012;123(4):615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovanlikaya A, Panigrahy A, Krieger MD et al. Untreated pediatric primitive neuroectodermal tumor in vivo: quantitation of taurine with MR spectroscopy. Radiology. 2005;236(3):1020–1025. [DOI] [PubMed] [Google Scholar]
- 18.Panigrahy A, Krieger MD, Gonzalez-Gomez I et al. Quantitative short echo time 1H-MR spectroscopy of untreated pediatric brain tumors: preoperative diagnosis and characterization. Am J Neuroradiol. 2006;27(3):560–572. [PMC free article] [PubMed] [Google Scholar]
- 19.Blüml S, Panigrahy A, eds. MR Spectroscopy of Pediatric Brain Disorders. New York: Springer; 2013. [Google Scholar]
- 20.Yuneva MO, Fan TWM, Allen TD et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012;15:157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margol A, Robison N, Gnanachandran J et al. Tumor-associated macrophages in SHH subgroup of medulloblastomas. Clin Cancer Res. 2015;21(6):1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. [DOI] [PubMed] [Google Scholar]
- 23.Graybill FA. Theory and Applications of the Linear Model. Boston: Cengage Learning; 2000. [Google Scholar]
- 24.Braga-Neto UM, Dougherty ER. Is cross-validation valid for small-sample microarray classification? Bioinformatics. 2004;20(3):374–380. [DOI] [PubMed] [Google Scholar]
- 25.Borgan E, Sitter B, Lingjærde OC et al. Merging transcriptomics and metabolomics--advances in breast cancer profiling. BMC Cancer. 2010;10(628):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibson P, Tong Y, Robinson G et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468(7327):1095–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pietsch T, Schmidt R, Remke M et al. Prognostic significance of clinical, histopathological, and molecular characteristics of medulloblastomas in the prospective HIT2000 multicenter clinical trial cohort. Acta Neuropathol. 2014;128(1):137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson PL, Muraszko KM, Holmes EJ et al. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children's Oncology Group. J Neurosurg. 2006;105(6 Suppl):444–451. [DOI] [PubMed] [Google Scholar]
- 29.Beckwitt Turkel S, Krieger MD, O'Neil S et al. Symptoms before and after posterior fossa surgery in pediatric patients. Pediatr Neurosurg. 2012;48(1):21–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.