Abstract
We previously reported that neonatal mice infected with influenza A virus (IAV) develop interstitial pneumonia characterized by reduced lung cytokine and chemokine responses. The failure of T cells to infiltrate the airways of neonates correlated with delayed clearance of sublethal IAV infections compared to adults. We hypothesized that negative regulators in the neonatal lungs such as cytokines or T regulatory (Treg) cells are responsible for these differences. Neonates either deficient in interleukin-10 (IL-10) or with T cells unresponsive to transforming growth factor-β signaling due to absence of SMAD family member 4 (Smad4) had similar IAV clearance kinetics to wild-type pups and no difference in T-cell responses. In contrast, functional depletion of Treg cells with anti-CD25 monoclonal antibody resulted in increased proportions of activated CD4+ T cells in the lungs, but failure to clear IAV. Similarly, scurfy pups (mutation in forkhead box P3 [Foxp3] rendering them deficient in Treg cells) had increased proportions of activated T cells in the lungs compared to littermate controls. Scurfy pups also had increased proportions of IL-13-producing CD4+ T cells. Interestingly, like anti-CD25-treated pups, scurfy pups had significantly elevated viral loads compared to controls. Based on these data, we conclude that Tregs are critical for clearance of IAV in neonatal mice.
Introduction
Influenza A virus (IAV) is a significant contributor to infant morbidity and mortality in the United States. Yearly influenza epidemics are particularly difficult for populations known to have deficiencies in immune function such as the very young and elderly. Very few studies have examined the neonatal immune response to IAV (21,47), but it has been appreciated for some time that neonatal T-cell responses to antigen are frequently biased toward T helper 2 (Th2) cytokines (1,30).
We previously reported that neonatal T cells migrated to the lung interstitium in response to IAV infection, but did not enter the airways (21). Interstitial pneumonia along with elevated interleukin (IL)-4 mRNA in the lungs corresponded with delayed clearance of IAV compared to adult mice (21). Neutrophils and eosinophils entering the airways of IAV-infected neonatal mice correlated with delayed type 1 cytokines and chemokines (21). Our efforts to draw neonatal T cells into the airways by intranasal administration of CCL2, interferon-γ (IFNγ), or CXCL9 were unsuccessful (21).
There is evidence that regulatory T cells (Tregs) also coordinate the trafficking of innate and adaptive immune cells to the primary site of infection. In adult mice, Tregs were shown to promote immune cell trafficking in CB6F1 hybrid mice infected with respiratory syncytial virus (RSV) (31) and in mucosal herpes simplex virus (HSV)-2 infection (22). Furthermore, Tregs altered the proportions of antigen-specific CD8+ T cells during IAV infection in adult mice (14) and may block entry into tissue sites by altering chemokine receptor expression (35). In a neonatal model of HSV type 2 infection, depletion of Tregs with the PC61 anti-CD25 antibody resulted in significant recruitment of CD8+ T cells into the draining lymph nodes and increased granzyme B and IFNγ expression by CD8+ T cells (9). The mechanism through which Tregs alter T effector cell migration is not clear and appears to vary depending on the model system.
In this study, we report that in IAV infection in neonatal mice, neither IL-10 nor transforming growth factor-β (TGFβ) signaling through Smad4 in T cells had an effect on the proportion of Tregs in the lungs or the migration of T effector cells into the airways. In the absence of functional Tregs, there was an enhanced proinflammatory response to IAV in neonatal lungs, although no difference in the migration of T cells into the alveolar spaces. While others have reported that adult mice depleted of Tregs had no differences in viral lung burden (3), we report here that the neonatal lungs had a significantly higher IAV burden after Treg depletion using the anti-CD25 antibody and in scurfy (Foxp3 mutant) mice. The viral burden corresponded with significantly increased expression of the Th2 cytokine, IL-13. Our data indicate that contrary to our initial hypothesis, Tregs contribute to the clearance of IAV in neonatal mice.
Materials and Methods
Mice
Wild-type (WT) C57BL/6 breeders were purchased from Taconic (Hudson, NY) or the Jackson Laboratory (Bar Harbor, ME). Breeder C57BL/6J, B.Cg-Foxp3sf (scurfy) heterozygous female mice were purchased from the Jackson Laboratory and bred with WT C57BL/6 males under specific pathogen-free conditions. Male pups were screened for Foxp3 expression in T cells by flow cytometry to verify the scurfy mutation, and nonscurfy littermates were used as controls. Breeder IL-10 knockout (KO) animals were obtained from the Jackson Laboratory and then maintained within the colony. Breeder B6.Cg-Tg(Lck-cre)548Jxm/J were purchased from the Jackson Laboratory and crossed with Smad4co/co mice, which were graciously provided by Dr. Chu-Xia Deng at the National Institutes of Health (NIH) (46). Control pups were obtained from Smad4co/co mice crossed with C57BL/6 mice. For each Lck-Smad4Co breeding or experiment, tail snips were performed and mice were genotyped by polymerase chain reaction (PCR). Primers used were Lck: 5′-gCggTCTggCAgTAAAAACTATC-3′ and 5′-gTgAAACAgCATTgCTgTCAC TT-3′; internal controls: 5′-CTAggCCACAgAATTgAAAgATCT-3′ and 5′-gTAggTggAAATTCTAgCATCATCC-3′. PCR conditions were according to the protocol provided by the Jackson Laboratory. For Smad4, PCR was done according to Yang et al. using the primers Smad4 B: 5′-gggCAgCgTAgCATATAAgA-3′ and Smad4 C: 5′-gACCCAAACgTCACCTTCAg-3′ (46). All animals were maintained at the Lexington Veterans Administration (VA) Medical Center or University of Kentucky, Division of Laboratory Animal Research facilities. All mouse studies were approved by the University of Kentucky and Lexington VA Institutional Animal Care and Use Committees (IACUC) and Institutional Biosafety Committees.
IAV infections and stocks
Influenza A/Puerto Rico/8/34 (PR8) was grown in the allantoic fluid of 10-day-old embryonated, specific pathogen-free chicken eggs as previously described (5). Viral stocks were tested for common mouse pathogens and were shown to contain only IAV. Mice were given intranasal (i.n.) inoculations of IAV under isoflurane anesthesia with a lethal dose (LD)10 of PR8 virus. This corresponded to 0.25 egg infectious dose (EID)50/g in 10 μL for pups and 2.5 EID50/g in 50 μL for adults. Multiple litters of neonatal mice were utilized with dates of birth within 48 h of one another and infected between postnatal days 2 and 4. Following infection, mice were monitored daily for body weight changes and were euthanized using criterion specified by the University of Kentucky and VA IACUCs.
Cell isolation
Lungs were lavaged with five washes of cold HBSS/3 mM EDTA to isolate alveolar cells. Supernatants from the first wash were frozen for subsequent analysis of albumin leakage using the Bromocresol Green Assay (Sigma-Aldrich, St. Louis, MO) according to the manufacturer's instructions. The lungs were perfused with HBSS through the right ventricle of the heart to remove blood from the lungs. The lungs and tracheobronchial lymph nodes (TBLN) were excised and minced. Lungs were digested at 37°C with 50 U/mL DNase (Sigma-Aldrich) and 1 mg/mL collagenase A (Sigma-Aldrich) in RPMI 1640 containing 3% FCS. The lungs and TBLN were pressed through 70-μm cell strainers (BD Biosciences, San Jose, CA) to yield single-cell suspensions. The red blood cells were lysed with ammonium chloride/potassium (ACK) lysis buffer and the remaining cells were washed and suspended in HBSS.
Flow cytometry
For surface staining, 5 × 105 to 106 cells were stained with fluorochrome-conjugated antibodies specific for murine CD4, CD8, CD44, CD62L, and CD25 (eBioscience, San Diego, CA) in phosphate-buffered saline containing 0.1% BSA and 0.02% NaN3 (PBA). Surface stained cells were fixed at room temperature in 10% formalin for 20 min and then washed with HBSS. For intracellular staining of nuclear Foxp3, cells were surfaced stained as above and were fixed and permeabilized with the Foxp3 Staining Buffer Set according to the manufacturer's protocol (eBioscience). For intracellular staining of cytoplasmic proteins, cells were stimulated for 4 h with 50 ng/mL PMA and 1 μg/mL of ionomycin. Brefeldin A (10 μg/mL) was added for the final 2 h of incubation to inhibit secretion of cytokines, and then cells were surfaced stained as above in PBA with 10 μg/mL of Brefeldin A. Cells were fixed and permeabilized as earlier, incubated with anti-CD16/CD32 (eBioscience) followed by antibodies for cytoplasmic proteins IFNγ, IL-17a, and IL-13 (eBioscience), and analyzed by flow cytometry. Multiparameter analysis was performed using a FACSCalibur or LSRII cytofluorometer (BD Biosciences, Mountain view, CA). Analysis was performed on 50,000 events collected from each sample utilizing FlowJo software (Tree Star, Inc., Ashland, OR).
Determination of lung virus titers
Lungs from infected mice were either unmanipulated or lavaged and then frozen at −80°C until analysis. Viral burdens were determined by plaque assay on Madin Darby Canine Kidney (MDCK) cells (ATCC, Manassas, VA). Cells were grown to confluence in six-well plates in Dulbecco's modified Eagle's medium (DMEM) (ATCC) supplemented with nonessential amino acids and 10% heat inactivated FCS (Atlas Biologicals, Fort Collins, CO). Tenfold dilutions of lung homogenate were incubated with the cells for 1 h at 37°C. The cells were then washed and overlaid with DMEM in 1% Bacto Agar with 1% trypsin (Sigma-Aldrich). Three days later, the cells were fixed with 20% acetic acid and the overlay removed. Plaques were visualized by staining with crystal violet and counted. The limit of detection was 10 PFU per lungs.
Determination of lung cytokine levels
Supernatants were collected from lung homogenates before processing for plaque assays. Supernatants were assayed for IL-4, IL-13, and IFNγ concentrations by ELISA (eBioscience). Cytokine concentrations were normalized by total protein content determined using an RC DC™ Protein Assay kit (Bio-Rad Laboratories, Inc., Hercules, CA).
Administration of neutralizing antibodies
To functionally inactivate Tregs, neonatal mice were given intraperitoneal (i.p.) injections of 100 μg of anti-CD25 clone PC-61.5.3 (BioXcell, West Lebanon, NH) in 50 μL HBSS on postnatal days 2 or 3, corresponding to either day 1 or 0 of infection. Control mice were given 100 μg of isotype control rat IgG1 (Sigma Aldrich).
Statistics
Data are expressed as the mean ± SD of 3–5 mice per group, and each experiment was repeated two or more times. Statistics was performed using SigmaPlot software (San Jose, CA). Data were tested for differences using Student's t-test or two-way analysis of variance (ANOVA) followed by the Holm-Sidak post hoc test for pairwise comparisons. If variance or normality tests failed, the Mann–Whitney rank sum test was performed or Kruskal–Wallis one-way ANOVA on ranks was performed at each individual time point followed by a Dunn's pairwise post hoc test. Differences were considered statistically significant with p < 0.05.
Results
The proportions of T regulatory cells are higher in the neonatal lung than in adult lungs during IAV infection
Treg cells are responsible for controlling inflammatory responses, maintaining peripheral tolerance to self-antigens and preventing autoimmunity (33). We previously published that there is a delay in the ability of neonatal mice to clear IAV infection that corresponded to the inability of T cells to infiltrate the alveolar space in neonatal lungs (21). TGFβ is known to be one of the factors important for the development of inducible Tregs, and since we and others have reported that there is elevated TGFβ in the postnatal developing lungs (2,17), we hypothesized that TGFβ could be driving Tregs that, in turn, modulate the neonatal T-cell immune response to IAV. We first examined the proportions of Tregs present in neonatal compared to adult lungs in response to an LD10 dose of IAV. The LD10 dose (0.25 and 2.5 EID/g for pups and adults, respectively) did not significantly alter pup body weights compared to uninfected pups, but resulted in a small transient drop in body weight in adults (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/vim).
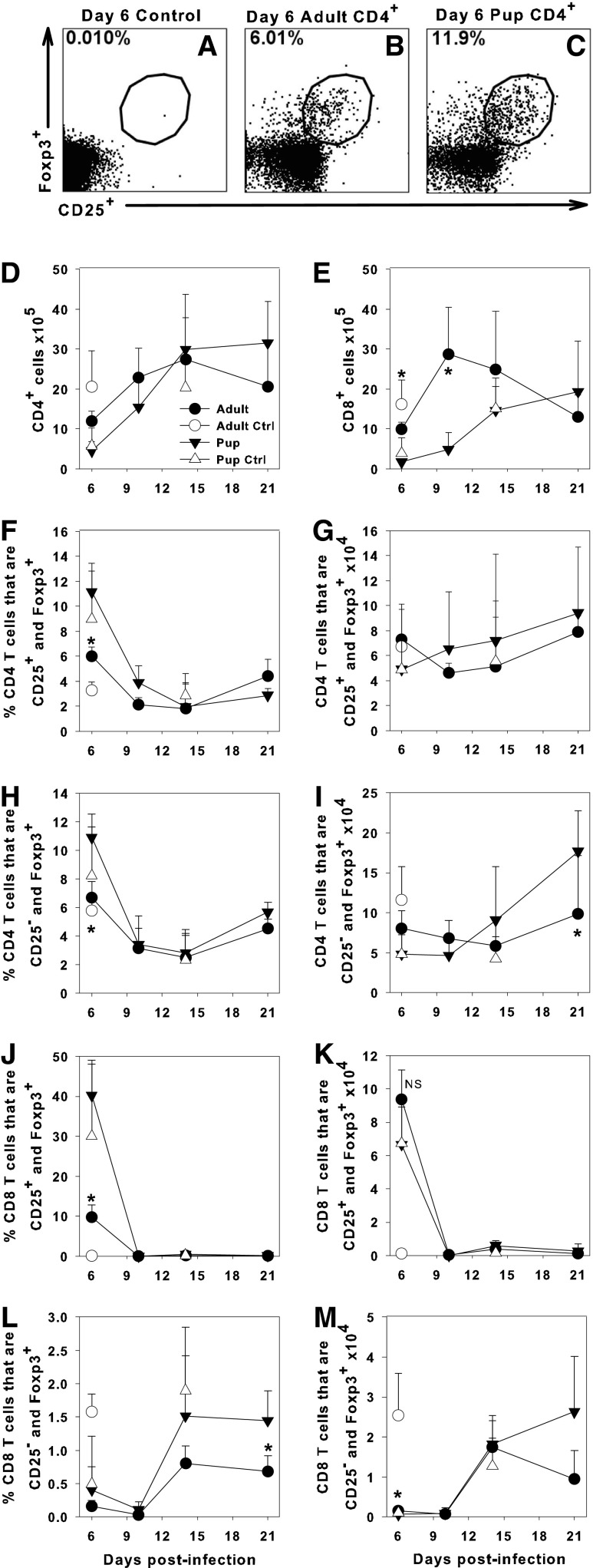
Tregs were defined as CD4+ T cells that express Foxp3 with or without expression of CD25. Figure 1A–C shows representative dot plots of CD4+ T cells with a region drawn around the Foxp3+CD25+ populations from adult and pup lung samples. The absolute numbers of CD4+ and CD8+ T cells in the lung digests for infected and uninfected pups and adults are shown in Figure 1D and E. IAV-infected neonatal mice had higher proportions of CD4+ T cells that were Foxp3+ in the lungs than adult mice at day 6 postinfection, but no significant differences at any other time point (Fig. 1F, H). There were either no significant differences or higher absolute numbers of Tregs in the lungs of neonates compared to adults after IAV infection despite the significant difference in size of the animals (Fig. 1G, I).
FIG. 1.
Proportions of T regulatory cells are elevated in the lungs of neonatal mice. Adult or 2-day-old C57BL/6 mice were infected i.n. with an LD10 dose of the PR8 strain of IAV. Representative flow cytometry dot plots showing expression of Foxp3 and CD25 on gated CD4+ cells are shown for an uninfected adult using isotype control antibodies (A) and Foxp3 and CD25 expression for an infected adult (B) and infected pup (C) at day 6 postinfection. Total numbers of CD4+ and CD8+ cells in the lungs (D, E) were determined by flow cytometry. The proportions (F, H, J, L) and numbers (G, I, K, M) of CD4+ (F–I) or CD8+ (J–M) cells expressing Foxp3 with or without CD25 in the lung digest of adults or pups were determined by flow cytometry. Data represent the mean ± SD of infected adults n = 4 and pups n = 5 and control adults n = 3 and pups n = 5. Data are representative of at least two separate experiments, *p < 0.05 comparing adult to pups at the same time point. IAV, influenza A virus; i.n., intranasal; NS, not significant.
Interestingly, we found that a significant proportion of CD8+ cells in the lungs of neonatal mice at day 6 postinfection were Foxp3+CD25+ (Fig. 1J), although the absolute numbers of these cells were slightly smaller in the neonatal compared to adult lungs (Fig. 1K). These cells disappeared by day 10 postinfection, replaced by a small proportion of CD8+ cells that were Foxp3+ and CD25− at day 14 postinfection (Fig. 1L, M). CD8+Foxp3+ cells have been shown to have a suppressive activity against tumor immunity and autoimmune cells (24,36). The relatively high numbers of Tregs in neonatal lungs suggest that they could be contributing to the significant reduction in T-cell entry into the airway and delayed clearance of IAV reported in neonatal mice (21).
Disruption of TGFβ signaling through Smad4 in T cells does not alter the T cell immune response to IAV
TGFβ is critical for the development and differentiation of inducible Foxp3+ Tregs (34,42). However, TGFβ is also important for lung development and homeostasis and is elevated during postnatal lung development (2,23). To address whether TGFβ has effects specifically on T cells during IAV infection, we used a conditional KO approach. We crossed transgenic mice expressing Smad4 with two loxP sites flanking exon 8 (Smad4co) with mice that carry the cre transgene downstream of the T cell-specific lck promoter (Lck-Cre) (15). Lck-Smad4co mice, with T cells that are not able to transmit TGFβ signals through Smad4 and Smad4co/+ controls, were infected with IAV to determine if TGFβ affects T-cell subset responses to the virus in neonates.
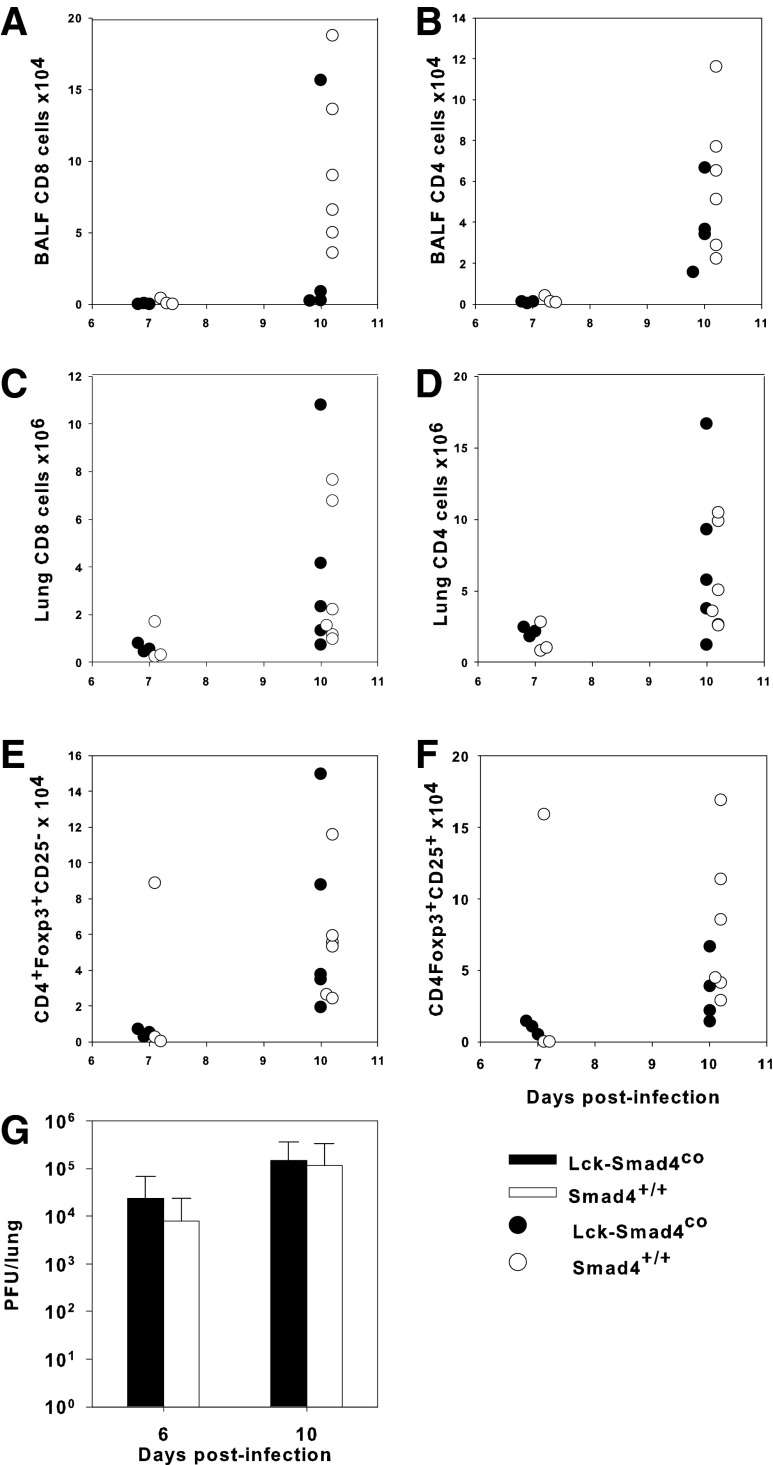
Our studies showed that there were no significant differences in the accumulation of CD8+ or CD4+ T cells in the bronchial alveolar lavage fluid (BALF) or in the lung digests between conditional KO and control pups (Fig. 2A–D). Likewise, there were no differences in the number of activated (CD44hiCD62Llo) CD8+ or CD4+ T cells in the BALF or lung digest between these two groups (data not shown). Surprisingly, the lack of TGFβ signaling through Smad4 did not significantly alter the number of either CD25− or CD25+ Tregs in the lungs compared with control animals (Fig. 2E, F). Finally, there were no differences in viral burden in the lungs of either group (Fig. 2G). This corresponded to no differences in body weight between the groups during the course of infection (Supplementary Fig. S2). These data suggest that TGFβ signaling through Smad4 in T cells is not necessary for the induction of Tregs and does not affect the IAV clearance mechanisms in the neonatal lungs.
FIG. 2.
Disrupting TGFβ signaling through Smad4 does not alter the immune response to IAV. Two-day-old Lck-Smad4co and Smad4co/+ pups were either infected i.n. with an LD10 dose of the PR8 strain of IAV or left uninfected. Flow cytometry was used to determine the number of CD8+ and CD4+ T cells in the BALF (A, B) or lung digest (C, D), and CD4 T cells that were CD25+Foxp3+ or CD25−Foxp3+ Tregs in lung digest (E, F). Viral burden in lung homogenates was determined by plaque assay on MDCK cells with the horizontal line representing the limit of detection (G). Data represent the mean ± SD of 3–6 mice per group and are representative of three independent experiments. No statistically significant differences between pups and adults were detected. BALF, bronchial alveolar lavage fluid; MDCK, Madin Darby Canine Kidney; TGFβ, transforming growth factor-β.
IL-10-deficient neonatal mice had similar T cell responses as WT neonatal mice
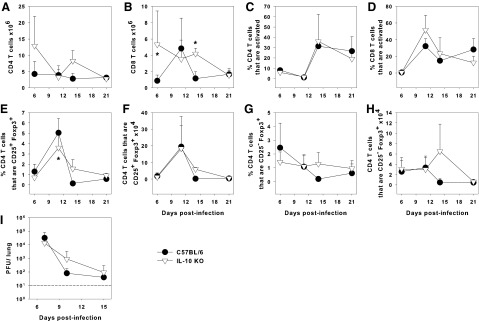
It has been shown that IL-10 acts on Tregs to maintain Foxp3 expression and suppressive functions in a model of experimental colitis (25). Moreover, we have previously published that IL-10 KO pups had elevated numbers of T cells infiltrating the lungs with expedited clearance of the fungal pathogen Pneumocystis murina compared to WT neonates (17). To determine whether IL-10 contributes to maintenance of Tregs and dampens the response to IAV, 2-day-old IL-10 KO pups and WT controls were infected with an LD10 of the PR8 strain of IAV. IL-10 did not have a significant effect on the migration of T cells into the alveolar spaces. As we have previously shown for neonatal mice (21), we found negligible numbers of T cells in the BALF of WT and IL-10 KO pups (data not shown). The numbers of CD8+, but not CD4+ cells, in the lungs of IL-10 KO pups were elevated at day 6 postinfection and remained at these levels through day 14 postinfection (Fig. 3A, B). In contrast, WT CD8+ cells peaked at day 10 and were significantly lower than in IL-10 KO pups by day 14 postinfection (Fig. 3B). However, there were no differences in the proportions of activated (CD44hiCD62Llo) CD4+ or CD8+ T cells in the lung digests over the course of the experiment (Fig. 3C, D).
FIG. 3.
IL-10 does not alter clearance of IAV in neonatal mice. Two-day-old IL-10 KO and C57BL/6 mice were infected i.n. with an LD10 dose of the PR8 strain of IAV or left uninfected. Flow cytometry was used to determine the number of CD4+ (A) or CD8+ (B) T cells, the proportion of CD4+ (C) or CD8+ (D) T cells that were activated (CD44hi CD62Llow), and the proportion and number of CD4+ T cells that expressed Foxp3+ with (E, F) or without (G, H) CD25 in the lung digest. Viral burden was determined by plaque assay in lung homogenates on MDCK cells (I). Data represent the mean ± SD of 3–11 mice per group and are representative of two independent experiments. *p < 0.05 compared to WT C57BL/6 pups at the same time point. IL, interleukin; WT, wild type.
There were no consistent differences in the proportions or numbers of CD4+ T cells in the lungs expressing CD25 and Foxp3 between the groups (Fig. 3E, F) and no significant difference in the number or proportion of CD25− Treg cells over time (Fig. 3G, H). Consistent with the lack of significant changes in the activated T-cell numbers in the lungs, there were no significant differences in the viral burden of IL-10 KO pups compared to WT controls (Fig. 3I) and no differences in body weight between the groups (Supplementary Fig. S3). These data indicate that IL-10 does not alter IAV clearance and has a minor effect on the numbers of T cells in the lungs of neonatal mice.
Depletion of Tregs delayed the clearance of IAV
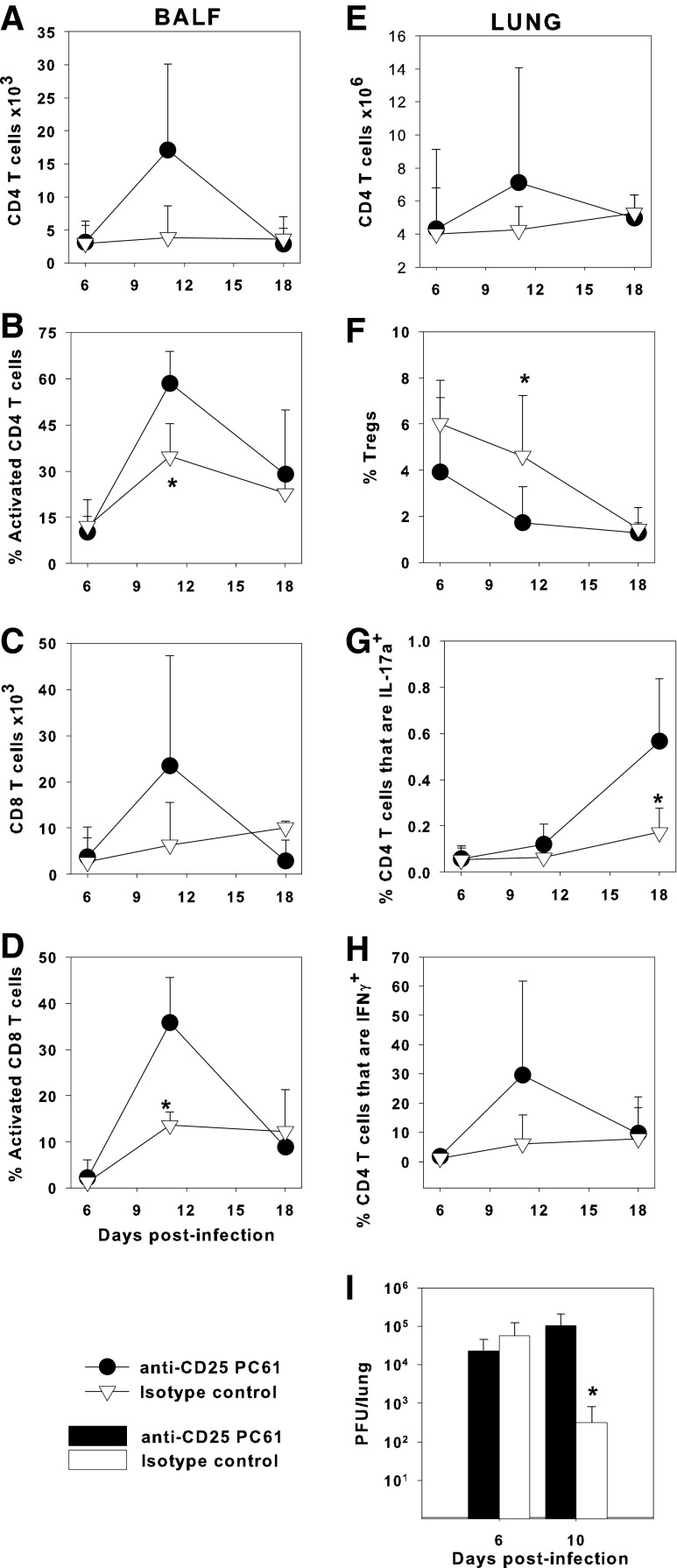
Since neither IL-10 nor TGFβ signaling through Smad4 had an effect on Tregs in neonatal lungs, we directly addressed the role of Tregs in the neonatal response to IAV by injecting 2-day-old neonatal C57BL/6 mice with 100 μg of anti-CD25 or an isotype control rat IgG1 at the time of infection with an LD10 dose of the PR8 strain of IAV. Intraperitoneal injections of anti-CD25 depletes Tregs without preventing generation of activated effector T cells in adult mouse models of HSV, vaccinia virus, RSV, and IAV infections (14,18,40). The number of CD4+ and CD8+ T cells in the airways was not different between the anti-CD25-treated pups and isotype control-treated pups (Fig. 4A, C). However, the proportion of CD4+ and CD8+ T cells that expressed activation markers CD44hi CD62Llo was significantly increased in the anti-CD25-treated pups compared to the control group at day 11 after infection (Fig. 4B, D). In the lung parenchyma, there were similar numbers of CD4+ T cells between both groups (Fig. 4E). The anti-CD25-treated animals had significantly fewer CD25+ Foxp3+ Tregs in the lung than isotype control-treated pups at day 11 postinfection (Fig. 4F). There was no difference in the number of CD4+CD25−Foxp3+ cells with anti-CD25 treatment (data not shown). After partial depletion of Tregs, the anti-CD25-treated pups had higher proportions of CD4+ T cells that were IL-17a+ at day 18, compared to isotype control-treated pups, although at this late time point Tregs were at their lowest point in anti-CD25-treated pups (Fig. 4G). We found no consistent differences in the Th2 cytokine IL-13, as measured by ELISA in the lungs of control versus anti-CD25-treated pups (Supplementary Fig. S4B).
FIG. 4.
Functional inactivation of T regulatory cells delays viral clearance. Two-day-old C57BL/6 mice were infected i.n. with an LD10 dose of the PR8 strain of IAV and treated i.p. with 100 μg anti-CD25 PC61 or rat IgG1 isotype control. The number of BALF CD4+ (A) or CD8+ (C) T cells and the proportion of CD4+ (B) or CD8+ (D) T cells that were activated (expressing CD44hi and CD62Llow) were determined by flow cytometry. The number of CD4+ (E) T cells, the proportion of CD4+ T cells that were CD25+ Foxp3+ Tregs (F), and the proportion of CD4+ T cells that expressed IL-17a (G) or IFNγ (H) in the lung digest were determined by flow cytometry. Lung viral burden was determined by plaque assay on MDCK cells (I). Data represent the mean ± SD of 6 mice per group for cell analysis and the mean ± SD of n = 4–6 mice for viral burden and are representative of three independent experiments. *p < 0.05 compared to infected anti-CD25-treated pups at the same time point. IFNγ, interferon-γ. i.p., intraperitoneal.
The anti-CD25-treated mice tended to have higher proportions of IFNγ+ CD4+ T cells, compared to control mice at day 11 postinfection (Fig. 4H). Although this was not statistically significant, the pattern was seen consistently for every experiment performed using anti-CD25 (data not shown). Strikingly, although the anti-CD25-treated mice had more activated T cells and more Th1 cells, there was a significantly higher viral burden at day 10 postinfection than in control pups (Fig. 4I). There were no significant differences in body weight between the two groups (Supplementary Fig. S4A). Overall, these data demonstrate that partial depletion of Tregs with the anti-CD25 antibody delayed IAV clearance from the lungs of neonatal mice despite an enhanced T-cell response in the lungs.
T cells in scurfy mice are more activated regardless of infection with IAV
Treatment with anti-CD25 did not completely deplete Treg cells and it has been suggested that the use of anti-CD25 could abrogate effector T-cell function (6). Therefore, we utilized neonatal mice with a natural mutation in the Foxp3 transcription factor, the master regulator of the Treg lineage, to validate the beneficial role of Treg in IAV clearance in the neonatal lung (10). To obtain the Foxp3-deficient (scurfy) pups, heterozygous females with the scurfy mutation were bred to WT C57BL/6 males. All of the pups were infected with an LD10 dose of IAV on day 2 of life, and at each time point the pups were screened for Foxp3 expression in CD4+ T cells by flow cytometry to determine which animals had the scurfy mutation and lacked Tregs. Mice with the scurfy mutation and littermates developed normally over the first 2 weeks of life during the experiments.
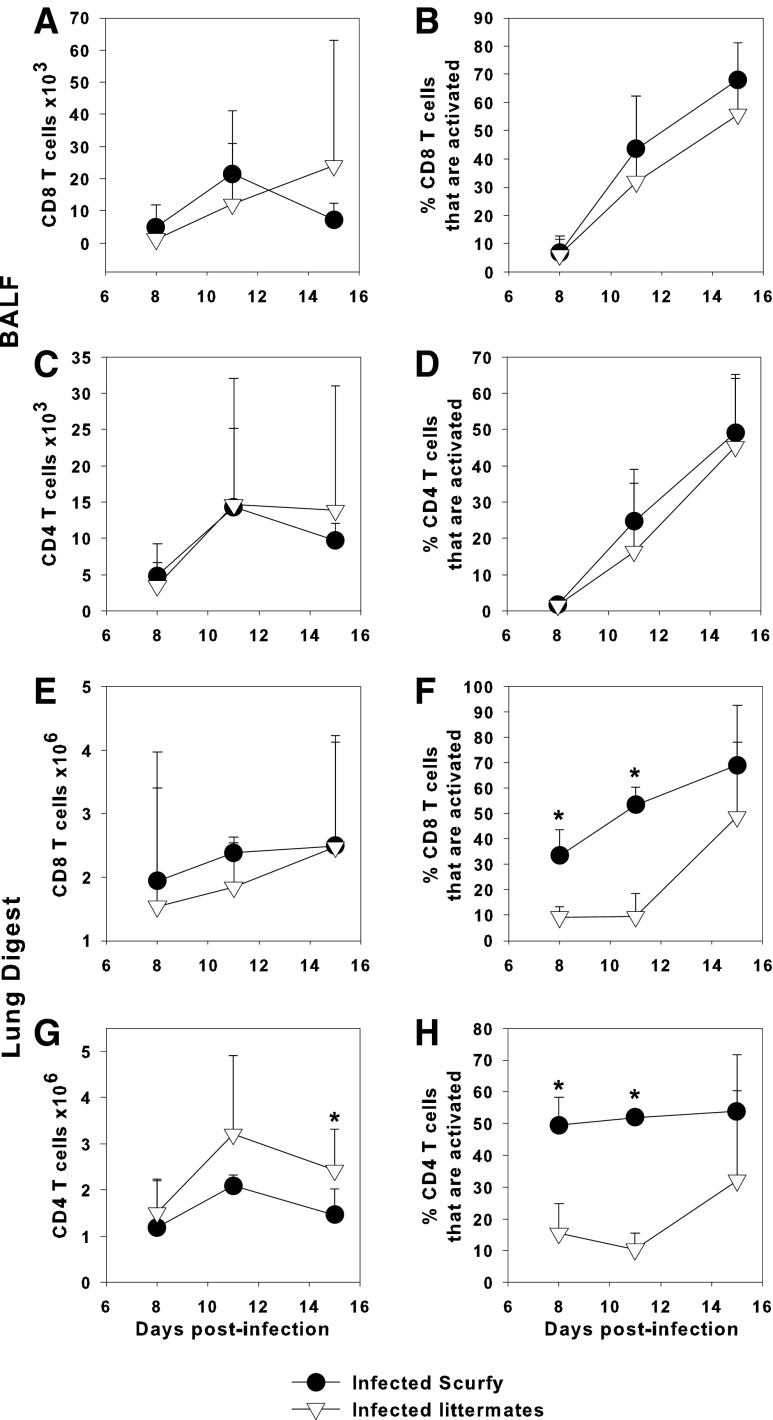
We found that IAV-infected scurfy pups and littermate controls had similar numbers of CD8+ and CD4+ T cells in the BALF (Fig. 5A, C) and the lung digest (Fig. 5E, G). The proportions of CD8+ and CD4+ T cells in the infected scurfy mice that were activated (CD44hi CD62Llo) were similar in the BALF, but significantly elevated in the lung digest compared to infected littermate control pups (Fig. 5B, F, D, H). Moreover, a higher proportion of CD8+ and CD4+ T cells from uninfected scurfy pups had an activated phenotype than the uninfected littermate controls (data not shown). In the lung digest, the proportion of CD8+ and CD4+ T cells that were activated in uninfected scurfy pups resembled infected scurfy and littermate control pups (data not shown). These data indicate that CD4+ and CD8+ T cells from scurfy mice are more highly activated regardless of infection status, which is consistent with the known predilection of Treg-deficient mice to develop a lymphoproliferative disease within a month of age (11). However, this did not correspond to leakage of blood into the lungs since we were unable to detect albumin in the BALF of mice from either group, suggesting that Tregs did not have a role in controlling lung damage in this model of neonatal infection (data not shown).
FIG. 5.
A higher proportion of T cells in scurfy mice are activated than heterozygous controls. Two-day-old scurfy or heterozygous littermate control mice were infected i.n. with an LD10 dose of the PR8 strain of IAV or left uninfected. The number of CD8+ (A, E) or CD4+ (C, G) T cells and the proportion of CD8+ (B, F) or CD4+ (D, H) T cells that were activated (CD44hi CD62Llow) in the BALF (A–D) or lung digest (E–H) were determined by flow cytometry. Data represent the mean ± SD of 3–9 mice per group and are representative of three independent experiments. *p < 0.05 between groups at the same time point.
Tregs inhibit the Th2 response to IAV
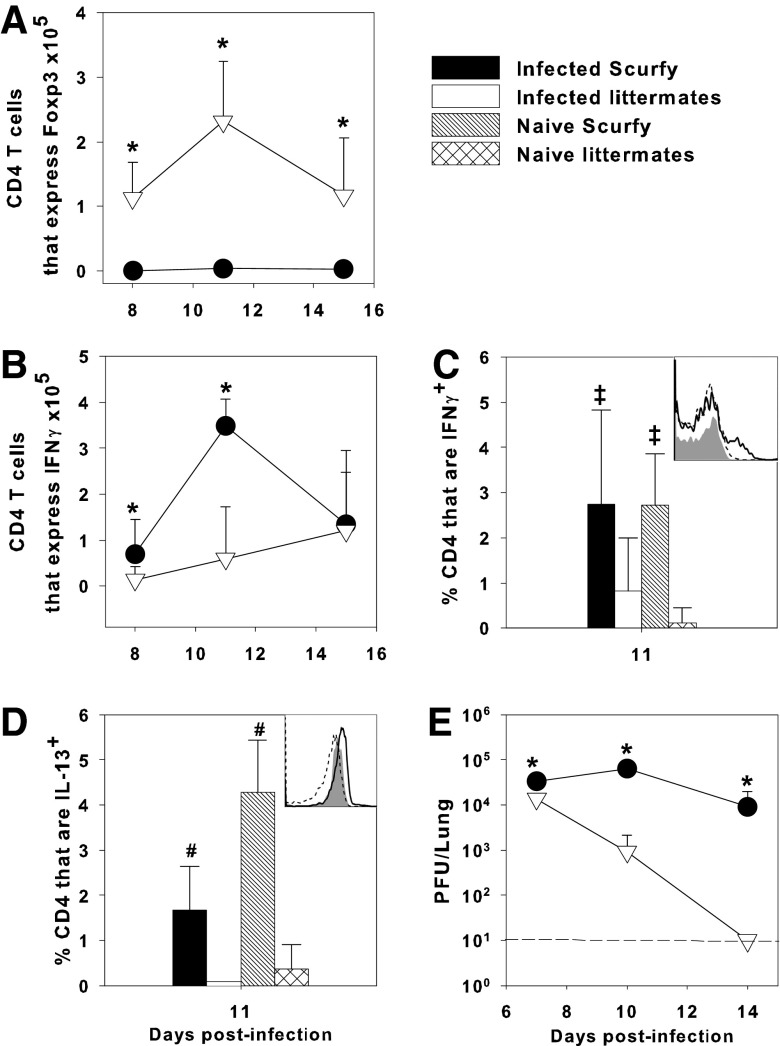
Unlike the anti-CD25-treated pups in which there was only a reduction in the number of Tregs (Fig. 4F), scurfy mice had no detectable Treg cells in the lungs (Fig. 6A). It has been previously reported that adult mice depleted of Tregs had increased IFNγ production by T cells (14). Similarly, we found that the number of CD4+ T cells expressing IFNγ in infected scurfy mice at day 11 postinfection was significantly elevated over infected littermate controls (Fig. 6B, C). Moreover, there was a 34% increase in the concentration of IFNγ in the lung homogenates of scurfy mice compared to littermate controls at day 11 postinfection, as measured by ELISA (data not shown). Uninfected (naïve) scurfy pups also had significant numbers of CD4+IFNγ+ cells in the lungs (Fig. 6C), consistent with the lymphoproliferative disease that begins at day 14 of life (11,12).
FIG. 6.
Tregs inhibit IL-13 production by CD4+ T cells and protect against IAV. Two-day-old scurfy or heterozygous littermate control mice were infected i.n. with an LD10 dose of the PR8 strain of IAV or left uninfected. Flow cytometry was used to determine the number of CD4+ Foxp3+ T cells in the lung digest (A) and the proportion of CD4+ T cells expressing IFNγ (B, C) or IL-13 (D) in the lungs over time. Insets (C, D) show representative histograms of IFNγ or IL-13 expression on CD4+ lung digest cells of scurfy (solid line), littermate controls (dotted line), and an isotype control (shaded histogram). Lung viral burden was determined by plaque assay on MDCK cells (E). Data represent the mean ± SD of 3–9 mice per group and are representative of three experiments. *p < 0.05 for infected scurfy compared to infected heterozygous littermate control, #p < 0.05 compared to all other groups, ‡p < 0.05 compared to naive littermates.
Because we previously found that Th2 cytokines are elevated in influenza virus-infected neonates compared to adults (21), we examined IL-4 and IL-13 protein levels in the lung homogenates of influenza virus-infected scurfy and littermate control mice. Unlike what we found in the anti-CD25-treated pups, scurfy mice had about a twofold higher concentration of IL-13 compared to littermates (10.3 ± 3.5 pg/mg protein vs 5.4 ± 0.7 pg/mg protein in scurfy and littermates, respectively), but there was no difference in the concentration of IL-4 (4.4 ± 2.9 and 3.5 ± 2.9 pg/mg protein, respectively) between the two groups at day 11 postinfection. The proportion of CD4+ T cells that expressed IL-13 was significantly higher in infected scurfy than infected littermates (Fig. 6D). This was not driven by IAV infection because naive scurfy pups also had a significantly higher proportion of CD4+IL-13+ cells than infected or naive littermate controls (Fig. 6D). Importantly, similar to our findings in anti-CD25 Treg-depleted pups, scurfy pups had a significantly higher viral burden than infected littermate controls (Fig. 6E). Together, these data demonstrate that Treg cells are important in facilitating clearance of IAV infection in neonatal mice.
Discussion
The main function of Treg cells is to modulate immune responses. For instance, these cells have been shown to prevent deleterious immune responses by dampening the response to self-antigens as well as turning off proinflammatory responses to foreign antigens (26,34,45). The beneficial anti-inflammatory activity of Treg cells is counterbalanced by the negative outcomes such as allowing viral escape and contributing to immune tolerance to tumors (26,34,45). Neonates have significant numbers of Treg cells in the lungs, which may suggest that they are strategically placed to help prevent inflammation during postnatal lung development. We hypothesized that Tregs would suppress migration of T cells into the lungs of neonatal mice that develop interstitial pneumonia in response to IAV. However, we found that in the absence of Tregs, migration of T cells into the airways was no better than in the presence of Tregs. Instead, we made the surprising discovery that Tregs are actually critical for promoting clearance of IAV in neonates.
Loss of activity of two regulatory cytokines, IL-10 and TGFβ (through Smad4 signaling), did not improve T-cell entry into the airways of neonatal mice (Figs. 2 and 3). TGFβ signaling can either be transduced through phosphorylation of Smad proteins or through less characterized alternative pathways through MAPK, PI3 kinase, or Rho GTPase (20,43). Phosphorylated Smad2/3 can also interact with either Smad4 or TIF1γ to regulate gene expression (43). It is possible that in the Lck-Smad4co pups, TGFβ could have exerted its immunoregulatory activity through an alternative pathway. In a neonatal mouse model of Pneumocystis infection, we found that antibody neutralization of TGFβ in IL-10 KO pups resulted in increased numbers of CD4+ cells in the airways, which suggested there are redundant mechanisms for controlling lymphocyte infiltration into the airways (17). Others have reported that conditional KO mice with the TGFβ receptor II deleted on CD11c+ cells lacked Tregs and exhibited a similar phenotype to scurfy mice (29). We have experiments planned using the conditional TGFβ receptor II KO mice that may provide definitive answers regarding the role of TGFβ in suppressing immune responses in neonatal mice.
Tregs can use IL-10 to dampen immune responses. Our laboratory has reported that neonatal mice infected with Pneumocystis carinii had significantly higher levels of IL-10 mRNA in the lungs than infected adult mice (28). In this study, we showed that there were no significant differences in T-cell activation or entry into the airway in IL-10 KO pups compared to controls (Fig. 3). In contrast to our findings, it has been shown that blocking the IL-10 receptor in adult mice in the midst of an immune response to a lethal dose of IAV caused severe inflammation in the lung and increased mortality (38). The same study showed that during pathogenic IAV infection, IL-10 was produced by effector T cells and was necessary to modulate the inflammatory response (38). Other reports demonstrated that IL-10 KO mice had increased IAV clearance due to anti-influenza antibody titers that depended on help from CD4+ T cells (39). Although antibody responses were not analyzed here, neonatal CD4+ T cells have been shown to be less capable of helping B cells in a humoral response due, in part, to reduced levels of CD40L (27). Our data indicate that anti-inflammatory cytokines IL-10 had no effect on Treg cell numbers or clearance of a sublethal dose of IAV from the lungs of neonatal mice.
Debate exists regarding whether the anti-CD25 antibody depletes or functionally inactivates Tregs (16,37,48). Regardless, studies have found that a systemic injection of the anti-CD25 antibody neutralizes the suppressive effects of Tregs (3,14), but may also inadvertently deplete activated T cells (41). We found that depleting Tregs using the anti-CD25 antibody significantly enhanced T-cell activation (Fig. 4B, D). The number of IFNγ-expressing CD4+ T cells in anti-CD25-treated pups was increased (although not statistically significant) at day 11 postinfection (Fig. 4H). Surprisingly, anti-CD25-treated pups had significantly higher lung IAV burdens over control-treated pups at day 10 postinfection despite the presence of higher proportions of activated T cells in the lungs of Treg-depleted pups. This is in contrast to reports indicating that anti-CD25 depletion in adult mice had no effect on IAV burden (3).
It has been reported that adult mice treated with the anti-CD25 antibody had partial depletion of Tregs but, unlike our neonatal mice, there were no differences in IAV clearance compared to isotype-control-treated adult mice (3). This could be due to the fact that the adult T cells responded to IAV with a Th1 biased immune response, whereas we have shown that neonatal T cells have a mixed Th1/Th2 response and delayed viral clearance compared to adults (21). It has been demonstrated that a Th2 biased response is not protective against IAV (13) and instead contributes to clustered T cells in the lung parenchyma (4). In this study, we observed that partial depletion of Tregs using anti-CD25 did not affect IL-13 production in the lungs; however, in the complete absence of Treg cells in scurfy pups, significantly more CD4+ T cells expressed IL-13 (Fig. 6D).
The Openshaw laboratory reported that adult mice with an inducible deletion of Foxp3 produced increased IL-13 responses to RSV infection, which corresponded with increased lung pathology (7). In adult models of RSV infection, depletion of Tregs using anti-CD25 increased the viral burden despite increased accumulation of RSV-specific CD8+ T cells early after infection (18,31,32). In contrast, Fernandez et al. found that depletion of Tregs in neonatal mice during herpes simplex 2 infection enhanced viral clearance (9). The strains of HSV-2 used in this study induced a Th1 response from neonatal mice, which could account for the differences in viral clearance between our systems (8).
Neonatal Tregs have been shown to inhibit conventional T-cell proliferation as efficiently as do natural adult Tregs (44). This is consistent with our findings of elevated proportions of activated T cells in both CD25-depleted and scurfy pups making it curious that these pups had difficulty clearing infection. A recent article from León et al. demonstrated that Treg cells promote T follicular helper (Tfh)-cell function and germinal center B-cell responses to IAV infection (19). They also showed that Tregs control excessive IL-2 signaling, which suppressed Tfh-cell differentiation. We did not examine Tfh- or B-cell function in the draining lymph nodes, but this is a potential mechanism we are interested in.
The original goal of this work was to determine whether Tregs were responsible for the inability of neonatal T cells to migrate efficiently to the airways as we have previously reported (21). However, we found that in the absence of Tregs, there were some differences in activation of T cells, but migration was no different than in the presence of Tregs. Instead, we demonstrated that Treg cells have an important role in promoting the control of IAV clearance in neonatal mice. Although we were unable to identify a mechanism for this activity of Tregs, we did show that it is not due to IL-10, TGFβ signaling through Smad4, or possibly by promoting IL-13 production. We did not examine the B-cell or antibody response in neonates and so future efforts will be in that direction.
Supplementary Material
Acknowledgments
This work was supported by the NIH Grant R21AI083528 to B.A.G. and a predoctoral fellowship from the American Heart Association to J.L.L. The authors thank Weihua Jiang for expert technical assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Adkins B, and Hamilton K. Freshly isolated, murine neonatal T cells produce IL-4 in response to anti-CD3 stimulation. J Immunol 1992;149:3448–3455 [PubMed] [Google Scholar]
- 2.Alejandre-Alcazar MA, Michiels-Corsten M, et al. TGF-β signaling is dynamically regulated during the alveolarization of rodent and human lungs. Dev Dyn 2008;237:259–269 [DOI] [PubMed] [Google Scholar]
- 3.Betts RJ, Ho AW, and Kemeny DM. Partial depletion of natural CD4(+)CD25(+) regulatory T cells with anti-CD25 antibody does not alter the course of acute influenza A virus infection. PLoS One 2011;6:e27849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerwenka A, Morgan TM, Harmsen AG, et al. Migration kinetics and final destination of type 1 and type 2 CD8 effector cells predict protection against pulmonary virus infection. J Exp Med 1999;189:423–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cottey R, Rowe CA, and Bender BS. Influenza virus. Curr Protoc Immunol 2011;42:19.11.11–19.11.32 [DOI] [PubMed] [Google Scholar]
- 6.Couper KN, Lanthier PA, Perona-Wright G, et al. Anti-CD25 antibody-mediated depletion of effector T cell populations enhances susceptibility of mice to acute but not chronic Toxoplasma gondii infection. J Immunol 2009;182:3985–3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durant LR, Makris S, Voorburg CM, et al. Regulatory T cells prevent Th2 immune responses and pulmonary eosinophilia during respiratory syncytial virus infection in mice. J Virol 2013;87:10946–10954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans IAC, and Jones CA. HSV induces an early primary Th1 CD4 T cell response in neonatal mice, but reduced CTL activity at the time of the peak adult response. Eur J Immunol 2005; 35:1454–1462 [DOI] [PubMed] [Google Scholar]
- 9.Fernandez MA, Puttur FK, Wang YM, et al. T regulatory cells contribute to the attenuated primary CD8+ and CD4+ T cell responses to herpes simplex virus type 2 in neonatal mice. J Immunol 2008;180:1556–1564 [DOI] [PubMed] [Google Scholar]
- 10.Fontenot JD, and Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol 2005;6:331–337 [DOI] [PubMed] [Google Scholar]
- 11.Godfrey VL, Wilkinson JE, Rinchik EM, et al. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: potential model for thymic education. Proc Natl Acad Sci U S A 1991;88:5528–5532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godfrey VL, Wilkinson JE, and Russell L. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am J Pathol 1991;138:1379–1386 [PMC free article] [PubMed] [Google Scholar]
- 13.Graham MB, Braciale VL, and Braciale TJ. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med 1994;180:1273–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haeryfar SMM, DiPaolo RJ, Tscharke DC, et al. Regulatory T cells suppress CD8+ T cell responses induced by direct priming and cross-priming and moderate immunodominance disparities. J Immunol 2005;174:3344–3351 [DOI] [PubMed] [Google Scholar]
- 15.Kim BG, Li C, Qiao W, et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature 2006;441:1015–1019 [DOI] [PubMed] [Google Scholar]
- 16.Kohm AP. JMcMahon JS, Podojil JR, et al. 2006. Cutting edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol 2006;176:3301–3305 [DOI] [PubMed] [Google Scholar]
- 17.Kurkjian C, Hollifield M, Lines JL, et al. Alveolar macrophages in neonatal mice are inherently unresponsive to Pneumocystis murina infection. Infect Immun 2012;80:2835–2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee DC, Harker JA, Tregoning JS, et al. CD25+ natural regulatory T cells are critical in limiting innate and adaptive immunity and resolving disease following respiratory syncytial virus infection. J Virol 2010;84:8790–8798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.León B, Bradley JE, Lund FE, et al. FoxP3+ regulatory T cells promote influenza-specific Tfh responses by controlling IL-2 availability. Nat Commun 2014;5:3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li MO, Wan YY, Sanjabi S, et al. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 2006;24:99–146 [DOI] [PubMed] [Google Scholar]
- 21.Lines JL, Hoskins S, Hollifield M, et al. The migration of T cells in response to influenza virus is altered in neonatal mice. J Immunol 2010;185:2980–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund JM, Hsing L, Pham TT, et al. Coordination of early protective immunity to viral infection by regulatory T cells. Science 2008;320:1220–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masui T, Wakefield LM, Lechner JF, et al. Type beta transforming growth factor is the primary differentiation-inducing serum factor for normal human bronchial epithelial cells. Proc Natl Acad Sci U S A 1986;83:2438–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKenna KC, and Previte DM. Influence of CD8+ T regulatory cells on intraocular tumor development. Front Immunol 2012;3:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murai M, Turovskaya O, Kim G, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol 2009;10:1178–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen TL, Sullivan NL, Ebel M, et al. Antigen-specific TGF-β-induced regulatory T cells secrete chemokines, regulate T cell trafficking, and suppress ongoing autoimmunity. J Immunol 2011;187:1745–1753 [DOI] [PubMed] [Google Scholar]
- 27.Nonoyama S, Penix IA, Edwards CP, et al. Diminished expression of CD40 ligand by activated neonatal T cells. J Clin Invest 1995;95:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qureshi MH, Cook-Mills J, Doherty DE, et al. TNF-α-dependent ICAM-1- and VCAM-1-mediated inflammatory responses are delayed in neonatal mice infected with Pneumocystis carinii. J Immunol 2003;171:4700–4707 [DOI] [PubMed] [Google Scholar]
- 29.Ramalingam R, Larmonier CB, Thurston RD, et al. Dendritic cell-specific disruption of TGF-beta receptor II leads to altered regulatory T cell phenotype and spontaneous multiorgan autoimmunity. J Immunol 2012;189:3878–3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roux X, Remot A, Petit-Camurdan A, et al. Neonatal lung immune responses show a shift of cytokines and transcription factors toward Th2 and a deficit in conventional and plasmacytoid dendritic cells. Eur J Immunol 2011;41:2852–2861 [DOI] [PubMed] [Google Scholar]
- 31.Ruckwardt TJ, Bonaparte KL, Nason MC, et al. Regulatory T cells promote early enflux of CD8+ T cells in the lungs of respiratory syncytial virus-infected mice and diminish immunodominance disparities. J Virol 2009;83:3019–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruckwardt TJ, Malloy AM, Gostick E, et al. Neonatal CD8 T-cell hierarchy is distinct from adults and is influenced by intrinsic T cell properties in respiratory syncytial virus infected mice. PLoS Pathog 20117:e1002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakaguchi S, Sakaguchi N, Asano M, et al. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995;155:1151–1164 [PubMed] [Google Scholar]
- 34.Sakaguchi S, Yamaguchi T, Nomura T, et al. 2008. Regulatory T cells and immune tolerance. Cell 2008;133:775–787 [DOI] [PubMed] [Google Scholar]
- 35.Sarween N, Chodos A, Raykundalia C, et al. 2004. CD4+CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J Immunol 2004;173:2942–2951 [DOI] [PubMed] [Google Scholar]
- 36.Singh RP, La Cava A, Wong M, et al. CD8+ T cell-mediated suppression of autoimmunity in a murine lupus model of peptide-induced immune tolerance depends on Foxp3 expression. J Immunol 2007;178:7649–7657 [DOI] [PubMed] [Google Scholar]
- 37.Stephens LA, and Anderson SM. Comment on “Cutting edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells”. J Immunol 2006;177:2036; author reply 2037–2038 [DOI] [PubMed] [Google Scholar]
- 38.Sun J, Madan R, Karp CL, et al. 2009. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med 2009;15:277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun K, Torres L, and Metzger DW. 2010. A detrimental effect of interleukin-10 on protective pulmonary humoral immunity during primary influenza A virus infection. J Virol 2010;84:5007–5014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suvas S, Kumaraguru U, Pack CD, et al. 2003. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med 2003;198:889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tenorio EP, Fernandez J, Olguin JE, et al. Depletion with PC61 mAb before Toxoplasma gondii infection eliminates mainly Tregs in BALB/c mice, but activated cells in C57BL/6J mice. FEMS Immunol Med Microbiol 2011;62:362–367 [DOI] [PubMed] [Google Scholar]
- 42.Tran DQ. TGF-beta: the sword, the wand, and the shield of FOXP3(+) regulatory T cells. J Mol Cell Biol 2012;4:29–37 [DOI] [PubMed] [Google Scholar]
- 43.Travis MA, and Sheppard D. TGF-β activation and function in immunity. Ann Rev Immunol 2014;32:51–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang G, Miyahara Y, Guo Z, et al. “Default” generation of neonatal regulatory T cells. J Immunol 2010;185:71–78 [DOI] [PubMed] [Google Scholar]
- 45.Wing JB, and Sakaguchi S. Multiple Treg suppressive modules and their adaptability. Front Immunol 2012;3:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang X, Li C, Herrera P-L, et al. Generation of Smad4/Dpc4 conditional knockout mice. Genesis 2002;32:80–81 [DOI] [PubMed] [Google Scholar]
- 47.You D, Ripple M, Balakrishna S, et al. Inchoate CD8+ T cell responses in neonatal mice permit influenza-induced persistent pulmonary dysfunction. J Immunol 2008;181:3486–3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zelenay S, and Demengeot J. Comment on “Cutting edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells”. J Immunol 2006;177:2036–2037; author reply 2037–2038 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.