SUMMARY
Wild-type D. melanogaster males innately possess the ability to perform a multi-step courtship ritual to conspecific females. The potential for this behavior is specified by the male-specific products of the fruitless (fruM) gene; males without fruM do not court females when held in isolation. We show that such fruM null males acquire the potential for courtship when grouped with other flies; they apparently learn to court flies with which they were grouped, irrespective of sex or species, and retain this behavior for at least a week. The male-specific product of the doublesex gene (dsxM) is necessary and sufficient for the acquisition of the potential for such experience-dependent courtship. These results reveal a process that builds, via dsxM and social experience, the potential for a more flexible sexual behavior, which could be evolutionarily conserved as dsx related genes that function in sexual development are found throughout the animal kingdom.
Graphical Abstract
INTRODUCTION
A fundamental goal of neuroscience is to elucidate how the neural circuits embodying the potential for particular behaviors are established. When this goal is considered from a neurogenetic perspective a salient feature of behaviors is that they can be categorized as falling across a spectrum ranging from innate (i.e. built into the nervous system during development) to learned (i.e. acquired by experience) with many behaviors having some aspects that are innate and other aspects that are learned. Such a categorization of behaviors highlights two topics central to our findings. The first concerns whether there are commonalties as to how the potentials for innate behaviors and learned behaviors are acquired by the nervous system, or, alternatively, are they established by different mechanisms. Second, the past decade has seen a heightened interest in innate behaviors. This interest is, in part, because innate behaviors by their very nature are built into the nervous system during development, which makes it likely that they are (at some level) genetically specified. Thus genetically tractable animal species offer the possibilities of identifying genes that specify innate behaviors and then employing these genes to generate reagents that can be used to probe issues related to this behavior, such as how the potential for that innate behavior is established in the nervous system (Baker et al., 2001).
Among innate behaviors, male courtship in Drosophila melanogaster is of particular interest because the genes responsible for establishing the potential for male courtship behavior have been identified (Baker et al., 2001; Ito et al., 1996; Ryner et al., 1996). These genes are the two terminal genes in the fly sex determination hierarchy, fruitless (fru) and doublesex (dsx), both of which encode sex-specific zinc finger transcription factors (Burtis and Baker, 1989; Ito et al., 1996; Ryner et al., 1996). The male-specific products of the P1 promoter of the fru gene (fruM) are expressed in a dispersed subset of ca. 2000 neurons, which are found mostly in small groups throughout the central and peripheral nervous systems (Cachero et al., 2010; Lee et al., 2000; Manoli et al., 2005; Stockinger et al., 2005; Usui-Aoki et al., 2000; Yu et al., 2010). fruM function is both necessary and sufficient for building the potential for nearly all aspects of male courtship behavior into the nervous system (Demir and Dickson, 2005; Manoli et al., 2005; Manoli et al., 2006). dsx encodes male- and female-specific DSX proteins (DSXM and DSXF, respectively) (Burtis and Baker, 1989), and DSXM is expressed in ca. 700 CNS neurons, the majority of which also express fruM (Billeter et al., 2006; Lee et al., 2002; Rideout et al., 2007; Rideout et al., 2010; Robinett et al., 2010; Sanders and Arbeitman, 2008). DSXM is neither necessary nor sufficient for the execution of nearly all steps comprising courtship behavior (Taylor et al., 1994; Villella and Hall, 1996), but is required for one aspect of courtship song—sine song production (Rideout et al., 2007; Villella and Hall, 1996). In addition, in the absence of dsx function there is a poorly understood general diminution in the level of male courtship (Villella and Hall, 1996).
fruM functions post-mitotically to endow the nervous system with the innate potential for male courtship behavior (Demir and Dickson, 2005; Lee et al., 2000; Manoli et al., 2005; Stockinger et al., 2005; Usui-Aoki et al., 2000). The gross neuroanatomical features of the fruM-expressing circuitry were previously found to be largely unaffected by the loss of fruM, as seen in fruM null males or wild-type females, leading to the conclusion that fruM largely functions to regulate fine neural connectivity or neural physiology (Cachero et al., 2010; Manoli et al., 2005; Stockinger et al., 2005). Indeed, imaging small groups of fruM-expressing neurons showed that the normal morphological development of many of these neurons requires fruM function (Cachero et al., 2010; Kimura et al., 2008; Kimura et al., 2005; Lee and Hall, 2001; Mellert et al., 2010).
While the proposal that fruM functions developmentally to specify the potential for male courtship is strongly supported by extant data, and has motivated and provided a framework for much of the recent work on courtship in Drosophila (Baker et al., 2001), there are features of the data on the role of fruM in courtship that suggest our understanding of the genetic specification of Drosophila male courtship is significantly incomplete. In particular, courtship-like behaviors in the absence of fruM function have been reported (Anand et al., 2001; Shirangi et al., 2006; Villella et al., 1997).
In the present study, we start by confirming the findings (Anand et al., 2001; Villella et al., 1997) that males lacking fruM when housed together (group-housed) for a day or more during adulthood display courtship-like behaviors toward one another (male chaining). We then extend these observations to show that group-housing fruM null males with either conspecific females or females of other Drosophila species also leads to the acquisition by these males of the potential for courtship. In addition, we show that such fruM-independent, experience-dependent courtship behavior requires fruM/dsx overlapping neurons acutely during adulthood. Furthermore, dsxM is both necessary in fruM null males and sufficient in the otherwise wild-type females for the experience-dependent acquisition of the potential for courtship (with both conspecifics and other species). Finally, we show that dsxM- and experience-dependent courtship has properties indicative of learning and memory in that (1) the ability to court acquired via group-housing is retained for at least a week after being removed from that group, and (2) the sex and species of the flies fruM null males are group-housed with influences the male’s courtship preferences in subsequent tests. Thus our findings uncover a dsxM- and experience-dependent pathway that is utilized by animals to acquire and modify courtship behavior based on their adult experiences. Some evolutionary implications of these findings are discussed.
RESULTS
Courtship Behavior of Isolated and Group-housed fruM Null Males
For typical male-female courtship assays individual males are collected at eclosion and subsequently housed in isolation for 4–6 days prior to a single-pair (10 min) courtship test. With this protocol (1) wild-type control males intensively court virgin females [Courtship Index (CI) > 60%, which is the fraction of observation time that males courted], but rarely court males, whereas (2) fruM null males (fruLexA/fru4-40) (Mellert et al., 2010) do not court females, and only rarely court males (Figure 1A). These observations replicate previous findings that contributed to the proposal that wild-type fruM function specifies the potential for male courtship (Anand et al., 2001; Demir and Dickson, 2005; Ito et al., 1996; Manoli et al., 2005; Ryner et al., 1996).
Figure 1.
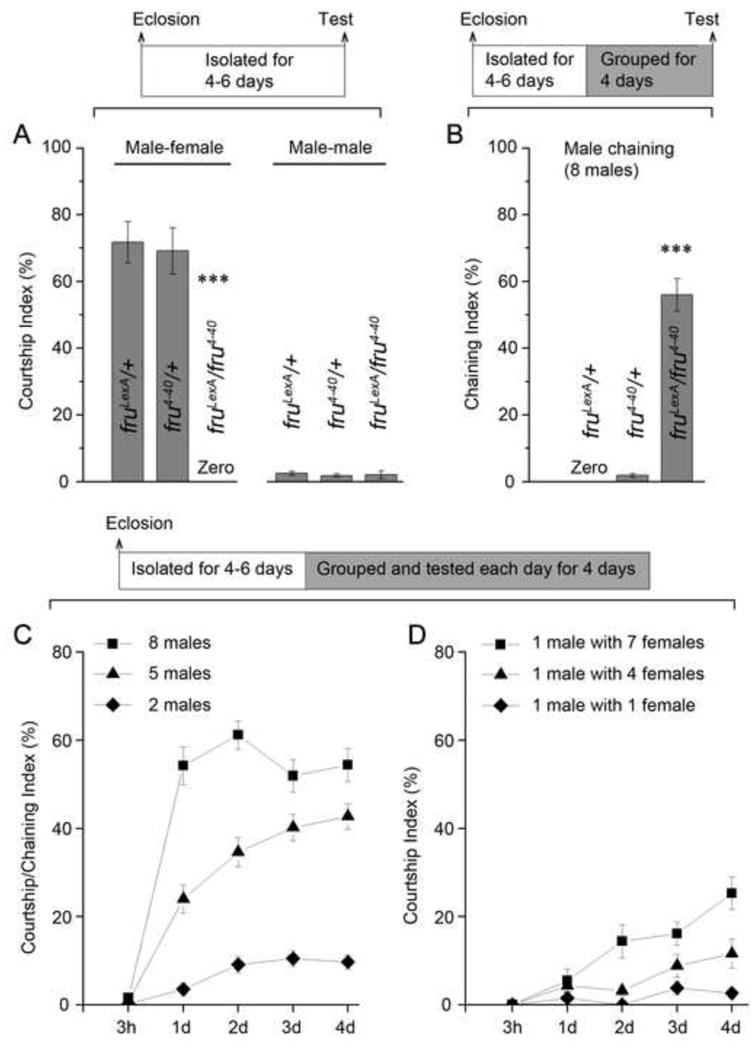
In the Absence of fruM Function D. melanogaster Males Acquire the Ability to Court as a Consequence of Being Group-housed with Other Flies. (A) Courtship indices of males that had been isolated since eclosion prior to a 10-min single-pair courtship test. ***p < 0.001, one-way ANOVA. (B) Chaining indices of groups of 8 males that had been housed together for 4 days. ***p < 0.001, one-way ANOVA. (C) Chaining indices of groups of 8 or 5 fruLexA/fru4-40 males and courtship indices between 2 fruLexA/fru4-40 males after grouping for 3 hours, 1 day, 2 days, 3 days, and 4 days. (D) Courtship indices of fruLexA/fru4-40 males to wild-type females after grouping (in groups of 1 male with 7 females, 1 male with 4 females, or 1 male with 1 female) from 3 hours to 4 days. n = 8~12 for chaining behavior, and n = 12~24 for others. n refer to number of pairs or groups, housing conditions are indicated in the top of each panel (apply to all figures). Error bars indicate SEM. Please see Figure S1, Movies S1 and S2.
In contrast, for assaying courtship-like interactions between multiple males, males are collected at eclosion, housed singly for 4–6 days, and then put together in groups of 8 males for 4 days during which they were assayed daily for male-male courtship within the group (Anand et al., 2001; Villella et al., 1997). With this protocol fruM null males showed intense courtship as quantified by a Chaining Index (ChI > 50%, the fraction of time at least 3 males in the group engaged in courtship together) (Figure 1B, see Movie S1), a phenotype that has also been observed in other fruM null genotypes (Anand et al., 2001; Villella et al., 1997). Groups of 8 wild-type control males that were treated identically did not exhibit chaining behavior (ChI ~0; Figure 1B).
Thus there is a striking difference between the courtship behaviors displayed by fruM null males housed singly and tested in a pair-wise courtship assay vs. housed in groups and tested in a chaining assay. Comparing the protocols for these two behavioral assays suggests that the contrasting results could be caused by (1) housing conditions (housed singly before test vs. housed in groups for 4 days before test), or (2) number of flies in a tested group (2 vs. 8), or (3) the target’s sex (male vs. female). The following experiments distinguish between these possibilities.
Acquisition of both Male-male and Male-female Courtship in fruM Null Males via Group-housing
We first tested chaining behavior in groups of 8 fruLexA/fru4-40 males daily across the 4 days subsequent to grouping them together. Chaining behavior was observed at a low level following grouping for 3 hours (ChI[3h] = 1.6 ± 0.4%), and reached a high level after grouping for one or more days (ChIs[1d, 2d, 3d, 4d] > 50%) (Figure 1C), consistent with previous findings (Anand et al., 2001; Villella et al., 1997). We then tested groups of either 5 or 2 fruM null males daily for 4 days, and also found increased levels of chaining (5 males) or male-male courtship (2 males) as a function of time they were grouped (Figure 1C). These results suggest that it is the group-housing experience that induces chaining/courtship in fruM null males.
We next inquired whether prolonged housing of fruM null males with females also led to elevated levels of male-female courtship. We therefore placed single fruLexA/fru4-40 males (4–6 day old adults kept in isolation since eclosion) together with 7 wild-type virgin females for 4 days during which male-female courtship was assayed daily. As expected these fruM null males did not court females after 3 hr (CI = 0); however, these males very substantially increased their courtship to females after grouping for one or more days (CIs[1d, 2d, 3d, 4d] are 5.5 ± 2.6%, 14.4 ± 3.8%, 16.1 ± 2.6%, and 25.3 ± 3.7% respectively, Figure 1D, see Movie S2). Increased courtship was also found when 1 male/4 females and 1 male/1 female combinations were housed together for varying periods of time prior to assaying courtship (Figure 1D). As male-male and male-female courtship behaviors (including chaining) were both observed in fruLexA/fru4-40 males, these behaviors are hereafter collectively referred to as courtship.
Examining courtship by males of 3 other fruM null genotypes (fruLexA/frusat15, fru4-40/frusat15, fru4-40/fruAJ96u3, with the latter two genotypes lacking both fru P1 and P2 products) (Anand et al., 2001; Mellert et al., 2010; Song et al., 2002) after grouping from 3 hours to 4 days replicated the above findings with fruLexA/fru4-40 males (Figure S1). These results establish that males without fruM function are able to court both male and female targets when group-housed for one or more days prior to testing.
FRUM-independent Courtship Behavior Requires the fruM/dsx Circuitry
As noted above the male CNS contains ~2000 fruM-expressing neurons as well as ~700 dsx-expressing neurons, with many dsx-expressing neurons also expressing fruM. The morphology of fruM and dsx overlapping neurons in both the brain and ventral nerve cord (VNC), as specifically labeled by the intersection of fruLexA and dsxGAL4(Δ2) expression (Figure 2A), was not grossly different in males with or without FRUM function (Figures 2B and 2C, respectively), although detailed differences could be found, such as there is no midline crossing from gustatory receptor neurons, compared to males expressing FRUM (Mellert et al., 2010) (Figures 2C).
Figure 2.
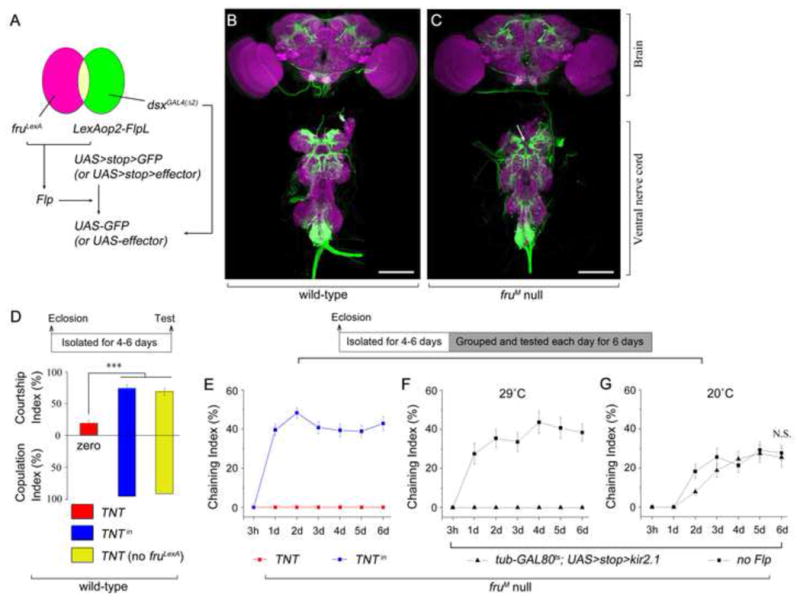
fruM and dsxM Overlapping Neurons Are Required for both Wild-type Courtship and fruM-independent Courtship. (A) A schematic of genetic strategy to label and manipulate fruM and dsx overlapping neurons. (B and C) Intersectional expression of fruLexA and dsxGAL4(Δ2) in wild-type (B) and fruM null (C) backgrounds (genotypes: LexAop2-FlpL/UAS>stop>myrGFP; dsxGAL4(Δ2) fruLexA/+ and LexAop2-FlpL/UAS>stop>myrGFP; dsxGAL4(Δ2) fruLexA/fru4-40, respectively). Arrow indicates no midline crossing by gustatory receptor neurons in fruM null background. Scale bars: 100 μm. (D) Courtship indices (upper panel) and copulation indices (lower panel) by males that have fruM function. ***p < 0.001, one-way ANOVA. n = 24 for each. (E–G) Chaining indices by fruM null males after grouping from 3 hours to 6 days. n = 12 for each. N.S., not significant, by comparing chaining indices of day 6. Detailed genotypes are described in text. Error bars indicate SEM.
Given the presence of fruM and dsx overlapping neurons in both wild-type males and males lacking fruM function, we asked whether (some of) these neurons were required for fruM -independent courtship behavior.
In males that have FRUM function, when fruM and dsx overlapping neurons were silenced by expressing tetanus toxin light chain (TNT) (LexAop2-FlpL/UAS>stop>TNT; dsxGAL4(Δ2) fruLexA/+), courtship of females was severely impaired (CI~ 20%), compared to control males expressing an inactive version of TNT (TNTin) in fruM and dsx overlapping neurons (LexAop2-FlpL/UAS>stop>TNTin; dsxGAL4(Δ2) fruLexA/+) or control males lacking the fruLexA driver (LexAop2-FlpL/UAS>stop>TNTin; dsxGAL4(Δ2)/+) (CIs~ 70%) (Figure 2D). Furthermore, TNT expressing males did not copulate with females (0 out of 24), while almost all control males copulated with females successfully (within 30 min) (Figure 2D). Thus in males that have FRUM function, synaptic transmission from fruM and dsx overlapping neurons is necessary for robust courtship behavior of females, and are also required for successful copulation.
When TNT was expressed in fruM and dsx overlapping neurons in fruM null males (LexAop2-FlpL/UAS>stop>TNT; dsxGAL4(Δ2) fruLexA/fru4-40), courtship was not observed even after 6 days of grouping (Figure 2E). Control fruM null males expressing TNTin in fruM and dsx overlapping neurons showed strong courtship after 1–6 days of grouping (ChIs ~40%) (Figure 2E).
To further test if the activity of fruM and dsx overlapping neurons is acutely required during adulthood for courtship by fruM null males, we used another neuronal silencer: the inward-rectifying potassium channel Kir2.1 (Baines et al., 2001; Pfeiffer et al., 2010), together with a temperature-sensitive GAL80ts that blocks Kir2.1 expression when flies are reared at lower temperature (18–20°C) but allows expression at 29°C. When LexAop2-FlpL/tub-GAL80ts; dsxGAL4(Δ2) fruLexA/UAS>stop>Kir2.1 fru4-40 males were reared at 18°C, isolated after eclosion at 18°C for 4–6 days, heat shocked at 29°C for 1 day, then tested in groups of 8 males for 6 days at 29°C, they did not show any courtship behavior. Control fruM null males lacking LexAop2-FlpL (tub-GAL80ts/+; dsxGAL4(Δ2) fruLexA/UAS>stop>Kir2.1 fru4-40) under the same conditions showed intensive courtship after grouping for 6 days (ChI ~40%, Figure 2F). When both experimental and control fruM null males were reared at 18°C, isolated after eclosion at 18°C for 5–7 days, then tested in groups of 8 males for 6 days at 20°C, they performed courtship after 1–6 days grouping, although in slightly reduced levels (ChIs 20–30%, Figure 2G), probably due to the lower temperature. These results indicate that activity of fruM and dsx overlapping neurons is necessary during adulthood for courtship acquisition in fruM null males.
dsxM is both Necessary and Sufficient for fruM-independent, Experience-dependent Courtship
That males lacking FRUM develop the potential for courtship when grouped, but wild-type females (which also lack FRUM) do not develop the potential for male courtship when grouped, suggests that non-fruM-specified sexual differences between males and females govern the ability to acquire the potential for fruM-independent courtship. If our understanding of the sex determination hierarchy is correct—that all aspects of somatic sex are controlled via the fruM and/or dsx branches of the hierarchy—then the ability to acquire male courtship behavior via group-housing must be governed by the dsx branch of the sex hierarchy. Thus chromosomal females (XX) that express DSXM in place of DSXF, but are otherwise wild-type, should be equivalent to chromosomal males (XY) that lack FRUM function but are otherwise wild-type (Figure 3A), and such females should be able to acquire the potential for male courtship behavior if grouped.
Figure 3.
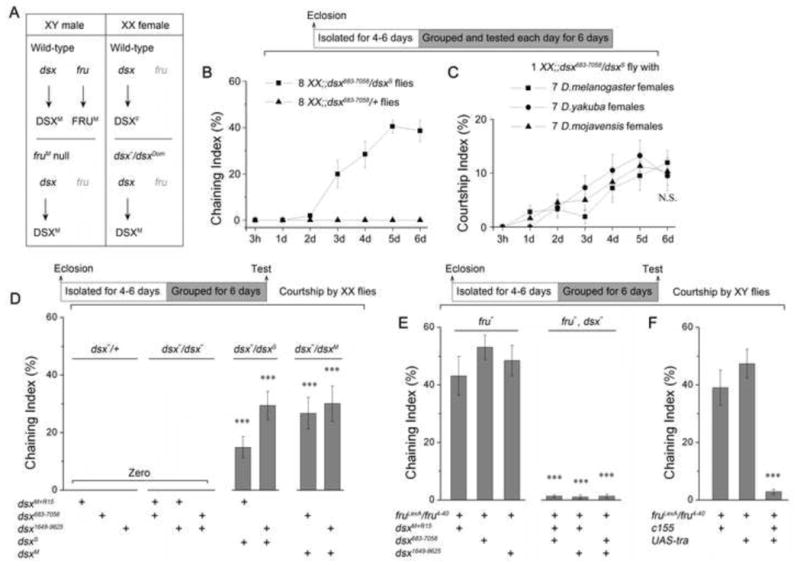
dsxM is Necessary and Sufficient in both XX and XY Flies for fruM-independent Courtship Behavior. (A) Expression of sex-specific products of fruM and dsx in wild-type males and females, as well as in fruM null males and dsx-masculinized females. (B) Chaining indices of groups of 8 XX;;dsx683-7058/dsxS flies after grouping from 3 hours to 6 days. (C) Courtship indices of individual XX;;dsx683-7058/dsxS flies to 7 wild-type females or females of other species (D. yakuba or D. mojavensis). N.S., not significant, by comparing courtship indices of day 6. (D) Chaining indices of groups of 8 XX flies after 6-day grouping. ***p < 0.001, compared with 0, one-sample t-test. (E–F) Chaining indices of groups of 8 XY flies after 6-day grouping. ***p < 0.001, one-way ANOVA. n = 8~12 for each. Genotypes indicated below. Error bars indicate SEM. Please see Movie S3.
To test this prediction, we used dsx alleles, dsxS, dsxM, or dsxD (collectively termed dsxDom), in which dsx pre-mRNA is constitutively spliced in the male pattern (Nagoshi and Baker, 1990), in combination with a dsx deficiency (either Df(3R)dsxf00683-d07058, Df(3R)dsxf01649-d09625 or Df(3R)dsxM+R15; hereafter referred to as dsx683-7058, dsx1649-9625, or dsxM+R15 respectively, and collectively termed dsx−) (Chatterjee et al., 2011; Mellert et al., 2012), to generate XX; dsx−/dsxDom individuals that express only the DSXM protein. The external sexual characteristics of these dsx−/dsxDom females are indistinguishable from those of wild-type males (Baker and Ridge, 1980). When singly housed for 4–6 days post eclosion all XX; dsx−/dsxDom combinations examined failed to court wild-type females in a 10 min single-pair courtship assay (data not shown), consistent with previous findings (Taylor et al., 1994).
We next grouped 8 XX; dsx683-7058/dsxS flies together and examined behavior daily for 6 days. No courtship was observed 3 hours after grouping, but strikingly, these XX individuals showed substantial levels of courtship behavior after grouping for 1–6 days (Figure 3B, see Movie S3). We then grouped 1 XX; dsx683-7058/dsxS fly with 7 wild-type (D. melanogaster) females or females of other species (D. yakuba or D. mojavensis). Strikingly, these DSXM expressing XX flies courted conspecific females as well as females of other species at similar levels when grouped for one or more days (Figure 3C). These results indicate that dsxM is sufficient in an otherwise wild-type female for courtship of both conspecific females or females of other species.
We further tested courtship in groups of 8 XX individuals using four other dsx−/dsxS and dsx−/dsxM allelic combinations, and observed significant courtship behavior in all four genotypes after grouping for 6 days (ChIs are 15% ~ 30%, Figure 3D). XX individuals of 2 dsx−/dsxD combinations examined only rarely courted after 6 days grouping (ChIs ~1%, data not shown), which may be due to inefficiency of dsxD or its genetic background. As controls, XX individuals with either dsx−/dsx− or dsx−/+ combinations did not court after grouping for 6 days (Figure 3D). These experiments further demonstrate that in both XX and XY flies the expression of the DSXM protein (and the concomitant lack of the DSXF and FRUM proteins) are sufficient for the acquisition of the potential for male courtship as a consequence of group-housing.
To examine whether DSXM is necessary for acquiring the potential for FRUM-independent courtship, we group-housed XY individuals that lack both fruM and dsx functions and subsequently tested their ability to court. fruM and dsx double mutant males with 3 different allele combinations rarely courted even after grouping for 6 days (ChIs ~2%), while control fruM null males that express DSXM courted intensively after grouping for 6 days (ChIs ~50%) (Figure 3E). A caveat is that the above manipulations affect dsx activity in all tissues, and thus could obscure its role in the CNS. Therefore we used a pan-neuronal GAL4 driver (c155) to feminize (UAS-tra) only the neurons in fruM null males. Such fruM null males (c155/Y; UAS-tra/+; fruLexA/fru4-40), which express DSXF in place of DSXM just in the nervous system, rarely courted even after grouping for 6 days (ChI ~3%), while control fruM null males expressing DSXM courted intensively after grouping for 6-days (ChIs ~40%), suggesting that DSXM function in the nervous system is necessary for the acquisition of the potential for courtship in fruM null males (Figure 3F). Thus DSXM is both necessary and sufficient for the FRUM-independent, experience-dependent courtship behavior.
Group-housing Induces Long-lasting Courtship Ability in fruM Null Males
We showed above that when fruM null males are grouped with wild-type females for 1–4 days, they courted those females in the chamber in which they were grouped (Figure 1D). Thus factors promoting this courtship could be either: (1) extrinsic (i.e. changes in the chamber itself such as pheromone levels) or (2) intrinsic (i.e. changes in flies’ nervous systems that confer the potential for courtship) to these fruM null males. To distinguish between these possibilities we grouped individual fruM null males with 10 wild-type females in food vials for up to 14 days, then individual males were gently aspirated into a fresh chamber, and a fresh wild-type virgin female was introduced into that chamber ~30 min later for a 10-min courtship test. fruM null males were able to court fresh females after 2 days of grouping (CI is 4.9 ± 3.5%), and such courtship increased and reached to its maximum after 10 days of grouping (CI is 39.7 ± 6.9%) (Figure 4A). fruM null males kept in isolation for 10 or 16 days did not court females at all (Figure 4A). Thus the potential for courtship by fruM null males, which arises consequent to their grouping with females, represents a change intrinsic to those males.
Figure 4.
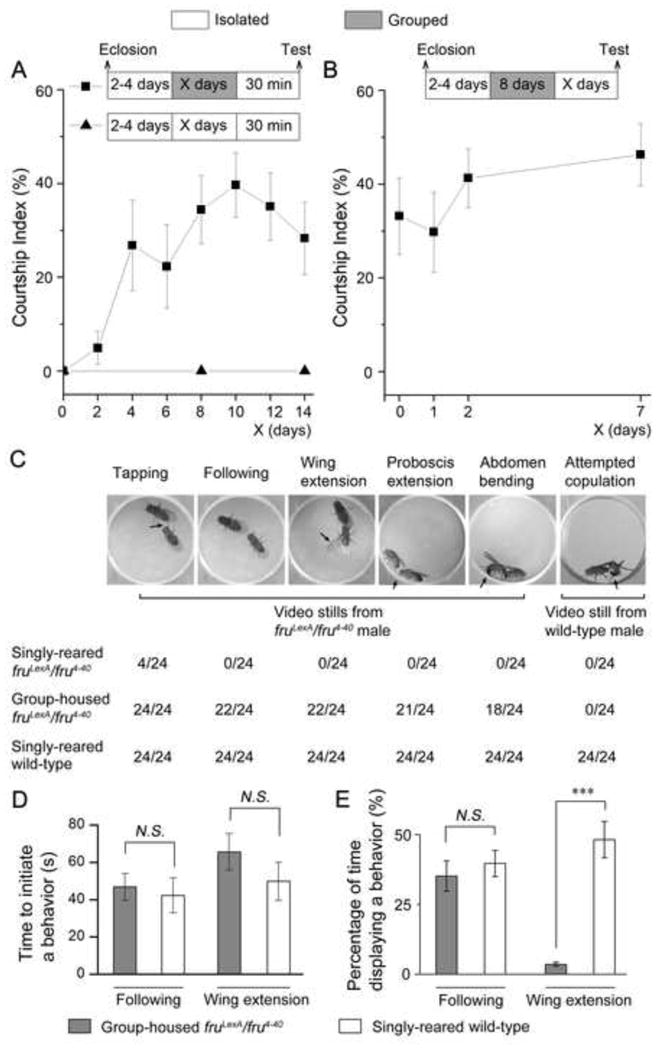
Grouping-induced Courtship Behavior in fruM null Males is Long-lasting. (A) Courtship indices of fruLexA/fru4-40 males, after being isolated (triangle) or grouped with wild-type females (square), to fresh wild-type females. X axis indicates number of days being isolated (triangle) or grouped (square) prior to testing. n = 12 for each. (B) Courtship indices of fruLexA/fru4-40 males, after being grouped with females for 8 days and then isolated from grouping for up to 7 days, to fresh females. X axis indicates number of days being isolated following the 8-day grouping and prior to testing. n = 12 for each. (C) Detail steps of courtship displayed by fruLexA/fru4-40 males after group-housing, compared with courtship by single-housed wild-type males. Arrows indicate tapping, wing extension, proboscis extension, abdomen bending and attempted copulation, respectively. n = 24 for each. (D) Latency of following and wing extension by fruM null males and wild-type males. (E) Percentage of time displaying following and wing extension by fruM null males and wild-type males. N.S., not significant; ***p < 0.001, two-sample t-test. Error bars indicate SEM. Please see Figure S2, Movie S4 and S5.
To examine how long a fruM null male retained the ability to court after removal from a group of females, individual fruM null males were first grouped with females for 8 days, then maintained in isolation for up to 7 days before testing courtship with a fresh female. We found that previously grouped fruM null males still intensively courted females after 7 days of isolation (CI is 46.2 ± 6.6%) (Figure 4B), indicating that a quasi-permanent courtship ability was established via their group-housing experience with females.
We next asked whether mushroom bodies, which are involved in many forms of learning including courtship conditioning in wild-type males (Keene and Waddell, 2007; Villella and Hall, 2008), are also required in the acquisition of the potential for male courtship in fruM null males. To do this we first group-housed individual fruM null males that broadly express the temperature sensitive shibirets1 (shits1) mutant (Kitamoto, 2001; Pfeiffer et al., 2012) in mushroom bodies (UAS-shits1/+; 19B03-GAL4 fruLexA/fru4-40 or UAS-shits1/+; 76D11-GAL4 fruLexA/fru4-40, Figures S2A and S2B) (Jenett et al., 2012; Pfeiffer et al., 2008) with females at permissive temperature (23°C) for 8 days, then isolated those males for a subsequent courtship test with fresh females at either permissive (25°C) or restrictive (30°C) temperature, and found that they courted intensively under both temperatures (Figure S2C), indicating that mushroom bodies are not required during courtship testing. We then silenced mushroom bodies in fruM null males by expressing Kir2.1 (tub-GAL80ts/+; 19B03-GAL4 fruLexA/UAS-kir2.1::GFP fru4-40 or tub-GAL80ts/+; 76D11-GAL4 fruLexA/UAS-kir2.1::GFP fru4-40) during adulthood including both group-housing and courtship testing, and found that these males still courted intensively (Figure S2D), indicating that mushroom bodies are not necessary during either group-housing or testing for the acquisition and manifestation of courtship by fruM null males.
Group-housing Induces Most, but Not All, Steps of Courtship in fruM Null Males
Although previously group-housed fruM null males courted fresh females intensively, they did not copulate and were thus sterile. Analysis of their courtship showed that more than 75% of tested males performed most courtship steps including tapping, following, wing extension (no courtship song was detected, data not shown), licking (proboscis extension) and abdomen bending, but none of them attempted to copulate (by fully curling their abdomen towards a female’s abdomen) (Figure 4C, see Movie S4). These fruM null males initiated following and wing extension as quickly as wild-type males did (Figure 4D); however, they showed a much lower level of wing extension comparing to wild-type courtship (Figure 4E). As fruM null males were isolated 2–4 days prior to grouping with females, we further tested males that were isolated from 0 to 6 days before grouping but did not observe any difference in courtship, suggesting that there is no sensitive period for acquiring courtship during grouping. Thus, group-housing experiences induce most aspects of courtship in fruM null males, but some aspects (such as attempted copulation) are still missing and may be purely fruM-dependent.
Courtship Preference of Group-housed fruM Null Males Depends on the Sex and Species of the Flies They Are Group-housed with
The preceding experiments have established that housing fruM null D. melanogaster males together with groups of either conspecific males or conspecific females leads to these fruM null males acquiring the potential to court the flies they have been housed with. Further, their acquisition of the ability to court is due to semi-permanent change(s) that are intrinsic to these males. We can envision two broad types of mechanisms underlying the manifestation of these changes. First, these changes could be the consequences of an elevated level of general stimulation/excitation/arousal generated by social interactions. Alternatively, these changes could be the consequences of sensory perceptions provided by grouping having specific effects on the courtship circuitry (i.e. learning).
To distinguish between these possibilities we inquired whether grouping fruM null males with different courtship targets (conspecific D. melanogaster males and females, and females of other species) would lead to differences in the quality of the courtship displayed by these males upon testing. Naïve fruM null males that had been isolated until the courtship test only rarely displayed courtship between 2 such males, and did not court D. melanogaster, D. yakuba or D. mojavensis females (Figure 5A). Control wild-type D. melanogaster males, regardless of whether isolated or grouped prior to a courtship test, all courted conspecific females intensively, but rarely courted conspecific males or females of other species (Figures 5F–5I). When fruM null males were housed together in food vials (11 males per vial) for 8 days and then isolated for a subsequent courtship test, 2 such males courted one another intensively (CI is 26.2 ± 3.1%), but rarely courted either conspecific females or females of other species (Figure 5B). In contrast, when individual fruM null males were housed with 10 D. melanogaster females in food vials for 8 days and then isolated for a subsequent courtship test, they courted D. melanogaster male and female targets intensively (CImale-male is 28.7 ± 3.9%, and CImale-female is 34.3 ± 7.3%), and D. yakuba females at a similar level (CI is 25.4 ± 4.5%), but D. mojavensis females at a reduced level (CI is 8.6 ± 1.3%) (Figure 5C). We further found that fruM null males that had been grouped with D. yakuba females courted D. yakuba females, as well as D. melanogaster males and females at similar levels, but D. mojavensis females at a reduced level (Figure 5D). Strikingly, fruM null males that had been grouped with D. mojavensis females courted females of D. mojavensis and D. yakuba, as well as D. melanogaster males at similar levels, but they courted conspecific D. melanogaster females at a reduced level (Figure 5E). These findings demonstrate that the quality of a fruM null male’s grouping experience determines at least some features of the courtship potential he acquires, and thus favor the proposition that group-housing experiences have specific learning effects on the courtship circuitry in fruM null males.
Figure 5.
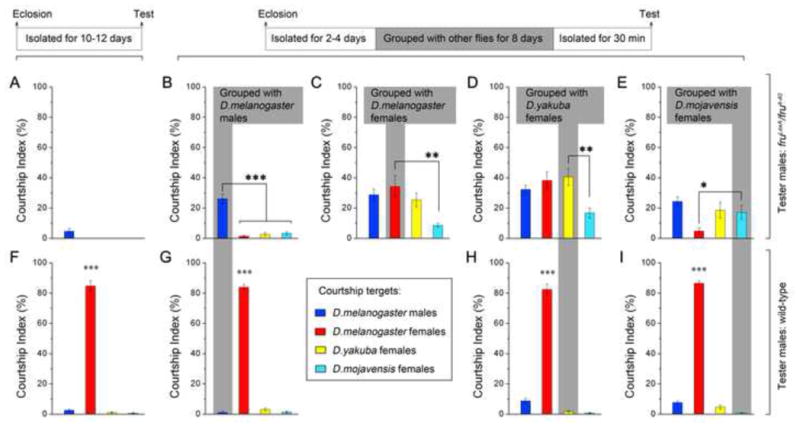
Flexibility of the Grouping-induced Courtship Behavior in fruM Null Males. (AI) All males were tested either between 2 males of the same genotype (blue), towards D. melanogaster females (red), D. yakuba females (yellow), or D. mojavensis females (cyan). (A–E) Courtship indices of fruLexA/fru4-40 males that had been isolated (A), or grouped with males of the same genotype (B), D. melanogaster females (C), D. yakuba females (D), or D. mojavensis females (E) for 8 days prior to testing. *p < 0.05, **p < 0.01 and ***p < 0.001, one-way ANOVA. (F–I) Courtship indices of wild-type males that had been isolated (F), or grouped with males of the same genotype (G), D. yakuba females (H), or D. mojavensis females (I) for 8 days prior to testing. ***p < 0.001, one-way ANOVA. n = 12~24 for each. Error bars indicate SEM.
Our results indicate that courtship by males that lack fruM function is, at least in part learned, as their mate preference is significantly dependent on prior experiences; while courtship by males that have fruM function is innate, and their mate choice is not significantly modifiable by our manipulations, suggesting that fruM may function to suppress dsxM-dependent courtship to inappropriate targets.
Contributions of Sensory Stimulation to the Acquisition of Courtship Behavior in fruM Null Males
The above data suggests that courtship by fruM null males is dependent on sensory perceptions during both (1) a multi-day grouping period that is crucial for courtship acquisition, and (2) a 10-min courtship test period. Here we present our initial experiments to examine the roles of individual sensory modalities in the acquisition and manifestation of courtship behavior in fruM null males.
We firstly examined the role(s) of vision during group-housing and the courtship test. When individual fruLexA/fru4-40 males were group-housed with 10 wild-type females in food vials with constant light for 8 days, then isolated for a subsequent courtship test with intact targets in the light, such males courted both male and female targets intensively (Figures 5C and 6A). When such males were tested with headless (largely stationary) targets, or intact targets in the dark, they rarely courted either male or female targets (Figures 6B and 6C), suggesting that visual information (especially motion detection) is required during testing for courtship by fruM null males, consistent with previous findings with respect to fruM null males with all dsx-expressing neurons activated (Pan et al., 2011). When individual fruLexA/fru4-40 males were group-housed with 10 wild-type females in food vials under constant dark condition for 8 days, then isolated for a subsequent courtship test with intact targets in the light, they did not court either male or female targets (Figures 6D), indicating that vision during group-housing is required for the acquisition of the potential for courtship behavior by fruM null males.
Figure 6.
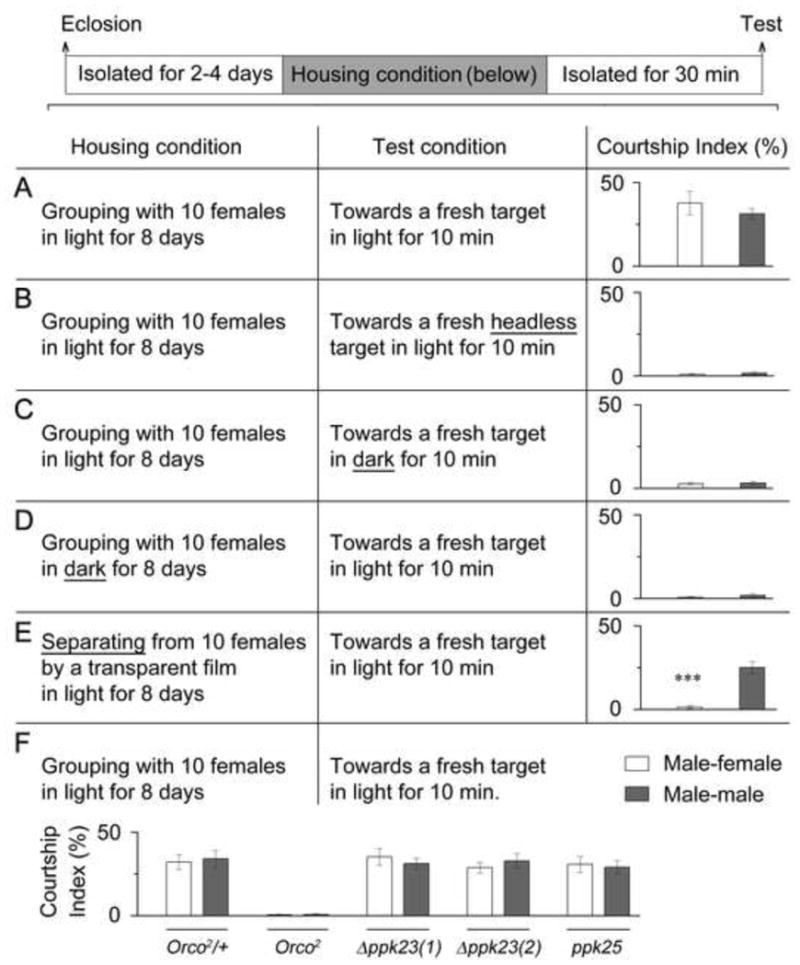
Sensory Basis of the Grouping-induced Courtship Behavior in fruM Null Males. (A–E) Courtship indices by fruLexA/fru4-40 males with male (gray) or female (white) targets. Manipulations of visual inputs during group-housing and/or courtship testing are described (highlighted by underlines). ***p < 0.001, two-sample t-test. (F) Courtship indices by fruLexA/fru4-40 males with Orco, ppk23 or ppk25 mutations. Detailed genotypes are described in text. n = 12~24 for each. Error bars indicate SEM. Please see Figure S2.
To further explore the role(s) of vision in these processes we next used 2-layered behavioral chambers in which the top and bottom halves of the chamber were separated by a transparent plastic sheet. The top half of the chamber housed individual fruLexA/fru4-40 males who were provided with a dollop of food at the edge of the chamber, while the bottom layer of the chamber housed 10 wild-type females on food. Flies were kept in these split chambers under constant light for 8 days (to allow only visual stimulation from females), and the males were then extracted for a subsequent courtship test with an intact target in the light. Surprisingly, these fruM null males courted males intensively, but rarely courted females (Figure 6E), suggesting that visual stimulation from females alone during group-housing is sufficient to induce the potential for male-male courtship behavior, while non-visual stimulation during group-housing may be required for the acquisition of the potential for male-female courtship.
We then tested the role of olfactory perception in eliciting the potential of courtship in fruM null males. The Orco2 mutant allele of the broadly expressed olfactory receptor gene Orco (aka Or83b) (Larsson et al., 2004) was introduced into a fruM null background (Orco2 fruLexA/Orco2 fru4-40), and such individual males were group-housed with 10 wild-type females in food vials in constant light for 8 days, then isolated for a subsequent courtship test with an intact target in the light. These males failed to court either male or female targets, while control males (Orco2 fruLexA/fru4-40) courted both male and female targets (Figure 6F) (similar findings in control fruLexA/Orco2 fru4-40 males, data not shown). These results indicate that olfactory perception is necessary during group-housing or courtship test, or in both phases, for courtship acquisition and manifestation in fruM null males. Silencing Orco-expressing neurons during just the test phase impaired courtship by fruM null males (UAS-shits1/Orco-GAL4; fruLexA/fru4-40) toward either male or female targets (Figure S2C and data not shown), indicating that olfactory perception is indeed necessary for courtship during testing. Taken together with the above results using split chambers, our results show that for male-male courtship, olfactory input is necessary during the test phase, but not necessary during group-housing phase if visual input is provided; for male-female courtship, olfactory input may be necessary in both group-housing phase and test phase.
We further tested the role of gustatory receptor genes ppk23 and ppk25, which have been recently reported to be involved in wild-type male courtship behavior (Lu et al., 2012; Starostina et al., 2012; Thistle et al., 2012; Toda et al., 2012), in the group-housing induced courtship by fruM null males, but did not observe any defect in these mutants in a fruM null background [genotypes are: Δppk23(1)/Y ;; fruLexA/fru4-40, Δppk23(2)/Y ;; fruLexA/fru4-40 and ppk25(Δ5-2)/ppk25(Δ5-22); fruLexA/fru4-40] (Figure 6F). Consistent with this, silencing ppk23- or ppk25-expressing neurons in fruM null males [genotypes are: UAS-shits1/+; ppk23-GAL4(1) fruLexA/fru4-40, UAS-shits1/ppk23-GAL4(2); fruLexA/fru4-40 and UAS-shits1/ppk25-GAL4; fruLexA/fru4-40] during just the test phase did not affect courtship behavior (Figure S2C). However, these results do not exclude the possible involvement of gustatory perception provided by other receptor genes in courtship by fruM null males.
DISCUSSION
For nearly 100 years male courtship behavior in D. melanogaster has been recognized as a robust, complex and largely innate behavior: a male fly is fully capable of performing all steps of courtship behavior when raised in complete isolation from egg to adulthood and then presented with a female fly as his first encounter with another creature. Thus male courtship has been used as a model system for the analysis of such topics as, how innate behaviors are elicited by specific environmental cues, and how sequential motor programs are coordinated (Baker et al., 2001; Greenspan and Ferveur, 2000).
One of the most significant findings with respect to courtship behavior during the last decade is that a single gene (fruM) is both necessary and sufficient for building the potential of courtship behavior into a dedicated courtship circuitry (Demir and Dickson, 2005; Manoli et al., 2005; Manoli et al., 2006; Stockinger et al., 2005). Here we show that while courtship behavior is abolished in fruM null males that are raised in isolation, a condition used by most studies in this field, many steps of courtship behavior can be alternatively established simply by group-housing fruM null males with either male or female flies for one or more days prior to testing. We further demonstrated that such fruM-independent, experience-dependent courtship is genetically specified by the dsx gene, whose expression significantly overlaps that of fruM in the CNS. Finally, we show that the experience-dependent acquisition of the potential for courtship has properties suggestive of learning and memory, but is independent of mushroom bodies. Integrating our results with previous findings deepens our understanding of both the genetic and neuronal underpinnings of courtship.
Numerous studies have contributed to identifying fruM as a dedicated regulatory gene that specifies the neural substrates of D. melanogaster male courtship (Demir and Dickson, 2005; Ito et al., 1996; Manoli et al., 2005; Ryner et al., 1996; Stockinger et al., 2005), and showing that fruM largely functions to regulate fine neural connectivity and/or neural physiology (Cachero et al., 2010; Kimura et al., 2008; Kimura et al., 2005; Manoli et al., 2005; Mellert et al., 2010; Stockinger et al., 2005). More recent findings have highlighted the importance of dsx-expressing neurons, and in particular those that also express fruM, in male courtship (Kimura et al., 2008; Pan et al., 2012; Pan et al., 2011; Rideout et al., 2007). Of particular relevance, in the light of our discovery of a fruM-independent, dsx- and experience-dependent courtship pathway, is the finding that artificial activation of all dsx neurons elicits courtship by males independent of whether they had functional fruM (Pan et al., 2011). About two thirds of all dsx-expressing CNS neurons are found in the ventral nerve cord, and in particular the abdominal ganglion, where they likely function in the execution of sexual behaviors. This leaves 5 bilaterally present clusters of dsx-expressing neurons in the brain (~300 neurons total counting both hemispheres) as likely containing the dsx neurons that mediate the acquisition of experience-dependent courtship. Of these 5 clusters of dsx-expressing neurons, the male-specific PC1 (also termed P1) cluster, which expresses both fruM and dsxM, is a particularly attractive candidate for having a significant role in experience-dependent courtship, based on its key role in initiating fruM-dependent courtship (Kimura et al., 2008; Kohatsu et al., 2011; Pan et al., 2012).
Our findings add significantly to understanding the role of dsxM in specifying male courtship behavior. Previous studies showed that in males that are wild-type for fruM one specific aspect of male courtship—sine song—is dependent on dsxM function (Villella and Hall, 1996). Thus, it is likely that the potential for sine song is innately established in CNS by dsxM in a manner analogous to how the potential for fruM-dependent aspects of courtship are innately established. Additionally, in dsx null males that are wild-type for fruM there is a poorly understood deficit in the overall level of courtship (as measured by the CI), but all steps of courtship occur, except for sine song and copulation itself, which is mechanically not possible due to dsx-dependent defects in genital development. Our results reveal additional roles of dsxM in the acquisition of the potential for courtship in the absence of fruM function. This reasoning suggests that dsxM functions both to facilitate acquisition of the potential for many aspects of courtship (in the absence of fruM) and to (in the presence of fruM) innately determine at least one aspect of courtship—sine song.
Specification of Courtship Circuitry during Development and in Adults
As we noted above, the sex determination genes fruM and dsxM in males function developmentally to build some aspects of courtship behavior into the CNS. Although the majority of neurons comprising the courtship circuitry are still present in fruM null males, they do not function effectively in transducing sensory cues to motor centers that execute courtship behavior. Strikingly, group-housing experience allows efficient transduction from sensory cues to motor centers when fruM is not expressed. Thus social experience acting via dsxM-mediated processes somehow compensates for many aspects of fruM function. We note that other aspects of courtship behavior, e.g. attempted copulation, are not observed in fruM null males, even after they have been group-housed, suggesting that the latter aspects of courtship are solely fruM-dependent.
How does social experience change the courtship circuitry in the absence of fruM? We note that many recent studies on flies have found that social experience can change gene expression, synaptic connectivity and/or pheromone profiles (Bushey et al., 2011; Carney, 2007; Donlea et al., 2009; Donlea et al., 2011; Farine et al., 2012; Krupp et al., 2008). As our study showed that when fruM null males that had been group housed were isolated and then singly housed for 7 days, they still courted fresh females intensively, it is unlikely that changed pheromone profiles, if any, play essential roles in establishing courtship behavior in fruM null males. Rather, we suggest that social experience induces courtship in fruM null males by changing gene expression and/or neuronal connectivity to allow efficient transduction from sensory perception to motor output. Whether social experience functioning through dsxM during adulthood, and fruM functioning during development, act through identical or synonymous mechanisms to specify the courtship circuitry is unknown and awaited further study. In this regard we note that the experience-dependent acquisition of the potential for male courtship behavior during adulthood provides a robust single fly paradigm for learning that may facilitate studies of learning at a variety of levels.
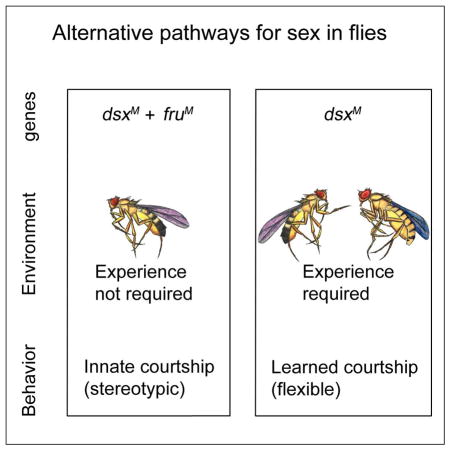
An Evolutionary Scenario
That two alternative systems for establishing the potential for male courtship co-exist in D. melanogaster raises the question of why it is genetically structured in this way (Figure 7). One possibility is that fruM’s specification of innate courtship in D. melanogaster represents a system that has evolved from an ancestral state in which the potential for courtship was acquired through social interactions and dsx. It has been hypothesized that learned behavioral adaptations evolutionarily precede innately specified forms of the same behaviors (Tierney, 1986). That fruM has not been identified outside of insects, whereas dsx related genes (DMRTs) that function in sexual development are found throughout the animal kingdom (Kopp, 2012) is consistent with this view. We also note that, since DMRTs are deeply conserved, the dsxM/social experience pathway may be functional in other species, including those such as humans that show more flexibility in their courtship rituals.
Figure 7.
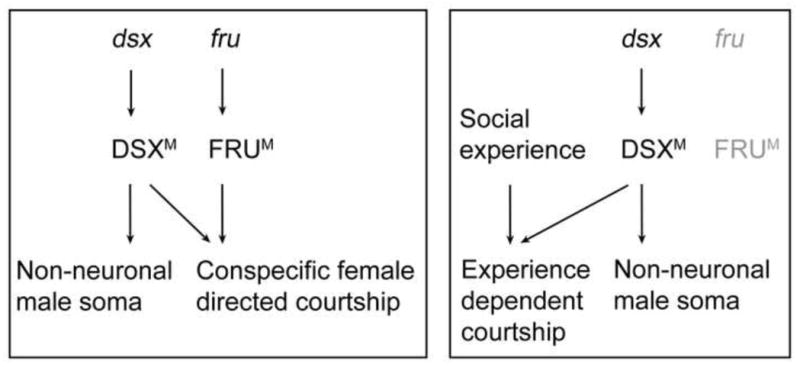
Alternative modes of specifying male courtship in Drosophila. As dsx is expressed in both non-neuronal somatic cells as well as neurons, we distinguish its role in the nervous system for male courtship from non-neuronal somatic development. In contrast, fruM is expressed exclusively in neurons. Left panel: FRUM and DSXM jointly specify wild-type courtship that is conspecific female directed. Right panel: in the absence of FRUM, social experience and DSXM jointly specify the experience-dependent courtship that is more flexible.
EXPERIMENTAL PROCEDURES
Fly Stocks
fruM null alleles used in this study include fruLexA, fru4-40, frusat15 and fruAJ96u3. dsx null alleles are dsx683-7058, dsx1649-9625 and dsxM+R15. dsx dominant alleles are dsxS, dsxM and dsxD. dsxGAL4(Δ2) was described in Pan et al., 2011. UAS>stop>TNT and UAS>stop>TNTin were provided by B. Dickson. LexAop2-FlpL, UAS>stop>myrGFP, UAS>stop>Kir2.1, UAS-shits1, UAS-kir2.1::GFP, 19B03-GAL4 and 76D11-GAL4 were gifts from G. Rubin. Δppk23 and ppk23-GAL4 lines were obtained independently from K. Scott [referred to as Δppk23(1) and ppk23-GAL4(1)] and Y. Ben-Shahar [referred to as Δppk23(2) and ppk23-GAL4(2)]. ppk25(Δ5-2), ppk25(Δ5-22) and ppk25-GAL4 were gifts from C. Pikielny. All crosses were performed and kept at room temperature (~23°C), unless stated otherwise.
Courtship Assays
Two courtship assays were used: (1) for assaying a one-time single-pair courtship (Figures 1A, 2D, 4–6 and S2), small round 2-layer chambers (diameter: 1 cm; height: 2.5 mm per layer) were used. Individual tester flies and target flies were gently aspirated into different layers and were separated by a plastic transparent barrier which was removed ~30 min later to allow the courtship test; (2) for assaying chaining/courtship behavior of multiple flies (Figures 1B–1D, 2E–2G, 3 and S1), large round 1-layer chambers were used (diameter: 4 cm; height: 3 mm). Groups of tester flies were briefly cooled on ice and loaded into the chamber. Tests were performed daily for up to 6 days (3 hours after grouping as day 0, then days 1–6). For both assays, tests were performed at 25°C on fly food that was at the bottom of behavioral chambers, unless stated otherwise. Each test was performed for 10 min. For detailed preparation of courtship tester and target flies, see SI.
Analyze of Courtship Behavior
Courtship index (CI), which is the percentage of observation time a fly performs any courtship step (Villella et al., 1997), was used to measure courtship to female targets or between 2 males. In the case there are multiple female targets (such as 1 male and 7 females in Figure 1D), CI represents courtship to all targets. Paired male-male courtship used 2 males of the same genotype, but focused on the male fly that first initiated courtship (courtship of the initiator to the other). Chaining index (ChI), which is the percentage of observation time at least 3 flies engaged in courtship together, was used to measure courtship in groups of 8 flies (and groups of 5 flies in Figure 1C). Comparison of two indices was made by two-sample t test, and comparison between multiple groups was made by one-way ANOVA.
Tissue Dissection, Staining and Imaging
CNSs were dissected at 4–6 days post eclosion (Figures 2A and 2B), unless stated otherwise (Figure S2E). Antibodies used were rabbit anti-GFP (Invitrogen A11122) 1:1000, mouse anti-Bruchpilot (Developmental Studies Hybridoma Bank nc82) 1:30, and secondary Alexa Fluor 488 and 568 antibodies (1:500). Samples were imaged at 20X magnification on a Zeiss 710 confocal microscope, and processed with Fiji software.
Supplementary Material
Highlights.
Naïve fruM null males do not court, but after group-housing they do court
dsxM is necessary and sufficient for such fruM-independent courtship
Socially experienced fruM null males retain the ability to court for a week or more
The mate preference of experienced fruM males is dependent on prior experiences
Acknowledgments
We thank the Fly Light project team in Janelia Farm for generating the expression pattern images shown in Figures S2A and S2B; G. Rubin, K. Scott, Y. Ben-Shahar, C. Pikielny, L. Vosshall and B. Dickson for fly stocks; G. Card for sharing high-speed cameras; V. Jayaraman, M. Reiser, J. Simpson, J. Truman, L. Riddiford, Y. Aso, T. Shirangi and members of the B. Baker lab for discussions. We also thank A. Howard for administrative support; members of the fly facility in Janelia Farm for technical support. This work was supported by the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anand A, Villella A, Ryner LC, Carlo T, Goodwin SF, Song HJ, Gailey DA, Morales A, Hall JC, Baker BS, et al. Molecular genetic dissection of the sex-specific and vital functions of the Drosophila melanogaster sex determination gene fruitless. Genetics. 2001;158:1569–1595. doi: 10.1093/genetics/158.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. The Journal of neuroscience. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BS, Ridge KA. Sex and the single cell. I. On the action of major loci affecting sex determination in Drosophila melanogaster. Genetics. 1980;94:383–423. doi: 10.1093/genetics/94.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BS, Taylor BJ, Hall JC. Are complex behaviors specified by dedicated regulatory genes? Reasoning from Drosophila. Cell. 2001;105:13–24. doi: 10.1016/s0092-8674(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Billeter JC, Villella A, Allendorfer JB, Dornan AJ, Richardson M, Gailey DA, Goodwin SF. Isoform-specific control of male neuronal differentiation and behavior in Drosophila by the fruitless gene. Curr Biol. 2006;16:1063–1076. doi: 10.1016/j.cub.2006.04.039. [DOI] [PubMed] [Google Scholar]
- Burtis KC, Baker BS. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell. 1989;56:997–1010. doi: 10.1016/0092-8674(89)90633-8. [DOI] [PubMed] [Google Scholar]
- Bushey D, Tononi G, Cirelli C. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 2011;332:1576–1581. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachero S, Ostrovsky AD, Yu JY, Dickson BJ, Jefferis GS. Sexual dimorphism in the fly brain. Curr Biol. 2010;20:1589–1601. doi: 10.1016/j.cub.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney GE. A rapid genome-wide response to Drosophila melanogaster social interactions. BMC genomics. 2007;8:288. doi: 10.1186/1471-2164-8-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee SS, Uppendahl LD, Chowdhury MA, Ip PL, Siegal ML. The female-specific doublesex isoform regulates pleiotropic transcription factors to pattern genital development in Drosophila. Development. 2011;138:1099–1109. doi: 10.1242/dev.055731. [DOI] [PubMed] [Google Scholar]
- Demir E, Dickson BJ. fruitless splicing specifies male courtship behavior in Drosophila. Cell. 2005;121:785–794. doi: 10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324:105–108. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea JM, Thimgan MS, Suzuki Y, Gottschalk L, Shaw PJ. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science. 2011;332:1571–1576. doi: 10.1126/science.1202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farine JP, Ferveur JF, Everaerts C. Volatile Drosophila cuticular pheromones are affected by social but not sexual experience. PLoS One. 2012;7:e40396. doi: 10.1371/journal.pone.0040396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan RJ, Ferveur JF. Courtship in Drosophila. Annu Rev Genet. 2000;34:205–232. doi: 10.1146/annurev.genet.34.1.205. [DOI] [PubMed] [Google Scholar]
- Ito H, Fujitani K, Usui K, Shimizu-Nishikawa K, Tanaka S, Yamamoto D. Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc Natl Acad Sci U S A. 1996;93:9687–9692. doi: 10.1073/pnas.93.18.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenett A, Rubin GM, Ngo TT, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell reports. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene AC, Waddell S. Drosophila olfactory memory: single genes to complex neural circuits. Nature reviews Neuroscience. 2007;8:341–354. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
- Kimura K, Hachiya T, Koganezawa M, Tazawa T, Yamamoto D. Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron. 2008;59:759–769. doi: 10.1016/j.neuron.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ote M, Tazawa T, Yamamoto D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature. 2005;438:229–233. doi: 10.1038/nature04229. [DOI] [PubMed] [Google Scholar]
- Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- Kohatsu S, Koganezawa M, Yamamoto D. Female Contact Activates Male-Specific Interneurons that Trigger Stereotypic Courtship Behavior in Drosophila. Neuron. 2011;69:498–508. doi: 10.1016/j.neuron.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Kopp A. Dmrt genes in the development and evolution of sexual dimorphism. Trends in genetics: TIG. 2012;28:175–184. doi: 10.1016/j.tig.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp JJ, Kent C, Billeter JC, Azanchi R, So AK, Schonfeld JA, Smith BP, Lucas C, Levine JD. Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr Biol. 2008;18:1373–1383. doi: 10.1016/j.cub.2008.07.089. [DOI] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Lee G, Foss M, Goodwin SF, Carlo T, Taylor BJ, Hall JC. Spatial, temporal, and sexually dimorphic expression patterns of the fruitless gene in the Drosophila central nervous system. J Neurobiol. 2000;43:404–426. doi: 10.1002/1097-4695(20000615)43:4<404::aid-neu8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Lee G, Hall JC. Abnormalities of male-specific FRU protein and serotonin expression in the CNS of fruitless mutants in Drosophila. The Journal of neuroscience. 2001;21:513–526. doi: 10.1523/JNEUROSCI.21-02-00513.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Hall JC, Park JH. Doublesex gene expression in the central nervous system of Drosophila melanogaster. Journal of neurogenetics. 2002;16:229–248. doi: 10.1080/01677060216292. [DOI] [PubMed] [Google Scholar]
- Lu B, LaMora A, Sun Y, Welsh MJ, Ben-Shahar Y. ppk23-Dependent chemosensory functions contribute to courtship behavior in Drosophila melanogaster. PLoS genetics. 2012;8:e1002587. doi: 10.1371/journal.pgen.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- Manoli DS, Meissner GW, Baker BS. Blueprints for behavior: genetic specification of neural circuitry for innate behaviors. Trends Neurosci. 2006;29:444–451. doi: 10.1016/j.tins.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Mellert DJ, Knapp JM, Manoli DS, Meissner GW, Baker BS. Midline crossing by gustatory receptor neuron axons is regulated by fruitless, doublesex and the Roundabout receptors. Development. 2010;137:323–332. doi: 10.1242/dev.045047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellert DJ, Robinett CC, Baker BS. doublesex functions early and late in gustatory sense organ development. PLoS One. 2012;7:e51489. doi: 10.1371/journal.pone.0051489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi RN, Baker BS. Regulation of sex-specific RNA splicing at the Drosophila doublesex gene: cis-acting mutations in exon sequences alter sex-specific RNA splicing patterns. Genes Dev. 1990;4:89–97. doi: 10.1101/gad.4.1.89. [DOI] [PubMed] [Google Scholar]
- Pan Y, Meissner GW, Baker BS. Joint control of Drosophila male courtship behavior by motion cues and activation of male-specific P1 neurons. Proc Natl Acad Sci U S A. 2012;109:10065–10070. doi: 10.1073/pnas.1207107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Robinett CC, Baker BS. Turning males on: activation of male courtship behavior in Drosophila melanogaster. PLoS One. 2011;6:e21144. doi: 10.1371/journal.pone.0021144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Jenett A, Hammonds AS, Ngo TT, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci U S A. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Ngo TT, Hibbard KL, Murphy C, Jenett A, Truman JW, Rubin GM. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Truman JW, Rubin GM. Using translational enhancers to increase transgene expression in Drosophila. Proc Natl Acad Sci U S A. 2012;109:6626–6631. doi: 10.1073/pnas.1204520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout EJ, Billeter JC, Goodwin SF. The sex-determination genes fruitless and doublesex specify a neural substrate required for courtship song. Curr Biol. 2007;17:1473–1478. doi: 10.1016/j.cub.2007.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout EJ, Dornan AJ, Neville MC, Eadie S, Goodwin SF. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat Neurosci. 2010;13:458–466. doi: 10.1038/nn.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinett CC, Vaughan AG, Knapp JM, Baker BS. Sex and the single cell. II. There is a time and place for sex. PLoS Biol. 2010;8:e1000365. doi: 10.1371/journal.pbio.1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Taylor BJ, Wasserman SA. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell. 1996;87:1079–1089. doi: 10.1016/s0092-8674(00)81802-4. [DOI] [PubMed] [Google Scholar]
- Sanders LE, Arbeitman MN. Doublesex establishes sexual dimorphism in the Drosophila central nervous system in an isoform-dependent manner by directing cell number. Dev Biol. 2008;320:378–390. doi: 10.1016/j.ydbio.2008.05.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirangi TR, Taylor BJ, McKeown M. A double-switch system regulates male courtship behavior in male and female Drosophila melanogaster. Nat Genet. 2006;38:1435–1439. doi: 10.1038/ng1908. [DOI] [PubMed] [Google Scholar]
- Song HJ, Billeter JC, Reynaud E, Carlo T, Spana EP, Perrimon N, Goodwin SF, Baker BS, Taylor BJ. The fruitless gene is required for the proper formation of axonal tracts in the embryonic central nervous system of Drosophila. Genetics. 2002;162:1703–1724. doi: 10.1093/genetics/162.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starostina E, Liu T, Vijayan V, Zheng Z, Siwicki KK, Pikielny CW. A Drosophila DEG/ENaC subunit functions specifically in gustatory neurons required for male courtship behavior. The Journal of neuroscience. 2012;32:4665–4674. doi: 10.1523/JNEUROSCI.6178-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger P, Kvitsiani D, Rotkopf S, Tirian L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Taylor BJ, Villella A, Ryner LC, Baker BS, Hall JC. Behavioral and neurobiological implications of sex-determining factors in Drosophila. Dev Genet. 1994;15:275–296. doi: 10.1002/dvg.1020150309. [DOI] [PubMed] [Google Scholar]
- Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell. 2012;149:1140–1151. doi: 10.1016/j.cell.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney AJ. The evolution of learned and innate behavior: contributions from genetics and neurobiology to a theory of behavioral evolution. Animal Learning & Behavior. 1986;14:339–348. [Google Scholar]
- Toda H, Zhao X, Dickson BJ. The Drosophila female aphrodisiac pheromone activates ppk23(+) sensory neurons to elicit male courtship behavior. Cell reports. 2012;1:599–607. doi: 10.1016/j.celrep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Usui-Aoki K, Ito H, Ui-Tei K, Takahashi K, Lukacsovich T, Awano W, Nakata H, Piao ZF, Nilsson EE, Tomida J, et al. Formation of the male-specific muscle in female Drosophila by ectopic fruitless expression. Nature cell biology. 2000;2:500–506. doi: 10.1038/35019537. [DOI] [PubMed] [Google Scholar]
- Villella A, Gailey DA, Berwald B, Ohshima S, Barnes PT, Hall JC. Extended reproductive roles of the fruitless gene in Drosophila melanogaster revealed by behavioral analysis of new fru mutants. Genetics. 1997;147:1107–1130. doi: 10.1093/genetics/147.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villella A, Hall JC. Courtship anomalies caused by doublesex mutations in Drosophila melanogaster. Genetics. 1996;143:331–344. doi: 10.1093/genetics/143.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villella A, Hall JC. Neurogenetics of courtship and mating in Drosophila. Adv Genet. 2008;62:67–184. doi: 10.1016/S0065-2660(08)00603-2. [DOI] [PubMed] [Google Scholar]
- Yu JY, Kanai MI, Demir E, Jefferis GS, Dickson BJ. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol. 2010;20:1602–1614. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


