DNA repair in plants is more complex than previously anticipated and is defined by different intrastrand cross-link repair pathways.
Abstract
DNA polymerase zeta catalytic subunit REV3 is known to play an important role in the repair of DNA damage induced by cross-linking and methylating agents. Here, we demonstrate that in Arabidopsis (Arabidopsis thaliana), the basic polymerase activity of REV3 is essential for resistance protection against these different types of damaging agents. Interestingly, its processivity is mainly required for resistance to interstrand and intrastrand cross-linking agents, but not alkylating agents. To better define the role of REV3 in relation to other key factors involved in DNA repair, we perform epistasis analysis and show that REV3-mediated resistance to DNA-damaging agents is independent of the replication damage checkpoint kinase ataxia telangiectasia-mutated and rad3-related homolog. REV3 cooperates with the endonuclease MMS and UV-sensitive protein81 in response to interstrand cross links and alkylated bases, whereas it acts independently of the ATP-dependent DNA helicase RECQ4A. Taken together, our data show that four DNA intrastrand cross-link subpathways exist in Arabidopsis, defined by ATP-dependent DNA Helicase RECQ4A, MMS and UV-sensitive protein81, REV3, and the ATPase Radiation Sensitive Protein 5A.
The DNA of all living organisms is constantly exposed to damaging factors, and therefore a number of DNA damage repair and bypass mechanisms have evolved. DNA lesions that interfere with the replication machinery constitute a particular challenge for cells (Schröpfer et al., 2014a); if not repaired in a timely manner, such damage can result in the stalling or collapse of replication forks, which in turn can lead to cell death. Furthermore, one-sided double-strand breaks (DSBs) can occur when the replication fork encounters a single-strand break. Lesions within one DNA strand, such as alkylations or DNA intrastrand cross links, can be bypassed by postreplicative repair (PRR), a process that is best understood in yeast (Saccharomyces cerevisiae). This mechanism does not lead to repair of the lesion but prevents fatal long-lasting stalling of the replication fork. PRR can be divided into two branches: the error-prone pathway and the error-free pathway (for review, see Goodman and Woodgate, 2013; Haynes et al., 2015; Jansen et al., 2015). It is known from yeast that both branches of PRR are controlled by the Radiation sensitivity protein6 (Rad6) and Mms-Ubc13 E3 ubiquitin-conjugating enzyme complexes, which ubiquitinate the replicative processivity factor Proliferating Cellular Nuclear Antigen1. The monoubiquitination of PCNA at Lys-164 by Rad6-Rad18 initiates the error-prone pathway, whereas polyubiquitination additionally requires Mms2, Ubc13, and Rad5, and triggers the error-free PRR branch (Hoege et al., 2002; Moldovan et al., 2007; Lee and Myung, 2008). There are two possible competing models postulated for the error-free bypass of lesions at the replication fork, both of which depend on template-switch mechanisms; if the lesion concerns only one of the two sister strands, the undamaged strand can be used as the template for bypassing the lesion. One of the two models features the so-called overshoot synthesis, whereby the newly synthesized strand on the undamaged parental strand is elongated further than the strand blocked by the lesion. Regression of the replication fork then leads to the formation of a special type of four-way junction called a chicken-foot structure. This regression mechanism is thought to be accomplished by helicases, such as the RecQ helicase Bloom Syndrome Protein (BLM) in humans (Croteau et al., 2014). AtRECQ4A is the respective BLM homolog in Arabidopsis (Arabidopsis thaliana), and this enzyme has the ability to regress replication forks in vitro (Hartung et al., 2007, 2008; Schröpfer et al., 2014b). The second error-free subpathway entails invasion of the newly synthesized strand on the blocked sister chromatid into the complementary newly replicated strand on the other sister chromatid. Such a step forms a displacement loop-like structure in which synthesis over the damaged region can occur. In yeast, both error-free pathways are dependent on the multifunctional protein Rad5, which is known to recruit PRR factors and also exhibits helicase activity itself (Blastyák et al., 2007). We previously identified AtRAD5A as a functional Arabidopsis homolog of Rad5 (Chen et al., 2008). Interestingly, AtRAD5A is required for efficient repair by homologous recombination via the synthesis-dependent strand-annealing mechanism, a pathway that in some steps is related to the invasion model of PRR (Mannuss et al., 2010).
The error-prone pathway is based on the function of translesion synthesis (TLS) polymerases, which promote replication through DNA lesions (Prakash et al., 2005). In a mechanism termed polymerase switch, the replicative polymerase is exchanged by such a TLS polymerase at a damaged site. After incorporation of a nucleotide opposite the damaged base by the TLS polymerase, a second polymerase switch exchanges the TLS polymerase for the replicative polymerase so that replication can proceed (Prakash and Prakash, 2002; Lehmann et al., 2007). TLS polymerases possess no 5′-3′-exonuclease activity, and therefore act in a potentially mutagenic manner. Nevertheless, depending on the damage incurred and the TLS polymerase used, damage bypass can be error free (Haracska et al., 2000; McCulloch et al., 2004).
Polymerases can be divided into at least six families based on their amino acid sequences and crystal structures: A, B, C, D, X, and Y. All of them share the common structure analogous to a right hand grasping DNA with palm, finger, and thumb domains (Steitz, 1999). The amino acid sequences of the finger and thumb domains of different polymerase families are highly variable, whereas the palm domains share high similarity. The palm domain forms the largest part of the polymerase active site and contains highly conserved Asp residues that have been postulated to be involved in the catalytic activity of the enzyme (Joyce and Steitz, 1995; Steitz, 1999).
Most TLS polymerases belong to the Y family of polymerases, a class of specially structured enzymes that catalyze replication over damaged templates (Ohmori et al., 2001; Sale et al., 2012). Although some polymerases of the A, B, or X family can also exhibit TLS activity, this is often not their primary function (Prakash et al., 2005). DNA Polymerase Zeta (POLζ) is a B family polymerase and consists of a DNA Polymerase Zeta subunit REV3-REV7 heterodimer, in which REV3 is the catalytic subunit with its accessory subunit and processivity factor REV7 (Nelson et al., 1996). Recent studies in yeast and human cells have shown that POLζ contains two additional subunits, Pol31 and Pol32 in yeast, orthologs to human POLD2 and POLD3, which are known to be accessory subunits of the replicative polymerase POLδ (Johnson et al., 2012; Lee et al., 2014). REV3 contains three regions that are highly conserved between organisms: an N-terminal region, a REV7 binding domain, and a B family-type polymerase domain. The polymerase domain carries the six common conserved regions, I to VI (IV-II-VI-III-I-V), of which I is the most and VI the least conserved region. The A (II), B (III), and C (I) motifs, located within regions I, II, and III (Wong et al., 1988), form the active site of the enzyme, and each harbors an essential Asp residue that coordinates two catalytic metal ions. Deficiency of REV3 in mice is embryo lethal (Bemark et al., 2000; Esposito et al., 2000), and vertebrate cells depleted in REV3 show hypersensitivity to various DNA-damaging agents, including UV and ionizing irradiation, cisplatin, MMS, and mitomycin C (MMC; Sonoda et al., 2003; Sharma and Canman, 2012). In Arabidopsis, rev3 mutants exhibit no obvious phenotype under standard growth conditions, but are hypersensitive to UV-B and gamma irradiation, MMC, MMS, and cisplatin (Sakamoto et al., 2003).
Our previous work demonstrated the existence of several different pathways in Arabidopsis involved in repairing the DNA damage induced by cross-linking and methylating agents. These independent pathways are defined by the ATPase RAD5A, the helicase RECQ4A, and MMS and UV-sensitive protein81 (MUS81; Mannuss et al., 2010). The structure-specific endonuclease MUS81 together with its noncatalytic subunit (Mms4 in yeast, Eme1 in Schizosaccharomyces pombe, and MMS4 or Crossover Junction Endonuclease (EME1) in humans and plants) functions in the rescue of stalled replication forks. The enzyme is able to cleave the stalled fork at the lesion site, which leads to a one-sided DSB that is repaired by homologous recombination (HR) to restore the stalled fork (Hanada et al., 2006). We previously showed that mus81 transfer DNA (T-DNA) insertion lines in Arabidopsis are highly sensitive to treatment with MMS, cisplatin, hydroxyurea, ionizing irradiation, and MMC. We also found that AtMUS81 can form a complex with its heterologous binding partners AtEME1A or AtEME1B that is able to process intricate DNA structures, such as nicked Holliday junctions, which might also form at stalled replication forks (Hartung et al., 2006; Geuting et al., 2009).
In the current study, we address whether fully functional polymerase activity is required for the repair of DNA damage induced by alkylating and cross-link-inducing agents. Moreover, we sought to clarify whether REV3 cooperates with other key factors identified in Arabidopsis in the repair of these different types of damage. Indeed, it has already been shown that AtREV3 and AtRAD5A do not cooperate in the repair of such DNA damage, confirming independent pathways of error-prone and error-free PRR in plants (Wang et al., 2011). However, as plants, animals, and yeast differ in their DNA cross-link repair machinery (e.g. Mannuss et al., 2010; Knoll et al., 2012; Dangel et al., 2014; Herrmann et al., 2015), it is of particular importance to define the role of REV3 in relation to MUS81 and RECQ4A.
RESULTS
Loss of the Highly Conserved Asp in Polymerase Motif A Leads to a Total Loss of REV3 Function, whereas an Asp in Motif C Is Not Essential for All REV3 Functions
It has previously been reported that, in Arabidopsis, REV3 is involved in the repair of DNA damage induced by cross-linking and methylated bases (Sakamoto et al., 2003; Takahashi et al., 2005). However, to date, whether a fully functional polymerase activity is required for this process has not been addressed. Therefore, we introduced into wild-type AtREV3 complementary DNA (cDNA) two different amino acid substitutions that should result in different types of functional defects. In motif A, we exchanged the Asp at position 1,385 with an Ala (D1385A). The respective Asp is known to be involved in Mg2+ binding, which is required for initiating the covalent linkage between the primer and the incoming deoxynucleotide (Patel and Loeb, 2000). Accordingly, this mutant enzyme should not have any polymerase activity. We also mutated an Asp to Ala in motif C (D1549A). Bacterial polymerases harbor an identical YGDTDS motif found in REV3, and biochemical studies have shown that the respective mutation in the α-like DNA polymerase of the phage Phi29 results in a partially active enzyme with reduced elongation activity (Bernad et al., 1990). Thus, we expected that this mutation should not result in a total knockout of REV3 function but mainly a defect in the translocation step, such that this enzyme should still be able to incorporate single nucleotides. As a consequence, the processivity of the polymerase should be disturbed.
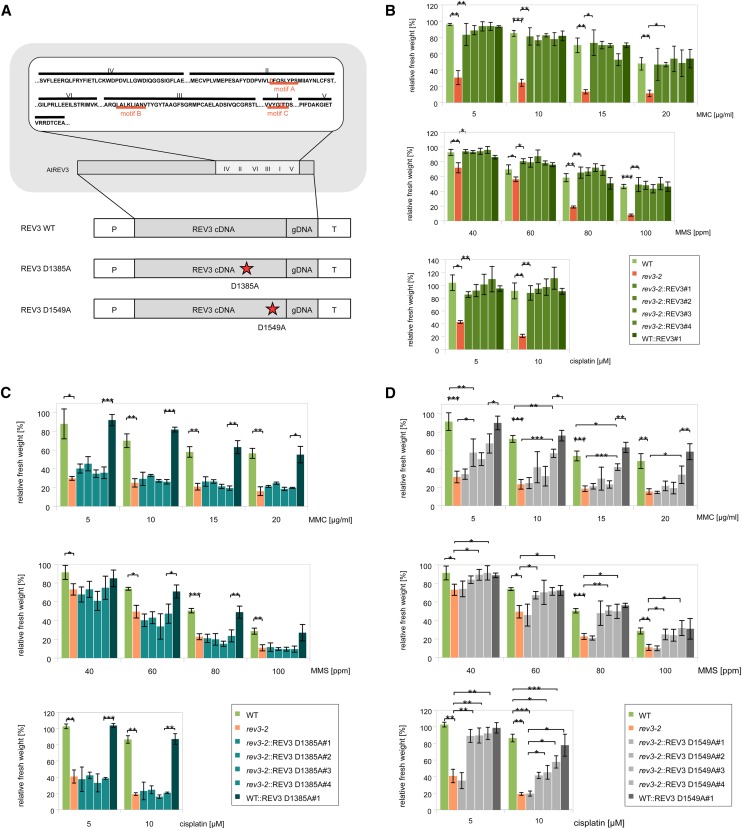
Utilizing Agrobacterium tumefaciens, we transformed the three different constructs into the well-characterized rev3-2 mutant line (Sakamoto et al., 2003) and Columbia-0 (Col-0) as a control (Fig. 1). Homozygous single-locus lines were established for four independent complementation lines with different integration sites in the rev3-2 background and one in the Col-0 background. Expression levels of the transformed REV3 ORFs in the lines were determined by quantitative PCR (Supplemental Fig. S1; Supplemental Method S1). These lines were used for sensitivity assays with genotoxic agents MMC, MMS, and cisplatin as well as root growth assays after UV-B irradiation. As shown in Figure 1B, full complementation of the hypersensitivity to MMC, MMS, and cisplatin was achieved by expression of the wild-type construct in rev3-2. In contrast, expression of the construct containing the D1385A amino acid substitution does not lead to complementation of the observed hypersensitivity to any of the tested chemical genotoxins; as it does not affect the fresh weight of the control line, negative complementation is excluded (Fig. 1C). Interestingly, sensitivity assays with the lines transformed with the D1549A construct reveal different results (Fig. 1D). Although hypersensitivity to MMS is fully restored to wild-type levels after expression of the construct in rev3-2 in all but one line, this is not the case for all tested concentrations of MMC and cisplatin. In this case, three out of four complementation lines show mostly an intermediate phenotype, with relative fresh weights between those of wild-type and the rev3-2 insertion line, which is most distinct after induction with 5 µg mL−1 MMC. In contrast, the fourth line (D1385A#4) resembles the mutant phenotype, which correlates with a lower REV3 mRNA level than in the other lines (P < 0.05; Supplemental Fig. S1).
Figure 1.
Mutations in REV3 motifs led to different effects in DNA damage repair. A, The depicted constructs were assembled from three fragments amplified from Col-0 genomic DNA (gDNA) and cDNA (promoter: 718-bp gDNA, middle fragment: 5,113 bp cDNA, 3′open reading frame (ORF)-terminator gDNA: 1,465 bp) and transformed in rev3-2 mutants. Expression of the REV3 wild-type (WT) construct leads to a protein identical to natural REV3. Point mutations were inserted into motif A and motif C, respectively, with substitution of an Asp residue in the motif to an Ala (marked red in the illustration). These motifs are part of the six conserved regions within the polymerase domain of REV3. The relative fresh weight of homozygous single-locus lines transformed with the wild-type construct of REV3 (B), D1385A (C), and D1549A (D) point mutations in comparison with the wild-type and rev3-2 plants after treatment with MMC, MMS, and cisplatin is shown. For each construct, four different transgenic lines with rev3-2 background are shown. Plantlets were treated with the different genotoxins, and their fresh weight was measured and calculated in relation to the untreated control plants of the same line. Each assay was performed at least three times for calculation of the sd (error bars). B, Hypersensitivity of rev3-2 can be complemented by expression of the wild-type construct, which is true for all tested chemical genotoxins. A control line expressing the construct in a wild-type background (WT::REV3#1) exhibits a relative fresh weight that does not differ from the untransformed wild-type fresh weight. C, Hypersensitivity of rev3-2 cannot be complemented by expression of the D1385A construct, which is true for all tested chemical genotoxins. A control line expressing the construct in a wild-type background (WT::REV3 D1385A#1) exhibits a relative fresh weight that does not differ from the untransformed wild-type fresh weight. Expression of the D1549A construct can complement sensitivity of rev3-2 after treatment with MMS, whereas hypersensitivity is not abolished after treatment with MMC or high concentrations of cisplatin. The line rev3-2::REV3 D1549A#1 behaves like rev3-2 in all tested conditions. A control line expressing the construct in a wild-type background (WT::REV3 D1549A#1) exhibits a relative fresh weight that does not differ from the untransformed wild-type fresh weight. ***, P < 0.001; **, 0.01 > P > 0.001; *, 0.05 > P > 0.01.
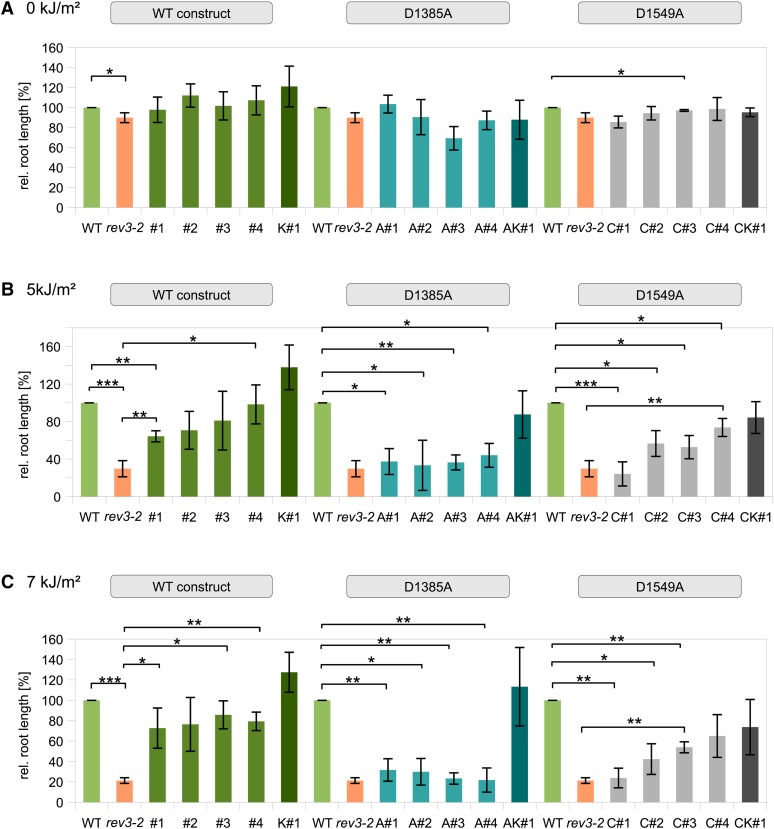
As translesion polymerases in plants have an important role in overcoming UV-B-induced DNA damage, we also determined the UV-B sensitivities of the transgenic lines using root growth assays. The root lengths of plantlets irradiated with 5 and 7 kJ m2 UV-B radiation were compared with roots grown under nonirradiated conditions (Fig. 2). In untreated seedlings, we found that rev3-2 root growth is already slightly but significantly limited compared with wild-type roots, whereas only one line with a slight, statistically significant difference in root growth could be found. However, this line, D1549A#3, shows only a marginal difference of not even 3% in mean relative root growth compared with wild-type roots. After irradiation with 5 and 7 kJ m2, expression of the wild-type construct complemented the hypersensitivity of rev3-2 to UV-B irradiation, which is not observed with the D1385A construct. Lines transformed with the D1549A construct showed an intermediate phenotype after UV-B treatment.
Figure 2.
Relative (rel.) root growth in plants transformed with the wild type (WT), D1385A, and D1549A construct, respectively. Root lengths were measured on untreated plants (A) and after irradiation with 5 kJ m2 UV-B (B), and 7 kJ m2 UV-B (C). Root length was measured and calculated relative to the length of the wild-type roots. The rev3-2 insertion line exhibits slightly reduced root growth without irradiation compared with the wild type, whereas a strong reduction is detectable after UV-B treatment. All other tested lines showed no particularly reduced root growth in comparison with the wild type without irradiation. After being irradiated with 5 and 7 kJ m2 UV-B, rev3-2 plants expressing the wild-type construct exhibit root lengths comparable with the wild type, whereas expression of the D1385A construct does not complement the sensitivity. As in assays with chemical genotoxins, expression of the D1549A construct leads only to partial complementation of the hypersensitivity. Control lines transformed with the wild-type construct in wild-type background (K#1) show less reduced root growth compared with the untransformed wild type. ***, P < 0.001; **, 0.01 > P > 0.001; *, 0.05 > P > 0.01.
REV3-Mediated DNA Repair Is Not Dependent on ATR
As Ataxia telangiectasia-mutated and Rad3-related homolog (ATR) is the master checkpoint kinase activated by replication stress, we addressed whether this kinase is involved in REV3-mediated resistance to cross-linking and methylating agents. Recently, it was reported that AtATR is also required for resistance to cross-linking (CL) agents (Aklilu et al., 2014), and in other organisms, ATR deficiency also leads to sensitivity to methylating agents (Collis et al., 2003).
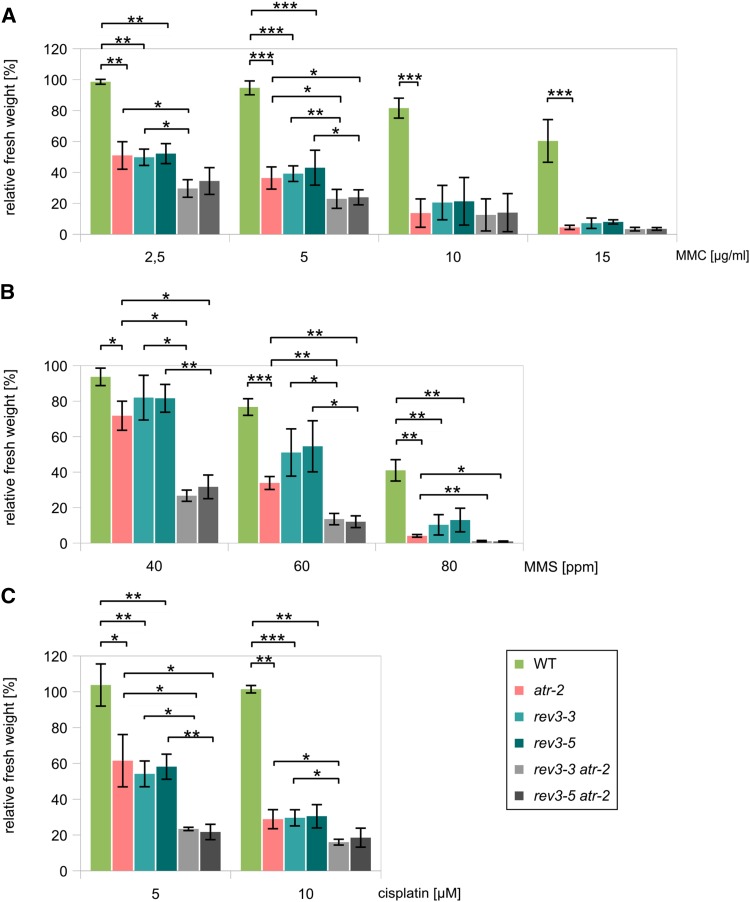
To determine whether ATR and REV3 cooperate in the stress response to DNA-damaging agents that affect the replication fork, we crossed mutants for two different alleles of REV3, rev3-3 and rev3-5, with atr-2. The double-mutant lines were tested in sensitivity assays using MMC, MMS, and cisplatin as genotoxic agents. All three genotoxins yield similar results (Fig. 3). After treatment with MMC, the single-mutant lines of rev3 and atr-2 show comparable sensitivities, and their relative fresh weights are approximately 50% of the wild type using 2.5 µg mL−1 MMC. In contrast, the double mutants display significantly higher hypersensitivities than the single mutants, with fresh weights of approximately 30% compared with the wild type. Treatment with 5 µg mL−1 MMC resulted in similar results, whereas no significant difference in the fresh weights of the single and double mutants was found at higher concentrations. Similar results were found using the alkylating agent MMS. As before, the rev3 mutant shows minor growth inhibition after treatment with MMS, which is also true for atr-2. The double-mutant lines, however, are dramatically more hypersensitive to MMS than any single-mutant line. The relative fresh weights of the single-mutant lines of rev3 and atr-2 correspond to 80% and 70% of the wild type, respectively, and those of the double mutants are 30% after induction with 40 ppm MMS. Similar results are obtained with higher concentrations. The results after induction with cisplatin are similar to those obtained with MMC: no significant difference is observed between the hypersensitivities of atr-2 and the rev3 insertion lines, although the double-mutant lines do show increased hypersensitivities to cisplatin compared with the single mutants. Thus, although both REV3 and ATR are key factors in the stress responses to the tested genotoxins, they appear to have some nonoverlapping functions.
Figure 3.
Relative fresh weight of rev3 atr-2 single and double mutants after MMC, MMS, and cisplatin treatment. Plantlets were treated with the different genotoxins, and their fresh weight was measured and calculated in relation to the untreated control plants of the same line. Each assay was performed at least three times for calculation of the sds (error bars). A, Relative fresh weight after treatment with 2.5, 5, 10, and 15 µg mL−1 MMC. B, After treatment with 40, 60, and 80 ppm MMS, atr-2 exhibits stronger hypersensitivity than rev3 single mutants, whereas the double-mutant lines show even stronger hypersensitivity than atr-2. C, The rev3 single mutants as well as atr-2 are significantly hypersensitive against cisplatin. Although their relative fresh weights are indistinguishable, corresponding double-mutant lines exhibit stronger hypersensitivity than the single-mutant lines. ***, P < 0.001; **, 0.01 > P > 0.001; *, 0.05 > P > 0.01. WT, Wild type.
Generation of Double Mutants of rev3 with the Nuclease mus81 and the DNA Helicase recq4a
In contrast to the situation in animals and fungi, the DNA helicases RECQ4A and RAD5A as well as the endonuclease MUS81 define three parallel pathways of DNA cross-link repair in Arabidopsis (Mannuss et al., 2010). Interestingly, RAD5A and REV3 act independently in the repair of interstrand and intrastrand cross links and base alkylation (Wang, 2007). To elucidate interactions between REV3 and these two factors, we established double-mutant lines of rev3 recq4a and rev3 mus81 to determine whether REV3 participates in the pathways involving MUS81 and RECQ4A. After crossing of the respective T-DNA insertion lines, we identified homozygous double-mutant lines in the F2 generation by PCR-based genotyping. The homozygous double mutant lines of rev3 recq4A-4 and rev3 mus81-1 display no obvious phenotypes under standard growth conditions.
REV3 Cooperates with MUS81 to Repair DNA Interstrand Cross Links
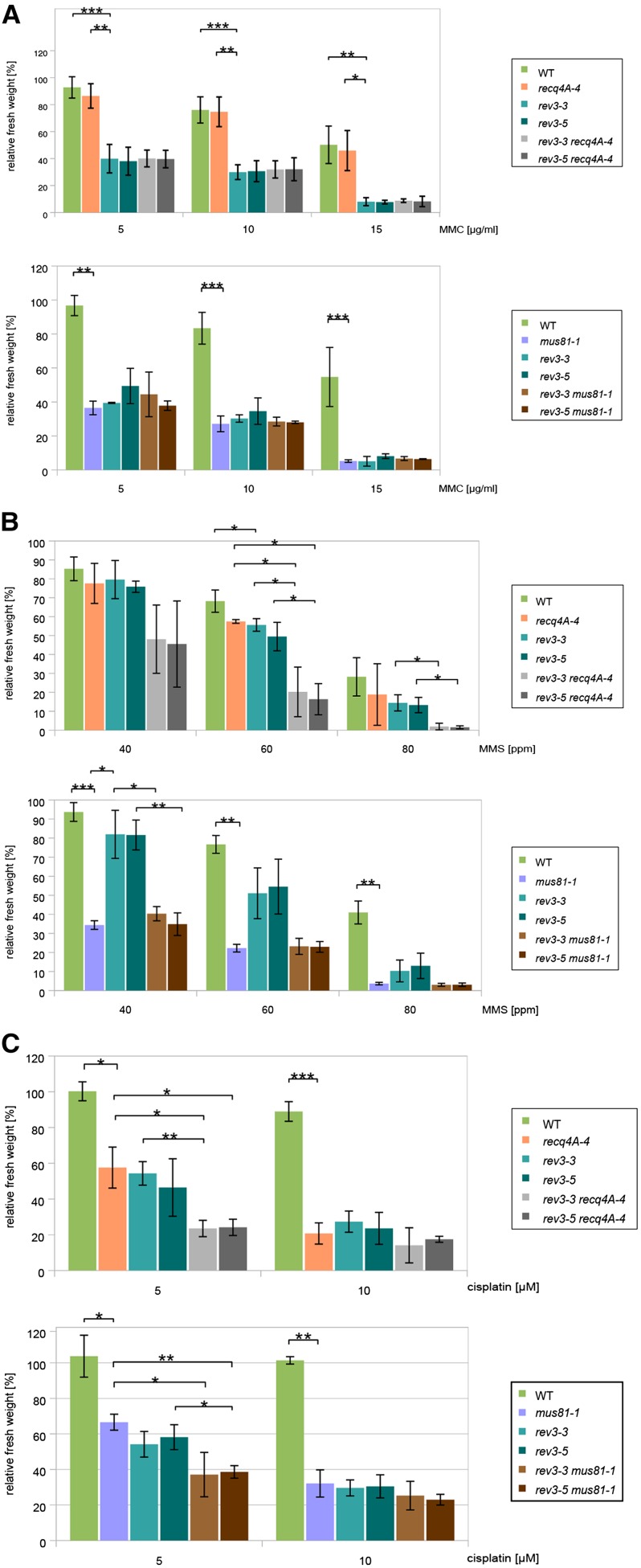
To determine the sensitivities of the mutant lines to interstrand cross links, four different concentrations of MMC were used: 5, 10, 15, and 20 µg mL−1. The insertion line recq4A-4 shows no hypersensitivity to MMC, whereas rev3 single-mutant lines and the mus81 single-mutant line show comparable hypersensitivities (Fig. 4A). The relative fresh weight of the double-mutant lines does not differ from the fresh weight of the rev3 or mus81-1 single mutant, which is approximately 20% to 40% of the wild type. Therefore, our results indicate that REV3 cooperates with MUS81 in response to interstrand cross links. Furthermore, our data exclude the possibility that REV3 is involved in interstrand cross-link repair in a pathway independent of RECQ4A, in contrast to what we previously demonstrated for MPH1-associated histone-fold protein1 (MHF1; Dangel et al., 2014).
Figure 4.
Relative fresh weights of rev3 recq4A-4 and rev3 mus81-1 single and double mutants after MMC (A), MMS (B), and cisplatin (C) treatment. Each assay was performed at least three times for calculation of the sds (error bars). A, Plantlets were treated with 5, 10, 15, and 20 µg mL−1 MMC, and their fresh weight was measured and calculated in relation to the untreated control plants of the same line. In contrast to rev3 mutants, recq4A-4 is not sensitive against MMC. The double mutant shows comparable sensitivity with the rev3 insertion lines. The mus81-1 and rev3 insertion lines as well as the double-mutant lines exhibit the same hypersensitivity against MMC. B, Plantlets were treated with 40, 60, and 80 ppm MMS, and their fresh weight was measured and calculated in relation to the untreated control plants of the same line. recq4A-4 and rev3 single mutants exhibit the same hypersensitivity against MMS, whereas both double-mutant lines show stronger sensitivities than the single-mutant lines. mus81-1 shows stronger sensitivity against MMS than rev3 single mutants, whereas double mutants of the corresponding lines are not distinguishable from mus81-1 single mutants. C, Plantlets were treated with 5 and 10 µm cisplatin, and their fresh weight was measured and calculated in relation to the untreated control plants of the same line. After treatment with 5 µm cisplatin, rev3 single mutants as well as the recq4A-4 single mutant show significantly reduced fresh weights compared with the wild type (WT). The double-mutant lines exhibit stronger hypersensitivity against cisplatin than the corresponding single-mutant lines. Treatment with 5 µm cisplatin leads to reduced fresh weights of mus81-1 and rev3 single mutants, whereas double-mutant lines show increased hypersensitivity compared with the single mutants. ***, P < 0.001; **, 0.01 > P > 0.001; *, 0.05 > P > 0.01;.
Repair of Alkylated Bases Requires Cooperation of REV3 and MUS81 but Not REV3 and RECQ4A
We used 40, 60, and 80 ppm of the alkylating genotoxin MMS in sensitivity assays. Both rev3 single-mutant lines and recq4A-4 show only moderate hypersensitivities to MMS that are similar to each other (Fig. 4B). In contrast, mus81-1 is highly hypersensitive to this genotoxin. The rev3 recq4A-4 double mutants show increased hypersensitivities compared with the single mutants, whereas the rev3 mus81-1 double mutants are as sensitive as the rev3 single mutants. These results indicate that, on the one hand, REV3 and MUS81 act in the same pathway as already demonstrated for interstrand cross linking; on the other hand, they clearly show that REV3 and RECQ4A function in different pathways of alkylated base DNA repair.
REV3 Defines a Unique, Fourth Pathway of Intrastrand Cross-Link Repair
To investigate the interplay of REV3 and RECQ4A as well as REV3 and MUS81 at intrastrand cross links, we performed sensitivity assays with 5 and 10 µm cisplatin. We obtained similar results for both experiments: rev3, recq4A-4, and mus81-1 are moderately hypersensitive to this genotoxin, and their fresh weights at 5 µm cisplatin are approximately 50% to 65% of the wild type (Fig. 4C). Interestingly, in both cases, the double-mutant line is more hypersensitive to treatment with cisplatin when compared with the respective single-mutant line, with fresh weights of approximately 30% for rev3 recq4A-4 and 40% for rev3 mus81-1. Thus, REV3 is not active in intrastrand cross-link repair pathways in which either RECQ4A or MUS81 is involved. Considering the previous results regarding RAD5A (Mannuss et al., 2010; Wang et al., 2011), we now have evidence for the existence of all four different subpathways of intrastrand cross-link repair in plants.
DISCUSSION AND CONCLUSION
The TLS polymerase POLζ is known to be involved in stress responses to several DNA-damaging agents. Knockout mutations of REV3, the catalytic subunit of POLζ, lead to embryonic lethality in mice, and rev3 deficiency in human cell lines and T-DNA insertion lines of rev3 in Arabidopsis shows hypersensitivities to MMC, MMS, cisplatin, and gamma- and UV-B irradiation (Bemark et al., 2000; Esposito et al., 2000; Sakamoto et al., 2003; Curtis and Hays, 2007). Here, we provide, to the best of our knowledge, new insights into the function of REV3 in Arabidopsis by demonstrating the variable involvement of REV3 in the repair of different types of DNA damage and by assigning REV3 to known DNA damage response pathways.
Processivity Requirements of REV3 Differ for Different Types of DNA Damage
Using complementation analysis, we were able to gain new insights, to the best of our knowledge, into the mode of action of REV3 in Arabidopsis. As a member of the B family of polymerases, REV3 contains a conserved polymerase domain. Amino acids located in motifs A, B, and C are known to form the active site (Joyce and Steitz, 1995). Point mutations in these motifs have been performed in other B family polymerases, revealing specific functions for these highly conserved residues. Although most substitutions in motif A, with the consensus sequence DxxxLYPS of B family polymerase I of Thermus aquaticus, preserve wild-type activity, substitution of the Asp results in the total loss of polymerase function (Patel and Loeb, 2000). The consensus sequence of motif C is YGDTDS, which is highly conserved among many eukaryotic and prokaryotic polymerases. Studies with the Bacillus subtilis phage Φ29 DNA polymerase and human polymerase alpha have indicated that mutations of the first Asp result in a partially functional polymerase with a deficiency in processivity. Motif C, however, is involved in both the initiation and elongation reactions (Bernad et al., 1990; Copeland and Wang, 1993).
As expected, we were able to show that expression of the wild-type REV3 construct complemented the hypersensitivity of rev3-2 to MMC-, MMS-, cisplatin-, and UV-B-induced DNA damage in Arabidopsis. It is of interest to note that an expression analysis revealed that less than 20% expression of the wild-type gene is sufficient for full complementation (Supplemental Fig. S1). Conversely, expression of the construct carrying the amino acid substitution in motif A (D1385A) did not complement the sensitivities of rev3-2 to MMC, MMS, cisplatin, or UV-B. The affected catalytic Asp is known to be involved in Mg2+ binding, which is necessary for polymerase activity (Patel and Loeb, 2000). Therefore, our results indicate that the function of REV3 at damaged sites is entirely dependent on its polymerase activity, as opposed to other functions, such as the recruitment of other repair factors.
Some striking results were obtained when we performed assays with plants expressing the D1549A construct mutated in motif C, for which the results varied depending on the genotoxin used. Expression of the construct fully complements hypersensitivity to MMS; however, after damage induction with MMC, cisplatin, and UV-B, we observed an intermediate phenotype for fresh weight and root length that differs from both the wild type and rev3-2. At first glance, these varying phenotypes between the lines might be attributed to different expression levels of the constructs. Indeed, one out of four lines shows no complementation at all. Expression analysis showed that this line has only 5% expression of the wild type, and this probably leads to an insufficient amount of protein to achieve any complementation at all (Supplemental Fig. S1). However, it is important to note that we see full complementation with respect to the DNA damage induced by MMS but not by CL-inducing agents for the three other lines. Therefore, apparently there is enough protein present in these lines to repair DNA damage, and we can see a qualitative difference in the response to different kinds of DNA damage for these three lines. As polymerases mutant for the substituted Asp in motif C are still active in short polymerase reactions, it can be assumed that minor obstacles, such as single methylated bases, can be overcome by the mutated REV3 protein. However, synthesis over DNA damage that is not restricted to a single base requires the processivity of the polymerase to be bypassed. It is most likely that the mutated REV3 is lost from the template before repair synthesis is complete, resulting in replication defects and slow growth. To complement our in vivo analysis, it would be interesting to test how the respective mutations change the biochemical behavior of an in vitro-expressed REV3 protein in detail.
REV3 Is Not Controlled by ATR in DNA Damage Repair
ATR is one of the master checkpoint kinases and is mainly activated upon replication stress. We found that, despite being key factors in response to stressed replication forks, ATR and REV3 do not function in a common pathway during the repair or bypass of DNA damage induced by MMC, MMS, or cisplatin in Arabidopsis. In human cells, recruitment of REV3 to sites of stalled replication forks is dependent on the monoubiquitination of PCNA, which is independent of ATR or Ataxia Telangiectasia Mutated (Brun et al., 2010). PCNA monoubiquitination is followed by a polymerase switch from an accurate replicative polymerase to a TLS polymerase to bypass the lesion. Our results show that TLS bypass of alkylations by MMS and cross links by cisplatin and MMC can also occur independently of ATR in Arabidopsis.
REV3 Cooperates with RECQ4A and MUS81 to Repair DNA Interstrand Cross Links
Arabidopsis possesses at least three parallel pathways for the repair of DNA cross-link damage. These pathways are characterized by the proteins RAD5A, RECQ4A, or MUS81, respectively (Mannuss et al., 2010). The RecQ helicase RECQ4A was thought to have no function in response to MMC-induced interstrand cross links because the single-mutant line is not hypersensitive to this genotoxin (Hartung et al., 2007). Nevertheless, recent findings revealed a hidden function of RECQ4A in interstrand cross-link repair because double mutants mhf1 recq4a and fancm recq4a are hypersensitive to MMC and the respective single mutants are not (Dangel et al., 2014). It was also reported that Arabidopsis REV3 functions independently of RAD5 in response to DNA damage caused by MMC, MMS, and cisplatin (Wang et al., 2011).
As we found in this study, double mutants of rev3 with recq4a or mus81 show the same hypersensitivity to MMC as the respective single mutants; therefore, one must assume that REV3 cooperates with both RECQ4A and MUS81 in response to interstrand cross links (Fig. 5A). Because the structure-specific endonuclease MUS81 is thought to be involved in the unhooking step of interstrand cross-link repair (Ciccia et al., 2008; Sengerová et al., 2011), which is followed by TLS activity to repair single-stranded gaps in DNA, one could speculate that MUS81 and REV3 are required for consecutive steps in a single pathway.
Figure 5.
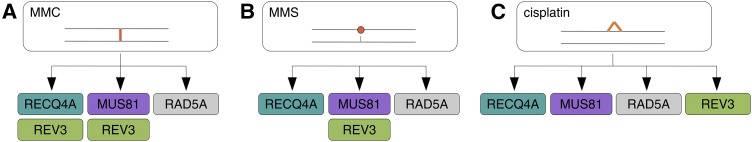
Assignment of REV3 to DNA damage response pathways. REV3 and RAD5 are known to participate in alternative pathways in reaction to MMC- (A), MMS- (B), and cisplatin-induced (C) damage. A, We found that, in response to MMC-induced interstrand cross links, REV3 functions in pathways containing either RECQ4A or MUS81, respectively. B, MMS-induced alkylated bases are repaired or bypassed by at least three alternate pathways. We were able to assign REV3 to the pathway containing MUS81, whereas RECQ4A is not epistatic to REV3. C, Cisplatin mainly causes intrastrand cross links. Recent work suggested at least three separate pathways in response to such cross links with the proteins RECQ4A, MUS81, and RAD5A as key factors. Here, we revealed a fourth pathway that is characterized by the participation of REV3.
During repair of an interstrand CL, a one-sided DSB might also arise, which needs to be repaired by HR. RecQ helicases, specifically RECQ4A and its yeast and human homologs Slow Growth Suppressor1 and BLM, have been shown to be involved in HR reactions. Indeed, it has been shown in human cells that REV3 is involved in HR (Sharma et al., 2012), making it likely that AtREV3 acts together with AtRECQ4A in a common HR pathway.
Repair of Alkylated Bases Requires the Cooperation of REV3 and MUS81 But Not REV3 and RECQ4A
In contrast to MMC-induced interstrand cross links, alkylated bases and intrastrand cross links affect only one of the two DNA strands. Therefore, other mechanisms should be involved in the repair or bypass of the damage. As mentioned above, template switching and TLS can be used if the replication fork encounters such a lesion. Our results indicate MUS81 and REV3 operate in a common pathway in response to MMS-induced alkylated bases (Fig. 5B), which is surprising because there is no known mechanism based on the function of an endonuclease and a TLS polymerase for bypassing or repairing alkylated substrates. One possibility might be that MUS81 generates double-strand breaks at stalled replication forks, which are then repaired by HR. A potential function of REV3 might be the elongation of the emerging displacement loop recombination intermediate. Because recent studies suggest a role for POLζ and other TLS polymerases in DNA synthesis during HR (Kawamoto et al., 2005; McIlwraith et al., 2005; Hirano and Sugimoto, 2006; Kane et al., 2012), this interplay of MUS81 and REV3 does not seem unlikely.
Our data indicate that RECQ4A and REV3 do not share a common pathway in response to alkylated bases. An important function of RecQ helicases is the regression of stalled replication forks, which has, for instance, been demonstrated in vitro for the human protein BLM (Ralf et al., 2006). A similar activity has been recently reported for Arabidopsis RECQ4A (Schröpfer et al., 2014b). As the DNA synthesis after fork regression occurs on a strand that has just been synthesized using the undamaged parental strand as the template, this strand is obviously damage free; therefore, no translesion polymerase is required for this synthesis. In contrast, REV3 might be directly involved in the translesion bypass of alkylated bases. Thus, two separate pathways exist in Arabidopsis for responses to alkylation damage that appear to involve either RECQ4A or REV3.
REV3 Resembles a Fourth Pathway in Response to Intrastrand Cross Links
Cisplatin mostly generates intrastrand cross links between adjacent bases on the same DNA strand. To date, there are three pathways described in response to intrastrand cross links in Arabidopsis, involving RECQ4A, MUS81, or RAD5A, respectively (Mannuss et al., 2010). Our results show that Arabidopsis REV3 does not cooperate with MUS81 and RECQ4A in the bypass or repair of intrastrand cross links. Because it is also known that REV3 and RAD5A do not share a common pathway regarding such damage (Wang et al., 2011), our findings reveal a fourth pathway in response to intrastrand cross links (Fig. 5C). Interestingly, in contrast to alkylated bases, intrastrand cross links do not appear to be repaired or bypassed in a pathway in which MUS81 and REV3 cooperate. It is likely that only basic translesional polymerase activity is required for bypassing intrastrand cross links. In this specific case, REV3 does not appear to be dependent on DNA repair-specific helicases or nucleases.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Col-0 was used as the wild-type control in this study. Three different mutant lines of REV3 were used in this study: the well-characterized mutant rev3-2 (SALK_029237; Sakamoto et al., 2003; Curtis et al., 2009) was used for the complementation analysis, whereas the rev3-3 (SALK_067237; Sakamoto et al., 2003) and rev3-5 (SALK_126789) mutant lines were used for the epistasis analysis. As the molecular nature of the rev3-5 allele was not known in detail, we characterized the insertion site by sequencing the locus in both the 5′ and 3′ direction of the insertion and found that the T-DNA is located in intron 8 (of 21), accompanied by short deletions at both left borders (8 and 2 bp). Furthermore, a deletion of 31 bp involving the intron adjacent to the 3′-oriented left border is present. To ensure that the T-DNA insertion in this line disrupts REV3 gene expression, total RNA of 2-week-old wild-type and rev3-5 plants was extracted and reverse transcribed. Using quantitative PCR, we measured REV3 expression at three positions of the gene: 5′ and 3′ of the insertion as well as across the insertion. Compared with the wild type, the expression level of REV3 is not altered in rev3-5 in the 5′ and 3′ orientations of the insertion. In contrast, expression (0.28% of the wild-type level) was barely detectable across the insertion, indicating that the T-DNA is not removed by splicing during gene expression. Due to the location of the insertion within the gene, translation of the putative transcript would lead to a very short protein harboring only the very N-terminal portion of REV3 and missing all of the domains important for TLS activity.
To generate double mutants with rev3, we used the lines mus81-1 (GABI_113F11), recq4A-4 (GABI_203C07), and atr-2 (SALK_032841, described in Hartung et al. [2006, 2007] and Culligan et al. [2004], respectively). Homozygous double mutants were identified in the F2 generation by PCR-based genotyping. For double-mutant generation and transformations, the plants were grown in a greenhouse in soil (1:1 mixture of Floraton 3 [Floragard] and vermiculite [1.3 mm; Deutsche Vermiculite Dämmstoff GmbH]) at 22°C with 16-h light and 8-h dark. For sensitivity assays and root growth assays, the plants were grown under axenic conditions. Seeds were surface sterilized for 5 min in 4% (w/v) sodium hypochlorite solution. After three washes in sterile water and an overnight stratification at 4°C, the seeds were sown on agar plates containing germination medium (GM: 4.9 g L−1l Murashige and Skoog including vitamins and MES [2 (N morpholino) ethanesulphonic acid; Duchefa Biochemie]), 10 g L−1 Suc, and 7.6 g L−1 agar (adjusted to pH 5.7 with KOH) and incubated in a CU-36L4 plant culture chamber (Percival Scientific, Inc.) with 16-h light at 22°C and 8-h dark at 20°C.
Primers Used for PCR-Based Genotyping of T-DNA Insertion Lines
To determine the genotypes of T-DNA insertion lines, two primer pairs for each mutant line were used. For detection of the wild-type locus of the respective gene, primers with binding sites upstream and downstream of the insertion site were used. One gene-specific and one T-DNA border-specific primer were used to identify T-DNA insertions. For rev3-5, wild-type PCR was performed using the gene-specific primers SK-75 (5′-GCCCTGAAGCCTTCCTTACTTG-3′) and SK-76 (5′-GAAGAGGCTAGTTCAAACGTCC-3′); for identification of T-DNA insertions, SK-75 was combined with the T-DNA-specific primer Lbd1 (5′-TCGGAACCACCATCAAACAG-3′). The lines mus81-1, recq4A-4, and atr-2 were genotyped as previously described (Culligan et al., 2004; Hartung et al., 2006).
Sensitivity Assays Using Chemical Genotoxic Agents
For sensitivity assays, seeds were sterilized and sown on solid GM. After 7 d of incubation in a CU-36L4 plant culture chamber, 10 plantlets were transferred into each well of six-well plates containing liquid GM; for untreated controls, 5 mL of GM was used, whereas 4 mL was used for samples treated with genotoxic agents. The next day, different concentrations of genotoxins, MMC (Duchefa Biochemie), MMS, and cisplatin (Sigma-Aldrich Chemie) were added in 1 mL of liquid GM. After another 14 d of incubation, the fresh weight of the seedlings in each well was determined; to exclude possible line-specific growth differences, the evaluation was made relative to the untreated samples of each line.
Root Growth Assays
To determine root growth after UV-B irradiation, the seeds were surface sterilized and sown on square culture plates containing solid GM. The seedlings were allowed to grow vertically on the plates in a CU-36L4 plant culture chamber for 3 d before they were exposed to 0, 5, or 7 kJ m2 UV-B in a BIO-LINK BLX UV-B chamber (Vilber Lourmat). After incubation in the dark for 1 d, the plates were again incubated in the CU-36L4 plant culture chamber for another 6 d. The length of root growth was measured using the SmartRoot Add-on of ImageJ (Rasband, 2008; Lobet and Draye, 2013). The evaluation of root growth of the different plant lines was then expressed as a percentage of the average length of wild-type roots.
Constructs and Plant Transformation
The wild-type complementation construct consisting of the cDNA and gDNA of REV3 was cloned into the multiple-cloning site of the binary vector pPZP221 (Hajdukiewicz et al., 1994). For this construct, three fragments were amplified: the natural promoter including the 5′ untranslated region (718 bp), the full-length ORF amplified from both cDNA (first 5,113 bp) and gDNA (945 bp), and the natural terminator including the 3′ untranslated region (520 bp). Using the In-Fusion Advantage PCR Cloning Kit (Clontech), this construct was introduced between the XmaI and SalI restriction sites of vector pPZP221. Using a site-directed mutagenesis approach, constructs carrying point mutations were created from the wild-type construct (Ho et al., 1989). The vector containing the wild-type construct was amplified with two complementary mutagenesis primers (GTTATTGTGTTGgctTTTCAATCTC and GAGATTGAAAagcCAACACAATAAC for D1385A, GAGTTGTATATGGTgccACTGATAGG and CCTATCAGTggcACCATATACAACTC for D1549A), and the original, methylated plasmid was digested with DpnI. The recombinant plasmids were introduced into Agrobacterium tumefaciens strain GV3101::pMP90 (Koncz et al., 1984) by electroporation, and then transformed into Arabidopsis Col-0 and rev3-2 by the floral dip method (Clough and Bent, 1998). Transgenic plants harboring the transformed T-DNA were isolated by selection on solid GM containing 75 mg L−1 gentamycin. T3 homozygous single-locus lines were used for sensitivity and root growth assays.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AtATR: At5g40820, AtMUS81: At4g30870, AtRAD5A: At5g22750, AtRECQ4A: At1g10930, and AtREV3: At1g67500.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Expression levels of the REV3, REV3 D1385A, and REV3 D1549A constructs
Supplemental Method S1. Method for Supplemental Figure S1.
Supplementary Material
Acknowledgments
We would like to thank Julia Kremer for technical assistance in the laboratory.
Glossary
- DSB
double-strand break
- PRR
postreplicative repair
- TLS
translesion synthesis
- MMC
mitomycin C
- HR
homologous recombination
- T-DNA
transfer DNA
- cDNA
complementary DNA
- Col-0
Columbia-0
- GM
germination medium
Footnotes
This work was supported by the European Research Council (grant no. ERC–2010–AdG_20100317 COMREC).
References
- Aklilu BB, Soderquist RS, Culligan KM (2014) Genetic analysis of the Replication Protein A large subunit family in Arabidopsis reveals unique and overlapping roles in DNA repair, meiosis and DNA replication. Nucleic Acids Res 42: 3104–3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemark M, Khamlichi AA, Davies SL, Neuberger MS (2000) Disruption of mouse polymerase zeta (Rev3) leads to embryonic lethality and impairs blastocyst development in vitro. Curr Biol 10: 1213–1216 [DOI] [PubMed] [Google Scholar]
- Bernad A, Lázaro JM, Salas M, Blanco L (1990) The highly conserved amino acid sequence motif Tyr-Gly-Asp-Thr-Asp-Ser in alpha-like DNA polymerases is required by phage phi 29 DNA polymerase for protein-primed initiation and polymerization. Proc Natl Acad Sci USA 87: 4610–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blastyák A, Pintér L, Unk I, Prakash L, Prakash S, Haracska L (2007) Yeast Rad5 protein required for postreplication repair has a DNA helicase activity specific for replication fork regression. Mol Cell 28: 167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun J, Chiu RK, Wouters BG, Gray DA (2010) Regulation of PCNA polyubiquitination in human cells. BMC Res Notes 3: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IP, Mannuss A, Orel N, Heitzeberg F, Puchta H (2008) A homolog of ScRAD5 is involved in DNA repair and homologous recombination in Arabidopsis. Plant Physiol 146: 1786–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccia A, McDonald N, West SC (2008) Structural and functional relationships of the XPF/MUS81 family of proteins. Annu Rev Biochem 77: 259–287 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Collis SJ, Swartz MJ, Nelson WG, DeWeese TL (2003) Enhanced radiation and chemotherapy-mediated cell killing of human cancer cells by small inhibitory RNA silencing of DNA repair factors. Cancer Res 63: 1550–1554 [PubMed] [Google Scholar]
- Copeland WC, Wang TS (1993) Mutational analysis of the human DNA polymerase alpha. The most conserved region in alpha-like DNA polymerases is involved in metal-specific catalysis. J Biol Chem 268: 11028–11040 [PubMed] [Google Scholar]
- Croteau DL, Popuri V, Opresko PL, Bohr VA (2014) Human RecQ helicases in DNA repair, recombination, and replication. Annu Rev Biochem 83: 519–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culligan K, Tissier A, Britt A (2004) ATR regulates a G2-phase cell-cycle checkpoint in Arabidopsis thaliana. Plant Cell 16: 1091–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Belcram K, Bollmann SR, Tominey CM, Hoffman PD, Mercier R, Hays JB (2009) Reciprocal chromosome translocation associated with TDNA-insertion mutation in Arabidopsis: genetic and cytological analyses of consequences for gametophyte development and for construction of doubly mutant lines. Planta 229: 731–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Hays JB (2007) Tolerance of dividing cells to replication stress in UVB-irradiated Arabidopsis roots: requirements for DNA translesion polymerases eta and zeta. DNA Repair (Amst) 6: 1341–1358 [DOI] [PubMed] [Google Scholar]
- Dangel NJ, Knoll A, Puchta H (2014) MHF1 plays Fanconi anaemia complementation group M protein (FANCM)-dependent and FANCM-independent roles in DNA repair and homologous recombination in plants. Plant J 78: 822–833 [DOI] [PubMed] [Google Scholar]
- Esposito G, Godindagger I, Klein U, Yaspo ML, Cumano A, Rajewsky K (2000) Disruption of the Rev3l-encoded catalytic subunit of polymerase zeta in mice results in early embryonic lethality. Curr Biol 10: 1221–1224 [DOI] [PubMed] [Google Scholar]
- Geuting V, Kobbe D, Hartung F, Dürr J, Focke M, Puchta H (2009) Two distinct MUS81-EME1 complexes from Arabidopsis process Holliday junctions. Plant Physiol 150: 1062–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MF, Woodgate R (2013) Translesion DNA polymerases. Cold Spring Harb Perspect Biol 5: a010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdukiewicz P, Svab Z, Maliga P (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25: 989–994 [DOI] [PubMed] [Google Scholar]
- Hanada K, Budzowska M, Modesti M, Maas A, Wyman C, Essers J, Kanaar R (2006) The structure-specific endonuclease Mus81-Eme1 promotes conversion of interstrand DNA crosslinks into double-strands breaks. EMBO J 25: 4921–4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haracska L, Yu SL, Johnson RE, Prakash L, Prakash S (2000) Efficient and accurate replication in the presence of 7,8-dihydro-8-oxoguanine by DNA polymerase eta. Nat Genet 25: 458–461 [DOI] [PubMed] [Google Scholar]
- Hartung F, Suer S, Bergmann T, Puchta H (2006) The role of AtMUS81 in DNA repair and its genetic interaction with the helicase AtRecQ4A. Nucleic Acids Res 34: 4438–4448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung F, Suer S, Knoll A, Wurz-Wildersinn R, Puchta H (2008) Topoisomerase 3alpha and RMI1 suppress somatic crossovers and are essential for resolution of meiotic recombination intermediates in Arabidopsis thaliana. PLoS Genet 4: e1000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung F, Suer S, Puchta H (2007) Two closely related RecQ helicases have antagonistic roles in homologous recombination and DNA repair in Arabidopsis thaliana. Proc Natl Acad Sci USA 104: 18836–18841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B, Saadat N, Myung B, Shekhar MP (2015) Crosstalk between translesion synthesis, Fanconi anemia network, and homologous recombination repair pathways in interstrand DNA crosslink repair and development of chemoresistance. Mutat Res Rev Mutat Res 763: 258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann NJ, Knoll A, Puchta H (2015) The nuclease FAN1 is involved in DNA crosslink repair in Arabidopsis thaliana independently of the nuclease MUS81. Nucleic Acids Res 43: 3653–3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y, Sugimoto K (2006) ATR homolog Mec1 controls association of DNA polymerase zeta-Rev1 complex with regions near a double-strand break. Curr Biol 16: 586–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77: 51–59 [DOI] [PubMed] [Google Scholar]
- Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S (2002) RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419: 135–141 [DOI] [PubMed] [Google Scholar]
- Jansen JG, Tsaalbi-Shtylik A, de Wind N (2015) Roles of mutagenic translesion synthesis in mammalian genome stability, health and disease. DNA Repair (Amst) 29: 56–64 [DOI] [PubMed] [Google Scholar]
- Johnson RE, Prakash L, Prakash S (2012) Pol31 and Pol32 subunits of yeast DNA polymerase δ are also essential subunits of DNA polymerase ζ. Proc Natl Acad Sci USA 109: 12455–12460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce CM, Steitz TA (1995) Polymerase structures and function: variations on a theme? J Bacteriol 177: 6321–6329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane DP, Shusterman M, Rong Y, McVey M (2012) Competition between replicative and translesion polymerases during homologous recombination repair in Drosophila. PLoS Genet 8: e1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T, Araki K, Sonoda E, Yamashita YM, Harada K, Kikuchi K, Masutani C, Hanaoka F, Nozaki K, Hashimoto N, et al. (2005) Dual roles for DNA polymerase eta in homologous DNA recombination and translesion DNA synthesis. Mol Cell 20: 793–799 [DOI] [PubMed] [Google Scholar]
- Knoll A, Higgins JD, Seeliger K, Reha SJ, Dangel NJ, Bauknecht M, Schröpfer S, Franklin FC, Puchta H (2012) The Fanconi anemia ortholog FANCM ensures ordered homologous recombination in both somatic and meiotic cells in Arabidopsis. Plant Cell 24: 1448–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C, Kreuzaler F, Kalman Z, Schell J (1984) A simple method to transfer, integrate and study expression of foreign genes, such as chicken ovalbumin and alpha-actin in plant tumors. EMBO J 3: 1029–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Myung K (2008) PCNA modifications for regulation of post-replication repair pathways. Mol Cells 26: 5–11 [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Gregory MT, Yang W (2014) Human Pol ζ purified with accessory subunits is active in translesion DNA synthesis and complements Pol ζ in cisplatin bypass. Proc Natl Acad Sci USA 11: 2954–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann AR, Niimi A, Ogi T, Brown S, Sabbioneda S, Wing JF, Kannouche PL, Green CM (2007) Translesion synthesis: Y-family polymerases and the polymerase switch. DNA Repair (Amst) 6: 891–899 [DOI] [PubMed] [Google Scholar]
- Lobet G, Draye X (2013) Novel scanning procedure enabling the vectorization of entire rhizotron-grown root systems. Plant Methods 9: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannuss A, Dukowic-Schulze S, Suer S, Hartung F, Pacher M, Puchta H (2010) RAD5A, RECQ4A, and MUS81 have specific functions in homologous recombination and define different pathways of DNA repair in Arabidopsis thaliana. Plant Cell 22: 3318–3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch SD, Kokoska RJ, Chilkova O, Welch CM, Johansson E, Burgers PMJ, Kunkel TA (2004) Enzymatic switching for efficient and accurate translesion DNA replication. Nucleic Acids Res 32: 4665–4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwraith MJ, Vaisman A, Liu Y, Fanning E, Woodgate R, West SC (2005) Human DNA polymerase eta promotes DNA synthesis from strand invasion intermediates of homologous recombination. Mol Cell 20: 783–792; erratum McIlwraith MJ, Vaisman A, Liu Y, Fanning E, Woodgate R, West SC (2006) Mol Cell 21: 445 [DOI] [PubMed] [Google Scholar]
- Moldovan GL, Pfander B, Jentsch S (2007) PCNA, the maestro of the replication fork. Cell 129: 665–679 [DOI] [PubMed] [Google Scholar]
- Nelson JR, Lawrence CW, Hinkle DC (1996) Thymine-thymine dimer bypass by yeast DNA polymerase zeta. Science 272: 1646–1649 [DOI] [PubMed] [Google Scholar]
- Ohmori H, Friedberg EC, Fuchs RPP, Goodman MF, Hanaoka F, Hinkle D, Kunkel TA, Lawrence CW, Livneh Z, Nohmi T, et al. (2001) The Y-family of DNA polymerases. Mol Cell 8: 7–8 [DOI] [PubMed] [Google Scholar]
- Patel PH, Loeb LA (2000) DNA polymerase active site is highly mutable: evolutionary consequences. Proc Natl Acad Sci USA 97: 5095–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S, Johnson RE, Prakash L (2005) Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu Rev Biochem 74: 317–353 [DOI] [PubMed] [Google Scholar]
- Prakash S, Prakash L (2002) Translesion DNA synthesis in eukaryotes: a one- or two-polymerase affair. Genes Dev 16: 1872–1883 [DOI] [PubMed] [Google Scholar]
- Ralf C, Hickson ID, Wu L (2006) The Bloom’s syndrome helicase can promote the regression of a model replication fork. J Biol Chem 281: 22839–22846 [DOI] [PubMed] [Google Scholar]
- Rasband WS. (2008) ImageJ. http://rsbweb. nih. gov/ij (November 6, 2015).
- Sakamoto A, Lan VTT, Hase Y, Shikazono N, Matsunaga T, Tanaka A (2003) Disruption of the AtREV3 gene causes hypersensitivity to ultraviolet B light and gamma-rays in Arabidopsis: implication of the presence of a translesion synthesis mechanism in plants. Plant Cell 15: 2042–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale JE, Lehmann AR, Woodgate R (2012) Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat Rev Mol Cell Biol 13: 141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröpfer S, Knoll A, Trapp O, Puchta H (2014a) DNA Repair and Recombination in Plants. In Howell SH, ed, Molecular Biology. Springer, New York, pp. 51-93. [Google Scholar]
- Schröpfer S, Kobbe D, Hartung F, Knoll A, Puchta H (2014b) Defining the roles of the N-terminal region and the helicase activity of RECQ4A in DNA repair and homologous recombination in Arabidopsis. Nucleic Acids Res 42: 1684–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengerová B, Wang AT, McHugh PJ (2011) Orchestrating the nucleases involved in DNA interstrand cross-link (ICL) repair. Cell Cycle 10: 3999–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Canman CE (2012) REV1 and DNA polymerase zeta in DNA interstrand crosslink repair. Environ Mol Mutagen 53: 725–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Hicks JK, Chute CL, Brennan JR, Ahn JY, Glover TW, Canman CE (2012) REV1 and polymerase ζ facilitate homologous recombination repair. Nucleic Acids Res 40: 682–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda E, Okada T, Zhao GY, Tateishi S, Araki K, Yamaizumi M, Yagi T, Verkaik NS, van Gent DC, Takata M, et al. (2003) Multiple roles of Rev3, the catalytic subunit of polzeta in maintaining genome stability in vertebrates. EMBO J 22: 3188–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz TA. (1999) DNA polymerases: structural diversity and common mechanisms. J Biol Chem 274: 17395–17398 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Sakamoto A, Sato S, Kato T, Tabata S, Tanaka A (2005) Roles of Arabidopsis AtREV1 and AtREV7 in translesion synthesis. Plant Physiol 138: 870–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wen R, Shi X, Lambrecht A, Wang H, Xiao W (2011) RAD5a and REV3 function in two alternative pathways of DNA-damage tolerance in Arabidopsis. DNA Repair (Amst) 10: 620–628 [DOI] [PubMed] [Google Scholar]
- Wang W. (2007) Emergence of a DNA-damage response network consisting of Fanconi anaemia and BRCA proteins. Nat Rev Genet 8: 735–748 [DOI] [PubMed] [Google Scholar]
- Wong SW, Wahl AF, Yuan PM, Arai N, Pearson BE, Arai K, Korn D, Hunkapiller MW, Wang TS (1988) Human DNA polymerase alpha gene expression is cell proliferation dependent and its primary structure is similar to both prokaryotic and eukaryotic replicative DNA polymerases. EMBO J 7: 37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.