Abstract
Objectives:
To test a collaborative care model for interfacing sleep specialists with primary care providers to enhance patients' sleep disorders management.
Methods:
This study used a randomized, parallel group, clinical intervention trial design. A total of 137 adult (29 women) VA outpatients with sleep complaints were enrolled and randomly assigned to (1) an intervention (INT) consisting of a one-time consultation with a sleep specialist who provided diagnostic feedback and treatment recommendations to the patient and the patient's primary care provider; or (2) a control condition consisting of their usual primary care (UPC). Provider-focused outcomes included rates of adherence to recommended diagnostic procedures and sleep-focused interventions. Patient-focused outcomes included measures taken from sleep diaries and actigraphy; Pittsburgh Sleep Quality Index (PSQI) scores; and self-report measures of sleepiness, fatigue, mood, quality of life, and satisfaction with health care.
Results:
The proportions of provider-initiated sleep-focused interventions were significantly higher in the INT group than in the UPC group for polysomnography referrals (49% versus 6%; P < 0.001) and mental health clinic referrals (19% versus 6%; P = 0.02). At the 10-mo follow up, INT recipients showed greater estimated mean reductions in diary total wake time (−17.0 min; 95% confidence interval [CI]: −30.9, −3.1; P = 0.02) and greater increases in sleep efficiency (+3.7%; 95% CI: 0.8, 6.5; P = 0.01) than did UPC participants. A greater proportion of the INT group showed ≥ 1 standard deviation decline on the PSQI from baseline to the 10-mo follow-up (41% versus 21%; P = 0.02). Moreover, 69% of the INT group had normal (≤ 10) Epworth Sleepiness Scale scores at the 10-mo follow-up, whereas only 50% of the UPC group fell below this clinical cutoff (P = 0.03).
Conclusions:
A one-time sleep consultation significantly increased healthcare providers' attention to sleep problems and resulted in benefits to patients' sleep/wake symptoms.
Clinical Trials Registration:
This study is registered with clinicaltrials.gov with identifier # NCT00390572.
Citation:
Edinger JD, Grubber J, Ulmer C, Zervakis J, Olsen M. A collaborative paradigm for improving management of sleep disorders in primary care: a randomized clinical trial. SLEEP 2016;39(1):237–247.
Keywords: collaborative care, primary and specialty care, sleep disorders
Significance.
Determining the optimal interface between primary and specialty care remains a challenge. Specifically it is often difficult to determine what level of specialty care involvement is needed to assist primary care providers in their management of the various disorders their patients present. The current trial entailed a novel, initial attempt to examine this question in regard to the management of sleep disorders. Specifically this study tested the usefulness of a single sleep specialist consultation that resulted in the specialist's guidance and advice conveyed to both the patient and the patient's provider. The highly promising results confirmed the usefulness and efficacy of this sort of intervention for guiding providers' practice patterns so as to more effectively address their patients' sleep complaints.
INTRODUCTION
Sleep disorders are widespread health problems that reduce quality of life, increase risks for psychiatric and medical disease, and raise health care utilization and costs among affected individuals worldwide.1–19 A subset of patients with sleep problems seek care from sleep specialists, but most such patients are seen in primary care settings where they are likely to receive suboptimal sleep-problem management. As noted by Gottschalk and Flocke20 during a typical primary care visit, the provider has only 10 to 15 min per patient to manage an average of two to three major medical problems that carry significant risk of morbidity and mortality; this leaves very little time to address whatever nonspecific sleep/wake complaints patients might present. Moreover, primary care providers often have limited knowledge of sleep disorders medicine. As such, sleep disorders may either go unrecognized or improperly treated. Thus, many sleep disordered patients seen in primary care settings fail to be properly diagnosed and receive effective, evidence-based therapies.13,21–23
In view of the prevalence, morbidity, and economic significance of sleep disorders, the accurate diagnosis of affected patients should be a priority for our health care system. Most patients with sleep disturbances would likely show improved clinical outcomes if diagnosis and treatment were shifted to sleep specialty care. However, such a proposal is hardly cost-effective, and the limited number of specialty sleep centers would be overwhelmed by the burden of patient care. What is needed is a mechanism whereby sleep specialists serve as consultants to the primary care setting, guiding diagnostic procedures, recommending further specialty care when indicated, and providing feedback to patients and/or providers to improve management of these conditions within the primary care setting.
Outside the sleep literature, there have been a number of efforts to enhance the interface between primary and specialty care. Provider-targeted interventions including focused training, specialist outreach, and financial incentives all improve primary care treatment outcomes and/or referral rates to specialty care.24 The incorporation of problem-focused structured interviews into patients' electronic medical records along with computer-generated clinical reminders for their periodic completion has shown efficacy for the detection and management of disorders such as clinical depression in primary care.25 One additional promising approach is the collaborative care model26 developed for evaluation and management of depressed patients. Collaborative care has mental health specialists providing patient education as well as a structured treatment plan to patients' primary care providers. This approach has proven superior to standard consult-liaison care by providing more rapid improvement in depressive symptoms and greater sustained gains in patients' overall mental health status.26
Recently, Strollo et al.27 proposed a collaborative role for sleep specialists within the context of the patient-centered medical home (PCMH) healthcare delivery model. With this approach, patient care is directed by the primary care physician (PCP), while the sleep specialist acts as a collaborating partner. In some instances, the sleep specialist's role is merely that of advisor to the PCP, whereas in other circumstances the sleep specialist participates in comanagement of the patient. Whereas this approach seems promising for optimizing the primary care-specialty care interface, it has yet to be tested in the management of sleep disorders. Thus, this study was initiated to test a collaborative care intervention (INT) for optimizing PCP's management of their patients' sleep complaints. The INT consisted of: (1) a comprehensive interview-based sleep disorder assessment conducted in the context of a single visit with a sleep specialist; (2) sharing resultant diagnostic impressions and relevant treatment recommendations with the patient; and (3) conveying diagnostic impressions, suggestions for additional diagnostic testing, and treatment recommendations to the patient's PCP. The primary study aims were to compare the INT to usual care for enhancing: (1) provider attention to sleep-focused diagnostic tests and management; and (2) patients' improvements in sleep, functional status, quality of life, and satisfaction with their health care overall.
METHOD
Design
The study employed a prospective, randomized, clinical intervention trial design to compare the INT and a control condition comprised of usual primary care (UPC) at three time points: a pre-treatment baseline and 5 and 10 mo after assignment to treatment conditions. The study was approved by the Institutional Review Board of the Durham VA Medical Center and all enrollees provided written informed consent. Participants received reimbursement for travel expenses as well as a maximum of $175.00 for completing study measures.
Participants
The study was conducted at a tertiary care, university-affiliated VA Medical Center (VAMC). Recruitment occurred from July 2007 to August 2009 through: (1) posted announcements; (2) solicitation letters sent to registered outpatients, and (3) periodic information tables within the outpatient clinic area wherein study staff discussed the study with interested patients and distributed printed study information. Eligible participants were enrolled in outpatient clinics at the VAMC, had active sleep complaints, and scored > 5 on a screening Pittsburgh Sleep Quality Index (PSQI). Exclusion criteria were: (1) an established sleep disorder diagnosis and/ or previous evaluation and treatment by a sleep disorders specialist (VA or Non-VA); (2) unstable physical health/terminal illness; (3) acute or highly unstable Axis I psychiatric condition; (4) mental incompetence (i.e. score < 24 on the Mini-Mental State Examination2; (5) unstable living environment/ homelessness; (6) judged inappropriate by the PCP.
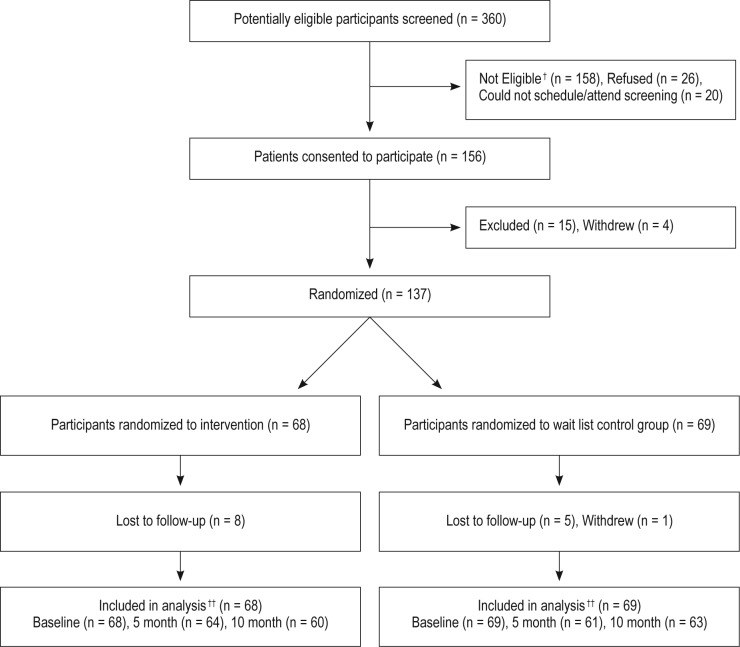
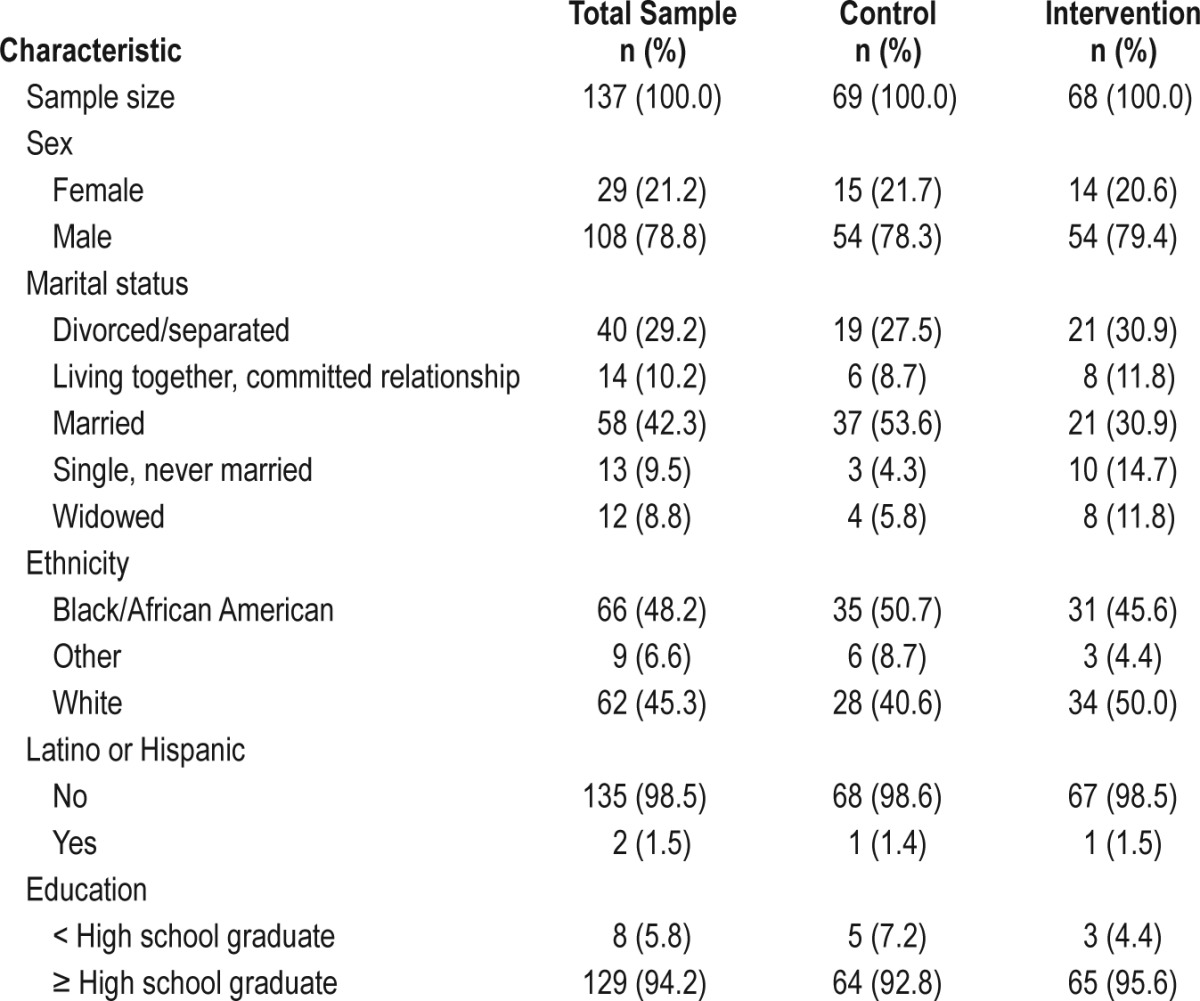
Of the 360 candidates screened, 158 were not eligible due to: prior specialty sleep treatment (n = 44); exclusionary Axis I diagnosis (n = 26); lacking a primary care provider at the medical center (n = 22); unstable health status (n = 21); unstable living situation/homelessness (n = 18); ongoing substance abuse treatment (n = 11); having no clear sleep problem (n = 10); or nonveteran status (n = 6). Additionally, 26 individuals refused enrollment and 20 individuals failed to schedule/attend their initial study visit. A total of 156 subjects were consented, but 15 were excluded before randomization for not meeting entry criteria and 4 withdrew because they found study procedures too demanding. The remaining 137 continued in the study and were randomly assigned to either the INT arm (n = 68) or the UPC arm (n = 69). Figure 1 shows the participant flow through the project, whereas Table 1 provides enrollees' demographic information.
Figure 1.
Sleep consultation study CONSORT flow chart. †Reasons for exclusions: prior specialty sleep treatment (n = 44); exclusionary Axis I diagnosis (n = 26); no primary care provider at the medical center (n = 22); unstable health status (n = 21); unstable living situation/homelessness (n = 18); ongoing substance abuse treatment (n = 11); no clear sleep problem (n = 10); non-veteran status (n = 6). ††Had survey, sleep log, and/or actiwatch data available.
Table 1.
Demographic characteristics of the sample.
Measures
Provider Outcomes
Measures were collected to assess provider adherence to INT recommendations and to compare the rates of new sleep-focused diagnoses, diagnostic procedures, and interventions initiated by providers of patients in both treatment arms. These provider-specific outcomes were collected during the 10-mo period following the patient's randomization to treatment condition. Provider outcomes included the provider's number of sleep laboratory referrals, referrals to other specialty clinics for evaluation/treatment of participant sleep problems, and presence of newly initiated sleep-focused therapies for study participants. Information about these outcomes was obtained via review of provider orders and information included in notes entered into the VA's computerized medical record system (CPRS).
Patients' Outcomes
The patient outcomes described in the next paragraphs were collected during three 2-w evaluation periods; at baseline and 5 and 10 mo postrandomization.
Personal Data Assistant Electronic Sleep Diary. Sleep diary measures were obtained nightly for 2 w at each assessment period using a Palm Pilot-style personal data assistant (PDA) containing an interactive program that automated the collection of subjective sleep data. The program, developed by our laboratory using Satellite Forms (Thacker Network Technologies, Inc, Lacombe, Alberta, Canada) software, presented common questions contained in a conventional paper sleep diary. Each question appeared on the PDA screen individually and solicited numerical entries designating the respondent's estimate of various sleep parameters. At the end of the entries for 1 d, the program automatically recorded a time stamp to verify the time and date that data were entered. The PDA was programmed such that respondents had only 24 h to input data for each night of sleep. The specific nightly measures derived from the PDA included total sleep time (TST), sleep onset latency (SOL), wake time after sleep onset (WASO), total wake time (TWT: SOL + WASO), and sleep efficiency (SE). Mean and standard deviation values of TST, TWT, and SE for each participant obtained at the prerandomization and postrandomization time points served as dependent measures.
Wrist Actigraphy. Mini-Mitter Actiwatch actigraphs (Mini-Mitter Co., Inc., Sun River, OR, USA) were used to derive nightly objective sleep measures for 2-w periods at prerandomization and postrandomization time points during this project. The Actiwatch contains a calibrated accelerometer and 32 K memory storage apparatus, housed in a casing that, in size and shape, resembles a wristwatch. The Actiwatch is designed to interface with a personal computer via a specially designed Reader/Interface unit. Windows-style software, accompanies the Actiwatch, and is used to program the recording unit, download data into storage, and engage a scoring algorithm, which provides estimates of various sleep parameters. Mean and standard deviation values of TST, TWT and SE obtained for each participant at the prerandomization and postrandomization time points served as actigraphic dependent measures in study analyses.
Outcome Questionnaires. Participants completed the 19-item PSQI28 to measure improvements in global sleep quality perceptions, the eight-item Epworth Sleepiness Scale (ESS)29 to assess reductions in daytime sleepiness, the nine-item Fatigue Severity Scale (FSS)30,31 to track reductions in daytime fatigue, the 65-item Profile of Mood States (POMS)32 to assess mood state improvements, the 36-item Veterans Short Form-36 (VSF-36)33 to measure changes in quality of life perceptions, and the three-item global evaluation of care domain from the Perceptions of Care Survey-Outpatient Version (OPOC; http://ebasis.org/pdf/OPOC.pdf) to assess changes in satisfaction with health care from baseline to the postrandomization assessments. The PSQI includes items that inquire about sleep over the past month and produces seven component scores, which are summed to provide a global sleep quality index. The ESS requires respondents to rate their likelihood (0 = “would never doze” to 3 = “high chance of dozing”) of falling asleep in common daytime situations with the total score across items conveying the overall level of daytime sleepiness. The FSS items assess the vitality-anergia continuum with respondents indicating agreement with each item using a 7-point scale (1 = “strongly disagree” to 7 = “strongly agree”). Averaged responses across the nine items comprise the total FSS score. The V-SF-36 is a variant of the MOS SF-3634 adapted for veterans. It measures eight quality-of-life indices and yields two component scores, the Physical Component Summary (PCS) and Mental Component Summary (MCS) scores that served as outcomes of interest herein. The POMS yields scores for six different mood state subscales as well as a summary score for the entire inventory. Only the summary score was considered for this study's purposes. Finally, global evaluation of care domain from the OPOC (http://ebasis.org/pdf/OPOC.pdf) included three tailored questions (“How much have you been helped by the care you received at the Durham VA?”, “Using any number from 0 to 10, where 0 = ”worst possible care” and 10 = ”best possible care”, what is your overall rating of the care you received at the Durham VA?”, and “Would you recommend the Durham VA to someone who needed treatment?”). A domain score was constructed following guidance provided in the Perceptions of Care Training Guide provided to the authors by McLean eBASIS/BASISplus (see Acknowledgments) and used in study analyses to assess changes in patient satisfaction across study time points.
Procedure
Study candidates first completed screening including: (1) an initial interview to obtain informed consent and determine eligibility; (2) a qualifying screening PSQI; and (3) the Folstein Mini-Mental State Examination. Consenting individuals who met entry criteria then underwent a baseline assessment including the aforementioned measures. Following baseline assessments, participants were stratified by sex, age (age < 55 y versus age ≥ 55 y), initial sleep symptom severity (PSQI ≤ 10 versus PSQI > 10), and sleep medication use upon enrollment (none versus one or more medications) and then assigned to the INT or UPC, using a modified randomization procedure known as the minimization method35,36 to balance treatment conditions in regard to stratification variables. The minimization method is particularly useful when it is important to balance treatment groups on a larger number of covariates.
Interventions
Those assigned to the INT group received a comprehensive sleep specialty consultation conducted in a single visit with each patient. The INT included a thorough assessment so as to ascertain the most likely causes and sleep disorder diagnoses underlying each patient's complaint. This assessment was conducted by a doctoral-level clinical psychologist (CU) who had special expertise in sleep medicine and received supervision from the study PI (JDE). The assessment itself consisted of a clinician-administered structured interview for sleep disorders, a review of a sleep history questionnaire and sleep diaries completed by the patient, and a review of medical/ psychiatric information in the patient's electronic medical record. Following this assessment, the participant was provided education regarding his/her primary sleep disorder diagnosis. INT recipients also were given recommendations for additional diagnostic tests and/or specific treatment suggestions. Relevant changes in lifestyle and cognitive behavioral treatment strategies were also discussed, and printed educational materials relevant to the patient's presumptive sleep disorder were provided. The patient's primary care provider was also informed of the INT-derived sleep diagnosis(es) and given recommendations for further evaluation/treatment of the specific sleep disorder(s) in question. This information was also recorded in the patient's electronic medical record, with the PCP identified as an additional cosigner. Most typically this note included templated recommendations for the sleep disorder(s) ascertained. In some cases, the recommendations suggested laboratory tests such as nocturnal sleep monitoring (e.g., for suspected sleep apnea), whereas in other instances, treatment strategies that could be implemented immediately were given. Below is an example of an INT treatment recommendation:
We recently evaluated one of your primary care patients as part of a research study on the diagnosis of primary sleep disorders in the VA. During the evaluation, the veteran reported symptoms consistent with a diagnosis of restless legs syndrome. We recommend first checking this patient's ferritin level, as RLS has been reported in association with iron-deficiency anemia. If the ferritin level is below 40, consider iron replacement therapy. If not medically contraindicated, a dopamine agonist such as pramipexole is often recommended for symptom relief. A typical starting dose is 0.125 mg at bedtime and can be titrated up to 1 mg depending on clinical response. Alternatively, you could refer this patient to the sleep neurology clinic for further evaluation and treatment. In CPRS, this is listed under Medicine Specialties, Neurology Consults, Sleep Disorder Clinic Evaluation.
Those assigned to UPC received treatment as usual; they did not receive any specialty assessment of their sleep complaints until after the 10-mo follow-up visit. Nonetheless, UPC participants' providers remained free to conduct whatever sleep-focused assessments and interventions they chose on their own without interference from the study team. UPC participants who elected to receive the INT after their 10-mo follow-ups were provided this service and received information about their probable sleep diagnoses and recommendations for their management.
Analyses
Sample size estimation was based on the primary hypothesis that INT recipients would have greater improvement in subjective/objective measures of TWT and SE as well as in their total scores on the PSQI at the 10-moh follow-up than would UPC recipients. Methods for the calculations were from Borm et al.37 using data from a previous randomized trial38 to estimate quantities for the baseline standard deviation and correlation between baseline and 10 mo. Baseline standard deviations were assumed to be 38.0 min, 8.1%, and 3.75 points for TWT, SE, and PSQI, respectively. Correlations between baseline and 10 mo were assumed to be 0.5, 0.7, and 0.6, for TWT, SE, and PSQI, respectively. All calculations assumed a type I error rate of 5%, a type II error rate of 20%, and a dropout rate of 10%. There were no adjustments in the type 1 error rate = 5% for the multiple statistical comparisons planned because primary and secondary outcomes as well as all analyses were specified a priori. To detect a 17-min change in TWT, a 3.3% change in SE, and a 1.4-point change in the PSQI, 136 subjects total (68 per arm) were needed.
Provider Outcomes
Research staff conducted reviews of participants' electronic medical records during the 10-mo postrandomization period to: (1) track provider adherence to study staff's diagnostic and treatment recommendations for INT participants and (2) compare the numbers of new sleep-focused consultations and interventions initiated by providers of participants in both the INT and UPC groups. Given the nature of this data extraction process, we assessed the reliability of the data obtained by having the two research staff (CU and JZ) involved in data acquisition each independently review the medical records of a subset (25% of the sample) of the participants and produce independent tallies of the specific diagnostic and treatment data of interest. One of these staff members (JZ) was the study coordinator who was kept blind to the group assignments of all study participants. Results showed high interrater agreement with an average kappa value = 0.89 across the seven specific sleep-focused diagnostic and treatment activities initiated by primary care providers. For each provider-initiated activity we: (1) examined our data for evidence of within-provider clustering by comparing generalized estimating equations of group differences, with and without inclusion of repeated measures based on provider, and (2) tested for INT versus UPC differences in each activity using the Fisher exact test.
Patient Outcomes
All patient-focused outcomes were collected by our blinded study coordinator. Our primary patient-focused outcomes included the PSQI global score along with sleep diary and actigraphy measures of TWT and SE. Our other secondary outcomes included diary and actigraphy TST, and the scores derived from the remaining outcome questionnaires. We used longitudinal data analysis models to examine INT effects on these primary and secondary outcomes at 5- and 10-mo follow-ups. Our models used an unstructured covariance to account for the correlation of patients' repeated measurements over time (PROC MIXED in SAS v9.2; SAS Institute, Cary, NC). Model parameters included a common intercept, baseline sleep medication use (using versus not using) centered around its mean, dummy-coded time, and intervention arm interacted with each follow-up time point (i.e., treatment × time is 2 df). Mean differences between the INT and UPC groups at 5 and 10 mo were calculated, along with corresponding 95% confidence intervals, using ESTIMATE statements. We also conducted two analyses to determine the clinical significance of INT and UPC outcomes. In the first analysis we compared the proportions of INT and UPC participants who showed ≥ 1 standard deviation decline on their PSQI scores from baseline to the 10-mo follow-up. In the second analysis we compared the proportions of INT and UPC participants who showed normal scores (≤ 10) on the ESS by this study endpoint. For these two final analyses, we tested for differences in the groups using the Fisher exact test.
RESULTS
Diagnoses Assigned to Intervention Group Participants
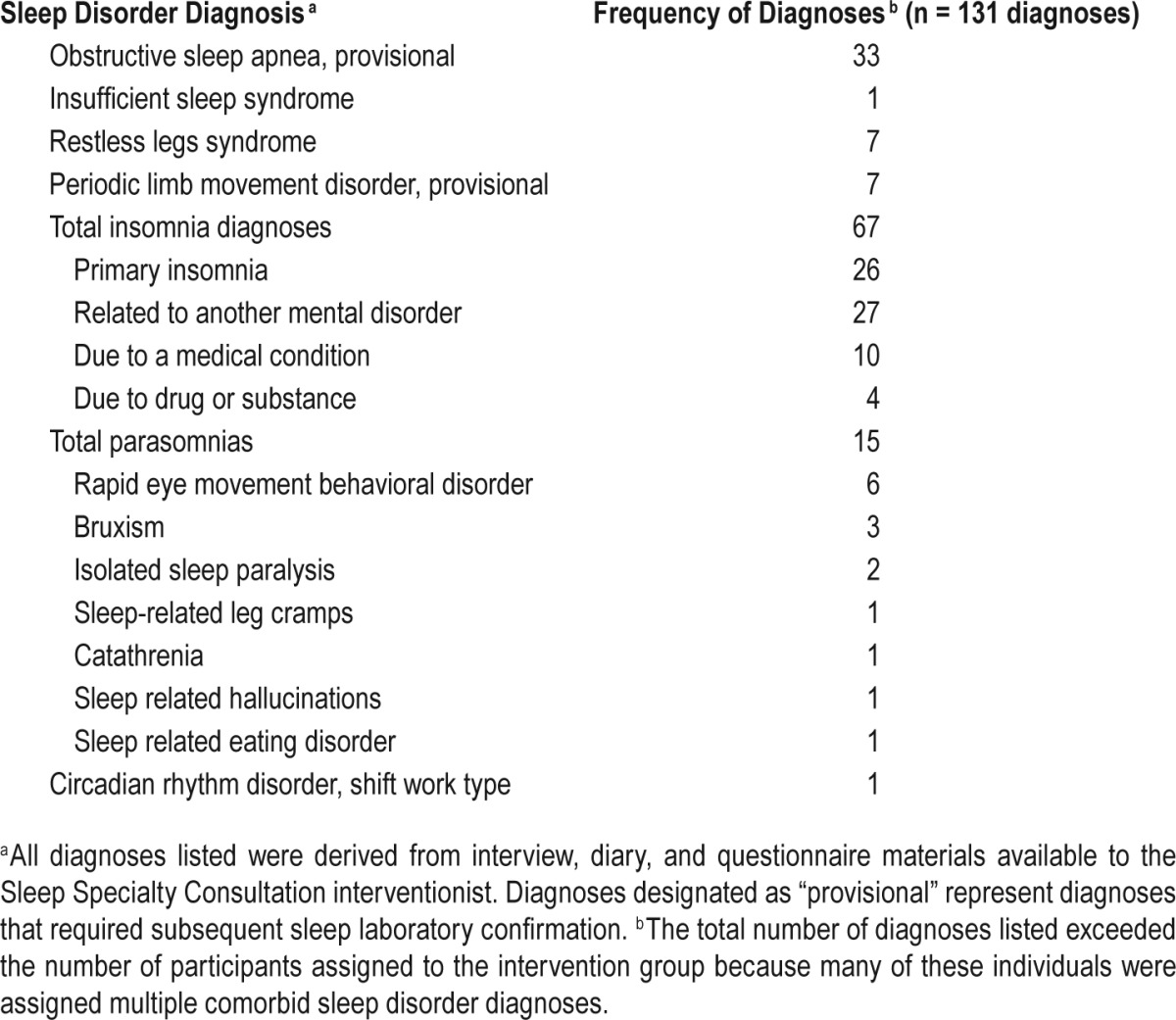
INT assessment resulted in the assignment of 131 sleep disorder diagnoses to the 68 INT recipients (see Table 2). Twenty-six patients (38.2%) received one diagnosis, 17 (25.0%) received 2 diagnoses, 20 (29.4%) had 3 or more diagnoses, and 5 individuals (7.4%) did not receive a sleep disorder diagnosis. Insomnia diagnoses were the most frequently assigned followed by obstructive sleep apnea as a “rule out” diagnosis contingent on the results of a recommended diagnostic polysomnogram. Parasomnias accounted for 11.5% of all diagnoses assigned, whereas restless legs syndrome and suspected periodic limb movement disorder each represented 5.3% of the diagnoses assigned.
Table 2.
Nature and frequency of sleep disorder diagnoses assigned to intervention group participants (n = 68).
Provider Outcomes
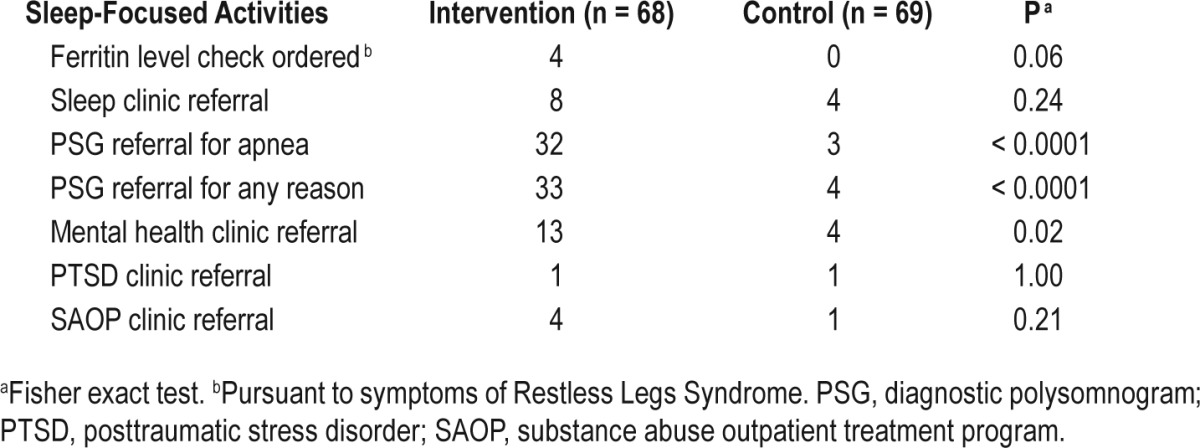
There were 60 PCPs for the 137 study participants. The maximum number of participants under the care of one PCP was 7. Forty-five INT participants and 9 UPC patients received one or more of the sleep-focused activities listed in Table 3 from their provider during the postrandomization time period. INT patients' providers generally were responsive to INT recommendations they received. Thirty-two PCPs initiated one or more sleep-focused activities for 45 INT participants. The maximum number of INT patients per initiating PCP was 4. When the INT recommendation entailed ordering a sleep laboratory study to rule out sleep apnea, the PCPs adhered to this recommendation at a rate of 95%. Adherence rates for referral to a substance abuse outpatient clinic and the PTSD specialty clinic were 100%. Lower adherence was found for referral to the mental health clinic for treatment of sleep-disruptive psychiatric problems (71%) and referral to the psychology sleep clinic for treatment of nightmares (67%). In the UPC group, eight PCPs initiated one or more sleep-focused activities for nine participants. Table 3 shows the frequencies of sleep-focused diagnostic tests and interventions ordered by providers of INT and UPC participants during the 10-mo post-randomization period. Analyses showed no evidence of clustering of sleep-focused activities within providers. Fisher exact tests showed that providers of INT patients initiated significantly more referrals for polysomnographic evaluation of suspected primary sleep disorders (P < 0.001) and mental health clinic referrals to address sleep-disruptive mental disorders (P = 0.02) than did providers for UPC patients. Hence, the INT had a significant influence on providers' attention to participants' sleep-focused complaints.
Table 3.
Sleep-focused activities initiated by primary care providers for intervention and wait list control participants subsequent to baseline interview.
Patient-Focused Outcomes
Of the 137 randomized patients, 123 completed the protocol. Eight INT participants were lost to follow-up, whereas 5 UPC participants were lost to follow-up and 1 withdrew. Data from one or more postrandomization time points were available for 64 INT participants and 66 UPC participants.
Table 4 shows the observed (raw) mean scores for diary, actigraphy and PSQI measures from baseline through the postrandomization period as well as the statistical model-estimated differences between the INT and UPC groups at 5 and 10 mo post randomization. Longitudinal models indicated that the treatment × time interaction for diary TWT was statistically significant (F [2, 125] = 3.19, P = 0.05). At the 10-mo follow-up, INT recipients showed greater model-estimated mean TWT reductions than did UPC recipients (−17.0 min; 95% confidence interval [CI]: −30.9, −3.1; P = 0.02). Changes for Actigraphy TWT mirrored these trends but were much smaller in magnitude. As such, no significant group × time interaction effects were found for actigraphy TWT in the longitudinal model. Analyses of diary SE showed a significant treatment × time interaction (F [2, 124] = 3.95, P = 0.02). At the 10-mo follow-up, INT recipients showed greater increases in sleep efficiency (+3.7%; 95% CI: 0.8, 6.5; P = 0.01) than did UPC participants. Again actigraphy data mirrored these trends but showed more modest and nonsignificant group differences. The INT group showed a 41.6-min increase in their observed average nightly TST on sleep diaries by the 10-mo follow-up, whereas the UPC group showed a 20.9-min increase in diary TST by the study endpoint (F [2, 119] = 2.46, P = 0.09) in the longitudinal model. When within-subject standard deviations of sleep measures were used as an index of night-to-night sleep variability in our models, we found significant group by time interactions for diary values of TWT (F [2, 124] = 5.02, P = 0.008) and SE (F [2, 125] = 6.31, P < 0.003). Both of these analyses showed INT participants had significantly greater reductions in the internight variability for these measures than did those in the UPC group. Similar analyses to examine sleep variability with actigraphy data showed no significant effects. Finally, the intervention group showed greater reductions in PSQI total scores than did the UPC group by the 10-mo follow-up, but these group × time differences did not reach statistical significance (F [1, 123] = 3.19, P = 0.08) in our model. Results of the longitudinal analyses conducted with our remaining questionnaire-based outcomes showed no significant group differences.
Table 4.
Observed and mixed models estimated values (95% confidence interval) of sleep diary, actigraphy and Pittsburgh Sleep Quality Index measures across time periods.
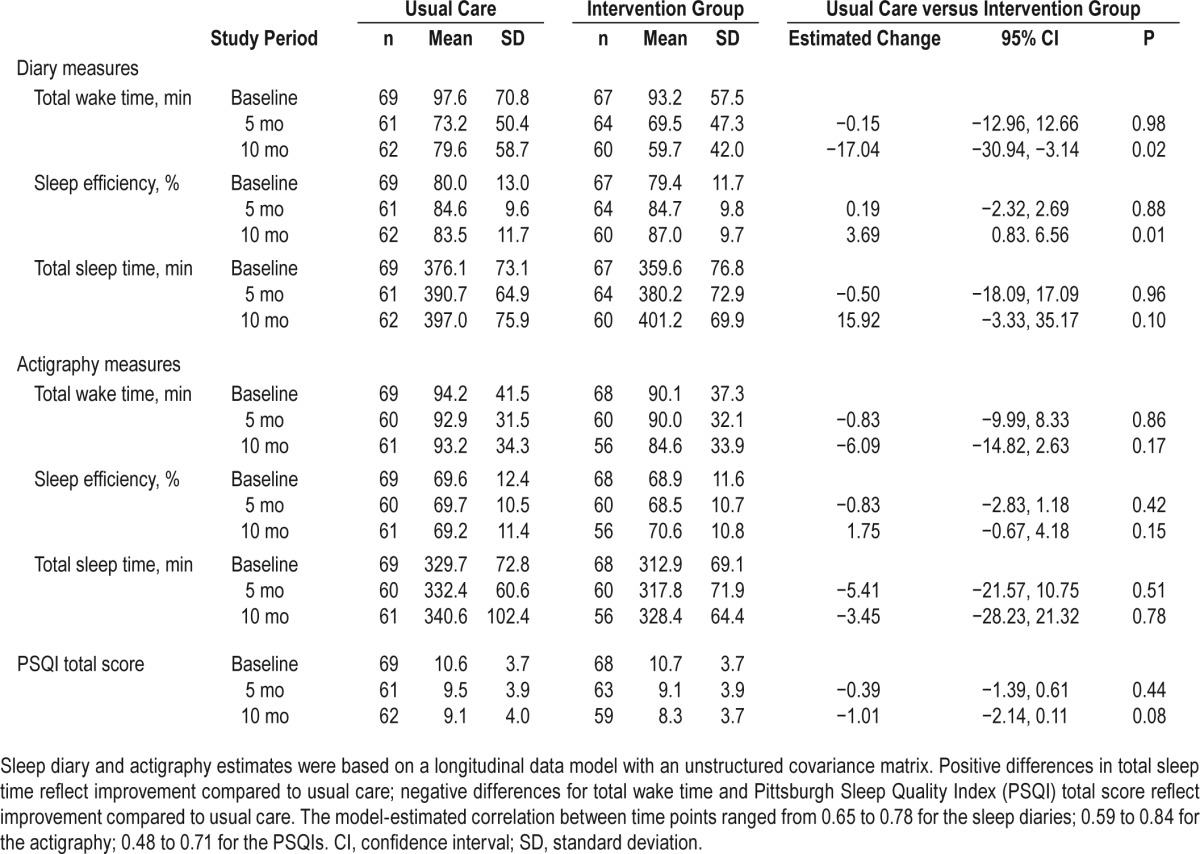
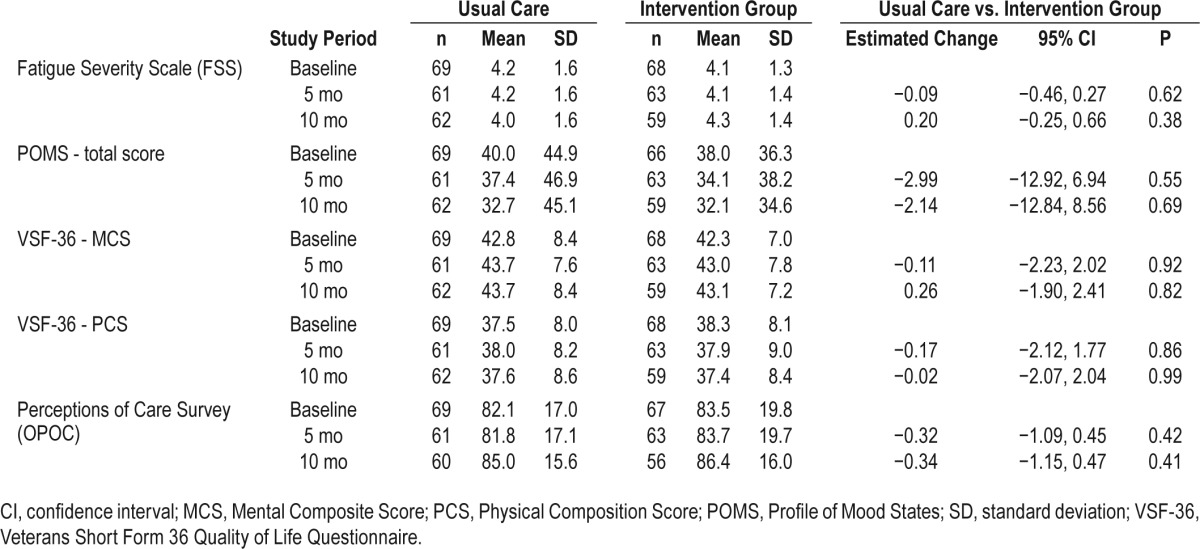
Table 5 shows observed mean and standard deviation values of the INT and UPC groups as well as model-estimated group differences for the various questionnaire measures used as secondary outcome measures. The statistical analyses showed no significant group, time or group by time interaction effects for any of these measures.
Table 5.
Observed and mixed-models estimated values (95% confidence interval) of questionnaire measures across time periods.
Tests of Clinically Significant Change
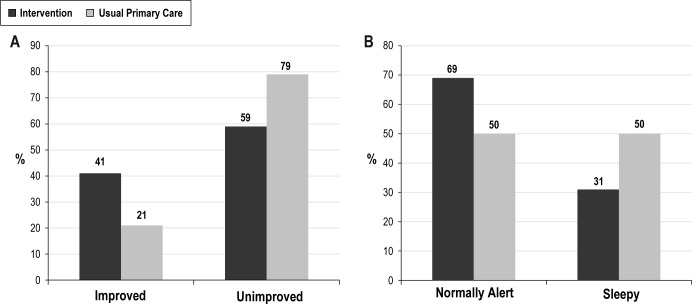
Figure 2 displays the proportions of INT and UPC participants showing clinically significant outcomes on the PSQI and ESS. A significantly greater proportion of the INT group achieved ≥ 1 standard deviation decline in PSQI scores from baseline to the 10-mo follow-up than did the UPC group (41.0% versus 21.0%; Fisher exact test P = 0.02). Also, a greater proportion of the INT group showed normal scores on the ESS by the 10-mo follow-up than did the UPC group (69.0% versus 50.0%; Fisher exact test P = 0.03). Thus, on these measures of sleep quality and daytime alertness, greater proportions of INT recipients showed clinically significant outcomes than did UPC recipients.
Figure 2.
Group comparisons across tests of clinically significant outcomes. (A) Proportions of intervention and usual primary care groups rated as improved or unimproved on the basis of their PSQI score changes. (B) Proportions of intervention and usual primary care groups showing normal daytime alertness and excessive sleepiness on the Epworth Sleepiness Scale by the 10-month follow-up.
DISCUSSION
This project was conducted to test a collaborative care model for managing patients' sleep complaints in a primary care setting. We utilized the VAMC's email system and electronic medical record, CPRS, for communication between sleep specialist and PCP, and demonstrated a high rate of follow-through by the PCPs scrutinized. Provider-focused outcomes showed that most INT recommendations communicated to providers were enacted. This was particularly true in the case of suspected primary sleep disorders such as sleep apnea. The finding of differential numbers of referrals across groups to assess for sleep apnea is noteworthy, given the significant cardiovascular risks associated with this condition. However, there also was a high rate of provider follow-through when a referral for mental health treatment was recommended to address a sleep-disruptive psychiatric condition. Moreover, it is clear that the INT increased sleep-focused interventions overall among PCPs of INT patients compared to those in the UPC condition. This finding is perhaps particularly notable when it is considered that none of these PCPs took part in the study design and planning, and all were blind to its aims.
The findings also suggested some notable positive patient outcomes. Perhaps the most promising findings were those for sleep diary data and our tests of clinical significance. The model-estimated improvements in diary TWT and SE were about twice as large in the intervention group as they were in the UPC group, and these group differences were statistically significant. Moreover, analyses of sleep variability indices showed that diary TWT and SE also became much more stable across nights for INT participants compared to controls. Our repeated-measures analysis of the PSQI suggested a small treatment effect for patients' overall sleep quality perceptions, yet we found a significantly greater proportion of INT recipients met our criterion for judging clinically significant outcomes in sleep quality and daytime alertness than did UPC recipients.
It should be mentioned that not all patient-focused outcome measures showed statistically significant differences between our intervention and control groups over the post-randomization period. The actigraphic sleep measures showed less marked differences between the INT and UPC groups over time than did comparable measures obtained from sleep diary. It should be noted that several previous studies39–42 have found actigraphy to show less impressive differences between active therapies and control conditions than did sleep diary data so it may be that actigraphic sleep estimates are less sensitive to a treatment signal than are diary data. However, none of our questionnaire measures used to provide secondary outcome measures showed significant differences between our active and control interventions. As such, it is possible that factors other than the sleep-targeted intervention provided influence the findings obtained from actigraphy and the responses obtained on the questionnaires employed. This speculation seems particularly credible in regard to the various study questionnaires employed because they measured various domains (i.e., mood, quality of life, satisfaction with care) that are not specifically targeted by sleep interventions and likely are influenced by an array of factors in the patients' day-to-day lives and interactions with the healthcare environment other than their nightly sleep quality.
The setting wherein this study was conducted offered a favorable infrastructure for evaluating the collaborative care model tested. The sleep specialist and PCPs involved were all colleagues in the same large medical center, so this fact likely facilitated the sort of collaboration the intervention encouraged. The VA's CPRS, which was available to study staff, enhanced communication between the specialist/interventionist and the INT study participants' PCPs. As such, selection of a VAMC test site provided the study team several advantages. It may be more difficult to effect collaborative care outside the VA system where use of and access to electronic medical records is more limited43 and sleep specialists and PCPs have independent and unaffiliated practices. For such situations, alternate strategies for enhancing collaborative care may be needed.
In considering our findings, it seems reasonable to question whether factors other than the study intervention influenced the results obtained. Because the study was conducted in an academic medical center, it is possible that the PCPs of the patients enrolled in the study were reasonably knowledgeable about sleep disorders and, hence, more prone to order sleep-focused tests and interventions for all their patients including those assigned to the study's control group. This possibility could have reduced the differences observed between the INT and UPC conditions herein. However, our experience would not support this speculation. In fact, impetus for the current investigation came from our prior experiences recruiting patients for our insomnia treatment study38 conducted in the same medical center wherein the current study was effected. In screening study candidates for that study, we were impressed by the large number of patients with obvious symptoms of sleep apnea and restless legs syndrome that had been undetected and unaddressed by their PCPs. Those observations suggested that even in an academic setting, front-line physicians may not be particularly knowledgeable about sleep disorders and might, thus, benefit from the structured interface with sleep specialists provided in this study to augment their management of patients with ongoing sleep disturbances.
It also seems reasonable to question if the PCPs considered in the current investigation had familiarity with the investigators and knowledge of which of their patients were enrolled in the study. If this were the case, it is possible that providers may have withheld treatments from patients assigned to the control group knowing that they eventually would be offered the INT and this practice, in turn, could have enhanced the differences between the INT and UCP conditions. However, it should be noted that a total of 60 different resident and staff physicians were considered in this project, reflecting the fact that there was ongoing provider turnover during the study due to physician rotational schedules. As a consequence, the investigators were unfamiliar with a majority of the providers considered. In addition, none of the physicians were aware of the aims of the study, so it is not likely that they would bias their patient care practices in favor of study hypotheses. Finally, the patients enrolled were self-referred to the study and, thus, it is unlikely that physicians were aware of which of their patients actually were assigned to the control condition. They only had awareness of those assigned to the INT after they received consultative feedback about the recommended sleep management strategies for them from study staff. Thus, it is unlikely that the physicians considered altered their practices in any way so as to favor study aims.
Admittedly the study had a number of limitations. The sample size was moderate and may have been insufficient to detect INT benefits on secondary outcomes. A related consideration is that this study was conducted at one university-affiliated VAMC. It may be more challenging to implement the intervention used herein in smaller facilities that lack sleep specialists, have fewer resources, and/or have a less accepting attitude toward systemic changes. Furthermore, the outcome measures chosen were limited in focus. We did not evaluate our INT's effects on participants' medical and psychiatric comorbidities, so we do not know if comorbid conditions benefitted from INT. Finally, the 10-mo postintervention follow-up period may have been too short to fully assess the long-term benefits INT patients derived. Thus, future studies of this nature may benefit by larger samples, scrutiny of intervention effects on comorbidities, and longer periods of postintervention follow-up. Given our encouraging findings, such future investigations seem warranted.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by the Department of Veterans Affairs, Health Services Research and Development Grant # IIR 05-213. The Durham VA Medical Center Institutional Review Board maintained oversight of the project to assure the safety of participants. The sponsor did not participate in the design or conduct of the study. The views expressed herein are exclusively those of the authors and do not represent the views of the Department of Veterans Affairs. Dr. Edinger received supplemental funding and a loan of sleep testing equipment from Philips Respironics, Inc. to support two NIMH-funded multisite clinical trials in which he served as site principal investigator. He also has current research funding from Merck Sharp & Dohme, Corp. The remaining authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge that McLean eBASIS/BASISplus provided us with the Perceptions of Care (Outpatient Version) survey and associated scoring. Contact information: http://ebasis.org/; McLean Hospital, 115 Mill Street, Belmont, MA 02478-9106; basisadmin@mclean.harvard.edu
REFERENCES
- 1.Zammit GK, Weiner J, Damato N, et al. Quality of life in people with insomnia. Sleep. 1999;22:S379–85. [PubMed] [Google Scholar]
- 2.Katz DA, McHorney CA. Clinical correlates of insomnia in patients with chronic illness. Arch Intern Med. 1998;158:1099–107. doi: 10.1001/archinte.158.10.1099. [DOI] [PubMed] [Google Scholar]
- 3.Kushida CA, Allen RP, Atkinson MJ. Modeling the causal relationships between symptoms associated with restless legs syndrome and the patient-reported impact of RLS. Sleep Med. 2004;5:485–8. doi: 10.1016/j.sleep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Partinen MHC. Epidemiology of sleep disorders. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia, PA: Elsevier/Saunders; 2005. pp. 226–47. [Google Scholar]
- 5.Kapur VK, Redline S, Nieto J, Young TB, Newman AB, Henderson JA. The relationship between chronically disrupted sleep and healthcare use. Sleep. 2002;25:289–96. [PubMed] [Google Scholar]
- 6.Weissman MM, Greenwald S, Nino-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. Gen Hosp Psychiatry. 1997;19:245–50. doi: 10.1016/s0163-8343(97)00056-x. [DOI] [PubMed] [Google Scholar]
- 7.Walsh JK, Engelhardt CL. The direct economic costs of insomnia in the United States for 1995. Sleep. 1999;22(Suppl 2):S386–93. [PubMed] [Google Scholar]
- 8.Hatoum HT, Kong SX, Kania CM, et al. Insomnia, health-related quality of life and healthcare resource consumption: a study of managed care organisation enrollees. Pharmacoeconomics. 1998;14:629–37. doi: 10.2165/00019053-199814060-00004. [DOI] [PubMed] [Google Scholar]
- 9.Mustafa M, Erokwu N, Ebose I, Strohl K. Sleep problems and the risk for sleep disorders in an outpatient veteran population. Sleep Breath. 2005;9:57–63. doi: 10.1007/s11325-005-0016-z. [DOI] [PubMed] [Google Scholar]
- 10.Peacock MD, Morris MJ, Houghland MA, Anders GT, Blanton HM. Sleep apnea-hypopnea syndrome in a sample of veterans of the Persian Gulf War. Milit Med. 1997;162:249–51. [PubMed] [Google Scholar]
- 11.Zucconi M, Ferini-Strambi L. Epidemiology and clinical findings of restless legs syndrome. Sleep Med. 2004;5:293–9. doi: 10.1016/j.sleep.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 12.National Institutes of Health. National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults, June 13-15, 2005. Sleep. 2005;28:1049–57. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 13.Reuveni H, Tarasiuk A, Wainstock T, Ziv A, Elhayany A, Tal A. Awareness level of obstructive sleep apnea syndrome during routine unstructured interviews of a standardized patient by primary care physicians. Sleep. 2004;27:1518–25. doi: 10.1093/sleep/27.8.1518. [DOI] [PubMed] [Google Scholar]
- 14.Nichols DA, Allen RP, Grauke JB, et al. Restless legs syndrome symptoms in primary care. Arch Intern Med. 2003;163:2323–9. doi: 10.1001/archinte.163.19.2323. [DOI] [PubMed] [Google Scholar]
- 15.Meissner H, Riemer A, Santiago SM, Stein M, Goldman MD, Williams AJ. Failure of physician documentation of sleep complaints in hospitalized patients. West J Med. 1998;169:146–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh JK, Uestuen TB. Prevalence and health consequences of insomnia. Sleep. 1999;22:S427–36. [Google Scholar]
- 17.Kapur VK, Blough DK, Sandblom RE, et al. The medical cost of undiagnosed sleep apnea. Sleep. 1999;22:749–55. doi: 10.1093/sleep/22.6.749. [DOI] [PubMed] [Google Scholar]
- 18.Kryger MH, Roos L, Delaive K, Walld R, Horrocks J. Utilization of health care services in patients with severe obstructive sleep apnea. Sleep. 1996;19:S111–6. doi: 10.1093/sleep/19.suppl_9.s111. [DOI] [PubMed] [Google Scholar]
- 19.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 20.Gottschalk A, Flocke SA. Time spent in face-to-face patient care and work outside the examination room. Ann Fam Med. 2005;3:488–93. doi: 10.1370/afm.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espie CA, Inglis SJ, Tessier S, Harvey L. The clinical effectiveness of cognitive behaviour therapy for chronic insomnia: implementation and evaluation of a sleep clinic in general medical practice. Behav Res Ther. 2001;39:45–60. doi: 10.1016/s0005-7967(99)00157-6. [DOI] [PubMed] [Google Scholar]
- 22.Nichols DA, Allen RP, Grauke JH, et al. Restless legs syndrome symptoms in primary care: a prevalence study. Arch Intern Med. 2003;163:2323–9. doi: 10.1001/archinte.163.19.2323. [DOI] [PubMed] [Google Scholar]
- 23.Edinger JD, Buysse DJ, Deriy L, et al. Quality measures for the care of patients with insomnia. J Clin Sleep Med. 2015;11:311–34. doi: 10.5664/jcsm.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faulkner A, Mills N, Bainton D, et al. A systematic review of the effect of primary care-based service innovations on quality and patterns of referral to specialist secondary care. Br J Gen Pract. 2003;53:878–84. [PMC free article] [PubMed] [Google Scholar]
- 25.Sherman SE, Chapman A, Garcia D, Braslow JT. Improving recognition of depression in primary care: a study of evidence-based quality improvement. Jt Comm J Qual Saf. 2004;30:80–8. doi: 10.1016/s1549-3741(04)30009-2. [DOI] [PubMed] [Google Scholar]
- 26.Hedrick S, Chaney E, Felker B, et al. Effectiveness of collaborative care depression treatment in Veterans' Affairs primary care. J Gen Intern Med. 2003;18:9–16. doi: 10.1046/j.1525-1497.2003.11109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strollo PJ, Badr S, Coppola MP, Fleishman SA, Jacobowitz O. The future of sleep medicine. Sleep. 2011;34:1613–9. doi: 10.5665/sleep.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buysse D, Reynolds C, Monk T, Berman S, Kupfer D. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.Johns M. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 30.Krupp LB, LaRocca NG, Muir-Nash J, Sternberg AD. The Fatigue Severity Scale: application to patients with multiple sclerosis and systemic lupus erythemasis. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 31.Lichstein K, Means M, Noe S, Aguillard R. Fatigue and sleep disorders. Behav Res Ther. 1997;35:733–40. doi: 10.1016/s0005-7967(97)00029-6. [DOI] [PubMed] [Google Scholar]
- 32.McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: EDITS; 1971. [Google Scholar]
- 33.Kazis L, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Internal Med. 1998;158:626–32. doi: 10.1001/archinte.158.6.626. [DOI] [PubMed] [Google Scholar]
- 34.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36): conceptual framework and item selection. Medical Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 35.Pocock S. Allocation of patients to treatment in clinical trials. Biometrics. 1979;35:183–97. [PubMed] [Google Scholar]
- 36.White SJ, Freedman LS. Allocation of patients to treatment groups in a controlled clinical study. Br J Cancer. 1978;37:849–857. doi: 10.1038/bjc.1978.124. Br J Cancer 1978;37:849-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60:1234–8. doi: 10.1016/j.jclinepi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Edinger JD, Olsen MK, Stechuchak KM, et al. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: a randomized clinical trial. Sleep. 2009;32:499–510. doi: 10.1093/sleep/32.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman L, Benson K, Noda A, et al. An actigraphic comparison of sleep restriction and sleep hygiene treatments for insomnia in older adults. J Geriatr Psychiatry Neurol. 2000;13:17–27. doi: 10.1177/089198870001300103. [DOI] [PubMed] [Google Scholar]
- 40.Espie CA, MacMahon KM, Kelly HL, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep. 2007;30:574–84. doi: 10.1093/sleep/30.5.574. [DOI] [PubMed] [Google Scholar]
- 41.Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR. Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial. Arch Intern Med. 2005;165:2527–35. doi: 10.1001/archinte.165.21.2527. [DOI] [PubMed] [Google Scholar]
- 42.Rybarczyk B, Lopez M, Benson R, Alsten C, Stepanski E. Efficacy of two behavioral treatment programs for comorbid geriatric insomnia. Psychol Aging. 2002;17:288–98. [PubMed] [Google Scholar]
- 43.Hing E, Hsiao CJ. Electronic medical record use by office-based physicians and their practices. Natl Health Statistics Rept. 2007;2010:1–11. [PubMed] [Google Scholar]