Abstract
Study Objectives:
Children with obstructive sleep apnea syndrome (OSAS) often experience periods of hypercapnia during sleep, a potent stimulator of cerebral blood flow (CBF). Considering this hypercapnia exposure during sleep, it is possible that children with OSAS have abnormal CBF responses to hypercapnia even during wakefulness. Therefore, we hypothesized that children with OSAS have blunted CBF response to hypercapnia during wakefulness, compared to snorers and controls.
Methods:
CBF changes during hypercapnic ventilatory response (HCVR) were tested in children with OSAS, snorers, and healthy controls using diffuse correlation spectroscopy (DCS). Peak CBF changes with respect to pre-hypercapnic baseline were measured for each group. The study was conducted at an academic pediatric sleep center.
Results:
Twelve children with OSAS (aged 10.1 ± 2.5 [mean ± standard deviation] y, obstructive apnea hypopnea index [AHI] = 9.4 [5.1–15.4] [median, interquartile range] events/hour), eight snorers (11 ± 3 y, 0.5 [0–1.3] events/hour), and 10 controls (11.4 ± 2.6 y, 0.3 [0.2–0.4] events/hour) were studied. The fractional CBF change during hypercapnia, normalized to the change in end-tidal carbon dioxide, was significantly higher in controls (9 ± 1.8 %/mmHg) compared to OSAS (7.1 ± 1.5, P = 0.023) and snorers (6.7 ± 1.9, P = 0.025).
Conclusions:
Children with OSAS and snorers have blunted CBF response to hypercapnia during wakefulness compared to controls. Noninvasive DCS blood flow measurements of hypercapnic reactivity offer insights into physiopathology of OSAS in children, which could lead to further understanding about the central nervous system complications of OSAS.
Citation:
Busch DR, Lynch JM, Winters ME, McCarthy AL, Newland JJ, Ko T, Cornaglia MA, Radcliffe J, McDonough JM, Samuel J, Matthews E, Xiao R, Yodh AG, Marcus CL, Licht DJ, Tapia IE. Cerebral blood flow response to hypercapnia in children with obstructive sleep apnea syndrome. SLEEP 2016;39(1):209–216.
Keywords: cerebral blood flow, child, humans, hypercapnia, obstructive, sleep apnea
Significance.
Children with obstructive sleep apnea syndrome are chronically exposed to hypercapnia during sleep. It is unknown, however, if their cerebral blood flow regulation during wakefulness is abnormal. This study provides evidence that suggests children with the obstructive sleep apnea syndrome and snorers have blunted cerebral blood flow response to hypercapnia during wakefulness.
INTRODUCTION
Increasingly, central nervous system (CNS) complications such as neurobehavioral deficits have been recognized in children with untreated obstructive sleep apnea syndrome (OSAS).1 OSAS affects 2% to 3% of children and is characterized by episodes of repetitive upper airway collapse during sleep, which may result in intermittent hypercapnia, hypoxemia, and/or arousal from sleep.2 Hypercapnia, in particular, is a powerful stimulator of cerebral blood flow (CBF),3,4 and in light of their chronic exposure to hypercapnia during sleep, it is possible that children with OSAS have abnormal CBF responses to hypercapnia even during wakefulness. Indeed, although OSAS occurs during sleep, the pathophysiology behind CNS manifestations of OSAS during wakefulness in children is still unknown and no polysomnographic measure has been shown to predict CNS outcomes.1 Therefore, it is desirable to investigate the utility of new noninvasive medical tools and new metrics for OSAS patients during wakefulness. Such methods could help to elucidate CNS complications of OSAS.
Near-infrared (650–950 nm) optical techniques such as diffuse optical and diffuse correlation spectroscopy (DOS and DCS) offer the potential to noninvasively probe cerebral oxygenation and cerebral blood flow. They both use the diffusion approximation for light transport to relate detected light on the head surface to physiological properties of underlying cortical tissues. Specifically, in contrast to traditional continuous-wave near infrared spectroscopy,5 frequency-modulated DOS accurately measures tissue scattering and absolute concentrations of tissue chromophores such as oxyhemoglobin and deoxyhemoglobin. DCS provides complementary measurements of microvascular blood flow by introducing long coherence length (∼20 m) light into tissue and monitoring temporal intensity fluctuations of the interference (speckle) pattern emerging from the tissue surface. As discussed in several recent reviews,5,6 the rate of intensity fluctuations is dependent on microvascular blood flow. When quantified by the temporal intensity correlation function, these fluctuations yield a tissue blood flow index (cm2/s). Changes in this blood flow index provide a metric of relative blood flow.5
Relative blood flow measurements with DCS have been extensively validated against Doppler ultrasound in the preterm infant brain,7 laser Doppler flowmetry in rat brain,8,9 fluorescent microspheres in piglet brains,10 xenon-computed tomography in adult traumatic brain injury,11 and arterial spin-labeled perfusion MRI in the healthy adult human brain12 and in infants with congenital heart disease.13 Recently, Buckley et al.14 showed in children (median age 2.8 y) that changes in DCS measured indices of tissue blood flow due to hypercapnia agreed with changes in CBF measured simultaneously with phase-encoded velocity mapping MRI. To the best of our knowledge, there are currently no reports of DCS measurements in school-aged children in the literature. In addition to these cerebral measurements, DCS has also been used to measure relative blood flow in many organs including brain, muscle, and breast.15–19 The nonionizing radiation used in both DOS and DCS is considered safe for long-term monitoring.
To date, few researchers have applied diffuse optical techniques in adult OSAS subjects, focusing on relative near-infrared spectroscopy (NIRS) measurements of cerebral hemoglobin concentration and oxygenation.20–22 Other groups have used frequency-modulated DOS to study OSAS23–26 and have suggested reduced autoregulation during breath holding,21 graded preservation of autoregulation in milder forms of OSAS,25 and alterations of low-frequency hemodynamic oscillations during wakefulness and sleep.25 However, most of these studies focused on sleeping subjects and did not monitor the persistent effects of OSAS into waking periods. Moreover, these measurements of hemoglobin concentration and oxygenation do not fully capture oxygen delivery to tissues, because they do not measure blood flow.
Hou et al.27 recently measured variations in cerebral hemodynamics in adult OSAS subjects, during sleep, with combined DOS/DCS similar to the device described. This group compared changes in CBF during nonobstructive and obstructive breathing. Each subject was his or her own control. They found that obstructive breathing resulted in CBF increases that correlated with OSAS severity. Authors attributed these CBF increases to hypercapnia; however, hypercapnia was not measured. It is important to note that none of these studies focused on children, in whom the CNS is undergoing significant development and may be uniquely vulnerable. Further, awake response to hypercapnia was not studied. Therefore, the aim of this research was to identify signatures of obstructive sleep apnea in the awake pediatric brain using cutting-edge noninvasive optical techniques. We hypothesized that children with OSAS have blunted CBF response to hypercapnia during wakefulness compared to nonsnoring and snoring age-matched controls.
METHODS
Study Group
Children aged 6–16 y were included. Participants with OSAS and snorers were recruited after a clinical polysomnogram; asymptomatic, healthy nonsnoring controls were recruited from the community by means of advertisements. All subjects underwent baseline polysomnography using standard pediatric techniques and scoring as previously described.28,29 Briefly, a respiratory event was scored as an obstructive hypopnea if there was a drop of the nasal pressure peak signal excursion of ≥ 30% of pre-event baseline, for at least the duration of two breaths, and associated with a 3% oxyhemoglobin desaturation from baseline and/or an arousal. In addition, snoring, increased inspiratory flattening of the nasal pressure signal compared to baseline breathing, or paradoxical inward rib cage motion during inspiration occurring during the event only, were present. The obstructive apnea-hypopnea index (AHI) was calculated as the sum of obstructive apneas, mixed apneas, and obstructive hypopneas divided by the hours of sleep. OSAS was defined as having an AHI ≥ 3/h. This criterion was selected to exclude children with very mild OSAS. Controls were included, if they were asymptomatic and had an AHI < 1.5/h. Snorers were included if their AHI was < 1.5/h.30 Exclusion criteria included craniofacial anomalies, genetic syndromes, history of adenotonsillectomy or upper airway surgery, continuous positive airway pressure use, persistent asthma, and positive Pediatric Sleep Questionnaire for controls only.31 The Institutional Review Board at the Children's Hospital of Philadelphia approved the study. Informed consent was obtained from parents or legal guardians of the subjects, and assent from subjects older than 7 y.
Optical Methods
This study used quantitative frequency domain DOS, rather than the more typical trend-monitoring devices (e.g., near-infrared spectroscopy). The concentrations of oxygenated and deoxygenated hemoglobin were extracted from tissue absorption at 688 and 826 nm. Total hemoglobin concentration (THC = [HbO2] + [Hb]) and tissue oxygen saturation (StO2 = [HbO2] / THC × 100%) were subsequently derived. DCS analysis used these optical properties to fit an effective Brownian diffusion coefficient (i.e., blood flow index) to the decay of the intensity autocorrelation function of a single speckle.
Hypercapnia Methods
Hypercapnia was used to modulate CBF through a modified Read rebreathing technique32,33 (schematic in Figure 1). Hence, optical measurements were taken throughout this hypercapneic ventilatory response (HCVR) test. Participants were seated comfortably, breathing through a mouthpiece. End-tidal carbon dioxide was measured by infrared capnometry (Novametrix Medical System, Inc., Wallingford, CT, USA). Flow was measured using a heated pneumotachograph (Hans Rudolph, Inc., Shawnee, KS, USA) and transducer (ADInstruments, Colorado Springs, CO, USA). Minute ventilation (V̇E) was obtained by analog integration of the flow signal. All outputs were recorded on a PowerLab system (ADInstruments). Participants rebreathed from a bag filled with 70 mL/kg of a gas mixture with the initial composition of 95% oxygen and 5% carbon dioxide. Subjects breathed room air through the mouthpiece for several minutes to establish baseline values. The inhalation valve was then switched at the end of a normal expiration so that participants rebreathed the hypercapnic mixture. They were asked to take three deep breaths after the valve was switched, and then breathe normally. They were encouraged to continue until end-tidal carbon dioxide reached 65 mmHg. Ventilatory responses to hypercapnia were expressed as the slope of minute ventilation versus end-tidal carbon dioxide concentration.
Figure 1.
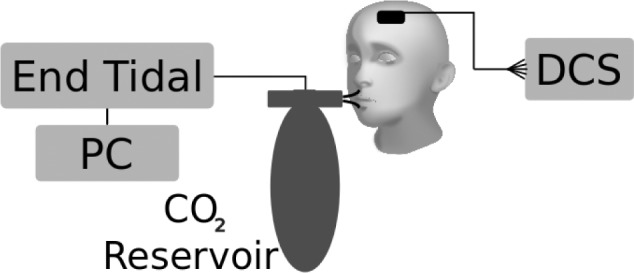
Schematic of hypercapnic ventilatory response apparatus. DCS, diffuse correlation spectroscopy.
Optical Measurements during HCVR
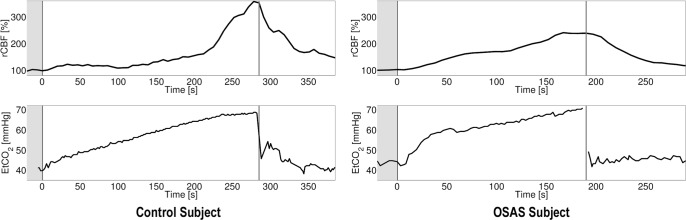
Optical measurements were performed during wakefulness in a related circadian rhythm period. Participants had similar sleep/wake cycles. DCS data was acquired continuously throughout the baseline, HCVR, and a post-HCVR recovery period (e.g., Figure 2). For the CBF baseline, we used data taken after insertion of the mouthpiece and while the children were breathing room air. We then computed blood flow changes relative to this baseline (time = 0, i.e., rCBF = CBF(t > 0) / CBF(t = 0) × 100%). A gaussian filter (width ∼18 s) was used to smooth the resulting curve in time. The maximum blood flow was defined as the peak of the smoothed curve and normalized to the prehypercapnic baseline measurement; this parameter was then compared across groups. To account for differences in individual baseline and peak end-tidal carbon dioxide, we compared peak rCBF normalized by the change in end-tidal carbon dioxide blood flow changes relative to a baseline breathing room air (time (t) = 0, i.e., rCBF = CBF(t > 0) / CBF(t = 0) × 100%) were computed. To account for differences in individual baseline and peak end-tidal carbon dioxide, we compared peak rCBF normalized by the change in end-tidal carbon dioxide (ΔEtCO2).
Figure 2.
Relative cerebral blood flow (rCBF, normalized to baseline period) versus time during hypercapnic ventilatory response for a single control and obstructive sleep apnea syndrome subject. Baseline period is designated by the region and the end of hypercapnia by the vertical line. The control subject had a short baseline with the end tidal monitoring. The horizontal axes are time in seconds; the vertical axes rCBF in % or end-tidal carbon dioxide in mmHg. OSAS, obstructive sleep apnea syndrome.
Statistical Analysis
Statistical analysis was performed with Matlab (Mathworks, MA, USA) and R.34 The Kolmogorov-Smirnov test was used to test for normality. Data are presented as mean ± standard deviation if normally distributed, and as median (interquartile range) otherwise. Categorical data were compared using the Fisher exact test. Analysis of variance or the Kruskal-Wallis test was used for three-way comparisons. Due to the small sample size, only one covariate of interest was included in the multiple regression model at each time, including age, sex, and body mass index (BMI). However, neither age nor sex was significantly associated with the outcome variable (rCBF% normalized by the change in end-tidal carbon dioxide) and therefore, none of them was included in the final regression model. A partial F-test in multiple linear regression was used to assess whether rCBF differed among groups adjusted for BMI z-score, and this model was compared to the one without adjusting for BMI z-score to confirm that obesity did not affect changes in rCBF. Pearson correlation was calculated between the polysomnographic measures and rCBF or rCBF/ΔETCO2, and between rCBF and ΔETCO2; a value of P < 0.05 was considered statistically significant.
RESULTS
Study Group
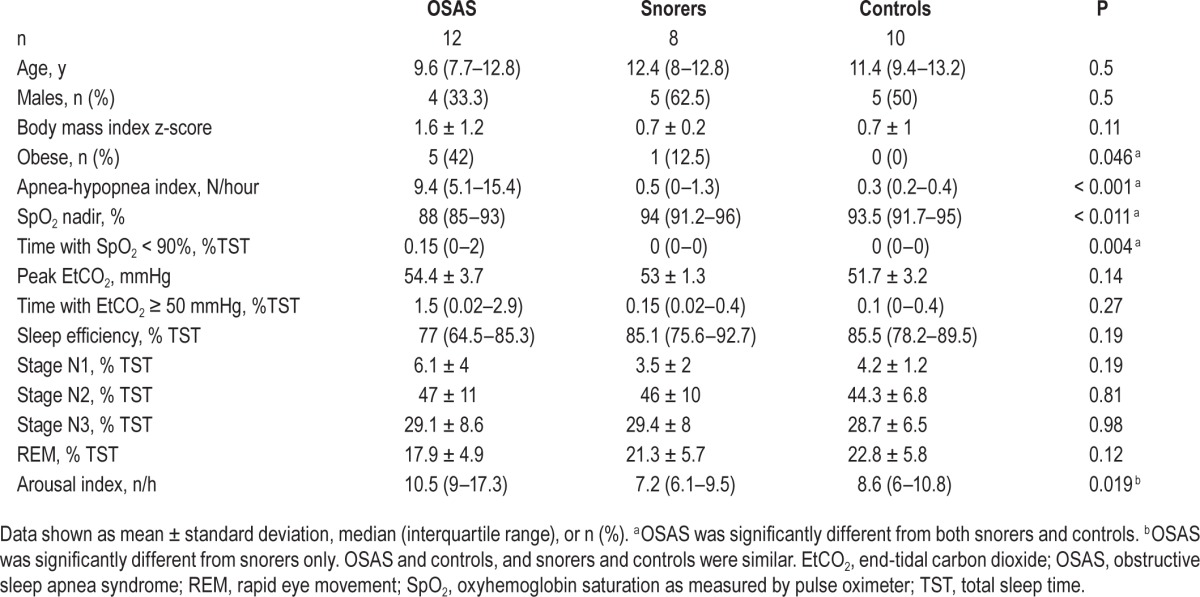
Fifteen children with OSAS, 13 snorers, and 15 controls were recruited. Nine subjects were excluded because they were unable to follow research procedures. Major challenges included motion during DOS/DCS data collection and carbon dioxide leaks due to insufficiently tight sealing around the mouthpiece. This level of compliance is expected for the young age group. Thus, the final data set included 12 children with OSAS, 8 snorers, and 10 controls. Table 1 contains the demographics and polysomnography study results of included subjects. Children with OSAS were more obese than the other groups. As expected, children with OSAS had a greater AHI and lower hemoglobin saturation nadir. OSAS participants exhibited a trend toward greater peak end-tidal carbon dioxide and percentage of sleep time with end-tidal carbon dioxide greater than 50 mmHg. OSAS participants had greater arousal index compared to snorers. Otherwise, sleep architecture was similar between the three groups.
Table 1.
Study group demographics and polysomnography results.
Hypercapnic Ventilatory Response
Results are shown in Table 2. There were no differences in end-tidal carbon dioxide dynamics between groups. Similarly, the slope of minute ventilation versus end-tidal carbon dioxide was similar across groups.
Table 2.
Hypercapnic ventilatory response results.
Cerebral Hemodynamics
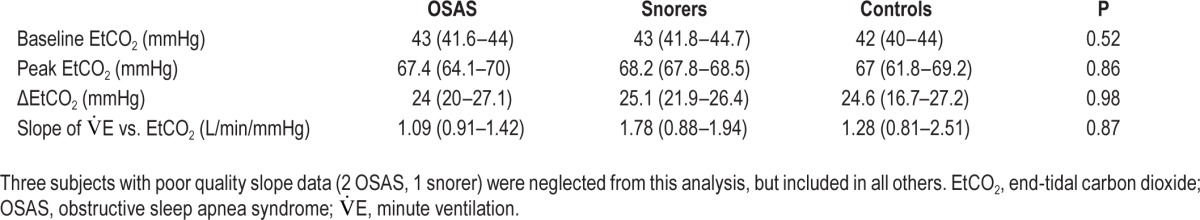
Results are shown in Table 3. No significant differences in cerebral blood oxygenation and tissue hemoglobin concentration were found between groups. However, the relative change in cerebral blood flow, normalized by the change in end-tidal carbon dioxide, was significantly greater in controls compared to children with OSAS and snorers (Figure 3). This result persisted even after controlling for obesity (OSAS versus controls, P = 0.007; snorers versus controls, P = 0.007). Figure 4 shows the rCBF response to hypercapnia in all participants. Of note, rCBF/ΔEtCO2 did not correlate significantly with polysomnographic measures such as AHI, peak end-tidal carbon dioxide, percentage of sleep time with end-tidal carbon dioxide greater than 50 mmHg, peripheral oxygen saturation (SpO2) nadir, percentage of sleep time with SpO2 < 90%, sleep efficiency, sleep stages, or arousal index when the 30 subjects were analyzed together (data not shown).
Table 3.
Optical measurements of cerebral physiological properties.
Figure 3.
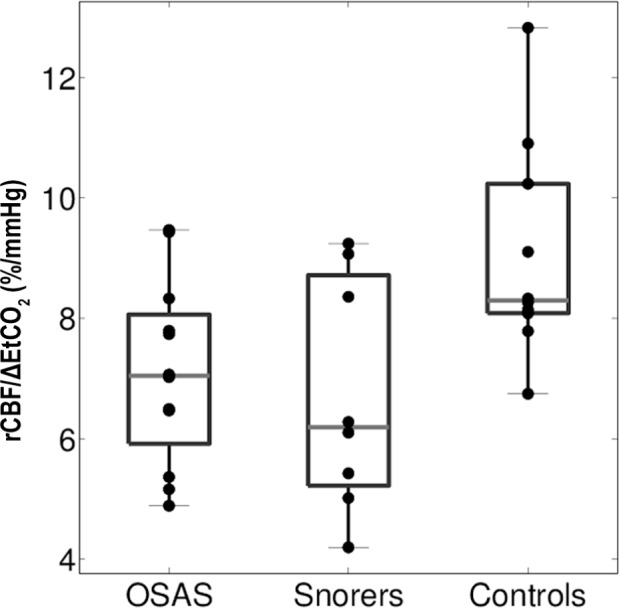
Relative change in cerebral blood flow normalized by end-tidal carbon dioxide change in response to hypercapnia in children with obstructive sleep apnea syndrome (OSAS), snorers, and controls. Relative change in cerebral blood flow (rCBF, vertical axis), normalized by end-tidal carbon dioxide change (ΔEtCO2, mmHg), in response to hypercapnia in children with OSAS, snorers, and controls (horizontal axis). Central line indicates median, the boxes define the area between the 25th and 75th percentiles, and the whiskers designate the most extreme points not considered outliers. rCBF/ΔEtCO2 was significantly different between the three groups. Specifically, controls had a greater response than OSAS (P = 0.028) and snorers (P = 0.025).
Figure 4.
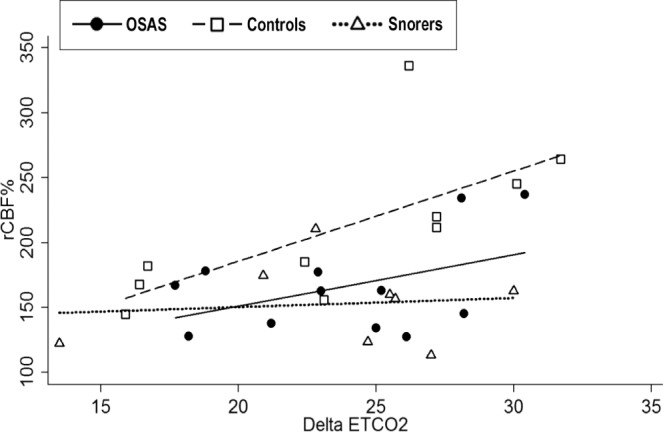
Relative change in cerebral blood flow versus delta end-tidal carbon dioxide (EtCO2) in children with OSAS, snorers, and controls. Regression lines per group are coded. Controls had a significant correlation between delta EtCO2 and relative changes in cerebral blood flow (r = 0.69, P = 0.027). OSAS and snorers did not have significant correlations.
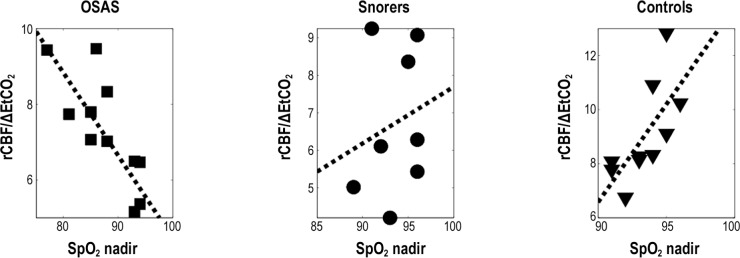
A subanalysis by diagnosis showed that children with OSAS had a significant correlation between the percentage of sleep time with SpO2 < 90% and rCBF/ΔEtCO2 (r = 0.65, P = 0.021). In contrast, controls exhibited a significant inverse correlation between sleep SpO2 nadir and rCBF/ΔEtCO2, and snorers did not have a significant correlation (Figure 5). No correlations between sleep architecture and rCBF/ΔEtCO2 were found in any group.
Figure 5.
Correlation between peripheral oxygen saturation (SpO2) nadir during sleep and relative change in cerebral blood flow/end-tidal carbon dioxide change (rCBF/ΔEtCO2) in children with OSAS, snorers, and controls. Horizontal axis is nadir of overnight SpO2 and vertical axis is relative cerebral blood flow normalized to change in end-tidal carbon dioxide. Participants with OSAS and controls had significant correlations. OSAS' r was −0.77 with P = 0.0034 and controls' r was 0.69, P = 0.026. Snorers' r was 0.21 with P = 0.62.
DISCUSSION
This study measured a blunted CBF response to hypercapnia in children with OSAS and snorers compared to healthy control subjects during wakefulness. This finding, which was independent of obesity, suggests that OSAS in children is associated with impaired cerebrovascular reactivity even during wakefulness. Snorers had a similar diminished response, which implies that even the milder range of sleep disordered breathing can be associated with diminished CBF. In addition, this study confirmed that children with OSAS have normal HCVR during wakefulness,35 and it provided new data on HCVR in snorers.
Cerebral Hemodynamics in OSAS
OSAS is associated with hypoxemia, hypercapnia, and sympathetic activation that result in blood pressure surges.36 All of these can adversely affect CBF regulation and lead to brain dysfunction during wakefulness. Hence, nocturnal intermittent hypoxemia, hypercapnia, and/or sleep fragmentation are potential pathogenic culprits for the neurocognitive and behavioral abnormalities seen in subjects with sleep disordered breathing. For example, a pathophysiologic link between nocturnal recurrent hypoxemia and brain damage has been suggested in adults by studies showing abnormalities of CNS white matter and cortical impairment in patients with OSAS, even in those without associated vascular risk factors.37 Other studies in adults with OSAS, carried out using techniques such as transcranial Doppler ultrasound, NIRS, and single pho ton emission computed tomography, have shown significant alterations in CNS blood flow during both wakefulness and sleep.38–40 The most common finding of these studies is CBF reduction. Recently, a study in young adults using blood oxygen level-dependent functional magnetic resonance imaging demonstrated decreased cerebrovascular reactivity during a breath-hold challenge in OSAS subjects, similar to our results.41 However, it is important to note that our subjects were young children with a shorter clinical course and a more plastic, rapidly developing CNS than those described in the aforementioned studies. Therefore, these results suggest potential mechanisms leading to CNS complications of OSAS in younger populations with a relatively short clinical course.
Considering we measured CBF percentage changes from baseline, it is possible that the blunted CBF response to hypercapnia observed in our subjects could be due to elevated baseline CBF, e.g., as a compensatory mechanism secondary to repetitive gas exchange abnormalities during sleep. If this is the case, children with OSAS would only be able to moderately increase their CBF compared to normal controls. Our data support this hypothesis. Importantly, rCBF/ΔEtCO2 showed a direct correlation with percentage of sleep time with SpO2 below 90% in OSAS. Similarly, rCBF/ΔEtCO2 showed an inverse correlation with SpO2 nadir during sleep in OSAS, whereas controls had a positive rCBF/ΔEtCO2 correlation with SpO2 nadir during sleep. The contrast in response may be due to the fact that SpO2 measurements were within the normal range of oxygenation in controls.
To the best of our knowledge, baseline absolute CBF has not been measured in children with OSAS. However, a study performed in children with mild sleep disordered breathing, defined as AHI ≤ 5 events per hour, showed increased middle cerebral artery blood flow velocity during wakefulness (measured by transcranial Doppler ultrasound) compared to controls.42 This technique is limited to measurements of blood velocity in large vessels and hence, measures macrovascular blood flow; by contrast, the DCS technique is sensitive to microvascular blood flow. Additionally, these subjects were lying quietly during measurements and did not perform a hypercapneic challenge. Further research is warranted to elucidate the relationship between these macrovascular and microvascular responses.
Snorers in our study had a blunted cerebrovascular response. Cerebral hemodynamics in snoring children have not been well studied. In fact, snorers were grouped together with children with mild OSAS in the few studies analyzing cerebral hemo-dynamics.42,43 Hence, it is not possible to draw snoring-specific conclusions from these publications. Studies in animals and adults have shown elevated inflammatory markers in snorers or induced in the laboratory by vibration.44,45 Snoring has also been associated with markers of endothelial dysfunction, such as reduced flow-mediated vasodilation of the brachial artery compared to nonsnoring healthy controls.46 The reasons for this are unknown as snorers typically have normal polysomnographic findings, including gas exchange and arousal indices. However, it has been hypothesized that snoring is part of a continuum that can progress to OSAS.47 Therefore, it is possible that snoring subjects with lower CBF response to hypercapnia may be closer to progress to OSAS than those with greater responses. Longitudinal studies may be useful for elucidating this relationship.48
DOS/DCS and Obesity
As expected, children with OSAS were more obese than snorers and controls.49 The DOS/DCS technique used here requires placement of the sources and detectors on the forehead over the scalp and skull. Hence, the detected light must travel through extracerebral tissues (skin, subcutaneous fat, scalp, and skull). It is possible that obese children had thicker layers of sub-cutaneous adipose tissue, which could potentially affect the DOS/DCS measurements. However, these factors are more likely to affect absolute rather than relative measurements such as rCBF. In addition, scalp fat deposition on the frontal area has not been demonstrated in children and a linear regression model controlling for obesity did not affect our results. Therefore, it is unlikely that obesity affected the DOS/DCS measurements in the study described here.
It is important to note that AHI has significant limitations as a diagnostic tool for OSAS. Specifically, the number of apneas and hypopneas are counted without consideration of duration and/or associated oxyhemoglobin desaturation, e.g., a 10-sec hypopnea with 4% desaturation counts the same as a 50-sec hypopnea with 15% desaturation. In addition, the AHI does not take into consideration the percentages of sleep time with SpO2 < 90% and end-tidal carbon dioxide greater than 50 mmHg. Thus, the current study and other studies that probe the CNS repercussion of OSAS may lead to improved metrics.
Limitations
The research procedures described here are challenging for young children because they must sit still while measurements are performed. We discarded ∼20% of our data, primarily due to motion artifacts. This motion could cause the subject's mouth to come off the mouthpiece, induce motion artifacts in the DCS data, and introduce leaks into the rebreathing apparatus. As expected, hyperpnea and motion were more common in the period near peak end-tidal carbon dioxide. Participants were at different stages of puberty, which may have affected the results. Tanner staging and sex hormones were not part of this protocol. The control group was not matched for BMI, which could potentially affect our findings, as the OSAS group had a greater obesity rate. However, results were not changed after controlling for BMI. The DOS/DCS analysis used a semi-infinite model of light propagation in tissue. Although the majority of cerebral DOS/DCS analyses use this model, it does not account for cerebral anatomy. In future work, more sophisticated data collection will permit use of more advanced models of light propagation and very recently developed techniques to reduce the effect of superficial blood flow on DCS measurements.50
CONCLUSION
DCS blood flow measurements show that awake children with OSAS and snorers have blunted CBF response to hypercapnia compared to healthy control subjects, independent of obesity. This finding suggests that even mild sleep disordered breathing is associated with deficits in cerebrovascular response. Ultimately, these techniques could be used in conjunction with polysomnography to reveal the CNS complications of OSAS. We have demonstrated that optical measurements of cerebral hemodynamics are feasible in young children and provide insight into the pathophysiology of pediatric OSAS.
DISCLOSURE STATEMENT
Funding was provided by the United States National Institute of Health through UL1-RR-024134, UL1-TR000003, R01-HL058585, R01-NS060653, P41-EB015893, R01-NS072338; American Sleep Medicine Foundation 93-JF-13; and The June and Steve Wolfson Family Foundation. DRB gratefully acknowledges support from the Whitaker award administered by IIE, Thrasher Research Fund Early Career Award, and NIH T32-HL007915. Dr. Busch has two pending patent applications relevant to this work. Dr. Marcus has received research support from Ventus and Philips Respironics in the form of loaned equipment only for investigator-initiated studies, not relevant to the current manuscript. Dr. Yodh has two patents relevant to this work (United States patents 8,082,015 and 6,076,010). The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the participants of this study and their families, as well as Dr. Wesley B. Baker, Dr. Igor Blanco, and Dr. Ashwin B. Parthasarathy for helpful discussions.
REFERENCES
- 1.Chervin RD, Ruzicka DL, Hoban TF, et al. Esophageal pressures, polysomnography, and neurobehavioral outcomes of adenotonsillectomy in children. Chest. 2012;142:101–10. doi: 10.1378/chest.11-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27:997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 3.Fortune JB, Bock D, Kupinski AM, Stratton HH, Shah DM, Feustel PJ. Human cerebrovascular response to oxygen and carbon dioxide as determined by internal carotid artery duplex scanning. J Trauma. 1992;32:618–27. discussion 27–8. [PubMed] [Google Scholar]
- 4.Brian JE., Jr Carbon dioxide and the cerebral circulation. Anesthesiology. 1998;88:1365–86. doi: 10.1097/00000542-199805000-00029. [DOI] [PubMed] [Google Scholar]
- 5.Durduran T, Choe R, Baker WB, Yodh AG. Diffuse optics for tissue monitoring and tomography. Rept Prog Phys. 2010;73:076701. doi: 10.1088/0034-4885/73/7/076701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boas DA, Campbell LE, Yodh AG. Scattering and imaging with diffusing temporal field correlations. Phys Rev Lett. 1995;75:1855–8. doi: 10.1103/PhysRevLett.75.1855. [DOI] [PubMed] [Google Scholar]
- 7.Buckley EM, Cook NM, Durduran T, et al. Cerebral hemodynamics in preterm infants during positional intervention measured with diffuse correlation spectroscopy and transcranial Doppler ultrasound. Opt Express. 2009;17:12571–81. doi: 10.1364/oe.17.012571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou C, Yu G, Furuya D, Greenberg J, Yodh A, Durduran T. Diffuse optical correlation tomography of cerebral blood flow during cortical spreading depression in rat brain. Opt Express. 2006;14:1125–44. doi: 10.1364/oe.14.001125. [DOI] [PubMed] [Google Scholar]
- 9.Culver JP, Durduran T, Cheung C, Furuya D, Greenberg JH, Yodh AG. Diffuse optical measurement of hemoglobin and cerebral blood flow in rat brain during hypercapnia, hypoxia and cardiac arrest. Adv Exp Med Biol. 2003;510:293–7. doi: 10.1007/978-1-4615-0205-0_48. [DOI] [PubMed] [Google Scholar]
- 10.Zhou C, Eucker SA, Durduran T, et al. Diffuse optical monitoring of hemodynamic changes in piglet brain with closed head injury. J Biomed Opt. 2009;14:034015. doi: 10.1117/1.3146814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MN, Durduran T, Frangos S, et al. Noninvasive measurement of cerebral blood flow and blood oxygenation using near-infrared and diffuse correlation spectroscopies in critically brain-injured adults. Neurocrit Care. 2010;12:173–80. doi: 10.1007/s12028-009-9305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durduran T. Noninvasive measurements of tissue hemodynamics with hybrid diffuse optical methods. Med Physics. 2004;31:2178. [Google Scholar]
- 13.Durduran T, Zhou C, Buckley EM, et al. Optical measurement of cerebral hemodynamics and oxygen metabolism in neonates with congenital heart defects. J Biomed Opt. 2010;15:037004. doi: 10.1117/1.3425884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckley EM, Hance D, Pawlowski T, et al. Validation of diffuse correlation spectroscopic measurement of cerebral blood flow using phase-encoded velocity mapping magnetic resonance imaging. J Biomed Opt. 2012;17:037007. doi: 10.1117/1.JBO.17.3.037007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durduran T, Choe R, Yu G, et al. Diffuse optical measurement of blood flow in breast tumors. Opt Lett. 2005;30:2915–7. doi: 10.1364/ol.30.002915. [DOI] [PubMed] [Google Scholar]
- 16.Sunar U, Quon H, Durduran T, et al. Noninvasive diffuse optical measurement of blood flow and blood oxygenation for monitoring radiation therapy in patients with head and neck tumors: a pilot study. J Biomed Opt. 2006;11:064021. doi: 10.1117/1.2397548. [DOI] [PubMed] [Google Scholar]
- 17.Yu G, Durduran T, Lech G, et al. Time-dependent blood flow and oxygenation in human skeletal muscles measured with noninvasive near-infrared diffuse optical spectroscopies. J Biomed Opt. 2005;10:024027. doi: 10.1117/1.1884603. [DOI] [PubMed] [Google Scholar]
- 18.Yu G, Durduran T, Zhou C, et al. Real-time in situ monitoring of human prostate photodynamic therapy with diffuse light. Photochem Photobiol. 2006;82:1279–84. doi: 10.1562/2005-10-19-RA-721. [DOI] [PubMed] [Google Scholar]
- 19.Zhou C, Choe R, Shah N, et al. Diffuse optical monitoring of blood flow and oxygenation in human breast cancer during early stages of neoadjuvant chemotherapy. J Biomed Opt. 2007;12:051903. doi: 10.1117/1.2798595. [DOI] [PubMed] [Google Scholar]
- 20.Hayakawa T, Terashima M, Kayukawa Y, Ohta T, Okada T. Changes in cerebral oxygenation and hemodynamics during obstructive sleep apneas. Chest J. 1996;109:916–21. doi: 10.1378/chest.109.4.916. [DOI] [PubMed] [Google Scholar]
- 21.Safonova LP, Michalos A, Wolf U, et al. Diminished cerebral circulatory autoregulation in obstructive sleep apnea investigated by near-infrared spectroscopy. Sleep Res Online. 2003;5:123–32. [Google Scholar]
- 22.Spielman AJ, Zhang G, Yang C-M, et al. Intracerebral hemodynamics probed by near infrared spectroscopy in the transition between wakefulness and sleep. Brain Res. 2000;866:313–25. doi: 10.1016/s0006-8993(00)02320-9. [DOI] [PubMed] [Google Scholar]
- 23.Virtanen J, Noponen T, Salmi T, Toppila J, Merilainen P. Impaired cerebral vasoreactivity may cause cerebral blood volume dip following obstructive sleep apnea termination. Sleep Breath. 2012;16:309–12. doi: 10.1007/s11325-011-0526-9. [DOI] [PubMed] [Google Scholar]
- 24.Olopade CO, Mensah E, Gupta R, et al. Noninvasive determination of brain tissue oxygenation during sleep in obstructive sleep apnea: a near-infrared spectroscopic approach. Sleep. 2007;30:1747. doi: 10.1093/sleep/30.12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pizza F, Biallas M, Wolf M, Werth E, Bassetti CL. Nocturnal cerebral hemodynamics in snorers and in patients with obstructive sleep apnea: a near-infrared spectroscopy study. Sleep. 2010;33:205–10. doi: 10.1093/sleep/33.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Näsi T, Virtanen J, Toppila J, Salmi T, Ilmoniemi RJ. Cyclic alternating pattern is associated with cerebral hemodynamic variation: a near-infrared spectroscopy study of sleep in healthy humans. PloS ONE. 2012;7:e46899. doi: 10.1371/journal.pone.0046899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou Y, Shang Y, Cheng R, et al. Obstructive sleep apnea-hypopnea results in significant variations in cerebral hemodynamics detected by diffuse optical spectroscopies. Physiol Meas. 2014;35:2135–48. doi: 10.1088/0967-3334/35/10/2135. [DOI] [PubMed] [Google Scholar]
- 28.Huang J, Marcus CL, Davenport PW, Colrain IM, Gallagher PR, Tapia IE. Respiratory and auditory cortical processing in children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2013;188:852–7. doi: 10.1164/rccm.201307-1257OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcus CL, Omlin KJ, Basinki DJ, et al. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992;146:1235–9. doi: 10.1164/ajrccm/146.5_Pt_1.1235. [DOI] [PubMed] [Google Scholar]
- 31.Chervin RD, Dillon JE, Bassetti C, Ganoczy DA, Pituch KJ. Symptoms of sleep disorders, inattention, and hyperactivity in children. Sleep. 1997;20:1185–92. doi: 10.1093/sleep/20.12.1185. [DOI] [PubMed] [Google Scholar]
- 32.Huang J, Pinto SJ, Yuan H, et al. Upper airway collapsibility and genioglossus activity in adolescents during sleep. Sleep. 2012;35:1345–52. doi: 10.5665/sleep.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Read DJ. A clinical method for assessing the ventilatory response to carbon dioxide. Aust Ann Med. 1967;16:20–32. doi: 10.1111/imj.1967.16.1.20. [DOI] [PubMed] [Google Scholar]
- 34.Team RC. R: A language and environment for statistical computing. 2013. Available from: http://www.R-project.org/
- 35.Yuan H, Pinto SJ, Huang J, et al. Ventilatory responses to hypercapnia during wakefulness and sleep in obese adolescents with and without obstructive sleep apnea syndrome. Sleep. 2012;35:1257–67. doi: 10.5665/sleep.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Driscoll DM, Horne RS, Davey MJ, et al. Increased sympathetic activity in children with obstructive sleep apnea: cardiovascular implications. Sleep Med. 2011;12:483–8. doi: 10.1016/j.sleep.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Alchanatis M, Deligiorgis N, Zias N, et al. Frontal brain lobe impairment in obstructive sleep apnoea: a proton MR spectroscopy study. Eur Respir J. 2004;24:980–6. doi: 10.1183/09031936.04.00127603. [DOI] [PubMed] [Google Scholar]
- 38.Joo EY, Tae WS, Han SJ, Cho JW, Hong SB. Reduced cerebral blood flow during wakefulness in obstructive sleep apnea-hypopnea syndrome. Sleep. 2007;30:1515–20. doi: 10.1093/sleep/30.11.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hajak G, Klingelhofer J, Schulz-Varszegi M, Sander D, Ruther E. Sleep apnea syndrome and cerebral hemodynamics. Chest. 1996;110:670–9. doi: 10.1378/chest.110.3.670. [DOI] [PubMed] [Google Scholar]
- 40.Hayakawa T, Terashima M, Kayukawa Y, Ohta T, Okada T. Changes in cerebral oxygenation and hemodynamics during obstructive sleep apneas. Chest. 1996;109:916–21. doi: 10.1378/chest.109.4.916. [DOI] [PubMed] [Google Scholar]
- 41.Buterbaugh J, Wynstra C, Provencio N, Combs D, Gilbert M, Parthasarathy S. Cerebrovascular reactivity in young subjects with sleep apnea. Sleep. 2015;38:241–50. doi: 10.5665/sleep.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hill CM, Hogan AM, Onugha N, et al. Increased cerebral blood flow velocity in children with mild sleep-disordered breathing: a possible association with abnormal neuropsychological function. Pediatrics. 2006;118:e1100–8. doi: 10.1542/peds.2006-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hogan AM, Hill CM, Harrison D, Kirkham FJ. Cerebral blood flow velocity and cognition in children before and after adenotonsillectomy. Pediatrics. 2008;122:75–82. doi: 10.1542/peds.2007-2540. [DOI] [PubMed] [Google Scholar]
- 44.Sun L, Pan A, Yu Z, et al. Snoring, inflammatory markers, adipokines and metabolic syndrome in apparently healthy Chinese. PLoS ONE. 2011;6:e27515. doi: 10.1371/journal.pone.0027515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almendros I, Acerbi I, Puig F, Montserrat JM, Navajas D, Farre R. Upper-airway inflammation triggered by vibration in a rat model of snoring. Sleep. 2007;30:225–7. doi: 10.1093/sleep/30.2.225. [DOI] [PubMed] [Google Scholar]
- 46.Li AM, Au CT, Chook P, Lam HS, Wing YK. Reduced flow-mediated vasodilation of brachial artery in children with primary snoring. Int J Cardiol. 2013;167:2092–6. doi: 10.1016/j.ijcard.2012.05.108. [DOI] [PubMed] [Google Scholar]
- 47.Friberg D, Ansved T, Borg K, Carlsson-Nordlander B, Larsson H, Svanborg E. Histological indications of a progressive snorers disease in an upper airway muscle. Am J Respir Crit Care Med. 1998;157:586–93. doi: 10.1164/ajrccm.157.2.96-06049. [DOI] [PubMed] [Google Scholar]
- 48.Immanuel SA, Pamula Y, Kohler M, et al. Heartbeat evoked potentials during sleep and daytime behavior in children with sleep-disordered breathing. Am J Respir Crit Care Med. 2014;190:1149–57. doi: 10.1164/rccm.201405-0920OC. [DOI] [PubMed] [Google Scholar]
- 49.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–76. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker WB, Parthasarathy AB, Busch DR, Mesquita RC, Greenberg JH, Yodh AG. Modified Beer-Lambert law for blood flow. Biomed Opt Express. 2014;5:4053–75. doi: 10.1364/BOE.5.004053. [DOI] [PMC free article] [PubMed] [Google Scholar]