Abstract
Repetitive transcranial magnetic stimulation (rTMS) has been proposed as a tinnitus treatment option. Promising results have been obtained by consecutive stimulation of lateral frontal and auditory brain regions. We investigated a combined stimulation paradigm targeting the anterior cingulate cortex (ACC) with double cone coil rTMS, followed by stimulation of the temporo-parietal junction area with a figure-of-eight coil. The study was conducted as a randomized, double-blind pilot trial in 40 patients suffering from chronic tinnitus. We compared mediofrontal stimulation with double-cone-coil, (2000 stimuli, 10 Hz) followed by left temporo-parietal stimulation with figure-of-eight-coil (2000 stimuli, 1 Hz) to left dorsolateral-prefrontal-cortex stimulation with figure-of-eight-coil (2000 stimuli, 10 Hz) followed by temporo-parietal stimulation with figure-of-eight-coil (2000 stimuli, 1 Hz). The stimulation was feasible with comparable dropout rates in both study arms; no severe adverse events were registered. Responder rates did not differ in both study arms. There was a significant main effect of time for the change in the TQ score, but no significant time x group interaction. This pilot study demonstrated the feasibility of combined mediofrontal/temporoparietal-rTMS-stimulation with double cone coil in tinnitus patients but failed to show better outcome compared to an actively rTMS treated control group.
Chronic Tinnitus Subjective tinnitus is characterized by the perception of sound in the absence of a corresponding sound source1. Most people have already experienced an occasional and transient perception of sound in their ears or head2. Typically, this perception is reversible and subsides approximately between a few seconds to a few days. However, in about 5 to 15% of the adult general population these phantom sounds are perceived chronically3,4. Approximately 1% of the general population report severe tinnitus-related impairment of daily living5 and seek medical help6. No evidence-based established treatments for curing chronic tinnitus or for reduction of its loudness are available7,8.
Transcranial magnetic stimulation
Transcranial magnetic stimulation (TMS) is a non-invasive tool for inducing electric currents in the brain9. Magnetic fields created by a short and strong electric current circulating within a coil, penetrate the skull painlessly and result in depolarization of superficial cortical neurons10. This technique has gained increasing attention as a potential clinical tool for the treatment of a variety of neuropsychiatric disorders (for an overview please see11) including chronic tinnitus12,13,14. Most, but not all sham-controlled studies revealed beneficial effects with this approach in the treatment of tinnitus (for the last five years e.g.15,16,17,18,19,20,21,22,23. TMS is regarded a safe technique with only few adverse effects24 and has been proven to be feasible for both in- and out-patient treatment25.
Boosting rTMS effects in chronic tinnitus by combined stimulation protocols – the involvement of the limbic system
However, rTMS treatment results are currently burdened by only moderate improvement and high inter-individual variability12,13,14 indicating the need for optimization strategies. As hypothesized already more than 20 years ago26 and confirmed by recent neuroimaging findings, tinnitus is related to (i) abnormal activity in both auditory and non-auditory brain regions27,28,29,30,31,32 and to (ii) abnormal functional connectivity between these regions33,34,35,36,37. Moreover, abnormal functional connectivity has been demonstrated in patients reporting bothersome tinnitus38, whereas normal functional connectivity was detected in tinnitus patients without bother39. Based on these results combined rTMS stimulation paradigms targeting both auditory and affect regulatory brain regions have been developed and given hopeful results up to now17,40,41.
Typically, the direct effect of the magnetic field produced by an rTMS coil remains limited to superficial brain areas and does not reach subcortical brain structures42. However, in the treatment of depression it has been postulated that the rTMS effects created by targeting the dorsolateral prefrontal cortex (DLPFC) rely on the modulation of the anterior cingulate cortex (ACC) via fronto-cingulate functional connectivity43. Moreover, the antidepressant response of rTMS over the DLPFC in the treatment of depression depends on baseline activity of the anterior cingulate44,45,46. Since the ACC is critically involved in tinnitus distress as well47 it may be hypothesized analogously, that a similar mode of action involving the ACC is the case in the combination treatment of chronic tinnitus targeting both the DLPFC and the auditory cortex17,41,48.
The newly developed double cone coil (DCC) exerts an expanded penetration depth based on its angled geometry. A positron emission tomography study revealed that mediofrontal TMS using a double-cone coil is apt to modulate deeper located brain areas such as the dorsal and subgenual ACC49. The ACC appears to be the critical link in the interaction of dorsal/cortical and ventral/limbic networks involved in the experience and regulation of emotion50. A subdivision of the ACC, the dorsal ACC (dACC) projects to the prefrontal cortex and plays a critical role in executive functions by influencing multiple cognitive processes51 while another subdivision of the ACC, the subgenual ACC (sgACC) has been shown to be involved in emotion experience and processing52.
As the ACC has been shown to be critically involved in depression53,54 anterior-cingulate-cortex-stimulation using a double cone coil (=ACDC-stimulation) has been investigated as an innovative approach for the treatment of affective disorders very recently and has given promising results so far55,56.
First clinical experience in tinnitus patients has been gained by Vanneste et al. applying double-cone-coil-rTMS to the dorsal frontal cortex of 78 patients57. The results indicated that both tinnitus intensity and tinnitus distress reduction following prefrontal double-cone-coil-rTMS were frequency dependent. The same group in Antwerp published a second study reporting differences between a single session and repeated sessions of 1Hz TMS by double-cone coil prefrontal stimulation for the improvement of tinnitus in 73 tinnitus patients receiving single or repetitive session(s) of TMS using a DCC placed over the prefrontal cortex. The results indicated that both single sessions as well as multiple sessions suppress tinnitus distress and tinnitus intensity transiently. It was further shown that multiple sessions of prefrontal double-cone-coil-rTMS generate a higher suppression effect in comparison to a single session and that more patients responded to repeated sessions of 1 Hz stimulation in comparison to a single session21.
Based on these previous results and the notion, that, in view of the involvement of both auditory and non-auditory brain regions in tinnitus27,28,29,30,31,32, combined stimulation protocols exert greater effects17,40,41 the aim of the present study was to investigate a combined stimulation paradigm primarily targeting the ACC by mediofrontal stimulation with double-cone-coil followed by conventional stimulation with figure-of-8-coil applied to the temporo-parietal junction area. Foci of interest were the evaluation of safety, feasibility, and clinical effectiveness of a combined anterior-cingulate-cortex-rTMS using a double-cone-coil in the treatment of patients with chronic tinnitus (TiCDC).
Methods and Materials
Study design
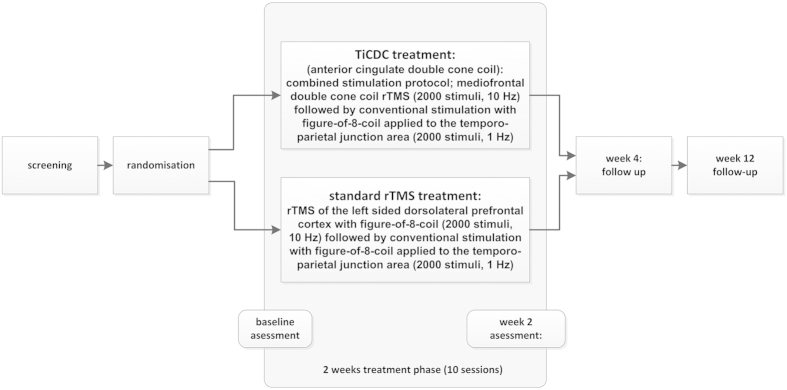
The study was conducted in a randomized, double-blind, parallel-group design with an active control group. Patients were assessed at screening, baseline, end of treatment (week 2), and two follow-up-visits taking place in week 4 and 12. We compared two combined study protocols consisting of medial frontal stimulation with double cone coil (study arm 1) vs. conventional prefrontal left DLPFC-stimulation (study arm 2/control group) both followed by stimulation of the left temporo-parietal junction area. Both study arms consisted of a total of 4000 stimuli per day. Detailed information on the study protocol and the applied stimulation parameters is provided in Fig. 1. The active control condition was chosen as this combined treatment approach to our current knowledge has proven the highest effectiveness compared to other approaches in larger samples up to now58.
Figure 1. Structure of the present “TiCDC” pilot trial; (for detailed information see methods’ section).
The study was approved by the Ethics Committee at the University of Regensburg. All participants gave written informed consent after a comprehensive explanation of the procedures. All study methods and procedures were carried out in accordance with the approved guidelines. The study had been previously registered with clinicaltrials.gov (identifier NCT01663311, date of registration: July 23, 2012).
Patient enrolment
Inclusion criterion was subjective tinnitus with duration of more than six months. Exclusion criteria comprise objective tinnitus (with a treatable cause), start of other treatments for tinnitus 3 months before study enrolment, presence of clinically relevant psychiatric comorbidities or unstable medical conditions, history or evidence of significant brain malformation or neoplasm, history of head injuries, cerebral vascular events, presence of irremovable metal objects in and around body, pregnancy, alcohol abuse or intake of illicit substances, and history of prior TMS treatment.
Patients were recruited for participation in the study after presentation in the outpatient clinic of the Interdisciplinary Tinnitus Centre at the University of Regensburg, Regensburg, Germany.
Outcome measures
Primary objective was the responder rate at week 2 evaluation (=end of rTMS treatment, see Fig. 1). Clinical response was defined as a minimal reduction of 5 points in the tinnitus questionnaire (TQ)59 as compared to baseline according to Adamchic et al.60. Accordingly, patients with a tinnitus reduction <5 TQ points were defined as “non-responders”. Secondary objectives were the assessment of adverse events and safety information for all available measurement time points as well as changes from baseline to final visit for the TQ, the Tinnitus Handicap Inventory (THI)61, the TBF-12-questionnaire scores62 (a short version of the THI consisting of 12 selected items), the Major Depression Inventory (MDI)63, the Clinical Global Impression (CGI-CHANGE), and quality of life measured by WHOQoL-Bref-questionnaire ratings64 where higher scores represent higher quality of life. Data were assessed according to international standards65,66 and registered in a tinnitus database following ICH-GCP-regulations67.
Study conduct
Participants of both study arms were treated applying combined stimulation paradigms. Conventional rTMS (study arm 2) of the left DLPFC was applied at 10 Hz frequency (2000 stimuli per session, 40 trains with 50 stimuli, intertrain interval 25 seconds) with a figure-of-eight (=butterfly) coil (Cool-B65, Magventure A/S, Denmark) followed by 1 Hz stimulation of the left temporoparietal junction area68,69 (2000 stimuli per session). TiCDC-stimulation (study arm 1) was performed over the medial frontal cortex at equal frequency using a double cone coil (Cool-D-B50, Magventure A/S, Denmark) followed by stimulation of the left temporo-parietal junction area in the same manner as in study arm 268,69. The coils were powered by a MagPro X100 stimulator (Magventure A/S, Denmark). Stimulation was performed at 110% resting motor threshold (RMT) for the butterfly coil applications, at 100% RMT for the double cone coil, or at 60% maximum stimulator output (MSO) when RMT exceeded 54% MSO (mean RMT = 45.8 ± 10.1% MSO). RMT was defined as the lowest intensity sufficient to produce left thenar muscle activation (magnetic evoked potentials >50 mV) with a single pulse delivered to the motor cortex in at least 5 out of 10 trials. For treatment of the left DLPFC the conventional butterfly-coil was positioned 6 cm anterior of the left motor hotspot in sagittal direction25, mediofrontal ACDC-stimulation followed the protocol described by Hayward et al. positioning the coil 1.5 cm anterior to one third of the distance from the nasion to the inion49. Treatment was started on Mondays and patients received a total of 4000 stimuli/day on 10 subsequent working days.
Statistical analyses
All data are displayed as mean ± standard deviation. In case of missing data the last observation was carried forward, dropout participants were excluded from analysis. Responder rates were compared by chi-square tests of independence. For secondary outcome measures we calculated analyses of variance (ANOVA) with the within-subjects factor time (baseline vs. final visit) and the between-subjects factor group (“TiCDC” vs. “standard rTMS” protocol). CGI scores were compared using chi-square tests. All statistical tests were conducted two-tailed, unadjusted for multiple comparisons due to the pilot study character of the trial, and a value of p < 0.05 was assumed as statistically significant. The sample size was set in a range similar to prior actively controlled pilot trials regarding rTMS-based treatment of chronic tinnitus (e.g.41,70), and was intended to a) estimate and gain first data regarding adverse effects and tolerability of the treatment, and b) provide orientation about clinical effectiveness of the treatment thus providing the basis for further statistical power calculations. Statistical data analysis was performed with IBM SPSS Statistics for Windows, Version 22.0 (released 2013. IBM Corp., Armonk, NY).
Results
Patient population
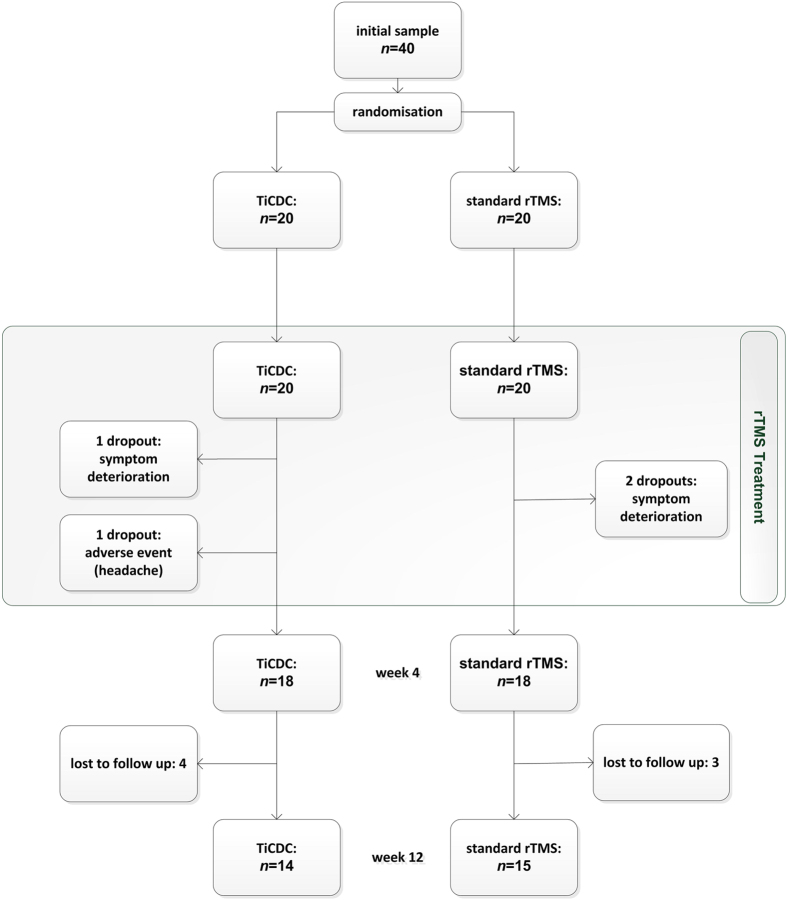
Forty patients were randomized for participation in the study. One participant aborted the stimulation due to headache (TiCDC-participant after session 7). Three study subjects withdrew their consent during the stimulation period due to deterioration of their tinnitus (one TiCDC-participant after session 2; two standard-group-participants after session 1 and session 5). These four subjects were not included in the per-protocol analysis resulting in an analytical sample of 36 patients. The comparison of participants of both treatment groups revealed no differences in the clinical and demographic baseline characteristics. Detailed information is provided in Table 1. Seven patients (standard rTMS: 3; TiCDC: 4) were lost to follow up between week 4 and week 12 (see Fig. 2).
Table 1. Baseline characteristics of patients.
| TiCDC(n = 18) | Standard rTMS(n = 18) | Statistics | |
|---|---|---|---|
| gender (female/male) | 5/13 | 6/12 | χ2(1) = 0.131,p = 0.717 |
| age (years) | 50.9 ± 10.7 | 54.4 ± 9.0 | t(34) = −1.054,p = 0.299 |
| tinnitus duration in months | 78.3 ± 79.7 | 97.0 ± 143.8 | t(34) = −0.483,p = 0.632 |
| tinnitus laterality (right/left/both) | 1/2/15 | 2/5/11 | χ2(5) = 3.562,p = 0.614 |
| TQ total score | 32.3 ± 19.3 | 33.8 ± 17.7 | t(34) = −0.243,p = 0.809 |
| THI total score | 38.3 ± 21.8 | 36.6 ± 21.8 | t(34) = 0.245,p = 0.808 |
| MDI | 7.1 ± 5.8 | 6.9 ± 6.3 | t(34) = 0.110,p = 0.913 |
TQ: Tinnitus Questionnaire, THI: Tinnitus Handicap Inventory, MDI: Major Depression Inventory.
Figure 2. TiCDC study patient flow.
Safety
No severe adverse events occurred during the course of the study. The incidence of adverse events was comparable in both study arms: six patients complained about headache (3 TiCDC/3 standard rTMS control group/1 dropout (TiCDC)), a deterioration of tinnitus was complained in one TiCDC-participant (leading to the second dropout of TiCDC-group) and two control-group-participants (2 dropouts), dizziness or a deterioration of pre-existing dizziness was reported by 1 patient of each group. Other adverse events included local pain (TiCDC: 1 report) and feelings of ear pressure on both sides (control group: 1 report).
Primary outcome
As a first step of the data analysis we aimed to compare the number of responders and non-responders in both treatment arms. The primary outcome criterion (responder rate week 2 vs. baseline) turned out non-significant (see Table 2) leading to acceptance of our null-hypothesis (no difference in responder rates comparing both study arms at end of treatment time point). The percentage of responders in the TiCDC arm was 6/18 or 33%, and the percentage of responders in the standard arm was 5/18 or 28%. This represents a difference of 5% with a 95% CI extending from −23% to 33%. For further detailed information see Table 2 (upper part).
Table 2. Responder Analysis.
| Week 2 visit (end of treatment)- primary outcome - | responder | non-responder |
|---|---|---|
| both groups | 11 | 25 |
| TiCDC | 6 | 12 |
| standard rTMS | 5 | 13 |
| statistics | χ2(1) = 0.131, p = 0.717 | |
| Week 12 visit (end of study) | ||
| both groups | 14 | 22 |
| TiCDC | 9 | 9 |
| standard rTMS | 5 | 13 |
| statistics | χ2(1) = 1.870; p = 0.171 | |
Secondary outcomes
Response rates at week 12 (=end of study) turned out non-significant (χ2(1) = 1.870, p = 0.171). With regard to the ANOVAs comparing secondary outcomes such as change in TQ, THI, TBF12, MDI, and WHOQoL-questionnaires, the main effect of time turned out significant for the change in the TQ score (baseline score: 33.08 ± 18.26; score at week 12: 29.89 ± 19.11) and marginally significant for the THI with effect sizes being moderate to high (for details see left part of Table 3). No significant changes over time were observed for the remaining outcome measures. The interaction effects between time and group turned out non-significant at uncorrected level (all p’s>.05). For detailed information see right part of Table 3. The observation that the two stimulation protocols did not differ in their clinical efficacy was met by the subjective evaluation of the patients: clinical global impression scales turned out non-significant between groups at week 2 visit and week 12 visit (see Table 4).
Table 3. Secondary outcomes: ANOVA with between subjects factor group (‘TiCDC’ vs. ‘standard rTMS’) and within subjects factor time (baseline vs. week 12).
| main effect time |
interaction effects for group x time (baseline vs. week 12) |
|||||
|---|---|---|---|---|---|---|
| F (1, 34) | p-value | part. Eta2 | F (1, 34) | p-value | part. Eta2 | |
| TQ | 5.085 | 0.031 | 0.130 | 1.431 | 0.240 | 0.040 |
| THI | 3.459 | 0.072 | 0.092 | 3.459 | 0.072 | 0.092 |
| TBF12 | 0.150 | 0.701 | 0.004 | 2.265 | 0.142 | 0.062 |
| MDI | 2.222 | 0.145 | 0.061 | 1.502 | 0.229 | 0.042 |
| WHOQOL1 | 1.956 | 0.171 | 0.054 | 3.412 | 0.073 | 0.091 |
| WHOQOL2 | 0.176 | 0.678 | 0.005 | 1.477 | 0.233 | 0.042 |
| WHOQOL3 | 0.103 | 0.751 | 0.003 | 1.499 | 0.229 | 0.042 |
| WHOQOL4 | 0.067 | 0.789 | 0.002 | 1.351 | 0.253 | 0.038 |
TQ: Tinnitus Questionnaire, THI: Tinnitus Handicap Inventory, MDI: Major Depression Inventory, WHOQoL: World Health Organization Quality of Life Questionnaire (Domain 1–4), TBF12: Tinnitus Impairment Questionnaire (short version of the THI consisting of 12 items).
Table 4. Clinical Global Impression (CGI) at week 2 and week 12.
| Week 2 visit(end of treatment) | much better | minimallybetter | no change | minimallyworse |
|---|---|---|---|---|
| both groups | 3 | 8 | 16 | 6 |
| TiCDC | 2 | 4 | 7 | 3 |
| standard rTMS | 1 | 4 | 9 | 3 |
| statistics | χ2(3, N = 33) = 0.554, p = 0.907 | |||
| Week 12 visit (end of study) | ||||
| both groups | 3 | 5 | 17 | 4 |
| TiCDC | 1 | 2 | 9 | 2 |
| standard rTMS | 2 | 3 | 8 | 2 |
| statistics | χ2(3, N = 29) = 0.558; p = 0.906 | |||
Graphical information on the TQ score at the different assessment time points is depicted in Fig. 3.
Figure 3. Tinnitus Questionnaire Scores (mean/sem).
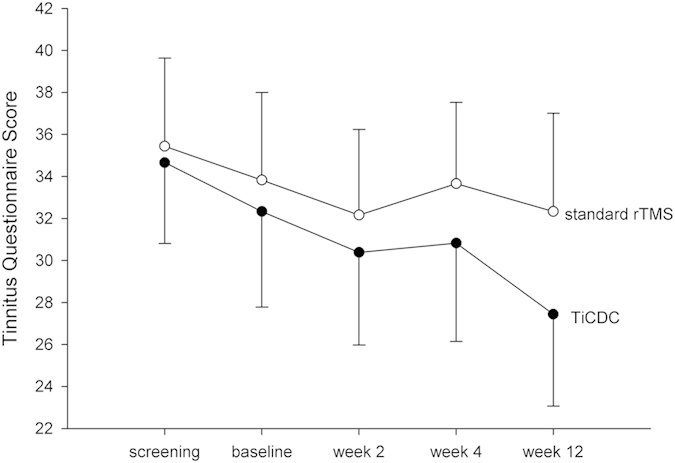
rTMS treatment took place between baseline and week 2 visit. Week 4 and 12 visits represent follow up.
Discussion
In our pilot study examining 10 sessions of TiCDC-stimulation of the anterior cingulate cortex combined with 1 Hz stimulation of the temporo-parietal junction area this paradigm was well tolerated by the majority of patients, adverse events and dropout rates/reasons were comparable among both study arms and matched prior observations24. TiCDC-stimulation turned out to be non-superior to standard rTMS combining butterfly-coil-stimulation of the left DLPFC followed by 1 Hz stimulation of the temporo-parietal junction area regarding both primary and secondary outcome measures.
Notably, the present study was a pilot study, which investigated the stimulation of the anterior cingulated cortex with a double cone coil following suggestions of Vanneste and colleagues21,57,71. An actively controlled parallel group study design has been used to investigate whether this approach is superior to an established protocol of combined frontal and temporal stimulation, which has shown promising results so far17,41,48. Active control conditions have especially been recommended in rTMS studies due to the inherent limitations of sham conditions72. For sure, the threshold of demonstrating superiority compared to an established and clinically efficient stimulation protocol was set high. Moreover, the treatment duration of 10 sessions was chosen for feasibility reasons, even if earlier studies especially regarding the treatment of depression have shown better results with an extended treatment duration up to 6 weeks73,74. Thus, in the interpretation of results the limited power of the study with its relatively small sample size of 2×20 participants, the contrast to an actively treated control group, and the short treatment duration of only 10 sessions rTMS have to be taken into account.
Pathophysiological considerations concerning the ACC’s role in chronic tinnitus
The anterior cingulate cortex is known to be critically involved in the attentional control of sensory processing75,76,77,78. In detail, top-down inhibitory mechanisms that originate in the prefrontal lobe have been shown to play an important role in auditory processing79 and in the generation of tinnitus80. Thus, it is tempting to speculate, that rTMS-mediated modulation of the ACC might strengthen deficient inhibitory top-down mechanisms in tinnitus patients. According to the model of chronic tinnitus originating from overlapping, concurrently-activated and interacting neural networks containing auditory, emotion regulatory, attention-processing and memory networks36 the ACC plays a central role in several of these functional networks. In detail, it has been proposed, that high-frequency, gamma band, synchronized neural activity in the sensory cortex representing the auditory hyperactivity in tinnitus patients becomes a conscious percept when connected to larger coactivated “(self-)awareness” and “salience” brain networks comprising the anterior cingulate cortex and the bilateral insula81. If tinnitus is stressful, this is reflected by a simultaneously co-activated nonspecific distress network consisting of again the anterior cingulate cortex, anterior insula, and amygdala36. Memory mechanisms play a role in the persistence of the awareness of the phantom percept, as well as in the reinforcement of the associated distress36. As part of the historical Papez circuit, the anterior cingulate cortex is critically involved in such memory processing exerting its connections to other brain areas such as the parahippocampal region that has been extinsively investigated regarding the pathophysiology of chronic tinnitus up to now82,83. Moreover, the ACC has also been shown to be involved in default mode network activity84,85, a fact, that – especially in consideration of reduced focality due to enhanced penetration depth in double cone coil rTMS – might have further distracted the results reported in this study.
Prior studies regarding double cone coil TMS
To our knowledge this is the first randomized and actively controlled clinical study applying double cone coil TMS in patients suffering from chronic tinnitus. A pubmed search with the term “double cone” AND “TMS” retrieved 26 hits (access date: 02.12.2014). Vanneste and colleagues reported the successful treatment of a patient suffering from a medication-resistant chronic depression by 10 sessions of ACDC-stimulation applying the same stimulation parameters as used in our pilot study (mediofrontal stimulation with double cone coil at 10 Hz, 2000 stimuli/session)55. We added 2000 stimuli of 1 Hz-rTMS to the temporo-parietal junction area68,69 applying a figure-of-8-coil transforming this treatment approach into a combined rTMS setting. The patient treated by Vanneste and colleagues was – although showing a relatively high RMT of 65% MSO – treated with a stimulation intensity of only 40% MSO because higher amplitudes were not well tolerated in this case. The Beck Depression Inventory score improved by 27%, and the two subscales of the Hospital Anxiety Depression Scale (HADS), namely depression (40%) and anxiety (33%) improved as well. Along with the clinical improvement source-localized electrophysiological resting state activity changed in the dACC and sgACC in this patient in comparison to a normative group55. Two large case series reporting stimulation of an almost identical target as in the first stimulation component of the TiCDC-protocol, the so-called dorsomedial prefrontal cortex (dmPFC) applying the same stimulation device, were very recently published by Salomons et al.86 and Downar et al.87. The former investigated whether resting-state functional connectivity predicted response to dmPFC-rTMS treatment in depressive patients. In twenty-five individuals with treatment-refractory MDD higher baseline cortico-cortical connectivity and lower cortico-thalamic, cortico-striatal, and cortico-limbic connectivity were associated with better treatment outcomes86. Downar and colleagues87 reported results of 20 rTMS sessions to the dorsomedial prefrontal cortex in 47 patients with a medication-resistant major depressive episode. Responders and non-responders showed opposite patterns of hemispheric lateralization in the connectivity of dorsomedial and dorsolateral regions to this same ventromedial region87. Very recently, the authors of the present study have published the results of a three-armed, randomized, double-blind clinical study applying ACDC-stimulation in a sample of inpatients suffering from major depression56. Forty-five patients suffering a moderate to severe depressive episode were randomized to receive 15 sessions of either conventional rTMS of the left DLPFC (“butterfly-rTMS”; 10 Hz; 2000 stimuli/day, RMT 110%), mediofrontal double cone coil stimulation of the anterior cingulate cortex (“ACDC-rTMS” with equal parameters), or sham-stimulation. There was a significant group x time interaction effect regarding the change in the 21-items Hamilton Rating Scale for Depression (HAMD) from baseline to the end of treatment, which served as primary outcome criterion. Post-hoc testing revealed a significant effect for the comparison ACDC vs. butterfly at week 3 (end of treatment). Equalling our present experience, no severe adverse events had occurred during the study, ACDC-stimulation was well tolerated by the majority of patients similar like butterfly-rTMS and sham-stimulation.
Work to do
Despite encouraging results of previous applications of mediofrontal double-cone-coil-rTMS in patients suffering from depression, the present study failed to confirm the alternative hypothesis of superiority as part of a combined treatment setting in subjects suffering from chronic tinnitus. This might be due to limited power of the present study, which would require comparisons to placebo-controlled study groups in larger samples. Apart from that, it is very likely that our understanding of stimulation settings is yet too mechanistic and in addition to the effect in the stimulated cortical area the impact of the stimulation on network activity and connectivity has to be taken into account in more detail88. Previous studies have already demonstrated that rTMS with a butterfly coil on the left DLPFC modulates the blood flow in the ACC89 via functional connectivity of the DLPFC to deeper located mood-regulatory brain structures. It remains to be investigated by further studies whether stimulation of the DLPFC with the butterfly-coil and dorsomedial double–cone-coil stimulation have a differential effect on ACC activity. The lack of a difference between the two protocols in our study could be either due to a similar effect of both protocols on the ACC or to the non-relevance of a potential differential effect for tinnitus treatment. Therefore, it is vital for a detailed understanding of stimulation sites and parameter optimization to systematically assess this network connectivity in tinnitus patients applying different techniques such as fMRI and source-localized EEG during stimulation. Moreover, these approaches could enable a deeper insight into the pathophysiological underpinnings of direct ACC modulation by double-cone-coil-rTMS taking into account the current uncertainty of optimum stimulation parameters.
However, despite these limitations of the present study and the negative results our data provide novel insights in the feasibility of double-cone-coil rTMS targeting the ACC in patients suffering from chronic tinnitus. Moreover, they allocate an estimation of clinical effects, thus allowing for further methodological adjustments and enabling power calculations for upcoming studies. In summary our pilot data confirm the potential of TiCDC-stimulation as a non-invasive, safe and well tolerated method of brain stimulation in the treatment of chronic tinnitus, but failed to demonstrate superiority compared to the clinical effectiveness of a combinatory rTMS study group addressing both the left DLPFC and the temporo-parietal junction area.
Additional Information
How to cite this article: Kreuzer, P. M. et al. Combined rTMS treatment targeting the Anterior Cingulate and the Temporal Cortex for the Treatment of Chronic Tinnitus. Sci. Rep. 5, 18028; doi: 10.1038/srep18028 (2015).
Acknowledgments
We thank Sandra Pfluegl, Ulrike Stadler, Helene Niebling, Jarmila Gerxhaliu-Holan, and Jan Brauner for their assistance in administering rTMS and data management. Most of all we want to thank our patients for participating in our studies and allowing us to use their data for analyses.
Footnotes
Author Contributions P.K., B.L., M.L. and R.R.: conception of the study, data interpretation and manuscript edition. A.L., W.S. and M.S.: statistics and data management. P.K., V.V. and T.B.P.: patient recruitment and conduction of clinical visits.
References
- Moller A. R. Pathophysiology of tinnitus. Otolaryngol Clin North Am 36, 249–266, v-vi (2003). [DOI] [PubMed] [Google Scholar]
- Eggermont J. J. & Roberts L. E. The neuroscience of tinnitus. Trends Neurosci 27, 676–682, 10.1016/j.tins.2004.08.010 (2004). [DOI] [PubMed] [Google Scholar]
- Axelsson A. & Ringdahl A. Tinnitus–a study of its prevalence and characteristics. Br J Audiol 23, 53–62, 10.3109/03005368909077819 (1989). [DOI] [PubMed] [Google Scholar]
- Hoffman, H. & Reed, G. In Tinnitus: Theory and Management (ed Snow J. B. ), Ch. 3, 16–41 (London: BC Decker, 2004). [Google Scholar]
- Harter M., Maurischat C., Weske G., Laszig R. & Berger M. [Psychological stress and impaired quality of life in patients with tinnitus]. HNO 52, 125–131, 10.1007/s00106-003-0889-8 (2004). [DOI] [PubMed] [Google Scholar]
- Coles R. R. Epidemiology of tinnitus: (1) prevalence. J Laryngol Otol Suppl 9, 7–15 (1984). [DOI] [PubMed] [Google Scholar]
- Hoare D. J., Kowalkowski V. L., Kang S. & Hall D. A. Systematic review and meta-analyses of randomized controlled trials examining tinnitus management. Laryngoscope 121, 1555–1564, 10.1002/lary.21825 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langguth B., Kreuzer P. M., Kleinjung T. & De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol 12, 920–930, 10.1016/s1474-4422(13)70160-1 (2013). [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature 406, 147–150, 10.1038/35018000 (2000). [DOI] [PubMed] [Google Scholar]
- Ridding M. C. & Rothwell J. C. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci 8, 559–567 (2007). [DOI] [PubMed] [Google Scholar]
- Lefaucheur J. P. et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 125, 2150–2206, 10.1016/j.clinph.2014.05.021 (2014). [DOI] [PubMed] [Google Scholar]
- Peng Z., Chen X. Q. & Gong S. S. Effectiveness of Repetitive Transcranial Magnetic Stimulation for Chronic Tinnitus: A Systematic Review. Otolaryngol Head Neck Surg, 10.1177/0194599812458771 (2012). [DOI] [PubMed] [Google Scholar]
- Meng Z., Liu S., Zheng Y. & Phillips J. S. Repetitive transcranial magnetic stimulation for tinnitus. Cochrane Database Syst Rev, CD007946, 10.1002/14651858.CD007946.pub2 (2011). [DOI] [PubMed] [Google Scholar]
- Soleimani R., Jalali M. M. & Hasandokht T. Therapeutic impact of repetitive transcranial magnetic stimulation (rTMS) on tinnitus: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol, 10.1007/s00405-015-3642-5 (2015). [DOI] [PubMed] [Google Scholar]
- Hoekstra C. E., Versnel H., Neggers S. F., Niesten M. E. & van Zanten G. A. Bilateral Low-Frequency Repetitive Transcranial Magnetic Stimulation of the Auditory Cortex in Tinnitus Patients Is Not Effective: A Randomised Controlled Trial. Audiol Neurootol 18, 362–373, 10.1159/000354977 (2013). [DOI] [PubMed] [Google Scholar]
- Kim B. G. et al. Comparison of the outcomes of repetitive transcranial magnetic stimulation to the ipsilateral and contralateral auditory cortex in unilateral tinnitus. Electromagn Biol Med 33, 211–215, 10.3109/15368378.2013.801353 (2014). [DOI] [PubMed] [Google Scholar]
- Langguth B. et al. Efficacy of different protocols of transcranial magnetic stimulation for the treatment of tinnitus: Pooled analysis of two randomized controlled studies. World J Biol Psychiatry 15, 276–285, 10.3109/15622975.2012.708438 (2014). [DOI] [PubMed] [Google Scholar]
- Lorenz I., Muller N., Schlee W., Langguth B. & Weisz N. Short-term effects of single repetitive TMS sessions on auditory evoked activity in patients with chronic tinnitus. J Neurophysiol 104, 1497–1505, 10.1152/jn.00370.2010 (2010). [DOI] [PubMed] [Google Scholar]
- Mennemeier M. et al. Variable changes in PET activity before and after rTMS treatment for tinnitus. Laryngoscope 121, 815–822, 10.1002/lary.21425 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S. & De Ridder D. The involvement of the left ventrolateral prefrontal cortex in tinnitus: a TMS study. Exp Brain Res 221, 345–350, 10.1007/s00221-012-3177-6 (2012). [DOI] [PubMed] [Google Scholar]
- Vanneste S. & De Ridder D. Differences between a single session and repeated sessions of 1 Hz TMS by double-cone coil prefrontal stimulation for the improvement of tinnitus. Brain Stimul 6, 155–159, 10.1016/j.brs.2012.03.019 (2013). [DOI] [PubMed] [Google Scholar]
- Lo Y. L. et al. A comparison study of repetitive transcranial magnetic stimulation for tinnitus treatment in an Asian population. Clin Neurol Neurosurg 119, 96–99, 10.1016/j.clineuro.2014.01.012 (2014). [DOI] [PubMed] [Google Scholar]
- Folmer R. L. et al. Repetitive Transcranial Magnetic Stimulation Treatment for Chronic Tinnitus: A Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg 141, 716–722, 10.1001/jamaoto.2015.1219 (2015). [DOI] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P. M. & Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin.Neurophysiol. 120, 2008–2039 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E. et al. Transcranial magnetic stimulation for the treatment of depression: feasibility and results under naturalistic conditions: a retrospective analysis. Eur.Arch.Psychiatry Clin.Neurosci. 261(4), 261–6 10.1007/s00406-010-0137-7 (2010). [DOI] [PubMed] [Google Scholar]
- Jastreboff P. J. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res 8, 221–254 (1990). [DOI] [PubMed] [Google Scholar]
- Golm D., Schmidt-Samoa C., Dechent P. & Kroner-Herwig B. Neural correlates of tinnitus related distress: an fMRI-study. Hear Res 295, 87–99, 10.1016/j.heares.2012.03.003 (2013). [DOI] [PubMed] [Google Scholar]
- Leaver A. M. et al. Dysregulation of limbic and auditory networks in tinnitus. Neuron 69, 33–43, 10.1016/j.neuron.2010.12.002 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver A. M. et al. Cortico-limbic morphology separates tinnitus from tinnitus distress. Front Syst Neurosci 6, 21, 10.3389/fnsys.2012.00021 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker J. P., Leaver A. M. & Muhlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron 66, 819–826, 10.1016/j.neuron.2010.04.032 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecklmann M. et al. Neural correlates of tinnitus duration and distress: a positron emission tomography study. Hum Brain Mapp 34, 233–240, 10.1002/hbm.21426 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanting C. P., de Kleine E. & van Dijk P. Neural activity underlying tinnitus generation: results from PET and fMRI. Hear Res 255, 1–13, 10.1016/j.heares.2009.06.009 (2009). [DOI] [PubMed] [Google Scholar]
- Schlee W., Hartmann T., Langguth B. & Weisz N. Abnormal resting-state cortical coupling in chronic tinnitus. BMC Neurosci 10, 11, 10.1186/1471-2202-10-11 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee W. et al. Mapping cortical hubs in tinnitus. BMC Biol 7, 80, 10.1186/1741-7007-7-80 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee W., Weisz N., Bertrand O., Hartmann T. & Elbert T. Using auditory steady state responses to outline the functional connectivity in the tinnitus brain. PLoS One 3, e3720, 10.1371/journal.pone.0003720 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D., Elgoyhen A. B., Romo R. & Langguth B. Phantom percepts: Tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci USA 108, 8075–8080, 10.1073/pnas.1018466108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamchic I., Langguth B., Hauptmann C. & Tass P. A. Abnormal cross-frequency coupling in the tinnitus network. Front Neurosci 8, 284, 10.3389/fnins.2014.00284 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H. et al. Altered networks in bothersome tinnitus: a functional connectivity study. BMC Neurosci 13, 3, 10.1186/1471-2202-13-3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wineland A. M., Burton H. & Piccirillo J. Functional connectivity networks in nonbothersome tinnitus. Otolaryngol Head Neck Surg 147, 900–906, 10.1177/0194599812451414 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner A. et al. Multisite rTMS for the treatment of chronic tinnitus: stimulation of the cortical tinnitus network–a pilot study. Brain Topogr 26, 501–510, 10.1007/s10548-012-0268-4 (2013). [DOI] [PubMed] [Google Scholar]
- Kleinjung T. et al. Combined temporal and prefrontal transcranial magnetic stimulation for tinnitus treatment: a pilot study. Otolaryngol Head Neck Surg 138, 497–501 (2008). [DOI] [PubMed] [Google Scholar]
- Siebner H. R. et al. Patients with focal arm dystonia have increased sensitivity to slow-frequency repetitive TMS of the dorsal premotor cortex. Brain 126, 2710–2725 (2003). [DOI] [PubMed] [Google Scholar]
- Paus T., Castro-Alamancos M. A. & Petrides M. Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci 14, 1405–1411 (2001). [DOI] [PubMed] [Google Scholar]
- Baeken C. et al. HF-rTMS treatment in medication-resistant melancholic depression: results from 18FDG-PET brain imaging. CNS Spectr 14, 439–448 (2009). [DOI] [PubMed] [Google Scholar]
- Hernandez-Ribas R. et al. Identifying brain imaging correlates of clinical response to repetitive transcranial magnetic stimulation (rTMS) in major depression. Brain Stimul 6, 54–61, 10.1016/j.brs.2012.01.001 (2013). [DOI] [PubMed] [Google Scholar]
- Langguth B. et al. Pre-treatment anterior cingulate activity as a predictor of antidepressant response to repetitive transcranial magnetic stimulation (rTMS). Neuro Endocrinol Lett 28, 633–638 (2007). [PubMed] [Google Scholar]
- Vanneste S. et al. The neural correlates of tinnitus-related distress. Neuroimage 52, 470–480, 10.1016/j.neuroimage.2010.04.029 (2010). [DOI] [PubMed] [Google Scholar]
- Lehner A. et al. Comparing single-site with multisite rTMS for the treatment of chronic tinnitus - clinical effects and neuroscientific insights: study protocol for a randomized controlled trial. Trials 14, 269, 10.1186/1745-6215-14-269 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G. et al. Exploring the physiological effects of double-cone coil TMS over the medial frontal cortex on the anterior cingulate cortex: an H2(15)O PET study. Eur J Neurosci 25, 2224–2233 (2007). [DOI] [PubMed] [Google Scholar]
- Phillips M. L., Drevets W. C., Rauch S. L. & Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry 54, 504–514 (2003). [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P. & Posner M. I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4, 215–222 (2000). [DOI] [PubMed] [Google Scholar]
- Ressler K. J. & Mayberg H. S. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 10, 1116–1124 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicak P. G. et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul 3, 187–199 (2010). [DOI] [PubMed] [Google Scholar]
- Herbsman T. et al. More lateral and anterior prefrontal coil location is associated with better repetitive transcranial magnetic stimulation antidepressant response. Biol Psychiatry 66, 509–515 (2009). [DOI] [PubMed] [Google Scholar]
- Vanneste S., Ost J., Langguth B. & De Ridder D. TMS by double-cone coil prefrontal stimulation for medication resistant chronic depression: a case report. Neurocase 20, 61–68, 10.1080/13554794.2012.732086 (2014). [DOI] [PubMed] [Google Scholar]
- Kreuzer P. M. et al. The ACDC Pilot Trial: Targeting the Anterior Cingulate by Double Cone Coil rTMS for the Treatment of Depression. Brain Stimul, 10.1016/j.brs.2014.11.014 (2014). [DOI] [PubMed] [Google Scholar]
- Vanneste S., Plazier M., Van de Heyning P. & De Ridder D. Repetitive transcranial magnetic stimulation frequency dependent tinnitus improvement by double cone coil prefrontal stimulation. J Neurol Neurosurg Psychiatry 82, 1160–1164, 10.1136/jnnp.2010.213959 (2011). [DOI] [PubMed] [Google Scholar]
- Langguth B. et al. Efficacy of different protocols of transcranial magnetic stimulation for the treatment of tinnitus: Pooled analysis of two randomized controlled studies. World J Biol Psychiatry, 10.3109/15622975.2012.708438 (2012). [DOI] [PubMed] [Google Scholar]
- Goebel G. & Hiller W. The tinnitus questionnaire. A standard instrument for grading the degree of tinnitus. Results of a multicenter study with the tinnitus questionnaire. HNO 42, 166–172 (1994). [PubMed] [Google Scholar]
- Adamchic I. et al. Linking the Tinnitus Questionnaire and the subjective Clinical Global Impression: Which differences are clinically important? Health Qual Life Outcomes 10, 79, 10.1186/1477-7525-10-79 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman C. W., Jacobson G. P. & Spitzer J. B. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg 122, 143–148 (1996). [DOI] [PubMed] [Google Scholar]
- Greimel K. V., Leibetseder M., Unterrainer J. & Albegger K. [Can tinnitus be measured? Methods for assessment of tinnitus-specific disability and presentation of the Tinnitus Disability Questionnaire]. HNO 47, 196–201 (1999). [DOI] [PubMed] [Google Scholar]
- Bech P., Rasmussen N. A., Olsen L. R., Noerholm V. & Abildgaard W. The sensitivity and specificity of the Major Depression Inventory, using the Present State Examination as the index of diagnostic validity. J Affect Disord 66, 159–164 (2001). [DOI] [PubMed] [Google Scholar]
- WHOQOL-BREF: Introduction, Administration, Scoring. World’s Health Organization. Geneva, Switzerland (1996) accessible via http://www.who.int/mental_health/media/en/76.pdf (date :October 1st, 2015).
- Langguth B. et al. Consensus for tinnitus patient assessment and treatment outcome measurement: Tinnitus Research Initiative meeting, Regensburg, July 2006. Prog Brain Res 166, 525–536 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgrebe M. et al. Methodological aspects of clinical trials in tinnitus: a proposal for an international standard. J Psychosom Res 73, 112–121, 10.1016/j.jpsychores.2012.05.002 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgrebe M. et al. The Tinnitus Research Initiative (TRI) database: a new approach for delineation of tinnitus subtypes and generation of predictors for treatment outcome. BMC Med Inform Decis Mak 10, 42 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo J. F. et al. Low-frequency repetitive transcranial magnetic stimulation to the temporoparietal junction for tinnitus. Arch Otolaryngol Head Neck Surg 137, 221–228, 10.1001/archoto.2011.3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo J. F. et al. Low-frequency repetitive transcranial magnetic stimulation to the temporoparietal junction for tinnitus: four-week stimulation trial. JAMA Otolaryngol Head Neck Surg 139, 388–395, 10.1001/jamaoto.2013.233 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer P. M. et al. Can Temporal Repetitive Transcranial Magnetic Stimulation be Enhanced by Targeting Affective Components of Tinnitus with Frontal rTMS? A Randomized Controlled Pilot Trial. Front Syst Neurosci 5, 88, 10.3389/fnsys.2011.00088 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S., van der Loo E., Plazier M. & De Ridder D. Parietal double-cone coil stimulation in tinnitus. Exp Brain Res 221, 337–343, 10.1007/s00221-012-3176-7 (2012). [DOI] [PubMed] [Google Scholar]
- Duecker F. & Sack A. T. Rethinking the role of sham TMS. Frontiers in Psychology 6, 10.3389/fpsyg.2015.00210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reardon J. P. et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 62, 1208–1216, 10.1016/j.biopsych.2007.01.018 (2007). [DOI] [PubMed] [Google Scholar]
- George M. S. et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry 67, 507–516, 10.1001/archgenpsychiatry.2010.46 (2010). [DOI] [PubMed] [Google Scholar]
- Crottaz-Herbette S. & Menon V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. J Cogn Neurosci 18, 766–780, 10.1162/jocn.2006.18.5.766 (2006). [DOI] [PubMed] [Google Scholar]
- Haupt S., Axmacher N., Cohen M. X., Elger C. E. & Fell J. Activation of the caudal anterior cingulate cortex due to task-related interference in an auditory Stroop paradigm. Hum Brain Mapp 30, 3043–3056, 10.1002/hbm.20731 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr C., Binkofski F., Erdmann C., Buchel C. & Helmchen C. The anterior cingulate cortex contains distinct areas dissociating external from self-administered painful stimulation: a parametric fMRI study. Pain 114, 347–357, 10.1016/j.pain.2004.12.036 (2005). [DOI] [PubMed] [Google Scholar]
- Rottmann S., Jung K., Vohn R. & Ellrich J. Long-term depression of pain-related cerebral activation in healthy man: an fMRI study. Eur J Pain 14, 615–624, 10.1016/j.ejpain.2009.10.006 (2010). [DOI] [PubMed] [Google Scholar]
- Grunwald T. et al. Neuronal substrates of sensory gating within the human brain. Biol Psychiatry 53, 511–519 (2003). [DOI] [PubMed] [Google Scholar]
- Norena A., Cransac H. & Chery-Croze S. Towards an objectification by classification of tinnitus. Clin Neurophysiol 110, 666–675 (1999). [DOI] [PubMed] [Google Scholar]
- White T. P., Joseph V., Francis S. T. & Liddle P. F. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr Res 123, 105–115, 10.1016/j.schres.2010.07.020 (2010). [DOI] [PubMed] [Google Scholar]
- De Ridder D. & Vanneste S. Targeting the parahippocampal area by auditory cortex stimulation in tinnitus. Brain Stimul 7, 709–717, 10.1016/j.brs.2014.04.004 (2014). [DOI] [PubMed] [Google Scholar]
- De Ridder D., Vanneste S. & Congedo M. The distressed brain: a group blind source separation analysis on tinnitus. PLoS One 6, e24273, 10.1371/journal.pone.0024273 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejad A. B., Fossati P. & Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Front Hum Neurosci 7, 666, 10.3389/fnhum.2013.00666 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P. & Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage 57, 1221–1233, 10.1016/j.neuroimage.2011.05.028 (2011). [DOI] [PubMed] [Google Scholar]
- Salomons T. V. et al. Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology 39, 488–498, 10.1038/npp.2013.222 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J. et al. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry 76, 176–185, 10.1016/j.biopsych.2013.10.026 (2014). [DOI] [PubMed] [Google Scholar]
- Fox M. D., Halko M. A., Eldaief M. C. & Pascual-Leone A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). Neuroimage 62, 2232–2243, 10.1016/j.neuroimage.2012.03.035 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. & Barrett J. Transcranial magnetic stimulation (TMS) of the human frontal cortex: implications for repetitive TMS treatment of depression. J Psychiatry Neurosci 29, 268–279 (2004). [PMC free article] [PubMed] [Google Scholar]