Significance
In virtually all human groups, differences in popularity induce social status and shape interactions. How do we recognize that certain individuals are popular—highly liked by the group—even when this collective preference differs from our own? Our results suggest that group members’ popularity is tracked by activity in neural valuation systems, which in turn engage social cognition systems that facilitate understanding others’ mental states. Popular participants’ valuation systems demonstrated enhanced sensitivity to differences among other group members’ popularity. These neural data offer insights into how status guides social behavior and reinforces social network structures, and why the affective valuation and social cognition systems are critical for navigating these networks and achieving high status within them.
Keywords: social status, fMRI, social network, popularity, social cognition
Abstract
Differences in popularity are a key aspect of status in virtually all human groups and shape social interactions within them. Little is known, however, about how we track and neurally represent others’ popularity. We addressed this question in two real-world social networks using sociometric methods to quantify popularity. Each group member (perceiver) viewed faces of every other group member (target) while whole-brain functional MRI data were collected. Independent functional localizer tasks were used to identify brain systems supporting affective valuation (ventromedial prefrontal cortex, ventral striatum, amygdala) and social cognition (dorsomedial prefrontal cortex, precuneus, temporoparietal junction), respectively. During the face-viewing task, activity in both types of neural systems tracked targets’ sociometric popularity, even when controlling for potential confounds. The target popularity–social cognition system relationship was mediated by valuation system activity, suggesting that observing popular individuals elicits value signals that facilitate understanding their mental states. The target popularity–valuation system relationship was strongest for popular perceivers, suggesting enhanced sensitivity to differences among other group members’ popularity. Popular group members also demonstrated greater interpersonal sensitivity by more accurately predicting how their own personalities were perceived by other individuals in the social network. These data offer insights into the mechanisms by which status guides social behavior.
Humans are a fundamentally social species, and the social networks in which we are embedded significantly determine our physical and psychological well-being (1). Effectively navigating interactions within these networks requires efficient mechanisms for processing social information about network members. This ability is so important that it may be among the foremost computational challenges that influenced primate evolution, particularly the dramatic development of our “social brains” (2, 3).
Differences in popularity reflect status inequalities that shape social interaction within virtually all human groups across an enormous array of contexts, from classrooms to military barracks to voluntary associations and beyond (4–8). For decades, social scientists have used sociometric assessment and social network analysis (SNA) to measure the organization of groups and individuals’ positions within them. Using these techniques, the extent to which each group member is collectively liked by group members—termed sociometric popularity—can be quantified (5, 9, 10). Highly likeable individuals attract group members and elicit their affiliation with warmth, altruism, and related traits like agreeableness (5, 10–12). Sociometric popularity disparities arising from asymmetries in group members’ liking ties are present in virtually all human groups and constitute a fundamental basis for status differentiation (4, 5).
The fact that differences in popularity have important behavioral consequences raises the question of how we recognize these differences in the first place. Consider, for example, that in our everyday social networks, we recognize that certain group members are collectively liked more than others, even when this consensus preference differs from our own. Adults and even children can perceive other group members’ asymmetric liking ties, detect differences in their relative popularity, and accordingly orient attention and affiliative behavior toward popular individuals (5–8). Achieving such acute sociometric awareness and attunement to popular group members might feel like second nature to us, yet little is known about the underlying neural mechanisms. Here, we combined functional MRI (fMRI) and SNA to investigate how the human brain tracks the popularity of members of real-world social networks.
To provide new insights into the neural mechanisms that undergird navigation of our complex social worlds, we addressed three interrelated questions. First, which brain systems track real-world popularity? Second, what is the functional organization of those systems? And third, does one’s own status predict more or less neural attunement to others’ status? Although no prior human research has investigated these questions, the extant literature suggests that two distinct types of brain systems may be involved in tracking popularity.
The first is comprised of the ventromedial prefrontal cortex (vmPFC), ventral striatum (VS), and amygdala. These densely interconnected regions (13), henceforth referred to collectively as the “valuation system,” are consistently implicated in processing the affective value and motivational significance of various stimuli, including other people (13–18). Although human neuroscience research has yet to investigate sociometric popularity, nonhuman primate researchers have found that neurons in these regions signal group members’ dominance rank (19–21) and proposed that the vmPFC, VS, and amygdala interact to encode, monitor, and signal other individuals’ social value (22). If tracking group members’ popularity depends on the motivational significance and social value attributed to them, then valuation system activity should track targets’ sociometric popularity.
The second network is comprised of the dorsomedial prefrontal cortex (dmPFC), temporoparietal junction (TPJ), and precuneus. These interconnected regions, henceforth referred to collectively as the “social cognition system,” are consistently activated in neuroimaging studies involving judgments about others’ psychological characteristics, mental states, and intentions (14, 18, 23), or the passive viewing of social stimuli—such as familiar faces—for which we might spontaneously make such attributions (24). Although no neuroscience work has asked how these systems might track sociometric popularity, behavioral research shows that people are particularly concerned with understanding high-status individuals’ mental states (especially how they are viewed by them) and predicting their intentions (25–28). If perceivers are preferentially motivated to understand popular (relative to unpopular) group members’ mental states, then social cognition system activity should scale with targets’ popularity.
Based on these findings, both the valuation and social cognition systems are candidate neural networks for tracking group members’ popularity. Our primary objective was to test these possibilities, recognizing that they are not mutually exclusive. Indeed, the two systems are functionally distinct but their interactions are often critical for diverse social behaviors (14).
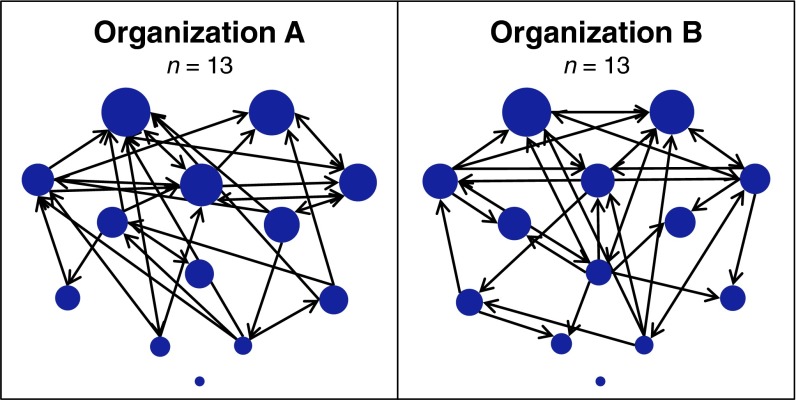
To address these questions, two different groups of well-acquainted participants were recruited from two voluntary student organizations with equivalent size and affiliation network structures (Methods, Fig. 1, and Table S1). Specifically, sociometric popularity was indexed by individuals’ degree prestige within the directed liking network, standardized by group. This measure of popularity aggregates liking ratings received by each group member and thus intuitively reflects how much individuals are collectively liked by their fellow group members (9) (see SI Text for alternative conceptualizations of popularity).
Fig. 1.
Social network structure of study participants (n = 26) in two voluntary student organizations (clubs; participant information detailed in Methods, Table S1, and SI Text). Each network was comprised of 13 well-acquainted members. Each node represents one person. Directional arrows represent group members’ directed liking relations (for visual clarity, only ties in the upper quartile are displayed). Node size reflects sociometric popularity: the extent to which the group collectively likes that person. Sociometric popularity was indexed by degree prestige, which we then standardized by group (Methods). Calculated by simply summing the weights of all liking ties received by an individual, this SNA metric represents an intuitive and straightforward index of popularity (9).
Table S1.
Tabulation of group members included in each study phase
| Study phase | Total | Organization A | Organization B |
| Nonparticipants | 2 (2 F) | 2 (2 F) | — |
| Participants | 26 (12 M, 14 F) | 13 (5 M, 8 F) | 13 (7 M, 6 F) |
| Targets: Incorporated in round-robin fMRI face-viewing task as stimuli | 25 (12 M, 13 F) | 13 (5 M, 8 F) | 12 (7 M, 5 F) |
| Perceivers: Incorporated in round-robin fMRI face-viewing task as subjects | 21 (10 M, 11 F) | 9 (3 M, 6 F) | 12 (7 M, 5 F) |
F, female; M, male.
To model everyday social encounters within face-to-face social networks, we developed a round-robin neuroimaging paradigm in which group members were both the target stimuli presented during the scan and the perceivers that viewed them. A cover task (29) guided perceivers to make simple judgments about briefly presented photographs of target faces.
To provide a strong test of our hypotheses about the neural systems tracking targets’ sociometric popularity, our primary analyses were based on independently identified valuation and social cognition networks that were localized using two additional tasks that participants completed in the same scanning session (Methods and SI Text). We then used combinations of multilevel regression and mediation analyses to ask how activity within each network tracked targets’ sociometric popularity during this face-viewing task, how activity in these systems interacted, and how perceivers’ own popularity impacted their sensitivity to differences in target popularity.
Results
Target Popularity Analyses.
ROI approach.
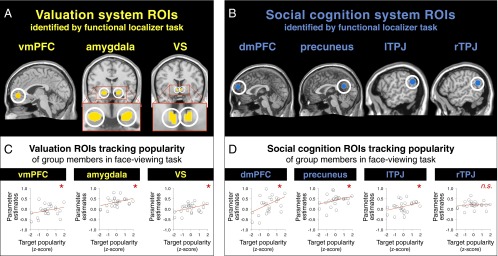
For our primary analysis, we first needed to independently localize regions-of-interest (ROIs) related to affective valuation and social cognition. Following the established analytic approach of previous neuroimaging studies, the monetary incentive delay (MID) task (30) was used to independently localize regions active during anticipation and receipt of monetary rewards (31, 32). The social cognition system localizer was a well-validated person judgment task (33) commonly used to identify regions involved in thinking about others’ mental states and traits, here adapted such that perceivers made judgments about target group members and predicted targets’ judgments of them. [As noted earlier, these are precisely the kinds of judgments that people are preferentially motivated to make about high-status (relative to low-status) targets.] For each functional localizer task we then defined 8-mm radius spherical ROIs surrounding activation peaks that fell within our a priori ROIs (Methods and SI Text). From the MID task we obtained anatomically constrained functional ROIs in the vmPFC, VS, and amygdala (Fig. 2A). The person judgment task revealed clusters with peaks in the dmPFC, precuneus, and bilateral TPJ (Fig. 2B). The activation peaks we found are consistent with previous neuroimaging studies using the MID (30–32, 34) and person judgment tasks (see ref. 23 for review).
Fig. 2.
Popularity of targets (group members presented as stimuli during the face-viewing task) predicted activity in each of the valuation and social cognition ROIs (all Ps < 0.05, as indicated by asterisks) except rTPJ (P > 0.5), even when controlling for perceivers’ own liking of target and other potential confounds (Results, SI Text, and Tables S2 and S3). Core brain regions underlying (A) valuation and (B) social cognition—and corresponding ROIs—were identified using two independent functional localizer tasks (Methods and SI Text). Each task identified a set of commonly coactivated and strongly interconnected regions that are referred to collectively as the valuation and social cognition systems, respectively. Illustrations of the parametric relationship between target popularity and βs extracted from (C) valuation system ROIs and (D) social cognition system ROIs. Note that activity is averaged across perceivers for visual clarity.
We then asked whether activation within these independently localized valuation and social cognition ROIs scaled with the popularity of targets presented in the face-viewing task. To answer this question we used multilevel models regressing activation parameter estimates (βs) extracted from each ROI against target popularity, controlling for each perceiver’s liking of targets to ensure that analyses reflect neural sensitivity to how much target group members are collectively liked by the group and not merely individually liked by the perceiver. These analyses (SI Text) revealed that target popularity was positively associated with activity in ROIs independently identified by the valuation (Fig. 2C) (vmPFC, amygdala, VS) and social cognition (Fig. 2D) [dmPFC, precuneus, left (l) TPJ] localizer tasks (Ps < 0.05). The only ROI in which activity did not track target popularity was the right (r) TPJ (P > 0.5), and was therefore not included in the subsequent analyses. To rule out alternative explanations, we conducted additional regression analyses controlling for perceiver–target relational characteristics (e.g., relationship duration, subjective interpersonal closeness) and target attributes (e.g., sex, facial attractiveness). The positive association between target popularity and βs from each ROI remained significant even when/after controlling for these potential confounds (Ps < 0.05), and did not differ between groups (Ps > 0.2) (see SI Text and Tables S2 and S3 for full list of potential confounds tested and regression results). Activity in valuation and social cognition systems was predicted by target popularity, whether or not perceiver’s own liking of target was partialled out (Ps < 0.01) (SI Text).
Table S2.
Valuation ROIs tracking target popularity during the face-viewing task: Results of linear mixed-effects models (one model for each ROI) regressing activation parameter estimates against target popularity and covariates (i.e., to rule out alternative explanations and potential confounds)
| Variable | vmPFC | amygdala | VS | |||||||||
| Est. | SE | df | P | Est. | SE | df | P | Est. | SE | df | P | |
| Target variables | ||||||||||||
| Popularity | 0.171 | 0.062 | 195.0 | 0.006 | 0.106 | 0.042 | 194.0 | 0.013 | 0.099 | 0.047 | 195.7 | 0.038 |
| Facial trustworthiness | −0.019 | 0.005 | 190.1 | 0.001 | −0.009 | 0.004 | 190.1 | 0.016 | −0.004 | 0.004 | 191.7 | 0.350 |
| Facial attractiveness | 0.012 | 0.005 | 189.2 | 0.013 | 0.007 | 0.003 | 189.5 | 0.049 | 0.003 | 0.004 | 190.9 | 0.394 |
| Male | −0.342 | 0.107 | 184.9 | 0.002 | −0.151 | 0.072 | 186.2 | 0.039 | −0.120 | 0.081 | 187.4 | 0.142 |
| Age | 0.019 | 0.024 | 185.3 | 0.436 | 0.017 | 0.017 | 186.6 | 0.311 | −0.006 | 0.019 | 187.8 | 0.729 |
| Relational variables | ||||||||||||
| Personal liking | −0.007 | 0.004 | 203.0 | 0.063 | −0.003 | 0.003 | 202.0 | 0.224 | 0.000 | 0.003 | 202.9 | 0.983 |
| Perceived closeness | 0.002 | 0.003 | 196.7 | 0.497 | 0.002 | 0.002 | 202.2 | 0.390 | 0.001 | 0.002 | 199.6 | 0.552 |
| Perceived similarity | 0.004 | 0.003 | 199.7 | 0.183 | 0.002 | 0.002 | 197.6 | 0.266 | 0.002 | 0.002 | 199.5 | 0.311 |
| Length of relationship | −0.012 | 0.014 | 188.8 | 0.414 | −0.002 | 0.010 | 199.6 | 0.838 | −0.015 | 0.011 | 194.4 | 0.189 |
| Hours per week spent together | 0.044 | 0.028 | 203.0 | 0.118 | 0.008 | 0.019 | 202.0 | 0.694 | 0.003 | 0.021 | 202.9 | 0.870 |
| Frequency of contact | 0.005 | 0.014 | 196.2 | 0.739 | −0.005 | 0.010 | 194.4 | 0.616 | −0.008 | 0.011 | 196.5 | 0.432 |
| (Intercept) | 0.106 | 0.715 | 192.1 | 0.882 | 0.106 | 0.486 | 194.8 | 0.828 | 0.300 | 0.546 | 194.2 | 0.583 |
Boldface values connote P < 0.05, two-tailed. df reflects degrees of freedom calculated using the Kenward–Roger method (57).
Table S3.
Social cognition ROIs tracking target popularity during the face-viewing task: Results of linear mixed-effects models (one model for each ROI) regressing activation parameter estimates against target popularity and covariates (i.e., to rule out alternative explanations and potential confounds)
| Variable | dmPFC | Precuneus | lTPJ | rTPJ | ||||||||||||
| Est. | SE | df | P | Est. | SE | df | P | Est. | SE | df | P | Est. | SE | df | P | |
| Target variables | ||||||||||||||||
| Popularity | 0.223 | 0.087 | 195.6 | 0.011 | 0.162 | 0.059 | 196.0 | 0.007 | 0.171 | 0.069 | 197.6 | 0.014 | 0.065 | 0.050 | 197.7 | 0.195 |
| Facial trustworthiness | −0.020 | 0.007 | 191.2 | 0.007 | −0.012 | 0.005 | 191.6 | 0.016 | −0.009 | 0.006 | 193.2 | 0.133 | −0.006 | 0.004 | 193.6 | 0.154 |
| Facial attractiveness | 0.015 | 0.007 | 190.3 | 0.034 | 0.012 | 0.005 | 190.7 | 0.011 | 0.007 | 0.005 | 192.2 | 0.177 | 0.005 | 0.004 | 192.7 | 0.200 |
| Male | −0.454 | 0.150 | 186.4 | 0.003 | −0.325 | 0.102 | 186.8 | 0.002 | −0.215 | 0.119 | 188.2 | 0.071 | −0.221 | 0.086 | 189.0 | 0.011 |
| Age | 0.016 | 0.034 | 186.9 | 0.640 | −0.025 | 0.023 | 187.2 | 0.292 | −0.042 | 0.027 | 188.4 | 0.123 | −0.025 | 0.020 | 189.3 | 0.209 |
| Relational variables | ||||||||||||||||
| Personal liking | −0.005 | 0.005 | 203.0 | 0.364 | −0.008 | 0.004 | 202.9 | 0.033 | −0.004 | 0.004 | 201.9 | 0.386 | −0.003 | 0.003 | 202.3 | 0.357 |
| Perceived closeness | 0.007 | 0.004 | 198.1 | 0.103 | 0.004 | 0.003 | 196.7 | 0.229 | 0.000 | 0.003 | 190.7 | 0.884 | 0.001 | 0.002 | 193.0 | 0.626 |
| Perceived similarity | 0.000 | 0.004 | 199.8 | 0.955 | 0.002 | 0.003 | 200.2 | 0.478 | 0.000 | 0.003 | 201.7 | 0.934 | 0.000 | 0.002 | 201.5 | 0.924 |
| Length of relationship | −0.002 | 0.020 | 191.4 | 0.931 | −0.002 | 0.014 | 189.1 | 0.901 | 0.020 | 0.016 | 179.7 | 0.214 | 0.002 | 0.011 | 183.5 | 0.859 |
| Hours per week spent together | 0.019 | 0.039 | 203.0 | 0.627 | 0.031 | 0.027 | 202.9 | 0.249 | −0.015 | 0.031 | 201.8 | 0.634 | 0.009 | 0.022 | 202.2 | 0.692 |
| Frequency of contact | −0.015 | 0.020 | 196.5 | 0.457 | 0.003 | 0.013 | 197.1 | 0.802 | 0.000 | 0.015 | 199.1 | 0.989 | 0.002 | 0.011 | 199.0 | 0.857 |
| (Intercept) | 0.329 | 1.004 | 193.2 | 0.744 | 1.542 | 0.685 | 193.1 | 0.025 | 1.589 | 0.793 | 193.0 | 0.046 | 1.179 | 0.574 | 193.8 | 0.041 |
Boldface values connote P < 0.05, two-tailed. df reflects degrees of freedom calculated using the Kenward–Roger method (57).
Whole-brain approach.
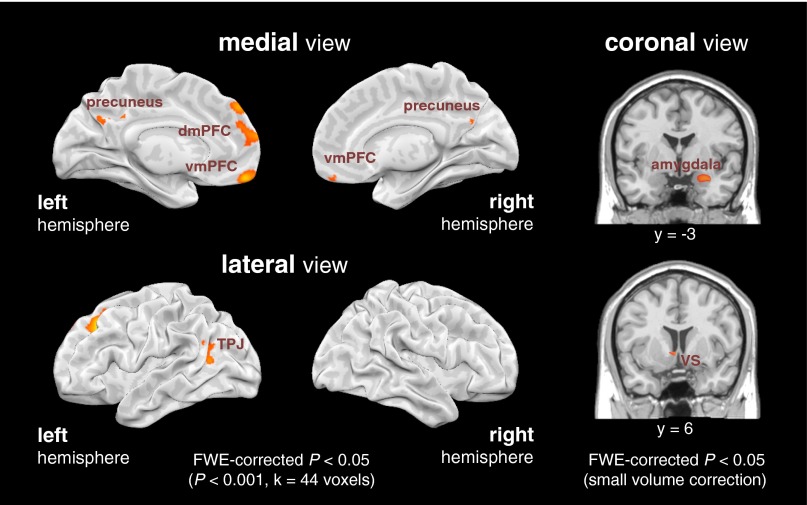
To validate these results and complement our hypothesis-driven ROI analyses with a data-driven analytic approach, we also conducted a random-effects, parametric whole-brain regression analysis at the group level. This analysis replicated the ROI-based analysis: the same core valuation (vmPFC, amygdala, VS) and social cognition (dmPFC, precuneus, lTPJ) regions tracked significantly with target popularity, even when controlling for the aforementioned potential confounds (SI Text, Fig. S1, and Table S4). It is worth noting that the whole-brain analysis used a two-tailed hypothesis to allow for testing of brain regions in which activity tracked negatively with target popularity; however, no such regions were found. (See Fig. S2 for comparison of the distinct neural correlates of target popularity and liking.)
Fig. S1.
Parametric whole-brain regression analysis isolating brain regions tracking target popularity during the face-viewing task (Table S4). Clusters were thresholded at P < 0.001, k = 44 voxels (whole-brain FWE-corrected, P < 0.05, two-tailed) except as noted below. For the VS and amygdala, subcortical structures of a priori interest, results were thresholded with small volume correction, FWE-corrected P < 0.05, two-tailed. Replicating the findings of the ROI-based approach, activity in the same core valuation (vmPFC, amygdala, VS) and social cognition (dmPFC, precuneus, lTPJ) regions tracked significantly with target popularity. The analysis also revealed a cluster in middle frontal gyrus, but was otherwise selective for our a priori hypothesized ROIs. Although the whole-brain analysis used a two-tailed hypothesis to allow for testing of brain regions in which activity tracked negatively with target popularity, no such regions were found.
Table S4.
Regions parametrically tracking target popularity during the face-viewing task, as identified by random-effects whole-brain analysis
| Region | MNI coordinates | t values | |||||
| x | y | z | k | Max | Mean | ||
| vmPFC/medial frontal gyrus (BA 11) | M | −6 | 51 | −15 | 81 | 6.76 | 4.61 |
| dmPFC/superior frontal gyrus (BA 10) | M | −9 | 60 | 18 | 54 | 6.19 | 4.41 |
| TPJ/superior temporal gyrus (BA 39) | L | −39 | −54 | 21 | 65 | 5.46 | 4.29 |
| Precuneus (BA 7) | M | 0 | −48 | 39 | 60 | 4.71 | 4.21 |
| Middle frontal gyrus (BA 8) | L | −24 | 30 | 39 | 189 | 7.43 | 4.84 |
| Amygdala* | R | 33 | −3 | −21 | — | 4.34 | 3.74 |
| Ventral striatum/caudate* | L | −6 | 3 | −3 | — | 4.36 | 3.34 |
Coordinates (in MNI space) refer to the peak activation in each cluster. All clusters were thresholded at P < 0.001, k = 44 voxels (whole-brain FWE-corrected P < 0.05, two-tailed), except as noted below. For subcortical structures of a priori interest, an asterisk reflects thresholding with small volume correction, FWE-corrected P < 0.05, two-tailed.
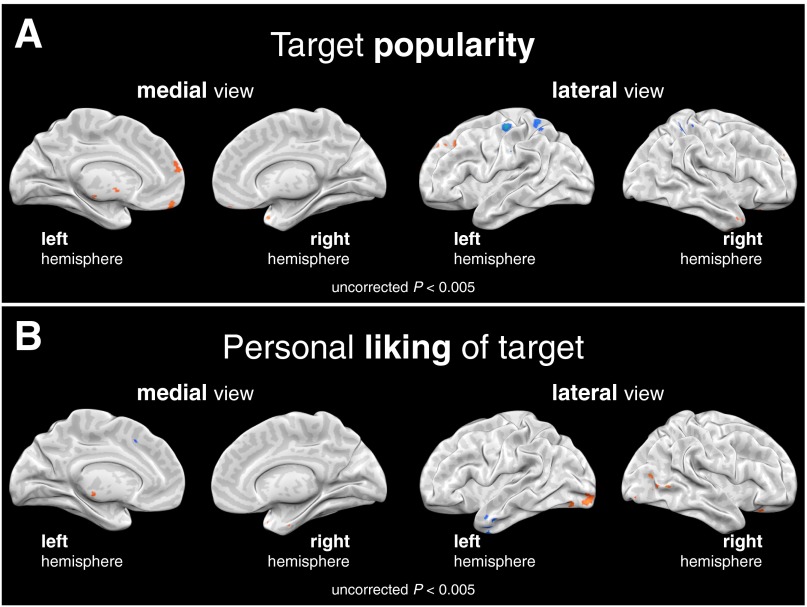
Fig. S2.
Primarily distinct patterns of neural activity associated with tracking (A) popularity of targets and (B) each perceiver’s own liking of targets during the face-viewing task. To enable more comprehensive comparison, parametric whole-brain regression analyses are presented with relaxed cluster threshold (uncorrected P < 0.005; orange and blue signify relative activation and deactivation, respectively). The results of this analysis provide empirical support for the conceptual distinction between sociometric popularity—the group’s collective preference—and the individual preferences from which it is comprised. This is further corroborated by the observation that the perceiver’s own liking of a target explains less than 5% of the variance in everyone else’s liking for that target.
Mediation Analyses.
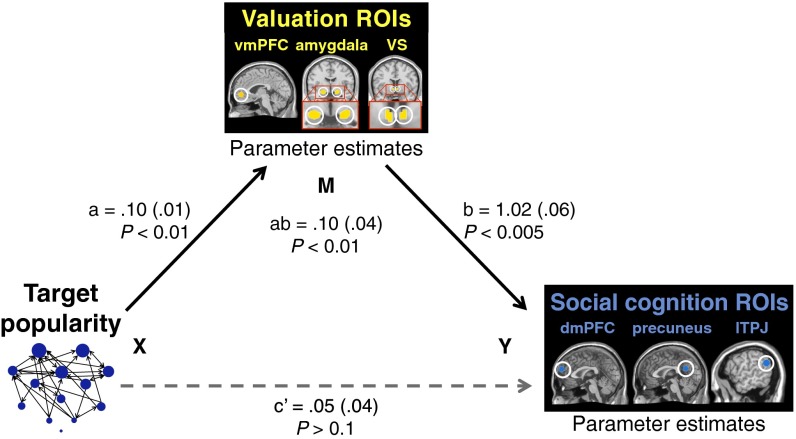
The observed correlations between target popularity and activity in valuation and social cognition regions confirmed our primary hypotheses, which led to our second question: Do the two systems track popularity in parallel (independently) or serially, with one system assuming a primary role that mediates the popularity–activity relationship for the other? We predicted the valuation system would function as mediator based on the aforementioned literatures in social psychology [i.e., it is high-status individuals’ social importance that motivates others to predict their mental states (25–28)] and nonhuman primate neurophysiology [i.e., neurons in valuation regions encode social value and signal presence of high-status group members (19–22)]. To test this prediction, we performed multilevel mediation analyses, assessing whether valuation system activity explains the observed relationship between target popularity and social cognition system activity. (βs extracted from the vmPFC, amygdala, and VS—ROIs that had been independently localized by the MID task—were averaged together to compute a composite measure of valuation system activity during the face-viewing task; likewise, βs extracted from the dmPFC, precuneus, and lTPJ—ROIs that had been independently localized by the person judgment task—were aggregated for a composite measure of social cognition activity.) We found that valuation activity did in fact significantly mediate this relationship (P < 0.01) (Fig. 3 and SI Text). [Moreover, additional analyses indicated this model had greater strength of evidence than did the sum total of (i) the alternative serial organization in which social cognition system activity operated as the mediator, and (ii) the parallel organization in which the two systems’ activity independently tracked target popularity (SI Text).] These results suggest that (i) a primary representation of sociometric popularity is value-based or motivational in nature, and (ii) social cognitive systems may be engaged in the presence of popular group members to the extent that valuation systems signal their motivational significance. In such cases, social cognition systems may ready perceivers for effective interaction by supporting retrieval of knowledge about what target individuals are like and how they view us (precisely the two kinds of judgments elicited by the social cognition functional localizer task). This knowledge is useful for predicting high-status individuals’ behavior and deciding how to act accordingly (25–28).
Fig. 3.
Activity in the valuation system (vmPFC, amygdala, and VS ROIs independently localized by the MID task) mediated the observed relationship between target popularity and social cognition system activity (dmPFC, precuneus, and lTPJ ROIs independently localized by the person judgment task), with 64.6% of the total effect mediated (P < 0.01). See Methods, Fig. 2, and SI Text for details on how these systems were defined and independently localized. Further analyses confirmed that the data supported this mediation model over both (i) the alternative serial organization in which social cognition system activity operated as the mediator, and (ii) the parallel organization in which the two systems’ activity independently tracked target popularity (Results and SI Text).
Perceiver Popularity Analyses.
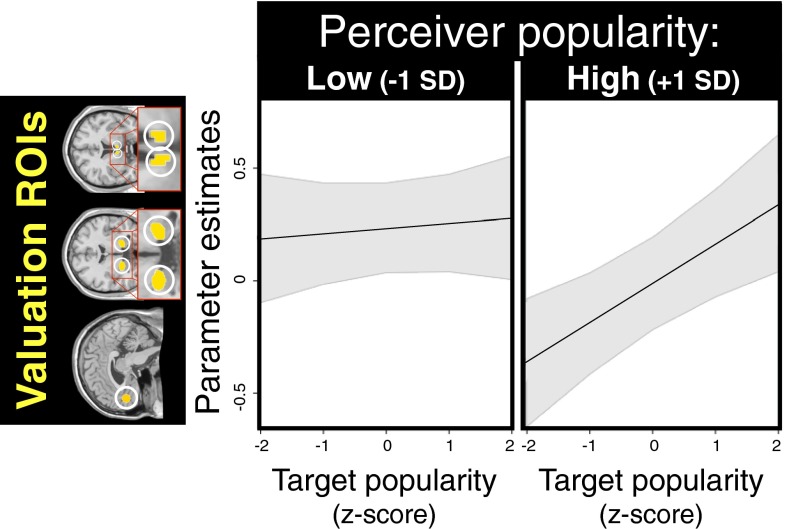
The finding that valuation system activity directly tracked target popularity led to our third question: Does the strength of this relationship (i.e., attunement to group members’ popularity differences) relate to one’s own popularity? In studies both of adults and children, popular individuals have more accurate perceptions of the affiliative social network structure that underlies differences in popularity (7, 35, 36). In addition, human and nonhuman primate experiments have shown that although low-status individuals pay attention to group members of any status, high-status group members attend selectively to one another (6, 37). Therefore, we hypothesized that (i) perceiver popularity would amplify the effect of target popularity on valuation system activity, [i.e., that valuation system activity of popular (relative to unpopular) perceivers would be more sensitive to status differences among group members], and (ii) this effect would be driven by popular perceivers’ attenuated responses to less popular targets.
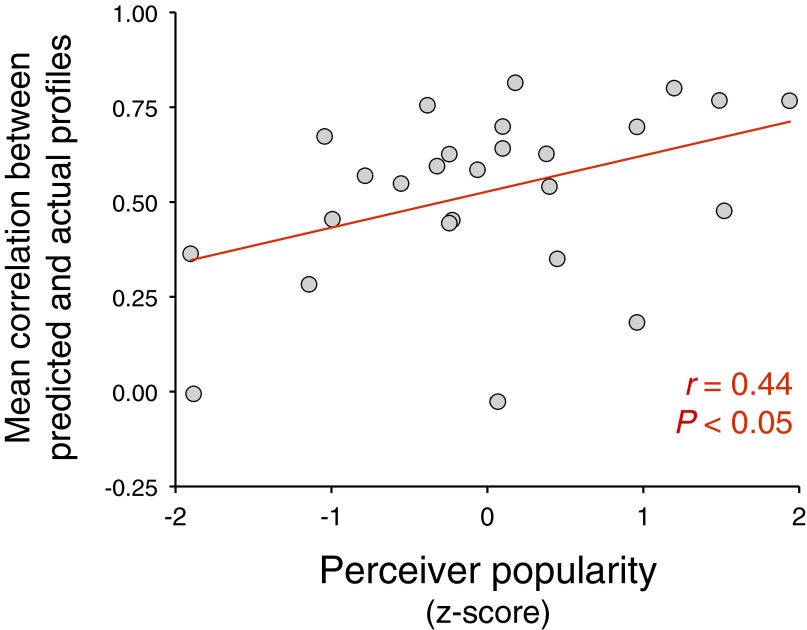
We tested this prediction with a multilevel model regressing valuation system βs against target popularity, perceiver popularity, and their interaction term (as well as additional models with the aforementioned covariates) (SI Text). We found that in addition to the main effect of target popularity (parameter estimate ± SE: 0.100 ± 0.037, P < 0.01), there was also an interaction such that the effect of target popularity on valuation activity was amplified for more popular perceivers (Fig. 4 and SI Text) (0.077 ± 0.037, P < 0.05). In other words, the valuation systems of popular perceivers were better calibrated to detecting the status differences among group members. This result is not an artifact of popular perceivers liking more popular targets. Consistent with our hypothesis and the aforementioned human and nonhuman primate findings (6, 37), the interaction effect was largely driven by an attenuation of responses to less-popular targets in popular, but not unpopular, perceivers (Fig. 4). Moreover, the main effect of perceiver popularity showed a nonsignificant trend in the opposite (i.e., negative) direction (0.122 ± 0.078, P = 0.13). Considered in tandem, these results suggest that popular individuals demonstrate enhanced interpersonal sensitivity (i.e., attunement to group members’ status differences), whereas unpopular individuals show more generalized interpersonal responsiveness (i.e., elevated valuation responses to all group members regardless of status). In support of the inference that popular perceivers have heightened interpersonal sensitivity (7, 35, 36), we also found that they were more accurate in predicting how each of the other group members perceived them across various personality attributes (SI Text and Fig. S3).
Fig. 4.
Interaction plot depicting popular (+1 SD, relative to −1 SD unpopular) perceivers’ enhanced attunement to group members’ status differences (shaded area represents 95% confidence interval). The main effect of target popularity on valuation activity (P < 0.01) was amplified for more popular perceivers (P < 0.05), suggesting their valuation systems were more sensitively calibrated to detecting status differences among group members. In contrast, there was a nonsignificant main effect trend of perceiver popularity in the opposite (i.e., negative) direction (P = 0.13), suggesting the valuation systems of unpopular individuals demonstrate greater generalized interpersonal responsiveness (i.e., elevated responses to all group members regardless of status). Additional details provided in Results and SI Text.
Fig. S3.
Popular perceivers were more accurate in their predictions of how individual members of their organization assessed their personality across all trait items (r = 0.44; P < 0.05, two-tailed; n = 26). We computed the Pearson correlation between each perceiver’s predicted personality profile (i.e., the perceiver’s predictions about how a specific group member would judge the perceiver on various personality attributes) and the corresponding individual’s actual personality profile of the perceiver (i.e., how that particular group member actually judged the perceiver on various personality attributes). In other words, a single correlation coefficient was computed for each pair of predicted-actual personality profiles across all trait items. These dyadic pairings of matched predicted-actual profile relationships were transformed from Pearson correlation coefficients to Fisher z-scores using Fisher’s r-to-z transformation and aggregated to compute each perceiver’s average profile relationship (across the perceiver’s 12 predicted-actual profile relationships, one for each of the other 12 group members). The resulting individual-difference measure of social acuity (i.e., perceiver mean correlations plotted along the y axis) reflects Fisher z-scores that have been transformed back into Pearson correlations to aid interpretation.
Discussion
Taken together, the present results provide, to our knowledge, the first examination of neural mechanisms tracking popularity. Using a naturalistic face-viewing task, we identified two kinds of neural systems activated during encounters with members of real-world social networks. Affective valuation regions may assign motivational significance to group members based on their sociometric popularity and, in turn, may mediate engagement of social cognition regions that support understanding their mental states.
This neural mechanism presents adaptive features for navigating interactions within complex social networks. Tracking group members’ status serves vital functions supported by valuation regions, such as assigning motivational importance to particular individuals, monitoring and detecting their presence, and signaling they deserve privileged status in attention and decision-making (17, 19–22). In an experimental demonstration of this principle, rhesus macaques were willing to sacrifice fruit juice to view faces of high-status group members, while requiring overpayment of juice to view low-status monkeys’ faces (38). Given the valuation system’s critical role in reward processing and reinforcement learning (13), this mechanism may also provide intrinsically rewarding reinforcement that motivates proximity and preferential attention to popular individuals as well as incentivizing interactions with them (5, 6, 8, 12, 22). At the group-level, this neural mechanism may help stabilize social networks over time, thereby contributing to the self-reinforcing nature of social status and hence the reproduction of social structure (39).
The mediation analysis suggests that the valuation system translates group members’ popularity into motivational value signals that mediate activation of social cognition systems critical for explicit attributions about group members’ psychological states and characteristics. Given our motivation to understand high-status individuals’ mental states and predict their behavior (25–28), this neural mechanism may be both adaptive and socially advantageous: upon observing popular group members, it could proactively set in motion social-cognitive processes that facilitate social interaction.
The social advantage of this neural mechanism is further suggested by the results of our individual-differences analysis showing that perceivers’ own popularity correlated with how strongly their valuation systems tracked network members’ popularity. These intriguing findings are consistent with two views of how perceivers’ own status relates to their perceptions of others. One view comes from the social psychological literature on power, which suggests that having low power or subordinate status imbues other people with heightened relevance that motivates more careful attention to them and their perspectives (25, 40, 41). Our data suggest that differences in popularity may function in a similar way: as illustrated in Fig. 4, unpopular perceivers (Fig. 4, Left) demonstrated elevated valuation responses to all group members regardless of their status; by contrast, popular individuals (Fig. 4, Right) demonstrated valuation responses that scaled with targets’ status. These results dovetail with evidence that although low-ranking monkeys and unpopular humans pay attention to group members of any status, their high-status counterparts attend selectively to one another (6, 37). Another view consistent with our data is that popular individuals achieve their status because they are particularly skilled social perceivers. At the behavioral level, heightened interpersonal acuity has been linked to popularity in social networks of children (7) and adults (35, 36), and we likewise found that popular individuals more accurately predicted how individual group members viewed them (Fig. S3). The findings in Fig. 4 could thus be interpreted as evidence at the neural level of popular individuals’ enhanced social attunement (i.e., that their valuation systems were better calibrated to the social structure). On this view, perceivers’ valuation responses to others might not reflect a consequence of perceivers’ own status, but rather a determinant of how much status they ultimately achieve.
Consistent with this account, which causally prioritizes valuation regions’ functioning as influencing status, primate and rodent studies have shown that lesions to the orbital PFC and amygdala resulted in disrupted social behavior and loss of status, and manipulation of serotonergic neurotransmission and synaptic efficacy in the mPFC influenced social skills, affiliative behavior, and changes in status (42). Although such experimental manipulations cannot be conducted in human research, the paradigm advanced here could be implemented longitudinally to investigate whether individual differences in the valuation system’s social sensitivity are important determinants or consequences of one’s ability and motivation to affiliate with group members and achieve status. Understanding the causal mechanisms underlying such individual differences in humans could have implications for clinical conditions such as depression and developmental disorders such as autism spectrum disorders, in which diminished interpersonal sensitivity, affiliative motivation, and social interaction have been linked to atypical valuation system structure and function (43, 44).
More broadly, our findings are consistent with prior research showing that other aspects of network membership may also relate to the structure and function of valuation and social cognition systems. Recent studies (reviewed in ref. 3) have reported that individuals’ social network size and complexity correlated with gray matter in the vmPFC (45, 46), amygdala (47, 48), and lTPJ (45). Moreover, individual macaques’ gray matter in the mPFC and regions approximating human TPJ covary with both social network size (which was experimentally assigned) and social status (49, 50). These findings support the proposition that affective valuation and social cognition systems are critical for navigating complex social networks and achieving high status within them.
Here it is important to note that prior neuroimaging studies examining processing of another dimension of social status—dominance—have not consistently implicated the valuation and social cognition systems observed here, but rather regions of the lateral PFC and inferior parietal lobe (51). These differing findings could reflect the possibility that the relative dominance and sociometric popularity of group members are represented by different types of brain systems. However, they could also reflect differences in methodology. Whereas our stimuli depicted members of participants’ real-world groups to study naturally occurring variability in social status, other human neuroimaging studies focusing on dominance have tended to experimentally manipulate social status with less naturalistic stimuli (see ref. 51 for review). [Note that the nonhuman primate studies in which valuation regions were found to track group members’ status (19–21) also used similarly naturalistic stimuli (i.e., faces of group members).] Our expectation is that for voluntary identity groups of comparable scale (ranging from 8–79), where similar structural dynamics are observed, the findings reported here should be robust (4, 52). As the scale increases, mutual observation becomes impossible. Consequently the structuring dynamics of networks change (53). Similarly, in groups with strong formal hierarchies, different dynamics may be observed. Future work could address these and other questions about the neural mechanisms that track popularity, specifically, and other kinds of social status in a wide range of social networks more generally.
In conclusion, this study advances an experimental paradigm that models group members’ everyday encounters using a naturalistic task and personalized stimuli. In so doing, we provide an interdisciplinary framework that integrates theories and methods from social psychology, neuroscience (fMRI), and sociology (SNA) to enable research on the brain mechanisms underlying person perception and social cognition processes in real-world, status-laden social networks.
Methods
Participants.
Participants were 26 healthy young adults (12 male, 14 female; mean age = 28.7 y, SD = 2.3) recruited from two different voluntary student club organizations with equivalent size and affiliation network structures (13 members from each) (Fig. 1 and Table S1) at a large university in the United States. Initial recruitment yielded 100% member response rate in both organizations, however not all met the inclusion criteria (detailed in SI Text) to participate in each of the study phases. Of 28 total individuals comprising both groups, 26 (93%) were eligible, willing, and able to participate in the study; among the 26 participants, all 26 (100%) completed the initial session in which the social network instruments were administered, 25 (96%) were photographed and incorporated as targets (face stimuli) in the subsequent fMRI face-viewing task, and 21 (81%) constituted perceivers who completed the fMRI scanning session (Table S1).
Beyond these core participants, 40 additional participants were recruited via Mechanical Turk to provide normative ratings of stimuli used in the fMRI face-viewing task (SI Text). All participants received monetary compensation and provided informed consent following the standards of the Columbia University Institutional Review Board. Additional recruitment and participant information is provided in SI Text.
Procedure and Design.
The study was comprised of two sessions. In a preliminary session, sociometric instruments and self-report questionnaires were administered, and photographs were taken of participants’ faces (to be used subsequently in the fMRI face-viewing task). In a second session, participants underwent fMRI scanning while completing several tasks described below. For all computerized tasks in both sessions, stimulus presentation and behavioral data acquisition were controlled using E-Prime 2.0 (Psychology Software Tools). For tasks completed in the fMRI scanning session, visual stimuli were displayed on a projection screen using a LCD projector and viewed via a rear-projecting mirror.
Sociometric Assessment and SNA.
Sociometric assessments of group members’ affiliative relations and resulting network structure were collected from participants during the first session. These assessments were conducted via a computerized peer-rating paradigm in which participants rated how much they liked each group member (presented in randomized order) on a sliding visual analog scale anchored by the labels “not very” and “very” on opposite ends. This sociometric instrument provided a continuous measure of personal liking (i.e., affiliation tie strength) between group members that was used as a covariate in analyses (Results and SI Text) and also to compute each group member’s popularity. Specifically, sociometric popularity was indexed by individuals’ degree prestige (alternatively referred to as “indegree centrality”) within the directed liking network (9), which we then standardized by group. In other words, liking ratings received by each group member were summed for that individual and then standardized to z-scores within group. Using these sociometric assessments and network analyses thus generated a popularity index that reflects how much individuals are collectively liked by their fellow group members.
Round-Robin fMRI Face-Viewing Task.
Stimuli for the fMRI face-viewing task were prepared from photographs of participants. During the preliminary session, participants’ faces were photographed with affectively neutral facial expression and gaze directed straight at the camera. These photographs were cropped and converted to grayscale images with equal luminance. In addition, a “ghost face” stimulus image representing the superimposition of all group members’ faces was prepared for each group following methods used in prior face perception research (29). The face-viewing task implemented a rapid event-related design that included 10 repetitions of each stimulus face presented in pseudorandomized order. Faces were presented for 1,000 ms and interstimulus intervals (ISIs) consisting of white fixation cross on black background were jittered between 1,500 ms and 11,500 ms (mean duration of ISI = 3,500 ms). Perceivers viewed faces of targets while performing a simple cover task (29) to maintain their alertness throughout. Specifically, participants were instructed to press a button with their pointer (second) finger each time a group member’s face was presented and a different button with their ring (fourth) finger each time a “ghost face” was presented (∼9% of total presentations).
Independent Functional Localizer Tasks for Valuation and Social Cognition Systems.
Two functional localizer tasks were completed at the end of the scanning session (methods detailed in SI Text). Participants completed the MID task (30) to independently identify valuation regions active during the anticipation and receipt of monetary rewards (31, 32). Trials in which participants won monetary rewards were contrasted with those in which they could not (win trials > neutral trials), encompassing both the anticipation and feedback phases of each trial. This analysis (thresholded at P < 0.05, corrected) revealed activation peaks consistent with previous studies using the MID task (30–32, 34) in regions of a priori interest: the vmPFC (−3, 48, −6), VS (0, 9, −3), and amygdala (−21, −6, −12 and 18, −3, −12). We then defined spherical ROIs with a radius of 8 mm around these peaks (31, 32) (Fig. 2A) [for the VS and amygdala, spherical ROIs were then anatomically constrained using structural masks obtained from FSL (SI Text)].
We used a well-validated person judgment task adapted from ref. 33 as an independent functional localizer to identify social cognition regions supporting two kinds of judgments relevant in interactions with group members: evaluating target group members’ mental states and traits [e.g., “to what extent is (target) helpful?”] and predicting how targets perceive them [e.g., “to what extent does (target) see me as lonely?”]. Specifically, we conducted a whole-brain conjunction analysis (thresholded at P < 0.05, corrected) to localize activation present in both you-about-other and other-about-you trials relative to active baseline curved line trials. This analysis revealed clusters with activation peaks in regions of a priori interest that were consistent with previous neuroimaging studies using similar social cognitive tasks (see ref. 23 for review): dmPFC (0, 60, 21), precuneus (−3, −57, 21), and left (−60, −60, 24) and right TPJ (54, −60, 21). As with the valuation localizer, we defined spherical ROIs with a radius of 8 mm around the observed activation peaks (Fig. 2B and SI Text).
Imaging Acquisition and Analysis.
Whole-brain fMRI data were acquired on a 1.5 Tesla GE system. High-resolution anatomical images with 1-mm × 1-mm × 1-mm resolution were acquired with a T1-sensitive SPGR sequence at the end of the scan session. Functional images were acquired with a T2*-sensitive EPI blood oxygenation-level dependent sequence. Scanning parameters and further details are included in SI Text.
SI Text
Participant Recruitment and Inclusion Criteria.
Participants were 26 healthy young adults (12 male, 14 female; mean age = 28.7 y, SD = 2.3) recruited from two different voluntary student club organizations with equivalent size and affiliation network structures (13 members from each) (Methods, Fig. 1, and Table S1) at a large university in the United States. Critically, this recruitment achieved several objectives. First, face-to-face organizations of this size were necessary to ensure that group members were sufficiently well-acquainted with one another (mean duration of relationship = 8.5 mo, SD = 5.0). Second, for groups of this size it was feasible for each participant’s face to be presented as a stimulus (target) with 10 repetitions (multiple repetitions being necessary to estimate blood flow responses to each face) during the face-viewing task in the scan session. Third, selecting two such groups with equivalent size and affiliation network structures enabled us to aggregate their data and analyze between-subject (perceiver) popularity effects.
All participants provided informed consent, were English-speaking, and had normal or corrected-to-normal vision. Participants were screened for a history of serious neuropsychiatric disorders, head injury, and other conditions that prevented scanning (e.g., a pacemaker, claustrophobia) before taking part in the fMRI scanning session.
Forty additional participants were recruited via Mechanical Turk to provide normative ratings of stimuli used in the fMRI face-viewing task. Twenty of these participants (7 male, 13 female; mean age = 35.9 y, SD = 14.6) rated faces based on attractiveness, and another 20 (10 male, 10 female; mean age = 34.8 y, SD = 12.9) rated faces based on trustworthiness. These normative ratings of stimulus faces could then be used as covariates (to rule out potential confounds associated with target facial attributes) in subsequent analyses described below and in Results.
Conceptualizing Sociometric Popularity as a Basis for Status.
The conceptualization of sociometric popularity (i.e., the extent to which each group member is collectively liked) as a basis for status dates back more than eight decades to Moreno’s pioneering work in sociometry (5) and has persisted since then in the research traditions of both sociometry and social network analysis (4, 7, 8, 10, 54–56). Another research tradition concerned primarily with adolescents has also investigated perceived popularity, the extent to which individuals are labeled as “popular” by their peers (54–56). Whereas sociometric popularity is achieved by attracting group members and eliciting their affiliation (5, 8, 10), perceived popularity—much like status based on dominance or power—can be attained by using relational and overt aggression to dominate peers (54–56).
In this study we chose to focus on sociometric popularity because it is a fundamental aspect of status that emerges organically (because of asymmetries in liking ties between group members) in virtually all human groups across an enormous array of contexts, from classrooms to military barracks to voluntary associations (4, 5). Our participants (mean age = 28.7 y, SD = 2.3) (Methods and Table S1) were beyond the stages of childhood and adolescence in which perceived popularity is typically studied (54–56).
Imaging Acquisition and Analysis.
Whole-brain fMRI data were acquired on a 1.5 Tesla GE system. Functional images were acquired with a T2*-sensitive EPI blood oxygenation-level dependent (BOLD) sequence using the following parameters: TR = 2,000 ms; TE = 34 ms; flip angle = 90°; field of view = 22.4 cm × 22.4 cm; matrix array size = 64 × 64; each volume consisted of 28 slices with slice thickness = 4 mm and no interslice gap. High-resolution anatomical images with 1-mm × 1-mm × 1-mm resolution were acquired with a T1-sensitive SPGR sequence at the end of the scan session.
In each of the two runs comprising the face-viewing task, 167 volumes (for participants in Organization A) and 157 volumes (for participants in Organization B) were acquired. (The difference in volumes acquired was because of the fact that the face task for Organization A included one more target than it did for Organization B.) For both groups, the person judgment task consisted of four runs of 230 volumes each, and the MID task consisted of one run of 115 volumes. The initial four “dummy” volumes of each run were discarded before analysis.
Functional images were preprocessed using SPM8 software (Wellcome Department of Cognitive Neurology, University College London), including slice-timing correction, motion-correction, realignment, coregistration between each participant’s functional and anatomical data, normalization to a standard template (Montreal Neurological Institute; MNI) using segmentation parameters, 3-mm isometric voxels, and spatial smoothing using a Gaussian kernel (full-width at half-maximum = 6 mm).
Data for each of the tasks were subjected to a first level of regression, separately for each subject, using an ordinary least-squares general linear model (GLM) implemented with Neuroelf v0.9c software (neuroelf.net). Task-based regressors are described below. Each of the GLMs included, in addition to the task-related regressors, the six motion parameters as estimated during realignment as well as a discrete cosine transform-based basis set covering low-frequency up to 1/80 Hz to account for signal variability introduced by head motion and temporal drifts. The GLM for the face-viewing task included one regressor for each target face (including the “ghost face” stimulus), representing the 10 repetitions of each respective face (12 repetitions for the ghost face). Each of these regressors was created by convolving the canonical hemodynamic response function (HRF) with a series of boxcars representing the 1,000-ms intervals during which a particular face was presented. The GLM for the MID task included three task regressors corresponding to three trial types: wins, misses, and neutral. Each of these regressors was created by convolving the canonical HRF with a series of boxcars representing the 3,000-ms interval encompassing anticipation (delay) and feedback phases of each trial. The GLM for the person judgment task included task regressors for each the 40 traits and 6 judgment types (i.e., you-about-you, you-about-other1, you-about-other2, other1-about-you, other2-about-you, and curved lines). Each of these task regressors was created by convolving the canonical HRF with a series of boxcars representing the 3,500-ms duration of judgment trials.
The output of these first-level regressions was a series of parameter estimate (β) maps used in the next group level of analyses. For the person judgment task, an additional intermediate step averaged a subset of these β-maps to obtain unbiased estimates of the BOLD response to a set of judgment trial types: you-about-other1 and you-about-other2 β-maps were combined into you-about-other β-maps; similarly, other1-about-you and other2-about-you were combined into other-about-you β-maps; additionally, the 40 individual trait β-maps were combined for each judgment type. For the face-viewing task, β-maps corresponding to trials on which participants viewed either the ghost face or themselves were discarded before the next level of analyses.
Independent Functional Localizer Tasks for Valuation and Social Cognition Systems.
Following the analytic strategy of several recent neuroimaging studies (31, 32), we used an MID task (30) to functionally localize components of the valuation system activated during anticipation and receipt of monetary rewards. The MID task included 30 trials in which it was possible to win a reward (reward-possible trials) intermixed with 15 trials in which winning a reward was not possible (neutral trials). Each trial of the MID task began with a 500-ms presentation of one of two cue symbols: a green square indicated that the current trial offered an opportunity to win $1 (reward-possible trial); a red square indicated that the current trial did not offer an opportunity to win money (neutral trial). Following the cue symbol, there was a delay interval (with randomly determined duration between 2,000 and 2,500 ms) and then a target stimulus (yellow star) was briefly presented. On reward-possible trials, participants would win $1 if they made a button press while the target stimulus was displayed and $0 if the button press was made before the target onset or after the target offset. On neutral trials, although no money could be earned, participants were instructed to still make a button press while the target stimulus was displayed. Following the target stimulus offset, feedback (i.e., the amount earned on that trial—either $0 or $1—and the total cumulative earnings) was presented for 500 ms. The duration of the target stimulus presentation was adjusted algorithmically (within the range of 150–550 ms) based on task performance up until that point; specifically, the algorithm was intended to generate a two-thirds success rate on reward-possible trials. The algorithm succeeded in adjusting task difficulty such that participants earned money on ∼20 of the 30 reward-possible trials (mean wins = 19.8). Five participants were unable to complete the MID task because of technical issues.
The social cognition system localizer was a modified person judgment task commonly used to identify regions involved in making judgments about others’ mental states and traits (adapted from ref. 33). The task implemented a rapid event-related design comprising 240 judgment trials lasting 3,500 ms each and intertrial intervals (ITIs) consisting of a white fixation cross on black background were jittered between 1,500 ms and 11,000 ms (mean duration of ITI = 4,000 ms). For each of 40 trait adjectives (20 positive, 20 negative), participants made six kinds of judgments using a five-point scale: you-about-you, you-about-other1, you-about-other2, other1-about-you, other2-about-you, and active baseline curved line judgments. On you-about-you trials, participants judged the extent to which the trait adjective described them (1 = not very; 5 = very). On you-about-other1 and you-about-other2 trials, participants judged the extent to which the adjectives described one of two group members. On other1-about-you and other2-about-you trials, participants predicted the extent to which one of two group members would judge the adjective as describing them (i.e., the participant). On curved line trials, as an active baseline task that matched nonsocial aspects of the other judgment trial types, participants judged the extent to which the trait word contained curved lines as opposed to straight lines (1 = very few, <20% curved lines; 5 = very many, >80% curved lines). Trial types were presented in a pseudorandom counterbalanced order and distributed across the task’s four runs such that each run included 10 trials (5 with positive traits, 5 with negative traits) of each judgment type. As detailed below, of interest for this study were activations common to both you-about-other and other-about-you judgment trials relative to active baseline curved line trials. Data relating to you-about-you trials were collected for other studies to be analyzed and reported separately.
Anatomically Constraining Spherical ROIs.
As detailed in Methods, for each of the two functional localizer tasks we defined spheres with a radius of 8 mm surrounding activation peaks that fell within our a priori ROIs. The resulting spherical ROIs were 2,109 voxels. For the subcortical structures (VS and amygdala), these spheres were then anatomically constrained using structural masks obtained from FSL: for the VS, the Oxford-GSK-Imanova Structural-Anatomical Striatal Atlas constrained the ROI to 39 voxels; for the amygdala, the Harvard-Oxford Atlas constrained the ROI to 1,592 voxels). For the dmPFC and lTPJ, the 8-mm radius spheres were anatomically constrained so as not to extend beyond the boundaries of the brain (resulting in ROIs comprised of 2,066 and 1,701 voxels, respectively).
Target Popularity Analyses.
ROI analyses.
To statistically evaluate the effect of target popularity on parameter estimates (βs) extracted from each ROI, linear mixed-effects models (lme4 and lmerTest packages for R) with random intercepts at the subject (perceiver) level were used. Separate models were run for each ROI, with the dependent measure comprising β-values for all voxels within the ROI averaged together across the 10 repetitions of each target face presented to each perceiver. The models included target popularity as the fixed-effect and perceivers’ personal liking ratings of targets as a covariate to ensure the analyses tested whether ROI activity specifically tracked targets’ sociometric popularity (i.e., the extent to which target group members were collectively liked by the group and not merely individually liked by the perceiver). [It is important to note that correlation between perceivers’ personal liking of targets and the rest of the group’s liking of those targets was relatively low (r = 0.22), corresponding to less than 5% of variance explained. Moreover, whether or not perceiver’s own liking of target was partialled out, target popularity predicted activity in both valuation and social cognition systems (Ps < 0.01; see below for details about system-level aggregation across valuation ROIs and social cognition ROIs, respectively).] All linear mixed-effect models were fitted using restricted maximum-likelihood estimation with the appropriate degrees of freedom calculated using the Kenward–Roger method (57) (Tables S2 and S3). All hypotheses tests were two-sided with a statistical significance level of 0.05.
The ROI analyses revealed that target popularity was positively associated with activity in each of the ROIs identified by the valuation localizer task (Fig. 2C) (vmPFC parameter estimate ± SE: 0.122 ± 0.059, P = 0.039; amygdala: 0.089 ± 0.038, P = 0.019; VS: 0.083 ± 0.041, P = 0.047) and social cognition localizer task (Fig. 2D) (dmPFC: 0.194 ± 0.079, P = 0.015; precuneus: 0.127 ± 0.055, P = 0.022; lTPJ: 0.124 ± 0.059, P = 0.038). The only ROI in which activity did not track target popularity was rTPJ (0.029 ± 0.045, P > 0.5), and was therefore was not included in the subsequent analyses.
To rule out alternative explanations—that activation in valuation and social cognition regions reflected variables potentially confounded with popularity rather than popularity per se—additional regression analyses were run that accounted for perceiver–target relational characteristics (i.e., length of relationship, frequency of contact, hours per week spent together, and subjective ratings of interpersonal closeness and similarity) and target attributes (i.e., age, sex, and normative ratings of facial attractiveness and trustworthiness) by including these covariates as fixed effects in the linear mixed-effect models described above. The positive association between target popularity and parameter estimates extracted from each ROI proved robust, remaining statistically significant after accounting for the effect of these potential confounds (all Ps < 0.05) (Tables S2 and S3).
To verify that these target popularity effects did not differ between groups, an additional model was run for each ROI that included all of the aforementioned parameters as well as two additional fixed effects for group membership (with −0.5 and 0.5 effect coding for each of the respective groups) and the interaction between group and target popularity. The main effect of target popularity on parameter estimates was robust to the inclusion of these additional parameters (all P’s < 0.05, two-tailed; for VS, P = 0.06, two-tailed); importantly, there was no main effect of group or interaction between group and target popularity for any ROI (all Ps > 0.2).
Whole-brain parametric analysis.
To validate these results, we also conducted a random-effects, parametric whole-brain regression analysis not constrained by a priori hypotheses. In this analysis, perceivers’ neural responses to group members (targets) during the face-viewing task were modeled as a function of targets’ popularity (as a subject-level random slope parameter), again controlling for each perceiver’s personal liking of individual targets (as a fixed effect) and including a random intercept for each perceiver. By fitting perceiver-specific random slopes and conducting a whole-brain search for regions in which these random slopes differed significantly from 0, this mixed-effects approach was best suited to answer the question: Are there any regions in which activity reliably (i.e., across perceivers) corresponds to target popularity?
This analysis replicated the ROI-based analysis: the same core valuation (vmPFC, amygdala, VS) and social cognition (dmPFC, precuneus, lTPJ) regions tracked significantly with target popularity, even when controlling for the aforementioned potential confounds (Fig. S1 and Table S4) [whole-brain family-wise error (FWE)-corrected P < 0.05 with uncorrected P < 0.001, k = 44 voxels; for the amygdala and VS, FWE-corrected P < 0.05 using small volume correction]. The analysis also revealed a cluster in middle frontal gyrus; as we had no specific predictions about the involvement of this region, the finding is reported without post hoc interpretation.
The clusters revealed by the whole-brain analysis were then subjected to the additional analyses conducted for the ROIs (described above) to rule out alternative explanations for the observed relationship and to verify that these target popularity effects did not differ between groups. We found that the positive association between target popularity and parameter estimates extracted from each cluster remained statistically significant even with the inclusion of fixed effects for all of the potential confounds listed above, as well as group membership and the interaction between group and target popularity (all Ps < 0.01); moreover, there was no main effect of group or interaction between group and target popularity for any of the clusters (all Ps > 0.2).
Mediation Analyses.
Next, we conducted multilevel mediation analyses to test whether valuation and social cognition systems track popularity in parallel (independently) or serially, with one system assuming a primary role that mediates the popularity–activity relationship for the other. Specifically, we performed formal mediation analyses to test whether valuation system activity explained the observed correlation between target popularity and social cognition system activity. First, parameter estimates extracted from the vmPFC, amygdala, and VS—ROIs that had been independently localized by the MID task—were averaged to compute a composite measure of valuation system activity during the face-viewing task. Parameter estimates from the dmPFC, precuneus, and lTPJ—ROIs that had been independently localized by the person judgment task—were likewise aggregated to compute a composite measure of social cognition activity while viewing group members’ faces. Multilevel mediation analyses were then implemented via the gsem (generalized structural equation model) estimation command in Stata 13 (StataCorp, 2013), with social cognition activity as the dependent “Y” variable, valuation activity as the mediator “M” variable, and target popularity as the predictor “X” variable. As with the linear mixed-effects models in the target popularity main effect analyses above, the generalized structural equation models included random intercepts at the subject (perceiver) level and perceivers’ personal liking ratings of targets as a covariate. Thus, the mediation analysis enabled us to quantify and statistically evaluate the extent to which increases in social cognition activity evoked by popular targets (independent of how much the individual perceiver liked them) were mediated by associated increases in valuation activity. The nlcom command in Stata 13, which computes “delta method” SEs, was used to conduct two-tailed significance tests of indirect paths.
We found that valuation activity mediated the association between target popularity and social cognition activity (Results and Fig. 3) (indirect effect parameter estimate ± SE: 0.100 ± 0.039, P < 0.01), with 64.60% of the total effect mediated. Moreover, as assessed by Akaike’s information criterion (AIC) and Bayesian information criterion (BIC), model fit was greater with valuation activity as the mediator (AIC = 734.43; BIC = 772.25) than with (i) the parallel organization in which the valuation activity and social cognition activity independently tracked target popularity (AIC = 902.10; BIC = 936.48), and (ii) the alternative serial organization in which social cognition activity operated as the mediator (AIC = 736.96; BIC = 774.78). To evaluate the relative strength of these models and aid interpretation, AIC and BIC raw values were transformed into AIC and BIC weights, respectively (58). According to either measure, the model with valuation activity as mediator had 3.54-times greater strength of evidence than did the other two models combined.
Perceiver Popularity Analyses.
Neural measure of interpersonal sensitivity.
Having found that valuation system activity directly tracked target popularity, we then examined whether individual differences in the strength of this relationship related to perceivers’ own popularity. Following the same analytic procedures as for the target popularity main effect analyses above, linear mixed-effects models (lme4 and lmerTest packages for R) with random intercepts at the subject (perceiver) level were used here to predict valuation activity parameter estimates. The models included fixed effects for target popularity, perceiver popularity, and their interaction term. As with the previous analyses, perceivers’ personal liking ratings of targets were included as a covariate and linear mixed-effect models were estimated using restricted maximum-likelihood estimation with the appropriate degrees of freedom calculated using the Kenward–Roger method (57).
Behavioral measure of interpersonal sensitivity.
Having established a link between perceiver popularity and a neural measure of interpersonal sensitivity, we examined whether this finding would be corroborated using a social psychological (behavioral) measure of social acuity. During the behavioral (first) session, the same participants assessed each of their group members on a range of personality traits; in addition, they predicted how each of their group members judged them on these same traits. In this computerized paradigm (E-Prime 2.0), participants used a sliding visual analog scale (anchored by the labels “not very” and “very” on opposite ends) to judge the extent to which trait adjectives described each group member and also predict how each group member would judge them on these traits.
We computed the Pearson correlation between each perceiver’s predicted personality profile (i.e., the perceiver’s predictions about how a specific group member would judge the perceiver on various personality attributes) and the corresponding individual’s actual personality profile of the perceiver (i.e., how that particular group member actually judged the perceiver on various personality attributes). In other words, a single correlation coefficient was computed for each dyadic pairing of matched predicted-actual personality profiles across all trait items. These dyadic predicted-actual profile relationships were transformed using Fisher’s r-to-z transformation (i.e., from Pearson correlation coefficients to Fisher z-scores) and aggregated to compute each perceiver’s average profile relationship (across the perceiver’s 12 predicted-actual profile relationships, one for each of the other 12 group members). The resulting individual-difference measure of social acuity, termed overall meta-accuracy (59), was then correlated with perceiver popularity. Consistent with our prediction, perceivers’ popularity was positively associated with their accuracy in predicting how individual group members assessed their personality across all items (r = 0.44; P < 0.05, two-tailed) (Fig. S3). This finding corroborates the neural perceiver popularity result and its interpretation as reflecting enhanced interpersonal sensitivity. Furthermore, it dovetails with previous research demonstrating that popular adults and children more accurately perceive network members’ interpersonal sentiments (7, 35, 36).
Acknowledgments
We thank the participants in this study; K. Tsvetkova, L. Bennett, Y. Jun, A. Radin for data collection assistance; and N. Bolger and K. Makovi for helpful advice and assistance with multilevel mediation and social network analyses, respectively. This work was supported by a Columbia University Interdisciplinary Center for Innovative Theory and Empirics seed grant (to P.S.B. and K.N.O.); National Institute of Child Health and Human Development Grant R01-HD069178 (to K.N.O.); and National Institute on Aging Grant R01-AG043463 (to K.N.O.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1511477112/-/DCSupplemental.
References
- 1.Smith KP, Christakis NA. Social networks and health. Annu Rev Sociol. 2008;34(1):405–429. [Google Scholar]
- 2.Silk JB. Social components of fitness in primate groups. Science. 2007;317(5843):1347–1351. doi: 10.1126/science.1140734. [DOI] [PubMed] [Google Scholar]
- 3.Dunbar RI. The social brain meets neuroimaging. Trends Cogn Sci. 2012;16(2):101–102. doi: 10.1016/j.tics.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Davis JA. Clustering and hierarchy in interpersonal relations: Testing two graph theoretical models on 742 sociomatrices. Am Sociol Rev. 1970;35:843–851. [Google Scholar]
- 5.Moreno JL. Who Shall Survive? A New Approach to the Problem of Human Interrelations. Beacon House; Beacon, NY: 1934. [Google Scholar]
- 6.Lansu TA, Cillessen AH, Karremans JC. Adolescents’ selective visual attention for high-status peers: The role of perceiver status and gender. Child Dev. 2014;85(2):421–428. doi: 10.1111/cdev.12139. [DOI] [PubMed] [Google Scholar]
- 7.Krantz M, Burton C. The development of the social cognition of social status. J Genet Psychol. 1986;147(1):89–95. [Google Scholar]
- 8.Vaughn BE, Waters E. Attention structure, sociometric status, and dominance: Interrelations, behavioral correlates, and relationships to social competence. Dev Psychol. 1981;17(3):275–288. [Google Scholar]
- 9.Wasserman S, Faust K. Social Network Analysis: Methods and Applications. Cambridge Univ Press; New York, NY: 1994. [Google Scholar]
- 10.Newcomb TM. Stabilities underlying changes in interpersonal attraction. J Abnorm Soc Psychol. 1963;66(4):376–386. doi: 10.1037/h0041059. [DOI] [PubMed] [Google Scholar]
- 11.Wiggins JS, Trapnell PD. A dyadic-interactional perspective on the five-factor model. In: Wiggins JS, editor. The Five-Factor Model of Personality: Theoretical Perspectives. Guilford Press; New York, NY: 1996. pp. 89–162. [Google Scholar]
- 12.Henrich J, Gil-White FJ. The evolution of prestige: Freely conferred deference as a mechanism for enhancing the benefits of cultural transmission. Evol Hum Behav. 2001;22(3):165–196. doi: 10.1016/s1090-5138(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 13.Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doré BP, Zerubavel N, Ochsner KN. Social cognitive neuroscience: A review of core systems. In: Mikulincer M, Shaver PR, Borgida D, Bargh J, editors. APA Handbook of Personality and Social Psychology, Vol 1: Attitudes and Social Cognition. American Psychological Association; Washington, DC: 2014. pp. 693–720. [Google Scholar]
- 15.Zink CF, et al. Know your place: Neural processing of social hierarchy in humans. Neuron. 2008;58(2):273–283. doi: 10.1016/j.neuron.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Güroğlu B, et al. Why are friends special? Implementing a social interaction simulation task to probe the neural correlates of friendship. Neuroimage. 2008;39(2):903–910. doi: 10.1016/j.neuroimage.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Krienen FM, Tu P-C, Buckner RL. Clan mentality: Evidence that the medial prefrontal cortex responds to close others. J Neurosci. 2010;30(41):13906–13915. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4(3):165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 19.Klein JT, Platt ML. Social information signaling by neurons in primate striatum. Curr Biol. 2013;23(8):691–696. doi: 10.1016/j.cub.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson KK, Platt ML. Social signals in primate orbitofrontal cortex. Curr Biol. 2012;22(23):2268–2273. doi: 10.1016/j.cub.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azzi JC, Sirigu A, Duhamel J-R. Modulation of value representation by social context in the primate orbitofrontal cortex. Proc Natl Acad Sci USA. 2012;109(6):2126–2131. doi: 10.1073/pnas.1111715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein JT, Shepherd SV, Platt ML. Social attention and the brain. Curr Biol. 2009;19(20):R958–R962. doi: 10.1016/j.cub.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci. 2012;24(8):1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45(1):32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Fiske ST. Social cognition and social perception. Annu Rev Psychol. 1993;44(1):155–194. doi: 10.1146/annurev.ps.44.020193.001103. [DOI] [PubMed] [Google Scholar]
- 26.Snodgrass SE. Women’s intuition: The effect of subordinate role on interpersonal sensitivity. J Pers Soc Psychol. 1985;49(1):146–155. [Google Scholar]
- 27.Snodgrass SE. Further effects of role versus gender on interpersonal sensitivity. J Pers Soc Psychol. 1992;62(1):154–158. [Google Scholar]
- 28.Dépret E, Fiske ST. Social cognition and power: Some cognitive consequences of social structure as a source of control deprivation. In: Weary G, Gleicher F, Marsh KL, editors. Control Motivation and Social Cognition. Springer; New York, NY: 1993. pp. 176–202. [Google Scholar]
- 29.Taylor MJ, et al. Neural correlates of personally familiar faces: Parents, partner and own faces. Hum Brain Mapp. 2009;30(7):2008–2020. doi: 10.1002/hbm.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knutson B, Westdorp A, Kaiser E, Hommer D. fMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- 31.Zaki J, Schirmer J, Mitchell JP. Social influence modulates the neural computation of value. Psychol Sci. 2011;22(7):894–900. doi: 10.1177/0956797611411057. [DOI] [PubMed] [Google Scholar]
- 32.Tamir DI, Mitchell JP. Disclosing information about the self is intrinsically rewarding. Proc Natl Acad Sci USA. 2012;109(21):8038–8043. doi: 10.1073/pnas.1202129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ochsner KN, et al. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28(4):797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 34.Hommer DW, et al. Amygdalar recruitment during anticipation of monetary rewards: An event-related fMRI study. Ann N Y Acad Sci. 2003;985:476–478. doi: 10.1111/j.1749-6632.2003.tb07103.x. [DOI] [PubMed] [Google Scholar]
- 35.Casciaro T. Seeing things clearly: Social structure, personality, and accuracy in social network perception. Soc Networks. 1998;20(4):331–351. [Google Scholar]
- 36.Bondonio D. Predictors of accuracy in perceiving informal social networks. Soc Networks. 1998;20(4):301–330. [Google Scholar]
- 37.Shepherd SV, Deaner RO, Platt ML. Social status gates social attention in monkeys. Curr Biol. 2006;16(4):R119–R120. doi: 10.1016/j.cub.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Deaner RO, Khera AV, Platt ML. Monkeys pay per view: Adaptive valuation of social images by rhesus macaques. Curr Biol. 2005;15(6):543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 39.Magee JC, Galinsky AD. Social hierarchy: The self‐reinforcing nature of power and status. Acad Management Ann. 2008;2(1):351–398. [Google Scholar]
- 40.Keltner D, Gruenfeld DH, Anderson C. Power, approach, and inhibition. Psychol Rev. 2003;110(2):265–284. doi: 10.1037/0033-295x.110.2.265. [DOI] [PubMed] [Google Scholar]
- 41.Galinsky AD, Magee JC, Inesi ME, Gruenfeld DH. Power and perspectives not taken. Psychol Sci. 2006;17(12):1068–1074. doi: 10.1111/j.1467-9280.2006.01824.x. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Kessels HW, Hu H. The mouse that roared: Neural mechanisms of social hierarchy. Trends Neurosci. 2014;37(11):674–682. doi: 10.1016/j.tins.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Healey KL, Morgan J, Musselman SC, Olino TM, Forbes EE. Social anhedonia and medial prefrontal response to mutual liking in late adolescents. Brain Cogn. 2014;89:39–50. doi: 10.1016/j.bandc.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis PA, Rezaie R, Brown R, Roberts N, Dunbar RI. Ventromedial prefrontal volume predicts understanding of others and social network size. Neuroimage. 2011;57(4):1624–1629. doi: 10.1016/j.neuroimage.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powell J, Lewis PA, Roberts N, García-Fiñana M, Dunbar R. Orbital prefrontal cortex volume predicts social network size: An imaging study of individual differences in humans. Proc Biol Sci. 2012;279(1736):2157–2162. doi: 10.1098/rspb.2011.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nat Neurosci. 2011;14(2):163–164. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanai R, Bahrami B, Roylance R, Rees G. Online social network size is reflected in human brain structure. Proc Biol Sci. 2012;279(1732):1327–1334. doi: 10.1098/rspb.2011.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noonan MP, et al. A neural circuit covarying with social hierarchy in macaques. PLoS Biol. 2014;12(9):e1001940. doi: 10.1371/journal.pbio.1001940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sallet J, et al. Social network size affects neural circuits in macaques. Science. 2011;334(6056):697–700. doi: 10.1126/science.1210027. [DOI] [PubMed] [Google Scholar]
- 51.Chiao JY. Neural basis of social status hierarchy across species. Curr Opin Neurobiol. 2010;20(6):803–809. doi: 10.1016/j.conb.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Hallinan MT. The Structure of Positive Sentiment. Elsevier; New York, NY: 1974. [Google Scholar]
- 53.Bearman P. Generalized exchange. Am J Sociol. 1997;102(5):1383–1415. [Google Scholar]
- 54.Parkhurst JT, Hopmeyer A. Sociometric popularity and peer-perceived popularity two distinct dimensions of peer status. J Early Adolesc. 1998;18(2):125–144. [Google Scholar]
- 55.Babad E. On the conception and measurement of popularity: More facts and some straight conclusions. Soc Psychol Educ. 2001;5(1):3–29. [Google Scholar]
- 56.Cillessen AH, Rose AJ. Understanding popularity in the peer system. Curr Dir Psychol Sci. 2005;14(2):102–105. [Google Scholar]
- 57.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53(3):983–997. [PubMed] [Google Scholar]
- 58.Wagenmakers E-J, Farrell S. AIC model selection using Akaike weights. Psychon Bull Rev. 2004;11(1):192–196. doi: 10.3758/bf03206482. [DOI] [PubMed] [Google Scholar]
- 59.Carlson EN, Furr RM, Vazire S. Do we know the first impressions we make? Evidence for idiographic meta-accuracy and calibration of first impressions. Soc Psychol Personal Sci. 2010;1(1):94–98. [Google Scholar]