This prospective, multicenter, single-blind, randomized, phase III study compares a combination of ramosetron, aprepitant, and dexamethasone (RAD) with a combination of ondansetron, aprepitant, and dexamethasone (OAD) to prove the noninferiority of RAD in controlling highly emetogenic chemotherapy-induced nausea and vomiting (CINV). The results indicated that RAD is as effective and tolerable as OAD for CINV prevention. Ramosetron could be considered one of the best partners for aprepitant.
Keywords: Ramosetron, Ondansetron, Aprepitant, Nausea, Vomiting, Chemotherapy
Abstract
Background.
A combination of serotonin receptor (5-hydroxytryptamine receptor type 3) antagonists, NK-1 receptor antagonist, and steroid improves the complete response (CR) of chemotherapy-induced nausea and vomiting (CINV) in cancer patients. Ramosetron’s efficacy in this triple combination regimen has not been investigated. This prospective, multicenter, single-blind, randomized, phase III study compares a combination of ramosetron, aprepitant, and dexamethasone (RAD) with a combination of ondansetron, aprepitant, and dexamethasone (OAD) to prove the noninferiority of RAD in controlling highly emetogenic CINV.
Methods.
Aprepitant and dexamethasone were orally administered for both arms. Ramosetron and ondansetron were intravenously given to the RAD and OAD groups. The primary endpoint was no vomiting and retching and no need for rescue medication during the acute period (day 1); the noninferiority margin was −15%.
Results.
A total of 299 modified intention-to-treat cancer patients who received RAD (144 patients) and OAD (155 patients) were eligible for the efficacy analysis. The CR rates of RAD versus OAD were 97.2% versus 93.6% during the acute period, 77.8% versus 73.6% during the delayed period (day 2–5), and 77.1% versus 71.6% during the overall period. Furthermore, RAD was noninferior to OAD in subgroups stratified by age, cancer type, chemotherapeutic agents, and schedule. Repeated measures analysis showed that in male patients, RAD was superior to OAD. Profiles of adverse events were similar in both groups.
Conclusion.
RAD is as effective and tolerable as OAD for CINV prevention in patients receiving highly emetogenic chemotherapy. Ramosetron could be considered one of the best partners for aprepitant.
Implications for Practice:
This is the first prospective, multicenter, randomized phase III study to show that ramosetron, a new 5-hydroxytryptamine receptor type 3 antagonist, is as effective and tolerable as ondansetron when administered in combination with aprepitant and dexamethasone for the prevention of chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is one of the worst fears of patients receiving chemotherapy [1]; it significantly affects quality of life and can decrease the likelihood of a patient continuing chemotherapy. New antiemetic agents, including antagonists of the serotonin receptor (5-hydroxytryptamine receptor type 3 [5-HT3R]) and the neurokinin-1 receptor (NK-1R), have been shown to be effective in decreasing CINV incidence and severity; a combination of NK-1R and 5-HT3R antagonists with dexamethasone is recommended for CINV prevention in patients receiving highly emetogenic chemotherapy (HEC) [2–4]. A triple-drug regimen consisting of 5-HT3R antagonists dolasetron, granisetron, ondansetron, or palonosetron has been reported to be highly efficacious [5–7].
Currently, palonosetron is the clinically preferred antiemetic for CINV [3, 8]; however the best 5-HT3R antagonists for use in a triple drug combination for HEC has not yet been determined in randomized phase III trials [5, 8]. Ramosetron, a 5-HT3R antagonist with an increased half-life compared with that of ondansetron, as well as increased receptor affinity compared with that of ondansetron and granisetron [9], has been widely used for CINV prevention in Asia. In several clinical trials, it showed efficacy and safety profiles similar to those of ondansetron and granisetron, whether administered alone or in combination with dexamethasone [10–12]. The authors reported a phase II study in which ramosetron in combination with aprepitant effectively prevented HEC-induced CINV [13]. However, ramosetron activity in the triple-drug regimen has not been compared with that of other 5-HT3R antagonists. The purpose of this study was to compare the efficacy and safety of the combination of ramosetron, aprepitant, and dexamethasone (RAD) with the efficacy and safety of the combination of ondansetron, aprepitant, and dexamethasone (OAD) in HEC-induced CINV.
Materials and Methods
This prospective, multicenter, single-blind, randomized phase III clinical trial was conducted in 17 institutions of the Korean Cancer Study Group. The study protocol was approved by the institutional review boards and registered with ClinicalTrials.gov (NCT01536691); all patients provided written informed consent. Eligible patients were more than 19 years old with pathologically confirmed malignant disease and were scheduled to receive HEC on the first day of treatment. The major exclusion criteria were as follows: medications, conditions, or procedures that could affect nausea or vomiting and previous chemotherapy within 12 months (all eligibility criteria are shown in the supplemental online Appendix and the protocol).
Patients were assigned to the RAD or OAD groups (1:1 ratio) according to a stratified block randomization table. Additional stratification factors were: chemotherapeutic regimen (cisplatin vs. noncisplatin), schedule (single-day vs. multiple-day chemotherapy), and institution. Aprepitant (125 mg, day 1, 1 hour prior to chemotherapy; 80 mg, days 2–3) and dexamethasone (12 mg, day 1, 30 min prior to chemotherapy; 8 mg, days 2–4) were administered orally. Ramosetron (0.3 mg, day 1) and ondansetron (16 mg, day 1) were administered intravenously to the RAD group and the OAD group, respectively, 30 min before chemotherapy. Rescue antiemetics for vomiting/severe nausea were administered at the request of the patient or upon recommendation by the attending physicians at any time during the study period; the type was determined by the physician.
A complete response (CR) was defined as no vomiting, including retching, and no requirement for rescue antiemetics, and complete control (CC) was defined as CR without nausea. Nausea severity was determined using the visual analog scale (VAS). During the overall period (days 1–5), patients were asked to record daily episodes of vomiting or retching, the degree of nausea, and the use of rescue medication in a diary form and in the Rhodes Index of Nausea and Vomiting Form-2 (INV-2) [14]. Tolerability was assessed based on clinical and laboratory adverse events between the start day and the day before the next chemotherapy cycle and evaluated according to the Common Toxicity Criteria for Adverse Events (CTCAE, version 4.0).
The primary endpoint of hypothesis was that the RAD CR rate during the acute period (first 24 h, as reported in the diary form) would not be inferior to OAD CR rate. Secondary endpoints were the CR rates in the delayed (days 2–5) and overall (days 1–5) periods and adverse events. The CINV incidents reported in the INV-2 form were separately evaluated.
In both groups, a sample size of 135 allowed an 80% power and one-sided α level of 0.025 to detect the CR noninferiority difference of −15% between the RAD and OAD groups, assuming the actual CR in each group to be 85% and 90%, respectively. Considering 20% of expected dropout rate (withdrawal of consents, missing data, and follow-up losses), sample size was increased to 169 patients in each group. Effectiveness was calculated in a modified intention-to-treat (m-ITT) population. The two groups were compared using the chi-square test, Fisher’s exact test, and Wilcoxon rank-sum test as appropriate. The daily efficacy difference was evaluated by the generalized estimating equations (GEE) approach [15]. The data were analyzed using SAS (version 9.3; SAS Institute, Cary, NC, http://www.sas.com).
Results
Patient Characteristics
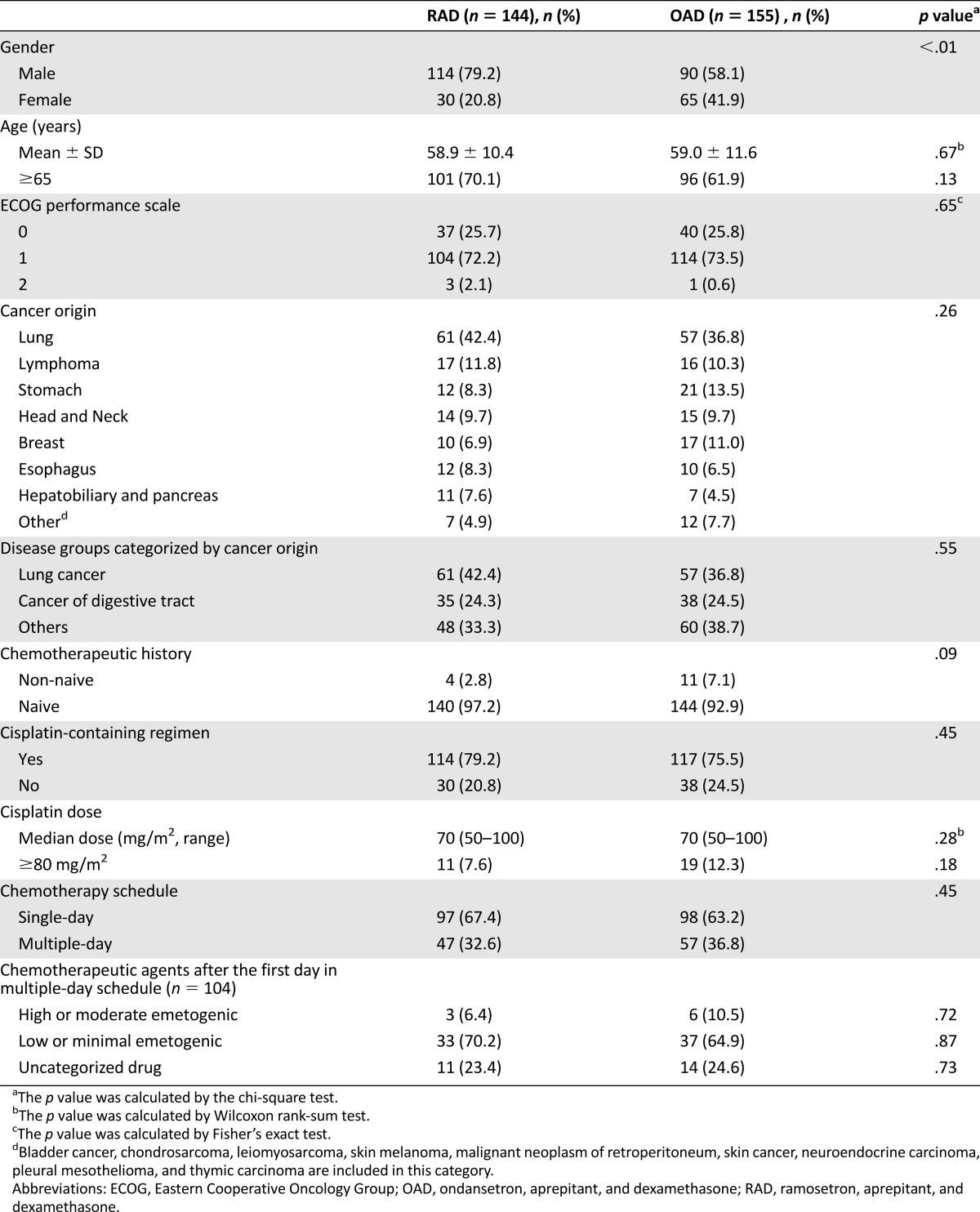
A total of 340 patients were screened and 338 enrolled between June 2011 and September 2012; an m-ITT population of 299 was subjected to the efficacy analysis (supplemental online Fig. 1). Patient characteristics, disease demographics, chemotherapeutic regimens, and chemotherapy schedules were well balanced between the two arms (Table 1); however, the proportion of male patients was significantly higher in the RAD group (p < .01).
Table 1.
Demographic and clinical characteristics of the modified intention-to-treat patients
Efficacy
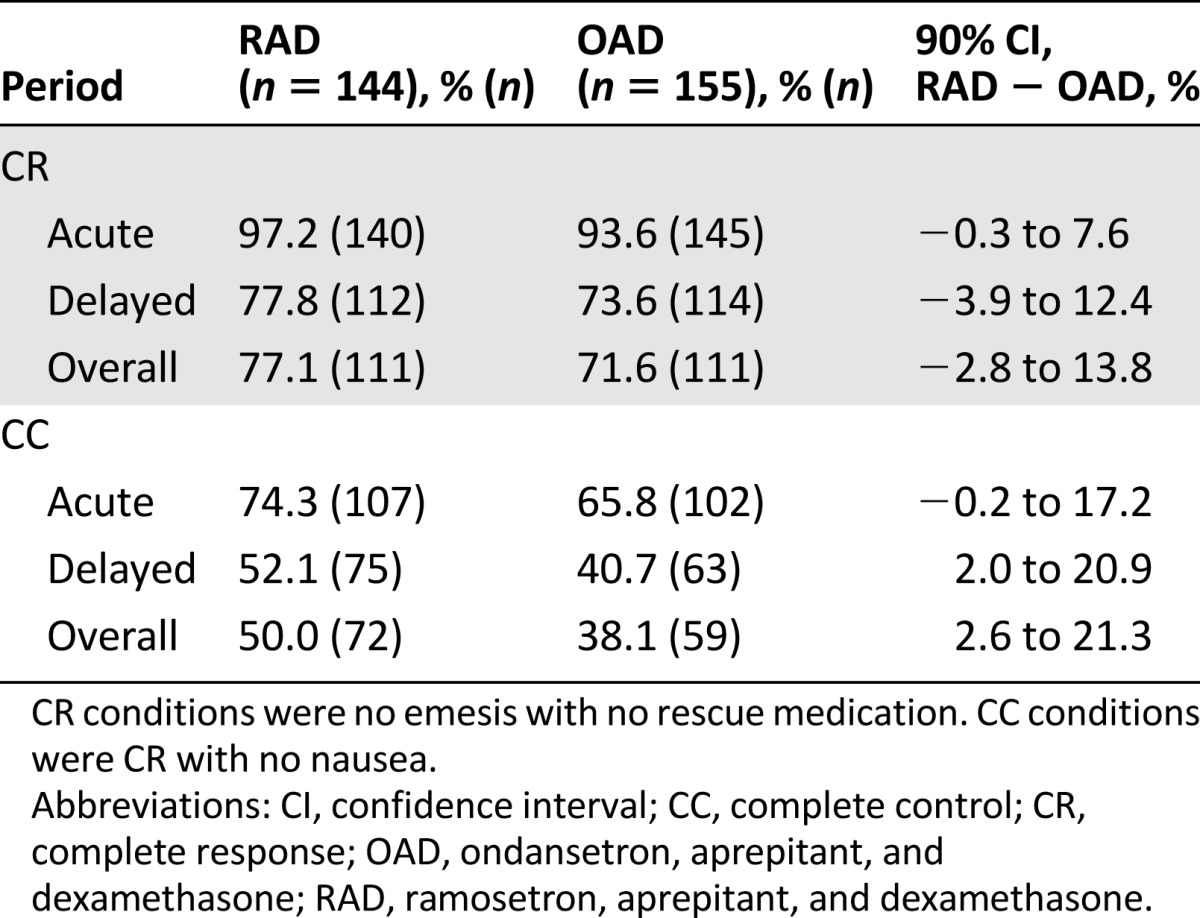
RAD was noninferior to OAD, as evidenced by CR rates in the acute, delayed, and overall periods (efficacy differences of 3.7%, 4.2%, and 5.5%, respectively) (Table 2). After gender adjustment (because more male patients were unintentionally assigned to the RAD group), CR in the RAD was still noninferior to that of the OAD, with efficacy differences of 4.1% (90% CI: 0.5%–7.7%), 4.5% (90% CI: −3.9%–12.8%), and 4.9% (90% CI: −3.6%–13.4%) for the acute, delayed, and overall periods, respectively.
Table 2.
CR and CC rates (modified intention-to-treat population)
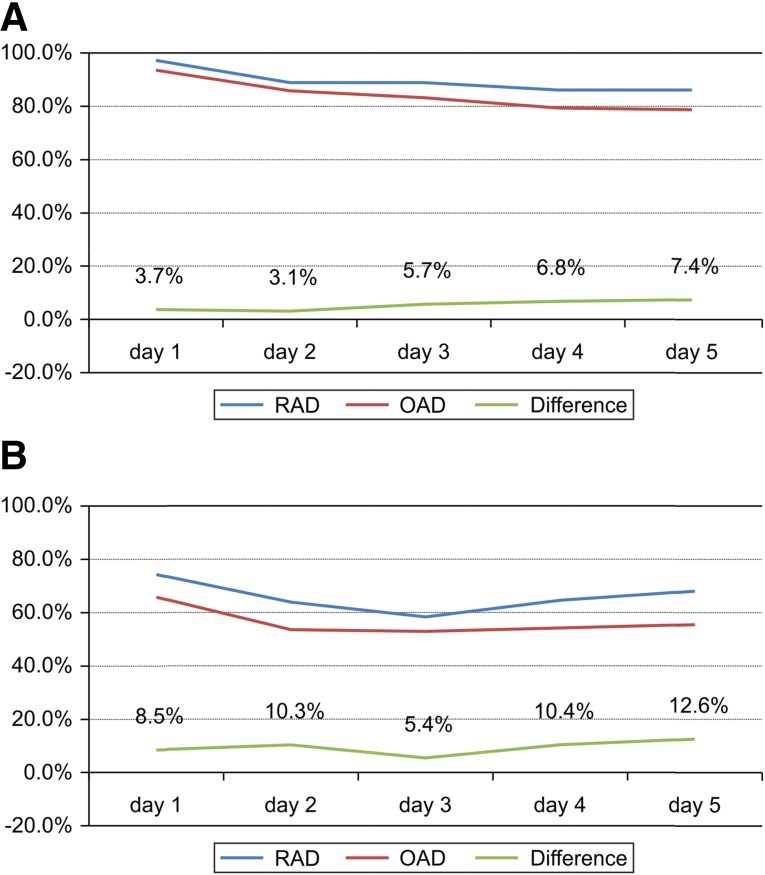
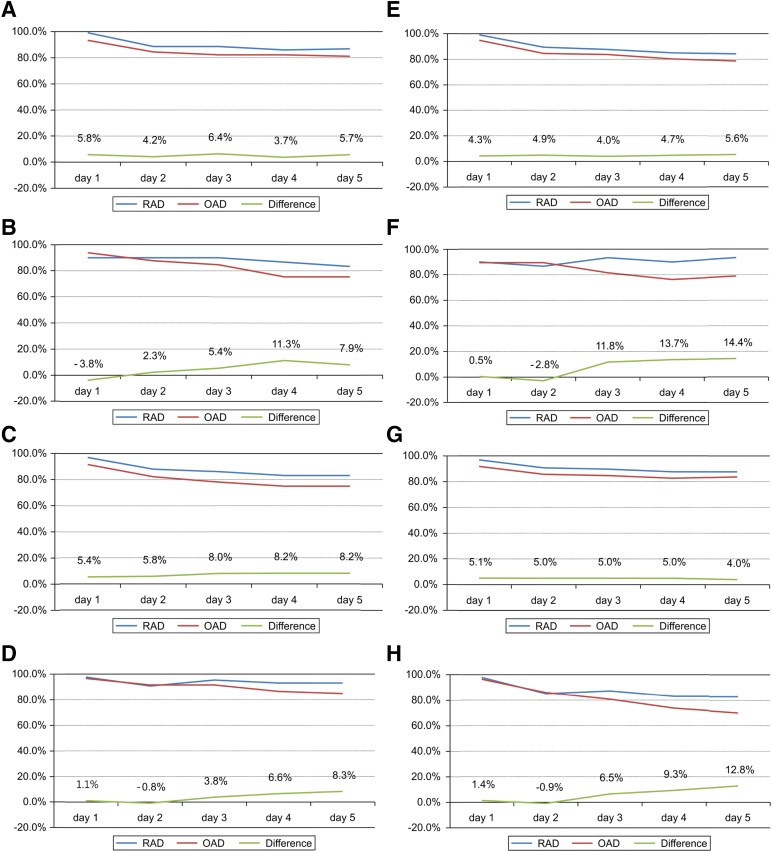
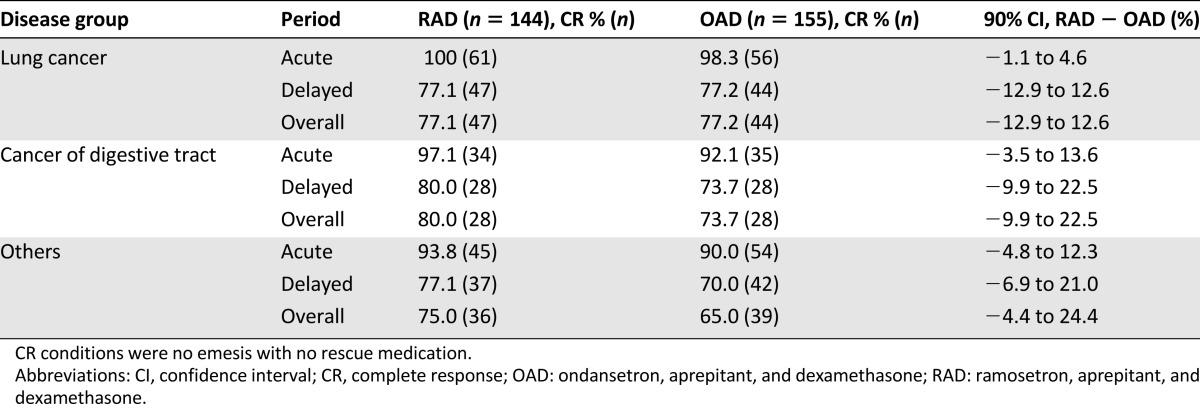
RAD was superior to OAD when compared on a daily basis using the GEE approach (efficacy difference, 7.8%; Fig. 1), and this superiority persisted after adjustment for gender (efficacy difference, 6.8%). The gender-stratified CR rates also revealed the superiority of RAD to OAD in men but did not show noninferiority in women (difference of 10.2%, 90% CI, 2.8%–17.0% for men and difference of −3.0%, 90% CI, −16.2%–10.1% for women; Fig. 2). The values that were stratified for disease groups categorized by cancer origin (lung vs. digestive tract vs. other origins), age (≥65 vs. <65 years), chemotherapeutic regimen (cisplatin vs. noncisplatin), and treatment schedule (single-day vs. multiday) also demonstrated RAD noninferiority to OAD (Table 3; Fig. 2).
Figure 1.
Complete response rate (A) and complete control rate (B) in the modified intention-to-treat population (n = 299) on a daily basis. (A): The risk difference between the two arms was 7.8% (90% confidence interval [CI], 1.4%–14.1%), and the value adjusted for gender was 6.8% (90% CI, 0.2%–13.3%). (B): The risk difference between the two arms was 9.8% (90% CI, 1.9%–17.6%), and the value adjusted for gender was 7.1% (90% CI, −0.9%–15.2%) using the generalized estimating equation model.
Abbreviations: OAD, ondansetron, aprepitant, and dexamethasone; RAD: ramosetron, aprepitant, and dexamethasone.
Figure 2.
Subgroup analyses of the complete response rate between the RAD and OAD group. In the modified intention-to-treat population (n = 299). The data were stratified for gender (male [A], female [B]), age (<65 years [C], ≥65 years [D]), chemotherapeutic agent (cisplatin-based [E], non-cisplatin-based regimen [F]), and schedule of chemotherapy (single-day chemotherapy [G], multiple-day chemotherapy [H]). RAD was noninferior to OAD in all subgroups except female. (A): Difference, 10.2%; 90% confidence interval (CI), 2.8%–17.0%. (B): Difference, −3.0%; 90% CI, −16.2%–10.1%. (C): Difference, 5.4%; 90% CI, −0.1%–10.8%. (D): Difference, 1.1%; 90% CI, −4.4%–6.5%. (E): Difference, 4.3%; 90% CI, 0.6%–7.9%. (F): Difference, 0.5%; 90% CI, −11.7%–12.7%. (G): Difference, 5.8%; 90% CI, −2.1%–13.7%. (H): Difference, 11.5%; 90% CI, 0.9%–22.1%. The risk difference between the two arms was analyzed using the generalized estimating equation model.
Abbreviations: OAD, ondansetron, aprepitant, and dexamethasone; RAD: ramosetron, aprepitant, and dexamethasone.
Table 3.
CR rates analyzed by the disease group of categorized by cancer origin (modified intention-to-treat population)
The efficacy difference in the CC rate confirmed RAD noninferiority (8.5%, 11.4%, and 11.9% during the acute, delayed, and overall periods, respectively; Table 2), even after the adjustment for gender (4.9%, 90% CI, −3.6%–13.5%; 9.3%, 90% CI, −0.4%–19.0%; and 9.2%, 90% CI, −0.5%–18.9%, respectively). On a daily basis, the difference in the CC rate as analyzed by the GEE approach was 9.8% (90% CI, 1.9%–17.6%; Fig. 1).
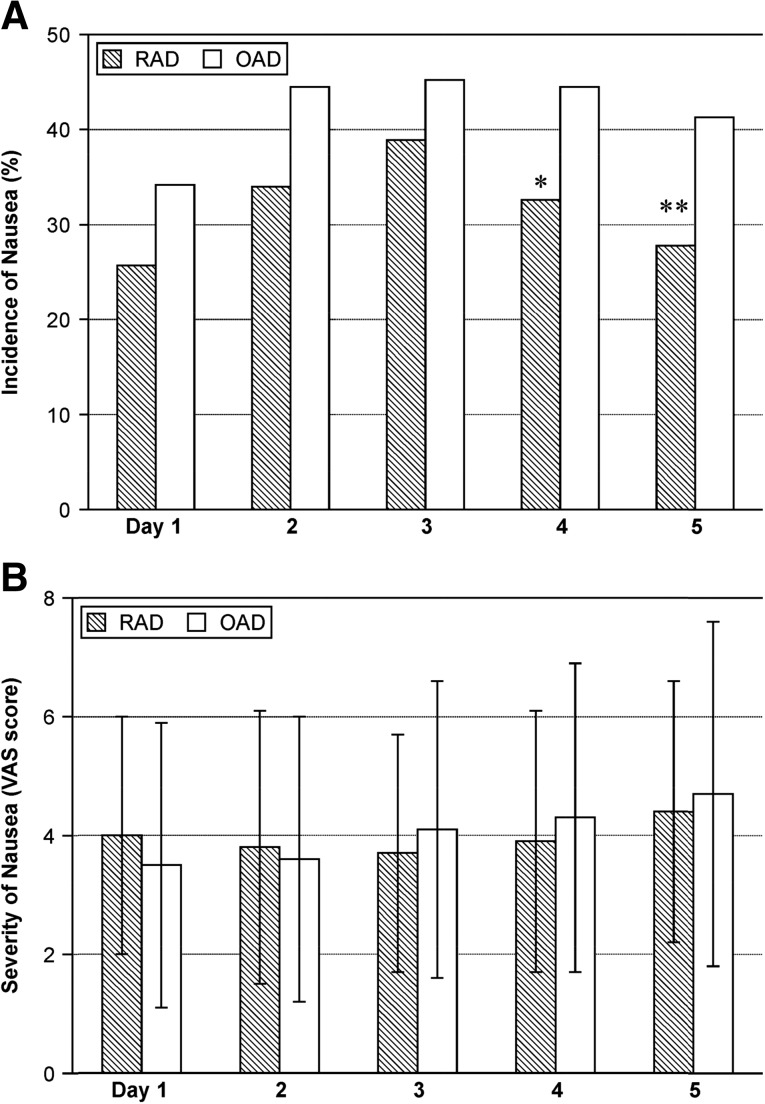
There was no significant difference between the two arms in frequency of vomiting and rescue medication usage (supplemental online Fig. 2). Nausea incidents were similar during the first 3 days but significantly lower in the RAD group than in the OAD group at days 4 and 5 (32.6% vs. 44.5%, p = .04; 27.8% vs. 41.3%, p = .02, respectively), and the severity as indicated by the VAS score was similar in both groups (Fig. 3). The frequency of nausea, vomiting, and retching incidents as measured by the INV-2 were similar in both groups (supplemental online Fig. 3). Body weight changes between baseline and the day before the next chemotherapy treatment were comparable in the RAD and OAD patients (−0.6 ± 5.6 and −0.4 ± 3.8 kg, respectively; p = .75), and there was no significant difference in the proportion of patients with > 5% weight loss.
Figure 3.
Incidence and severity of nausea in the modified intention-to-treat population (n = 299). There is a significant difference in the incidence of nausea at days 4 and 5, but the severity was similar in both groups (Wilcoxon rank-sum test). ∗, p = .04; ∗∗, p = .02 (chi-square test).
Abbreviations: OAD, ondansetron, aprepitant, and dexamethasone; RAD: ramosetron, aprepitant, and dexamethasone; VAS, visual analog scale.
Adverse Events
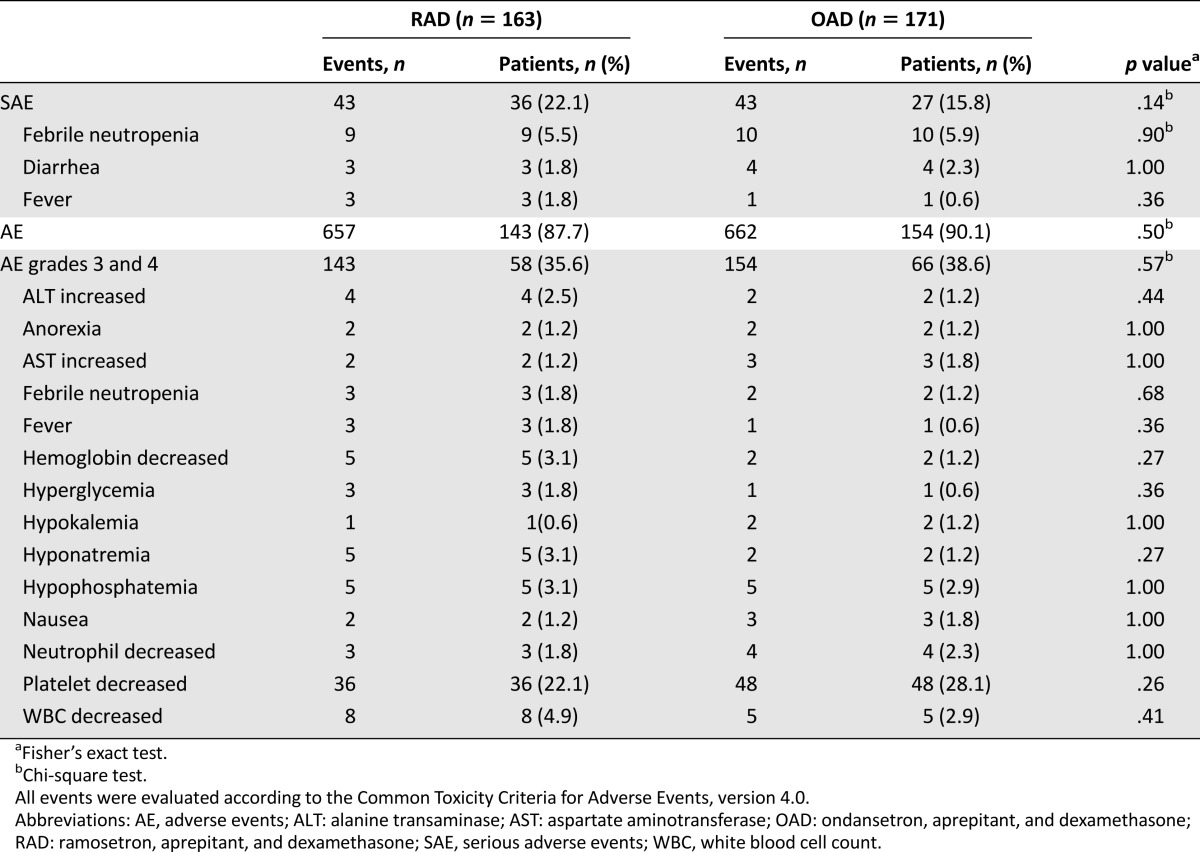
Safety analysis was performed for all patients except for four subjects with incomplete medical records. In the OAD group, only one drug-related serious adverse event (diarrhea) was reported, which was spontaneously resolved without any complications. Three deaths of OAD patients during the study were considered unrelated to the medication. Because the drugs were used together with chemotherapy, it was difficult to differentiate between drug-related events and other adverse events (AE); therefore, total grade 3 or 4 AEs occurring in >1% of patients were scored (Table 4). There was no significant difference in the safety profiles between the two arms.
Table 4.
Summary of adverse events with incidence ≥1% (safety population)
Discussion
The current study demonstrated that RAD was noninferior to OAD in the prevention of HEC-induced nausea and vomiting, irrespective of patient age or the type of cancer or chemotherapeutic regimen. RAD demonstrated its efficacy in the acute, delayed, and overall periods; for male patients, RAD was more effective than OAD.
Ramosetron is structurally different from the first generation 5-HT3R antagonists; it has higher receptor affinity, a slower dissociation rate, and a half-life twice as long as ondansetron [9]. Ramosetron administered alone was as effective as granisetron or ondansetron for CINV prevention in clinical trials [10–12]. In combination with dexamethasone, ramosetron showed a 77% CR rate in acute period, which is comparable to that of granisetron (82%) [16]. After the development of the NK-1R antagonists, a triple-drug regimen consisting of aprepitant, dexamethasone, and 5-HT3R antagonists showed more efficacy in CINV prevention than double-drug combinations, especially in delayed emesis. Thus, CR rates to aprepitant, dexamethasone, and ondansetron were 88%–89%, 74%–75%, and 72%–73% in the acute, delayed, and overall periods, respectively [6, 17]; the combination with palonosetron demonstrated similar results (98%, 73%, and 70%, respectively) [7]. Based on the efficacy of the triple regimen in clinical trials, it was recommended as a standard treatment for HEC-induced CINV [2–4]. Previous antiemetic guidelines, which were used during the study design period, stated that the antiemetic activities of 5-HT3R antagonists were similar at equivalent doses. Based on the meta-analysis of various 5-HT3R antagonists in double regimens, the National Comprehensive Cancer Network (NCCN) guideline has suggested palonosetron as a preferred 5-HT3R antagonist in the triple antiemetic drug combination [3, 8]. In Asia, RAD is one of the most popular treatments for HEC-treated cancer patients. However, the lack of clinical studies has precluded the recommendation of RAD as a standard regimen for HEC-induced CINV. In our previous study, RAD showed high CR rates of 95%, 92%, and 92% in the acute, delayed, and overall periods, respectively, in chemotherapy-naïve patients receiving high-dose cisplatin [13]. Based on the information described above, the current study was designed to provide scientific support for the prescription of RAD by comparing its efficacy with that of OAD. Antiemetic guidelines had informed that the antiemetic activities of 5-HT3R antagonists used in the clinic were similar at equivalent doses. Therefore, OAD, a standard treatment regimen, was chosen as a control arm in this study.
The characteristics of this study were as follows: (a) A heterogeneous patient population (chemotherapy-naïve vs. non-naïve patients, single day vs. multiple-day schedule, cisplatin vs. noncisplatin chemotherapy, and various cancer types: lung vs. digestive tract vs. other origins) that reflected real clinical situations. Although, in contrast to conventional studies on antiemetic effects, this study enrolled patients with various diseases and chemotherapeutic regimens, the presence of an adequate number of patients and the appropriately planned stratification factors would yield statistically significant results. (b) CINV assessment that was based on the patients’ dual recordings of the symptoms in the diary and the INV-2 form [14], both of which revealed that RAD was as effective as OAD, supporting our hypothesis. (c) The use of the GEE approach (used for longitudinal modeling in regression analysis of correlated data) to evaluate the relationship between covariates and repeatedly measured outcome at different time points.
Traditionally, female gender is considered a high risk factor for CINV; however, a recent study revealed that adding an NK-1R antagonist to a double regimen may negate the adverse gender-related prognostic effect [18]. In our study, gender was not used as a randomization factor, and the proportion of female patients in the RAD (20.8%) was, unintentionally, significantly lower than that in the OAD (41.9%). Nonetheless, RAD was consistently noninferior, even after gender adjustment and even superior to OAD in the male patients. In addition, RAD significantly reduced nausea incidents than in the OAD at days 4 and 5. Further investigation is required into gender-related and prolonged efficacy of ramosetron in CINV.
Despite recent improvement in antiemetic strategies, nausea remains an unsolved problem in clinical oncology. Similar to previous findings achieved using triple-emetic regimens [6, 17], RAD and OAD in this study failed to completely abolish HEC-induced nausea, and more effective treatment strategies are needed. Olanzapine, an antipsychotic drug blocking the action of multiple neurotransmitters, is known to be effective in controlling CINV, especially the nausea component [19]. Therefore, a novel ramosetron-based combination regimen with olanzapine might be a promising strategy through which to improve the control of CINV.
In this trial, there was no difference in the incidence of serious AEs or AEs graded higher than 3 between the treatment groups. In the middle of the study, the U.S. Food and Drug Administration announced that ondansetron may cause QT prolongation and recommended that a single intravenous dose should not exceed 16 mg [20]. In this study, all investigators were required to check baseline QT intervals, to correct electrolyte imbalances, and to be cautious about QT prolongation during treatment. One RAD patient with normal baseline electrocardiogram exhibited grade 3 arterial fibrillations on day 2, which was normalized immediately after electric cardioversion. To date, there has been no report on causality between ramosetron and QT prolongation.
Conclusion
This study provides evidence that RAD is at least as effective and tolerable as OAD for the prevention of HEC-induced CINV in all treatment periods. RAD noninferiority to OAD was independent of age, cancer type, chemotherapeutic agents, or schedule; furthermore, it was superior to OAD for male patients. RAD could be one of the standard regimens for the prevention of HEC-induced CINV.
See http://www.TheOncologist.com for supplemental material available online.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Supplementary Material
Acknowledgments
We thank Jinjoo Hong at the Korean Cancer Study Group data center for collecting data from each of the participating centers and Jeonghyeon Ju for her help in preparation for the meetings. We are also grateful to Prof. Young Jae Kim (Chosun Nursing College) for allowing us to use the Korean version of the INV-2 form. This study was supported by the Korean Cancer Study Group.
Author Contributions
Conception/Design: Hyo Jung Kim, Sang Won Shin, Jin Seok Ahn, Hwa Jung Kim, Jin-Hyoung Kang
Provision of study material or patients: Hyo Jung Kim, Sang Won Shin, Eun-Kee Song, Na-Ri Lee, Jun Suk Kim, Jin Seok Ahn, Hwan-Jung Yun, Yo-Han Cho, Keon Uk Park, Si-Young Kim, Joung Soon Jang, Sang-We Kim, Hyun Woo Lee, Se Ryeon Lee, Yang Soo Kim, Soon Nam Lee, Yoon Ho Ko, Jin-Hyoung Kang
Collection and/or assembly of data: Hyo Jung Kim, Sang Won Shin, Eun-Kee Song, Na-Ri Lee, Jun Suk Kim, Jin Seok Ahn, Hwan-Jung Yun, Yo-Han Cho, Keon Uk Park, Si-Young Kim, Joung Soon Jang, Sang-We Kim, Hyun Woo Lee, Se Ryeon Lee, Yang Soo Kim, Soon Nam Lee, Yoon Ho Ko, Hwa Jung Kim
Data analysis and interpretation: Hyo Jung Kim, Jin Seok Ahn, Hwa Jung Kim, Jin-Hyoung Kang
Final approval of manuscript: Hyo Jung Kim, Sang Won Shin, Eun-Kee Song, Na-Ri Lee, Jun Suk Kim, Jin Seok Ahn, Hwan-Jung Yun, Yo-Han Cho, Keon Uk Park, Si-Young Kim, Joung Soon Jang, Sang-We Kim, Hyun Woo Lee, Se Ryeon Lee, Yang Soo Kim, Soon Nam Lee, Yoon Ho Ko, Hwa Jung Kim, Jin-Hyoung Kang
Disclosures
Jin-Hyoung Kang: Pfizer, Ono Pharmaceutical Co., Boehringer Ingelheim, Eli Lilly (C/A), Eli Lilly, AstraZeneca (RF), Boehringer Ingelheim, AstraZeneca (H); Jin Seok Ahn: AstraZeneca, Lilly, Roche, Boehringer Ingelheim (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Hickok JT, Roscoe JA, Morrow GR, et al. Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemetics: A University of Rochester James P. Wilmot Cancer Center Community Clinical Oncology Program Study of 360 cancer patients treated in the community. Cancer. 2003;97:2880–2886. doi: 10.1002/cncr.11408. [DOI] [PubMed] [Google Scholar]
- 2.Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: Results of the Perugia consensus conference. Ann Oncol. 2010;21(suppl 5):v232–v243. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger DS, Armstrong DK, Barbour S, et al. Antiemesis. J Natl Compr Canc Netw. 2012;10:456–485. doi: 10.6004/jnccn.2012.0047. [DOI] [PubMed] [Google Scholar]
- 4.Basch E, Prestrud AA, Hesketh PJ, et al. doi: 10.1200/JCO.2010.34.4614. Antiemetics: American Society of Clinical Oncology clinical practice guideline update [published correction appears in: J Clin Oncol 2014;32:2117]. J Clin Oncol 2011;29:4189–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan K, Hinke A, Grothey A, et al. A meta-analysis comparing the efficacy of four 5-HT3-receptor antagonists for acute chemotherapy-induced emesis. Support Care Cancer. 2007;15:1023–1033. doi: 10.1007/s00520-006-0186-7. [DOI] [PubMed] [Google Scholar]
- 6.Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: A multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin: The Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21:4112–4119. doi: 10.1200/JCO.2003.01.095. [DOI] [PubMed] [Google Scholar]
- 7.Longo F, Mansueto G, Lapadula V, et al. Palonosetron plus 3-day aprepitant and dexamethasone to prevent nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer. 2011;19:1159–1164. doi: 10.1007/s00520-010-0930-x. [DOI] [PubMed] [Google Scholar]
- 8.Botrel TE, Clark OA, Clark L, et al. Efficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: Systematic review and meta-analysis. Support Care Cancer. 2011;19:823–832. doi: 10.1007/s00520-010-0908-8. [DOI] [PubMed] [Google Scholar]
- 9.Rabasseda X. Ramosetron, a 5-HT3 receptor antagonist for the control of nausea and vomiting. Drugs Today (Barc) 2002;38:75–89. doi: 10.1358/dot.2002.38.2.820104. [DOI] [PubMed] [Google Scholar]
- 10.Koizumi W, Tanabe S, Nagaba S, et al. A double-blind, crossover, randomized comparison of granisetron and ramosetron for the prevention of acute and delayed cisplatin-induced emesis in patients with gastrointestinal cancer: Is patient preference a better primary endpoint? Chemotherapy. 2003;49:316–323. doi: 10.1159/000074533. [DOI] [PubMed] [Google Scholar]
- 11.Cheirsilpa A, Sinthusake T, Songsakkaesorn A, et al. Comparison of ramosetron and granisetron for the prevention of acute and delayed emesis in Cisplatin-based chemotherapy: A randomized controlled trial. Jpn J Clin Oncol. 2005;35:695–699. doi: 10.1093/jjco/hyi192. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y, He X, Yang S, et al. doi: 10.1159/000098418. Ramosetron versus ondansetron in the prevention of chemotherapy-induced gastrointestinal side effects: A prospective randomized controlled study [published correction appears in: Chemotherapy 2007;53:308]. Chemotherapy 2007;53:44–50. [DOI] [PubMed] [Google Scholar]
- 13.Jang G, Song HH, Park KU, et al. A phase II study to evaluate the efficacy of ramosetron, aprepitant, and dexamethasone in preventing cisplatin-induced nausea and vomiting in chemotherapy-naïve cancer patients. Cancer Res Treat. 2013;45:172–177. doi: 10.4143/crt.2013.45.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhodes VA. Criteria for assessment of nausea, vomiting, and retching. Oncol Nurs Forum. 1997;24(suppl):13–19. [PubMed] [Google Scholar]
- 15.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 16.Ho CL, Su WC, Hsieh RK, et al. A randomized, double-blind, parallel, comparative study to evaluate the efficacy and safety of ramosetron plus dexamethasone injection for the prevention of acute chemotherapy-induced nausea and vomiting. Jpn J Clin Oncol. 2010;40:294–301. doi: 10.1093/jjco/hyp169. [DOI] [PubMed] [Google Scholar]
- 17.Schmoll HJ, Aapro MS, Poli-Bigelli S, et al. Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol. 2006;17:1000–1006. doi: 10.1093/annonc/mdl019. [DOI] [PubMed] [Google Scholar]
- 18.Hesketh PJ, Grunberg SM, Herrstedt J, et al. Combined data from two phase III trials of the NK1 antagonist aprepitant plus a 5HT 3 antagonist and a corticosteroid for prevention of chemotherapy-induced nausea and vomiting: Effect of gender on treatment response. Support Care Cancer. 2006;14:354–360. doi: 10.1007/s00520-005-0914-4. [DOI] [PubMed] [Google Scholar]
- 19.Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: A randomized phase III trial. J Support Oncol. 2011;9:188–195. doi: 10.1016/j.suponc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 20.FDA drug safety communication: New information regarding QT prolongation with ondansetron (Zofran). Available at http://www.fda.gov/Drugs/DrugSafety/ucm310190.htm. Accessed October 8, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.