The survival benefit of combining sorafenib and transarterial chemoembolization (TACE) therapy was compared with sorafenib monotherapy for patients with advanced hepatocellular carcinoma and main portal vein tumor thrombosis. Disease control rate, median time to progression, median overall survival, and occurrence of adverse events were similar between patients in the sorafenib-TACE group and those treated with sorafenib monotherapy.
Keywords: Hepatocellular carcinoma, Main portal vein tumor thrombosis, Transarterial chemoembolization, Sorafenib
Abstract
Background.
The survival benefit of combining sorafenib and transarterial chemoembolization (TACE) therapy compared with sorafenib monotherapy for patients with advanced hepatocellular carcinoma (HCC) and main portal vein tumor thrombosis (MPVTT) is unclear.
Methods.
Between January 2009 and June 2013, 183 consecutive patients with advanced HCC (Barcelona Clinic Liver Cancer stage C) and MPVTT were retrospectively reviewed. Of these, 89 patients with advanced HCC and MPVTT were enrolled in this study: 45 were treated with combination therapy (sorafenib-TACE group), and the other 44 treated with sorafenib monotherapy (sorafenib group).
Results.
The mean number of TACE sessions per patient was 2.6 (range: 1–5). The median duration of sorafenib in the sorafenib-TACE group and sorafenib group was 5.6 months and 5.4 months, respectively. The disease control rate was similar between the two groups. Median time to progression was 3.0 months (95% confidence interval [CI]: 2.2, 3.7) in the sorafenib-TACE group, and 3.0 months (95% CI: 2.1, 3.8) in the sorafenib group (p = .924). Median overall survival was 7.0 months (95% CI: 6.1, 7.8) and 6.0 months (95% CI: 4.7, 7.3) in the sorafenib-TACE group and the sorafenib group, respectively (p = .544). The adverse events related to sorafenib were comparable between the two groups. Twenty-one adverse events of grade 3–4 related to TACE occurred in 12 patients (26.7%), and 2 of them died (4.4%).
Conclusion.
This study demonstrated no advantage of combination therapy over sorafenib monotherapy. Considering the patients’ morbidity after TACE, sorafenib monotherapy is appropriate for managing patients with advanced HCC and MPVTT.
Implications for Practice:
For patients with advanced hepatocellular carcinoma (HCC) and main portal vein tumor thrombosis (MPVTT), no benefit was seen in this study in terms of disease control rate, time to progression, and overall survival for patients receiving sorafenib and transarterial chemoembolization compared with those receiving sorafenib monotherapy. Considering the patients’ morbidity after combination therapy, monotherapy is appropriate for managing patients with advanced HCC and MPVTT.
Abstract
摘要
背景. 在晚期肝细胞癌(HCC)合并门静脉主干癌栓(MPVTT)患者中,索拉非尼联合动脉化疗栓塞(TACE)治疗与索拉非尼单药相比的生存获益尚不明确。
方法. 我们回顾了2009年1月至2013年6月期间的183例连续性晚期HCC(巴塞罗那临床肝癌分期C期)合并MPVTT患者的资料。研究纳入了其中89例晚期HCC合并MPVTT患者:45例接受了联合治疗(索拉非尼-TACE组),另外44例接受了索拉非尼单药治疗(索拉非尼组)。
结果. 患者平均接受2.6次TACE(范围:1 ∼ 5)。索拉非尼-TAEC组和索拉非尼组的中位索拉非尼治疗时间分别为5.6个月和5.4个月。两组的疾病控制率相似。索拉非尼-TACE组的中位至疾病进展时间为3.0个月[95%置信区间(CI):2.2 ∼ 3.7],索拉非尼组为3.0个月(95%CI:2.1 ∼ 3.8,P = 0.924)。索拉非尼-TACE组和索拉非尼组的中位总生存分别为7.0个月(95%CI:6.1 ∼ 7.8)和6.0个月(95%CI:4.7 ∼ 7.3, P = 0.544)。两组的索拉非尼相关性不良事件发生率相似。12例患者(26.7%)发生了21宗3/4级TACE相关性不良事件,其中2例患者死亡(4.4%)。
结论. 本研究证实联合治疗与索拉非尼单药治疗相比没有优势。考虑到患者在接受TACE后的病损情况,对于晚期HCC合并MPVTT患者采用索拉非尼单药治疗是适当的。The Oncologist 2015;20:1417–1424
对临床实践的提示:对于晚期肝细胞癌(HCC)合并门静脉主干癌栓(MPVTT)患者而言,本研究未观察到索拉非尼联合动脉化疗栓塞与索拉非尼单药治疗相比在疾病控制率、至疾病进展时间和总生存方面的获益。考虑到晚期HCC合并MPVTT患者在接受联合治疗后的病损情况,单药治疗是较为适当的选择。
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third leading cause of cancer-related death worldwide [1]. Hepatic resection, liver transplantation, and percutaneous ablation are the main radical treatments for HCC. Unfortunately, only 30%–40% of patients with early-stage disease are amenable to such curative therapies, and about 40% of all HCCs are diagnosed at an advanced stage (Barcelona Clinic Liver Cancer [BCLC] stage C), so these patients must rely on palliative therapy to prolong their survival time [2–4]. Sorafenib and transarterial chemoembolization (TACE) are important and common palliative treatments for most patients with advanced HCC in the Asia-Pacific region [2–7].
Although regular screening programs for high-risk patients are being developed, advanced HCCs with main portal vein tumor thrombosis (MPVTT) are still often seen at diagnosis. Patients with advanced HCC and MPVTT bear a poor prognosis with a median survival of 2.6 months with supportive care [8]. MPVTT has been considered a contraindication for TACE because of the risk of impacting liver function due to ischemic liver damage [2, 3, 9]. Therefore, based on the BCLC staging system and treatment strategy, sorafenib monotherapy is recommended for patients with advanced HCC and MPVTT [2, 3]. However, some reports suggested that TACE is a safe treatment for patients with advanced HCC and MPVTT if the patient has good liver function and collateral circulation around the MPV [8, 10]. Moreover, there has been increased focus on combining TACE with sorafenib to potentially improve the efficacy for patients with unresectable/advanced HCC [11–15]. Therefore, patients with advanced HCC and MPVTT treated with the combination therapy of sorafenib and TACE are expected to show improved survival time.
The survival benefit of the combination therapy for the specific subgroup of patients with advanced HCC and MPVTT is still unclear. This is because these recently published cohort studies consist of a mixed group of patients with different degrees of vascular invasion and/or extrahepatic metastasis; some studies exclude the subgroup of patients with MPVTT [11–15]. Therefore, we conducted a retrospective analysis of 89 patients with advanced HCC and MPVTT to evaluate the safety of combination therapy and compare this treatment group’s time to progression (TTP) and overall survival (OS) with those of patients receiving sorafenib monotherapy.
Patients and Methods
Patient Selection
The protocol was approved by ethics committees of the First Affiliated Hospital, Sun Yat-sen University. Written informed consent was obtained from each participant in accordance with the Declaration of Helsinki. Between January 2009 and June 2013, consecutive patients with advanced HCC and MPVTT were retrospectively analyzed at our center. Inclusion criteria were that all patients had pathologically or radiologically (contrast-enhanced computed tomography [CT] scan) confirmed advanced HCC (BCLC stage C) based on the European Association for the Study of the Liver diagnostic criteria [3], and the presence of MPVTT confirmed with the demonstration of a low-attenuation intraluminal mass expanding the portal vein or filling defects in the MPV on contrast-enhanced CT scan [16]. Exclusion criteria were patients who had a Child-Pugh score of greater than 8 or massive ascites; those with a secondary malignancy; those that had undergone liver surgery, transplantation, or systemic chemotherapy; and those with missing data.
According to the therapeutic strategy, those patients were divided into two groups. One received the combination therapy of sorafenib and TACE; the other received sorafenib monotherapy.
TACE Procedure
Briefly, for the TACE procedure, 10–20 mL lipiodol (Guerbet, Paris, France, http://www.guerbet.com) was mixed with 20–40 mg epirubicin (Pfizer, New York, NY, http://www.pfizer.com) to create an emulsion. Depending on the tumor size and liver function, 2–20 mL of the emulsion was infused into the liver tumor through a catheter. Subsequently, embolization using gel foam was carried out. When blood flow slowed or a vascular cast was observed, the injection was stopped. The tumor-feeding artery was selected, as much as possible, through the lobar, segmental, or subsegmental region of the liver tumor, depending on the tumor distribution [15].
Sorafenib Treatment
Patients in the sorafenib group began to take sorafenib after the diagnosis of HCC. In the sorafenib-TACE group, sorafenib treatment was started 1–3 days after TACE, and administration was suspended on the day a repeated TACE procedure was performed. The initial dose of oral sorafenib was 400 mg given twice daily. Doses were modified depending on the toxicity, according to version 3.0 of the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) [17]. Continued administration of sorafenib was encouraged if the side effects were manageable.
Follow-Up
In the sorafenib-TACE group, contrast-enhanced CT scan of the liver, liver function tests, and α-fetoprotein (AFP) measurement were performed 1 month after TACE to evaluate the effect of the treatment. If there was still residual viable tumor or new lesions had formed, and there was preserved liver function, additional TACE was performed. In the sorafenib group, enhanced CT scan of the liver, liver function tests, and AFP measurement were performed every month for the first 3 months to evaluate the effect of the treatment. The follow-up interval was extended to every 2 months starting at 3 months after treatment.
Assessments
Tumor response was assessed based on radiological evaluation according to modified RECIST [18]. AFP response was classified into the following: (a) complete response (normalization), (b) partial response (decrease by >50% of the baseline value), (c) stable (between −50% and +50% of baseline value), or (d) progression (more than +50% of baseline value) [19].
The complications of TACE that occurred within 4 weeks were recorded according to CTCAE [17]. TTP was defined as the time from the baseline radiological data to disease progression. OS was defined as the time from the start of treatment until death or the last follow-up.
Statistical Analysis
All statistical analyses were performed using SPSS software (version 16.0; IBM Corp., Chicago, IL, http://www-01.ibm.com). For baseline characteristics, continuous variables are described as median ± SD and categorical variables are expressed as frequencies and percentages. The Student t test was used to compare continuous variables between the two groups. The chi-square test was used to compare categorical variables between the two groups. The Kaplan-Meier method was used to calculate the TTP and OS between groups. Univariate analyses were performed with the log-rank test. Variables with a p value <.1 in univariate analysis were entered into a multivariate analysis. A multivariate Cox model was used to identify risk factors that affected overall survival. All statistical tests were two-sided, and p < .05 was considered statistically significant.
Results
Study Population
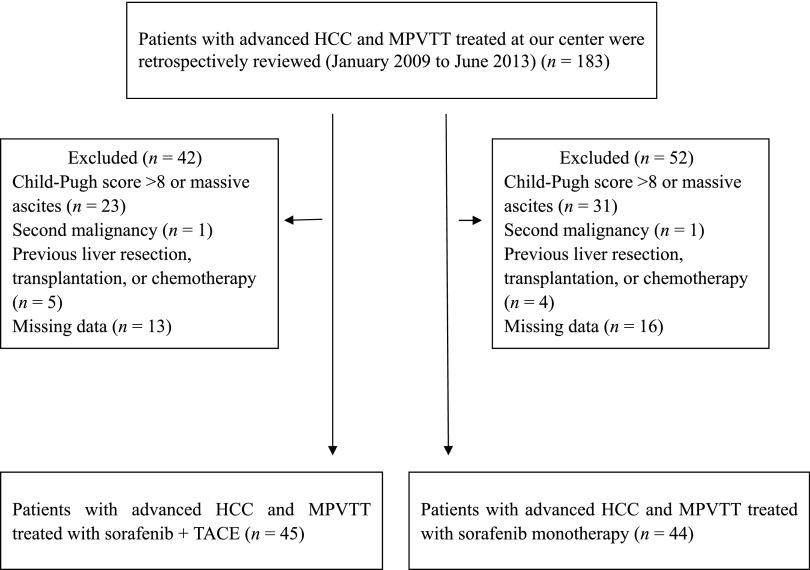
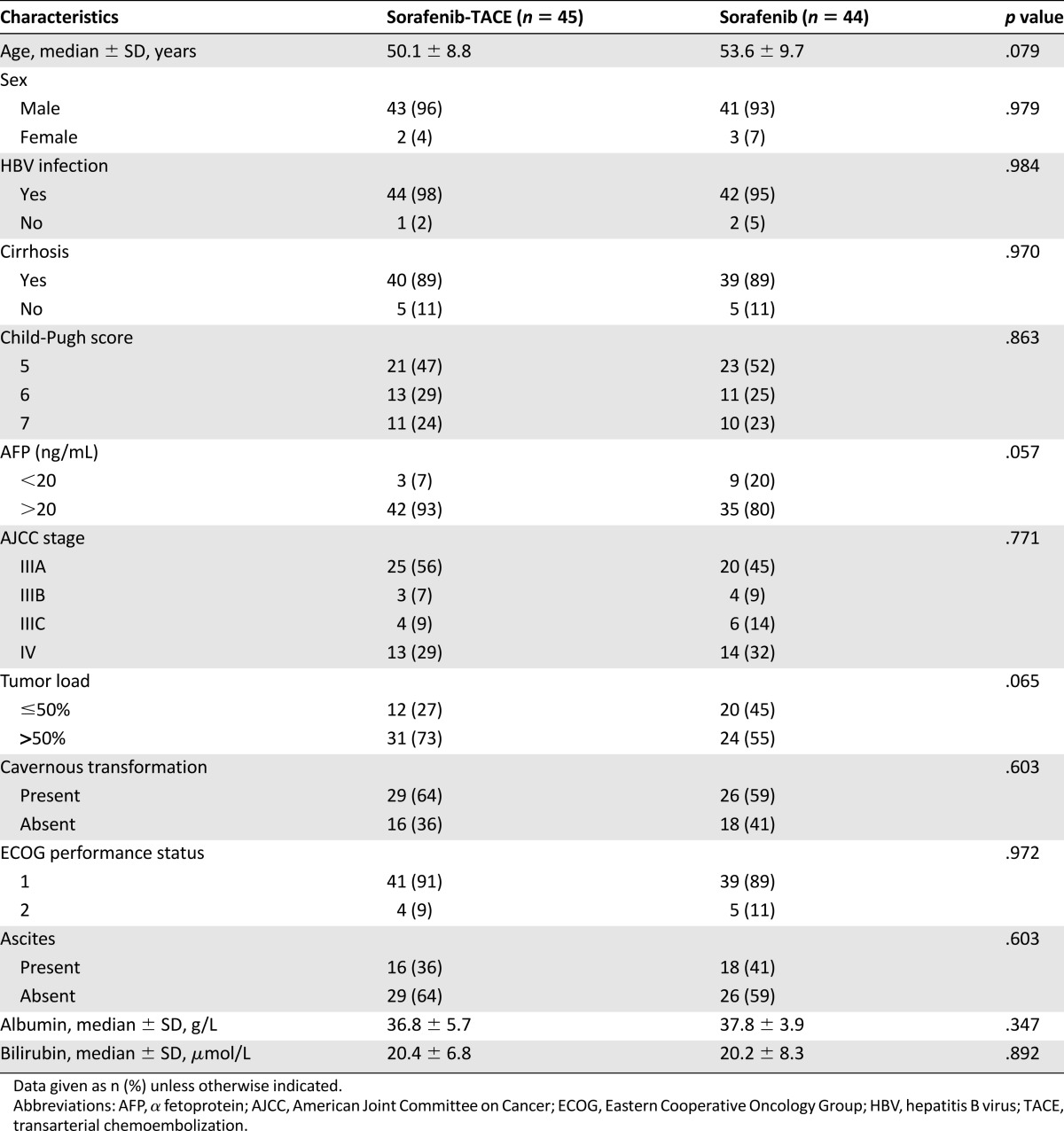
In total, between January 2009 and June 2013, 183 consecutive patients with advanced HCC and MPVTT were retrospectively observed during the study period. A total of 94 patients were excluded from the analysis because they did not satisfy the inclusion criteria. As a result, 89 patients with advanced HCC and MPVTT were enrolled in this analysis; 45 of them received the combination therapy of sorafenib and TACE, and the other 44 received sorafenib monotherapy (Fig. 1). The baseline characteristics of all patients are shown in Table 1. There was no significant difference between the two groups in terms of the cause of liver disease, liver function, and tumor characteristics. The majority of the patients were male, and hepatitis B virus and cirrhosis were the most common underlying diseases.
Figure 1.
Flow diagram of patient selection.
Abbreviations: HCC, hepatocellular carcinoma; MPVTT, main portal vein tumor thrombosis; TACE, transarterial chemoembolization.
Table 1.
Comparison of baseline patient characteristics
Treatment
The mean number of TACE procedures per patient in the sorafenib-TACE group was 2.6 (range: 1–5). The median duration of sorafenib administration in the sorafenib-TACE group and sorafenib groups was 5.6 months (range: 1–18 months) and 5.4 months (range: 1–17 months), respectively.
Treatment Outcome
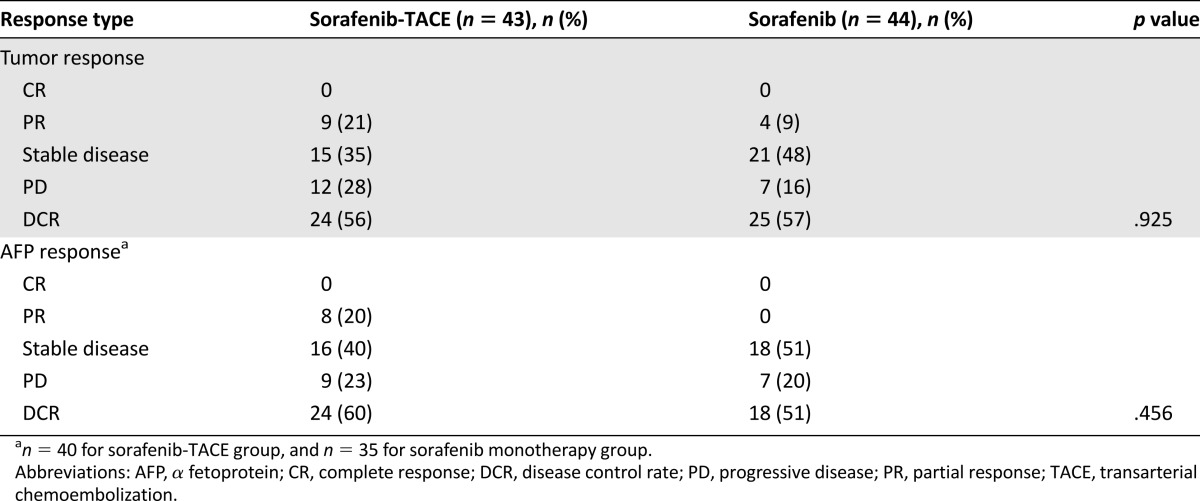
Follow-up data at month 3 were available for 34 of 43 patients (79%) in the sorafenib-TACE group and 32 of 44 patients (73%) in the sorafenib group. In the sorafenib-TACE group, 9 patients (21%) had a partial response (PR), 15 (35%) had stable disease, and 10 (23%) had progressive disease (PD). The disease control rate (DCR) was 56%. In the sorafenib group, 4 patients (9%) had a PR, 21 (48%) had stable disease, and 7 (16%) had PD. The DCR was 57%.
Twelve patients had a normal AFP level at the initial treatment. Therefore, AFP response was assessed in 58 patients at month 3. In the sorafenib-TACE group, 8 patients (20%) had a PR, 16 (40%) had stable disease, and 9 (23%) had PD. The DCR was 60%. In the sorafenib group, 18 (51%) patients had stable disease, and 7 (20%) had PD. The DCR was 51%. The details of treatment response are shown in Table 2.
Table 2.
Treatment response evaluated at month 3 in both treatment groups
Survival
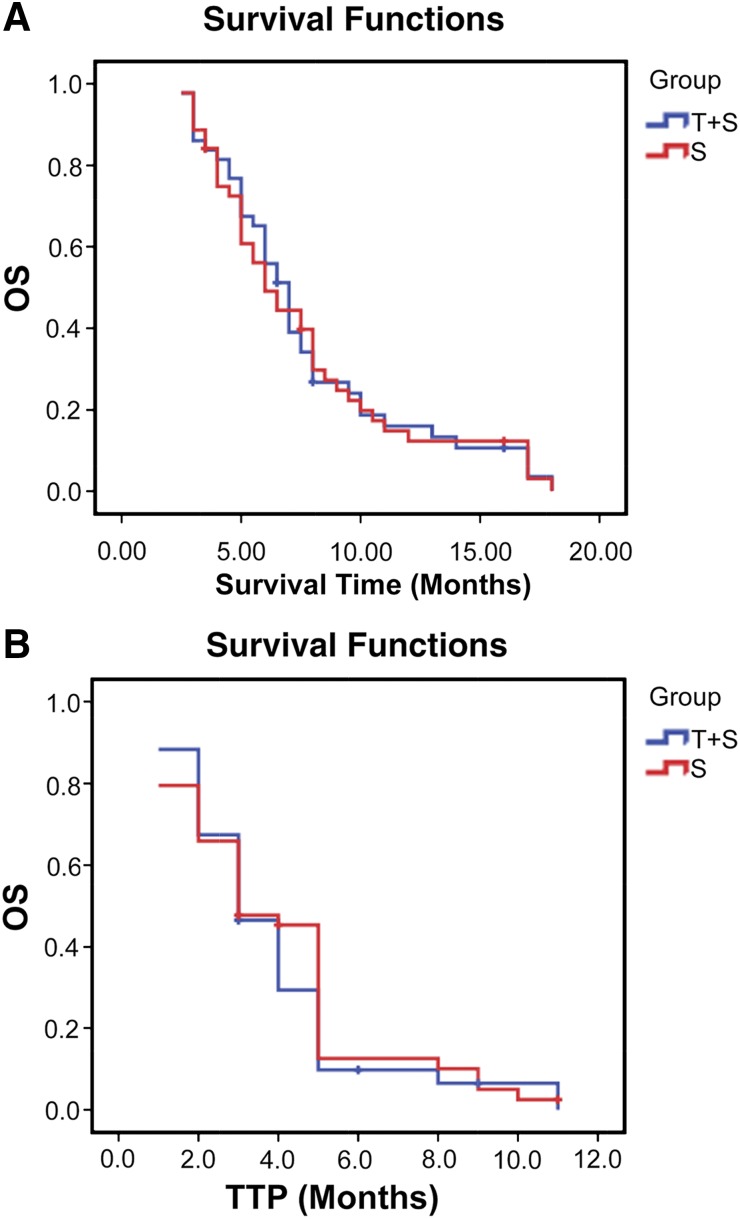
At the end of follow-up (December 2014), all patients in both groups had died. The median follow-up for these patients was 7.3 months (range: 2–18 months). The median OS was 7.0 months (95% CI: 6.1, 7.8 months) in the sorafenib-TACE group and 6.0 months (95% CI: 4.7, 7.3 months) in the sorafenib group. The difference between the 2 groups was not significant (p = .924). The median TTP was 3.0 months (95% CI: 2.2, 3.7 months) in the sorafenib-TACE group and 3.0 months (95% CI: 2.1, 3.8 months) in the sorafenib group; there was no significant difference between them (p = .544) (Fig. 2A, 2B).
Figure 2.
Kaplan-Meier curves for patients with advanced hepatocellular carcinoma and main portal vein tumor thrombosis for the T+S and S groups. (A): The median OS for the T+S group (n = 43) was 7.0 months, and 6.0 months for the S group (n = 44) (p = .924). (B): The median TTP for the T+S group (n = 43) and the S group was 3.0 months for both groups (p = .544).
Abbreviations: OS, overall survival; S, sorafenib monotherapy; T+S, combination sorafenib plus transarterial chemoembolization; TTP, time to progression.
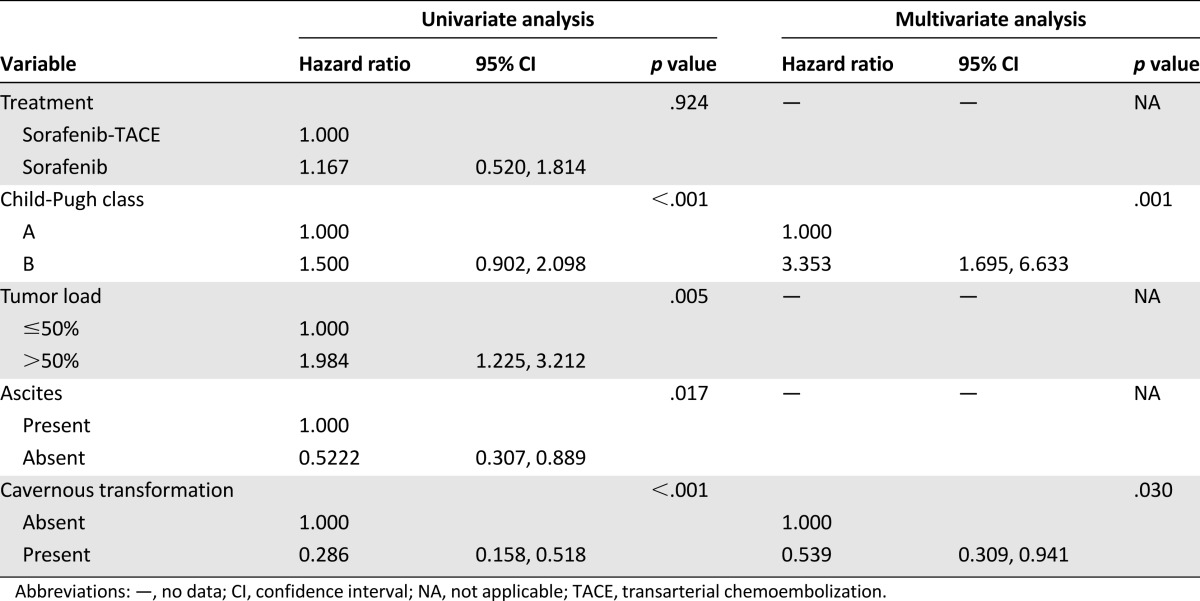
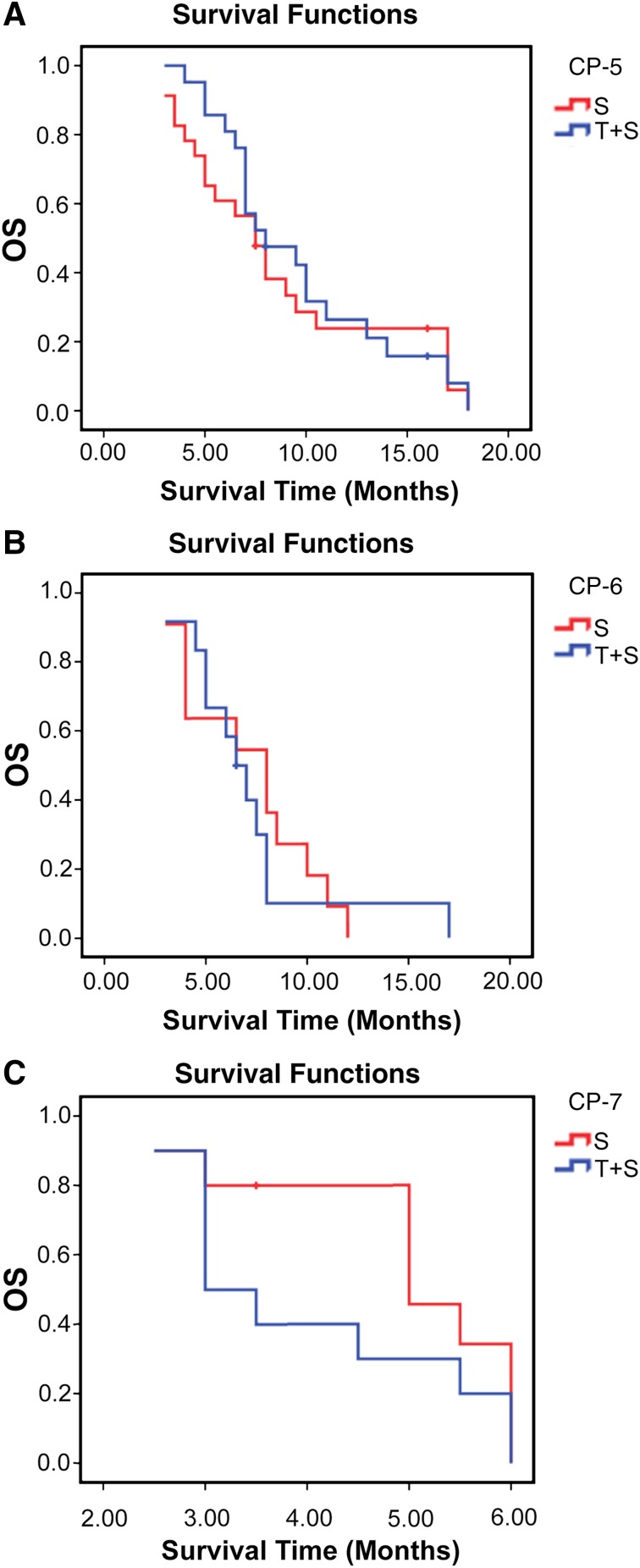
At univariate analysis, Child-Pugh class, tumor load, ascites, and cavernous transformation were significantly associated with overall survival. At multivariate Cox analysis, Child-Pugh class (hazard ratio (HR): 3.353; 95% CI: 1.695, 6.633; p = .001) and cavernous transformation (HR: 0.539; 95% CI: 0.309, 0.941; p = .030) were demonstrated as independent prognostic factors for overall survival (Table 3). The Child-Pugh (CP) score is a known predictor of survival of patients with HCC. Therefore, we further divided patients into CP-5 (those with a score of 5) CP-6 (a score of 6), and CP-7 (a score of 7) subgroups and compared their OS. The median OS of patients in subgroups CP-5, CP-6, and CP-7 was 8.0 months, 6.5 months, and 3.0 months, respectively, for sorafenib-TACE group (n = 21, 12, and 10) and 7.5 months, 8.0 months, and 5.0 months for sorafenib group (n = 23, 11, and 10; p = .669, .778, .233), respectively (Fig. 3A–C).
Table 3.
Univariate and multivariate analysis of prognostic factors
Figure 3.
Kaplan-Meier curves for patients with advanced hepatocellular carcinoma and main portal vein tumor thrombosis for the T+S and S groups. (A): Patients with CP-5: The median OS for T+S group (n = 21) and the S group (n = 23) was 8.0 months and 7.5 months, respectively (p = .669). (B): Patients with CP-6: The median OS for the T+S group (n = 12) and the S group (n = 11) was 6.5 months and 8.0 months, respectively (p = .778). (C): Patients with CP-7: The median OS for the T+S group and the S group (n = 10 for both) was 3.0 months and 5.0 months, respectively (p = .233).
Abbreviations: CP, Child-Pugh score; OS, overall survival; S, sorafenib monotherapy; T+S, combination sorafenib plus transarterial chemoembolization.
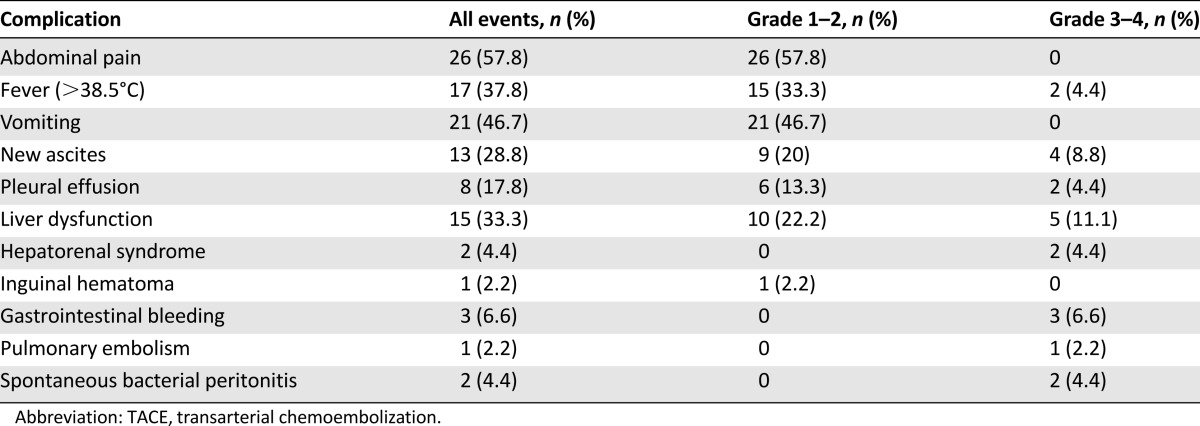
Safety and Toxicity
Side effects in patients treated with TACE are shown in Table 4. The most common adverse events were postembolization syndrome and liver dysfunction. Twenty-one grade 3 or higher adverse events were observed in 12 patients (26.7%) and 2 of them (4.4%) died within 2 weeks after the first TACE procedure (1 of liver failure; 1 of pulmonary embolism).
Table 4.
Complications of TACE in the sorafenib-TACE group
Adverse events related to sorafenib for the 2 groups are detailed in Table 5. Eight temporary reductions in sorafenib dose were made in the sorafenib-TACE group and six in the sorafenib group, due to sorafenib toxicity. The others received the full dose of sorafenib without interruption due to toxicity. The most common adverse events related to sorafenib included hand-foot skin reaction (HFSR), alopecia, and diarrhea. Fourteen grade 3 or higher adverse events were observed in 11 patients (12.3%) in the sorafenib-only group. The most common grade 3 or higher adverse events were HFSR and diarrhea.
Table 5.
Adverse events related to sorafenib administration in both groups
Discussion
We assessed the safety and efficacy of combination therapy of sorafenib and TACE and of sorafenib monotherapy for a specific subgroup of patients with advanced HCC and MPVTT. To our knowledge, this is the first study focusing on the outcome of combination therapy versus sorafenib monotherapy for the specific subgroup of patients.
In the present study, we observed a similar median OS between the sorafenib-TACE group and the sorafenib monotherapy group, which was likely due to the similar disease control rate and TTP in these two groups. This contradicts studies showing the effectiveness of TACE combined with sorafenib for advanced HCC [13–15]. However, the inclusion of less advanced-stage patients with MPVTT in those studies could be a possible explanation for the contradictions. On the other hand, we confirmed the results of another report [14] showing that TACE plus sorafenib has no benefits compared with TACE in the subgroup with MPVTT (the subgroup consisted of only 10 patients vs. 11 patients). The possible explanations were (a) the combination therapy may decrease collateral circulation of liver parenchyma from hepatic arteries and this decreased circulation exacerbates liver ischemic damage in patients with MPVTT [14]; and (b) vascular endothelial growth factor (VEGF) may contribute to native collateral formation and arteriogenesis [20], and sorafenib can inhibit the VEGF receptor, which is important for reconstruction of hepatic cells. Therefore, we suggest that the combination therapy may not benefit patients with MPVTT.
The median OS of 7.0 months for the sorafenib-TACE group in the present study is significantly shorter than that of other studies [11–15]. The shorter survival time might be related to the increased number of patients with MPVTT. The OS of 6.0 months for sorafenib monotherapy is also lower than that reported in SHARP (Sorafenib in Advanced Hepatocellular Carcinoma) trial (OS: 10.7 months), but that study cohort only consisted of 36% patients with macroscopic vascular invasion [5]. The increased number of patients with MPVTT and underlying disease (hepatitis B and cirrhosis) in the present study might account for the poor survival, since those factors are known negative predictors for OS [14, 21, 22].
Safety is the priority in the management of every disease. However, every therapy poses risks that must be balanced against the benefits. Although our results show moderate side effects, which were mostly manageable after TACE, some of the patients suffered from irreversible incidents that might have required urgent treatment or lead to death. The adverse events relating to sorafenib were comparable between the two groups. The data also demonstrate that the combination therapy has no benefit in term of DCR, TTP, or OS compared with sorafenib monotherapy. Considering all the side effects observed in the sorafenib-TACE group and its effectiveness, we believe that sorafenib monotherapy is a better approach than combination therapy for managing this specific subgroup of patients with advanced HCC and MPVTT.
In this study focusing on advanced HCC with MPVTT, cavernous transformation and Child-Pugh class were identified as independent prognostic factors for OS. Cavernous transformation of the portal vein is known to serve as a bypass route between the portal and the splanchnic veins [23]. The presence of a cavernous transformation was identified as a good prognostic factor because (a) adequate collateral circulation around an occluded portal vein prevents hepatic deterioration and (b) the presence of cavernous transformation might be the result of a slow-growing, less-aggressive tumor [24].
The Child-Pugh score is a known predictor of survival of patients with HCC. Moreover, TACE might aggravate liver function in patients with PVTT, especially in patients with MPVTT [2]. Therefore, we compared further the OS among the subgroups CP-5, CP-6, and CP-7. We found that the OS for patients receiving sorafenib monotherapy was longer than that for those receiving combination therapy in the subgroup CP-6 and CP-7. The difference persisted for 2 months but was not statistically significant. The most likely interpretation was the small sample size in the subgroups CP-6 and CP-7 (CP-6: n = 12 vs. n = 11; CP-7: n = 10 vs. n = 10). Those results demonstrated a trend indicating that sorafenib monotherapy might be more suitable for HCC patients with MPVTT and in the CP-6 or CP-7 subgroups than combination therapy of sorafenib and TACE, for the following reasons: (a) The combination therapy may exacerbate liver ischemic damage in patients with MPVTT; (b) a poor hepatic reserve increases the risk for irreversible hepatotoxicity after TACE caused by chemotherapy drugs and lipiodol or embolization agents, especially in the patients with MPV obstruction [9, 25, 26]; (c) patients with a CP score of 6 or 7 tolerated sorafenib well and benefited from it [27]. This finding is important and a large prospective trial should be explored to confirm it.
This study has some limitations. First, it is a retrospective analysis and the data came from a single center. The reason we analyzed data from the single center is that the success of TACE strongly depends on the applicator’s experience. In South China, this center had the largest population of patients with liver cancer and had considerable experience with TACE. Second, the sample size is relatively small. Third, therapeutic options (combination therapy or monotherapy) for patients were determined by the attending physician, which likely led to selection bias in our population. However, the bias was limited by choosing similar baseline characteristics between the two groups.
Conclusion
The results of this study suggest that there is no benefit in terms of DCR, TTP, and OS for combination therapy in patients with advanced HCC and MPVTT. Considering the patients’ morbidity after TACE, sorafenib monotherapy is appropriate for managing patients with advanced HCC and MPVTT. Additional and larger prospective studies are needed to confirm this conclusion.
Acknowledgments
We thank Ziping Li, Shiting Feng (Department of Radiology, the First Affiliated Hospital, Sun Yat-sen University, Guangzhou) for imaging assistance, and Yuantao Hao (School of Public Health, Sun Yat-sen University, Guangzhou) for statistical assistance in the report. Grant support was provided by the Natural Science Foundation of China (Grants 81371653 and 81171441), the Natural Science Foundation of Guangdong Province (Grant S2012020010904), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (Grant 20111139).
Author Contributions
Conception/Design: Yingqiang Zhang, Wenzhe Fan, Yu Wang, Jianyong Yang, Jiaping Li
Provision of study material or patients: Yingqiang Zhang, Wenzhe Fan, Yu Wang, Ligong Lu, Sirui Fu, Yonghui Huang, Jiaping Li
Collection and/or assembly of data: Yingqiang Zhang, Wenzhe Fan, Yu Wang, Ligong Lu, Sirui Fu, Wang Yao
Data analysis and interpretation: Yingqiang Zhang, Wenzhe Fan, Yu Wang, Ligong Lu, Sirui Fu
Manuscript writing: Yingqiang Zhang, Jiaping Li
Final approval of manuscript: Yingqiang Zhang, Wenzhe Fan, Yu Wang, Ligong Lu, Sirui Fu, Jianyong Yang, Yonghui Huang, Wang Yao, Jiaping Li
Disclosures
The authors indicated no financial relationships.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. European Organisation for Research and Treatment of Cancer EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: The BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 7.Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung GE, Lee JH, Kim HY, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258:627–634. doi: 10.1148/radiol.10101058. [DOI] [PubMed] [Google Scholar]
- 9.Lladó L, Virgili J, Figueras J, et al. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer. 2000;88:50–57. doi: 10.1002/(sici)1097-0142(20000101)88:1<50::aid-cncr8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Lee HS, Kim JS, Choi IJ, et al. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79:2087–2094. [PubMed] [Google Scholar]
- 11.Park JW, Koh YH, Kim HB, et al. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol. 2012;56:1336–1342. doi: 10.1016/j.jhep.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Cabrera R, Pannu DS, Caridi J, et al. The combination of sorafenib with transarterial chemoembolisation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;34:205–213. doi: 10.1111/j.1365-2036.2011.04697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y, Wang WJ, Guan S, et al. Sorafenib combined with transarterial chemoembolization for the treatment of advanced hepatocellular carcinoma: A large-scale multicenter study of 222 patients. Ann Oncol. 2013;24:1786–1792. doi: 10.1093/annonc/mdt072. [DOI] [PubMed] [Google Scholar]
- 14.Zhu K, Chen J, Lai L, et al. Hepatocellular carcinoma with portal vein tumor thrombus: Treatment with transarterial chemoembolization combined with sorafenib—a retrospective controlled study. Radiology. 2014;272:284–293. doi: 10.1148/radiol.14131946. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Zhang F, Yang J, et al. Combination of individualized local control and target-specific agent to improve unresectable liver cancer managements: A matched case-control study. Target Oncol. 2015;10:287–295. doi: 10.1007/s11523-014-0338-5. [DOI] [PubMed] [Google Scholar]
- 16.Chung JW, Park JH, Han JK, et al. Hepatocellular carcinoma and portal vein invasion: Results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol. 1995;165:315–321. doi: 10.2214/ajr.165.2.7618547. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute. Common terminology criteria for adverse events, version 3.0. http://ctep.cancer.gov/reporting/ctc.html. 2006.
- 18.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Srivastava DN, Chau TT, et al. Inoperable hepatocellular carcinoma: Transarterial 188Re HDD-labeled iodized oil for treatment—prospective multicenter clinical trial. Radiology. 2007;243:509–519. doi: 10.1148/radiol.2432051246. [DOI] [PubMed] [Google Scholar]
- 20.Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res. 2008;103:1027–1036. doi: 10.1161/CIRCRESAHA.108.181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 22.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 23.Song B, Min P, Oudkerk M, et al. Cavernous transformation of the portal vein secondary to tumor thrombosis of hepatocellular carcinoma: Spiral CT visualization of the collateral vessels. Abdom Imaging. 2000;25:385–393. doi: 10.1007/s002610000057. [DOI] [PubMed] [Google Scholar]
- 24.Pentecost MJ, Daniels JR, Teitelbaum GP, et al. Hepatic chemoembolization: Safety with portal vein thrombosis. J Vasc Interv Radiol. 1993;4:347–351. doi: 10.1016/s1051-0443(93)71873-4. [DOI] [PubMed] [Google Scholar]
- 25.Garwood ER, Fidelman N, Hoch SE, et al. Morbidity and mortality following transarterial liver chemoembolization in patients with hepatocellular carcinoma and synthetic hepatic dysfunction. Liver Transpl. 2013;19:164–173. doi: 10.1002/lt.23552. [DOI] [PubMed] [Google Scholar]
- 26.Bahirwani R, Reddy KR. Drug-induced liver injury due to cancer chemotherapeutic agents. Semin Liver Dis. 2014;34:162–171. doi: 10.1055/s-0034-1375957. [DOI] [PubMed] [Google Scholar]
- 27.Chiu J, Tang YF, Yao TJ, et al. The use of single-agent sorafenib in the treatment of advanced hepatocellular carcinoma patients with underlying Child-Pugh B liver cirrhosis: A retrospective analysis of efficacy, safety, and survival benefits. Cancer. 2012;118:5293–5301. doi: 10.1002/cncr.27543. [DOI] [PubMed] [Google Scholar]