Abstract
Pulmonary tuberculosis (TB) remains one of the leading causes of infectious disease death despite widespread usage of the BCG vaccine. A number of new TB vaccines have moved into clinical evaluation to replace or boost the BCG vaccine including ID93+GLA-SE, an adjuvanted subunit vaccine. The vast majority of new TB vaccines in trials are delivered parenterally even though intranasal delivery can augment lung-resident immunity and protective efficacy in small animal models. Parenteral immunization with the adjuvanted subunit vaccine ID93+GLA-SE elicits robust TH1 immunity and protection against aerosolized Mycobacterium tuberculosis in mice and guinea pigs. Here we describe the immunogenicity and efficacy of this vaccine when delivered intranasally. Intranasal delivery switches the CD4 T cell response from a TH1 to a TH17 dominated tissue-resident response with increased frequencies of ID93-specific cells in both the lung tissue and at the lung surface. Surprisingly these changes do not affect the protective efficacy of ID93+GLA-SE. Unlike intramuscular immunization, ID93+GLA does not require the squalene-based oil-in-water emulsion SE to elicit protective CD4 T cells when delivered intranasally. Finally we demonstrate that TNF and the IL-17 receptor are dispensable for the efficacy of the intranasal vaccine suggesting an alternative mechanism of protection.
Keywords: tuberculosis, mucosal, TH17, ID93+GLA-SE, intranasal
Introduction
Mycobacterium tuberculosis (Mtb) infects one-third of the world population and causes eight million cases of tuberculosis (TB) annually [1]. Several new vaccines have entered Phase 1 and 2 clinical testing to prevent infection or disease [2]. The first efficacy trial of one of these candidates, Modified Vaccinia virus Ankara expressing Ag85A (MVA85A), failed to reach either of these important benchmarks, reinforcing the need for new TB vaccine candidates [3]. We have developed a novel fusion protein antigen, designated ID93, consisting of four Mtb proteins, Rv1813, Rv2608, Rv3619, and Rv3620, and adjuvanted with the synthetic TLR4 agonist glucopyranosyl lipid adjuvant (GLA) formulated in a stable oil-in-water emulsion (SE). ID93+GLA-SE elicits a multi-functional TH1 response when delivered intramuscularly and limits both pulmonary and disseminated infection following exposure to aerosolized Mtb [4-6]. Intramuscular ID93+GLA-SE is currently undergoing Phase 2 immunogenicity and efficacy testing.
Immune responses to vaccine antigens can be shaped by the choice of vaccine adjuvant, the specific formulation of the antigen and adjuvant combination, and the route of vaccine delivery. Without the GLA-SE adjuvant ID93 elicits a TH2 response and does not protect against aerosolized Mtb in mice or guinea pigs [7]. When combined with the synthetic TLR4 agonist GLA in an oil-in-water stable nano-emulsion (SE) the vaccine drives robust TH1 responses and is protective. However, ID93+GLA formulated in an aqueous nanosuspension lacking an oil component (ID93+GLA-AF) fails to elicit protective TH1 responses, highlighting the importance of formulation effects on vaccine immunogenicity [6].
Mtb is primarily controlled by CD4 T cells, with both IFN-γ and TNF being implicated as critical effector cytokines [8]. Additionally IL-17 producing TH17 cells can contribute to vaccine efficacy in some circumstances by recruiting TH1 cells to the site of infection [9]. Mtb is acquired following aerosol exposure; thus, inducing robust pulmonary immunity may augment vaccine efficacy. Therefore there is considerable interest in developing TB vaccines that preferentially induce lung-resident CD4 T cell responses where they can react quickly. Intranasal delivery of either virally vectored TB vaccines such as MVA85A or adjuvanted protein vaccines can enhance lung-resident immunity and efficacy against Mtb [10-12]. Besides enhancing lung-resident immunity, intranasally administered vaccines may facilitate various practical benefits of high importance in the developing world, such as eliminating the risk of disease transmission through improper needle use as well as the need for sharps waste containment, and increasing compliance among end users while reducing the need for trained medical personnel to administer the vaccine [13]. We previously reported that intramuscular immunization with ID93+GLA-SE elicits TH1 responses in both the spleen and lung which may account for its protective efficacy [6]. In the current paper we assess whether intranasal delivery of ID93+GLA-SE enhances lung-resident immunity and subsequent efficacy against aerosolized Mtb challenge.
Materials and Methods
Mice and immunizations
Wild type C57Bl/6 and TNF-/- mice on the C57Bl/6 background were purchased from Jackson Laboratories (Bar Harbor, ME). IL-17R-/- mice on the C57Bl/6 background were a gift from Amgen, Seattle [14]. Mice were immunized with ID93 (0.5 μg) adjuvanted with GLA-SE or GLA-AF (5 μg) via intramuscular injection or intranasal delivery to both nostrils (25 μL total volume). Adjuvants were prepared as described previously [6]. Mice were anesthetized with ketamine and xylazine prior to intranasal immunization. Mice were immunized 3 times at 3 week intervals. All mice were maintained in specific pathogen-free conditions. After infection animals were maintained in BL3 containment. All procedures were approved by the IDRI institutional animal care and use committee.
Isolation of lymphocytes
Lymphocytes from BALF were isolated by lavaging 3mL of cold PBS into the lungs via the trachea twice. Cells were concentrated by centrifugation and resuspended in RPMI1640 and 10% FCS for subsequent analysis. Mice were then perfused with 10 mL of cold PBS into the right ventricle. Perfused lungs were collected, digested for 30 minutes with Liberase TM (Roche), and dissociated using a GentleMACS system (Miltenyi) to isolate lung lymphocytes. Splenocytes were isolated by dissociation and red blood cells were lysed using Red Blood Cell Lysis Buffer (eBioscience).
In vivo labeling of T cells
In some experiments 5μg of PE-Cy7 conjugated α-CD45.2 (clone 104) (BioLegend) was injected intravenously 3 minutes prior to euthanasia [15]. Lung lymphocytes were then isolated as described above and stained for CD4 (clone RM4-5), CD44 (clone IM7), CD69 (clone H1.2F3) and an I-Ab tetramer presenting the dominant epitope of Rv3619 (VIYEQANAHGQ), one of the components of ID93. APC labeled tetramers were provided by the NIH Tetramer Core Facility. Up to 106 events were collected on an LSR Fortessa flow cytometer (BD Biosciences). Data were analyzed with FlowJo v9. Cells were gated as singlets > lymphocytes > CD4+ > CD44+ tetramer+ > CD90.2.
Intracellular cytokine staining
Cells were plated at 2×106 cells/well and stimulated for 2 hours at 37°C with ID93 (10 μg/mL) or unstimulated. GolgiPlug (BD Biosciences) was added and the cells were incubated for an additional 8 hours at 37°C. Cells were washed and surface stained with fluorochrome-labeled antibodies to CD4 and CD8 (clone 53-6.7) (BioLegend and eBioscience) in the presence of anti-CD16/32 (clone 2.4G2) for 20 minutes. Cells were washed and permeabilized with Cytofix/Cytoperm (BD Biosciences) for 20 minutes. Cells were washed with Perm/Wash (BD Biosciences) and stained intracellularly with fluorochrome-labeled antibodies to CD154 (clone MR1), IFN-γ (clone XMG-1.2), IL-2 (clone JES6-5H4), TNF (clone MP6-XT22), GM-CSF (clone MP1-22E9), and IL-17A (clone TC11-18H10.1) (BioLegend and eBioscience) for 20 minutes. Cells were washed and resuspended in PBS. Up to 106 events were collected on an LSRFortessa flow cytometer (BD Biosciences). Data were analyzed with FlowJo v9. Cells were gated as singlets > lymphocytes > CD4+ CD8- > cytokine positive. ID93 specific response frequencies were determined by subtracting the frequency of response positives of unstimulated cells from ID93 stimulated cells in matched samples.
Multiplex quantitation of secreted cytokines
Cells were plated at 2×105 cells/well and stimulated for 48 hours at 37°C with ID93 (10 μg/mL) or unstimulated. Supernatants were stored at -20°C until analysis. Supernatants were analyzed in duplicate for secreted IFN-γ, IL,4, IL-5, IL-6, IL-10, IL-17A, IL-17F, IL-21, IL22, IL-23, GM-CSF, MIP-1α, and MIP1β using a custom ProcartaPlex Multiplex Immunoassay (eBioscience) according to the manufacturer's instructions. Alternatively supernatants were acid treated and analyzed for secreted active TGFβ1 using a custom ProcartaPlex Simplex Immunoassay (eBioscience) according to the manufacturer's instructions. Samples were read on a Luminex 200 (LuminexCorp) with MasterPlex Software (Hitachi Solutions America, Ltd.).
Mtb aerosol challenge and enumeration
Four weeks after the last immunization mice were aerogenically infected with M. tuberculosis H37Rv (ATCC No. 35718; American Type Culture Collection) using a GlasCol aerosol generator calibrated to deliver 50–100 bacteria into the lungs. Protection was determined at the indicated times after challenge by harvesting the lungs and spleens, homogenizing the tissue in 0.1% PBS–Tween 80, and plating 5-fold serial dilutions on 7H10 agar plates (Molecular Toxicology) for bacterial growth. Bacterial colony forming units (CFU) were counted after incubation at 37°C with 5% CO2 for 14 days.
Statistics
Bacterial counts were log-transformed prior to analysis. ANOVA analysis with the Bonferroni correction for multiple comparisons was used to determine significant reductions in bacterial burden vs the unimmunized, genotype-matched controls. Statistical analyses were performed using Prism software (GraphPad Software, Inc., La Jolla, CA).
Results
Vaccination route dictates durable TH1 or TH17 responses
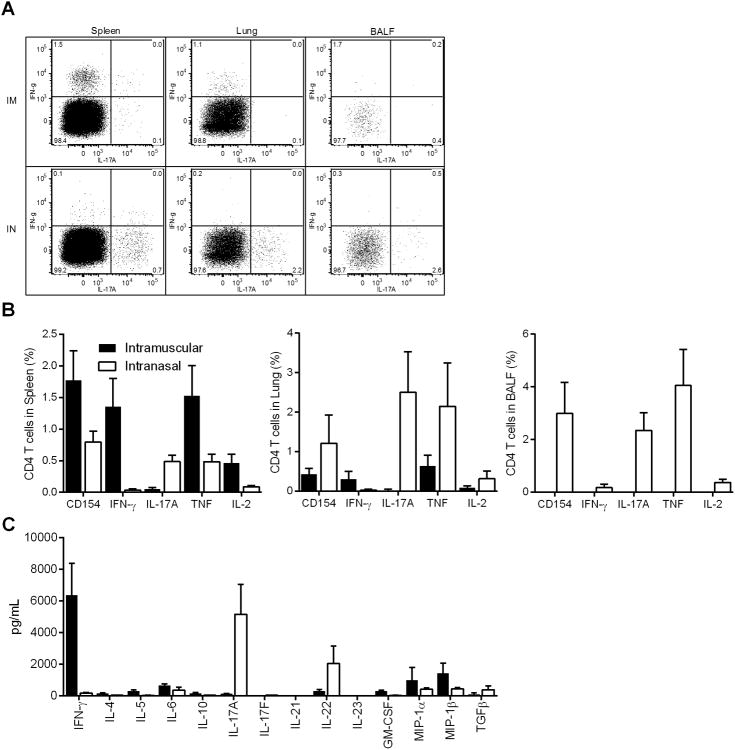
Intramuscular immunization with ID93+GLA-SE elicits a robust TH1 response and limits Mtb in mice and guinea pigs. To determine whether intranasal immunization can enhance the immunogenicity of ID93+GLA-SE we immunized C57Bl/6 mice by intramuscular or intranasal delivery. Four weeks after the third immunization we found that intranasal immunization elicited a significantly different quality of CD4 T cell response. Intramuscular immunization elicited a classical TH1 immune response characterized by IFN-γ producing ID93-specific CD4 T cells in the spleen and lung. Intranasal immunization switched this response to a TH17 response with significant production of IL-17A, but not IFN-γ, by CD4 T cells (Figure 1A). Additionally intranasal immunization elicited ID93-specific cells that localized to the lung surface as determined by cells recovered from bronchoalveolar lavage fluid (BALF). ID93-specific CD4 T cells elicited by intranasal or intramuscular immunization also expressed CD154, TNF, and IL-2 upon antigen stimulation. Intramuscular immunization preferentially elicited systemic CD4 T cells as evidenced by higher CD4 T cell responses in the spleen. Conversely intranasal immunization preferentially elicited tissue resident CD4 T cells as there were higher frequencies of antigen-specific cells in both the lung and BALF (Figure 1B). In addition to IL-17A intranasal immunization also generated splenocytes that secrete IL-22, but not IL-17F, whereas intramuscular immunization promoted GM-CSF, MIP-1α and MIP-1β secreting cells (Figure 1C). Cytokine production by CD8 T cells was not evident in the spleen, lung, or BALF following either vaccination regimen.
Figure 1. Immunization route with ID93+GLA-SE determines TH1 or TH17 induction.
B6 mice were immunized with ID93+GLA-SE either by intramuscular injection or intranasal instillation. One month after the third immunization lymphocytes were isolated and stimulated with media or ID93. (A) Representative FACS plots of CD4 T cell responses following ID93 stimulation of lymphocytes from the spleen, lung and BALF of mice immunized intramuscularly (top row) or intranasally (bottom row). (B) CD4 T cell responses upon ID93 stimulation. (C) Cytokines secreted by stimulated splenocytes. N = 5 mice/group. Data are representative of five experiments with similar results. Mean + s.d. is shown.
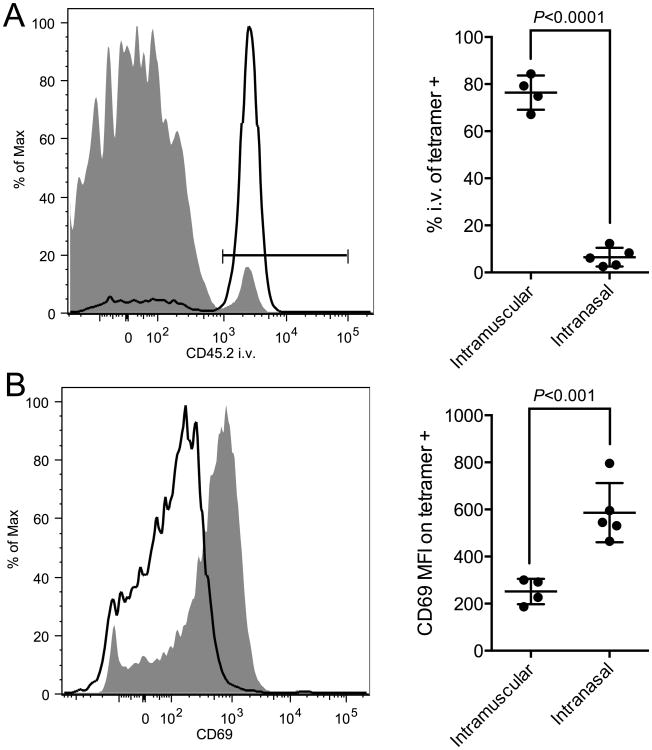
Up to 70% of T cells isolated from mouse lungs may be located in the vasculature rather than in the lung parenchyma despite perfusion [15]. To determine whether the ID93-specific CD4 T cells isolated from the lungs of immunized animals resided in the vasculature or parenchyma we intravenously injected fluorochrome labeled a-CD45.2 immediately before euthanasia. This technique labels cells within the bloodstream but not within tissues [15]. Vaccine specific CD4 T cells were then identified with an I-Ab tetramer bound to the dominant epitope of Rv3619. 80% of the tetramer binding cells in the lungs of animals receiving intramuscular immunization were labeled with the i.v. antibody indicating that they were located in the lung vasculature (Figure 2A). Conversely only 5% of the tetramer binding cells in the lungs of animals receiving intranasal immunization were labeled with the i.v. antibody indicating that they were residing in the lung tissue (Figure 2B). Further the lung-resident cells elicited by intranasal vaccination had a higher expression of CD69, compared to the cells elicited by intramuscular immunization, further confirming that intranasal immunization preferentially drives tissue-resident memory cells (Figure 2C).
Figure 2. Intranasal vaccination elicits CD4 T cells that reside exclusively in the lung parenchyma.
B6 mice were immunized with ID93+GLA-SE either by intramuscular injection or intranasal instillation. One month after the third immunization blood resident T cells were labeled by intravenous injection of α–CD45.2 prior to euthanasia of the animals. (A) Vaccine specific CD4 T cells in the lungs were identified by tetramer staining. (B) Intravenous CD45.2 labeling vaccine specific CD4 T cells from intramuscular (black line) or intranasal (grey fill) vaccinated animals is shown. The bar indicates the CD45.2+ gate. (C) CD69 staining of tetramer labeled cells is shown for intramuscular (black line) and intranasal (grey fill) vaccinated animals. Means ± s.d. are shown for 4-5 mice/group. The data are representative of three experiments with similar results.
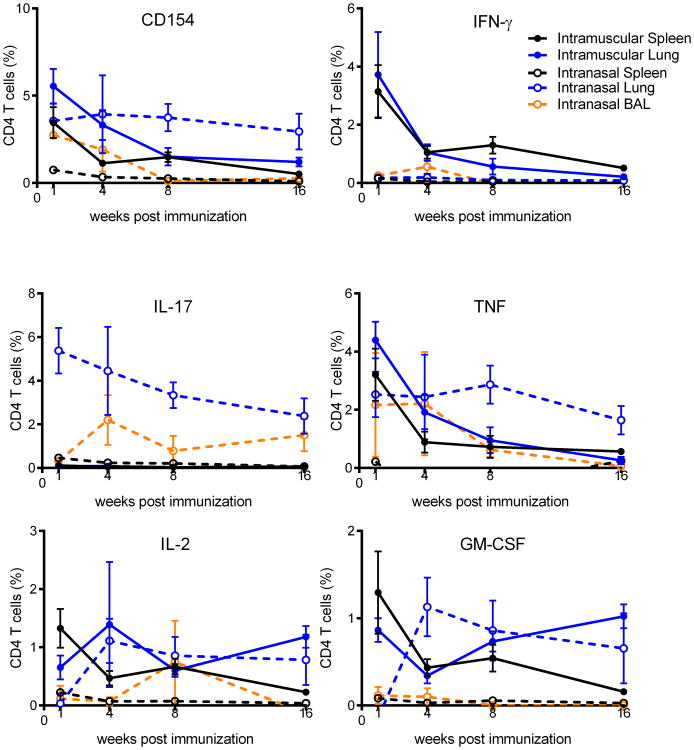
To determine whether this switch from TH1 to TH17 induction by intranasal vaccination elicited long-term stable memory populations we examined the kinetics of the CD4 T cell response from one week to four months after the final immunization. The cytokine production profile of CD4 T cells from the spleen, lung, and BALF elicited by intranasal (TH17) or intramuscular (TH1) immunization remained unchanged from one week to four months post-infection indicating that the CD4 T cell programming was stable in the absence of further stimulation. Notably the magnitude of the CD4 T cell response showed the same gradual loss of antigen-specific cells over time, regardless of immunization route (Figure 3). This was surprising as it had been previously reported that TH17 cells elicited by intranasal infection with Listeria monocytogenes failed to persist, whereas TH1 cells elicited by parenteral infection established long-lived memory responses [16]. Taken together, these data show that intranasal immunization with ID93+GLA-SE can elicit long-lived TH17 responses that preferentially reside in the lung, both in the tissue and at the lung surface.
Figure 3. Kinetics of memory CD4 T cell responses following intranasal or intramuscular immunization.
B6 mice were immunized three times with ID93+GLA-SE by the intranasal (dashed lines) or intramuscular (solid lines) route. 1,4, 8 or 16 weeks after the final immunization ID93 specific CD4 T cell responses from the spleen (black lines), lung (blue lines), and BALF (orange lines) were assessed by intracellular staining for CD154, IFN-γ, TNF, IL-17A, IL-2, and GM-CSF upon ID93 stimulation as indicated. N = 5 mice/group/timepoint. Data are representative of two experiments with similar results. Mean + s.d. is shown.
TH1 and TH17 vaccine-elicited immunity are similarly protective against Mtb
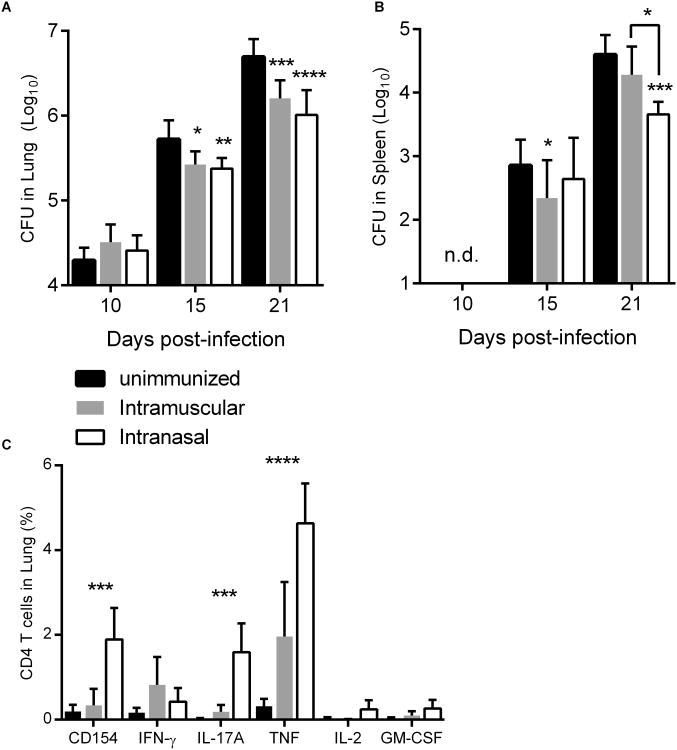
To determine whether the increased frequency of ID93-specific cells in the lung parenchyma and at the lung surface could enhance control of aerosolized Mtb we infected mice which were either unimmunized or immunized by the intranasal or intramuscular route. Ten days after challenge there were no significant differences in Mtb burden in the lung. By fifteen days post infection there was a modest, but significant, reduction of lung bacterial burden in both immunized groups. Protective efficacy in the lung was further enhanced at three weeks of infection, however there were no differences in efficacy in the lung with intranasal or intramuscular vaccine delivery (Figure 4A). Notably intranasal immunization did slow the dissemination of Mtb from the lung to the spleen and this was more substantial than the splenic protection elicited by intramuscular immunization (Figure 4B). Three weeks after Mtb infection ID93-specific CD4 T cells retained the cytokine production profile imprinted by intranasal (TH17) or intramuscular (TH1) vaccination (Figure 4C). Overall intranasal immunization elicited greater frequencies of ID93 specific cells post-infection. These data demonstrate that either TH1 or TH17 ID93-specific cells are sufficient to limit Mtb infection via prophylactic immunization.
Figure 4. ID93+GLA-SE immunization by either the intramuscular or intranasal route protects against aerosolized Mtb.
B6 mice were immunized three times with ID93+GLA-SE by the intranasal (white bars) or intramuscular (grey bars) route or left unimmunized (black bars). Four weeks after the final immunization mice were infected with a low dose of aerosolized Mtb. 10, 15, and 21 days later Mtb burden in the (A) lungs and (B) spleens were determined. N = 7 mice/group/timepoint. (C) 21 days after infection lung lymphocytes were assessed by intracellular staining for CD154, IFN-γ, TNF, IL-17A, IL-2, and GM-CSF upon ID93 stimulation as indicated. Data are representative of two experiments with similar results. Mean + s.d. is shown. *,**,***, and **** indicate P < 0.05, 0.01, 0.001 and 0.0001, respectively. (A and B) Statistically significant differences from unimmunized are shown unless otherwise indicated. (C) Statistically significant differences between intramuscular and intranasal immunization are indicated.
IL-17 and TNF are dispensable for intranasal vaccine efficacy
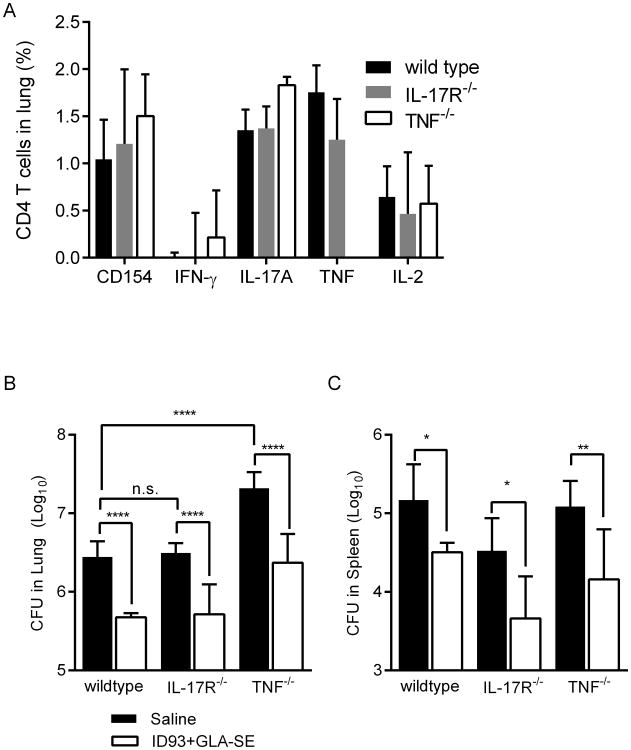
IFN-γ is critical for the control of primary Mtb infection in both patients and experimentally infected animals, therefore we were surprised that intranasal immunization with ID93+GLA-SE limited Mtb burden despite not eliciting substantial numbers of IFN-γ producing CD4 T cells (Figure 3). One possible explanation for this is the large frequency of ID93-specific cells that produce TNF, which is also critical for control of Mtb, is sufficient to limit infection [17]. Another possibility is that IL-17A production is sufficient to control Mtb in the absence of vaccine elicited IFN-γ producing cells [9]. To determine if either IL-17 responsiveness or TNF were necessary for the efficacy of intranasal ID93+GLA-SE immunization we immunized wild type, IL-17R-/-, or TNF-/- mice with intranasal ID93+GLA-SE. Immunization of any of the three genotypes resulted in very similar ID93-specific TH17 responses in the lungs, with the exception that TNF-/- mice did not generate TNF-producing cells, as expected (Figure 5A). Following Mtb infection unimmunized TNF-/- mice exhibited substantially higher bacterial burdens in their lungs compared to unimmunized wild type mice, in line with previous reports (Figure 5B) [17]. However ID93+GLA-SE was as protective in IL-17R-/- or TNF-/-mice as in wild type mice indicating that these cytokines are not critical for control of Mtb by ID93+GLA-SE, even though there are few IFN-γ producing cells elicited by vaccination (Figure 5B and C). Therefore although intranasal immunization with ID93+GLA-SE induces TNF and IL-17A secreting cells, these cytokines are not necessary for vaccine efficacy against aerosolized Mtb.
Figure 5. The protective efficacy of intranasally delivered ID93+GLA-SE does not require TNF or IL-17R.
Wildtype, IL-17R-/-, TNF-/- were immunized with saline or intranasal ID93+GLA-SE. Four weeks after the final immunization, ID93-specific CD4 T cell responses from the (A) lungs were assessed by intracellular staining for CD154, IFN-γ, IL-17A, and IL-2 upon ID93 stimulation. N = 5 mice/group. Four weeks after the final immunized mice were infected with a low dose of aerosolized Mtb. Three weeks later Mtb burden in the (B) lungs and (C) spleens were determined. N = 7 mice/group. Data are representative of two experiments with similar results. Mean + s.d. is shown. *,**, and **** indicate P < 0.05, 0.01, and 0.0001, respectively.
Formulation of GLA in an oil-in-water emulsion is not necessary for T cell induction and efficacy by intranasal vaccination
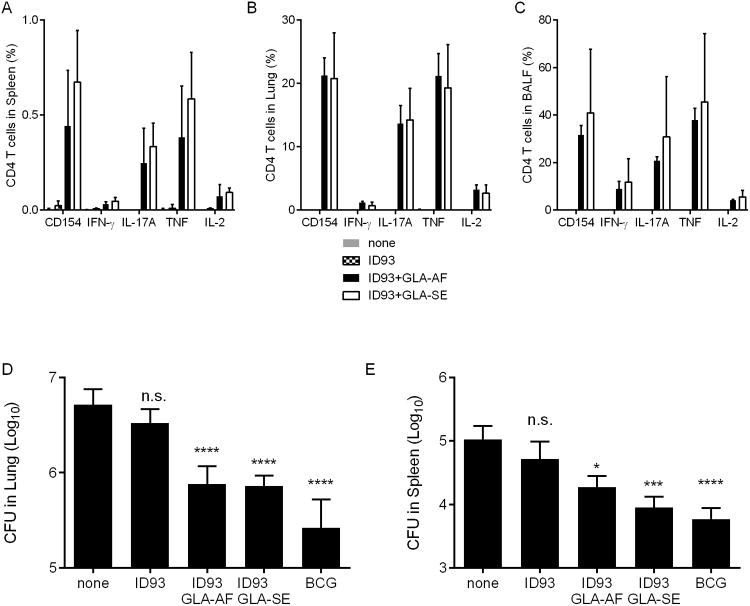
We previously found that formulating ID93 and GLA in a squalene based oil-in-water nanoemulsion (GLA-SE) was necessary to induce a high frequency of TH1 cells and limit Mtb burden. ID93 with an aqueous suspension of GLA lacking an oil component (GLA-AF) failed to elicit TH1 responses or limit Mtb burden when delivered intramuscularly [6]. To determine whether the oil-in-water emulsion system was also necessary for intranasal vaccination we immunized mice with ID93 alone or adjuvanted with either GLA-AF or GLA-SE. Intranasal immunization with ID93 was insufficient to induce ID93-specific CD4 T cell responses in the spleen, lung, or BALF confirming that a GLA-containing adjuvant was necessary for CD4 T cell induction (Figure 6A-C). To our surprise, both the oil-in-water emulsion (GLA-SE) and aqueous nanosuspension (GLA-AF) formulations of GLA elicited robust TH17 responses in the spleen, lung, and BALF when administered together with ID93. The quality and magnitude of the ID93-specific CD4 T cell responses were indistinguishable between the two formulations. Moreover, intranasal immunization with ID93+GLA-AF or ID93+GLA-SE elicited similar levels of protection against aerosolized Mtb, whereas intranasal ID93 alone did not impact bacterial burden in the lungs or spleen (Figure 6D and E). Thus in contrast to intramuscular immunization, ID93+GLA does not require an oil-in-water emulsion to be immunogenic or efficacious when delivered intranasally.
Figure 6. Stable nanoemulsions and nanosuspension formulations of ID93+GLA elicit similar TH17 responses and protective efficacy.
B6 mice were immunized with ID93 alone (stippled bars) or adjuvanted GLA-SE (white bars) or GLA-AF (grey bars) by intranasal delivery or left unimmunized. Four weeks after the final immunization, ID93-specific CD4 T cell responses from the (A) spleen, (B) lung, and (C) BALF were assessed by intracellular staining for CD154, IFN-γ, IL-17A, IL-2, and GM-CSF upon ID93 stimulation. N = 5 mice/group. Four weeks after the final immunized mice were infected with a low dose of aerosolized Mtb. Three weeks later Mtb burden in the (D) lungs and (E) spleens were determined. A cohort of mice that received a single immunization with BCG was used as a positive control for vaccine efficacy. N = 7 mice/group. Data are representative of two experiments with similar results. Mean + s.d. is shown. * and **** indicate P < 0.05 and 0.0001, respectively.
Discussion
In the present study we evaluated whether intranasal delivery of the clinical stage vaccine ID93+GLA-SE would augment the immunogenicity or efficacy against aerosolized Mtb as has been found for other TB vaccines. Compared to parenteral immunization, intranasal immunization altered the response to vaccination from TH1 to TH17 dominated responses. These TH17 responses were long-lasting and stable upon Mtb infection. Unlike other TB vaccine candidates, intranasal delivery of ID93+GLA-SE did not enhance its protective efficacy over intramuscular delivery. The reasons for this are unclear but could be related to the switch from TH1 to TH17 or limitations of the mouse system employed. Although CD4 T cells elicited by vaccination primarily produced TNF and IL-17A, neither of these effector functions was necessary for vaccine efficacy. Finally, unlike with intramuscular immunization the squalene-based oil-in-water formulation was not required for the immunogenicity or efficacy of ID93+GLA when delivered intranasally.
Intranasal immunization has proven a tractable means of eliciting durable immune responses at mucosal surfaces including the lung which is the site of Mtb infection. Therefore there has been considerable discussion of whether any new TB vaccine should be delivered intranasally to maximize immune responses at the mucosal surfaces. In support of this several studies have shown that intranasal delivery of TB vaccines provides superior protection to that provided by parenteral immunization with the same vaccine. This has held true for adjuvanted subunit vaccines, BCG, and vectored vaccines. In some cases this improved efficacy was correlated with an increased frequency of vaccine specific CD4 T cells in the lung or at the lung surface [10-12, 18-22]. However unlike many of these candidates we find substantial frequencies of ID93-specific CD4 T cells in both the lung parenchyma and vasculature following parenteral immunization with ID93+GLA-SE. This may account for the efficacy of parenteral ID93+GLA-SE immunization and a lack of detectable benefit of switching to an intranasal delivery route.
The rational development of TB vaccines would be greatly aided by the identification of mechanisms of vaccine efficacy. To date most TB vaccine development has focused on eliciting IFN-γ-producing CD4 T cells as defects in IFN-γ production or signaling are detrimental to control of Mtb in both humans and experimental animals [23-26]. More recently there has been considerable focus on inducing multi-functional CD4 T cells that make combinations of cytokines including IFN-γ, TNF, and IL-2. Thus we were surprised that despite not eliciting IFN-γ producing CD4 T cells intranasal immunization with ID93+GLA-SE controlled Mtb infection. Even more surprisingly this held true for vaccinated TNF-/- mice which are highly susceptible to Mtb [17], suggesting that neither IFN-γ nor TNF are necessary for vaccine efficacy against TB. We recently found that the protective efficacy of intramuscular ID93+GLA-SE required antigen specific CD4 T cells, but was independent of both TNF and IFNγR, suggesting a novel or redundant mechanism of protection [27]. Similarly Gallegos et al. found that in vitro primed ESAT-6 specific CD4 TCR transgenic cells could protect against TB in a manner independent of IFN-γ and TNF {Gallegos, 2011 #119}. Previous work suggested that vaccine elicited TH17 cells could contribute to control of Mtb infection by recruiting IFN-γ producing CD4 T cells into the infection site [9]. However this is unlikely to be the mechanism of protection for intranasal ID93+GLA vaccination as there were very few ID93-specific CD4 T cells capable of making IFN-γ after infection. Additionally IL-17 signaling through the IL-17R was not required for vaccine efficacy. This suggests that there is either a highly redundant network of immune responses sufficient to control Mtb following vaccination and/or there are alternative mechanisms of protection that remain to be defined. Recent work has suggested that the majority of IFN-γ producing CD4 T cells in an Mtb-infected mouse lung are located in the lung vasculature, not in the parenchyma and that these cells do not contribute to the bulk of Mtb control by CD4 T cells [28]. Identifying mechanisms of efficacy or at least correlates of protection, for new TB vaccines would aid in the clinical development of these candidates. It is possible that such mechanisms will be different for each vaccine candidate modality (e.g. intranasal vs parenteral immunization or adjuvanted subunit vaccine vs viral vectored vaccine).
One potential concern with intranasal immunization is possible adverse side effects since administered components could be transported to the central nervous system [13]. Indeed, intranasal delivery of a non-toxic mutant of E. coli heat labile toxin (LTK63) induced facial paralysis in a subset of volunteers who received it as part of a vaccine candidate, most likely because the toxin-based adjuvant accessed the CNS via binding to ganglioside receptors in the olfactory epithelium [29]. However, live-attenuated influenza vaccines currently available commercially have demonstrated acceptable safety profiles and even enhanced efficacy in children compared to parenteral vaccines; indeed, the U.S. Advisory Committee on Immunization Practices preferentially recommended that children aged 2-8 years old receive intranasal live attenuated influenza vaccine, and uptake has been increasing in recent years [30]. It is also important to note that GLA is a synthetic, molecularly defined, highly pure compound representing the next generation of vaccine immune potentiators that are based on rational structural design and well defined components which thus far have not been reported to cause safety concerns following intranasal administration such as those seen with the toxin-based adjuvants. In this regard it is interesting to note that the oil-free formulation of ID93+GLA was as immunogenic and protective in the present study as the emulsion-based formulation. While no safety concerns have been reported with oil-in-water emulsion based intranasal formulations [31], the aqueous suspension formulation of GLA contains much less excipient content, thereby reducing manufacturing cost as well as the potential for any reactogenicity due to the oil and emulsifier excipients present in the emulsion. Moreover, previous reports of intranasally administered aqueous suspension of the TLR4 ligand MPL have shown promising safety and immunogenicity results in preclinical and clinical testing [32-34]. Thus, it may be possible to move the oil-free vaccine (ID93+GLA-AF) forward into clinical testing.
To date GLA containing vaccines have been delivered to hundreds of subjects via parenteral immunization for a number of diseases without serious adverse events. The data presented here support the concept of intranasal immunization with a GLA-containing adjuvant, particularly for a pulmonary infection such as TB. It will be important to determine whether the TH17 induction upon intranasal vaccination will be recapitulated in human subjects as well as the augmentation of lung resident T cells. If so it may be instructive to compare the protective efficacy of parenteral and intranasal ID93+GLA immunization in humans as they elicit significantly different immune profiles to the same antigen. The results of such a clinical comparison would be important to inform future TB vaccine development as it could point to potential immune correlates of protection. Additionally intranasal delivery of a vaccine adjuvanted with GLA or other TLR4 agonist containing adjuvants may be beneficial for other diseases controlled by TH17 cells. For example a new pertussis vaccine may benefit from this approach as TH17 cells contribute to control of this pathogen [35, 36].
Acknowledgments
We thank Traci Mikasa, Molly Blust, Susan Lin, and Tony Phan for preparing adjuvants for these studies and Jeff Guderian for technical assistance with the multiplex cytokine analysis. This work was conducted with support from National Institutes of Health grant AI-078054 and contract HHSN272200800045C to R.N.C.
Footnotes
Disclosure: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–70. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen P, Kaufmann SH. Novel vaccination strategies against tuberculosis. Cold Spring Harbor perspectives in medicine. 2014:4. doi: 10.1101/cshperspect.a018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381:1021–8. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertholet S, Ireton GC, Kahn M, Guderian J, Mohamath R, Stride N, et al. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J Immunol. 2008;181:7948–57. doi: 10.4049/jimmunol.181.11.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, et al. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med. 2010;2:53ra74. doi: 10.1126/scitranslmed.3001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orr MT, Fox CB, Baldwin SL, Sivananthan SJ, Lucas E, Lin S, et al. Adjuvant formulation structure and composition are critical for the development of an effective vaccine against tuberculosis. J Control Release. 2013;172:190–200. doi: 10.1016/j.jconrel.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldwin SL, Bertholet S, Reese VA, Ching LK, Reed SG, Coler RN. The importance of adjuvant formulation in the development of a tuberculosis vaccine. J Immunol. 2012;188:2189–97. doi: 10.4049/jimmunol.1102696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 10.Santosuosso M, Zhang X, McCormick S, Wang J, Hitt M, Xing Z. Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J Immunol. 2005;174:7986–94. doi: 10.4049/jimmunol.174.12.7986. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich J, Andersen C, Rappuoli R, Doherty TM, Jensen CG, Andersen P. Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette-Guerin immunity. J Immunol. 2006;177:6353–60. doi: 10.4049/jimmunol.177.9.6353. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Thorson L, Stokes RW, Santosuosso M, Huygen K, Zganiacz A, et al. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J Immunol. 2004;173:6357–65. doi: 10.4049/jimmunol.173.10.6357. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen G, Cox R. The mucosal vaccine quandary: intranasal vs. sublingual immunization against influenza. Hum Vaccin Immunother. 2012;8:689–93. doi: 10.4161/hv.19568. [DOI] [PubMed] [Google Scholar]
- 14.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 2014;9:209–22. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11:83–9. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–72. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 18.Wong YL, Sampson S, Germishuizen WA, Goonesekera S, Caponetti G, Sadoff J, et al. Drying a tuberculosis vaccine without freezing. Proc Natl Acad Sci U S A. 2007;104:2591–5. doi: 10.1073/pnas.0611430104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyadova IV, Vordermeier HM, Eruslanov EB, Khaidukov SV, Apt AS, Hewinson R, Intranasal BCG. vaccination protects BALB/c mice against virulent Mycobacterium bo and accelerates production of IFN-gamma in their lungs. Clin Exp Immunol. 2001;126:274–9. doi: 10.1046/j.1365-2249.2001.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamasur B, Haile M, Pawlowski A, Schroder U, Williams A, Hatch G, et al. Mycobacterium tuberculosis arabinomannan-protein conjugates protect against tuberculosis. Vaccine. 2003;21:4081–93. doi: 10.1016/s0264-410x(03)00274-3. [DOI] [PubMed] [Google Scholar]
- 21.Haile M, Schroder U, Hamasur B, Pawlowski A, Jaxmar T, Kallenius G, et al. Immunization with heat-killed Mycobacterium bovis bacille Calmette-Guerin (BCG) in Eurocine L3 adjuvant protects against tuberculosis. Vaccine. 2004;22:1498–508. doi: 10.1016/j.vaccine.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Todoroff J, Lemaire MM, Fillee C, Jurion F, Renauld JC, Huygen K, et al. Mucosal and systemic immune responses to Mycobacterium tuberculosis antigen 85A following its co-delivery with CpG, MPLA or LTB to the lungs in mice. PLoS One. 2013;8:e63344. doi: 10.1371/journal.pone.0063344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jouanguy E, Lamhamedi-Cherradi S, Lammas D, Dorman SE, Fondaneche MC, Dupuis S, et al. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat Genet. 1999;21:370–8. doi: 10.1038/7701. [DOI] [PubMed] [Google Scholar]
- 26.Filipe-Santos O, Bustamante J, Chapgier A, Vogt G, de Beaucoudrey L, Feinberg J, et al. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18:347–61. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Orr MT, Windish HP, Beebe EA, Argilla D, Huang PW, Reese VA, et al. Interferon gamma and Tumor Necrosis Factor Are Not Essential Parameters of CD4+ T-Cell Responses for Vaccine Control of Tuberculosis. J Infect Dis. 2015 doi: 10.1093/infdis/jiv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakai S, Kauffman KD, Schenkel JM, McBerry CC, Mayer-Barber KD, Masopust D, et al. Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J Immunol. 2014;192:2965–9. doi: 10.4049/jimmunol.1400019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riese P, Sakthivel P, Trittel S, Guzman CA. Intranasal formulations: promising strategy to deliver vaccines. Expert Opin Drug Deliv. 2014;11:1619–34. doi: 10.1517/17425247.2014.931936. [DOI] [PubMed] [Google Scholar]
- 30.Rodgers L, Pabst LJ, Chaves SS. Increasing uptake of live attenuated influenza vaccine among children in the United States, 2008-2014. Vaccine. 2015;33:22–4. doi: 10.1016/j.vaccine.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong PT, Wang SH, Ciotti S, Makidon PE, Smith DM, Fan Y, et al. Formulation and characterization of nanoemulsion intranasal adjuvants: effects of surfactant composition on mucoadhesion and immunogenicity. Mol Pharm. 2014;11:531–44. doi: 10.1021/mp4005029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldridge JR, Yorgensen Y, Ward JR, Ulrich JT. Monophosphoryl lipid A enhances mucosal and systemic immunity to vaccine antigens following intranasal administration. Vaccine. 2000;18:2416–25. doi: 10.1016/s0264-410x(99)00572-1. [DOI] [PubMed] [Google Scholar]
- 33.Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, et al. Norovirus vaccine against experimental human Norwalk Virus illness. N Engl J Med. 2011;365:2178–87. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldman M. Translational mini-review series on Toll-like receptors: Toll-like receptor ligands as novel pharmaceuticals for allergic disorders. Clin Exp Immunol. 2007;147:208–16. doi: 10.1111/j.1365-2249.2006.03296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen AC, Mills KH. Improved pertussis vaccines based on adjuvants that induce cell-mediated immunity. Expert Rev Vaccines. 2014;13:1253–64. doi: 10.1586/14760584.2014.936391. [DOI] [PubMed] [Google Scholar]
- 36.Arias MA, Van Roey GA, Tregoning JS, Moutaftsi M, Coler RN, Windish HP, et al. Glucopyranosyl Lipid Adjuvant (GLA), a Synthetic TLR4 agonist, promotes potent systemic and mucosal responses to intranasal immunization with HIVgp140. PLoS One. 2012;7:e41144. doi: 10.1371/journal.pone.0041144. [DOI] [PMC free article] [PubMed] [Google Scholar]