Abstract
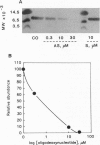
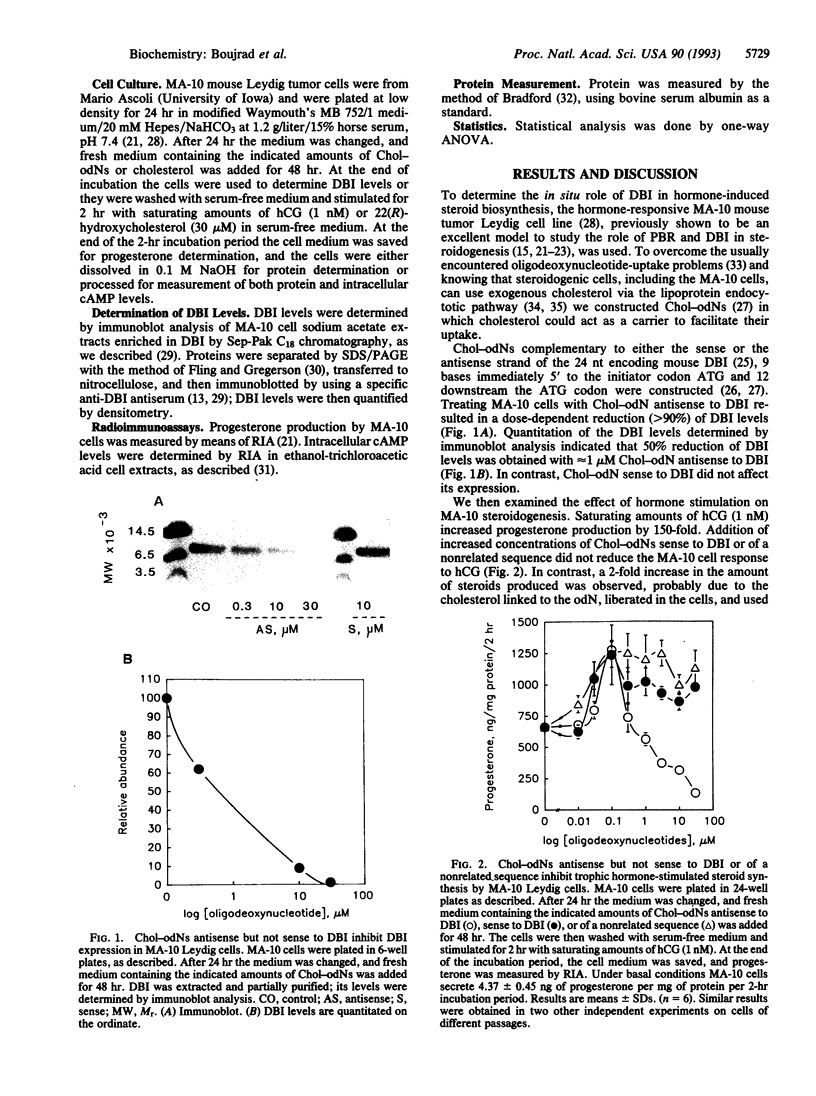
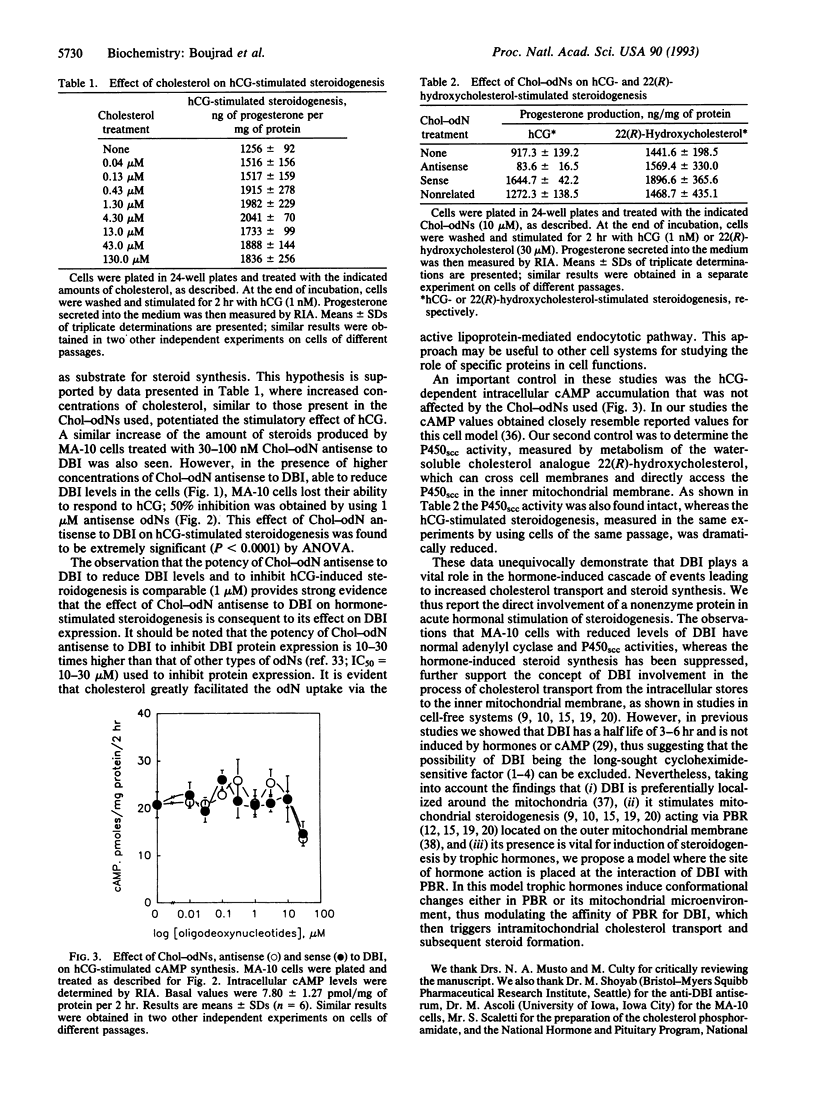
The polypeptide diazepam-binding inhibitor (DBI) has been previously shown to stimulate testicular Leydig, adrenocortical, and glial-cell mitochondrial steroidogenesis in vitro. To assess the in situ role of DBI in trophic hormone-stimulated steroidogenesis, we suppressed DBI levels in the hormone-responsive MA-10 Leydig tumor cells, using a cholesterol-linked phosphorothioate oligodeoxynucleotide (Chol-odN) antisense to DBI. Treating MA-10 cells with Chol-odN antisense to DBI resulted in a dose-dependent reduction of DBI levels (ED50 = 1 microM). In contrast, Chol-odN sense to DBI did not affect its expression. Saturating amounts of human choriogonadotropin (hCG) increased MA-10 progesterone production by 150-fold. Addition of increased concentrations of Chol-odNs sense to DBI or of a nonrelated sequence did not reduce the MA-10 response to hCG. However, in the presence of Chol-odN antisense to DBI that could reduce DBI levels, MA-10 cells lost their ability to respond to hCG (ED50 = 1 microM). In these studies the hCG-stimulated cAMP levels and cytochrome P450 side-chain cleavage activity, as measured by metabolism of 22(R)-hydroxycholesterol, were not affected by the Chol-odNs used. These observations provide unequivocal evidence that DBI plays a vital role in the acute stimulation of steroidogenesis by trophic hormones.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam A., Suh B. S. An inducible functional peripheral benzodiazepine receptor in mitochondria of steroidogenic granulosa cells. Endocrinology. 1991 Jul;129(1):503–510. doi: 10.1210/endo-129-1-503. [DOI] [PubMed] [Google Scholar]
- Anholt R. R., Pedersen P. L., De Souza E. B., Snyder S. H. The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. J Biol Chem. 1986 Jan 15;261(2):576–583. [PubMed] [Google Scholar]
- Ascoli M. Characterization of several clonal lines of cultured Leydig tumor cells: gonadotropin receptors and steroidogenic responses. Endocrinology. 1981 Jan;108(1):88–95. doi: 10.1210/endo-108-1-88. [DOI] [PubMed] [Google Scholar]
- Ascoli M., Euffa J., Segaloff D. L. Epidermal growth factor activates steroid biosynthesis in cultured Leydig tumor cells without affecting the levels of cAMP and potentiates the activation of steroid biosynthesis by choriogonadotropin and cAMP. J Biol Chem. 1987 Jul 5;262(19):9196–9203. [PubMed] [Google Scholar]
- Besman M. J., Yanagibashi K., Lee T. D., Kawamura M., Hall P. F., Shively J. E. Identification of des-(Gly-Ile)-endozepine as an effector of corticotropin-dependent adrenal steroidogenesis: stimulation of cholesterol delivery is mediated by the peripheral benzodiazepine receptor. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4897–4901. doi: 10.1073/pnas.86.13.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovolin P., Schlichting J., Miyata M., Ferrarese C., Guidotti A., Alho H. Distribution and characterization of diazepam binding inhibitor (DBI) in peripheral tissues of rat. Regul Pept. 1990 Jul 30;29(2-3):267–281. doi: 10.1016/0167-0115(90)90089-f. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Kovanen P. T., Goldstein J. L. Receptor-mediated uptake of lipoprotein-cholesterol and its utilization for steroid synthesis in the adrenal cortex. Recent Prog Horm Res. 1979;35:215–257. doi: 10.1016/b978-0-12-571135-7.50009-6. [DOI] [PubMed] [Google Scholar]
- Dym M., Lamsam-Casalotti S., Jia M. C., Kleinman H. K., Papadopoulos V. Basement membrane increases G-protein levels and follicle-stimulating hormone responsiveness of Sertoli cell adenylyl cyclase activity. Endocrinology. 1991 Feb;128(2):1167–1176. doi: 10.1210/endo-128-2-1167. [DOI] [PubMed] [Google Scholar]
- Fling S. P., Gregerson D. S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 1986 May 15;155(1):83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Freeman D. A., Ascoli M. The low-density lipoprotein pathway of cultured Leydig tumor cells. Utilization of low-density lipoprotein-derived cholesterol for steroidogenesis. Biochim Biophys Acta. 1983 Nov 1;754(1):72–81. doi: 10.1016/0005-2760(83)90083-8. [DOI] [PubMed] [Google Scholar]
- Garnier M., Boujrad N., Oke B. O., Brown A. S., Riond J., Ferrara P., Shoyab M., Suarez-Quian C. A., Papadopoulos V. Diazepam binding inhibitor is a paracrine/autocrine regulator of Leydig cell proliferation and steroidogenesis: action via peripheral-type benzodiazepine receptor and independent mechanisms. Endocrinology. 1993 Jan;132(1):444–458. doi: 10.1210/endo.132.1.8380386. [DOI] [PubMed] [Google Scholar]
- Guarneri P., Papadopoulos V., Pan B., Costa E. Regulation of pregnenolone synthesis in C6-2B glioma cells by 4'-chlorodiazepam. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5118–5122. doi: 10.1073/pnas.89.11.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A., Forchetti C. M., Corda M. G., Konkel D., Bennett C. D., Costa E. Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3531–3535. doi: 10.1073/pnas.80.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. F. Trophic stimulation of steroidogenesis: in search of the elusive trigger. Recent Prog Horm Res. 1985;41:1–39. doi: 10.1016/b978-0-12-571141-8.50005-6. [DOI] [PubMed] [Google Scholar]
- Kimura T. Transduction of ACTH signal from plasma membrane to mitochondria in adrenocortical steroidogenesis. Effects of peptide, phospholipid, and calcium. J Steroid Biochem. 1986 Nov;25(5B):711–716. doi: 10.1016/0022-4731(86)90299-2. [DOI] [PubMed] [Google Scholar]
- Knudsen J., Højrup P., Hansen H. O., Hansen H. F., Roepstorff P. Acyl-CoA-binding protein in the rat. Purification, binding characteristics, tissue concentrations and amino acid sequence. Biochem J. 1989 Sep 1;262(2):513–519. doi: 10.1042/bj2620513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger K. E., Papadopoulos V. Peripheral-type benzodiazepine receptors mediate translocation of cholesterol from outer to inner mitochondrial membranes in adrenocortical cells. J Biol Chem. 1990 Sep 5;265(25):15015–15022. [PubMed] [Google Scholar]
- MacKellar C., Graham D., Will D. W., Burgess S., Brown T. Synthesis and physical properties of anti-HIV antisense oligonucleotides bearing terminal lipophilic groups. Nucleic Acids Res. 1992 Jul 11;20(13):3411–3417. doi: 10.1093/nar/20.13.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme-Johnson N. R. Distinctive properties of adrenal cortex mitochondria. Biochim Biophys Acta. 1990 Dec 6;1020(3):213–231. doi: 10.1016/0005-2728(90)90151-s. [DOI] [PubMed] [Google Scholar]
- Ostenson C. G., Ahrén B., Karlsson S., Sandberg E., Efendic S. Effects of porcine diazepam-binding inhibitor on insulin and glucagon secretion in vitro from the rat endocrine pancreas. Regul Pept. 1990 Jul 30;29(2-3):143–151. doi: 10.1016/0167-0115(90)90077-a. [DOI] [PubMed] [Google Scholar]
- Owens G. P., Sinha A. K., Sikela J. M., Hahn W. E. Sequence and expression of the murine diazepam binding inhibitor. Brain Res Mol Brain Res. 1989 Nov;6(2-3):101–108. doi: 10.1016/0169-328x(89)90043-0. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V., Berkovich A., Krueger K. E., Costa E., Guidotti A. Diazepam binding inhibitor and its processing products stimulate mitochondrial steroid biosynthesis via an interaction with mitochondrial benzodiazepine receptors. Endocrinology. 1991 Sep;129(3):1481–1488. doi: 10.1210/endo-129-3-1481. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V., Mukhin A. G., Costa E., Krueger K. E. The peripheral-type benzodiazepine receptor is functionally linked to Leydig cell steroidogenesis. J Biol Chem. 1990 Mar 5;265(7):3772–3779. [PubMed] [Google Scholar]
- Papadopoulos V., Nowzari F. B., Krueger K. E. Hormone-stimulated steroidogenesis is coupled to mitochondrial benzodiazepine receptors. Tropic hormone action on steroid biosynthesis is inhibited by flunitrazepam. J Biol Chem. 1991 Feb 25;266(6):3682–3687. [PubMed] [Google Scholar]
- Pedersen R. C., Brownie A. C. Steroidogenesis-activator polypeptide isolated from a rat Leydig cell tumor. Science. 1987 Apr 10;236(4798):188–190. doi: 10.1126/science.3563495. [DOI] [PubMed] [Google Scholar]
- Pon L. A., Orme-Johnson N. R. Acute stimulation of steroidogenesis in corpus luteum and adrenal cortex by peptide hormones. Rapid induction of a similar protein in both tissues. J Biol Chem. 1986 May 15;261(14):6594–6599. [PubMed] [Google Scholar]
- Schultz R., Pelto-Huikko M., Alho H. Expression of diazepam binding inhibitor-like immunoreactivity in rat testis is dependent on pituitary hormones. Endocrinology. 1992 Jun;130(6):3200–3206. doi: 10.1210/endo.130.6.1597138. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Gentry L. E., Marquardt H., Todaro G. J. Isolation and characterization of a putative endogenous benzodiazepineoid (endozepine) from bovine and human brain. J Biol Chem. 1986 Sep 15;261(26):11968–11973. [PubMed] [Google Scholar]
- Simpson E. R., Waterman M. R. Regulation by ACTH of steroid hormone biosynthesis in the adrenal cortex. Can J Biochem Cell Biol. 1983 Jul;61(7):692–707. doi: 10.1139/o83-088. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Cohen J. S. Oligodeoxynucleotides as inhibitors of gene expression: a review. Cancer Res. 1988 May 15;48(10):2659–2668. [PubMed] [Google Scholar]
- Stocco D. M., Kilgore M. W. Induction of mitochondrial proteins in MA-10 Leydig tumour cells with human choriogonadotropin. Biochem J. 1988 Jan 1;249(1):95–103. doi: 10.1042/bj2490095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne P. A., Greenfield N. J., Lieberman S. Modulation of the kinetics of cholesterol side-chain cleavage by an activator and by an inhibitor isolated from the cytosol of the cortex of bovine adrenals. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1877–1881. doi: 10.1073/pnas.80.7.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagibashi K., Ohno Y., Kawamura M., Hall P. F. The regulation of intracellular transport of cholesterol in bovine adrenal cells: purification of a novel protein. Endocrinology. 1988 Oct;123(4):2075–2082. doi: 10.1210/endo-123-4-2075. [DOI] [PubMed] [Google Scholar]
- Yanagibashi K., Ohno Y., Nakamichi N., Matsui T., Hayashida K., Takamura M., Yamada K., Tou S., Kawamura M. Peripheral-type benzodiazepine receptors are involved in the regulation of cholesterol side chain cleavage in adrenocortical mitochondria. J Biochem. 1989 Dec;106(6):1026–1029. doi: 10.1093/oxfordjournals.jbchem.a122958. [DOI] [PubMed] [Google Scholar]