Abstract
This study evaluated whether the relation between subjective memory complaints and cognitive performance is influenced by the presence of hypertension in the elderly. One hundred and five healthy older adults, 70-89 years of age, with and without hypertension treatment or diagnosis, completed a scale of subjective memory complaints. Participants were divided into those with mild memory concerns and those with minimal or no complaints. All participants completed a battery of neuropsychological tests including measures of verbal and non-verbal memory. After controlling for differences in age, gender, education, and overall intellectual ability, there were significant main effects for memory concerns and significant interactions for memory complaints and hypertension on several measures of memory performance. There were no main effects for hypertension on memory performance. Simple effects analyses of the interactions showed that the hypertensive complainers demonstrated poorer performance on measures of long-term memory and greater reliance on short-term recall than the hypertensive non-complainers. There were no differences in memory performance for the non-hypertensive groups. Among healthy elderly community-dwelling adults, those with mild subjective memory complaints in the context of hypertension demonstrated greater objective cognitive difficulties than those without hypertension as well as a greater reliance on a less efficient learning strategy. These findings suggest that memory concerns in the presence of hypertension may be important when evaluating treatment efficacy in these individuals and for identifying differences in cognitive aging.
Keywords: cognitive aging, cognition, blood pressure, memory, aging
Introduction
With advancing age, older adults are more likely to subjectively report memory problems and have an increased prevalence of cerebrovascular risk factors, such as hypertension (Amariglio, Townsend, Grodstein, Sperling, & Rentz, 2011). Previous research has demonstrated that differences in cognitive function are associated with healthy aging, and performance on measures of memory, executive functions, and processing speed are most often affected (Alexander et al., 2012; Glisky, 2007; Salthouse, 1992; Zec, 1995). However, there is a wide range of cognitive performance among healthy older adults, which contributes to the complexity in assessing the validity of memory complaints. Despite age-related differences in memory performance, it remains unclear whether these expressed memory concerns have clinical significance for future cognitive decline. Moreover, hypertension may also contribute to the heterogeneity seen in cognitive performance, as this health factor has been associated with cognitive decline, particularly affecting memory, executive functions, and processing speed (Hudak, Edwards, Athilingam, & McEvoy, 2013; Saxby, Harrington, McKeith, Wesnes, & Ford, 2003; Waldstein, Manuck, Ryan, & Muldoon, 1991). The present study sought to investigate the effects of the combination of subjective memory complaints and hypertension on neuropsychological test performance in a neurologically healthy, community-dwelling elderly sample, ages 70-89, with mild self-reported memory concerns.
Subjective memory complaints and cognition
Subjective memory complaints are commonly defined as self-reported, self-evaluative concerns of memory decline relative to memory capabilities earlier in life (Jonker, Geerlings, & Schmand, 2000). These memory complaints can occur in conjunction with objective memory decline but can also be present when memory performance is within the normal limits for a person’s age and educational level. In a study of adults aged 65 and older who endorsed memory concerns, the frequency of memory complaints increased with age from 43% of those aged 65 to 74, to 51% of those aged 75 to 84, and to 88% of those aged 85 and older (Bassett & Folstein, 1993). Given this association between the rising number of memory complaints and older age, subjective memory complaints often prompt concerns about current and future cognitive decline as well as subsequent development of dementia (Albert et al., 2011).
The association between subjective memory complaints and memory performance has been previously investigated in non-demented older adults, but the findings remain unclear (Reid & MacLullich, 2006). Memory concerns have been associated with poorer objective memory performance in healthy older adults (Amariglio et al., 2011; Dufouil, Fuhrer, & Alpérovitch, 2005; Jonker, Launer, Hooijer, & Lindeboom, 1996; Schofield et al., 1997; Wang et al., 2000). Moreover, a community-based sample of older adults without dementia, who were characterized as having normal cognition based on Mini-Mental State Exam scores of 26-30 at baseline, had memory complaints that were predictive of dementia following an average of 3.2 years later (Geerlings, Jonker, Bouter, Adèr, & Schmand, 1999).
Other investigators have found that subjective memory complaints have been associated with depression and selective personality traits rather than with objective memory performance in healthy older adults (Jorm et al., 2004; Kliegel, Zimprich, & Eschen, 2005; Minett, Dean, Firbank, English, & O'Brien, 2005; Smith, Petersen, Ivnik, Malec, & Tangalos, 1996). Poor memory and concentration, symptoms often seen with depression, and lowered self-esteem, may contribute to a depressed individual’s concerns about memory (Reid & MacLullich, 2006). Differences in methodology, including the definition and measurement of subjective memory complaints and objective memory performance, undefined ranges of severity of memory concerns, and inclusion or exclusion of individuals with mood disorders, may have contributed to the differences observed across studies for the association between subjective memory complaints and memory performance.
Effects of hypertension on cognition
Essential hypertension is typically defined as sustained systolic blood pressure greater than or equal to 140mm Hg and diastolic blood pressure greater than or equal to 90mm Hg with no known cause (Burt et al., 1995). Among the elderly, hypertension has been associated with cognitive deficits in attention, executive function, learning and memory, visuospatial abilities, processing speed, and abstract reasoning (Elias, Wolf, D'Agostino, Cobb, & White, 1993; Harrington, Saxby, McKeith, Wesnes, & Ford, 2000; Waldstein et al., 1991; Waldstein, Giggey, Thayer, & Zonderman, 2005).
Uncontrolled hypertension has been associated with poorer performance on neuropsychological tests of executive functioning, processing speed, and memory, and these cognitive deficits have not been merely attributable to age-related changes in cognition (Brady et al., 2005; Harrington et al., 2000). In contrast, treatment of hypertension with antihypertensive drugs has been associated with a relatively decreased risk for cognitive decline and may provide some cognitive benefits and preservation of functions compared to those with untreated hypertension (Dufouil et al., 2001; Papademetriou, 2005). Brady, Spiro, & Gaziano (2005) evaluated cognitive performance in hypertensive and non-hypertensive men aged 52-85 years and found no differences in cognitive performance between individuals with controlled hypertension and those without a hypertension diagnosis. Conversely, the uncontrolled hypertensives demonstrated poorer memory performance than both other groups. These and related findings have suggested an inverse relation between blood pressure level and cognition, with individuals who have higher blood pressure above the normal range generally demonstrating poorer cognitive function, particularly for individuals with less than optimal hypertension treatment. However, the inverse relation is not always the case. In a 10-year longitudinal study of advanced elderly (age > 90 years), hypertension was not a risk factor for dementia, and a hypertension onset in older age (80-89 years) was associated with a decreased risk of dementia compared to those without a history of hypertension (Corrada et al., 2014).
Although antihypertensive medications may provide some cognitive protection for older adults, it remains unclear whether current treatment regimens are optimal for preventing cognitive decline, particularly in the memory domain. Researchers focusing on the cognitive effects of controlled and uncontrolled hypertension have also shown associations between deficits in both verbal (Elias et al., 1993; Waldstein et al., 1991) and nonverbal memory (André-Petersson, Hagberg, Janzon, & Steen, 2001; Harrington et al., 2000; Swan et al., 1998) and high blood pressure in both groups. This suggests that controlled hypertension can also be associated with cognitive changes in older adults, although the extent of deficits may not be as pronounced.
Present study
Despite the extensive literature exploring the individual effects of subjective memory complaints and hypertension status on objective memory performance, previous research has not considered the combined effects of these two factors on memory in healthy older adults and especially in community-dwelling elderly with only a mild range of self-reported memory concerns, with or without a history of hypertension diagnosis. We focused on mild memory complaints and hypertension status within a sample of elderly adults screened to exclude overt neurological disorders and dementia. We hypothesized that subjective memory complaints combined with a history of hypertension would lead to poorer performance on memory measures than non-complaining hypertensive and non-hypertensive older adults.
Method
Participants and procedure
Participants were 105 adults, 70-89 years of age, who were drawn from a cohort of 210 neurologically healthy older adults, ages 50-89, as part of a longitudinal study on healthy cognitive aging. For this study, we focused on older participants due to the consistently higher prevalence of memory complaints and hypertension within this age range. The sample was predominately Caucasian (97.0%), with 52 female participants (49.5%). The average education was 16.4 years (SD = 2.9), and the average Mini Mental Status Exam (MMSE; Folstein, Folstein, & McHugh, 1975) score was 28.6 (SD = 1.3).
To exclude significant neurological and psychiatric disorders that could affect cognitive performance, participants underwent an extensive medical screen before they were enrolled in the study. They provided information regarding their medical history and medication status and underwent a physical and neurological examination by a neurologist (GAH) who specializes in aging. Participants were administered questionnaires and rating scales assessing their functional capacity (Lawton & Brody, 1969), family history of dementia, sleep quality and habits (Buysse, Reynolds III, Monk, Berman, & Kupfer, 1989), and current depressive symptoms with the Hamilton Depression Rating Scale (HAM-D; Hamilton, 1960). To additionally screen for possible dementia, subjects completed the MMSE. Participants were excluded if they had a MMSE score < 26 or a HAM-D score ≥ 10. The data were collected as part of a University of Arizona Institutional Review Board approved protocol, and participants provided informed written consent to participate.
Hypertension status
Participants reported their hypertension status and were grouped as hypertensives and non-hypertensives based on history of diagnoses and current antihypertensive medication use. The hypertensive group contained 44 participants who were previously diagnosed with hypertension and currently taking antihypertensive medications. They reported the number of antihypertensive medications they were taking and the duration of their hypertension diagnosis. The hypertensive group’s mean blood pressure obtained during the clinic screening visit was a systolic blood pressure of 149.51mm Hg (SD = 15.57) and diastolic blood pressure of 83.0mm Hg (SD = 11.45). The non-hypertensive group contained 61 participants who had never been diagnosed with hypertension and were not currently taking antihypertensive medications. Their mean clinical blood pressure was systolic blood pressure of 143.08mm Hg (SD = 16.80) and diastolic blood pressure of 78.15mm Hg (SD = 7.95). The groups significantly differed on systolic (t(102) = −1.98, p = 0.05) and diastolic (t(102) = −2.4, p = 0.019) blood pressure, with the hypertensive group having overall higher clinic blood pressure.
However, the hypertensive and non-hypertensive groups did not differ in other clinic and demographic characteristics, including gender distribution (male/female hypertensives (n) = 24/20, male/female non-hypertensives (n) = 29/32, χ2(1) = 0.50, p = 0.48), age (M(SD) hypertensives = 79.05(4.97), M(SD) non-hypertensives = 78.18(5.13), t(103) = −0.86, p = 0.39), HAM-D scores (M(SD) hypertensives = 1.64(1.81), M(SD) non-hypertensives = 1.56(1.63), t(103) = −0.23, p = 0.82), MMSE scores (M(SD) hypertensives = 28.59(1.32), M(SD) non-hypertensives = 28.61(1.37), t(103) = 0.06, p = 0.95), and Wechsler Adult Intelligence Scale-Fourth edition, Full Scale Intelligence Quotient (WAIS-IV FSIQ; Wechsler, 2008; M(SD) hypertensives = 113.43(11.75), M(SD) non-hypertensives = 114.30(11.98), t(103) = 0.37, p = 0.71).Years of education reached significance, with the hypertensives having more years of education than the non-hypertensives (M(SD) hypertensives = 17.02(3.11), M(SD) non-hypertensives = 15.89(2.72), t(103) = −1.99, p = 0.05).
Subjective memory complaints
Memory complaints were measured using a portion of the Memory Functioning Questionnaire (MFQ), which has been shown to have high internal consistency and is considered a reliable measure for evaluating subjective memory complaints (Gilewski, Zelinski, & Schaie, 1990). For the purpose of this study, we focused on the general question asking participants to rate their overall problems with memory, if at all, on a 1-7 scale (Gilewski et al., 1990). Participants’ scores ranged from mild to minimal or no complaints. Those who endorsed a rating of mild/minor memory complaints (rating of 3-5) were included in the complaints group (n = 72; M(SD) memory complaint rating = 4.36(0.70)), while those who endorsed minimal or no memory complaints (rating of 6-7) were included in the non-complaints group (n = 33; M(SD) memory complaint rating = 6.30(0.47)). No participants endorsed ratings below 3, indicating that none had reported major memory concerns.
Neuropsychological battery
Cognitive testing was performed using a comprehensive neuropsychological battery. The battery included an assessment of general cognitive and intellectual function, verbal and visual memory, visuospatial skills, complex attention and executive function, working memory and processing speed, language ability, planning and abstract reasoning, and motor ability.
For the purpose of this study, we focused on memory measured by the 12-item, 12-trial version of the Selective Reminding Test (SRT; Buschke, 1973) and the Rey Complex Figure Test (RCFT; Rey, 1964). Both memory tests included an immediate and 30-minute delay recall. The SRT consisted of learning a list of 12 unrelated words over a span of 12 trials and immediately recalling the words after each trial. Participants were selectively reminded of words that were not recalled on each trial. After a 30-minute delay, participants were asked to recall the 12 words and were subsequently given a recognition test. Scores were determined for several aspects of verbal memory, including Long-term Storage, Long-term Retrieval, Continuous Long-term Retrieval, Short-term Retrieval, Sum Recall, Delayed Recall, and Recognition (Buschke, 1973). The RCFT required copying a complex line drawing, and subsequently recalling the figure from memory after 3-minute immediate and 30-minute delayed recall conditions (Rey, 1964). Participants were then given an RCFT recognition test. In addition, we used general intellectual function assessed by the WAIS-IV FSIQ as a covariate to evaluate group differences in memory performance after we controlled for individual differences in intellectual abilities.
Statistical analyses
Differences in demographic and clinical variables for memory complaint status were evaluated using independent t tests. Gender and hypertension differences were compared with chi square tests. Among the hypertensives, differences in clinic blood pressure, duration of hypertension and number of antihypertensive medications were evaluated using an independent t test and chi square. Additionally, interactions between memory complaint status and hypertension status for demographic and clinical characteristics were performed using a chi square test and a two-factor analysis of variance (ANOVA), where appropriate.
A two-factor analysis of covariance (ANCOVA) with hypertension status and memory complaint status as between-group factors was performed. Age, gender, and years of education were included as initial covariates, as these demographic characteristics can be associated with differences in memory performance. To additionally control for overall intelligence in the sample, a two-factor analysis of covariance (ANCOVA) with hypertension status and memory complaint status as between-group factors and WAIS-IV FSIQ as an added covariate was performed. Simple effects for significant two-way interactions were tested using pairwise ANCOVAs, with the corresponding covariates. To account for hypertension severity, the number of antihypertensive medications and hypertension duration were included separately as covariates in the pairwise ANCOVAs, as appropriate. Significance for omnibus and simple effects was taken at p < 0.05. All analyses were conducted using Statistical Package for the Social Sciences (SPSS; Windows version 18, Chicago, IL, USA).
Results
Demographic and clinical characteristics
Group demographic and clinical characteristics for the memory complainers and non-complainers are shown in Table 1 (Table 1 about here). There were no significant effects for memory complaint status on demographic and clinical characteristics, including gender distribution (χ2(1) = 0.49, p = 0.49), age (t(103) = 0.29, p = 0.78), years of education (t(103) = −0.08, p = 0.94), measures of diastolic blood pressure (t(102) = −0.48, p = 0.64 ), MMSE scores (t(103) = −1.13, p = 0.26), WAIS-IV FSIQ scores (t(102) = −1.02, p = 0.31), and hypertension status (χ2(1) = 0.005, p = 0.94). There were trends for higher clinic systolic blood pressure in the non-complainers (t(102) = −1.74, p = 0.08) and higher HAM-D scores in the complainers (t(103) = 1.81, p = 0.07). However, the HAM-D scores were well within the normal range, and the groups did not differ in diastolic blood pressure.
Table 1.
Subject demographic and clinical characteristics by memory complaint status
| Complainers | Non- complainers |
p values | |
|---|---|---|---|
| n | 72 | 33 | |
| Age (years) | 78.64 (4.86) | 78.33 (5.53) | 0.78 |
| Education (years) | 16.35 (2.77) | 16.39 (3.29) | 0.94 |
| Clinic SBP | 143.88 (16.71) | 149.94 (15.57) | 0.08 |
| Clinic DBP | 79.85 (9.89) | 80.84 (9.72) | 0.64 |
| HAM-D | 1.79 (1.79) | 1.15 (1.40) | 0.07 |
| MMSE | 28.50 (1.33) | 28.82 (1.36) | 0.26 |
| WAIS-IV FSIQ | 113.13 (11.14) | 115.67 (13.21) | 0.31 |
| Gender (M/F) | 38/34 | 15/18 | 0.49 |
| Hypertension (Y/N) | 30/42 | 14/19 | 0.94 |
Notes. Means and standard deviations for complaint status are presented, with the exception of gender and hypertension status. Both groups did not significantly differ in subject characteristics (p’s > 0.05). SBP = systolic blood pressure; DBP = diastolic blood pressure; HAM-D = Hamilton Depression Rating Scale; MMSE = Mini Mental Status Exam; WAIS-IV FSIQ = Wechsler Adult Intelligence Scale-Fourth edition, Full Scale Intelligence Quotient.
Among the hypertensive group, memory complainers and non-complainers did not significantly differ in duration of hypertension (t(33) = 0.09, p = 0.93) and number of antihypertensive medications (χ2(4) = 5.37, p = 0.25). Furthermore, the hypertensive complainers and non-complainers did not significantly differ in clinic systolic blood pressure (χ2(27) = 30.36, p = 0.30) and diastolic blood pressure (χ2(25) = 27.20, p = 0.35).
There were no significant memory complaint group by hypertension status interactions for gender distribution (χ2(1) = 0.005, p = 0.94), age (F(1,101) = 0.41, MSE = 25.98, p = 0.52), years of education (F(1,101) = 0.69, MSE = 8.43, p = 0.41), systolic blood pressure (F(1,100) = 0.04, MSE = 262.59, p = 0.85), diastolic blood pressure (F(1,100) = 0.32, MSE = 95.50, p = 0.58), HAM-D scores (F(1,101) = 0.04, MSE = 2.87, p = 0.84), and MMSE scores (F(1,101) = 0.05, MSE = 1.83, p = 0.83). There was a trend for higher WAIS-IV FSIQ scores in non-hypertensive non-complainers compared to all other groups (F(1,100) = 3.47, MSE = 137.77, p = 0.07).
Memory test differences
Main and interactive group effects when accounting for age, gender, and years of education
After we controlled for age, gender, and education, significant main effects for memory complaints were observed for SRT verbal memory scores, with the memory complainers showing poorer memory performance than the non-complainers on Sum Recall (F(1,97) = 5.49, MSE = 238.24, p = 0.021), Long-term Storage (F(1,97) = 4.87, MSE = 557.27, p = 0.03), Long-term Retrieval (F(1,97) = 5.80, MSE = 561.41, p = 0.018), Consistent Long-term Retrieval (F(1,97) = 4.92, MSE = 739.86, p = 0.029), and Short-term Retrieval (F(1,97) = 4.56, MSE = 99.05, p = 0.035). The main effect of memory concerns did not differ in performance on SRT delayed recall (F(1,97) = 1.07, MSE = 6.70, p = 0.30) and recognition (F(1,97) = 1.37, MSE = 0.80, p = 0.25). Performance on nonverbal memory, as measured by RCFT immediate recall (F(1,97) = 4.17, MSE = 37.67, p = 0.044) and RCFT recognition (F(1,97) = 4.37, MSE = 4.62, p = 0.039), was also poorer in the complaint group. There was a trend for the complainers showing poorer performance than the non-complainers on RCFT delayed free recall (F(1,97) = 3.67, MSE = 40.09, p = 0.058). Main effects for hypertension status were not significant for any measure of memory performance.
When accounting for age, gender, and education, interactive effects for memory concerns and hypertension status on SRT sum scores were observed for the following verbal SRT measures: Sum Recall (F(1,97) = 5.37, MSE = 238.24, p = 0.023), Long-term Storage (F(1,97) = 4.52, MSE = 557.27, p = 0.036), Long-term Retrieval (F(1,97) = 5.66, MSE = 561.41, p = 0.019), Consistent Long-term Retrieval (F(1,97) = 7.46, MSE = 739.86, p = 0.007), and Short-term Retrieval (F(1,97) = 4.03, MSE = 99.05, p = 0.048). The hypertensive complainers demonstrated poorer memory performance compared to the hypertensive non-complainers and the non-hypertensive groups (see Table 2). SRT delay (F(1,97) = 0.001, MSE = 6.70, p = 0.97) and recognition (F(1,97) = 0.93, MSE = 0.80, p = 0.34), RCFT immediate (F(1,97) = 0.14, MSE = 37.67, p = 0.71) and long delay recall (F(1,97) = 0.93, MSE = 40.09, p = 0.34), and RCFT recognition (F(1,97) = 0.85, MSE = 4.62, p = 0.36) did not show significant hypertension by memory complaints interactions.
Table 2.
Analysis of covariance for memory measures by memory complaint and hypertension status
| Without Hypertension |
With Hypertension |
|
|
|||
|---|---|---|---|---|---|---|
| Neuropsychological Tests | Complainers | Non-complainers | Complainers | Non-complainers | CS A | CS × HTN B |
| SRT Sum Recall | 95.32 (19.36) | 99.42 (19.57) | 88.43 (20.73)1,2,3 | 102.43 (19.57) 1,2,3 | 0.033* | 0.002* |
| SRT LTS | 83.44 (28.09) | 89.79 (29.46) | 73.63 (30.39) 1,2,3 | 93.00 (25.05) 1,2,3 | 0.049* | 0.009* |
| SRT LTR | 73.83 (29.65) | 80.00 (28.74) | 62.90 (31.13) 1,2,3 | 84.86 (26.57) 1,2,3 | 0.030* | 0.003* |
| SRT CLTR | 48.29 (36.16) | 50.79 (28.50) | 35.63 (32.80) 1,2,3 | 63.07 (32.80) 1,2,3 | 0.049* | 0.0003** |
| SRT STR | 21.41 (11.63) | 19.11 (10.93) | 25.60 (11.97) 1,2,3 | 17.71 (8.22) 1,2,3 | 0.05* | 0.02* |
| SRT Delay | 7.32 (3.05) | 8.32 (2.85) | 6.90 (2.45) | 7.29 (2.92) | 0.43 | 0.62 |
| SRT Recognition | 11.59 (0.92) | 11.74 (0.73) | 11.37 (1.22) | 11.71 (0.47) | 0.29 | 0.28 |
| RCFT Immediate Recall | 12.67 (6.09) | 15.58 (7.58) | 12.52 (5.51) | 15.14 (6.78) | 0.07* | 0.60 |
| RCFT Delay | 12.14 (6.30) | 15.58 (8.18) | 12.83 (5.95) | 14.71 (6.09) | 0.10 | 0.88 |
| RCFT Recognition | 19.17 (2.14) | 19.68 (2.85) | 19.14 (1.96) | 20.57 (0.94) | 0.07* | 0.11 |
Notes. Results are presented as means (M) and standard deviations (SD). SRT = Selective Reminding Test, LTS = Long-term Storage, LTR = Long-term Retrieval, CLTR = Consistent Long-term Retrieval, STR = Short-term Retrieval, RCFT = Rey Complex Figure Test.
No significant main effects for hypertension (p’s > .05) were observed for any memory measures.
p-value of ANCOVA main effects of complaining status (CS) for measures of memory performance, controlling for age, gender, education, and WAIS-IV FSIQ.
p-value of ANCOVA interactive effects of complaining status (CS) and hypertension (HTN) for measures of memory performance, controlling for age, gender, education, and WAIS-IV FSIQ.
Significant effects at p < 0.05 for ANCOVA main and interactive effects, controlling for age, gender, and education only.
significant effects at p < 0.01 for ANCOVA main and interactive effects, controlling for age, gender, and education only.
Pairwise ANCOVA simple effects of CS and HTN show significant group differences between hypertensive complainers and hypertensive non-complainers after controlling for age, gender, and, education.
Pairwise ANCOVA simple effects of CS and HTN show significant group differences between hypertensive complainers and hypertensive non-complainers after controlling for age, gender, education, and WAIS-IV FSIQ.
Pairwise ANCOVA simple effects of CS and HTN show significant group differences between hypertensive complainers and hypertensive non-complainers after controlling for age, gender, education, WAIS-IV FSIQ, HTN duration, and number of HTN medications.
All other pairwise comparisons were not significant.
Main and interactive group effects when accounting for age, gender, years of education, and intellectual ability
After additionally controlling for WAIS-IV Full-Scale IQ scores, the main effects remained significant on verbal SRT memory tests (see Table 2) (Table 2 about here): Sum Recall (F(1,95) = 4.67, MSE = 203.39, p = 0.033), Long-term Storage (F(1,95) = 3.99, MSE = 522.93, p = 0.049), Long-term Retrieval (F(1,95) = 4.84, MSE = 505.36, p = 0.03), Consistent Long-term Retrieval (F(1,95) = 3.99, MSE = 619.60, p = 0.049), and Short-term Retrieval (F(1,95) = 3.75, MSE = 96.27, p = 0.05). SRT delayed recall (F(1,95) = 0.63, MSE = 6.22, p = 0.43) and recognition (F(1,95) = 1.12, MSE = 0.80, p = 0.29) were not significantly related to memory complaints. There was a trend for memory complainers showing poorer performance than non-complainers on nonverbal RCFT immediate recall (F(1,95) = 3.27, MSE = 29.54, p = 0.074) and recognition (F(1,95) = 3.47, MSE = 4.09, p = 0.066), but no significant effects of delayed recall (F(1,95) = 2.73, MSE = 31.87, p = 0.102) on memory complaints. There were no significant main effects for hypertension status on any measure of memory performance.
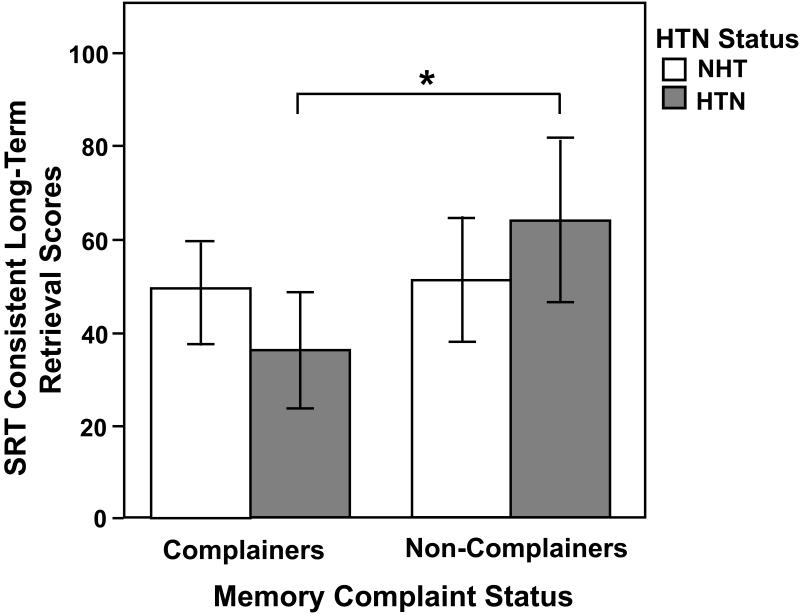
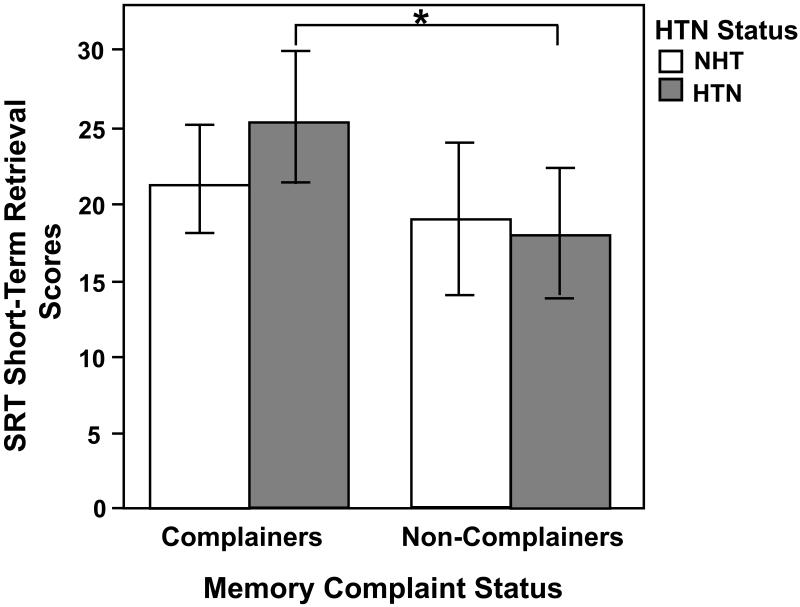
The interactive effects when additionally controlling for WAIS-IV Full-Scale IQ continued to be robust for the following SRT measures: Sum Recall (F(1,95) = 10.32, MSE = 203.39, p = 0.002), Long-term Storage (F(1,95) = 7.13, MSE = 522.93, p = 0.009), Long-term Retrieval (F(1,95) = 9.58, MSE = 505.36, p = 0.003), Consistent Long-term Retrieval (F(1,95) = 13.98, MSE = 619.60, p = 0.0003; see Figure 1), and Short-term Retrieval (F(1,95) = 5.61, MSE = 96.27, p = 0.02; see Figure 2) (Figure 1 and 2 about here), with the hypertensive complainers showing poorer memory performance than all other groups. There were no significant interactive effects for SRT delayed recall (F(1,95) = 0.25, MSE = 6.22, p = 0.62) and recognition (F(1,95) = 1.17, MSE = 0.80, p = 0.28), RCFT immediate (F(1,95) = 0.28, MSE = 29.54, p = 0.60) and delayed recall (F(1,95) = 0.02, MSE = 31.87, p = 0.88), and RCFT recognition (F(1,95) = 2.65, MSE = 4.09, p = 0.11). These effects are shown in Table 2.
Figure 1.
Subjective memory complaint status and hypertension (HTN) interactive effects for Selective Reminding Test (SRT) Consistent Long-Term Retrieval scores after controlling for age, gender, education, and Wechsler Adult Intelligence Scale-Fourth edition, Full Scale Intelligence Quotient. There were significantly higher long-term memory scores in the non-complaints group than in the complaints group (p = 0.049), and a subjective memory complaint status by hypertension group interaction effect (p = 0.0003). The hypertensive complainers showed poorer scores on this long-term memory measure than the hypertensive non-complainers. The main effect for hypertension was not significant. *p < 0.001, differs from hypertensive non-complainers. NHT = non-hypertensives; HTN = hypertensives.
Figure 2.
Subjective memory complaint status and hypertension (HTN) interactive effects for Selective Reminding Test (SRT) Short-Term Retrieval scores after controlling for age, gender, education, and Wechsler Adult Intelligence Scale-Fourth edition, Full Scale Intelligence Quotient. There were significantly higher short-term memory scores in the complaints group than in the non-complaints group (p = 0.05), and a subjective memory complaint status by hypertension group interaction effect (p = 0.02). The hypertensive complainers showed higher scores on this short-term memory measure than the hypertensive non-complainers, which may indicate the use of less efficient learning strategies. The main effect for hypertension was not significant. *p < 0.05, differs from hypertensive non-complainers. NHT = non-hypertensives; HTN = hypertensives.
Pairwise simple effects analyses for interactions when accounting for age, gender, years of education, and intellectual ability
Follow-up pairwise simple effects analyses for the significant interactions when controlling for age, gender, and education demonstrated that the hypertensive complainers had poorer performance than the hypertensive non-complainers on the following SRT measures: Sum Recall (F(1,39) = 9.34, MSE = 257.68, p = 0.004), Long-term Storage (F(1,39) = 9.06, MSE = 532.98, p = 0.005), Long-term Retrieval (F(1,39) = 10.33, MSE = 563.43, p = 0.003), and Consistent Long-term Retrieval (F(1,39) = 11.12, MSE = 711.88, p = 0.002; see Table 2). The hypertensive complainers showed higher scores for SRT Short-term Retrieval (F(1,39) = 8.32, MSE = 90.87, p = 0.006) than the hypertensive non-complainers. The non-hypertensive complainers and non-complainers did not significantly differ on SRT Sum Recall (F(1,55) = 0.02, MSE = 229.11, p = 0.89), Long-term Storage (F(1,55) = 0.02, MSE = 592.67, p = 0.88), Long-term Retrieval (F(1,55) = 0.01, MSE = 579.34, p = 0.92), Consistent Long-term Retrieval (F(1,55) = 0.12, MSE = 784.29, p = 0.74), and Short-term Retrieval (F(1,55) = 0.02, MSE = 108.98, p = 0.90).
Moreover, simple effects analyses for the significant interactions that additionally controlled for WAIS-IV Full-Scale IQ also revealed that the hypertensive complainers had poorer memory performance than the hypertensive non-complainers on the following SRT measures: Sum Recall (F(1,38) = 10.82, MSE = 238.72, p = 0.002), Long-term Storage (F(1,38) = 9.32, MSE = 534.85, p = 0.004), Long-term Retrieval (F(1,38) = 11.12, MSE = 548.79, p = 0.002), and Consistent Long-term Retrieval (F(1,38) = 13.85, MSE = 619.70, p = 0.001; see Table 2). The hypertensive complainers showed higher scores for SRT Short-term Retrieval (F(1,38) = 8.19, MSE = 93.05, p = 0.007) than the hypertensive non-complainers. The non-hypertensive complainers and non-complainers did not significantly differ on SRT Sum Recall (F(1,53) = 0.82, MSE = 187.34, p = 0.37), Long-term Storage (F(1,53) = 0.44, MSE = 530.85, p = 0.51), Long-term Retrieval (F(1,53) = 0.71, MSE = 494.98, p = 0.40), Consistent Long-term Retrieval (F(1,53) = 1.86, MSE = 653.11, p = 0.18), and Short-term Retrieval (F(1,53) = 0.28, MSE = 101.85, p = 0.60).
Pairwise simple effects for interactions when accounting for age, gender, years of education, intellectual ability, and hypertension severity
Pairwise ANCOVAs showed that significant effects remained between the hypertensive complainers and non-complainers after separately controlling for hypertension duration (SRT Sum Recall (F(1,28) = 7.64, MSE = 279.35, p = 0.01), Long-term Storage (F(1,28) = 7.59, MSE = 560.91, p = 0.01), Long-term Retrieval (F(1,28) = 8.43, MSE = 617.77, p = 0.007), Consistent Long-term Retrieval (F(1,28) = 9.56, MSE = 762.22, p = 0.004), and Short-term Retrieval (F(1,28) = 7.37, MSE = 100.34, p = 0.01)) and number of antihypertensive medications (SRT Sum Recall (F(1,37) = 12.64, MSE = 233.65, p = 0.001), Long-term Storage (F(1,37) = 11.22, MSE = 520.80, p = 0.002), Long-term Retrieval (F(1,37) = 12.76, MSE = 540.01, p = 0.001), Consistent Long-term Retrieval (F(1,37) = 13.73, MSE = 631.48, p = 0.001), and Short-term Retrieval (F(1,37) = 9.16, MSE = 92.95, p = 0.004)).
Discussion
In this cohort of neurologically healthy, community-dwelling older adults, we found that those who had hypertension and memory complaints demonstrated poorer cognitive performance than hypertensive non-complainers. In contrast, there were no differences in memory performance between the non-hypertensive complainers and non-complainers. Together, these findings indicate that among those with a history of treated hypertension, self-reported memory concerns, even when they are mild, reflect performance differences, suggesting that elderly hypertensive individuals who report mild problems with memory may be accurately reporting greater memory difficulties. These findings suggest that hypertension may contribute to the heterogeneity in cognitive performance within older adults and also to differences in memory performance in those with and without reported memory concerns. Follow-up longitudinal studies are needed to determine if this poorer performance indicates a greater risk for subsequent decline in cognitive functioning.
Previous research has found that cerebrospinal fluid markers of Alzheimer’s disease pathology were common in participants with memory complaints and without cognitive impairment (Visser et al., 2009). These findings suggest that individuals with memory concerns, but without overt clinical symptoms of dementia may be experiencing greater development of underlying Alzheimer’s disease pathology. Given that hypertension is a risk factor for both cerebrovascular and Alzheimer’s dementia, future longitudinal studies are needed to further investigate whether memory complaints in the context of hypertension is associated with a greater risk for developing cerebrovascular and Alzheimer-related pathology, as well as dementia.
In addition to poorer memory performance, the hypertensive complainers demonstrated higher scores on the SRT Short-term Retrieval measure, indicating that these individuals may be utilizing different memory strategies compared to the hypertensive non-complainers. Both groups did not differ on SRT delayed recall performance, but the hypertensive complainers relied on a short-term store rather than a long-term store to learn the words over time. This strategy of utilizing repetition of words over multiple trials may have benefitted the hypertensive complainers’ delayed recall performance, but this group may be less efficiently consolidating information into longer-term memory. This short-term learning strategy may be contributing to their overall poorer memory performance and subsequent memory complaints, and over time, may contribute to diminished ability to perform daily tasks that require consistent long-term storage and retrieval of information.
Brain changes that occur within the context of aging and hypertension may lead to the use of less than optimal learning strategies. The combination of aging with hypertension has been associated with volume reductions in temporal regions, which are important for learning and memory (Strassburger et al., 1997), as well as deterioration of white matter integrity, which reflects axonal damage that can lead to a decline in cognitive function (Burgmans et al., 2010). The disruption of white matter tracts may affect the connectivity needed to support the transfer between short-term and long-term consolidation of information. The hypertensive complainers’ reliance on less efficient learning strategies may reflect such brain changes, and further work is needed to evaluate these findings in relation to structural and functional brain imaging.
The effect of hypertension status on memory performance did not show overall differences between the hypertensives and the non-hypertensives despite higher clinic blood pressure in the hypertensives. There have been contrasting findings regarding the effects of antihypertensive medications on memory performance. Murray et al. (2002) found that antihypertensive medications were associated with reduced risk of cognitive impairment in older adults, whereas Raz, Rodrigue, & Acker (2003) found that controlled hypertension was associated with more perseverative errors on tasks assessing memory compared to individuals without a hypertension diagnosis. Although hypertension status alone did not have an effect on memory performance within our sample, the combination of treated hypertension and memory complaints resulted in differences in memory scores. Conversely, there were no memory performance differences within the non-hypertensive complainers and non-complainers. Clinic blood pressure and severity of hypertension, including hypertension duration and number of antihypertensive medications, did not differ between the hypertensive complainers and non-complainers. Together, these findings indicate that treated hypertension in the whole cohort did not have an adverse effect on memory performance, but that memory concerns in the context of treated hypertension may be an important indicator of memory differences.
Additionally, our findings suggest that evaluation of antihypertensive treatment efficacy may benefit from assessments of subjective memory complaints. Memory concerns may serve as an additional factor in identifying more beneficial treatments for hypertensive patients. Some treatments may be more effective in reducing cognitive decline and subsequent dementia risk by offering protective effects on cognition beyond peripheral blood pressure control. Centrally active angiotensin converting enzyme (ACE) inhibitors that cross the blood brain barrier may have anti-inflammatory benefits and may improve cerebral blood flow beyond other antihypertensive medications. In community-dwelling older adults with hypertension, Sink et al. (2009) found that ACE inhibitors that crossed the blood brain barrier were associated with a 65% reduction in cognitive decline per year over the course of several years. Conversely, ACE inhibitors that did not cross the blood brain barrier resulted in a 73% greater risk of incident dementia over several years of exposure. Memory complaints may therefore indicate a need for re-evaluation of antihypertensive treatment efficacy in hypertensive patients.
There are potential limitations of this study. First, because our sample was comprised mainly of Caucasian participants, our findings may not extend to other groups. The combined effects of hypertension and memory complaints on memory performance should be further evaluated in larger, ethnically diverse samples, as the prevalence of hypertension and its detrimental effects vary across different ethnic groups. Second, by utilizing a single question to assess overall subjective memory problems rather than using multiple questions to assess self-reported memory function, we may not be assessing the full breadth and depth of memory complaints. However, previous investigators have found an association between memory complaints and objective cognitive performance when utilizing one general question in older adult samples (Bassett & Folstein, 1993; Gagnon et al., 1994; Schofield et al., 1997; Wang et al., 2000). The single question used in the present study was derived from an established questionnaire that assessed memory problems (MFQ) and was able to differentiate those who were experiencing mild memory complaints from those endorsing no memory concerns. Third, given our inclusion criteria for hypertension status (i.e., previous hypertension diagnosis and use of antihypertensive medications), it is possible that the non-hypertensive group included some individuals with untreated hypertension. Although such inclusion of participants would not influence the findings in the hypertensive group, future studies may benefit from including blood pressure readings taken at multiple times during study. Hypertension diagnoses typically require multiple blood pressure readings at various time points to obtain the best estimate of blood pressure status, which can also be influenced by individual differences in response to clinic visits (e.g., white coat hypertension). Fourth, although depressive symptoms did not account for the observed effects in our sample, those with mild memory complaints may have experienced some stress- and anxiety-related symptoms that could potentially contribute to decreased memory performance. Future research investigating the potential role of psychological stress and anxiety on subjective memory complaints in the context of hypertension may be warranted.
Moreover, self-reported memory complaints may be limited in capturing actual cognitive impairment in individuals with severe brain pathology compared to those with subtle or mild cognitive problems. In a meta-analysis assessing memory complaints in dementia and mild cognitive impairment patients, Mitchell (2008) found that only 40% of participants with dementia and mild cognitive impairment diagnoses acknowledged problems with memory, suggesting decreased awareness of actual deficits in those with more significant cognitive impairment. In line with these findings, Graham, Kunik, Doody, and Snow (2005) found that participants with mild-moderate and moderate-severe dementia over-estimated their performance on neuropsychological tests at a significantly higher rate than normal controls and may have consequently lacked insight for their declining cognitive function. Together, these findings suggest that differences in the level of cognitive impairment may in part explain the differences in the sensitivity of cognitive complaints in reflecting test performance across studies, highlighting the limitations of subjective self-report measures when there is greater severity of cognitive dysfunction.
We assessed memory concerns and hypertension status at only one time point and used hypertension duration and number of antihypertensive medications as measures of hypertension severity. Future research may benefit from assessing the role of longitudinal variability in blood pressure control, as well as differences for other measures of hypertension severity in affecting the relation between subjective memory complaints and memory performance. Follow-up longitudinal assessments are also needed to provide the temporal relation between memory complaints, hypertension status, and performance on neuropsychological tests of memory in order to assess the impact of self-reported memory differences over time in otherwise healthy aging.
In this sample of neurologically healthy elderly, memory performance was diminished in those with a combination of hypertension and mild subjective memory complaints. Conversely, mild memory concerns in the absence of hypertension did not have the same impact on memory performance. By using a less efficient learning strategy that relied to a greater extent on short-term stores and consistent reminding, the hypertensive complainers performed equivalently in delayed recall of a word list compared to the hypertensive non-complainers. Although these less efficient methods of learning information may be a compensatory strategy, they may reflect underlying brain differences, which may lead to increased effort and the potential for more memory problems over time. Memory concerns in the presence of hypertension may indicate the need to further evaluate antihypertensive treatment for some patients. Overall, these findings suggest that even mild memory complaints are important in hypertension and may reflect differences with memory that may have an impact on daily function and age-related cognitive decline. Further, it is possible that these effects reflect very early or subtle cognitive changes that may be indicative of incipient neuropathology, in addition to the effects of cognitive aging. Future research is needed to directly evaluate brain changes that may be occurring in elderly adults who are hypertensive and endorse such mild memory concerns, as well as their potential effects on other cognitive domains, to better understand the longer-term implication of mild memory complaints in the context of hypertension and aging.
Acknowledgments
Funding
This work was supported by the National Institute on Aging (grant number AG025526); the Advanced Research Institute for Biomedical Imaging; the McKnight Brain Research Foundation; and the State of Arizona and Arizona DHS.
Footnotes
The authors have reported no conflict of interest.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. doi:10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Ryan L, Bowers D, Foster TC, Bizon JL, Geldmacher DS, Glisky EL. Characterizing cognitive aging in humans with links to animal models. Frontiers in Aging Neuroscience. 2012;4:1–18. doi: 10.3389/fnagi.2012.00021. doi:10.3389/fnagi.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amariglio RE, Townsend MK, Grodstein F, Sperling RA, Rentz DM. Specific subjective memory complaints in older persons may indicate poor cognitive function. Journal of the American Geriatrics Society. 2011;59:1612–1617. doi: 10.1111/j.1532-5415.2011.03543.x. doi: 10.1111/j.1532-5415.2011.03543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André-Petersson L, Hagberg B, Janzon L, Steen G. A comparison of cognitive ability in normotensive and hypertensive 68-Year-Old Men: results from population study "Men Born in 1914," in Malmö, Sweden. Experimental Aging Research. 2001;27:319–340. doi: 10.1080/03610730109342352. doi:10.1080/03610730109342352. [DOI] [PubMed] [Google Scholar]
- Bassett SS, Folstein MF. Memory complaint, memory performance, and psychiatric diagnosis: a community study. Journal of Geriatric Psychiatry and Neurology. 1993;6:105–111. doi: 10.1177/089198879300600207. doi:10.1177/089198879300600207. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Lindgren KN, Bonaccorsy C, Bleecker ML. Memory complaints in older adults: Fact or fiction? Archives of Neurology. 1991;48(1):61–64. doi: 10.1001/archneur.1991.00530130069022. doi:10.1001/archneur.1991.00530130069022. [DOI] [PubMed] [Google Scholar]
- Brady CB, Spiro A, III, Gaziano JM. Effects of age and hypertension status on cognition: the Veterans Affairs Normative Aging Study. Neuropsychology. 2005;19:770–777. doi: 10.1037/0894-4105.19.6.770. doi:10.1037/0894-4105.19.6.770. [DOI] [PubMed] [Google Scholar]
- Burgmans S, van Boxtel MP, Gronenschild EHBM, Vuurman EFPM, Hofman P, Uylings HBM, Raz N. Multiple indicators of age-related differences in cerebral white matter and the modifying effects of hypertension. Neuroimage. 2010;49:2083–2093. doi: 10.1016/j.neuroimage.2009.10.035. doi:10.1016/j.neuroimage.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt VL, Cutler JA, Higgins M, Horan MJ, Labarthe D, Whelton P, Roccella EJ. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population. Data from the health examination surveys, 1960 to 1991. Hypertension. 1995;26(1):60–69. doi: 10.1161/01.hyp.26.1.60. doi:10.1161/01.HYP.26.1.60. [DOI] [PubMed] [Google Scholar]
- Buschke H. Selective reminding for analysis of memory and learning. Journal of Verbal Learning and Verbal Behavior. 1973;12:543–550. doi:10.1016/S0022-5371(73)80034-9. [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. doi:10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Corrada M, Hayden KM, Bullain SS, Paganini-Hill A, DeMoss J, Aguirre C, Kawas C. Age of onset of hypertension and risk of dementia in the oldest-old: The 90+ Study. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2014;10:P501. doi: 10.1016/j.jalz.2016.09.007. doi:10.1016/j.jalz.2014.05.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufouil C, de Kersaint–Gilly A, Besancon V, Levy C, Auffray E, Brunnereau L, Tzourio C. Longitudinal study of blood pressure and white matter hyperintensities The EVA MRI Cohort. Neurology. 2001;56:921–926. doi: 10.1212/wnl.56.7.921. doi:10.1212/WNL.56.7.921. [DOI] [PubMed] [Google Scholar]
- Dufouil C, Fuhrer R, Alpérovitch A. Subjective cognitive complaints and cognitive decline: consequence or predictor? The epidemiology of vascular aging study. Journal of the American Geriatrics Society. 2005;53:616–621. doi: 10.1111/j.1532-5415.2005.53209.x. doi:10.1111/j.1532-5415.2005.53209.x. [DOI] [PubMed] [Google Scholar]
- Elias MF, Wolf PA, D'Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. American Journal of Epidemiology. 1993;138:353–364. doi: 10.1093/oxfordjournals.aje.a116868. PMID: 8213741. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state": a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. doi:10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gagnon M, Dartigues JF, Mazaux J, Dequae L, Letenneur L, Giroire JM, Barberger-Gateau P. Self-reported memory complaints and memory performance in elderly French community residents: results of the PAQUID Research Program. Neuroepidemiology. 1994;13:145–154. doi: 10.1159/000110373. doi:10.1159/000110373. [DOI] [PubMed] [Google Scholar]
- Geerlings MI, Jonker C, Bouter LM, Adèr HJ, Schmand B. Association between memory complaints and incident Alzheimer's disease in elderly people with normal baseline cognition. American Journal of Psychiatry. 1999;156:531–537. doi: 10.1176/ajp.156.4.531. PMID: 10200730. [DOI] [PubMed] [Google Scholar]
- Gilewski MJ, Zelinski EM, Schaie KW. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychology and Aging. 1990;5:482–490. doi: 10.1037//0882-7974.5.4.482. doi:10.1037/0882-7974.5.4.482. [DOI] [PubMed] [Google Scholar]
- Glisky EL. Changes in Cognitive Function in Human Aging. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton (FL): 2007. doi:10.1201/9781420005523.sec1. [Google Scholar]
- Graham DP, Kunik ME, Doody R, Snow AL. Self-reported awareness of performance in dementia. Cognitive Brain Research. 2005;25(1):144–152. doi: 10.1016/j.cogbrainres.2005.05.001. doi:10.1016/j.cogbrainres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. Journal of the American College of Cardiology. 2012;60:599–606. doi: 10.1016/j.jacc.2012.04.026. doi:10.1016/j.jacc.2012.04.026. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. doi:10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington F, Saxby BK, McKeith IG, Wesnes K, Ford GA. Cognitive performance in hypertensive and normotensive older subjects. Hypertension. 2000;36:1079–1082. doi: 10.1161/01.hyp.36.6.1079. doi:10.1161/01.HYP.36.6.1079. [DOI] [PubMed] [Google Scholar]
- Hudak EM, Edwards JD, Athilingam P, McEvoy CL. A Comparison of Cognitive and Everyday Functional Performance Among Older Adults With and Without Hypertension. Clinical Gerontologist. 2013;36:113–131. doi: 10.1080/07317115.2012.749322. doi:10.1080/07317115.2012.749322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. International Journal of Geriatric Psychiatry. 2000;15:983–991. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. doi:10.1002/1099-1166(200011)15:11<983::AID-GPS238>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Jonker C, Launer LJ, Hooijer C, Lindeboom J. Memory complaints and memory impairment in older individuals. Journal of the American Geriatrics Society. 1996;44(1):44–49. doi: 10.1111/j.1532-5415.1996.tb05636.x. PMID: 8537589. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Butterworth P, Anstey KJ, Christensen H, Easteal S, Maller J, Sachdev P. Memory complaints in a community sample aged 60–64 years: associations with cognitive functioning, psychiatric symptoms, medical conditions, APOE genotype, hippocampus and amygdala volumes, and white-matter hyperintensities. Psychological Medicine. 2004;34:1495–1506. doi: 10.1017/s0033291704003162. doi:10.1017/S0033291704003162. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Christensen H, Korten AE, Henderson AS, Jacomb PA, Makinnon A. Do cognitive complaints either predict future cognitive decline or reflect past cognitive decline? A longitudinal study of an elderly community sample. Psychological Medicine. 1997;27(1):91–98. doi: 10.1017/s0033291796003923. doi:10.1017/S0033291796003923. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Zimprich D, Eschen A. What do subjective cognitive complaints in persons with aging-associated cognitive decline reflect? International Psychogeriatrics. 2005;17:499–512. doi: 10.1017/s1041610205001638. doi:10.1017/S1041610205001638. [DOI] [PubMed] [Google Scholar]
- Larrabee GJ, Crook TH. Estimated prevalence of age-associated memory impairment derived from standardized tests of memory function. International Psychogeriatrics. 1994;6(1):95–104. doi: 10.1017/s1041610294001663. doi:10.1017/S1041610294001663. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontology. 1969;9:179–186. doi:10.1093/geront/9.3_Part_1.179. [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. doi:10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minett TS, Dean JL, Firbank M, English P, O'Brien JT. Subjective memory complaints, white-matter lesions, depressive symptoms, and cognition in elderly patients. The American Journal of Geriatric Psychiatry. 2005;13:665–671. doi: 10.1176/appi.ajgp.13.8.665. doi:10.1097/00019442-200508000-00005. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. International Journal of Geriatric Psychiatry. 2008;23:1191–1202. doi: 10.1002/gps.2053. doi:10.1002/gps.2053. [DOI] [PubMed] [Google Scholar]
- Murray MD, Lane KA, Gao S, Evans RM, Unverzagt FW, Hall KS, Hendrie H. Preservation of cognitive function with antihypertensive medications. Archives of Internal Medicine. 2002;162:2090–2096. doi: 10.1001/archinte.162.18.2090. doi:10.1001/archinte.162.18.2090. [DOI] [PubMed] [Google Scholar]
- Papademetriou V. Hypertension and cognitive function. Blood pressure regulation and cognitive function: a review of the literature. Geriatrics. 2005;60(1):20–24. PMID: 15700945. [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behavioral Neuroscience. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. doi:10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Reid LM, MacLullich AM. Subjective memory complaints and cognitive impairment in older people. Dementia and Geriatric Cognitive Disorders. 2006;22:471–485. doi: 10.1159/000096295. doi:10.1159/000096295. [DOI] [PubMed] [Google Scholar]
- Rey A. L'Examen clinique en psychologie. Presses Universitaires de France; Paris: 1964. [Google Scholar]
- Salthouse TA. Influence of processing speed on adult age differences in working memory. Acta Psychologica. 1992;79:155–170. doi: 10.1016/0001-6918(92)90030-h. doi:10.1016/0001-6918(92)90030-H. [DOI] [PubMed] [Google Scholar]
- Saxby BK, Harrington F, McKeith IG, Wesnes K, Ford GA. Effects of hypertension on attention, memory, and executive function in older adults. Health Psychology. 2003;22:587–591. doi: 10.1037/0278-6133.22.6.587. doi:10.1037/0278-6133.22.6.587. [DOI] [PubMed] [Google Scholar]
- Schofield PW, Marder K, Dooneief G, Jacobs DM, Sano M, Stern Y. Association of subjective memory complaints with subsequent cognitive decline in community-dwelling elderly individuals with baseline cognitive impairment. American Journal of Psychiatry. 1997;154:609–615. doi: 10.1176/ajp.154.5.609. PMID: 9137114. [DOI] [PubMed] [Google Scholar]
- Sink KM, Leng X, Williamson J, Kritchevsky SB, Yaffe K, Kuller L, Goff DC. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Archives of Internal Medicine. 2009;169:1195–1202. doi: 10.1001/archinternmed.2009.175. doi:10.1001/archinternmed.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GE, Petersen RC, Ivnik RJ, Malec JF, Tangalos EG. Subjective memory complaints, psychological distress, and longitudinal change in objective memory performance. Psychology and Aging. 1996;11:272–279. doi: 10.1037//0882-7974.11.2.272. doi:10.1037/0882-7974.11.2.272. [DOI] [PubMed] [Google Scholar]
- Strassburger TL, Lee HC, Daly EM, Szczepanik J, Krasuski JS, Mentis MJ, Alexander GE. Interactive effects of age and hypertension on volumes of brain structures. Stroke. 1997;28:1410–1417. doi: 10.1161/01.str.28.7.1410. doi:10.1161/01.STR.28.7.1410. [DOI] [PubMed] [Google Scholar]
- Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51:986–993. doi: 10.1212/wnl.51.4.986. doi:10.1212/WNL.51.4.986. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Miller TP, Tinklenberg JR. Correlates of memory decline: a 4-year longitudinal study of older adults with memory complaints. Psychology and Aging. 1992;7:185–193. doi: 10.1037//0882-7974.7.2.185. doi:10.1037/0882-7974.7.2.185. [DOI] [PubMed] [Google Scholar]
- Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund LO, Freund-Levi Y, Blennow K. Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. The Lancet Neurology. 2009;8:619–627. doi: 10.1016/S1474-4422(09)70139-5. doi:10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension. 2005;45:374–379. doi: 10.1161/01.HYP.0000156744.44218.74. doi:10.1161/01.HYP.0000156744.44218.74. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Manuck SB, Ryan CM, Muldoon MF. Neuropsychological correlates of hypertension: review and methodologic considerations. Psychological Bulletin. 1991;110:451–468. doi: 10.1037/0033-2909.110.3.451. doi:10.1037/0033-2909.110.3.451. [DOI] [PubMed] [Google Scholar]
- Wang PN, Wang SJ, Fuh JL, Teng EL, Liu CY, Lin CH, Liu HC. Subjective memory complaint in relation to cognitive performance and depression: a longitudinal study of a rural Chinese population. Journal of the American Geriatrics Society. 2000;48:295–299. doi: 10.1111/j.1532-5415.2000.tb02649.x. PMID: 10733056. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale – Fourth Edition. The Psychological Corporation; San Antonio, TX: 2008. [Google Scholar]
- Zec RF. The neuropsychology of aging. Experimental Gerontology. 1995;230:431–442. doi: 10.1016/0531-5565(94)00066-c. doi:10.1016/0531-5565(94)00066-C. [DOI] [PubMed] [Google Scholar]