Abstract
Several eye diseases are associated with axonal injury in the optic nerve, which normally leads to degeneration of retinal ganglion cells (RGCs) and subsequently to loss of vision. There is experimental evidence that some members of the small heat shock protein family (HspBs) are upregulated upon optic nerve injury (ONI) in the retina and sufficient to promote RGC survival. These data raise the question as to whether other family members may play a similar role in this context. Here, we performed a comprehensive comparative study comprising all HspBs in an experimental model of ONI. We found that five HspBs were expressed in the adult rat retina at control conditions but only HspB1 and HspB5 were upregulated in response to ONI. Furthermore, HspB1 and HspB5 were constitutively phosphorylated in Müller cells at serine 15 and serine 59, respectively. In RGCs, phosphorylation was stimulated by ONI and occurred at serine 86 of HspB1 and at serine 19 and 45 of HspB5. These data suggest that of all small heat shock proteins, only HspB1 and HspB5 might be of protective value for RGCs after ONI and that this process might be regulated by phosphorylation at serine 86 of HspB1 and serine 19 and serine 45 of HspB5. The molecular targets of phosphoHspB1 and phosphoHspB5 remain to be identified.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-015-0650-8) contains supplementary material, which is available to authorized users.
Keywords: Heat shock protein, HspB, Retina, Retinal ganglion cells, Neuroprotection, Optic nerve injury
Introduction
Damage of retinal ganglion cell (RGC) axons in the optic nerve head is one of the causes for irreversible loss of vision in glaucoma or after traumatic injuries (Diekmann et al. 2013; Fischer and Leibinger 2012). Therapeutic strategies aim at protection of RGCs and increase of their survival during pathological conditions. The group of heat shock proteins is one general, powerful endogenous cytoprotective effector. In this study, we focus on small heat shock proteins characterized by their molecular weight between 12 and 43 kDa, which are members of a common protein family now called heat shock proteins B comprising ten family members (HspB1 to HspB10) (Kappe et al. 2003). This new nomenclature replaced the older one, where the proteins were named according to their molecular weight or the tissue where they were first identified. The protein Hsp16.2 (alternatively named HSPC034, C1orf41, PP25, IFT20, HspB11) has been suggested to be included into this protein family because of its homology, induction upon heat stress, and chaperone activity (Bellyei et al. 2007; Kampinga et al. 2009); however, it lacks a clear alpha-crystallin domain, which is the hallmark of all HspB proteins (Kappe et al. 2010).
HspBs prevent protein denaturation and aggregation, have anti-apoptotic and anti-inflammatory properties, and interact with several cytoskeletal proteins thereby increasing cell viability under cellular stress conditions (Garrido et al. 2001; Golenhofen et al. 1998, 2002; Gusev et al. 2002; Haslbeck et al. 2005; Ousman et al. 2007; Shao et al. 2013; Sun and MacRae 2005; Verschuure et al. 2002).
Most HspBs are preferentially expressed in muscle tissue but show additionally lower ubiquitous expression. A cardioprotective role has clearly been identified for several HspBs (Golenhofen et al. 2006; Morrison et al. 2004; Ray et al. 2001) and there is increasing evidence for their neuroprotective potential (Brownell et al. 2012; Golenhofen and Bartelt-Kirbach 2015; Reddy and Reddy 2015). In the brain, ΗspΒs are expressed to a lower extent and are found in glial cells as well as in neurons (Bartelt-Kirbach and Golenhofen 2011; Plumier et al. 1997; Quraishe et al. 2008). Some HspBs, especially HspB1 (Hsp25) and HspB5 (αB-crystallin), have also been investigated in the retina. HspB1 is upregulated in RGCs following axotomy (Krueger-Naug et al. 2002). Furthermore, intravitreal injection of HspB4 and HspB5 or overexpression of these two proteins in RGCs enhanced the survival of RGCs subjected to axotomy and increased axonal regeneration (Munemasa et al. 2009; Ying et al. 2008, Wu et al. 2014). These data clearly indicate a protective function of some HspBs in RGCs (reviewed in Thanos et al. 2014). Whether this holds true for all members of the HspB family has not yet been investigated, as well as it is not clear which molecular mechanisms are involved therein.
HspBs not only become upregulated in response to stress conditions, but at least some of them will also be phosphorylated. Phosphorylation increases the cytoprotective effect in several cell types (Arrigo and Welch 1987; Ito et al. 1997; Landry et al. 1991, 1992). In the peripheral nervous system, overexpression and phosphorylation of HspB1 is of protective value in an experimental axotomy model (Benn et al. 2002). Mouse and rat HspB1 have two major phosphorylation sites at serine (Ser) 15 and Ser 86 (Gaestel et al. 1991; Hoffert et al. 2006) whereas HspB5 has three phosphorylation sites at Ser 19, Ser 45, and Ser 59 (Chiesa et al. 1987; Hoffert et al. 2006, Ito et al. 1997) which are conserved in several species. Recently, we showed in cultured hippocampal neurons a phosphorylation-dependent recruitment of HspB1 and ΗspΒ5 to different cellular compartments, i.e., axons, dendrites, and synapses (Schmidt et al. 2012). These data suggest that neuronal HspB functions might also be regulated by phosphorylation.
In the current study, we systematically and comprehensively investigated the expression and phosphorylation of HspBs including HspB11 in the naive rat retina and upon optic nerve injury (ONI). ONI in rats is a useful model for damage of RGCs in several eye diseases. We found that five HspBs were expressed at control conditions but only expression of HspB1 and HspB5 was upregulated in response to ONI. HspB1 and HspB5 were constitutively phosphorylated in Müller cells at Ser 15 and Ser 59, respectively. In contrast, in RGCs, phosphorylation was only identified after ONI and occurred at different sites, i.e., at Ser 86 of HspB1 and at Ser 19 and 45 of HspB5. Thus, of all HspBs, only HspB1 and HspB5 are involved in the response of RGCs to axotomy.
Methods
Animals and optic nerve injury (ONI)
All animal experiments were performed in compliance with the guidelines for the welfare of experimental animals issued by the Federal Government of Germany. All experimental animal procedures were approved by the local ethics committee (Regierungspräsidium, Tübingen, Germany, permit number: 87–51.04.2011.A062) and performed as described previously (Hauk et al. 2010). Adult female Sprague–Dawley rats (Charles-River Lab. Int. Inc., Wilmington, MA, USA) were housed in the animal facility of the university under specific pathogen-free conditions. Up to four animals were housed per cage and were maintained on a 12-h light/dark cycle with ad libitum access to food and water. Health monitoring was performed daily and no adverse advents were observed. At each experimental day, rats were randomized into three groups with at least one animal in each group: (1) rats after cutting of the optic nerve, (2) sham-operated rats which underwent the same surgical procedure without cutting of the nerve, and (3) untreated rats. The surgical procedure was performed as follows. Rats were anesthetized by intraperitoneal injections of ketamine (75 mg/kg) and xylazine (15 mg/kg). A 1- to 1.5-cm incision was made in the skin above the right orbit. The optic nerve was surgically exposed under an operating microscope, the dural sheath was longitudinally opened, and the nerve was completely cut 1 mm behind the eye avoiding injury to the retinal artery. The vascular integrity of the retina was verified by funduscopic examination after surgery. Five days after surgery, animals were sacrificed and retinae dissected and processed for different purposes, i.e., RNA isolation, Western blotting, or immunohistochemistry. Analysis of samples of animals of the different groups was performed in parallel. In total, each experimental group consisted of at least three animals for each different further processing. RNA was isolated to assess the expression levels of HspBs, Western blot analysis to measure the protein amount, and immunohistochemistry to investigate the localization of the respective proteins in the retina. In total, 11 control animals, 11 sham-operated animals, and 12 animals subjected to ONI were used.
In addition, four untreated Sprague–Dawley rats were killed by CO2 inhalation and decapitation, retinae and brains dissected on ice and shock-frozen in liquid nitrogen, and samples stored at −80 °C. These tissues were used for isolation of RNA and comparison of the expression pattern of HspBs in retina versus brain.
Immunohistochemistry
Animals were anesthetized as described above and perfused with 4 % formaldehyde in phosphate-buffered saline (PBS). Eyes were postfixed for several hours, transferred to 30 % sucrose in PBS overnight (4 °C), frozen, and stored at −80 °C. Cryosections of the eyes of 14-μm thickness were longitudinally cut, mounted onto coated glass slides (0.1 mg/ml poly-L-lysine, Sigma-Aldrich, Steinheim, Germany), and air-dried. Sections were blocked for unspecific binding with 3 % bovine serum albumin and 1 % normal goat serum in PBS for 30–60 min at ambient temperature. The primary antibodies were applied at 4 °C overnight. Rabbit anti-HspB1 (Stressgen SPA-801, Victoria, Canada), anti-HspB5 (Stressgen SPA-223), anti-phospho-HspB1 (Ser15) (Acris, San Diego, CA, USA), anti-phospho-HspB1(Ser86) (Acris), anti-phospho-HspB5(Ser19) (Stressgene), anti-phospho-HspB5(Ser45) (Stressgene), and anti-phospho-HspB5(Ser59) (Stressgene) were used at a dilution of 1:200. Mouse anti-glutamine synthetase (Millipore, Temecula, CA, USA) and anti-β-III-tubulin (Hiss Diagnostics, Freiburg, Germany) were diluted 1:500. After washing three times for 10 min with PBS, sections were incubated with goat anti-rabbit Alexa Fluor 488 and goat anti-mouse Alexa Fluor 568 (final dilutions 1:900, Molecular Probes, USA) for 45 min at ambient temperature. After final rinsing for three times for 10 min with PBS, sections were mounted in Mowiol 4.88 (Hoechst, Germany) containing 1 μg/ml DAPI (Applichem, Darmstadt, Germany). Images were acquired on a Zeiss Axioscope2 microscope equipped with an AxioCam MRm camera (Zeiss, Oberkochen, Germany) and analyzed using AxioVision software (Zeiss). An identical exposure time was used for sections of all three experimental groups stained with the same antibody. Representative picture details were cut and assembled to the figures using Adobe Photoshop (Adobe Systems, USA). Representative images from at least three independent experiments are shown. Controls were performed by omitting the primary antibody and in case of the phospho-specific antibodies additionally by preabsorption of the antibodies with their respective antigens (see next paragraph).
Preabsorption of antibodies with phospho-peptides
Specificity of the phospho-specific antibodies was proven by preabsorption of the antibodies with the respective antigens. The following peptides were used: NH2-LLRGP(pS)WDPFRC-CONH2 (pHspB1-S15, Acris), NH2-RQL(pS)SGVSEIR-CONH2 (pHspB1-S86, CASLO Laboratories, Lyngby, Denmark), NH2-FFPFH(pS)PSRLFD-CONH2 (pHspB5-S19, ABGENT, San Diego, CA, USA), NH2-L(pS)PFYLRPPSF-CONH2 (pHspB5-S45, ABGENT), and NH2-FLRAP(pS)WIDTG-CONH2 (pHspB5-S59, ABGENT). Ten micrograms of phosphopeptide was incubated with 1 μg of the corresponding antibody in 100 μl Tris-buffered saline (TBS) and incubated for 2 h at RT. Afterward, 100 μl of blocking solution (3 % bovine serum albumin and 1 % normal goat serum in TBS) was added (final dilution of the antibody 1:200) and the preabsorbed antibody used for immunostaining of rat eye sections as described above. In parallel, antibodies not preabsorbed to their antigen but otherwise treated in the same way were used as positive controls for immunostaining.
Western blot
Retinal lysates were prepared by adding Laemmli protein sample buffer (Laemmli 1970) to the retinal tissue with subsequent sonication and heating to 95 °C for 5 min. Protein concentration was measured with amidoblack staining (Dieckmann-Schuppert and Schnittler 1997; Heinzel et al. 1965). Equal amounts of protein were separated on 10–15 % SDS-polyacrylamide gels followed by blotting onto Hybond PVDF membranes (Amersham, GE Healthcare, Freiburg, Germany) with 90 V for 150 min at 4 °C. Following blotting, membranes were incubated for 2–5 min in Ponceau S solution (Sigma, Steinheim, Germany) to confirm equal protein loading and blotting. After washing, membranes were blocked with 5 % (w/v) low fat milk in TBST (50 mM Tris, 150 mM NaCl, pH 7.5 containing 0.05 % (v/v) Tween 20) for 1 h, followed by incubation with the primary antibody (diluted in the blocking solution) at 4 °C overnight. Rabbit anti-HspB1 (Stressgen, SPA-801), anti-HspB5 (Stressgen, SPA-223), anti-HspB8 (Cell Signaling), anti-HspB11 (kind gift of Prof. Dr. Bellyei, Dept. of Oncotherapy, University of Pécs, Hungary), mouse anti-HspB6 (Acris), and anti-Hsp70i (Stressgene) were all applied at a dilution of 1:1000. After washing three times for 10 min in TBST, membranes were incubated with secondary antibodies, i.e., horseradish-peroxidase-labeled goat anti-rabbit or anti-mouse IgG (Jackson Immunoresearch, West Grove, PA) at a dilution of 1:3000 in the blocking solution for 1 h at RT. After final washing steps, bound secondary antibodies were detected with the enhanced chemiluminescence system. Blots of at least three independent experiments are shown.
Quantitative RT-PCR
RNA isolation, cDNA synthesis, and quantitative RT-PCR was performed as described previously (Bartelt-Kirbach and Golenhofen 2011) with slight modifications. RNA was isolated from rat retinae or brain with the RNeasy system (Qiagen, Hilden, Germany) according to the manufacturer’s protocol and digested with DNase I (Fermentas, St. Leon-Rot, Germany) at 37 °C for 1 h. To ensure complete removal of contaminating DNA, 1 μl of DNase treated RNA was subsequently amplified with primers for HspB3, a gene which carries no introns (Table 1). Afterward, 10 μl of RNA was reverse transcribed with the SuperScript III First Strand Synthesis System (Invitrogen, Darmstadt, Germany) and oligo-d(T) primers according to the manufacturer’s protocol. The resulting cDNA was diluted 1:10 and 2 μl of this dilution amplified in a LightCycler 1.0 (Roche Applied Science, Mannheim, Germany) with the QuantiTect SYBR Green PCR Kit (Qiagen) using the primers designated in Table 1. Cycling conditions were 15 min of initial denaturation at 94 °C and 50 subsequent cycles of 15 s at 94 °C, 30 s at 55 °C, and 30 s at 72 °C. To assess reference gene stability, the expression of the eight different genes hprt1 (hypoxanthine guanine phosphoribosyltransferase 1), actb (beta-actin), gapdh (glyceraldehyde-3-phosphate dehydrogenase), hmbs (hydroxymethylbilane synthase), 18 s rRNA (18 s subunit ribosomal RNA), cyc a (cyclophilin A), rpl13a (ribosomal protein L13A), and ywhaz (tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta) was determined in retinae of control rats and rats subjected to ONI. The geNorm algorithm (Vandesompele et al. 2002) was employed to select the two most stable reference genes, actb and ywhaz, whose mean expression values were then used for normalization of HspB expression data. Analysis of the real-time PCR data was carried out as described (Bartelt-Kirbach and Golenhofen 2011). The expression threshold below which a gene was defined as not expressed was set to 0.1 % of the mean of the reference genes.
Table 1.
Primer pairs for real-time PCR
| Gene | Primer | Product length (bp) |
|---|---|---|
| HspB1 (Hsp25) | Fwd: CGGCAACTCAGCAGCGGTGTCT | 160 |
| Rev: CATGTTCATCCTGCCTTTCTTCGTG | ||
| HspB2 (MKBP) | Fwd: CCGAGTACGAATTTGCCAACCC | 191 |
| Rev: AAATGCCTGGAACTTGCCTTCACT | ||
| HspB3 | Fwd: AACCAGAATGGACGAACACGG | 104 |
| Rev: CATGACAGAGGATGGCAGACAAAT | ||
| HspB4 (αA-crystallin) | Fwd: AGATGGCTGCGGGTTCA | 154 |
| Rev: GGCTCTTGGGGTTGGTTAG | ||
| HspB5 (αB-crystallin) | Fwd: CTTCTACCTTCGGCCACCCTC | 164 |
| Rev: GCACCTCAATCACGTCTCCC | ||
| HspB6 (Hsp20) | Fwd: CCATGTGGAGGTCCATGCTCG | 195 |
| Rev: GCAGGTGGTGACGGAAGTGAG | ||
| HspB7 (cvHsp) | Fwd: CCACCTCCAACAACCACATCG | 100 |
| Rev: ACATCCTCTGGTAGCTGGCACTT | ||
| HspB8 (Hsp22) | Fwd: CCGGAAGAACTGATGGTAAAGAC | 166 |
| Rev: CCTCTGGAGAAAGTGAGGCAAATAC | ||
| HspB9 | Fwd: GCCCTTTCTGAACGAAATCAAGC | 159 |
| Rev: GCCATCTATCCGCACCACCA | ||
| HspB10 (ODF1) | Fwd: AACGTCTGCGGCTTTGAACCT | 195 |
| Rev: ACTGCCGAGCCCGTAGGAGTAGGTC | ||
| HspB11 (Hsp16.2) | Fwd: ATTATGGCACGAGATGGCTACG | 128 |
| Rev: TGTAATCACTAAGGAAGACTTGAGACTG | ||
| Hsp70i | Fwd: GGTCATCTCCTGGCTGGACTCTAACACG | 212 |
| Rev: GCCAGAAAAGCCTCTAATCCACCTCC | ||
| ACTB | Fwd: TCCGTAAAGACCTCTATGCCAACA | 104 |
| Rev: GCTAGGAGCCAGGGCAGTAATCT | ||
| Ywhaz | Fwd: TTGAGCAGAAGACGGAAGGT | 136 |
| Rev: GAAGCATTGGGGATCAAGAA |
Hsp70i inducible form of Hsp70, ACTB beta-actin, Ywhaz tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta
Statistics
Data are presented as means ± standard error of the mean. The non-parametric Mann–Whitney U test was used for statistical analysis. This test does not require a Gaussian distribution of the data which could not be postulated. Statistical significance was assumed for p < 0.05.
Results
Expression of HspBs in the adult rat retina
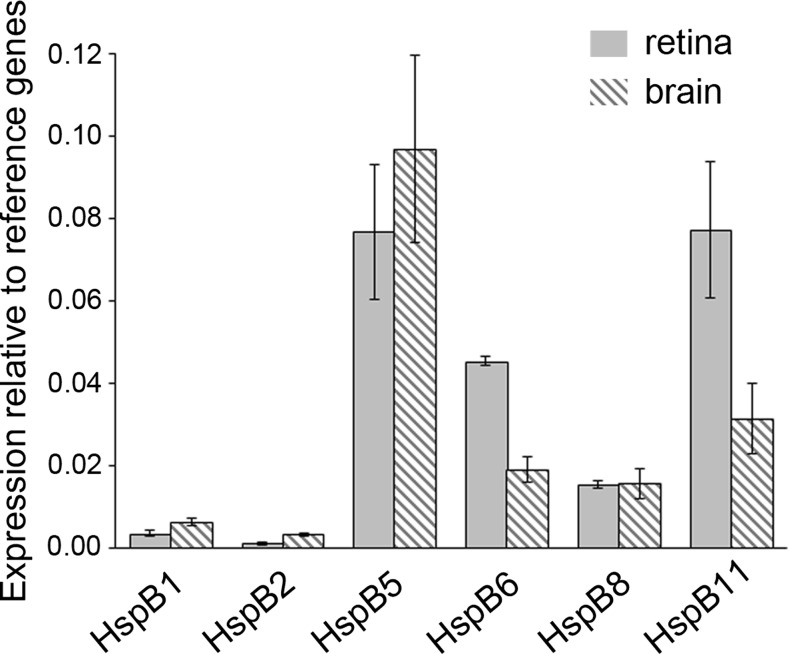
To investigate the expression pattern of HspBs, we comparatively measured the messenger RNA (mRNA) levels of all eleven HspBs by quantitative RT-PCR in the whole retina and brain. Table 1 depicts the primer pairs used. The expression of HspBs relative to the mean of the reference genes actb and ywhaz is illustrated in Fig. 1. Five HspBs were clearly expressed in the retina as well as in the brain, namely HspB1 (Hsp25), HspB5 (αB-crystallin), HspB6 (Hsp20), HspB8 (Hsp22), and HspB11 (Hsp16.2). HspB2 (MKBP) was expressed in the brain but near or sometimes below the expression threshold in the retina (0.11 ± 0.04 % of reference genes). The other five HspBs were not expressed in brain as well as in retina as defined by values <0.1 % of reference genes. Thus, in the retina, the expression pattern of HspBs was similar to that observed in the brain.
Fig. 1.
Expression pattern of HspBs in the retina compared to the brain. mRNA amount of all 11 sHsps was measured in rat retina (n = 3) and whole brain (n = 4) by quantitative RT-PCR in relation to the mean of the reference genes actb and ywhaz. Five sHsps were consistently expressed in the retina as well as in the brain, namely, HspB1, HspB5, HspB6, HspB8, and HspB11. HspB2 was near the detection threshold in the retina. The other five sHsps were not expressed, defined by values <0.1 % of reference genes
Retinal expression of HspBs upon optic nerve injury
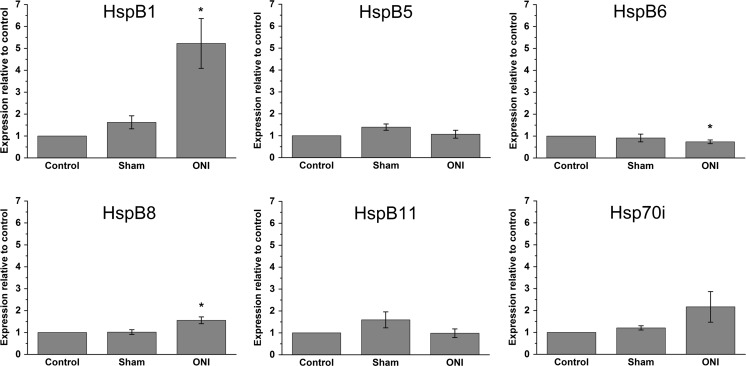
To identify HspBs that might potentially be involved in protection of axotomized retinal ganglion cells (RGCs) under pathophysiological conditions, we determined their expression in retina after optic nerve injury (ONI), i.e., cutting of the optic nerve which contains the axons from the retinal ganglion cells. Three groups of rats were used: (1) untreated rats, (2) sham-operated rats, and (3) rats subjected to ONI. Five days thereafter, retinal RNA was isolated for quantitative RT-PCR analysis or lysates of the retina were prepared for Western blot analysis. Virtually all axotomized RGCs reportedly survive for 5 days and undergo apoptosis afterward resulting in rapid cell loss with less than 10 % of RGCs surviving at day 14 (Berkelaar et al. 1994). Thus, 5-day post-surgical treatment seemed to be an optimal time point for analysis since RGCs could be assumed to have reacted to axotomy but would be still vital. All eleven HspBs were investigated on the mRNA level. The five HspBs that were not expressed in the naive retina were not expressed after ONI either (Table 2). Also, HspB2 expression was below or close to the expression threshold during all experimental conditions and, thus, was considered to be not expressed (Table 2). Retinal mRNA levels of the other five HspBs expressed and of Hsp70i for comparison are shown in Table 2 and Fig. 2. Interestingly, an increased expression after ONI could only be observed for HspB1 and HspB8. HspB1 mRNA increased 5.2 ± 1.1-fold and HspB8 mRNA 1.6 ± 0.2-fold compared to control. HspB5 and HspB11 mRNA did not show any significant alterations, and HspB6 mRNA slightly decreased after ONI. Surprisingly, also Hsp70i mRNA expression was not significantly altered after ONI although this Hsp is reported to be induced strongly in many different cell types including neurons after several kinds of stresses (Brown 1990; Kiang and Tsokos 1998).
Table 2.
Expression of HspBs after optic nerve injury
| Gene | Control | Sham | ONI |
|---|---|---|---|
| HspB1 | 1 | 1.6 ± 0.3 | 5.2 ± 1.1 |
| HspB2 | n.e. | n.e. | n.e. |
| HspB3 | n.e. | n.e. | n.e. |
| HspB4 | n.e. | n.e. | n.e. |
| HspB5 | 1 | 1.4 ± 0.1 | 1.1 ± 0.2 |
| HspB6 | 1 | 0.9 ± 0.2 | 0.74 ± 0.1 |
| HspB7 | n.e. | n.e. | n.e. |
| HspB8 | 1 | 1.0 ± 0.1 | 1.6 ± 0.2 |
| HspB9 | n.e. | n.e. | n.e. |
| HspB10 | n.e. | n.e. | n.e. |
| HspB11 | 1 | 1.6 ± 0.4 | 1.0 ± 0.2 |
| Hsp70i | 1 | 1.2 ± 0.1 | 2.2 ± 0.7 |
mRNA levels of all 11 HspBs in rat retinae were measured by quantitative RT-PCR of control rats, sham-operated rats, and rats after cutting of the optic nerve (ONI). Data are presented in relation to control. No expression (<0.1 % of the mean of the reference genes) is depicted by “n.e.” Control, sham n = 5, ONI n = 6
Fig. 2.
Expression of HspBs after optic nerve injury. mRNA levels of HspBs in rat retinae were measured by quantitative RT-PCR of control rats, sham-operated rats, and rats 5 days after cutting of the optic nerve (ONI). Note a significantly increased expression of HspB1 and HspB8 in response to ONI. Control, sham n = 5, ONI n = 6, *p < 0.05
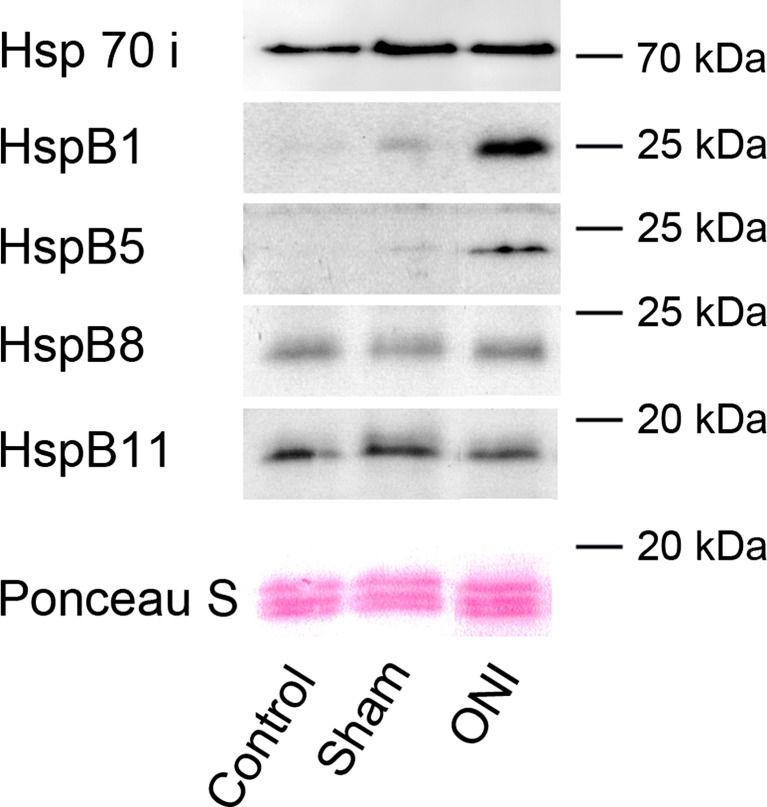
We used Western blot analysis to test whether differential mRNA expression was also reflected on the protein level. As indicated in Fig. 3, ONI-induced upregulation of HspB1 was confirmed on the protein level whereas the increase of HspB8 mRNA was not. Interestingly, a clear upregulation of HspB5 protein was observed, despite no significant differences of mRNA levels (see Fig. 2). These differences might indicate a posttranscriptional regulation of HspB5 under these conditions. HspB11 and Hsp70i protein amount was not altered. Moreover, HspB6 protein could not be detected, which might be due to the very low expression level or low sensitivity of the antibody.
Fig. 3.
Protein amount of HspBs after optic nerve injury. Protein amount was determined by Western blotting for HspBs of retinae of control, sham-operated rats, and rats subjected to ONI (5 days thereafter). HspB1 and HspB5 protein were upregulated in response to ONI whereas no changes in the protein amount of HspB8, HspB11, or Hsp70i could be observed. Representative blots of three experiments are shown. Ponceau S staining confirms equal loading of total protein per lane
Thus, ONI clearly induced HspB1 and HspB5 protein expression in the retina.
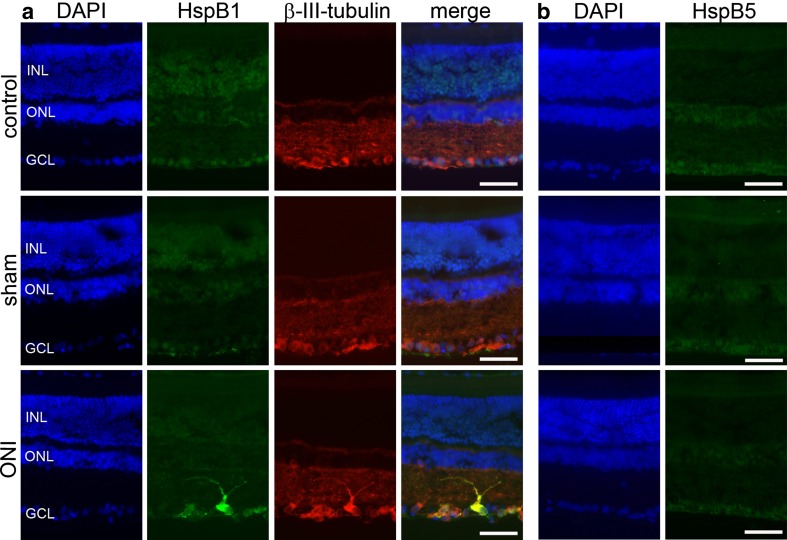
Localization of HspB1 and HspB5 in the retina after ONI
To identify the retinal cells causing the determined upregulation of HspB1 and HspB5, we immunohistochemically analyzed cryosections of eyes from either control, sham-operated rats, or rats that had been subjected to ONI (Fig. 4). Sections were double stained for the respective HspB and βIII-tubulin (a known marker for RGCs; Hauk et al. 2009). Cell nuclei were visualized using DAPI. For HspB1, no specific immunosignal could be observed in control and sham-operated rats. However, after ONI, prominent labeling of most but not all cells of the ganglion cell layer occurred. HspB1 was clearly identified in βIII-tubulin-positive RGCs (Fig. 4). In contrast to HspB1, HspB5 showed no clear localization in any retinal cell type in all three groups investigated. This might be due to a diffuse distribution of HspB5 or a low sensitivity of the antibody.
Fig. 4.
Localization of HspB1 and HspB5 of rat retinae of control, sham-operated rats, and rats subjected to ONI. Cryosections of eyes of the three experimental groups (as indicated on the left) were immunostained for a HspB1 or b HspB5 and β-III-tubulin. For simplification, β-III-tubulin stainings are not shown in the case of HspB5. Cell nuclei were visualized with DAPI. Note the localization of HspB1 in response to ONI in retinal ganglion cell layer which could be identified by β-III-tubulin labeling. In contrast, HspB5 showed no clear localization in any cell type of the retina in all three groups investigated. Representative images of three independent experiments are shown. INL inner nuclear layer, ONL outer nuclear layer, GCL ganglion cell layer, bar: 50 μm
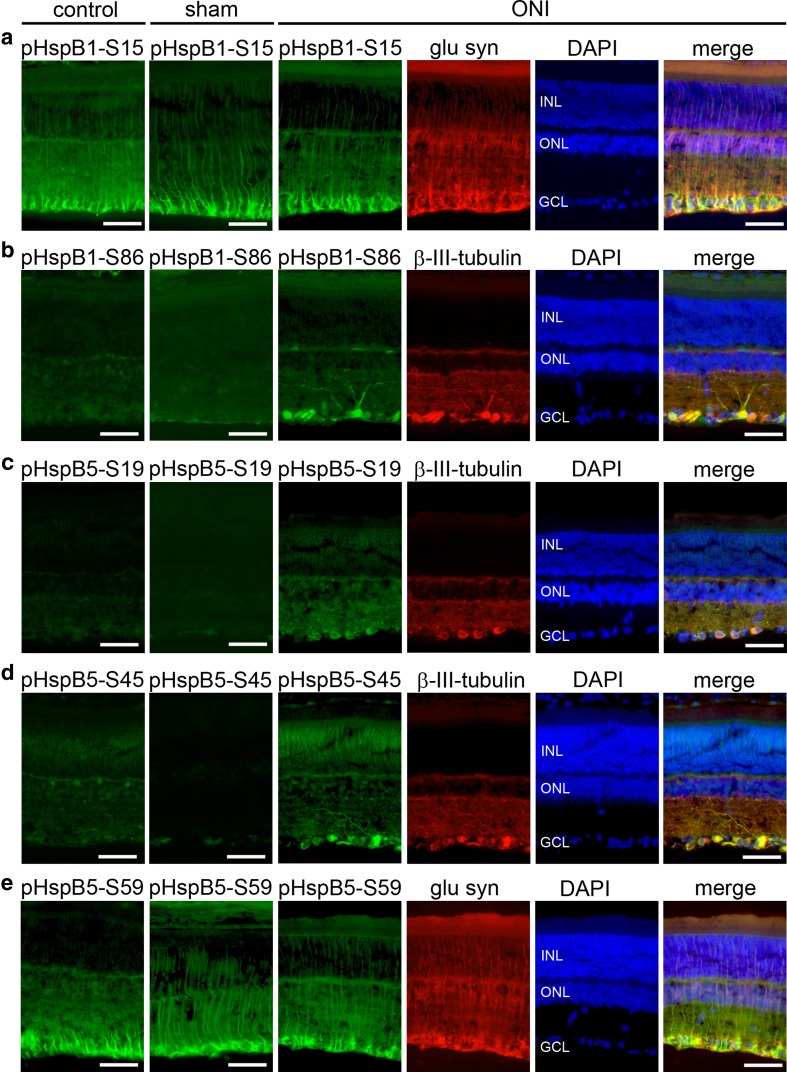
ONI induced phosphorylation of HspB1 and HspB5 in the retina
HspB1 and HspB5 are known to be phosphorylated in response to various kinds of cellular stress in several cell types and phosphorylation regulates the cytoprotective function of these HspBs (Ito et al. 1997; Landry et al. 1991, 1992). HspB1 has two phosphorylation sites at Ser 15 and 86 and HspB5 has three phosphorylation sites at Ser 19, 45, and 59. To investigate if these two HspBs were phosphorylated after ONI in the retina, we stained retinal sections with phospho-specific antibodies against each phosphorylation site. We found a radial staining pattern of the retina for pHspB1-S15 in all three experimental groups (Fig. 5a). Double labeling with glutamine synthetase, a marker for Müller cells, showed that the pHspB1-S15 and glutamine synthetase immunosignals colocalized (shown for ONI in Fig. 5a). Thus, HspB1 was phosphorylated in Müller cells at Ser 15 constitutively as well as after ONI. pHspB1-S86, however, was not detected in control and sham-operated rats but was clearly localized in RGCs after ONI as shown by double labeling with βIII-tubulin (Fig. 5b). These data demonstrate that HspB1 was constitutively phosphorylated at Ser 15 in Müller cells whereas it was phosphorylated in response to ONI in RGCs at Ser 86.
Fig. 5.
Localization of phosphoHspB1 and phosphoHspB5 of rat retinae of control, sham-operated rats, and rats subjected to ONI. Cryosections of eyes of the three experimental groups (as indicated at the top) were immunostained for a pHspB1-S15, b pHspB1-S86, c pHspB5-S19, d pHspB5-S45, e pHspB59, and β-III-tubulin or glutamine synthetase as marked. pHspB1-S15 and pHspB5-S59 (a, e) showed a radial staining pattern of the retina in all three experimental groups which colocalized with glutamine synthetase demonstrating localization of these phosphoforms in Müller cells. In contrast, pHspB1-S86, pHspB5-S19, and pHspB5-S45 were not detected in control and sham-operated rats but were clearly localized in ganglion cells after ONI as shown by double labeling with β-III-tubulin (b, c, d). Representative images of three independent experiments are shown. INL inner nuclear layer, ONL outer nuclear layer, GCL ganglion cell layer, bar: 50 μm
For pHspB5-S19 and -S45, a weak, most likely unspecific staining was observed under control and sham-operated conditions whereas βIII-tubulin-positive RGCs were strongly labeled after ONI (Fig. 5c, d). In contrast, HspB5 was found to be phosphorylated at S59 in Müller cells, labeled by glutamine synthetase, already at control conditions as well as after sham operation and ONI (Fig. 5e).
Specificity of the phospho-specific antibodies was verified by preabsorption with the respective phosphopeptides in cultured hippocampal neurons as shown previously (Schmidt et al. 2012) as well as in the retina in this study (Electronic Resource 1).
Taken together, HspB1 was constitutively phosphorylated at Ser 15 and HspB5 at Ser 59 in Müller cells. ONI led to phosphorylation of HspB1 at Ser 86 and of HspB5 at Ser 19 and 45 in RGCs. At first sight, these results do not seem to be in line with the data obtained with the pan-HspB1 and pan-HspB5 antibodies, which should detect the unphosphorylated as well as the phosphorylated forms of the proteins (Fig. 4) and, thus, one would expect that the pan-antibodies would label Müller cells as well as RGCs. However, one has to take into account that under control conditions, only very little amount of the protein is phosphorylated and therefore the pan-antibody will recognize mostly the unphosphorylated protein. The phospho-specific antibodies can be assumed to display a much higher sensitivity leading to the characteristic labeling observed.
In conclusion, upregulation of HspB protein was restricted to HspB1 and HspB5 in axotomized RGCs suggesting that these two proteins may be the most likely candidates involved in protection of axotomized RGCs. In addition, phosphorylation of HspB1 at S86 and of HspB5 at S19 and S45 might regulate their function during this process.
Discussion
Axonal injury caused by either optic nerve damage or glaucoma normally leads to degeneration of RGCs and finally loss of vision. Intravitreal injection of HspB4 (αA-crystallin) together with HspB5 (αB-crystallin) or their overexpression in RGCs reportedly promotes RGC survival (Munemasa et al. 2009; Wu et al. 2014). Whether other HspBs might also be involved in neuroprotective processes is unknown. To get a first indication, the current study systematically and comprehensively investigated the expression of all members of the small heat shock protein family after experimental ONI. Furthermore, we addressed the role of HspB phosphorylation in this context, which could potentially be a part of a regulatory mechanism. Interestingly, we found that ONI induced only HspB1 and HspB5 protein and no other HspB in the retina and that phosphorylation of HspB1 at S86 and of HspB5 at S19 and S45 might regulate their function during this process.
Expression and upregulation of HspBs in the retina
To our knowledge, this is the first comprehensive comparative study analyzing all members of the HspB family in the retina after ONI. By quantitative PCR, we found consistent expression of HspB1, HspB5, HspB6, HspB8, and HspB11 in the rat retina. In contrast, HspB3, HspB4, HspB7, HspB9, and HspB10 were not detected and HspB2 was near the detection threshold. Retinal expression of HspB1, HspB4, and HspB5 was reported previously by other studies focusing on only one or some HspBs (Lee et al. 2006; Munemasa et al. 2009; O'Reilly et al. 2010; Xi et al. 2003). In contrast to Xi et al. (2003) and Munemasa et al. (2009), we did not find HspB4 expression in the retina. The reason for this different finding is unclear. One explanation might be the species or rat strain used. Xi et al. reported HspB4 expression in mouse retina and Munemasa et al. used Wistar rats whereas Sprague–Dawley rats were used in this study.
Moreover, we found that the retinal expression pattern of HspBs was similar to that in the whole brain (Kirbach and Golenhofen 2011; Quraishe et al. 2008; and this study), suggesting that it might be therefore characteristic for the CNS in general.
One general characteristic feature of Hsps is their upregulation in response to cellular stress conditions, which is thought to be important for development of stress tolerance and their cytoprotective effect (Welch 1992). We studied expression of HspBs in the retina 5 days after ONI. This time point was chosen to investigate RGCs affected by ONI but still being alive. The response of RGCs to axotomy is a rather progressing process over several days/weeks (Berkelaar et al. 1994), where the first RGCs start to undergo apoptosis after 6 days, others after 10 days, while others survive for several weeks. Interestingly, ONI led to upregulation of only HspB1 and HspB5 protein, but no other HspB. The regulation pattern was different on the mRNA compared to the protein level. Whereas ONI-induced upregulation of HspB1 was reflected on the protein level the increase of HspB8 mRNA was not. Maybe the increase of HspB8 mRNA was too small (even though significant) to be reflected on the protein level. In the case of HspB5, no significant differences of mRNA levels were observed but a prominent upregulation of HspB5 protein. These differences might indicate a posttranscriptional regulation of HspB5 under these conditions. Posttranscriptional regulation of HspB5 was reported previously in cultured hippocampal neurons after heat shock and hyperosmotic stress (Bartelt-Kirbach and Golenhofen 2014; Kirbach and Golenhofen 2011). By which mechanism HspB5 is posttranscriptionally regulated remains to be determined. Consistent with our data, others previously reported an ONI-induced expression of HspB1 specifically in RGCs, more precisely in a subset of RGCs (Krueger-Naug et al. 2002). Furthermore, Krueger-Naug et al. (2002) reported that 14 and 28 days after optic nerve transaction, there was a significant increase in the percentage of surviving RGCs that were HspB1-positive. These data suggest that HspB1 may promote cell survival in this subset of RGCs. Our finding that HspB5 is increased upon ONI seems at first glance contradictory to a previous study (Munemasa et al. 2009). However, in that study, protein levels were analyzed 14 days after ONI, a time point at which most RGCs have already died. For this reason, the measured overall reduction in HspB5 reported in that study may be due to the cell death. It is therefore most likely that RGCs upregulate HspB1 and HspB5 upon ONI as part of a cellular stress response. As mentioned above, previous studies demonstrated that the survival of RGCs correlates with the expression level of HspB1 (Krueger-Naug et al. 2002) and that overexpression of HspBs in RGCs is neuroprotective (Munemasa et al. 2009). These data suggest that the ONI-mediated induction of HspB1 and HspB5 in RGCs has protective functions.
Phosphorylation of HspBs
Besides their induction, HspB1 and HspB5 are phosphorylated in response to various kinds of stresses in many different cell types. This phosphorylation is thought to be important for the exertion of their protective function (Arrigo and Welch 1987; Ito et al. 1997; Landry et al. 1991). Rat HspB1 displays two potential phosphorylation sites at Ser 15 and Ser 86; HspB5 has three phosphorylation sites at Ser 19, Ser 45, and Ser 59 (see Introduction). Interestingly, we could show that in Müller cells, HspB1 was constitutively phosphorylated at Ser 15 and HspB5 at Ser 59. In contrast, phosphorylation was induced by ONI in RGCs, i.e., of HspB1 at Ser 86 and of HspB5 at Ser 19 and Ser 45. Thus, we could observe a cell-type-specific phosphorylation pattern of HspB1 and HspB5. Whereas the stress-induced phosphorylation of HspBs in general is well documented, little is yet known about different potential functions of phospho-HspBs phosphorylated at different sites. Phosphorylation of HspBs after ONI has not been investigated before. However, increased phosphorylation of HspB1 at Ser 86 in RGCs (as well as in glia) was described previously in an experimental model of glaucoma (Huang et al. 2007b). Phosphorylation at Ser 15 or of HspB1 was not investigated in that study. Nevertheless, phosphorylation of HspB1 at Ser 15 could be shown in the peripheral nervous system, i.e., after transection of the sciatic nerve (Benn et al. 2002). In the same study, overexpression of wild-type HspB1 as well as a non-phosphorylatable mutant (all phosphorylation sites mutated) revealed that phosphorylation of HspB1 is of protective value. Additional experiments are needed to identify the specific role of the two HspB1 phosphorylation sites.
To our knowledge, phosphorylation of HspB5 in RGCs (or other types of neurons) subjected to axotomy has been investigated for the first time in this study. However, one study using an experimental diabetes rat model showed the importance of phosphorylation of HspB5 at Ser 45 and Ser 59 but not at Ser 19 for its anti-apoptotic function in RGCs (Kim et al. 2007). Maybe HspB5 exerts another function in axotomized RGCs, which is regulated by phosphorylation at different sites. In non-neuronal cell types, phosphorylation of Ser 59 seems to be most important. For instance, mimicking phosphorylation at this site protects cardiac myocytes from apoptosis (Morrison et al. 2003).
Phosphorylation of HspBs is known to affect their quaternary structure. HspBs assemble into higher-order oligomers with molecular weights up to 800 kDa. Their structural plasticity with dynamic subunit exchanges is an important part in regulation of substrate binding (Cheng et al. 2008; Jaya et al. 2009). Using phospho-mimicking mutants of HspB5, it could be shown that pseudophosphorylated forms lead to smaller oligomer sizes and enhanced subunit exchange which resulted in increased chaperone activity (Peschek et al. 2013). The amount of introduced negative charges (pseudophosphorylated residues) correlated with the degree of quarternary dynamics; however, effects of site-specific phosphorylation have not been investigated.
Our data point to the importance of phosphorylation at Ser 86 of HspB1 and at Ser 19 and Ser 45 of HspB5 in RGCs after axotomy. The different phosphorylation patterns of HspBs may be evoked in a stress- and cell-type-dependent manner. Specific phosphorylation patterns might finally control interactions of HspBs with specific target proteins and thus explain different functions of phospho-HspBs.
Outlook
Several studies have shown that intravitrealinjection of HspB4 and HspB5 together protects RGCs or increases axonal regeneration (Wu et al. 2014, Ying et al. 2008), which is one important therapeutic goal of various eye diseases in humans. Most studies were restricted to these two HspBs probably because of their lenticular origin (originally named α-crystallins) and the fact that lens injury is one well known factor leading to RGC protection (Fischer et al. 2000, 2001). Besides the effects of extracellularly applied HspBs, the function of endogenous HspBs in RGCs might be even more important. The retina displays the same expression pattern of HspBs as the brain; however, only HspB1 and HspB5 seem to be involved in the stress response induced by ONI in RGCs. Other HspBs are reportedly induced in neurons (HspB1, HspB5, HspB6, and HspB8) by various different types of stresses, suggesting that all HspBs act together in cytoprotection (Bartelt-Kirbach and Golenhofen 2014). It remains to be elucidated if HspB6 and HspB8 play a constitutive role in the retina or if they are upregulated by other types of cellular stresses in this tissue. In general, the neuroprotective function of HspB1 and HspB5 has been well documented in the brain. For example, ΗspΒ5-deficient mice show significant higher infarct sizes after an experimental stroke and more intense inflammation in response to experimental autoimmune encephalomyelitis compared to wild-type mice (Arac et al. 2011; Ousman et al. 2007). However, the retina has neither been investigated in this mouse model nor in the HspB1 knockout mouse (Huang et al. 2007a). Furthermore, it is remarkable that HspB1 and HspB5 were phosphorylated in RGCs at some specific but not all possible phosphorylation sites. In cultured hippocampal neurons, phosphorylated HspB1 and HspB5 were found to be localized in different subcellular compartments (dendrites, axons, synapses). These data suggest different molecular targets of phosphorylated HspBs depending on which specific site was phosphorylated (Schmidt et al. 2012). Identification of the targets of phosphorylated HspB1 and HspB5 in general and in RGCs in particular will be an important step in unraveling the molecular mechanisms of the neuroprotective function of these two proteins.
Electronic supplementary material
(GIF 380 kb)
Acknowledgments
We thank Diana Reinhardt for her excellent technical assistance and the International Graduate School in Molecular Medicine (University of Ulm, Germany) of which Thomas Schmidt was a member.
References
- Arac A, Brownell SE, Rothbard JB, Chen C, Ko RM, Pereira MP, Albers GW, Steinman L, Steinberg GK. Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proc Natl Acad Sci U S A. 2011;108:13287–13292. doi: 10.1073/pnas.1107368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo AP, Welch WJ. Characterization and purification of the small 28,000-dalton mammalian heat shock protein. J Biol Chem. 1987;262:15359–15369. [PubMed] [Google Scholar]
- Bartelt-Kirbach B, Golenhofen N. Differential expression and induction of small heat shock proteins in rat brain and cultured hippocampal neurons. J Neurosci Res. 2011;89:162–175. doi: 10.1002/jnr.22536. [DOI] [PubMed] [Google Scholar]
- Bartelt-Kirbach B, Golenhofen N. Reaction of small heat-shock proteins to different kinds of cellular stress in cultured rat hippocampal neurons. Cell Stress Chaperones. 2014;19:145–153. doi: 10.1007/s12192-013-0452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellyei S, Szigeti A, Pozsgai E, Boronkai A, Gomori E, Hocsak E, Farkas R, Sumegi B, Gallyas F., Jr Preventing apoptotic cell death by a novel small heat shock protein. Eur J Cell Biol. 2007;86:161–171. doi: 10.1016/j.ejcb.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Benn SC, Perrelet D, Kato AC, Scholz J, Decosterd I, Mannion RJ, Bakowska JC, Woolf CJ. Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron. 2002;36:45–56. doi: 10.1016/S0896-6273(02)00941-8. [DOI] [PubMed] [Google Scholar]
- Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994;14:4368–4374. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown IR. Induction of heat shock (stress) genes in the mammalian brain by hyperthermia and other traumatic events: a current perspective. J Neurosci Res. 1990;27:247–255. doi: 10.1002/jnr.490270302. [DOI] [PubMed] [Google Scholar]
- Brownell SE, Becker RA, Steinman L. The protective and therapeutic function of small heat shock proteins in neurological diseases. Front Immunol. 2012;3:74. doi: 10.3389/fimmu.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Basha E, Wysocki VH, Vierling E. Insights into small heat shock protein and substrate structure during chaperone action derived from hydrogen/deuterium exchange and mass spectrometry. J Biol Chem. 2008;283:26634–26642. doi: 10.1074/jbc.M802946200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa R, Gawinowicz-Kolks MA, Kleiman NJ, Spector A. The phosphorylation sites of the B2 chain of bovine alpha-crystallin. Biochem Biophys Res Commun. 1987;144:1340–1347. doi: 10.1016/0006-291X(87)91457-4. [DOI] [PubMed] [Google Scholar]
- Dieckmann-Schuppert A, Schnittler HJ. A simple assay for quantification of protein in tissue sections, cell cultures, and cell homogenates, and of protein immobilized on solid surfaces. Cell Tissue Res. 1997;288:119–126. doi: 10.1007/s004410050799. [DOI] [PubMed] [Google Scholar]
- Diekmann H, Leibinger M, Fischer D. Do growth-stimulated retinal ganglion cell axons find their central targets after optic nerve injury? New insights by three-dimensional imaging of the visual pathway. Exp Neurol. 2013;248:254–257. doi: 10.1016/j.expneurol.2013.06.021. [DOI] [PubMed] [Google Scholar]
- Fischer D, Leibinger M. Promoting optic nerve regeneration. Prog Retin Eye Res. 2012;31:688–701. doi: 10.1016/j.preteyeres.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Fischer D, Pavlidis M, Thanos S. Cataractogenic lens injury prevents traumatic ganglion cell death and promotes axonal regeneration both in vivo and in culture. Invest Ophthalmol Vis Sci. 2000;41:3943–3954. [PubMed] [Google Scholar]
- Fischer D, Heiduschka P, Thanos S. Lens-injury-stimulated axonal regeneration throughout the optic pathway of adult rats. Exp Neurol. 2001;172:257–272. doi: 10.1006/exnr.2001.7822. [DOI] [PubMed] [Google Scholar]
- Gaestel M, Schroder W, Benndorf R, Lippmann C, Buchner K, Hucho F, Erdmann VA, Bielka H. Identification of the phosphorylation sites of the murine small heat shock protein hsp25. J Biol Chem. 1991;266:14721–14724. [PubMed] [Google Scholar]
- Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun. 2001;286:433–442. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]
- Golenhofen N, Bartelt-Kirbach B (2015) “HspB5/alpha-B-crystallin in the brain”. The Big Book of Small Heat Shock Proteins. R. M. Tanguay and L. E. Hightower, Springer International Publishing AG, pp 365-381
- Golenhofen N, Ness W, Koob R, Htun P, Schaper W, Drenckhahn D. Ischemia-induced phosphorylation and translocation of stress protein alpha B-crystallin to Z lines of myocardium. Am J Physiol. 1998;274:H1457–H1464. doi: 10.1152/ajpheart.1998.274.5.H1457. [DOI] [PubMed] [Google Scholar]
- Golenhofen N, Arbeiter A, Koob R, Drenckhahn D. Ischemia-induced association of the stress protein alpha B-crystallin with I-band portion of cardiac titin. J Mol Cell Cardiol. 2002;34:309–319. doi: 10.1006/jmcc.2001.1513. [DOI] [PubMed] [Google Scholar]
- Golenhofen N, Redel A, Wawrousek EF, Drenckhahn D. Ischemia-induced increase of stiffness of alphaB-crystallin/HSPB2-deficient myocardium. Pflugers Arch. 2006;451:518–525. doi: 10.1007/s00424-005-1488-1. [DOI] [PubMed] [Google Scholar]
- Gusev NB, Bogatcheva NV, Marston SB. Structure and properties of small heat shock proteins (sHsp) and their interaction with cytoskeleton proteins. Biochemistry (Mosc) 2002;67:511–519. doi: 10.1023/A:1015549725819. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- Hauk TG, Leibinger M, Muller A, Andreadaki A, Knippschild U, Fischer D. Stimulation of axon regeneration in the mature optic nerve by intravitreal application of the toll-like receptor 2 agonist Pam3Cys. Invest Ophthalmol Vis Sci. 2009;51:459–464. doi: 10.1167/iovs.09-4203. [DOI] [PubMed] [Google Scholar]
- Hauk TG, Leibinger M, Muller A, Andreadaki A, Knippschild U, Fischer D. Stimulation of axon regeneration in the mature optic nerve by intravitreal application of the toll-like receptor 2 agonist Pam3Cys. Invest Ophthalmol Vis Sci. 2010;51:459–464. doi: 10.1167/iovs.09-4203. [DOI] [PubMed] [Google Scholar]
- Heinzel W, Vogt A, Kallee E, Faller W. A new method for the quantitative determination of antibody and antigen protein, with a sensitivity to five micrograms. J Lab Clin Med. 1965;66:334–340. [PubMed] [Google Scholar]
- Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci U S A. 2006;103:7159–7164. doi: 10.1073/pnas.0600895103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Min JN, Masters S, Mivechi NF, Moskophidis D. Insights into function and regulation of small heat shock protein 25 (HSPB1) in a mouse model with targeted gene disruption. Genesis. 2007;45:487–501. doi: 10.1002/dvg.20319. [DOI] [PubMed] [Google Scholar]
- Huang W, Fileta JB, Filippopoulos T, Ray A, Dobberfuhl A, Grosskreutz CL. Hsp27 phosphorylation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2007;48:4129–4135. doi: 10.1167/iovs.06-0606. [DOI] [PubMed] [Google Scholar]
- Ito H, Okamoto K, Nakayama H, Isobe T, Kato K. Phosphorylation of alphaB-crystallin in response to various types of stress. J Biol Chem. 1997;272:29934–29941. doi: 10.1074/jbc.272.47.29934. [DOI] [PubMed] [Google Scholar]
- Jaya N, Garcia V, Vierling E. Substrate binding site flexibility of the small heat shock protein molecular chaperones. Proc Natl Acad Sci U S A. 2009;106:15604–15609. doi: 10.1073/pnas.0902177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappe G, Franck E, Verschuure P, Boelens WC, Leunissen JA, de Jong WW. The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperones. 2003;8:53–61. doi: 10.1379/1466-1268(2003)8<53:THGECS>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappe G, Boelens WC, de Jong WW. Why proteins without an alpha-crystallin domain should not be included in the human small heat shock protein family HSPB. Cell Stress Chaperones. 2010;15:457–461. doi: 10.1007/s12192-009-0155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/S0163-7258(98)00028-X. [DOI] [PubMed] [Google Scholar]
- Kim YH, Choi MY, Kim YS, Han JM, Lee JH, Park CH, Kang SS, Choi WS, Cho GJ. Protein kinase C delta regulates anti-apoptotic alphaB-crystallin in the retina of type 2 diabetes. Neurobiol Dis. 2007;28:293–303. doi: 10.1016/j.nbd.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Kirbach BB, Golenhofen N. Differential expression and induction of small heat shock proteins in rat brain and cultured hippocampal neurons. J Neurosci Res. 2011;89:162–175. doi: 10.1002/jnr.22536. [DOI] [PubMed] [Google Scholar]
- Krueger-Naug AM, Emsley JG, Myers TL, Currie RW, Clarke DB. Injury to retinal ganglion cells induces expression of the small heat shock protein Hsp27 in the rat visual system. Neuroscience. 2002;110:653–665. doi: 10.1016/S0306-4522(01)00453-5. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landry J, Chretien P, Laszlo A, Lambert H. Phosphorylation of HSP27 during development and decay of thermotolerance in Chinese hamster cells. J Cell Physiol. 1991;147:93–101. doi: 10.1002/jcp.1041470113. [DOI] [PubMed] [Google Scholar]
- Landry J, Lambert H, Zhou M, Lavoie JN, Hickey E, Weber LA, Anderson CW. Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem. 1992;267:794–803. [PubMed] [Google Scholar]
- Lee J, Kim H, Lee JM, Shin T. Immunohistochemical localization of heat shock protein 27 in the retina of pigs. Neurosci Lett. 2006;406:227–231. doi: 10.1016/j.neulet.2006.07.067. [DOI] [PubMed] [Google Scholar]
- Morrison LE, Hoover HE, Thuerauf DJ, Glembotski CC. Mimicking phosphorylation of alphaB-crystallin on serine-59 is necessary and sufficient to provide maximal protection of cardiac myocytes from apoptosis. Circ Res. 2003;92:203–211. doi: 10.1161/01.RES.0000052989.83995.A5. [DOI] [PubMed] [Google Scholar]
- Morrison LE, Whittaker RJ, Klepper RE, Wawrousek EF, Glembotski CC. Roles for alphaB-crystallin and HSPB2 in protecting the myocardium from ischemia-reperfusion-induced damage in a KO mouse model. Am J Physiol Heart Circ Physiol. 2004;286:H847–H855. doi: 10.1152/ajpheart.00715.2003. [DOI] [PubMed] [Google Scholar]
- Munemasa Y, Kwong JM, Caprioli J, Piri N. The role of alphaA- and alphaB-crystallins in the survival of retinal ganglion cells after optic nerve axotomy. Invest Ophthalmol Vis Sci. 2009;50:3869–3875. doi: 10.1167/iovs.08-3138. [DOI] [PubMed] [Google Scholar]
- O’Reilly AM, Currie, RW, Clarke, DB (2010) HspB1 (Hsp 27) Expression and Neuroprotection in the Retina. Mol Neurobiol [DOI] [PubMed]
- Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O'Connor KC, Hafler DA, Sobel RA, Robinson WH, Steinman L. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- Peschek J, Braun N, Rohrberg J, Back KC, Kriehuber T, Kastenmuller A, Weinkauf S, Buchner J. Regulated structural transitions unleash the chaperone activity of alphaB-crystallin. Proc Natl Acad Sci U S A. 2013;110:E3780–E3789. doi: 10.1073/pnas.1308898110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumier JC, Hopkins DA, Robertson HA, Currie RW. Constitutive expression of the 27-kDa heat shock protein (Hsp27) in sensory and motor neurons of the rat nervous system. J Comp Neurol. 1997;384:409–428. doi: 10.1002/(SICI)1096-9861(19970804)384:3<409::AID-CNE7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Quraishe S, Asuni A, Boelens WC, O'Connor V, Wyttenbach A. Expression of the small heat shock protein family in the mouse CNS: differential anatomical and biochemical compartmentalization. Neuroscience. 2008;153:483–491. doi: 10.1016/j.neuroscience.2008.01.058. [DOI] [PubMed] [Google Scholar]
- Ray PS, Martin JL, Swanson EA, Otani H, Dillmann WH, Das DK. Transgene overexpression of aB crystallin confers simultaneous protection against cardiomyocyte apoptosis and necrosis during myocardial ischemia and reperfusion. FASEB J. 2001;15:393–402. doi: 10.1096/fj.00-0199com. [DOI] [PubMed] [Google Scholar]
- Reddy VS, Reddy GB. Emerging role for alphaB-crystallin as a therapeutic agent: pros and cons. Curr Mol Med. 2015;15:47–61. doi: 10.2174/1566524015666150114112853. [DOI] [PubMed] [Google Scholar]
- Schmidt T, Bartelt-Kirbach B, Golenhofen N. Phosphorylation-dependent subcellular localization of the small heat shock proteins HspB1/Hsp25 and HspB5/alphaB-crystallin in cultured hippocampal neurons. Histochem Cell Biol. 2012;138:407–418. doi: 10.1007/s00418-012-0964-x. [DOI] [PubMed] [Google Scholar]
- Shao W, Zhang SZ, Tang M, Zhang XH, Zhou Z, Yin YQ, Zhou QB, Huang YY, Liu YJ, Wawrousek E, et al. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature. 2013;494:90–94. doi: 10.1038/nature11748. [DOI] [PubMed] [Google Scholar]
- Sun Y, MacRae TH. The small heat shock proteins and their role in human disease. FEBS J. 2005;272:2613–2627. doi: 10.1111/j.1742-4658.2005.04708.x. [DOI] [PubMed] [Google Scholar]
- Thanos S, Bohm MR, Meyer zu Horste M, Prokosch-Willing V, Hennig M, Bauer D, Heiligenhaus A. Role of crystallins in ocular neuroprotection and axonal regeneration. Prog Retin Eye Res. 2014;42:145–161. doi: 10.1016/j.preteyeres.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034 [DOI] [PMC free article] [PubMed]
- Verschuure P, Croes Y, van den IJssel PR, Quinlan RA, de Jong WW, Boelens WC. Translocation of small heat shock proteins to the actin cytoskeleton upon proteasomal inhibition. J Mol Cell Cardiol. 2002;34:117–128. doi: 10.1006/jmcc.2001.1493. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- Wu N, Yu J, Chen S, Xu J, Ying X, Ye M, Li Y, Wang Y. alpha-Crystallin protects RGC survival and inhibits microglial activation after optic nerve crush. Life Sci. 2014;94:17–23. doi: 10.1016/j.lfs.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Xi J, Farjo R, Yoshida S, Kern TS, Swaroop A, Andley UP. A comprehensive analysis of the expression of crystallins in mouse retina. Mol Vis. 2003;9:410–419. [PubMed] [Google Scholar]
- Ying X, Zhang J, Wang Y, Wu N, Wang Y, Yew DT. Alpha-crystallin protected axons from optic nerve degeneration after crushing in rats. J Mol Neurosci. 2008;35:253–258. doi: 10.1007/s12031-007-9010-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(GIF 380 kb)