Abstract
Tobacco smoke contains thousands of compounds in addition to nicotine, a known neuroteratogen. We evaluated the developmental neurotoxicity of tobacco smoke extract (TSE) administered to pregnant rats starting preconception and continued through the second postnatal week. We simulated nicotine concentrations encountered with second-hand smoke, an order of magnitude below those seen in active smokers, and compared TSE with an equivalent dose of nicotine alone, and to a 10-fold higher nicotine dose. We conducted longitudinal evaluations in multiple brain regions, starting in adolescence (postnatal day 30) and continued to full adulthood (day 150). TSE exposure impaired presynaptic cholinergic activity, exacerbated by a decrement in nicotinic cholinergic receptor concentrations. Although both nicotine doses produced presynaptic cholinergic deficits, these were partially compensated by hyperinnervation and receptor upregulation, effects that were absent with TSE. TSE also produced deficits in serotonin receptors in females that were not seen with nicotine. Regression analysis showed a profound sex difference in the degree to which nicotine could account for overall TSE effects: whereas the 2 nicotine doses accounted for 36%–46% of TSE effects in males, it accounted for only 7%–13% in females. Our results show that the adverse effects of TSE on neurodevelopment exceed those that can be attributed to just the nicotine present in the mixture, and further, that the sensitivity extends down to levels commensurate with second-hand smoke exposure. Because nicotine itself evoked deficits at low exposures, “harm reduction” nicotine products do not eliminate the potential for neurodevelopmental damage.
Keywords: acetylcholine systems, developmental neurotoxicity, nicotine, serotonin systems: tobacco smoke
Maternal smoking during pregnancy substantially raises the risk of neurodevelopmental disorders (Gaysina et al., 2013; Pauly and Slotkin, 2008), reflecting in large measure the adverse effects of nicotine on brain development (Pauly and Slotkin, 2008; Slikker et al., 2005; Slotkin, 2008). Because neurotransmitters mediate essential steps in brain assembly (Dreyfus, 1998), inappropriate stimulation of nicotinic acetylcholine receptors (nAChRs) by nicotine preempts normal developmental signals required for the formation of neural circuits (Pauly and Slotkin, 2008; Slotkin, 2008). Nevertheless, tobacco smoke contains thousands of other potentially harmful chemicals, and with alternative nicotine delivery devices, it becomes important to distinguish their contributions from those of nicotine. A number of animal studies used cigarette smoke exposure in inhalation chambers and identified neurodevelopmental defects resembling those of nicotine (Fuller et al., 2012; Golub et al., 2007; Gospe et al., 2009; Slotkin et al., 2006a, b). Although these models reinforce the resemblance between smoke exposure and the effects of nicotine, they do not distinguish between them. Additionally, repetitive, involuntary confinement in a smoke-filled chamber is likely to elicit stress, a confound not ordinarily present in nicotine exposure models that use other forms of delivery.
We recently showed with an in vitro model that tobacco smoke extract (TSE) is much more potent than nicotine in perturbing neurodifferentiation (Slotkin et al., 2014). Specifically, TSE promoted the transition from neural cell replication to neurodifferentiation, resulting in deficits in cell numbers. Additionally, TSE altered neurodifferentiation outcomes, suppressing emergence of the acetylcholine (ACh) phenotype while promoting the monoaminergic phenotype. These results suggested that the other components of tobacco smoke contribute to developmental neurotoxicity either through their own actions or through potentiating the effects of nicotine. This study tests that hypothesis in vivo, comparing the effects of perinatal TSE exposure with those of an equivalent regimen of nicotine alone, or to a 10-fold higher nicotine dose level. The TSE and low-dose nicotine treatments were chosen to produce maternal nicotine plasma levels comparable to the low levels seen with second-hand smoke exposure, whereas the higher dose produces levels commensurate with active maternal smoking (Fewell et al., 2001; Trauth et al., 2000). To avoid stress-related confounds, the agents were delivered by osmotic minipumps, implanted prior to mating, and set to deliver TSE or nicotine throughout gestation and into the first 2 postnatal weeks; because rats are altricial, the postnatal exposure period corresponds to stages of brain development in the late second to third trimester human fetus. We then evaluated the impact on synaptic development from adolescence through full adulthood, focusing on ACh systems as well as serotonin (5-hydroxytryptamine, 5HT), a monoaminergic transmitter whose developmental profile is likewise known to be targeted by nicotine in vivo (Slikker et al., 2005; Slotkin et al., 2007).
We assessed the impact on brain regions comprising the major ACh and 5HT projections and their corresponding cell bodies. For ACh, we evaluated the activity of choline acetyltransferase (ChAT), the concentration of presynaptic high-affinity choline transporters (hemicholinium-3 [HC3] binding) and the concentration of α4β2 nAChRs. ChAT and high-affinity choline transporters are both constitutive components of ACh nerve terminals but they differ in their regulatory mechanisms and hence in their functional significance. ChAT is the enzyme that synthesizes ACh, but is not regulated by nerve impulse activity, so that its presence provides an index of the development of ACh projections (Slotkin, 2008). In contrast, HC3 binding to the choline transporter is directly responsive to neuronal activity (Klemm and Kuhar, 1979), so that comparative effects on HC3 binding and ChAT enable the characterization of both the development of cholinergic innervation and presynaptic impulse activity. For that determination, we calculated the HC3/ChAT ratio as an index of presynaptic activity relative to the number of cholinergic nerve terminals (Slotkin, 2008). The α4β2 nAChR is the most abundant nAChR subtype in the mammalian brain and regulates the ability of ACh systems to release other neurotransmitters involved in reward, cognition, and mood (Dani and De Biasi, 2001). For 5HT systems, we focused on 5HT1A and 5HT2 receptors, the subtypes that are known to be major targets for developmental neurotoxicity of nicotine (Slikker et al., 2005; Slotkin et al., 2007) and that play major roles in 5HT-related mental disorders, including depression (Maes and Meltzer, 1995).
MATERIALS AND METHODS
Tobacco smoke extract
TSE was prepared from Kentucky Reference cigarettes (KY3R4F) on a rotary smoke machine under International Organization for Standardization (ISO) smoke conditions. The smoke condensate was collected on 92-mm filter pads, which were then extracted by shaking for 20 min with undiluted dimethylsulfoxide, to obtain a solution of approximately 20 mg of condensate per milliliter. Condensate aliquots were stored in amber vials at −80°C until used. Two cigarettes were smoked to produce each milliliter of extract and the final product contained 0.8 mg/ml nicotine (determined by the manufacturer).
Animal treatments
All experiments were carried out humanely and with regard for alleviation of suffering, with protocols approved by the Duke University Animal Care and Use Committee and in accordance with all federal and state guidelines. Sprague-Dawley rats were shipped by climate-controlled truck (transportation time < 1 h) and were allowed to acclimate to the housing facility for 2 weeks prior to treatment. Type 2ML4 Alzet osmotic minipumps were implanted under anesthesia (60 mg/kg ketamine + 0.15–0.50 mg/kg dexmedetomidine given ip; followed postimplant by 0.15 mg/kg atipemezole + 5 mg/kg ketoprofen given sc and topical bupivicaine), and the animals were allowed to recover for 3 days. Mating was then initiated by including a male rat in the cage for a period of 5 days. Although the pumps are marketed as a 4-week delivery device, it actually takes approximately 39 days for the reservoir to be exhausted completely (information supplied by the manufacturer) and thus the infusions terminated on postnatal day (PN) 12 ± 2 days (the insemination date varied among different mating pairs). In earlier work, we measured plasma nicotine levels to confirm the termination of nicotine absorption coinciding with the calculated values (Trauth et al., 2000).
There were 4 treatment groups, each comprising 12–17 dams: control (dimethylsulfoxide (DMSO) vehicle), TSE, and 2 different dose levels of nicotine bitartrate dissolved in DMSO, with the nicotine groups calibrated to deliver 0.2 mg/kg/day or 2 mg/kg/day of nicotine free base at the start of the infusion period. Because body weights increased with gestation, the dose rate fell by approximately one-third by the end of gestation and then rose back toward the original values with the postpartum weight loss. Thus, the nicotine dose rate for the 2 mg/kg group remained well within the range that produces nicotine plasma levels similar to those in moderate smokers, compared with the much lower exposures in the low-dose group (Fewell et al., 2001; Trauth et al., 2000). The initial TSE dose rate (based on the nicotine concentration in the TSE preparation) corresponded to 0.18 mg/kg/day of nicotine, comparable to the levels in the group receiving low-dose nicotine.
Parturition occurred during gestational day 22, which was also taken as PN0, and litters were culled to 8–10 pups to ensure standard nutrition. Weaning occurred on PN21. On PN30, 60, 100, and 150, animals were decapitated and brain regions were dissected for determination of ACh and 5HT synaptic markers: frontal/parietal cortex, temporal/occipital cortex, hippocampus, striatum, midbrain, and brainstem. The 2 cortical regions were sectioned at the midline and the right half used for the current determinations. The left halves of the cortical regions were reserved for future studies, along with the cerebellum, which is sparse in ACh and 5HT projections. Tissues were frozen in liquid nitrogen and stored at −80°C until assayed. Each treatment group comprised 12 animals at each age point, equally divided into males and females, with each litter contributing no more than 1 male and 1 female to any of the treatment groups.
Assays and materials
Assays were conducted on each individual tissue, so that each determination represented a value from the corresponding brain region of 1 animal. Tissues were thawed in 79 volumes of ice-cold 10 mM sodium-potassium phosphate buffer (pH 7.4) and homogenized with a Polytron (Brinkmann Instruments, Westbury, New York). Aliquots of the homogenate were assayed for ChAT using established procedures (Qiao et al., 2003). Each tube contained 50 µM [14C]acetyl-coenzyme A as a substrate and activity was determined as the amount of labeled ACh produced relative to tissue protein (Smith et al., 1985). For measurements of HC3 binding, the cell membrane fraction was prepared from an aliquot of the same tissue homogenate by sedimentation at 40 000 × g for 15 min. The pellet was resuspended and washed, and the resultant pellet was assayed, using a ligand concentration of 2 nM [3H]HC3 with or without 10 µM unlabeled HC3 to displace specific binding (Qiao et al., 2003). Determinations of nAChR binding were carried out in another aliquot, each assay containing 1 nM [3H]cytisine with or without 10 µM nicotine to displace specific binding (Slotkin et al., 2008). Similarly, we determined binding to 5HT receptors with established techniques (Aldridge et al., 2004), using 1 nM [3H]8-hydroxy-2-(di-n-propylamino) tetralin for 5HT1A receptors, and 0.4 nM [3H]ketanserin for 5HT2 receptors. For 5HT1A receptors, specific binding was displaced by addition of 100 µM 5HT; for 5HT2 receptors, we used 10 µM methylsergide for displacement. Ligand binding was calculated relative to the membrane protein concentration.
Some of the regions had insufficient amounts of tissue to permit all assays to be performed. Accordingly, we did not obtain values for nAChRs in the striatum, nor for the 5HT receptors in either the striatum or hippocampus.
Data analysis
The initial statistical comparisons were conducted by a global analysis of variance (ANOVA) (data log-transformed because of heterogeneous variance among regions, measures, and ages) incorporating all the variables and measurements in a single test so as to avoid an increased probability of type 1 errors that might otherwise result from multiple tests of the same data set. The variables in the global test were prenatal treatment (vehicle, TSE, nicotine 0.2 mg/kg/day, nicotine 2 mg/kg/day), brain region, age, and sex, with multiple dependent measures (hereafter, designated simply as “measures”): ChAT, HC3 binding, and nAChR binding for the ACh synaptic makers; 5HT1A and 5HT2 receptor binding for the 5HT synaptic markers. For both transmitter systems, the dependent measures were treated as repeated measures, because all the determinations were derived from the same sample. Where we identified interactions of treatment with the other variables, data were then subdivided for lower-order ANOVAs to evaluate treatments that differed from the corresponding control or from each other. As permitted by the interaction terms, individual treatments that differed from control were identified with Fisher’s Protected Least Significant Difference Test. However, where treatment effects were not interactive with other variables, we report only the main treatment effects without performing lower-order analyses of individual values. Significance was assumed at the level of P < .05.
Data were compiled as means and standard errors. To enable ready visualization of treatment effects across different regions, ages, and measures, the results are given as the percent change from control values, but statistical procedures were always conducted on the original data, with log transforms because of heterogeneous variance as noted earlier. In addition, the log-transform evaluates the treatment differences as a proportion to control values rather than as an arithmetic difference. This was important because of technical limitations: on any single day, we could conduct assays for all treatment groups and both sexes, but for only 1 region at 1 age point. Accordingly, representing the data as proportional differences (percent control) enables a full comparison of treatment effects and treatment interactions with all the other variables, even though absolute values for the controls cannot be compared across regions and ages (because assays for each region and age point were run on separate days). Graphs were scaled to encompass the different dynamic ranges of the changes in the various parameters. The absolute values for each set of determinations appear in the Supplementary Tables.
Materials
TSE was prepared by Arista Laboratories (Richmond, Virginia). Animals were purchased from Charles River Laboratories (Raleigh, North Carolina) and osmotic minipumps were from Durect Corp (Cupertino, California). PerkinElmer Life Sciences (Boston, Massachusetts) was the source for [3H]HC3 (specific activity, 125 Ci/mmol), [3H]cytisine (specific activity 35 Ci/mmol), [3H]8-hydroxy-2-(di-n-propylamino) tetralin (specific activity, 135 Ci/mmol), [3H]ketanserin (63 Ci/mmol), and [14C]acetyl-coenzyme A (specific activity 6.7 mCi/mmol). Methylsergide was obtained from Sandoz Pharmaceuticals (E. Hanover, New Jersey) and all other chemicals came from Sigma Chemical Co (St Louis, Missouri).
RESULTS
TSE Bioequivalence With Nicotine
Before commencing the developmental studies, we validated the bioequivalence of nicotine delivered by TSE when compared with nicotine alone. Non-pregnant, adult female rats were implanted with minipumps set to deliver TSE (0.18 mg/kg/day nicotine equivalent), 0.2 or 2 mg/kg/day of nicotine (n = 5–6 per group). After 28 days, the cerebral cortex was analyzed to look for nAChR upregulation (Supplementary Fig. S1). TSE produced a statistically significant increase in nAChR binding that was indistinguishable from that of the low-dose nicotine group but less than that of the high-dose group.
Maternal, Litter, and Growth Effects
There were no significant effects of any of the treatments on maternal weight gain (data not shown). Likewise, we did not see a significant difference in the proportion of dams giving birth (12 out of 12 controls, 13 out of 17 TSE, 12 out of 14 nicotine 0.2 mg/kg/day, 12 out of 12 nicotine 2 mg/kg/day; not significant by Fisher’s Exact Test). Litter sizes were equivalent (control 13.0 ± 0.6, TSE 13.1 ± 0.6, nicotine 0.2 mg/kg/day 13.7 ± 0.5, nicotine 2 mg/kg/day 13.7 ± 0.5), as were the sex ratios of the offspring (control 52% ± 4% male, TSE 47% ± 4%, nicotine 0.2 mg/kg/day 54% ± 3%, nicotine 2 mg/kg/day 47% ± 4%). There were small, but statistically significant effects on growth of the offspring (Supplementary Table S1), with the TSE group showing approximately a 4% lower overall weight compared with the other 3 groups. However, brain region weights were not significantly different (Supplementary Table S1).
ACh Synaptic Markers
Multivariate ANOVA incorporating all 4 treatment groups, sex, age, brain region, and the 3 ACh synaptic measures (ChAT activity, HC3 binding, and nAChR binding) indicated the main effect of the treatments (P < .0001) that interacted with all the other variables: P < .0001 for treatment × measure, P < .009 for treatment × age, P < .0001 for treatment × measure × age and P < .0004 for treatment × measure × age × sex. The main treatment effect reflected the fact that each treatment group differed significantly from the control (TSE P < .0001, nicotine 0.2 mg/kg/day P < .0001, nicotine 2 mg/kg/day P < .008) and also that the TSE group differed from either of the nicotine groups (P < .0001 for each). In light of the strong interaction of treatment × measure, we subdivided the data into the individual groups and then separated the measures, looking for treatment effects and interactions of treatment with the remaining variables (sex, age, and region).
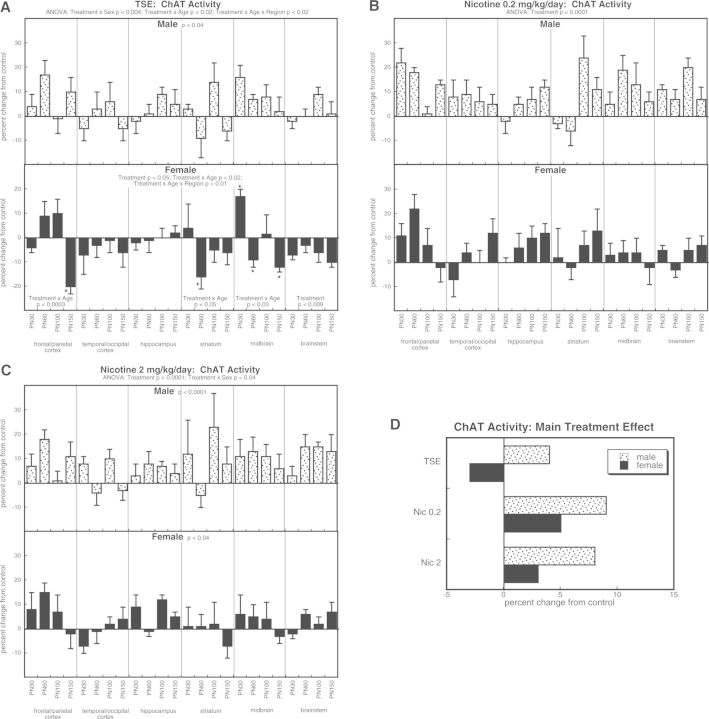
For ChAT activity, ANOVA incorporating all treatment groups indicated a main effect of treatment (P < .0001) that depended on sex (treatment × sex, P < .04). TSE exposure evoked an overall increase in ChAT activity in males (Fig. 1A). In contrast, females showed a net reduction in ChAT, accompanied by selective regional and temporal effects. For animals exposed to the low dose of nicotine, we found an elevation of ChAT activity, without distinction by sex (Fig. 1B). At the higher nicotine dose, both sexes showed significant increases, with a greater effect in males than in females (Fig. 1C). To illustrate the differences in the main treatment effects among the 3 groups, we calculated the mean values for effects on ChAT activity, collapsed across region and age (Fig. 1D). This simplified picture dilutes the effects seen for specific regions or ages by averaging them with data points for which there was no effect or an opposite effect, so that the absolute magnitude becomes smaller. Despite these limitations, there was an obvious overall pattern: whereas TSE evoked increases in males and decreases in females, either dose of nicotine produced increases in both sexes, with a more pronounced effect in males than females. Additionally, the effect of either dose of nicotine was significantly greater than that of TSE (P < .0001 and P < .004, respectively).
FIG. 1.
Effects of TSE (A), nicotine 0.2 mg/kg/day (B), and nicotine 2 mg/kg/day (C) on ChAT activity. Data represent means and standard errors, presented as the percent change from control values; complete original data are shown in Supplementary Table S2. Multivariate ANOVA for each treatment appears at the top of the panels. Lower-order tests for each sex were carried out only where there was a treatment × sex interaction (A, C). Effects on specific regions and ages are shown where justified by interactions with treatment; for females in panel A, there was an interaction of treatment with both age and region, so asterisks denote individual values that differ from control. Panel (D) shows the simple main treatment effects, collapsed across age and region.
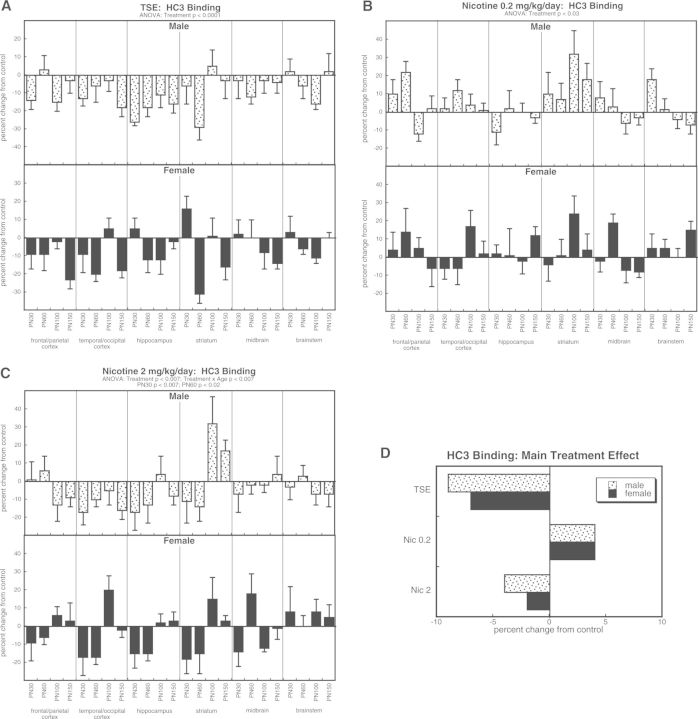
For HC3 binding, ANOVA incorporating all treatments indicated a main effect of treatment (P < .0001) that depended upon age (treatment × age, P < .0003) but not sex or region. TSE exposure elicited overall decrements in HC3 binding (Fig. 2A), whereas nicotine 0.2 mg/kg/day produced the opposite effect, a significant increase (Fig. 2B). Like TSE, the higher dose of nicotine evoked a net decrease in HC3 binding but with a temporal component (treatment × age interaction) reflecting more prominent effects in adolescence than in adulthood (Fig. 2C). Again, to illustrate the main treatment effects, we collapsed the values across age and region (Fig. 2D). Although TSE elicited a decrease, the low dose of nicotine produced an increase, and the high dose showed a decrease of significantly lesser magnitude than that seen with TSE (P < .004).
FIG. 2.
Effects of TSE (A), nicotine 0.2 mg/kg/day (B), and nicotine 2 mg/kg/day (C) on HC3 binding. Data represent means and standard errors, presented as the percent change from control values; complete original data are shown in Supplementary Table S3. Multivariate ANOVA for each treatment appears at the top of the panels. Lower-order tests for each sex were not carried out because of the absence of treatment × sex interactions. Effects at specific ages are shown where justified by interactions with treatment. Panel (D) shows the simple main treatment effects, collapsed across age and region.
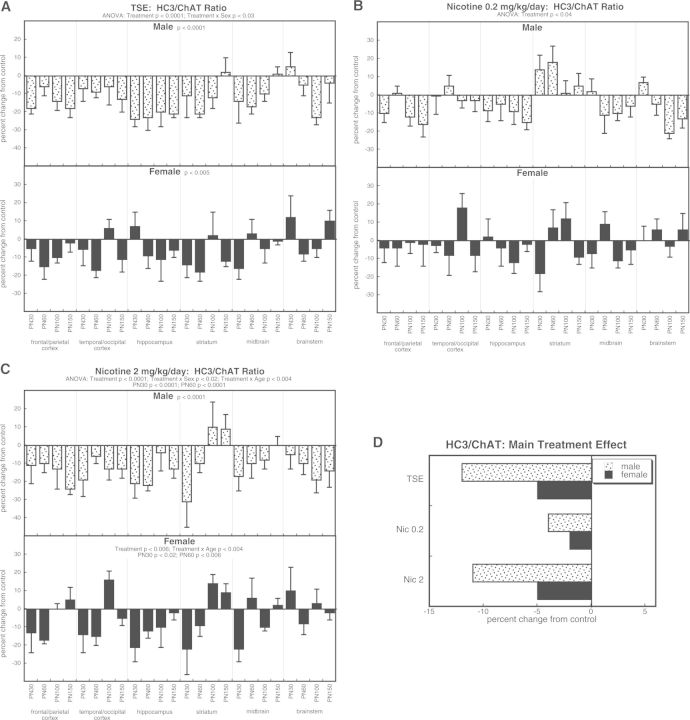
The HC3/ChAT ratio displayed main treatment effects across all the treatment groups (P < .0001) that interacted with both sex (P < .05 for treatment × sex) and age (P < .0002 for treatment × age). TSE exposure produced overall decreases in the ratio, with a treatment × sex interaction reflecting greater effects in males than females (Fig. 3A). Likewise, decrements in HC3/ChAT were found for both the low dose of nicotine (Fig. 3B) and the high dose of nicotine (Fig. 3C), with the latter showing significant sex differences just like TSE. The high dose of nicotine again displayed a temporal pattern (treatment × age interaction) with greater effects in adolescence than in adulthood. The main treatment effects, collapsed across age and region, showed a uniform reduction in the ratio with all treatments, preferentially in males. Notably, the TSE group closely matched the nicotine 2 mg/kg/day group and both of these were significantly more affected than with the lower nicotine dose (P < .0008 for TSE vs nicotine 0.2 mg/kg/day; P < .0002 for nicotine 2 mg/kg/day vs nicotine 0.2 mg/kg/day).
FIG. 3.
Effects of TSE (A), nicotine 0.2 mg/kg/day (B), and nicotine 2 mg/kg/day (C) on the HC3/ChAT ratio. Data represent means and standard errors, presented as the percent change from control values; complete original data are shown in Supplementary Table S4. Multivariate ANOVA for each treatment appears at the top of the panels. Lower-order tests for each sex were carried out only where there was a treatment × sex interaction (A, C). Effects at specific ages are shown where justified by interactions with treatment. Panel (D) shows the simple main treatment effects, collapsed across age and region.
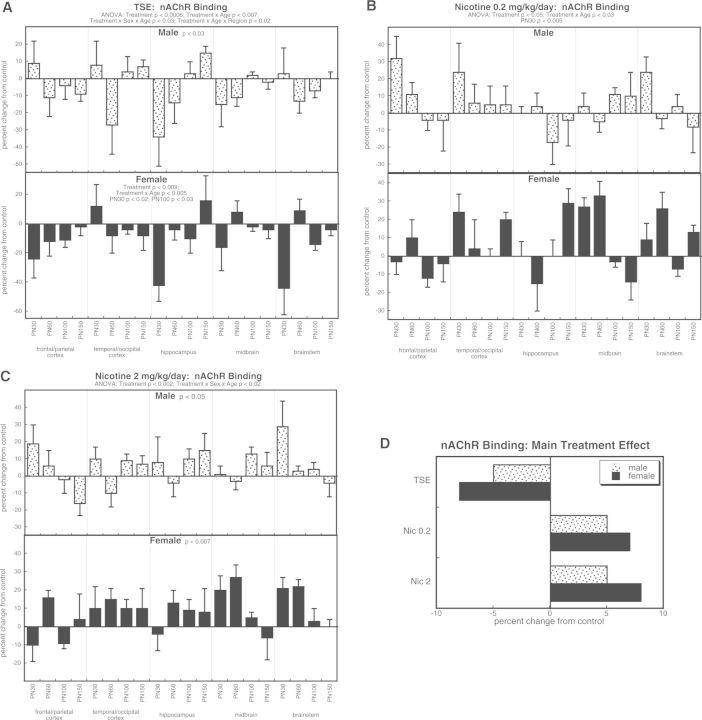
For nAChR binding, ANOVA identified significant main treatment effects (P < .0001) that interacted with all the other factors: P < .0001 for treatment × age, P < .05 for treatment × sex × age, and P < .03 for treatment × age × region. TSE exposure reduced nAChR binding in both sexes, with females showing a relatively greater overall effect (significant treatment × sex interaction), as well as selective reductions at PN30 and PN100 (Fig. 4A). In contrast, either the low (Fig. 4B) or high (Fig. 4C) dose of nicotine elicited an opposite effect, namely an overall increase in nAChR binding; again, this was greater for females than males. These results are more easily illustrated by the values collapsed across age and region (Fig. 4D). TSE produced decreases in nAChR binding, whereas nicotine elicited increases, and in each case, effects in females were greater regardless of the direction of change.
FIG. 4.
Effects of TSE (A), nicotine 0.2 mg/kg/day (B), and nicotine 2 mg/kg/day (C) on nAChR binding. Data represent means and standard errors, presented as the percent change from control values; complete original data are shown in Supplementary Table S5. Multivariate ANOVA for each treatment appears at the top of the panels. Lower-order tests for each sex were carried out only where there was a treatment × sex interaction (A, C). Effects at specific ages are shown where justified by interactions with treatment. Panel (D) shows the simple main treatment effects, collapsed across age and region.
5HT Receptors
Multivariate ANOVA incorporating all 4 treatment groups, sex, brain region, and the two 5HT receptor measurements (5HT1A and 5HT2 subtypes) indicated a main effect of treatment (P < .0001) that interacted with measure (treatment × subtype, P < .0005) and sex (treatment × sex, P < .0004). Again, the main effects reflected differences for the treated groups versus control (TSE P < .0003, nicotine 0.2 mg/kg/day P < .01) as well as for the TSE group compared with either nicotine group (P < .0001 vs nicotine 0.2 mg/kg/day; P < .004 vs nicotine 2 mg/kg/day). Given the interaction of treatment × measure, we subdivided the data into the individual groups and then separated the receptor subtypes, looking for treatment effects and interactions of treatment with the remaining variables (sex, age, and region).
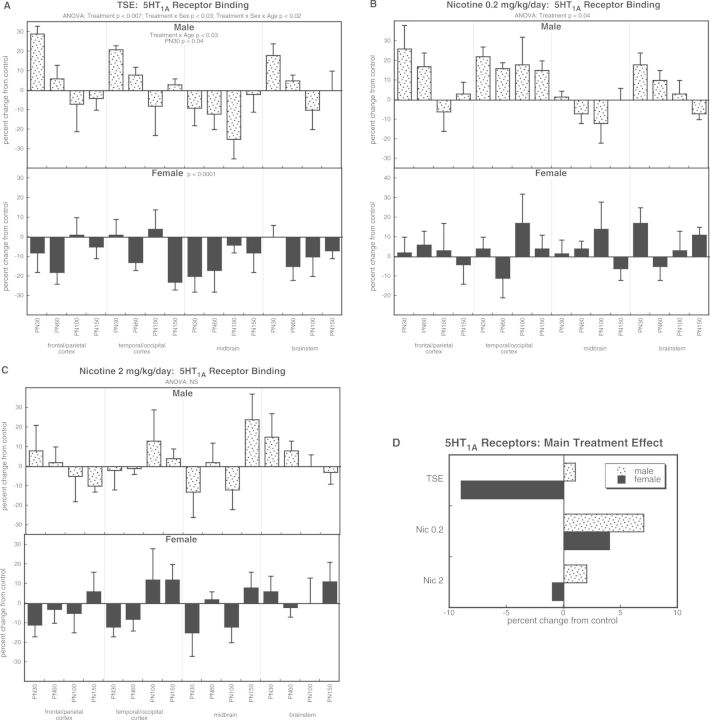
With the 5HT1A subtype, the overall ANOVA indicated main treatment effects (P < .0001) that were highly dependent on sex (treatment × sex, P < .0001). TSE exposure produced age-dependent changes in males, reflecting an increase in adolescence that regressed in adulthood (Fig. 5A); however, this should be interpreted with caution, because the overall ANOVA (all treatment groups) did not show a treatment × age interaction. In contrast, females showed a net reduction in 5HT1A receptor binding. The low dose of nicotine elicited significant overall increases without distinction between the sexes (Fig. 5B) but we did not see comparable effects at the higher dose (Fig. 5C). Thus the main effects (Fig. 5D) reflected a TSE-induced decrease in females that was not seen with nicotine at either dose and in fact, the low dose of nicotine had an opposite effect (overall increase in 5HT1A receptor binding).
FIG. 5.
Effects of TSE (A), nicotine 0.2 mg/kg/day (B), and nicotine 2 mg/kg/day (C) on 5HT1A receptor binding. Data represent means and standard errors, presented as the percent change from control values; complete original data are shown in Supplementary Table S6. Multivariate ANOVA for each treatment appears at the top of the panels. Lower-order tests for each sex were carried out only where there was a treatment × sex interaction (A). Effects on specific ages are shown where justified by interactions with treatment. Panel (D) shows the simple main treatment effects, collapsed across age and region. Abbreviation: NS, not significant.
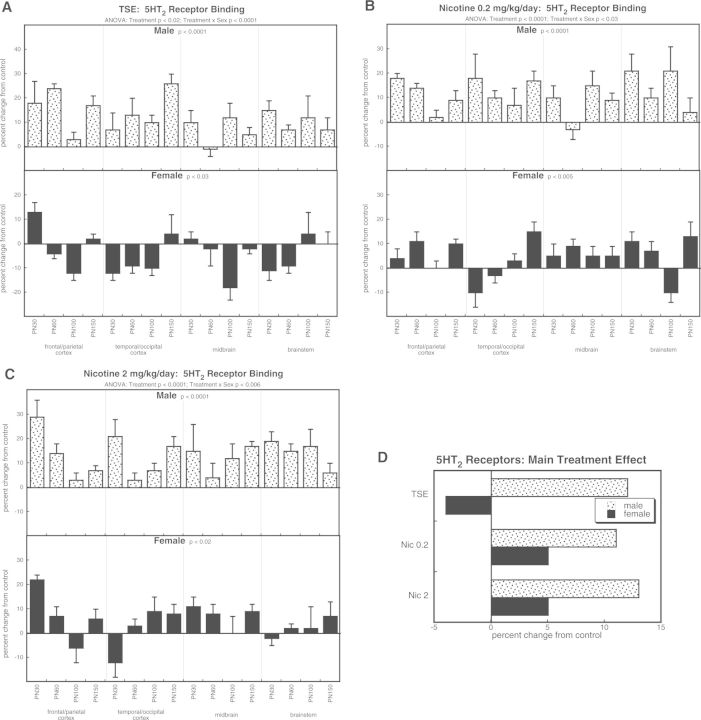
The 5HT2 receptor subtype likewise showed significant treatment effects (P < .0001) that depended on sex (treatment × sex, P < .0001). For TSE exposure, values increased in males but decreased in females (Fig. 6A). In males, treatment with either dose of nicotine produced effects similar in magnitude and direction to those seen with TSE (Fig. 6B and 6C). However, females exposed to nicotine displayed increases in 5HT2 receptor binding, an effect opposite to that of TSE. Again, these relationships were readily apparent with values collapsed across age and region (Fig. 6D). The effects of TSE and either dose of nicotine matched closely in males, whereas in females, TSE caused reductions but nicotine evoked increases, albeit to a smaller extent than in males.
FIG. 6.
Effects of TSE (A), nicotine 0.2 mg/kg/day (B), and nicotine 2 mg/kg/day (C) on 5HT2 receptor binding. Data represent means and standard errors, presented as the percent change from control values; complete original data are shown in Supplementary Table S6. Multivariate ANOVA for each treatment appears at the top of the panels. Lower-order tests for each sex were carried out for each treatment because of a significant treatment × sex interaction. Effects on specific regions or ages were not carried out because of the absence of their interactions with treatment. Panel (D) shows the simple main treatment effects, collapsed across age and region.
DISCUSSION
Our results show that the adverse effects of TSE on neurodevelopment exceed those that can be attributed to just the nicotine present in the mixture, and further, that the sensitivity extends down to levels commensurate with second-hand smoke exposure. Although both TSE and nicotine impaired ACh presynaptic activity (reduced HC3/ChAT ratio), the magnitude of the effect was greater for TSE; a comparable effect for nicotine was obtained only by raising the dose to 10 times that present in the TSE mixture. An even greater dichotomy was apparent for the impact on nAChRs. Receptor levels increased with nicotine alone, an effect that could provide partial compensation for the loss of presynaptic input. However, with TSE exposure, nAChRs decreased, an effect that would exacerbate the functional consequences of a presynaptic deficit.
Further differences were apparent in the cellular mechanisms underlying the reductions in ACh presynaptic activity with the different treatments. With nicotine, the reduction in presynaptic activity was associated with increases in ChAT superimposed on either a smaller increase in HC3 binding (0.2 mg/kg/day nicotine) or on a slight decrease in binding (2 mg/kg/day nicotine). Because ChAT is a constitutive marker of ACh nerve terminals (Slotkin, 2008), this implies that nicotine elicits ACh hyperinnervation, an effect that, like nAChR upregulation, could offset reduced presynaptic function. In contrast, TSE produced a much smaller ChAT increase in males, and a reduction in females, in the face of much larger decrements in HC3 binding; again, this points to a lack of compensatory changes with TSE, and instead, alterations that augment the functional deficits in presynaptic activity. The same pattern was seen for 5HT systems. For 5HT1A receptors, TSE elicited a decrease that was not seen with either nicotine treatment, and indeed, the low nicotine dose actually elicited an opposite effect. For 5HT2 receptors, males showed TSE-induced upregulation comparable to that seen with either dose of nicotine, but for females, TSE reduced the receptor concentration whereas nicotine increased it.
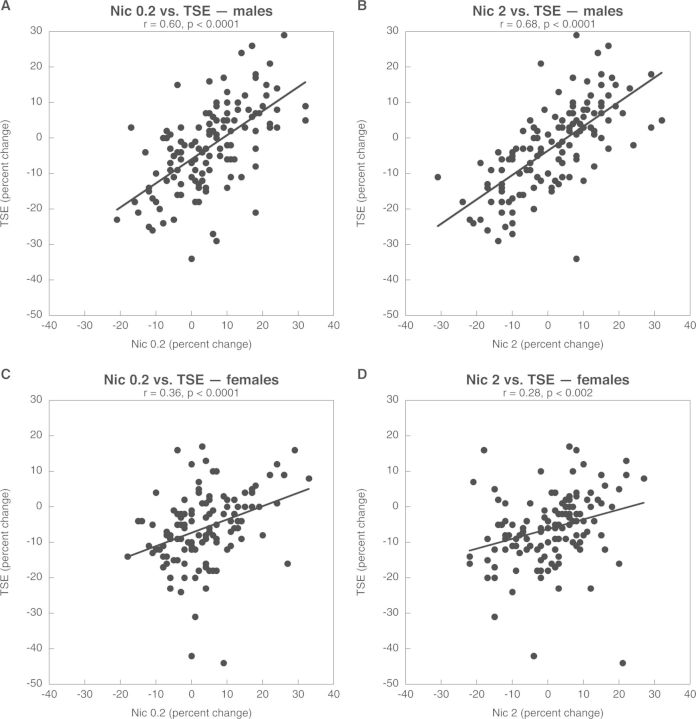
Thus, the effects of TSE appear to be a combination of an intensified response to nicotine, where the TSE reduction in HC3/ChAT resembles a 10-fold higher nicotine dose, as well as effects that are not associated with nicotine, and thus represent contributions from the thousands of other chemicals present in TSE. To distinguish these 2 components quantitatively, we performed regression analysis, including all of the regions, ages, and dependent measures simultaneously, so as to determine the extent to which the overall effects of nicotine can account for those of TSE; we separated the analyses for males and females because of the significant treatment × sex interactions seen for many of the variables. For males, the effects of the low dose of nicotine were significantly correlated with those of TSE and accounted for 36% (determined by r2) of the TSE effect (Fig. 7A). The higher dose of nicotine accounted for an even greater proportion of TSE effects (46%, Fig. 7B), reinforcing the interpretation of intensification of nicotine’s contributions in the presence of other TSE components. For females, the relationship was still significant but less strong, with nicotine accounting for only 13% of the effect at the low dose (Fig. 7C) and just 7% at the higher dose (Fig. 7D). The contributions of nicotine to TSE effects in females were significantly smaller than for males (P < .02 and P < .0002 by analysis of covariance, respectively). In turn, these differences provide a ready interpretation of the biological outcomes. The sex differences in TSE effects reflect a greater contribution of nicotine in males and an exacerbation of those effects by the other TSE components. However, in females, there is a lesser contribution of nicotine to the net outcome, and no exacerbation of nicotine’s effects by the other compounds. Thus, nicotine accounts for more of the TSE effect in males than females, and this in turn contributes to the overall sex differences in synaptic deficits. Studies are now underway to ascertain whether these relationships at the synaptic level lead to corresponding sex differences in behavioral deficits elicited by TSE when compared with nicotine. Given our findings for ACh and 5HT systems, these studies are focusing on both cognitive and emotional behaviors.
FIG. 7.
Correlations across all parameters for TSE effects with nicotine 0.2 mg/kg/day (A, C) or with nicotine 2 mg/kg/day (B, D), contrasted for males (A, B) and females (C, D). ANCOVA indicates a significantly higher correlation for males than females at either nicotine dose (P < .02 and P < .002, respectively).
As with all in vivo exposure models, our results do not provide the specific underlying mechanisms by which TSE leads to persistent deficits in synaptic function. However, in earlier work with an in vitro model of neurodifferentiation, we reached similar conclusions (Slotkin et al., 2014, 2015): TSE effects resembled those of 10-fold higher concentrations of nicotine but also included unique effects not seen with nicotine. Even a binary mixture of nicotine with one TSE component, benzo[a]pyrene, showed synergism as well as effects unrelated to nicotine alone (Slotkin et al., 2013). In concert with the present findings, this indicates that at least some of the differences between TSE and nicotine reflect direct effects on neurodifferentiation, rather than secondary actions on endocrine or metabolic status, or on maternal–fetal physiology.
Our study design involved thousands of measurements and potential intergroup comparisons, and thus it was vital to protect against the possibility that any individual difference might represent a random finding. Our approach was to use multifactor ANOVA to consider only those differences that were reinforced by consistency across multiple brain regions and time points. However, there are trade-offs in focusing only on main treatment effects compiled from large groupings of data points. First, this conservative statistical approach reduces the apparent magnitude of effects by combining data points showing large changes with those showing smaller changes. Second, it masks potentially important regional and temporal differences in the treatment effects. In fact, our results identified highly significant temporal effects (treatment × age interactions) for a number of the measured parameters. In each of those cases, there was a decline from peak effects in adolescence to lesser effects in adulthood, implying that there are adaptive changes that occur long after the termination of nicotine or TSE exposure. It is therefore likely that adverse effects are even stronger in juvenile stages, and it would be worthwhile to expand these studies to include additional developmental stages.
In conclusion, our results indicate that the complex mixture of compounds in tobacco smoke contributes significantly to developmental neurotoxicity, over and above the effects of nicotine. TSE lacks some of the volatile components of tobacco smoke, such as carbon monoxide and hydrogen cyanide, and thus the present model underestimates the potential for developmental neurotoxicity. Further, sex differences in the impact of TSE on brain development reflect differing contributions of nicotine in males and females, with nicotine responsible for a greater proportion of the adverse effect in males, and with TSE components exacerbating the impact of nicotine in males but not females. Nevertheless, these findings do not provide reassurance of so-called “harm reduction” for nicotine alternatives. First, that a single compound in TSE, nicotine, can account for as much as 35%–45% of the overall effect on cholinergic and serotonergic systems is a stark reminder of nicotine’s potency as a neuroteratogen. Second, we found significant adverse effects of nicotine alone at levels commensurate with second-hand smoke exposure, 10-fold below those of active smokers. This points to the importance of avoiding second-hand smoke exposure in pregnancy and to the liabilities of alternatives such as e-cigarettes, which, like cigarettes, expose both the user and bystanders to significant amounts of nicotine (Ballbe et al., 2014).
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
This work was supported by the National Institutes of Health (grant number ES022831) and by the U.S. Environmental Protection Agency (grant number 83543701). Environmental Protection Agency (EPA) support neither signifies that the contents reflect the views of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Supplementary Material
ACKNOWLEDGMENTS
T.A.S. has received consultant income in the past 3 years from the following firms: Acorda Therapeutics (Ardsley New York), Carter Law (Peoria Illinois), Pardieck Law (Seymour, Indiana), Tummel & Casso (Edinburg, Texas), and Chaperone Therapeutics (Research Triangle Park, North Carolina).
REFERENCES
- Aldridge J. E., Seidler F. J., Slotkin T. A. (2004). Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: Critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ. Health Perspect. 112, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballbe M., Martinez-Sanchez J. M., Sureda X., Fu M., Perez-Ortuno R., Pascual J. A., Salto E., Fernandez E. (2014). Cigarettes vs. e-cigarettes: Passive exposure at home measured by means of airborne marker and biomarkers. Environ. Res. 135, 76–80. [DOI] [PubMed] [Google Scholar]
- Dani J. A., De Biasi M. (2001). Cellular mechanisms of nicotine addiction. Pharmacol. Biochem. Behav. 70, 439–446. [DOI] [PubMed] [Google Scholar]
- Dreyfus C. F. (1998). Neurotransmitters and neurotrophins collaborate to influence brain development. Perspect. Dev. Neurobiol. 5, 389–399. [PubMed] [Google Scholar]
- Fewell J. E., Smith F. G., Ng V. K. Y. (2001). Threshold levels of maternal nicotine impairing protective responses of newborn rats to intermittent hypoxia. J. Appl. Physiol. 90, 1968–1976. [DOI] [PubMed] [Google Scholar]
- Fuller B. F., Cortes D. F., Landis M. K., Yohannes H., Griffin H. E., Stafflinger J. E., Bowers M. S., Lewis M. H., Fox M. A., Ottens A. K. (2012). Exposure of rats to environmental tobacco smoke during cerebellar development alters behavior and perturbs mitochondrial energetics. Environ. Health Perspect. 120, 1684–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaysina D., Fergusson D. M., Leve L. D., Horwood J., Reiss D., Shaw D. S., Elam K. K., Natsuaki M. N., Neiderhiser J. M., Harold G. T. (2013). Maternal smoking during pregnancy and offspring conduct problems: Evidence from three independent genetically-sensitive research designs. JAMA Psychiat. 70, 956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub M. S., Slotkin T. A., Pinkerton K. E. (2007). Visual recognition memory and auditory brainstem response in infant rhesus monkeys exposed perinatally to environmental tobacco smoke. Brain Res. 1151, 102–106. [DOI] [PubMed] [Google Scholar]
- Gospe S. M., Jr, Joyce J. A., Siebert J. R., Jack R. M., Pinkerton K. E. (2009). Exposure to environmental tobacco smoke during pregnancy in rats yields less effect on indices of brain cell number and size than does postnatal exposure. Reprod. Toxicol. 27, 22–27. [DOI] [PubMed] [Google Scholar]
- Klemm N., Kuhar M. J. (1979). Post-mortem changes in high affinity choline uptake. J. Neurochem. 32, 1487–1494. [DOI] [PubMed] [Google Scholar]
- Maes M., Meltzer H. (1995). The serotonin hypothesis of major depression. In Psychopharmacology: The Fourth Generation of Progress (Bloom F. E., Kupfer D. J., Bunney B. S., Ciaranello R. D., Davis K. L., Koob G. F., Meltzer H. Y., Schuster C. R., Shader R. I., Watson S. J., Eds.), pp. 933–944. Raven Press, New York. [Google Scholar]
- Pauly J. R., Slotkin T. A. (2008). Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Pædiatr. 97, 1331–1337. [DOI] [PubMed] [Google Scholar]
- Qiao D., Seidler F. J., Tate C. A., Cousins M. M., Slotkin T. A. (2003). Fetal chlorpyrifos exposure: Adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood. Environ. Health Perspect. 111, 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slikker W., Xu Z. A., Levin E. D., Slotkin T. A. (2005). Mode of action: Disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction — developmental neurotoxicity of nicotine. Crit. Rev. Toxicol. 35, 703–711. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A. (2008). If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol. Teratol. 30, 1–19. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Bodwell B. E., Levin E. D., Seidler F. J. (2008). Neonatal exposure to low doses of diazinon: Long-term effects on neural cell development and acetylcholine systems. Environ. Health Perspect. 116, 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A., Card J., Seidler F. J. (2013). Adverse benzo[a]pyrene effects on neurodifferentiation are altered by other neurotoxicant coexposures: Interactions with dexamethasone, chlorpyrifos, or nicotine in PC12 cells. Environ. Health Perspect. 121, 825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A., Card J., Stadler A., Levin E. D., Seidler F. J. (2014). Effects of tobacco smoke on PC12 cell neurodifferentiation are distinct from those of nicotine or benzo[a]pyrene. Neurotoxicol. Teratol. 43, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A., Pinkerton K. E., Seidler F. J. (2006a). Perinatal environmental tobacco smoke exposure in Rhesus monkeys: Critical periods and regional selectivity for effects on brain cell development and lipid peroxidation. Environ. Health Perspect. 114, 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A., Pinkerton K. E., Tate C. A., Seidler F. J. (2006b). Alterations of serotonin synaptic proteins in brain regions of neonatal Rhesus monkeys exposed to perinatal environmental tobacco smoke. Brain Res. 1111, 30–35. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Ryde I. T., Tate C. A., Seidler F. J. (2007). Lasting effects of nicotine treatment and withdrawal on serotonergic systems and cell signaling in rat brain regions: Separate or sequential exposure during fetal development and adulthood. Brain Res. Bull. 73, 259–272. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Skavicus S., Card J., Levin E. D., Seidler F. J. (2015). Amelioration strategies fail to prevent tobacco smoke effects on neurodifferentiation: Nicotinic receptor blockade, antioxidants, methyl donors. Toxicology 333, 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. (1985). Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76–85. [DOI] [PubMed] [Google Scholar]
- Trauth J. A., Seidler F. J., Slotkin T. A. (2000). An animal model of adolescent nicotine exposure: Effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 867, 29–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.