Abstract
Background
Tendon pain occurs in individuals with extreme cholesterol levels (familial hypercholesterolaemia). It is unclear whether the association with tendon pain is strong with less extreme elevations of cholesterol.
Objective
To determine whether lipid levels are associated with abnormal tendon structure or the presence of tendon pain.
Methods
We conducted a systematic review and meta-analysis. Relevant articles were found through an electronic search of 6 medical databases—MEDLINE, Cochrane, AMED, EMBASE, Web of Science and Scopus. We included all case–control or cross-sectional studies with data describing (1) lipid levels or use of lipid-lowering drugs and (2) tendon structure or tendon pain.
Results
17 studies (2612 participants) were eligible for inclusion in the review. People with altered tendon structure or tendon pain had significantly higher total cholesterol, low-density lipoprotein cholesterol and triglycerides, as well as lower high-density lipoprotein cholesterol; with mean difference values of 0.66, 1.00, 0.33, and −0.19 mmol/L, respectively.
Conclusions
The results of this review indicate that a relationship exists between an individual’s lipid profile and tendon health. However, further longitudinal studies are required to determine whether a cause and effect relationship exists between tendon structure and lipid levels. This could lead to advancement in the understanding of the pathoaetiology and thus treatment of tendinopathy.
Keywords: Cholesterol
Introduction
Tendinopathy is the term used to describe painful tendon conditions.1 While the exact pathoaetiology of tendinopathy remains unclear,2 overuse is considered as a major contributing factor.3 Despite this link, approximately one-third of cases occur in non-active individuals.4 Obesity and fat distribution have been associated with tendinopathy,5–7 with one explanation being that higher body mass causes increased tendon loading (mechanical hypothesis).7 However, increased body mass index (BMI) has also been linked with tendinopathy in the non-weight-bearing upper limbs,8 which suggests that a mechanical hypothesis does not sufficiently explain changes to tendon structure or onset of tendon pain.6 There is growing evidence for a metabolic hypothesis linking BMI and tendinopathy.
A well-known comorbidity of obesity is hypercholesterolaemia, an established risk factor for coronary heart disease.9 Cholesterol also accumulates in tendons, where it may be involved in structural disruption of the collagen matrix, similar to that seen in tendinopathy.10–12 This has been demonstrated in patients with familial hypercholesterolaemia (FH), a genetic lipid metabolism disorder that is characterised by lifelong elevation of serum cholesterol.13 Substantial cholesterol deposition occurs in FH and is known as tendon xanthoma.14 While many medical textbooks state that tendon xanthomas are asymptomatic, data show a sixfold increase in the lifetime incidence of Achilles tendon pain associated with FH.15
Chronic low-grade inflammation (para-inflammation) is a critical driver of cardiovascular disease (CVD) and is also a predominant feature of FH.16 17 The instigating event in CVD is retention of apolipoprotein-B containing lipoproteins beneath the endothelial layer of the blood vessel wall18 where they are irreversibly modified via interaction with extracellular matrix proteoglycans.19 Subendothelial lipoprotein retention triggers the release of chemokines, which promote monocyte recruitment and conversion to macrophages.17 As these macrophages ingest and process retained lipoproteins, they accumulate cytoplasmic lipid droplets, and under the microscope these droplets look like soap bubbles, leading to the term ‘foam cells’.20 Eventually cholesterol esterification and efflux mechanisms are overwhelmed and the excess cholesterol becomes cytotoxic.21 Each of these steps contributes to the para-inflammation present in CVD and FH.22
Recent studies of non-ruptured tendinopathy tissue indicate subtle increases in mast cells and macrophages23 24 although other studies find no such increase.25 26 When the clinician is considering this new information, it is critically important to remember that para-inflammation is triggered by subtle alterations in tissue homeostasis rather than infection and trauma, which are the familiar triggers of acute (triphasic) inflammation.27 Therefore, para-inflammation is not triggered by a bout of unresolved acute inflammation but is a response to tissue stress that is maintained over an extended period of time.27 Similarly, archetypal proinflammatory cytokines (eg, tumour necrosis factor alpha) can have anti-inflammatory effects via alternate signalling pathways.26
Other studies have identified associations between cholesterol and tendon rupture28 29 and chronic tendinopathy.6 Together, these data build support for the metabolic hypothesis as an explanation for the presentation of tendinopathy among sedentary individuals.
A systematic review by Gaida et al7 revealed that a relationship exists between adiposity and tendinopathy. As an abnormal lipid profile is commonly associated with obesity,30 this research has provided a basis for a closer examination of this relationship. Thus, the primary aim of this systematic review was to investigate whether there is an association between abnormal lipid levels and changes in tendon structure or tendon pain. A secondary aim was to describe the association that lipid-lowering drugs have with tendon structure or tendon pain. The review only considered individuals not diagnosed with FH.
Methods
Search strategy
Six electronic databases (MEDLINE, Cochrane, AMED, Web of Science, Scopus and EMBASE) were searched in April 2014. Medical subject headings used in the search included (1) Tendons, Tendon Injuries, Tendinopathy, Xanthomatosis AND (2) Lipids, Lipoproteins, Cholesterol, Hydroxymethylglutaryl-CoA Reductase Inhibitors and Lovastatin. Free text terms were also searched with appropriate truncation, and included (1) tendon structure, tendon thickness, tend#nopathy, tend#nos#s, tendon pain and (2) lipid, cholesterol, high-density lipoprotein, low-density lipoprotein, triglyceride, statin, hydroxymethylglutaryl-CoA reductase inhibitors (for complete list of search terms, see online supplementary appendix A). All records were then imported into reference management software (Endnote version X5).
Eligibility criteria
One author (BT) applied predefined eligibility criteria to the title and abstracts of the retrieved records. To be included studies had to provide data on both lipid levels or lipid-lowering drugs and tendon structure or tendon pain. We excluded case reports, case series and retrospective studies, reviews, letters, conference abstracts, comments, editorials, non-English articles, and studies of non-human, cadaver or biopsy material. Papers that involved participants with cerebrotendinous xanthomas, inflammatory or systemic arthritis, or FH were excluded. However, FH papers were included if they presented tendon data from a non-FH control group. In the second round of reviewing, the full text was read to determine whether the article met the inclusion and exclusion criteria.
Quality assessment
Included papers were assessed for methodological quality to identify potential sources of bias. A tool was developed by the authors based on the quality assessment criteria developed by Downs and Black (see online supplementary appendix B).31 The tool consisted of 11 criteria, each addressing a different source of bias. An explicit decision rule was developed for each criterion to improve precision. Quality assessment results were converted to a percentage score as not all studies could be marked on all criteria.
Data extraction and study analysis
Data were extracted by one author (BT) and included study details, participant demographics, tendon outcomes, lipid outcomes, and any results presented (ie, effect size, odds ratio (OR)). If data for the lipid levels of participants were presented in milligrams per decilitre (mg/dL), these units were converted to millimole per litre (mmol/L) using appropriate calculations (see online supplementary appendix C). When necessary, data were extracted from figures using Engauge Digitilizer (Mitchell, M; V.4.1) (see online supplementary appendix C).
Meta-analysis was conducted on appropriate data using RevMan (The Cochrane Collaboration; V.5.1). A random effects model was used with inverse variance weighting, and results presented as mean difference (MD). A meta-analysis was conducted for each of the four lipid measurements (total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG)), and their individual association with tendon structure or tendon pain.
Results
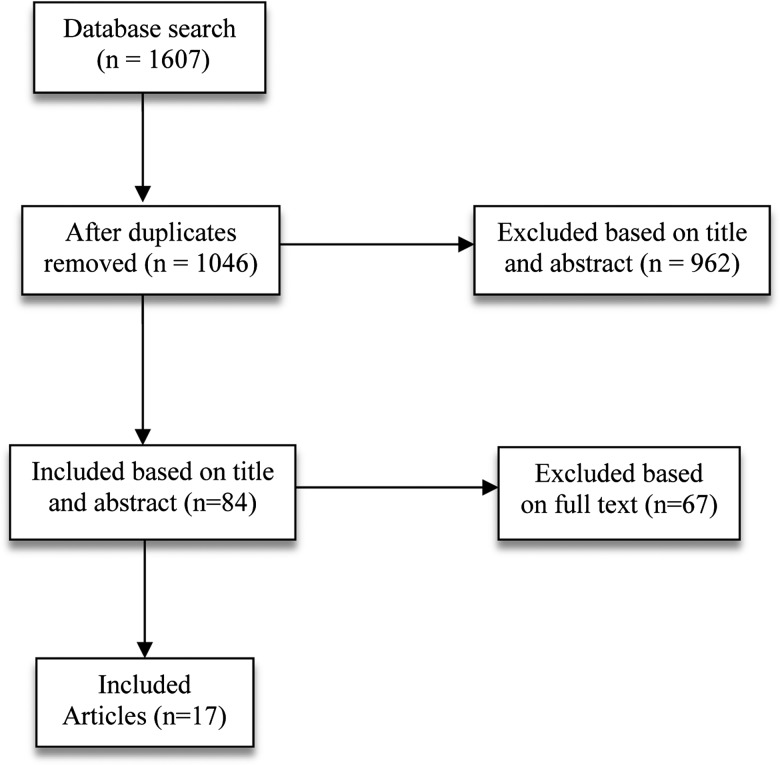
The databases search yield of 1607 papers was imported into Endnote and 561 duplicates were removed. The title and abstract of 1046 papers were reviewed and 962 studies were removed. The full text version of 84 papers was reviewed and 67 studies were removed. In the end, 17 studies were included in the review (figure 1).
Figure 1.
Flow chart of search yield and study selection.
The quality of the included papers showed considerable variation, with the mean quality assessment score being 62% (range 20–82%), suggesting that the current literature in this area is of moderate quality (see online supplementary appendix D). The majority of studies reported their method of participant recruitment; however, these participants were not commonly representative of the population from which they were recruited. The case–control nature of all but one of these studies explains why the two groups were often taken from different populations. With the exception of Ozgurtas et al,29 authors reported that controls were free from disease. The majority of authors presented a control group that was matched to the case group with respect to potential confounding factors. Reporting the method used to assess tendon structure was a common criteria met by these papers, however, these methods were not always identical for cases and controls. Only one study6 reported assessor blinding, with the remainder of the studies having the potential for assessor bias.
Fifteen of the included papers used a case–control study design, and two used a cross-sectional design (table 1). Imaging was used to define tendon structure in 14 studies—7 used ultrasound, 2 used MRI, 1 used CT and 4 used radiography. A variety of methods were used to assess tendon symptoms, including palpation, clinical history and a visual analogue scale for pain. The tendons examined included rotator cuff (n=4) and Achilles tendon (n=13).
Table 1.
Study details
| Author (year) | Study design | Country where the study was performed | Primary recruitment criteria (ie, tendon pain/structure or lipid level) | Was an upper limb (UL) or lower limb (LL) tendon assessed | Was tendon structure and/or tendon pain assessed |
|---|---|---|---|---|---|
| Abate (2014) | Cross-sectional study | Italy | Neither—referred for LL disease | UL | Structure |
| Abboud (2010) | Case–control study | USA | Tendon structure | UL | Structure |
| Beri (2009) | Case–control study | USA | Tendon structure | Both | Structure |
| Descamps (2001) | Case–control study | Belgium | Lipid levels | LL | Structure |
| Durrington (1982) | Case–control study | UK | Lipid levels | LL | Structure |
| Gaida (2009) | Case–control study | Australia/Sweden | Tendon pain | LL | Both |
| Gattereau (1973) | Case–control study | Canada | Lipid levels | LL | Structure |
| Junyent (2005) | Case–control study | Spain | Lipid levels | LL | Structure |
| Klemp (1993) | Case–control study | South Africa | Lipid levels | Both | Both |
| Kwak (2013) | Case–control study | Korea | Lipid levels | LL | Structure |
| Longo (2010) | Case–control study | UK | Tendon structure | Both | Both |
| Mabuchi (1977) | Case–control study | Japan | Lipid levels | LL | Structure |
| Mabuchi (1978) | Case–control study | Japan | Lipid levels | LL | Structure |
| Ozgurtas (2003) | Case–control study | Turkey | Tendon structure | LL | Structure |
| Rechardt (2013) | Cross-sectional study | Finland | Tendon pain | UL | Pain |
| Tsouli (2009) | Case–control study | Greece | Lipid levels | LL | Structure |
| Yuzawa (1989) | Case–control study | Japan | Lipid levels | LL | Structure |
Among the case–control papers, there were five studies that recruited the case group based on tendon structure or tendon pain,6 29 32–34 and three studies that recruited the case group based on lipid abnormalities.35–37 There were seven studies of FH where only the control group was included.13 14 38–42 Two studies examined the cross-sectional relationship between tendon health and lipid profiles.8 43 In three of the five studies that recruited based on tendon structure or tendon pain, significant differences in lipids or statin use were identified. In two of the three studies that recruited on lipid levels, significant differences in tendon pain or structure were identified.
Data were extracted from all 17 papers in the review (see online supplementary appendix E), of which five were pooled for meta-analysis.6 8 29 32 34 These papers used lipid levels as the continuous variable, for both case and control tendon health groups. The non-FH control groups from the seven FH studies13 14 38–42 were not included in the meta-analysis. Achilles tendon thickness (ATT) was used as a continuous outcome variable in two studies that each had a high and a low lipid group,35 37 however one of these investigated men and women separately and so the two could not be compared against each other. Two studies provided dichotomous data on tendon pathology; however one paper investigated the association with lipid levels,36 while the other examined the association with statin use,33 thus the papers were presented separately. Finally, one paper was a cross-sectional study and so could not be compared to the case–control studies.43
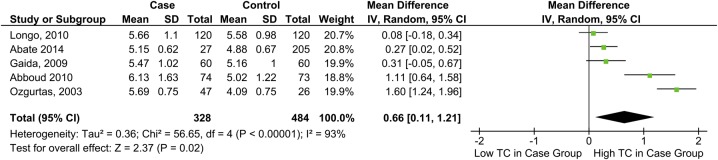
TC and tendon pathology
TC was significantly higher among individuals with tendon pain or rupture, MD=0.66 mmol/L, 95% CI 0.11 to 1.21 (figure 2).
Figure 2.
Forest plot showing the relationship between total cholesterol levels and tendon pathology (TC, total cholesterol).
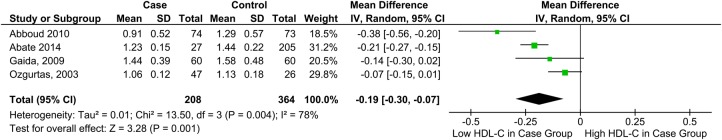
HDL-C and tendon pathology
HDL-C was significantly lower among individuals with tendon pain or rupture, MD=−0.19 mmol/L, 95% CI −0.30 to −0.07 (figure 3).
Figure 3.
Forest plot showing the relationship between high-density lipoprotein cholesterol levels and tendon pathology (HDL-C, high-density lipoprotein cholesterol).
LDL-C and tendon pathology
LDL-C was significantly higher among individuals with tendon pain or rupture, MD=1.00 mmol/L 95% CI 0.23 to 1.77 (figure 4).
Figure 4.
Forest plot showing the relationship between low-density lipoprotein cholesterol levels and tendon pathology (LDL-C, low-density lipoprotein cholesterol).
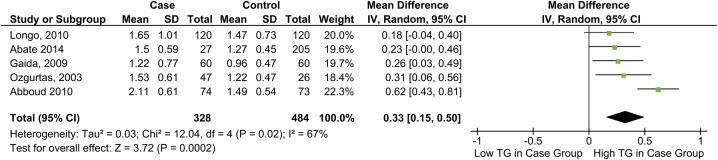
TG and tendon pathology
TG was significantly higher among individuals with tendon pain or rupture, MD=0.33 mmol/L, 95% CI 0.15 to 0.50 (figure 5).
Figure 5.
Forest plot showing the relationship between triglyceride levels and tendon pathology (TG, triglycerides).
Lipid level and tendon pathology
One paper36 recorded the prevalence of tendon pathology (history of Achilles tendinitis or tendon xanthomas) in a group with mixed hyperlipidaemia and a normolipidaemic control group. Tendon pathology was present in 18 (75%) of those with mixed hyperlipidaemia, compared with none of the normolipidaemic participants.
Lipid level and ATT
Tendon thickness was used as the continuous variable in two studies,35 37 looking for differences between the hyperlipidaemic case group and the normolipidaemic control group. However, the paper by Gattereau et al35 analysed men and women separately and did not provide data for the population as a whole, and so the results could not be compared with the paper by Kwak et al.37 Interestingly, as the former split the participants into men and women, this paper allowed an investigation of whether or not this association was greater in one sex compared with the other.
Gattereau et al35 used cut-off values for TC and TG of 240 and 140 mg/dL, respectively, to separate the hyperlipidaemic group from the normolipidaemic group. Hyperlipidaemic subjects were found to have a significantly increased ATT compared to the normolipidaemic controls. This was evident for both men (standardised MD (SMD)=0.95, 95% CI 0.19 to 1.71) and women (SMD=0.86, 95% CI 0.13 to 1.58). A significant positive correlation between cholesterol and ATT was also found, with correlation coefficients of 0.85 for women and 0.63 for men.
Kwak et al37 used the European Society of Cardiology definition for dyslipidaemia (any one of the following: TC >240 mg/dL, TG >200 mg/dL, LDL-C >160 mg/dL, HDL-C <40 mg/dL). There was no significant difference in ATT between the dyslipidaemic (0.44±0.04 cm) and control group (0.45±0.02 cm, p=0.783). There was also no significant correlation between ATT and lipid parameters. The coefficients for TC was −0.09 (p=0.36), HDL-C −0.06 (p=0.50), LDL-C −0.03 (p=0.69) and TGs −0.04 (p=0.63).
Statins and tendon rupture
One paper33 examined the association between statin therapy and tendon structure. Statin use was the same in those with and without tendon rupture, however, preplanned subgroup analysis showed an increase in tendon rupture in women taking statins, OR=3.09 (95% CI 1.04 to 9.75). No increased risk was identified in men, OR=0.62 (95% CI 0.3 to 1.3).
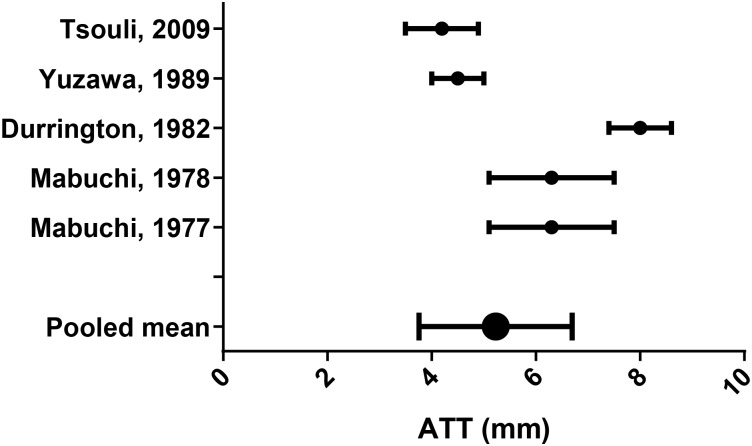
ATT and lipid levels in FH studies
Seven FH papers with a non-FH control group were included.13 14 38–42 Each paper presented data on ATT and lipid profiles, but only one40 analysed this relationship in the control group, finding a moderate positive correlation (r=0.454, p<0.01).
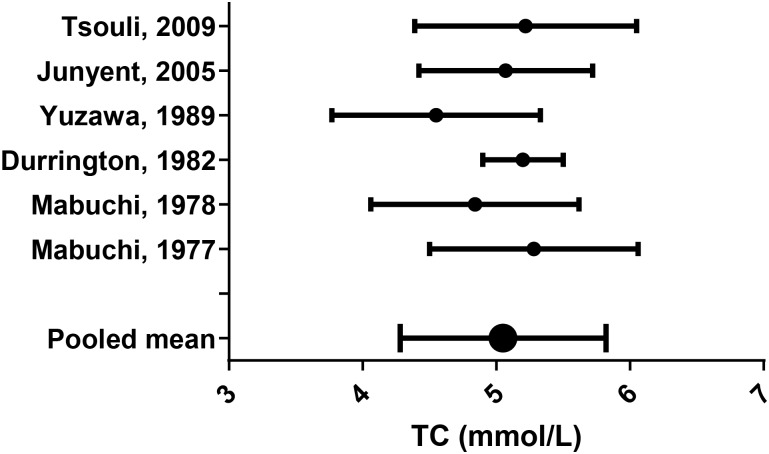
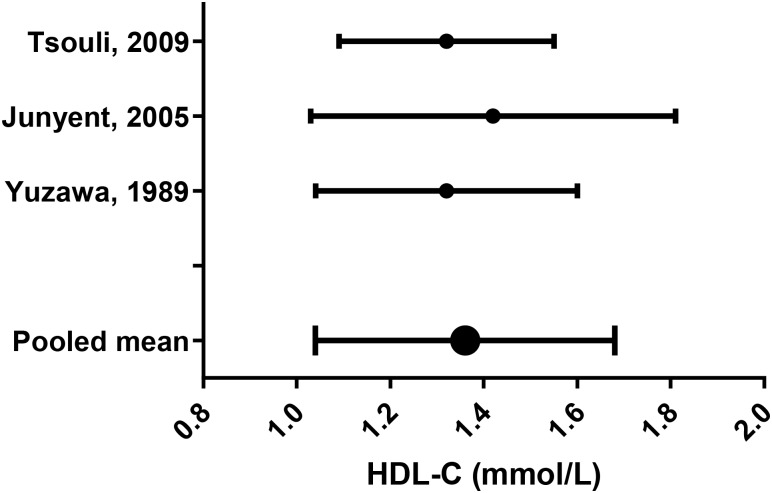
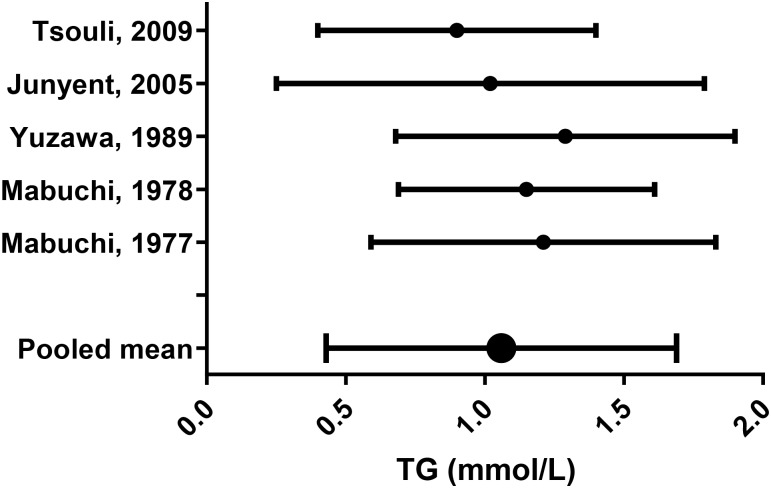
Lipid parameters from the remaining studies were pooled using appropriate equations (see online supplementary appendix F;44 figures 6–10).
Figure 6.
Achilles tendon thickness (ATT) mean (SD).
Figure 7.
Total cholesterol (TC) mean (SD).
Figure 8.
High-density lipoprotein cholesterol (HDL-C) mean (SD).
Figure 9.
Low-density lipoprotein cholesterol (LDL-C) mean (SD).
Figure 10.
Triglycerides (TG) mean (SD).
Lipid levels and pain intensity
There was only one study43 that used pain intensity as an outcome. This was a study of the association of obesity and metabolic parameters with upper extremity pain. Age-adjusted and gender-adjusted ORs of pain intensity was calculated for TC, HDL-C, LDL-C and TG. A high pain intensity was associated with both low HDL-C levels (OR 2.7, 95% CI 1.2 to 6.3) and high TG levels (OR 2.8, 95% CI 1.3 to 6.6; table 2).
Table 2.
OR of pain intensity according to TC, HDL-C, LDL-C and TG
| Characteristic | OR | 95% CI |
|---|---|---|
| TC (mmol/L) | ||
| <4.7 | 1 | 0.4 to 2.7 |
| 4.7–5.3 | 1.0 | 0.8 to 4.0 |
| >5.3 | 1.8 | |
| HDL-C (mmol/L) | ||
| >1.83 | 1 | 0.4 to 2.2 |
| 1.48–1.83 | 0.9 | 1.2 to 6.3 |
| <1.48 | 2.7 | |
| LDL-C (mmol/L) | ||
| <2.5 | 1 | 0.4 to 2.6 |
| 2.5–3.3 | 1.1 | 0.7 to 4.2 |
| >3.3 | 1.7 | |
| TG (mmol/L) | ||
| <0.72 | 1 | 0.7 to 4.0 |
| 0.72–1.08 | 1.7 | 1.3 to 6.6 |
| >1.08 | 2.8 |
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides.
Discussion
This systematic review showed with meta-analysis that people with altered tendon structure had significantly higher TC, LDL-C and TG, as well as lower HDL-C, than people with normal tendons. Individuals with an adverse lipid profile were much more likely to have tendon pathology, and to have higher pain intensity associated with upper limb musculoskeletal injury. Statin use was associated with Achilles tendon rupture in women but not men. Finally, two of the three studies that correlated ATT with lipid parameters found a significant positive correlation. Together, these findings provide significant support for a metabolic hypothesis of tendon injury and implicate lipid parameters as a potential link.
While the data in this review indicate a link between lipid parameters and tendon health, causation cannot be established from cross-sectional data. As tendon injury limits physical activity, reverse causation—tendon injury causing elevated lipids—is possible given that metabolic parameters rapidly deteriorate with complete bed rest45 and reduced daily steps.46 However, observational and mechanistic studies suggest that adverse lipid parameters can be harmful to tendons. For example, individuals with FH have altered tendon structure in childhood and adolescence,47 and aggressive lipid treatment reduces tendon thickness.14 Furthermore, mechanistic studies show that hyperlipidaemia alters the mechanical properties of tendon across several species.48 49 Together, these data provide plausibility for lipid parameters leading to reduced tendon health, suggesting that reverse causation is unlikely.
High cholesterol promotes the accumulation of cholesterol in macrophages and other immune cells, which drives para-inflammation. Downstream signalling pathways trigger a reduction in cholesterol efflux,22 which increases cholesterol accumulation and maintains the para-inflammation state.22 These processes provide a potential explanation for a link between hypercholesterolaemia and tendinopathy. Supporting this hypothesis is the increased number of mast cells and macrophages shown in those with tendinopathy, compared with a healthy control population.23 24 Additionally, the strong inflammatory phenotype of FH16 suggests that a state of para-inflammatory change may be involved in the mechanism of high cholesterol leading to tendinopathy.16 It is unclear whether these processes also occur in tendons, particularly given the magnitude of hypercholesterolaemia noted in association with tendinopathy (eg, 5.7±1.2, pooled mean of cases from figure 2) is far less than that observed in FH (eg, 8.3±1.0 mmol/L).14
A secondary aim of this review was to examine whether statin use was correlated with tendon structure. One eligible paper33 investigated this association and found, in an a priori subgroup analysis, that women taking statins had an increased risk of tendon rupture. Recent evidence suggests this effect may be mediated by changes in the profile of metalloproteinase enzymes within the tendon.50 These enzymes are involved in remodelling the extracellular matrix of tendon and have been linked with tendon rupture.51
Pain intensity was used as an outcome in one paper43 and was found to be associated with low HDL-C and high TG among individuals with upper extremity soft tissue disorders. This study pooled participants with a range of diagnoses, including tendinopathy of the rotator cuff, lateral elbow and wrist flexor tendons. Grouping diagnoses is, in one sense, a limitation of the study; however, this approach allowed the authors to collect a large sample of participants with a recent onset of pain (<1 month). The strong association of LDL-C and TG with recent onset pain provides strong, but obviously not definitive, evidence against reverse causation.
The lipid changes associated with tendon injury parallel those associated with CVD, namely, TC, LDL-C and TG are increased while HDL-C is decreased. This suggests that tendon and artery respond to their metabolic environments in similar ways. Both are collagen-based tissues, capable of responding to their loading environment. For example, branch points and bifurcations of arteries are exposed to shear forces created by turbulence,52 have an altered proteoglycan content, and are prone to atherosclerosis development.53 The pathogenesis of atherosclerosis is driven by long-standing para-inflammation, involving the recruitment and conversion of monocytes to macrophages.54 The macrophages accumulate cholesterol esters to form ‘foam cells’, which play a central role in the formation of atherosclerotic lesions.54 In tendons, shear and compression forces alter the proteoglycan profile,55 and these areas are prone to pathology. There is evidence that proteoglycan (particularly those with a chondroitin sulfate side chain) precipitates cholesterol accumulation within both tendon and artery through its strong affinity for cholesterol.56 57 In short, things that are bad for your heart also seem to be bad for tendons.
There are limitations of this review that should be acknowledged. One limitation was that 17 potentially relevant non-English studies were excluded. Language bias has been demonstrated for randomised trials58—studies with significant findings are more likely to be published in English-language journals—and may also affect observation studies such as those included in this review. Limitations are also inherent in the data set used for this review. For example, the included studies used a variety of methods to assess tendon structure or tendon pain. Blood lipid analysis is a standardised laboratory procedure with little potential for systematic bias. Blood collection procedures, however, may introduce bias. For example, in the study by Ozgurtas et al,29 blood samples were collected from the case group within 6 h of tendon rupture, while those from the control group were collected after an overnight fast. While this is not ideal, large datasets show minimal difference between fasting and non-fasting lipid measurements.59
Conclusion
The review comprehensively identified all available data on the potential link that lipid fractions have with tendon pain and structure. The meta-analysis supports an association for TC, HDL-C, LDL-C and TG. A potential mechanism for this link is the para-inflammation that is present in FH. Ongoing research is required to determine whether similar processes occur in the presence of less extreme cholesterol elevations associated with lifestyle factors. Notwithstanding this, the current work indicates that there is indeed an association between unfavourable changes in lipid parameters and tendinopathy.
Summary box.
This review identified 17 studies on the topic of tendon health and lipid profile. Five studies compared lipid profile between individuals with and without tendon pain/abnormality, and three studies compared tendon health between individuals with and without lipid abnormality. The cross-sectional relationship between tendon health and lipid profile was examined in two studies. Seven studies on familial hypercholesterolaemia provided data from their healthy control group.
Meta-analysis of appropriate studies showed significantly higher total cholesterol, low-density lipoprotein cholesterol and triglyceride, and lower high-density lipoprotein cholesterol in individuals with tendon pain/abnormality.
Two of the three studies that examined the correlation between tendon thickness and lipid levels found a significant positive relationship.
Statin use was associated with Achilles tendon rupture in women but not men.
Supplementary Material
Footnotes
Twitter: Follow James Gaida at @tendonresearch
Competing interests: None declared.
Funding: University of Canberra, Research Institute for Sport and Exercise (UC-RISE); University of Canberra, Faculty of Health; Monash University, Department of Physiotherapy.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cook JL, Khan KM Maffulli N et al. . Overuse tendinosis, not tendinitis part 2: applying the new approach to patellar tendinopathy. Physician Sportsmed 2000;28:31–46. [DOI] [PubMed] [Google Scholar]
- 2.Khan KM, Cook JL, Taunton JE, et al. Overuse tendinosis, not tendinitis part 1: a new paradigm for a difficult clinical problem. Physician Sportsmed 2000;28:38–48. [DOI] [PubMed] [Google Scholar]
- 3.Kujala UM, Sarna S, Kaprio J. Cumulative incidence of Achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med 2005;15:133–5. 10.1097/01.jsm.0000165347.55638.23 [DOI] [PubMed] [Google Scholar]
- 4.Rolf C, Movin T. Etiology, histopathology, and outcome of surgery in achillodynia. Foot Ankle Int 1997;18:565–9. 10.1177/107110079701800906 [DOI] [PubMed] [Google Scholar]
- 5.Holmes GB, Lin J. Etiologic factors associated with symptomatic Achilles tendinopathy. Foot Ankle Int 2006;27:952–9. [DOI] [PubMed] [Google Scholar]
- 6.Gaida JE, Alfredson L, Kiss ZS, et al. Dyslipidemia in Achilles tendinopathy is characteristic of insulin resistance. Med Sci Sports Exerc 2009;41:1194–7. 10.1249/MSS.0b013e31819794c3 [DOI] [PubMed] [Google Scholar]
- 7.Gaida JE, Ashe MC, Bass SL, et al. Is adiposity an under-recognized risk factor for tendinopathy? A systematic review. Arthritis Rheum 2009;61:840–9. 10.1002/art.24518 [DOI] [PubMed] [Google Scholar]
- 8.Abate M, Schiavone C, Di Carlo L, et al. Prevalence of and risk factors for asymptomatic rotator cuff tears in postmenopausal women. Menopause 2014;21:275–80. 10.1097/GME.0b013e31829638e3 [DOI] [PubMed] [Google Scholar]
- 9.Gordon T, Castelli WP, Hjortland MC, et al. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 1977;62:707–14. 10.1016/0002-9343(77)90874-9 [DOI] [PubMed] [Google Scholar]
- 10.Adams CW, Bayliss OB, Baker RW, et al. Lipid deposits in ageing human arteries, tendons and fascia. Atherosclerosis 1974;19:429–40. 10.1016/S0021-9150(74)80007-9 [DOI] [PubMed] [Google Scholar]
- 11.Finlayson R, Woods SJ. Lipid in the Achilles tendon. A comparative study. Atherosclerosis 1975;21:371–89. 10.1016/0021-9150(75)90050-7 [DOI] [PubMed] [Google Scholar]
- 12.Jozsa L, Reffy A, Balint JB. The pathogenesis of tendolipomatosis; an electron microscopical study. Int Orthop 1984;7:251–5. 10.1007/BF00266836 [DOI] [PubMed] [Google Scholar]
- 13.Junyent M, Gilabert R, Zambon D, et al. The use of Achilles tendon sonography to distinguish familial hypercholesterolemia from other genetic dyslipidemias. Arterioscler Thromb Vasc Biol 2005;25:2203–8. 10.1161/01.ATV.0000183888.48105.d1 [DOI] [PubMed] [Google Scholar]
- 14.Tsouli SG, Xydis V, Argyropoulou MI, et al. Regression of Achilles tendon thickness after statin treatment in patients with familial hypercholesterolemia: an ultrasonographic study. Atherosclerosis 2009;205:151–5. 10.1016/j.atherosclerosis.2008.10.032 [DOI] [PubMed] [Google Scholar]
- 15.Beeharry D, Coupe B, Benbow EW, et al. Familial hypercholesterolaemia commonly presents with Achilles tenosynovitis. Ann Rheum Dis 2006;65:312–15. 10.1136/ard.2005.040766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holven KB, Narverud I, Lindvig HW, et al. Subjects with familial hypercholesterolemia are characterized by an inflammatory phenotype despite long-term intensive cholesterol lowering treatment. Atherosclerosis 2014;233:561–7. 10.1016/j.atherosclerosis.2014.01.022 [DOI] [PubMed] [Google Scholar]
- 17.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011;145:341–55. 10.1016/j.cell.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 1995;15:551–61. 10.1161/01.ATV.15.5.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gustafsson M, Boren J. Mechanism of lipoprotein retention by the extracellular matrix. Curr Opin Lipidol 2004;15:505–14. 10.1097/00041433-200410000-00003 [DOI] [PubMed] [Google Scholar]
- 20.Rader DJ, Pure E. Lipoproteins, macrophage function, and atherosclerosis: beyond the foam cell? Cell Metab 2005;1:223–30. 10.1016/j.cmet.2005.03.005 [DOI] [PubMed] [Google Scholar]
- 21.Tabas I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J Clin Invest 2002;110:905–11. 10.1172/JCI0216452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol 2015;15:104–16. 10.1038/nri3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kragsnaes MS, Fredberg U, Stribolt K, et al. Stereological quantification of immune-competent cells in baseline biopsy specimens from Achilles tendons: results from patients with chronic tendinopathy followed for more than 4 years. Am J Sports Med 2014;42:2435–45. 10.1177/0363546514542329 [DOI] [PubMed] [Google Scholar]
- 24.Scott A, Lian O, Bahr R, et al. Increased mast cell numbers in human patellar tendinosis: correlation with symptom duration and vascular hyperplasia. Br J Sports Med 2008;42:753–7. 10.1136/bjsm.2007.040212 [DOI] [PubMed] [Google Scholar]
- 25.Fearon AM, Twin J, Dahlstrom JE, et al. Increased substance P expression in the trochanteric bursa of patients with greater trochanteric pain syndrome. Rheumatol Int 2014;34:1441–8. 10.1007/s00296-014-2957-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaida JE, Bagge J, Purdam C, et al. Evidence of the TNF-alpha system in the human Achilles tendon: expression of TNF-alpha and TNF receptor at both protein and mRNA levels in the tenocytes. Cells Tissues Organs 2012;196:339–52. 10.1159/000335475 [DOI] [PubMed] [Google Scholar]
- 27.Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454:428–35. 10.1038/nature07201 [DOI] [PubMed] [Google Scholar]
- 28.Mathiak G, Wening JV, Mathiak M, et al. Serum cholesterol is elevated in patients with Achilles tendon ruptures. Arch Orthop Trauma Surg 1999;119:280–4. 10.1007/s004020050410 [DOI] [PubMed] [Google Scholar]
- 29.Ozgurtas T, Yildiz C, Serdar M, et al. Is high concentration of serum lipids a risk factor for Achilles tendon rupture? Clin Chim Acta 2003;331:25–8. 10.1016/S0009-8981(03)00075-5 [DOI] [PubMed] [Google Scholar]
- 30.Franssen R, Monajemi H, Stroes ES, et al. Obesity and dyslipidemia. Endocrinol Metab Clin North Am 2008;37:623–33, viii 10.1016/j.ecl.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 31.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84. 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abboud JA, Kim JS. The effect of hypercholesterolemia on rotator cuff disease. Clin Orthop Relat Res 2010;468:1493–7. 10.1007/s11999-009-1151-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beri A, Dwamena FC, Dwamena BA. Association between statin therapy and tendon rupture: a case-control study. J Cardiovasc Pharmacol 2009;53:401–4. 10.1097/FJC.0b013e3181a0ce8b [DOI] [PubMed] [Google Scholar]
- 34.Longo UG, Franceschi F, Spiezia F, et al. Triglycerides and total serum cholesterol in rotator cuff tears: do they matter? Br J Sports Med 2010;44:948–51. 10.1136/bjsm.2008.056440 [DOI] [PubMed] [Google Scholar]
- 35.Gattereau A, Davignon J, Langelier M, et al. An improved radiological method for the evaluation of Achilles tendon xanthomatosis. CMAJ 1973;108:39–42. [PMC free article] [PubMed] [Google Scholar]
- 36.Klemp P, Halland AM, Majoos FL, et al. Musculoskeletal manifestations in hyperlipidaemia: a controlled study. Ann Rheum Dis 1993;52:44–8. 10.1136/ard.52.1.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwak M, Yoon S, Cho Y, et al. The correlation between Achilles tendon thickness and cardiovascular risk factors. J Lipid Atheroscler 2013;2:77–83. 10.12997/jla.2013.2.2.77 [DOI] [Google Scholar]
- 38.Descamps OS, Leysen X, Van Leuven F, et al. The use of Achilles tendon ultrasonography for the diagnosis of familial hypercholesterolemia. Atherosclerosis 2001;157:514–18. 10.1016/S0021-9150(01)00533-0 [DOI] [PubMed] [Google Scholar]
- 39.Durrington PN, Adams JE, Beastall MD. The assessment of Achilles tendon size in primary hypercholesterolaemia by computed tomography. Atherosclerosis 1982;45:345–58. 10.1016/0021-9150(82)90235-0 [DOI] [PubMed] [Google Scholar]
- 40.Mabuchi H, Ito S, Haba T. Discrimination of familial hypercholesterolemia and secondary hypercholesterolemia by Achilles’ tendon thickness. Atherosclerosis 1977;28:61–7. 10.1016/0021-9150(77)90199-X [DOI] [PubMed] [Google Scholar]
- 41.Mabuchi H, Tatami R, Haba T, et al. Achilles tendon thickness and ischemic heart disease in familial hypercholesterolemia. Metabolism 1978;27:1672–9. 10.1016/0026-0495(78)90289-5 [DOI] [PubMed] [Google Scholar]
- 42.Yuzawa K, Yamakawa K, Tohno E, et al. An ultrasonographic method for detection of Achilles tendon xanthomas in familial hypercholesterolemia. Atherosclerosis 1989;75:211–18. 10.1016/0021-9150(89)90178-0 [DOI] [PubMed] [Google Scholar]
- 43.Rechardt M, Shiri R, Lindholm H, et al. Associations of metabolic factors and adipokines with pain in incipient upper extremity soft tissue disorders: a cross-sectional study. BMJ Open 2013;3:e003036 10.1136/bmjopen-2013-003036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borenstein M, Hedges L, Higgins J, et al. Introduction to meta-analysis. John Wiley & Sons, 2008. [Google Scholar]
- 45.Hamburg NM, McMackin CJ, Huang AL, et al. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol 2007;27:2650–6. 10.1161/ATVBAHA.107.153288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olsen RH, Krogh-Madsen R, Thomsen C, et al. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA 2008;299:1261–3. 10.1001/jama.299.11.1259 [DOI] [PubMed] [Google Scholar]
- 47.Yamakawa K, Yanagi H, Saku K, et al. Family studies of the LDL receptor gene of relatively severe heriditary hypercholesterolemia associated with Achilles tendon xanthomas. Hum Genet 1991;86:445–9. 10.1007/BF00194631 [DOI] [PubMed] [Google Scholar]
- 48.Beason DP, Abboud JA, Kuntz AF, et al. Cumulative effects of hypercholesterolemia on tendon biomechanics in a mouse model. J Orthop Res 2011;29:380–3. 10.1002/jor.21255 [DOI] [PubMed] [Google Scholar]
- 49.Beason DP, Hsu JE, Marshall SM, et al. Hypercholesterolemia increases supraspinatus tendon stiffness and elastic modulus across multiple species. J Shoulder Elbow Surg 2013;22:681–6. 10.1016/j.jse.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Oliveira LP, Vieira CP, Da Re Guerra F, et al. Statins induce biochemical changes in the Achilles tendon after chronic treatment. Toxicology 2013;311:162–8. 10.1016/j.tox.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 51.Pasternak B, Schepull T, Eliasson P, et al. Elevation of systemic matrix metalloproteinases 2 and 7 and tissue inhibitor of metalloproteinase 2 in patients with a history of Achilles tendon rupture: pilot study. Br J Sports Med 2010;44:669–72. 10.1136/bjsm.2008.049411 [DOI] [PubMed] [Google Scholar]
- 52.Ku D. Blood flow in arteries. Annu Rev Fluid Mechanics 1997;29:399–434. 10.1146/annurev.fluid.29.1.399 [DOI] [Google Scholar]
- 53.Manley G, Hawksworth J. Distribution of mucopolysaccharides in the human vascular tree. Nature 1965;206:1152–3. 10.1038/2061152a0 [DOI] [PubMed] [Google Scholar]
- 54.Linton MF, Fazio S. Macrophages, inflammation, and atherosclerosis. Int J Obes Relat Metab Disord 2003;27(Suppl 3):S35–40. 10.1038/sj.ijo.0802498 [DOI] [PubMed] [Google Scholar]
- 55.Benjamin M, Qin S, Ralphs JR. Fibrocartilage associated with human tendons and their pulleys. J Anat 1995;187(Pt 3):625–33. [PMC free article] [PubMed] [Google Scholar]
- 56.Adams CW, Bayliss OB. Acid mucosubstances underlying lipid deposits in ageing tendons and atherosclerotic arteries. Atherosclerosis 1973;18:191–5. 10.1016/0021-9150(73)90100-7 [DOI] [PubMed] [Google Scholar]
- 57.Volker W, Schmidt A, Oortmann W, et al. Mapping of proteoglycans in atherosclerotic lesions. Eur Heart J 1990;11(Suppl E):29–40. 10.1093/eurheartj/11.suppl_E.29 [DOI] [PubMed] [Google Scholar]
- 58.Egger M, Zellweger-Zahner T, Schneider M, et al. Language bias in randomised controlled trials published in English and German. Lancet 1997;350:326–9. 10.1016/S0140-6736(97)02419-7 [DOI] [PubMed] [Google Scholar]
- 59.Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation 2008;118:2047–56. 10.1161/CIRCULATIONAHA.108.804146 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.