Abstract
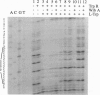
Highly purified preparations of trp repressor (TrpR) protein derived from Escherichia coli strains that were engineered to overexpress this material were found to contain another protein, of 21 kDa. The second protein, designated WrbA [for tryptophan (W) repressor-binding protein] remained associated with its namesake through several sequential protein fractionation steps. The N-terminal amino acid sequence of the WrbA protein guided the design of two degenerate oligonucleotides that were used as probes in the cloning of the wrbA gene (198 codons). The WrbA protein, in purified form, was found by several criteria to enhance the formation and/or stability of noncovalent complexes between TrpR holorepressor and its primary operator targets. The formation of an operator-holorepressor-WrbA ternary complex was demonstrated by gel mobility-shift analysis. The WrbA protein alone does not interact with the trp operator. During the stationary phase, cells deficient in the WrbA protein were less efficient than wild type in their ability to repress the trp promoter. It is proposed that the WrbA protein functions as an accessory element in blocking TrpR-specific transcriptional processes that might be physiologically disadvantageous in the stationary phase of the bacterial life cycle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidson D. N., Shapiro M., Youderian P. Mutant tryptophan aporepressors with altered specificities of corepressor recognition. Genetics. 1991 May;128(1):29–35. doi: 10.1093/genetics/128.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass S., Sorrells V., Youderian P. Mutant Trp repressors with new DNA-binding specificities. Science. 1988 Oct 14;242(4876):240–245. doi: 10.1126/science.3140377. [DOI] [PubMed] [Google Scholar]
- Benevides J. M., Weiss M. A., Thomas G. J., Jr Design of the helix-turn-helix motif: nonlocal effects of quaternary structure in DNA recognition investigated by laser Raman spectroscopy. Biochemistry. 1991 May 7;30(18):4381–4388. doi: 10.1021/bi00232a003. [DOI] [PubMed] [Google Scholar]
- Borden K. L., Bauer C. J., Frenkiel T. A., Beckmann P., Lane A. N. Sequence-specific NMR assignments of the trp repressor from Escherichia coli using three-dimensional 15N/1H heteronuclear techniques. Eur J Biochem. 1992 Feb 15;204(1):137–146. doi: 10.1111/j.1432-1033.1992.tb16616.x. [DOI] [PubMed] [Google Scholar]
- Brennan R. G., Roderick S. L., Takeda Y., Matthews B. W. Protein-DNA conformational changes in the crystal structure of a lambda Cro-operator complex. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8165–8169. doi: 10.1073/pnas.87.20.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier D., Roovers M., Van Vliet F., Boyen A., Cunin R., Nakamura Y., Glansdorff N., Piérard A. Arginine regulon of Escherichia coli K-12. A study of repressor-operator interactions and of in vitro binding affinities versus in vivo repression. J Mol Biol. 1992 Jul 20;226(2):367–386. doi: 10.1016/0022-2836(92)90953-h. [DOI] [PubMed] [Google Scholar]
- Chou W. Y., Matthews K. S. Serine to cysteine mutations in trp repressor protein alter tryptophan and operator binding. J Biol Chem. 1989 Nov 5;264(31):18314–18319. [PubMed] [Google Scholar]
- Fernando T., Royer C. Role of protein--protein interactions in the regulation of transcription by trp repressor investigated by fluorescence spectroscopy. Biochemistry. 1992 Apr 7;31(13):3429–3441. doi: 10.1021/bi00128a018. [DOI] [PubMed] [Google Scholar]
- Hurlburt B. K., Yanofsky C. Enhanced operator binding by trp superrepressors of Escherichia coli. J Biol Chem. 1990 May 15;265(14):7853–7858. [PubMed] [Google Scholar]
- Kelley R. L., Yanofsky C. Mutational studies with the trp repressor of Escherichia coli support the helix-turn-helix model of repressor recognition of operator DNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):483–487. doi: 10.1073/pnas.82.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klig L. S., Carey J., Yanofsky C. trp repressor interactions with the trp aroH and trpR operators. Comparison of repressor binding in vitro and repression in vivo. J Mol Biol. 1988 Aug 20;202(4):769–777. doi: 10.1016/0022-2836(88)90557-8. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kumamoto A. A., Miller W. G., Gunsalus R. P. Escherichia coli tryptophan repressor binds multiple sites within the aroH and trp operators. Genes Dev. 1987 Aug;1(6):556–564. doi: 10.1101/gad.1.6.556. [DOI] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Templin A., Clark A. J. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci U S A. 1971 Apr;68(4):824–827. doi: 10.1073/pnas.68.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane A. N., Jardetzky O. NMR studies of the trp repressor from Escherichia coli. Characterisation and assignments of residue types. Eur J Biochem. 1985 Oct 15;152(2):395–404. doi: 10.1111/j.1432-1033.1985.tb09210.x. [DOI] [PubMed] [Google Scholar]
- Lehnherr H., Velleman M., Guidolin A., Arber W. Bacteriophage P1 gene 10 is expressed from a promoter-operator sequence controlled by C1 and Bof proteins. J Bacteriol. 1992 Oct;174(19):6138–6144. doi: 10.1128/jb.174.19.6138-6144.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi B. F., Sigler P. B. The stereochemistry and biochemistry of the trp repressor-operator complex. Biochim Biophys Acta. 1990 Apr 6;1048(2-3):113–126. doi: 10.1016/0167-4781(90)90047-6. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z., Schevitz R. W., Zhang R. G., Lawson C. L., Joachimiak A., Marmorstein R. Q., Luisi B. F., Sigler P. B. Crystal structure of trp repressor/operator complex at atomic resolution. Nature. 1988 Sep 22;335(6188):321–329. doi: 10.1038/335321a0. [DOI] [PubMed] [Google Scholar]
- Paluh J. L., Yanofsky C. High level production and rapid purification of the E. coli trp repressor. Nucleic Acids Res. 1986 Oct 24;14(20):7851–7860. doi: 10.1093/nar/14.20.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Yanofsky C. Interaction of the operator of the tryptophan operon with repressor. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3134–3138. doi: 10.1073/pnas.71.8.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha R., Banik U., Bandopadhyay S., Mandal N. C., Bhattacharyya B., Roy S. An operator-induced conformational change in the C-terminal domain of the lambda repressor. J Biol Chem. 1992 Mar 25;267(9):5862–5867. [PubMed] [Google Scholar]
- Schevitz R. W., Otwinowski Z., Joachimiak A., Lawson C. L., Sigler P. B. The three-dimensional structure of trp repressor. 1985 Oct 31-Nov 6Nature. 317(6040):782–786. doi: 10.1038/317782a0. [DOI] [PubMed] [Google Scholar]
- Schweizer H. P. The pUC18CM plasmids: a chloramphenicol resistance gene cassette for site-directed insertion and deletion mutagenesis in Escherichia coli. Biotechniques. 1990 Jun;8(6):612-3, 616. [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Shimizu N., Hayashi M. In vitro repression of transcription of the tryptophan operon by trp repressor. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1990–1994. doi: 10.1073/pnas.70.7.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville R. The Trp repressor, a ligand-activated regulatory protein. Prog Nucleic Acid Res Mol Biol. 1992;42:1–38. doi: 10.1016/s0079-6603(08)60572-3. [DOI] [PubMed] [Google Scholar]
- Squires C. L., Lee F. D., Yanofsky C. Interaction of the trp repressor and RNA polymerase with the trp operon. J Mol Biol. 1975 Feb 15;92(1):93–111. doi: 10.1016/0022-2836(75)90093-5. [DOI] [PubMed] [Google Scholar]
- Squires C. L., Rose J. K., Yanofsky C., Yang H. L., Zubay G. Tryptophanyl-tRNA and tryptophanyl-tRNA synthetase are not required for in vitro repression of the tryptophan operon. Nat New Biol. 1973 Oct 3;245(144):131–133. doi: 10.1038/newbio245131a0. [DOI] [PubMed] [Google Scholar]
- Staacke D., Walter B., Kisters-Woike B., von Wilcken-Bergmann B., Müller-Hill B. How Trp repressor binds to its operator. EMBO J. 1990 Jun;9(6):1963–1967. doi: 10.1002/j.1460-2075.1990.tb08324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Tan S., Richmond T. J. DNA binding-induced conformational change of the yeast transcriptional activator PRTF. Cell. 1990 Jul 27;62(2):367–377. doi: 10.1016/0092-8674(90)90373-m. [DOI] [PubMed] [Google Scholar]
- Tasayco M. L., Carey J. Ordered self-assembly of polypeptide fragments to form nativelike dimeric trp repressor. Science. 1992 Jan 31;255(5044):594–597. doi: 10.1126/science.1736361. [DOI] [PubMed] [Google Scholar]
- Tsapakos M. J., Haydock P. V., Hermodson M., Somerville R. L. Ligand-mediated conformational changes in Trp repressor protein of Escherichia coli probed through limited proteolysis and the use of specific antibodies. J Biol Chem. 1985 Dec 25;260(30):16383–16394. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Velleman M., Heinzel T., Schuster H. The Bof protein of bacteriophage P1 exerts its modulating function by formation of a ternary complex with operator DNA and C1 repressor. J Biol Chem. 1992 Jun 15;267(17):12174–12181. [PubMed] [Google Scholar]
- Zubay G., Morse D. E., Schrenk W. J., Miller J. H. Detection and isolation of the repressor protein for the tryptophan operon of Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1100–1103. doi: 10.1073/pnas.69.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]