Abstract
Punta Toro virus (PTV), a member of the PTV complex, is a relatively common causative agent of febrile illness in Panama that is often misdiagnosed as ‘dengue’ or ‘influenza’. Currently, only two named members make up this species complex, PTV and Buenaventura virus (BUEV). Genomic and antigenic characterization of 17 members of the PTV complex, nine of which were isolated from human acute febrile illness cases, reveals that this species complex is composed of six distant viruses. We propose to add four additional new viruses, designated Leticia virus, Cocle virus, Campana virus and Capira virus.
The family Bunyaviridae is currently divided into five genera: Orthobunyavirus, Nairovirus, Hantavirus, Phlebovirus and Tospovirus (Nichol et al., 2005) comprising more than 350 different virus species. Human pathogens are found in each of the genera, except for the tospoviruses, which only infect plants. Genomes from Bunyaviridae include three unique molecules of negative or ambisense ssRNA, designated L (large), M (medium) and S (small) with a combined length of 11–19 kb. Viruses in each genus share similar segment and structural protein sizes and have characteristic terminal sequences at the 3′ and 5′ ends of each segment. As with other segmented virus families, genetic reassortment is frequent and has been demonstrated among related bunyaviruses both in vitro and in vivo (Briese et al., 2013; Henderson et al., 1995; Li et al., 1995; Pringle et al., 1984; Rodriguez et al., 1998).
The genus Phlebovirus comprises approximately 70 named viruses that are classified (based on their antigenic, genomic and/or vector relationships) into two broad groups: the Sandfly fever group, which includes Rift Valley fever and Toscana viruses and is transmitted by phlebotomine sandflies and mosquitoes; and the Uukuniemi group (Nichol et al., 2005), which are tick-borne and include three newly emerging viruses of public health importance, severe fever with thrombocytopenia syndrome (Yu et al., 2011), Heartland (McMullan et al., 2012) and Bhanja viruses (Matsuno et al., 2013). Recently, a third distinct lineage (group) within the genus Phlebovirus was described and is composed of two mosquito-specific viruses, Gouleako virus (Marklewitz et al., 2011) and Cumuto virus (Auguste et al., 2014). Because of the public health importance of some viruses in the genus Phlebovirus and in an effort to develop a more precise taxonomic system for classification of the phleboviruses, we have attempted to sequence all of the available named viruses in the genus in order to determine their phylogenetic relationships. The current report is the sixth in a series of publications describing this work (Palacios et al., 2011a, b, 2013a, b, 2014), and it covers members of the Punta Toro (PTV) species complex.
The viruses from the PTV species complex used in this study were obtained from the World Reference Center for Emerging Viruses and Arboviruses at the University of Texas Medical Branch (Table 1). The Balliet strain of PTV was isolated in 1966 from the blood of a febrile soldier involved in jungle warfare training in Colon Province, in the former Panama Canal Zone (Centers for Disease Prevention and Control, 2015). A second isolate of PTV, designated the Adames strain, was isolated in 1972 from the blood of an entomologist who developed a febrile illness during a collecting trip to a forested area of Darien Province (R. B. Tesh, unpublished data). Both of these individuals had illnesses characterized by sudden onset of fever, headache, weakness, back and retroorbital pain of 3–4 days duration, symptoms similar to that of classical sandfly or phlebotomus fever (Bartelloni & Tesh, 1976). PTV strains PaAR 2381, GML 902876 and GML 902878 were isolated from sandflies and sentinel hamsters during arbovirus field studies by Gorgas Memorial Institute in the Bayano district of Panama in 1975–1976. The remaining six PTV strains were obtained between 1992 and 2004 from sera of febrile patients attending clinics in and around Panama City, as part of dengue surveillance programs. Few isolates of the other five Punta Toro complex viruses are available, thus consequently less information is available about them. Two isolates of Buenaventura virus (BUEV) were obtained from sandflies (Lutzomyia sp.) collected in forested areas on the Pacific Coast of Colombia near the city of Buenaventura during arbovirus field studies in 1964 and 1984 (Centers for Disease Prevention and Control, 2015; Tesh et al., 1986). A single isolate of Leticia virus (LETV) was obtained from sandflies collected in another forested area near the city of Leticia, Amazonas department in the south-east corner of Colombia. A single isolate of Cocle virus (CCLV) was made from the serum of a febrile patient in Penonome, Cocle province, Panama, during a dengue surveillance program. Single isolates of Campana virus (CMAV) and Capira virus (CAPV) were made in 1970 from sandflies collected in a shaded coffee farm adjacent to the community of El Aguacate near the Altos de Compana National Park and Biological Reserve in Panama, during arbovirus field studies (Tesh et al., 1974).
Table 1. Names, abbreviations, strain numbers, sources, dates and locality of isolation and accession numbers of the viruses used in this study.
| Virus name | Abbreviation | Strain | Year of isolation | Source of isolate | Location | Accession numbers |
|---|---|---|---|---|---|---|
| Buenaventura virus | BUEV | CoAr 170255 | 1984 | Sandfly (Lutzomia) | Buenaventura, Valle del Cauca, Colombia | HM566149–HM566151 |
| Buenaventura virus | BUEV | CoAr 3319 | 1964 | Sandfly | Rio Raposo, Buenaventura, Valle del Cauca, Colombia | KP272001–KP272003 |
| Punta Toro virus | PTV | Adames | 1972 | Human | Darien, Panama | KP272028–KP272030 |
| Punta Toro virus | PTV | Balliet | 1966 | Human | Colon, Panama | KP272022–KP272024 |
| Punta Toro virus | PTV | GML 488778 | 2004 | Human | Panama | KP272037–KP272039 |
| Punta Toro virus | PTV | GML 488831 | 2004 | Human | Panama | KP272031–KP272033 |
| Punta Toro virus | PTV | GML 902876 | 1976 | Sentinel hamster | Bayano, Panama Pr., Panama | KP272010–KP272012 |
| Punta Toro virus | PTV | GML 902878 | 1976 | Sentinel hamster | Bayano, Panama Pr., Panama | KP272019–KP272021 |
| Punta Toro virus | PTV | PaAR 2381 | 1975 | Sandfly | Bayano, Panama Pr., Panama | KP272004–KP272006 |
| Punta Toro virus | PTV | PAN 472868 | 1996 | Human | Panama Pr., Panama | KP272025–KP272027 |
| Punta Toro virus | PTV | PAN 478718 | 1998 | Human | San Miguelito, Panama Pr., Panama | KP272016–KP272018 |
| Punta Toro virus | PTV | PAN 479603 | 1999 | Human | Panama Pr., Panama | KP272013–KP272015 |
| Punta Toro virus | PTV | PAN 483391 | 2000 | Human | San Miguelito, Panama Pr., Panama | KP272007–KP272009 |
| Leticia virus | LETV | CoAr 171616 | 1987 | Sandfly | Leticia, Amazonas, Colombia | HM566152–HM566154 |
| Cocle virus | CCLV | GML 244915 | 2009 | Human | Penonome, Cocle, Panama | KP272034–KP272036 |
| Campana virus | CMAV | VP-334K | 1970 | Sandfly | El Aguacate, Panama Pr., Panama | KP272042–KP272042 |
| Capira virus | CMAV | VP-366G | 1970 | Sandfly | El Aguacate, Panama Pr., Panama | KP272043–KP272045 |
Whole genome sequencing was completed for all viruses in Table 1 using viral stocks prepared in Vero cells. RNA was extracted using TRIzol LS (Invitrogen). Amplification of cDNA was completed as previous described (Palacios et al., 2008) and was sequenced on a 454 Genome Sequencer FLX without fragmentations (Cox-Foster et al., 2007; Margulies et al., 2005; Palacios et al., 2008). Sequence gaps were completed by PCR by using primers based on pyrosequencing data and sequenced on an ABI Prism 3700 DNA Analysers (Perkin-Elmer Applied Biosystems). For the termini of each segment, a primer with the 8 nt conserved sequence was used for a specific reverse transcription reaction with additional arbitrary nucleotides on the 5′ end (5′-AAGCAGTGGTATCAACGCAGAGTACACACAAAG-3′; the boldface portion indicates the conserved nucleotides). This primer is designed to bind to the 3′ end of the genomic RNA and the 3′ end of the mRNA. The sequences of the genomes were verified by classical dideoxy sequencing by using primers designed from the draft sequence to create products of 1000 bp with 500 bp overlap.
The sequencing data revealed that the genome organization of the 17 Punta Toro complex (PTC) viruses is consistent with other members of the genus Phlebovirus. The genomes encode six proteins: an RNA polymerase (L segment), two glycoproteins and a non-structural protein (GN, GC and NSm; M segment), and the nucleocapsid protein (N) and, in an ambisense orientation, a second non-structural protein (NSs) (S segment). The 3′ terminal sequence was obtained for 44 segments (16 different viruses) and the 5′ terminal sequence was obtained for 43 segments (16 different viruses). In all cases, the ten most terminal nucleotides were identical to those that have previously been reported for the genus (Plyusnin et al., 2012). The L ORF ranged in size from 6255 to 6264 nt. The M segment ranged in size from 3852 to 3942 nt. The size of the N protein was 732 nt, while the NSs ORF ranged from 753 to 795 nt. A similar pattern of conservation was observed among areas of the RNA-dependent RNA polymerase, signal sequences, transmembrane domains, cleavage sites for the cellular signal signallase protease and Golgi retention signals for the GN and GC, in comparison with all other phleboviruses, confirming an association with function (Palacios et al., 2011b, 2013a, b in comparison with all other phleboviruses, confirming an asso).
For phylogenetic analysis, a set of phlebovirus sequences (145 for the L segment, 187 for the M segment, 210 for the N gene, and 167 for the NS gene) comprising all nucleotide (partial or complete) sequences from GenBank available on 1 November 2013 were aligned, along with our sequences, using the clustal algorithm (as implemented in the mega package version 5) at the amino acid level with additional manual editing to ensure the highest possible quality of alignment. Neighbour-joining (NJ) analysis at the amino acid level was performed due to the observed high variability of the underlying nucleotide sequences. Given the saturation observed in all the alignments, the phylogenetic trees obtained by analysis of all members of the genus were used to define the species complexes; while additional phylogenetic analysis restricted to the PTC virus sequences was used to resolve the fine topology of the group. The statistical significance of tree topology was evaluated by bootstrap resampling of the sequences 1000 times. Phylogenetic analyses were performed by using mega software (Tamara et al., 2011).
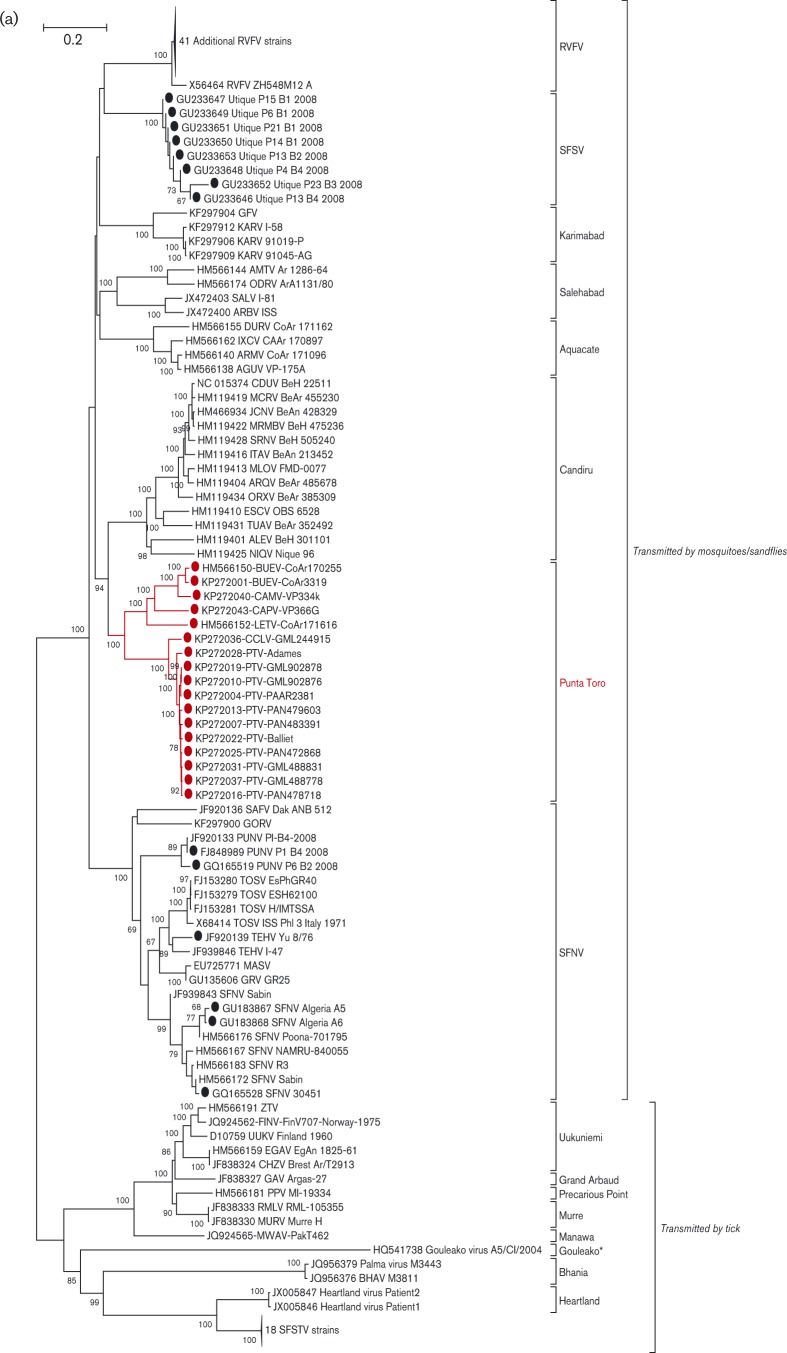
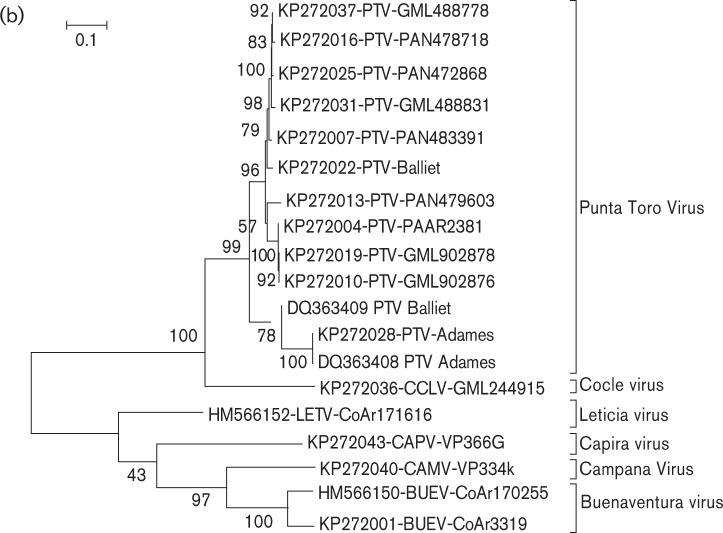
Phylogenetic analyses of the L, M and S gene segment sequences of the 17 PTC viruses (strains CoAr 171616, CoAr 170255, CoAr 3319, Adames, Baillet, GML 488778, GML488831, GML902876, GML902878, PaAr2381, PAN472868, PAN478718, PAN479603, PAN483391, VP334K, GML244915 and VP366G) are consistent with earlier reports, confirming that phleboviruses belonging to the same species complex cluster together (Charrel et al., 2009; Collao et al., 2010). As anticipated, based on their cross-reactivity in complement fixation (CF) tests (Bishop et al., 1980), members of the Punta Toro species complex generally cluster together (Figs. 1a, and S1a–c available with the online Supplementary Material). Based on L-, M- and S-segment sequences, four of the viruses sequenced here (VP334K, VP366K, GML244915 and CoAr171616) are distinct from the PTV or BUEV clades of PTC viruses (Figs. 1b and S1a–c). In fact, when compared with the sequence data now available for other phleboviruses, they exhibit similar levels of divergence to other named viruses in the Phlebovirus genus. No genus-wide framework has yet been proposed for determining genetically how phleboviruses should be uniquely named; however, based on the levels of genetic divergence among currently named viruses in this genus, VP334K; VP366G; GML244915 and CoAr 171616 should probably be assigned their own unique names. Accordingly, we propose the following names and abbreviations for the four viruses: VP334K to be named Campana virus (CMAV) for Altos de Campara National Park and Biological Reserve near where the virus was discovered; VP-366G to be named Capira virus (CAPV) for the Panamanian district of Capira where the virus was found; GML 244915 to be named Cocle virus for the Cocle province in Panama where the patient yielding the virus lived; and CoAr 171616 to be named Leticia virus (LETV) for the town in Colombia near where the infected sandflies were collected.
Fig. 1. Phylogenetic analysis of the available sequences of phlebovirus L ORF. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (100 replicates) are shown next to the branches. (a) The evolutionary distances are in the units of number of amino acid substitutions per site. Sequences marked with black dots corresponded to partial sequences. Sequences marked with red dots corresponded to sequences obtained during this work. Only partial (when only available for the species) or complete ORF sequences were included in the analysis. Non-coding regions were excluded. Bar, 0.2. *Gouleako virus was actually recovered from mosquitoes. (b) The evolutionary distances are in the units of number of nucleic acids substitutions per site. Phylogenetic analysis of all members of the Punta Toro species complex L segments by maximum-likelihood method. The evolutionary history was inferred by using the maximum-likelihood method based on the General Time Reversible model (Tamara et al., 2011). The tree with the highest log-likelihood ( − 6244.0302) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. A discrete Gamma distribution was used to model evolutionary rate differences among sites [five categories (+G, parameter = 1.2055)]. The rate variation model allowed for some sites to be evolutionarily invariable (+I, 49.3028 % sites). Bar, 0.1.
Systematic screening for the presence of recombination patterns was pursued by using the nucleotide alignments and the Recombination Detection Program (RDP) (Martin & Rybicki, 2000), Bootscan (Salminen et al., 1995), MaxChi (Smith, 1992), Chimeara (Posada & Crandall, 2001), lard (Holmes, 1998) and phylip Plot (Felsenstein, 1989). Segment reassortment in bunyaviruses has been reported with increasing frequency, especially in the genus Orthobunyavirus (Bowen et al., 2001; Briese et al., 2006, 2007; Burt et al., 2009; Collao et al., 2010; Iroegbu & Pringle, 1981; Kondiah et al., 2010; Nunes et al., 2005; Saeed et al., 2001; Yanase et al., 2006, 2010). Previously, we reported that the frequency of reassortment in the Candiru species complex of the genus Phlebovirus (5 of 13 named viruses) was unprecedented (Palacios et al., 2011b). In contrast, our analysis of members of the Uukuniemi group did not indicate any reassortment events (Palacios et al., 2013b). No evidence of PTV reassortment was found in topological analysis of phylogenetic trees (Figs. 1b and S2a–c) or by RDP, Bootscan, MaxChi, lard and phylip Plot analysis (data not shown).
In addition to whole genome sequencing, CF tests were also performed with eight of the PTC viruses (Table 2). Antigens used in CF tests were prepared from infected newborn mouse brains by the sucrose/acetone extraction method (Beaty et al., 1989) or from frozen harvests of infected cultures of Vero cells. Antigens for preparing hyperimmune ascitic fluids (HIAF) against the PTC viruses were 10 % crude suspensions of homogenized infected newborn mouse brain mixed with Freund's adjuvant. The immunization schedule consisted of four intraperitoneal injections given at weekly intervals. Sarcoma 180 cells were given with the final immunization to induce ascites formation. Since some PTC viruses were not lethal to newborn born, it was not possible to prepare ‘clean’ HIAF, and only a one-way CF test could be done with a Vero cell antigen. All animal work was carried out under an animal protocol approved by the University of Texas Medical Branch IACUC committee. CF tests were performed by a microtitre technique (Beaty et al., 1989) using 2 U of guinea pig complement and overnight incubation of the antigen and antibody at 4 °C. CF titres were recorded as the highest dilutions giving 3+ or 4+ fixation of complement (0–25 % haemolysis). By this method, there was broad cross-reaction among the various antigens and antibodies and no distinctive pattern could be determined. Nevertheless, given that the CF tests correlate mostly with the N protein reactivity, the antigen–antiserum relationships between the BUEV and CAMV, and PTV and CCLV, viruses appears to correlate with their phylogenetic positioning.
Table 2. Results of CF tests with selected Punta Toro complex virus strains.
Bold indicates same species and italics indicates cross-reactivtiy
| Antigen | Antibody | ||||
|---|---|---|---|---|---|
| BUEV | BUEV | PTV | PTV | LETV | |
| Co Ar 3319 | Co Ar 170255 | Balliet | Adames | Co Ar 171616 | |
| BUEV Co Ar 3319 | 1024* | 512 | 512 | 32 | 128 |
| BUEV Co Ar 170255 | 1024 | 512 | 512 | 32 | 128 |
| CAMV VP 334K | 1024 | 256 | 512 | 32 | 128 |
| PTV Balliet | 256 | 32 | 1024 | 128 | 256 |
| PTV Adames | 256 | 16 | 1024 | 128 | 138 |
| CCLV GML244915 | 256 | 32 | 1024 | 64 | 128 |
| LETV Co Ar 171616 | 256 | 32 | 512 | 32 | 512 |
| CAPV VP 366G | 256 | 32 | 512 | 32 | 256 |
Reciprocal of serum titre at optimal dilution of antigen.
We provide here the full genomes of 17 members of the Punta Toro species complex. It is significant that all of these viruses replicate and produce viral cytopathic effect in cultures of Vero cells. Nine of the total isolates (PTV and CCLV only) were isolated from humans with acute febrile illness (Table 1), and of these most were obtained during dengue surveillance programs from acute phase sera of suspected dengue cases. Since most dengue infections in tropical America are diagnosed clinically and laboratory confirmation is not done, it seems likely that human infections with PTC viruses in Panama and probably in Colombia are more frequent than is now being recognized. In summary, our studies indicate that the Punta Toro phlebovirus complex consists of six related viruses that occur in Panama and Colombia. From a public health perspective, PTV is by far the most important, and the full genomes of other PTC viruses will help in addressing whether these viruses are also having an impact on public health.
Acknowledgements
Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the U.S. Army. This work was supported by the Defense Threat Reduction Agency no. 1881290, and the United States Department of Defense, Google.org, National Institutes of Health award AI57158 (North-east Biodefence Center – Lipkin), and USAID PREDICT funding source code 07-301-7119-52258 (Center for Infection and Immunity). R. T., A. T. R. and H. G. were supported by NIH contract HHSN272201000040I/HHSN27200004/D04. J. P. C. was supported by Secretaria Nacional de Ciencia y Tecnologia e Inovación, Panama code: 60-4-FID09-103.
Supplementary Data
Supplementary Data
References
- Auguste A.J., Carrington C.V., Forrester N.L., Popov V.L., Guzman H., Widen S.G., Wood T.G., Weaver S.C., Tesh R.B. (2014). Characterization of a novel Negevirus and a novel Bunyavirus isolated from Culex (Culex) declarator mosquitoes in Trinidad J Gen Virol 95 481–485 10.1099/vir.0.058412-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelloni P.J., Tesh R.B. (1976). Clinical and serologic responses of volunteers infected with phlebotomus fever virus (Sicilian type) Am J Trop Med Hyg 25 456–462 . [DOI] [PubMed] [Google Scholar]
- Beaty B., Calisher C.H., Shope R.E. (1989). Arboviruses. In Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections, pp. 979–1005. Edited by Schmidt N. J., Emmons R. W. Washington, DC: American Public Health Association. [Google Scholar]
- Bishop D.H., Calisher C.H., Casals J., Chumakov M.P., Gaidamovich S.Y., Hannoun C., Lvov D.K., Marshall I.D., Oker-Blom N., other authors (1980). Bunyaviridae Intervirology 14 125–143 10.1159/000149174 . [DOI] [PubMed] [Google Scholar]
- Bowen M.D., Trappier S.G., Sanchez A.J., Meyer R.F., Goldsmith C.S., Zaki S.R., Dunster L.M., Peters C.J., Ksiazek, other authors (2001). A reassortant bunyavirus isolated from acute hemorrhagic fever cases in Kenya and Somalia Virology 291 185–190 10.1006/viro.2001.1201 . [DOI] [PubMed] [Google Scholar]
- Briese T., Bird B., Kapoor V., Nichol S.T., Lipkin W.I. (2006). Batai and Ngari viruses: M segment reassortment and association with severe febrile disease outbreaks in East Africa J Virol 80 5627–5630 10.1128/JVI.02448-05 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briese T., Kapoor V., Lipkin W.I. (2007). Natural M-segment reassortment in Potosi and Main Drain viruses: implications for the evolution of orthobunyaviruses Arch Virol 152 2237–2247 10.1007/s00705-007-1069-z . [DOI] [PubMed] [Google Scholar]
- Briese T., Calisher C.H., Higgs S. (2013). Viruses of the family Bunyaviridae: are all available isolates reassortants? Virology 446 207–216 10.1016/j.virol.2013.07.030 . [DOI] [PubMed] [Google Scholar]
- Burt F.J., Paweska J.T., Ashkettle B., Swanepoel R. (2009). Genetic relationship in southern African Crimean-Congo haemorrhagic fever virus isolates: evidence for occurrence of reassortment Epidemiol Infect 137 1302–1308 10.1017/S0950268808001878 . [DOI] [PubMed] [Google Scholar]
- Centers for Disease Prevention and Control (2015). The International Catalogue of Arboviruses Including Certain Other Viruses of Vertebrates https://wwwn.cdc.gov/arbocat/default.aspx. [Google Scholar]
- Charrel R.N., Moureau G., Temmam S., Izri A., Marty P., Parola P., da Rosa A.T., Tesh R.B., de Lamballerie X. (2009). Massilia virus, a novel Phlebovirus (Bunyaviridae) isolated from sandflies in the Mediterranean Vector Borne Zoonotic Dis 9 519–530 10.1089/vbz.2008.0131 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collao X., Palacios G., de Ory F., Sanbonmatsu S., Pérez-Ruiz M., Navarro J.M., Molina R., Hutchison S.K., Lipkin W.I., other authors (2010). Granada virus: a natural phlebovirus reassortant of the sandfly fever Naples serocomplex with low seroprevalence in humans Am J Trop Med Hyg 83 760–765 10.4269/ajtmh.2010.09-0697 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox-Foster D.L., Conlan S., Holmes E.C., Palacios G., Evans J.D., Moran N.A., Quan P.L., Briese T., Hornig M., other authors (2007). A metagenomic survey of microbes in honey bee colony collapse disorder Science 318 283–287 10.1126/science.1146498 . [DOI] [PubMed] [Google Scholar]
- Felsenstein J. (1989). phylip - phylogeny inference package (version 3.2) Cladistics 5 164–166. [Google Scholar]
- Henderson W.W., Monroe M.C., St Jeor S.C., Thayer W.P., Rowe J.E., Peters C.J., Nichol S.T. (1995). Naturally occurring Sin Nombre virus genetic reassortants Virology 214 602–610 10.1006/viro.1995.0071 . [DOI] [PubMed] [Google Scholar]
- Holmes E.C. (1998). Molecular epidemiology of dengue virus—the time for big science Trop Med Int Health 3 855–856 10.1046/j.1365-3156.1998.00332.x . [DOI] [PubMed] [Google Scholar]
- Iroegbu C.U., Pringle C.R. (1981). Genetic interactions among viruses of the Bunyamwera complex J Virol 37 383–394 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondiah K., Swanepoel R., Paweska J.T., Burt F.J. (2010). A simple-probe real-time PCR assay for genotyping reassorted and non-reassorted isolates of Crimean-Congo hemorrhagic fever virus in southern Africa J Virol Methods 169 34–38 10.1016/j.jviromet.2010.06.010 . [DOI] [PubMed] [Google Scholar]
- Li D., Schmaljohn A.L., Anderson K., Schmaljohn C.S. (1995). Complete nucleotide sequences of the M and S segments of two hantavirus isolates from California: evidence for reassortment in nature among viruses related to hantavirus pulmonary syndrome Virology 206 973–983 10.1006/viro.1995.1020 . [DOI] [PubMed] [Google Scholar]
- Margulies M., Egholm M., Altman W.E., Attiya S., Bader J.S., Bemben L.A., Berka J., Braverman M.S., Chen Y.J., other authors (2005). Genome sequencing in microfabricated high-density picolitre reactors Nature 437 376–380 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklewitz M., Handrick S., Grasse W., Kurth A., Lukashev A., Drosten C., Ellerbrok H., Leendertz F.H., Pauli G., Junglen S. (2011). Gouleako virus isolated from West African mosquitoes constitutes a proposed novel genus in the family Bunyaviridae J Virol 85 9227–9234 10.1128/JVI.00230-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D., Rybicki E. (2000). RDP: detection of recombination amongst aligned sequences Bioinformatics 16 562–563 10.1093/bioinformatics/16.6.562 . [DOI] [PubMed] [Google Scholar]
- Matsuno K., Weisend C., Travassos da Rosa A.P., Anzick S.L., Dahlstrom E., Porcella S.F., Dorward D.W., Yu X.J., Tesh R.B., Ebihara H. (2013). Characterization of the Bhanja serogroup viruses (Bunyaviridae): a novel species of the genus Phlebovirus and its relationship with other emerging tick-borne phleboviruses J Virol 87 3719–3728 10.1128/JVI.02845-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullan L.K., Folk S.M., Kelly A.J., MacNeil A., Goldsmith C.S., Metcalfe M.G., Batten B.C., Albariño C.G., Zaki S.R., other authors (2012). A new phlebovirus associated with severe febrile illness in Missouri N Engl J Med 367 834–841 10.1056/NEJMoa1203378 . [DOI] [PubMed] [Google Scholar]
- Nichol S.T., Beaty B.J., Elliott R.M., Goldbach R., Plyusin A., Schmaljohn C., Tesh R. (2005). Bunyaviridae. In Virus Taxonomy. Eight Report of the International Committee on Taxonomy of Viruses, pp. 695–716. Edited by Fauquet C. M., Mayo M. A., Maniloff J., Desselberger U., Ball L. A. San Diego, CA: Elsevier Academic Press. [Google Scholar]
- Nunes M.R., Travassos da Rosa A.P., Weaver S.C., Tesh R.B., Vasconcelos P.F. (2005). Molecular epidemiology of group C viruses (Bunyaviridae Orthobunyavirus) isolated in the Americas J Virol 79 10561–10570 10.1128/JVI.79.16.10561-10570.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios G., Druce J., Du L., Tran T., Birch C., Briese T., Conlan S., Quan P.L., Hui J., other authors (2008). A new arenavirus in a cluster of fatal transplant-associated diseases N Engl J Med 358 991–998 10.1056/NEJMoa073785 . [DOI] [PubMed] [Google Scholar]
- Palacios G., da Rosa A.T., Savji N., Sze W., Wick I., Guzman H., Hutchison S., Tesh R., Lipkin W.I. (2011a). Aguacate virus, a new antigenic complex of the genus Phlebovirus (family Bunyaviridae) J Gen Virol 92 1445–1453 10.1099/vir.0.029389-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios G., Tesh R., Travassos da Rosa A., Savji N., Sze W., Jain K., Serge R., Guzman H., Guevara C., other authors (2011b). Characterization of the Candiru antigenic complex (Bunyaviridae Phlebovirus), a highly diverse and reassorting group of viruses affecting humans in tropical America J Virol 85 3811–3820 10.1128/JVI.02275-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios G., Savji N., Travassos da Rosa A., Desai A., Sanchez-Seco M.P., Guzman H., Lipkin W.I., Tesh R. (2013a). Characterization of the Salehabad virus species complex of the genus Phlebovirus (Bunyaviridae) J Gen Virol 94 837–842 10.1099/vir.0.048850-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios G., Savji N., Travassos da Rosa A., Guzman H., Yu X., Desai A., Rosen G.E., Hutchison S., Lipkin W.I., Tesh R. (2013b). Characterization of the Uukuniemi virus group (Phlebovirus Bunyaviridae): evidence for seven distinct species J Virol 87 3187–3195 10.1128/JVI.02719-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios G., Tesh R., Savji N., Travassos da Rosa A.P., Guzman H., Bussetti A.V., Desai A., Ladner J., Sanchez-Seco M., Lipkin W.I. (2014). Characterization of the Sandfly fever Naples species complex (genus Phlebovirus, family Bunyaviridae) J Gen Virol 95 292–300 10.1099/vir.0.056614-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plyusnin A., Beaty B.J., Elliott R.M., Goldbach R., Kormelink R., Lundkvist A., Schmaljohn C.S., Tesh R.B. (2012). Bunyaviridae . In Virus Taxonomy. Ninth Report of the International Committee on Taxonomy of Viruses, pp. 725–741. Edited by King A. M. Q., Adams M. J., Carstens E. B., Lefkowitz E. J. San Diego, CA: Elsevier Academic Press. [Google Scholar]
- Posada D., Crandall K.A. (2001). Evaluation of methods for detecting recombination from DNA sequences: computer simulations Proc Natl Acad Sci U S A 98 13757–13762 10.1073/pnas.241370698 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C.R., Lees J.F., Clark W., Elliott R.M. (1984). Genome subunit reassortment among Bunyaviruses analysed by dot hybridization using molecularly cloned complementary DNA probes Virology 135 244–256 10.1016/0042-6822(84)90134-X . [DOI] [PubMed] [Google Scholar]
- Rodriguez L.L., Owens J.H., Peters C.J., Nichol S.T. (1998). Genetic reassortment among viruses causing hantavirus pulmonary syndrome Virology 242 99–106 10.1006/viro.1997.8990 . [DOI] [PubMed] [Google Scholar]
- Saeed M.F., Wang H., Suderman M., Beasley D.W., Travassos da Rosa A., Li L., Shope R.E., Tesh R.B., Barrett A.D. (2001). Jatobal virus is a reassortant containing the small RNA of Oropouche virus Virus Res 77 25–30 10.1016/S0168-1702(01)00262-3 . [DOI] [PubMed] [Google Scholar]
- Salminen M.O., Carr J.K., Burke D.S., McCutchan F.E. (1995). Identification of breakpoints in intergenotypic recombinants of HIV type 1 by bootscanning AIDS Res Hum Retroviruses 11 1423–1425 10.1089/aid.1995.11.1423 . [DOI] [PubMed] [Google Scholar]
- Smith J.M. (1992). Analyzing the mosaic structure of genes J Mol Evol 34 126–129 10.1007/BF00182389 . [DOI] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011). mega5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods Mol Biol Evol 28 2731–2739 10.1093/molbev/msr121 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh R.B., Chaniotis B.N., Peralta P.H., Johnson K.M. (1974). Ecology of viruses isolated from Panamanian phlebotomine sandflies Am J Trop Med Hyg 23 258–269 . [DOI] [PubMed] [Google Scholar]
- Tesh R.B., Boshell J., Young D.G., Morales A., Corredor A., Modi G.B., Ferro de Carrasquilla C., de Rodriquez C., Gaitan M.O. (1986). Biology of Arboledas virus, a new phlebotomus fever serogroup virus (Bunyaviridae Phlebovirus) isolated from sand flies in Colombia Am J Trop Med Hyg 35 1310–1316 . [DOI] [PubMed] [Google Scholar]
- Yanase T., Kato T., Yamakawa M., Takayoshi K., Nakamura K., Kokuba T., Tsuda T. (2006). Genetic characterization of Batai virus indicates a genomic reassortment between orthobunyaviruses in nature Arch Virol 151 2253–2260 10.1007/s00705-006-0808-x . [DOI] [PubMed] [Google Scholar]
- Yanase T., Aizawa M., Kato T., Yamakawa M., Shirafuji H., Tsuda T. (2010). Genetic characterization of Aino and Peaton virus field isolates reveals a genetic reassortment between these viruses in nature Virus Res 153 1–7 10.1016/j.virusres.2010.06.020 . [DOI] [PubMed] [Google Scholar]
- Yu X.J., Liang M.F., Zhang S.Y., Liu Y., Li J.D., Sun Y.L., Zhang L., Zhang Q.F., Popov V.L., other authors (2011). Fever with thrombocytopenia associated with a novel bunyavirus in China N Engl J Med 364 1523–1532 10.1056/NEJMoa1010095 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data