Abstract
Resealing allows cells to mend damaged membranes rapidly when plasma membrane (PM) disruptions occur. Models of PM repair mechanisms include the “lipid-patch”, “endocytic removal”, and “macro-vesicle shedding” models, all of which postulate a dependence on local increases in intracellular Ca2+ at injury sites. Multiple calcium sensors, including synaptotagmin (Syt) VII, dysferlin, and apoptosis-linked gene-2 (ALG-2), are involved in PM resealing, suggesting that Ca2+ may regulate multiple steps of the repair process. Although earlier studies focused exclusively on external Ca2+, recent studies suggest that Ca2+ release from intracellular stores may also be important for PM resealing. Hence, depending on injury size and the type of injury, multiple sources of Ca2+ may be recruited to trigger and orchestrate repair processes. In this review, we discuss the mechanisms by which the resealing process is promoted by vesicular Ca2+ channels and Ca2+ sensors that accumulate at damage sites.
Keywords: TRPML1, Ca2+, lysosomal exocytosis, calcium sensor, membrane repair
Introduction
Plasma membrane (PM) disruptions occur in most cells, especially in those residing in mechanically-active environments, such as skeletal and cardiac muscle [1, 2]. Resealing is a repair process that allows cells to mend damaged membranes, preventing the loss of terminally-differentiated cells [1, 2]. Recent studies have suggested that damaged cells are able to restore the lipid bilayer barrier by adding membrane components from intracellular vesicles to the cell surface [3].
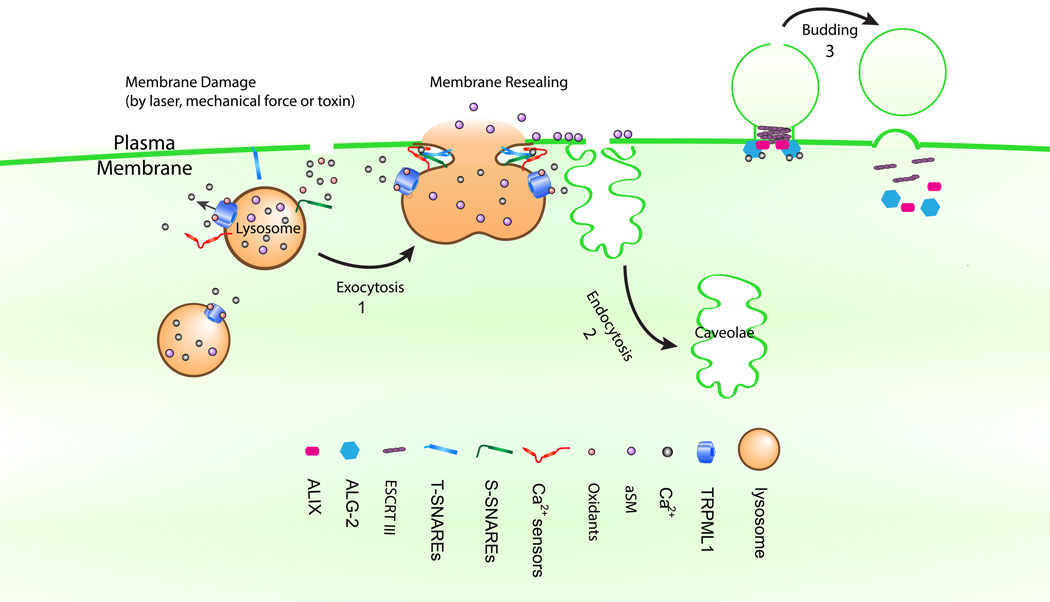
Three distinct mechanisms of PM lesion repair have been described (see Fig. 1). The first is the so-called “lipid-patch” model in which intracellular vesicles fuse with one another to form membrane patches, after which the patches fuse with the PM, thereby mending lesions [4, 5]. Among the intracellular vesicles, lysosomes are the primary candidate [4, 6]. The second mechanism is the so-called “endocytic removal” model, in which membrane lesions are removed through endocytosis [2, 7, 8]. Upon injury, acid sphingomyelinase (aSMase) is secreted to the extracellular space through lysosome exocytosis [2, 7, 9], and then aSMase-mediated hydrolysis of sphingomyelins (SMs) triggers ceramide-driven membrane invagination, mediating lesion removal [2, 7, 9]. The third mechanism is the recently-reported “macro-vesicle shedding” model, in which the damaged membranes undergo “outward” shedding upon injury [10–12]. This process involves the assembly of endosomal sorting complex required for transport (ESCRT) machinery [10, 13] to generate an outward curvature [14]. Depending on cell type, injury size, and type of injury, one or more of the aforementioned repair mechanisms may be recruited.
Fig. 1. Three working models for membrane repair.
In the “lipid-patch” model (1), TRPML1, Syt-VII, dysferlin, and SNAREs participate in membrane repair. Upon the incursion of membrane damage, an influx of oxidants and Ca2+ promotes TRPML1 conducted lysosomal Ca2+ release, activating Syt-VII and other Ca2+ sensors. Subsequently, lysosomal exocytosis is triggered to reseal the disrupted membranes. In the “endocytic removal” model (2), lysosomal exocytosis mediates the release of aSMase to catalyze ceramide-dependent rapid lesion removal by caveolar endocytosis. In the “macro vesicle shedding” model (3), an injury-triggered Ca2+ surge recruits ALG-2 to the injury site. Accumulation of ALG-2 facilitates the assembly of ALIX and ESCRT III at the injury site, resulting in the cleavage and shedding of the damaged span of membrane.
All three of the aforementioned repair process models entail a strict dependence on Ca2+ [1, 2, 7, 8, 10–12]. Membrane damage causes a significant increase in intracellular calcium concentration ([Ca2+]injury) at PM injury sites; [15–17]), and preventing the [Ca2+]injury response with calcium chelators has been shown to block PM repair [15–17]. Furthermore, multiple calcium sensors, including synaptotagmin (Syt) VII and dysferlin, have been shown to promote lysosomal exocytosis in repair models 1 and 2 [4, 6]. In support of repair model 3, the Ca2+-binding protein apoptosis-linked gene-2 (ALG-2) was shown to be essential for recruitment of ESCRT to damage sites [10, 13]. Cytosolic calcium ion concentration [Ca2+] is kept low at rest (~100 nM) in most cells. Conversely, the [Ca2+] in the extracellular space and in intracellular stores [e.g., in endoplasmic reticulum (ER) and endolysosomes] are maintained at 2 mM and 0.5–1 mM, respectively [18, 19]. Both the extracellular space and intracellular stores could contribute to [Ca2+]injury fluxes. However, almost all previous studies have focused on extracellular Ca2+. Very recently, Ca2+ release from intracellular stores was also shown to be important [20]. Hence, depending on injury size and the type of injury, one or both sources of Ca2+ may be used to trigger repair processes. In this review, we discuss the mechanisms by which PM resealing processes are promoted by intracellular Ca2+ channels and Ca2+ sensors.
Calcium acts locally
Virtually all aspects of cellular life are affected by Ca2+, which is appreciated for being an evolutionarily conserved cellular signaling molecule with key functions in synaptic transmission, muscle contraction, granule secretion, gene expression, and membrane repair [21]. Ca2+ adds charge to Ca2+-binding proteins, thereby initiating conformational changes and switching Ca2+ sensor protein functions “on” and “off” [19] [21]. There exist hundreds of Ca2+ sensor proteins with binding affinities in the nM to mM range that are known to trigger a wide variety of Ca2+-sensitive cellular processes [19] [21]. There are 5,000- to 20,000-fold Ca2+ concentration gradients between the cytosol (~100 nM) and extracellular space (~2 mM) or intracellular compartments (0.5–1 mM) [18, 19]. These gradients are established by primary and secondary Ca2+ transporters localized at the cell surface or on the membranes of intracellular organelles [22]. Upon cellular stimulation, Ca2+ enters the cytosol through PM Ca2+ channels and organellar Ca2+ release channels [22].
To activate regulated signaling transduction involving cascades at specific cellular sites, intracellular Ca2+ signaling is shaped by diffusion and cytosolic buffering. Cytosolic buffers restrict the spread of a Ca2+ signal such that it remains close to source channels by reducing and localizing transient [Ca2+] increases [19, 23–25]. Cytosolic buffering can produce ~10 µM to ~100 nM drops in intracellular [Ca2+] over a distance of 30 nm within a few milliseconds [19, 23–25]. Hence, steep [Ca2+] gradients around entry and release sites result in non-homogeneous activation of Ca2+ sensor proteins.
Sources of intracellular calcium flux
Extracellular space
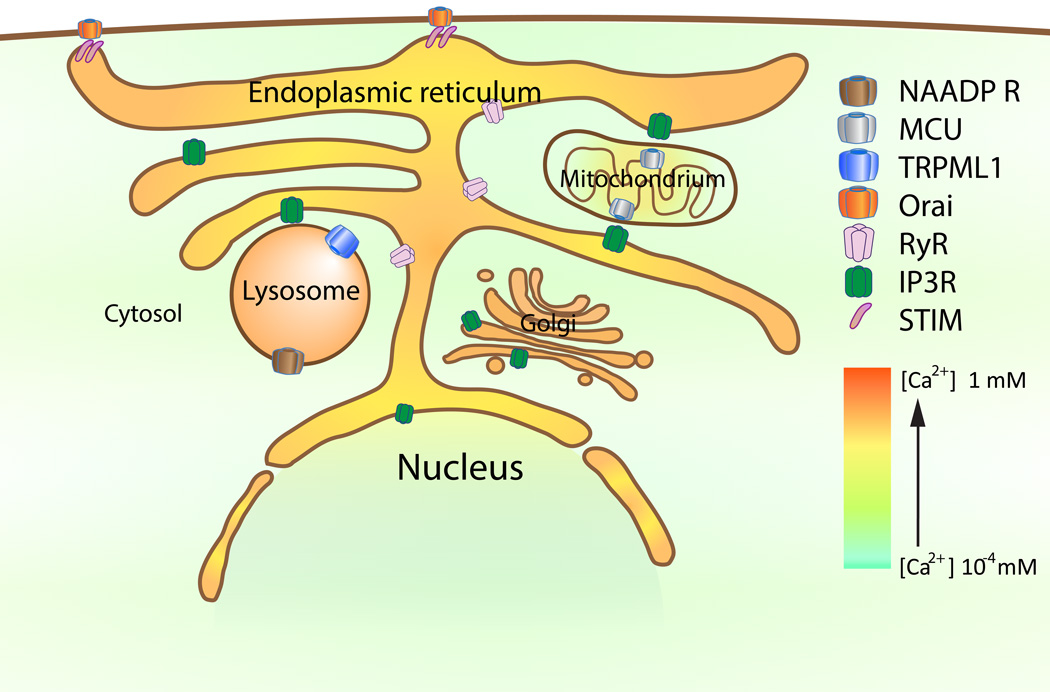
The extracellular space provides a virtually unlimited supply of Ca2+ (~2 mM). Under physiological conditions, PM Ca2+ channels mediate Ca2+ influx upon stimulation. Under certain pathological conditions, PM disruptions may also cause Ca2+ influxes, resulting in [Ca2+]injury [1, 2]. Such increases are also localized and transient due to cytosolic buffering and rapid resealing of membranes [1, 2]. Hence, the extracellular space is assumed to be the primary source of [Ca2+]injury. However, Ca2+ can also be released from specialized Ca2+ storage organelles [19], including the ER, endosomes, lysosomes, Golgi apparatus, and mitochondria (see Fig. 2).
Fig. 2. Intracellular Ca2+ signaling pathways.
ER Ca2+ depletion induces cytosolic entry of Ca2+ through the store-operated Ca2+ influx pathway, which is formed by the ER-localization stromal-interacting molecule 1 (STIM1) and the plasma membrane-localized Orai channels. Ca2+-mediated exchange between ER and mitochondria is dependent on the IP3R and the mitochondria Ca2+ uniporter (MCU). Ca2+ released through lysosomal TRPML1–3 channels and NAADP receptors may activate ER IP3R and RyRs to release more Ca2+.
ER
The ER, the largest intracellular Ca2+ store, is a heterogeneous organelle with a non-uniform distribution of Ca2+ [18, 19]. ER luminal [Ca2+] (0.3–1 mM) is established and maintained by the sarcoendoplasmic reticulum Ca2+ (SERCA) pump [26]. ER Ca2+ channels include ryanodine receptors (RyRs) and inositol 1,4,5-triphosphate receptors (IP3Rs) [27]. Activation of phopspholipase C-coupled, G-protein-coupled receptors induces IP3-mediated Ca2+ release from the ER lumen. Additionally, both IP3Rs and RyRs are activated by cytosolic Ca2+, a mechanism known as Ca2+-induced Ca2+ release [28]. Hence, ER Ca2+ may play an amplifying role by mediating a rapid augmentation of [Ca2+]injury. ER Ca2+ stores can be refilled through Ca2+ release-activated Ca2+ entry (see Fig. 2). Upon ER Ca2+ depletion, the so-called EF-hand domain in stromal-interacting molecule-1 proteins are oligomerized to activate Orai channels in the PM [29]. In the muscle cells, the sarcoendoplasmic reticulum is the primary source of Ca2+ [18, 19]. Given that the nuclear envelope is continuous with the ER membrane, the nucleus may also be viewed as a Ca2+ store [18].
Mitochondria
Mitochondria buffer Ca2+ released from the ER [29]. Recent studies have shown that mitochondria interact physically with the ER through mitochondria-associated membranes [30]. The mitochondria Ca2+ uniporter, which is localized to the inner mitochondrial membrane, conducts Ca2+ released from the ER Ca2+ into the mitochondrial matrix (see Fig. 2).
Lysosomes
Lysosomes are also Ca2+ stores with a luminal concentration of ~0.5 mM [31, 32]. Channels known to be involved in lysosomal Ca2+ release include the transient receptor protein mucolipin channel (TRPML; see below) and the as yet unconfirmed receptor for the second messenger nicotinic acid adenine dinucleotide phosphate (NAADP). Two-pore channel proteins are strong candidate NAADP receptors [33–38]. The ER and endolysosomes may cross-talk with each such that endolysosomal Ca2+ release may activate Ca2+-induced Ca2+ release from the ER [39]; lysosomes may also sequester Ca2+ released from the ER [40].
Role of Ca2+ influx in membrane repair
Cells that reside in a mechanically-active environment, such as skeletal and cardiac muscle cells [1, 2], face a “reseal or die” challenge when they are injured. It has been long known that resealing of disrupted cell membranes of sea urchin embryo cells and 3T3 fibroblasts requires external Ca2+ [15–17]. It has been known for decades that resealing can be blocked by Ca2+ chelators, such as ethylene glycol tetraacetic acid (EGTA) and 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA) [41]. Follow-up studies performed in various in vitro damage/repair assays and cell types have shown that removal of extracellular Ca2+ impairs the resealing [15–17]. The [Ca2+]injury influx is rendered transient by quick resealing, perhaps owing to extracellular Ca2+ triggers the rapid delivery, docking, and fusion of intracellular vesicles at the injury sites [41]. If resealing is blocked or delayed, uncontrolled rises in [Ca2+]injury may trigger calpain-mediated cell death [42]. Hence, whether Ca2+ triggers resealing or cell death may be determined by the magnitude of [Ca2+]injury flux.
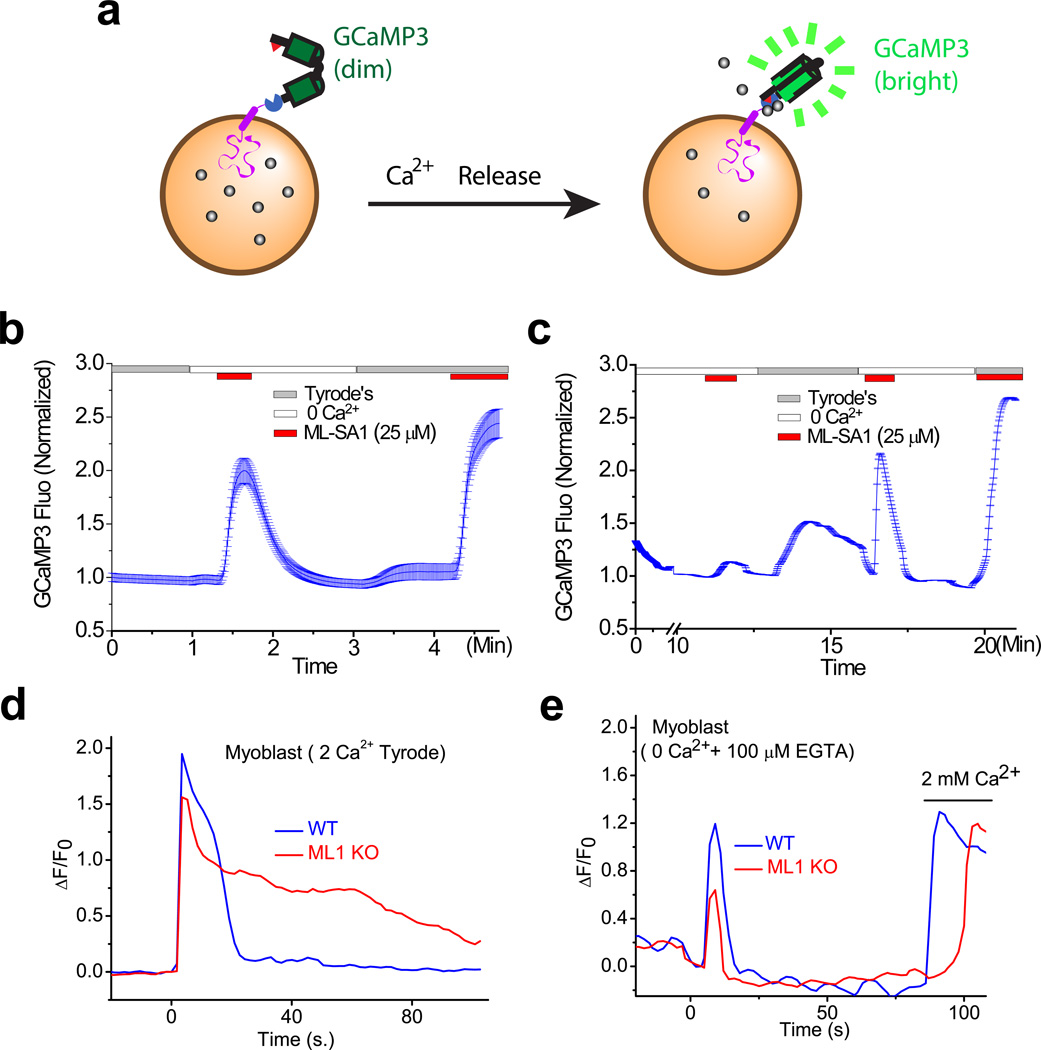
The simple interpretation of PM resealing’s extracellular Ca2+-dependence is that Ca2+ influx is required for [Ca2+]injury. However, it is questionable whether extracellular calcium is the sole source of calcium during membrane resealing. Passive depletion of ER Ca2+ stores triggers ER Ca2+ refilling through Ca2+ release-activated Ca2+ [29], extracellular Ca2+ is also required for the maintenance of intracellular Ca2+ stores (see Fig. 3). In a very recent study designed to examine the molecular mechanisms underlying lysosomal Ca2+ depletion and refilling (Garrity et al., in preparation), we found evidence indicating that extracellular Ca2+ plays an important role in the refilling of lysosome Ca2+ stores. We employed a lysosome-targeted genetically-encoded Ca2+ indicator, GCaMP3-ML1, to measure lysosomal Ca2+ release in intact cells, wherein transient Ca2+ increases, reflected by GCaMP3 fluorescence changes, are induced by an ML1-specific synthetic agonist in a Ca2+-free external solution [43]. Remarkably, pre-incubation in Ca2+-free solution for only 10 min resulted in a dramatic (~90%) reduction of lysosomal Ca2+ release, and lysosomal Ca2+ stores could be restored by exposure to a 2 mM Ca2+ external solution for a few minutes (Fig. 3). These results suggest that lysosomal Ca2+ may contribute, at least partially, to the Ca2+-dependence of PM resealing. Hence, one or both sources of Ca2+ may be used to trigger membrane repair depends on the type and size of PM injury.
Fig. 3. Extracellular Ca2+-dependence of lysosomal Ca2+ stores and membrane damage induced [Ca2+] change.
(a) Regulation of lysosomal Ca2+ store depletion and refilling by extracellular Ca2+. Lysosomal Ca2+ release is detected by a genetically-encoded Ca2+ indicator (GCaMP3) fused directly to the C-terminus of Lamp1 (Lamp1-GCaMP3) or the N-terminus of TRPML1 (GCaMP3-ML1). (b) TRPML1-mediated lysosomal Ca2+ release is triggered by the TRPML1-specfic synthetic agonist ML-SA1 in a zero Ca2+ external solution. (c) After a 10-min incubation period in the zero Ca2+ external solution, the ML-SA1-induced Ca2+ response was dramatically reduced. Re-incubation in Tyrode’s solution (2 mM Ca2+) for several minutes restored ML-SA1-induced responses. (d, e) Membrane damage induces intracellular [Ca2+] changes in the presence and absence of extracellular Ca2+ in Lamp1-GCaMP3-transfected wild-type and TRPML1 knockout myoblasts. Panels d and e are re-plotted from Ref. [20] with permission.
Role of Ca2+ release in membrane repair
Local and rapid [Ca2+] increases are critical for signal transduction and membrane trafficking [31, 44–46]. In cell-free vesicle fusion assays, late endosome-lysosome fusion is inhibited by the fast Ca2+ chelator BAPTA, but not by the slower chelator EGTA [45, 47]; the calculated association constant is 0.3 µs for BAPTA versus 1.2 ms for EGTA [48]. It has been estimated that a millisecond-elevation of Ca2+ within approximately 20 nm is required to trigger vesicle fusion [49]. These tight temporal and spatial requirements suggest that the vesicles themselves may provide the Ca2+ required for membrane fusion [46, 47]. Hence, PM injury may induce intracellular Ca2+ release to trigger membrane fusion and lysosomal exocytosis.
There are three ways to test the importance of intracellular Ca2+ release in membrane resealing: first, if intracellular Ca2+ release is critical for PM resealing, then depletion of specific intracellular stores would affect resealing; second, incubating cells with both membrane-permeable and non-permeable Ca2+ chelators would impair resealing; and third, and most telling, intracellular Ca2+ release channels would be required for membrane repair. These conditions were examined in a recent study in our laboratory [20]. First, we demonstrated that selective depletion of lysosome Ca2+ stores (with glycyl-L-phenylalanine-beta-naphthylamide) prevented PM resealing [20]. Second, we found that PM resealing was impaired in cells incubated with BAPTA-AM, a membrane permeable Ca2+ chelator [20]. And finally, we showed that PM resealing requires functional TRPML1, a lysosomal Ca2+ channel [20]. Although the dystrophin-glycoprotein complex and the known membrane repair proteins were expressed normally in TRPML1-null muscle fibers or when TRPML1-mediated lysosomal Ca2+ release was pharmacologically inhibited, membrane resealing was defective [20]. Hence, this work demonstrated that lysosomal Ca2+ channels and Ca2+ stores are both essential for membrane resealing. Furthermore, lysosomal Ca2+ imaging revealed that damage-induced [Ca2+]injury flux from intracellular Ca2+ stores was reduced in TRPML1 knockout cells (Fig. 3). However, elevated [Ca2+]injury lasted much longer in TRPML1 knockout cells when the cells were in 2 mM Ca2+ solution (Fig. 3), suggesting that PM resealing contributes to [Ca2+]injury kinetics. Moreover, together these findings suggest that intracellular Ca2+ release may also play an important role in regulating the PM resealing process.
Given the observed influence of extracellular Ca2+ availability on lysosome Ca2+ stores, it is difficult to dissect the relative contributions of extracellular Ca2+ flux versus lysosomal Ca2+ release in membrane repair. Membrane repair is a complicated process involving a rapid (within seconds) initial resealing response followed by a prolonged (up to tens of minutes) remodeling phase [50]. Massive Ca2+ influx peaks within 10–20 s of a PM breach (Fig. 3). However, the fusion of lysosomes with other vesicles and the PM of the cell takes place tens of minutes after injury [51]. Live imaging in primary human myotubes showed that membrane repair and remodeling may last more than 10 min [52]. Hence, it is likely that both extracellular and intracellular store Ca2+ plays multiple roles in membrane repair. Consistently, even for sarcolemma repair, multiple Ca2+ sensors have been identified, including dysferlin, Syt-VII, myoferlin, and the annexins A1 and A2.
Intracellular Ca2+ release channels in membrane repair
TRPML1 was the first identified Ca2+ release channel in the lysosome to be implicated in resealing. It remains to be investigated whether other lysosome channels, such as TRPML3 and the two-pore channels, contribute to [Ca2+]injury fluxes. Finally, ER Ca2+ channels could be involved in Ca2+-induced Ca2+ release, further increasing [Ca2+]injury.
TRPML1
The mucolipin subfamily of TRP channels comprises three members in mammals, namely TRPML1 (Mucolipin 1, MCOLN1), TRPML2 (MCOLN2), and TRPML3 (MCOLN3). Like other TRP channels, TRPML channels consist of six putative transmembrane-spanning domains (S1–S6) with the N- and C- termini facing the cytosol [53]. While TRPML1 is expressed ubiquitously in almost every tissue and cell type, TRPML2 and TRPML3 are expressed only in particular cell types [53, 54]. TRPML1–3 channels are localized predominately on the membranes of late endosomes and lysosomes [53, 55]. It is worth noting that TRPML1 is so important physiologically, presumably in large part because of its well-known role in lysosome-endosome trafficking of cellular constituents, that loss-of-function TRPML1 mutations result in Mucolipidosis type IV, a severe lysosomal storage disorder that manifests as a severe neurodegenerative disease characterized by psychomotor disabilities [56–58].
TRPML1 is a non-selective cation channel that conducts Ca2+ out of the endolysosomal lumen. Phosphatidylinositol 3,5-bisphosphate [PI(3,5)P2], which is localized on endosomes and lysosomes, activates whole-lysosome TRPML1 currents [59]. In contrast, PI(4,5)P2 and SMs in the PM inhibit TRPML1 [43, 60]. Besides TRPML1’s endogenous activators, several synthetic small molecule activators of TRPML1 have been identified [43, 61], including ML-SA1 and ML-SA3, which activate TRPML1 robustly at low micromolar concentrations, producing a response comparable to that produced by PI(3,5)P2 [43, 62].
Growing evidence support a direct role of TRPML1 in lysosomal exocytosis. The permeation and gating properties of TRPML1 suggest that the channel function of TRPML1 is to release Ca2+ from the lysosome lumen in response to various cellular cues [53, 63]. Consistent with a role of TRPML1 in lysosomal trafficking, several fusion defects were observed in TRPML1 knockout cells [64, 65]. Furthermore, several lines of evidence suggest that the role of TRPML1 in lysosomal exocytosis is likely to be direct. First, a gain-of-function mutation in TRPML1 results in enhanced lysosomal exocytosis in cells [66]. Second, acute ML-SA1 treatment has been shown to induce cell surface expression of lysosomal-associated membrane protein 1 (Lamp1) and lysosomal enzyme release in wild-type, but not TRPML1 knockout, macrophages [67]. And third, TRPML1 mediated lysosomal exocytosis is impaired in TRPML1 knockout or pharmacologically inhibited cells upon PM damage [20]. Importantly, TRPML1 knockout mice exhibit muscle repair defects and develop a muscular dystrophy phenotype [20].
The mechanism by which TRPML1 is activated by membrane damage is not known. Membrane damage causes influx of Ca2+ and oxidants [16]. It is conceivable that an event at injury sites may activate TRPML1; however, TRPML1 is unlikely to be directly activated by Ca2+ [59]. Oxidant levels are known to be increased at damage sites [16]. It remains to be determined whether intracellular reactive oxygen species may modulate TRPML1.
TRPML3
Like TRPML1, TRPML3 is also an endolysosomal Ca2+ release channel that is activated by PI(3,5)P2 [59]. However, the restricted expression pattern of TRPML3 would seem to limit its role in resealing to specialized cell types. An interesting property of TRPML3 is that its channel activity is inhibited under low-pH conditions [68]. When biliary epithelial cells are infected by uropathogenic E. coli (UPEC), TRPML3 senses the UPEC-mediated lysosome neutralization and releases Ca2+, triggering lysosome exocytosis to expel the exosome-encased UPEC [69]. Given the similarities in membrane repair and elimination of lysosome-localized pathogens, it is possible that TRPML3 may also play a role in membrane repair.
Calcium sensors in membrane repair
Consistent with a crucial role of Ca2+ in PM resealing, a number of Ca2+-sensor proteins have been shown to be involved in resealing in various cell types, including Syts, ferlins, annexins, and ALG-2. Most Ca2+ sensor proteins contain one or more Ca2+-binding domains, such as a C2 domain or an EF hand [70]. Ca2+ binding to a C2 domain may promote its interactions with phospholipids, altering the fusogenic potential of the lipid bilayer [70]. Likewise, EF hand-containing proteins may also be involved in membrane fusion [19].
Syts
The Syts are a group of transmembrane proteins with double C2 domains in their cytosolic regions [71, 72]. Syt-I, the most common isoform, functions as a Ca2+ sensor in synaptic vesicle exocytosis [73]. The plant homolog of Syt-I is required for the maintenance of membrane integrity in Arabidopsis thaliana [74, 75]. Several mammalian Syts, including Syt-VII, are expressed ubiquitously [76, 77]. The discovery that Syt-VII is a Ca2+ sensor that regulates lysosomal exocytosis is consistent with the possibility that it may play a role in membrane repair [17]. Indeed, Syt-VII is activated in Ca2+ triggered lysosomal exocytosis during membrane repair [7, 8, 78] via a mechanism that involves an interaction with SNARE proteins [73] (see Fig. 1).
Ferlins
Mammals have six ferlin proteins, including dysferlin, otoferlin, and myoferlin [79–82]. Ferlins, which share many structural and functional properties with Syts [83, 84], contain six C2 domains within their cytosolic regions [80] that confer Ca2+-dependent phophospholipid-binding activity [83, 85].
Mutations in the dysferlin gene DYSF cause limb girdle muscular dystrophy type 2B and Miyoshi myopathy [86–88], and dysferlin knockout mice exhibit defects in muscle repair [89]. Dysferlin is proposed to mediate the fusion of intracellular vesicles with the PM [89], most likely via Ca2+-dependent binding of the first C2A domain of dysferlin to phospholipids [90]. Full-length dysferlin is cleaved by calpains that are activated by injury specifically, resulting in mini-dysferlinC72 (i.e. C2E~C2F) [91]. Mini-dysferlinC72-postive vesicles are recruited rapidly to PM injury sites and fuse with PM compartments decorated with MG53, a potential oxidant sensor that accumulates at injury sites [15, 16]. Cleavage of dysferlin has been shown to be Ca2+-dependent, suggesting that Ca2+ may play multiple roles in the PM resealing process [91, 92].
Myoferlin is widely distributed in mammalian cells, with very high expression in myoblasts and particularly marked enrchment at sites where myoblasts are in contact with each other [93, 94]. Upon muscle injury, myoferlin levels become dramatically increased [79, 93, 95]. Like dysferlin, both myoferlin and otoferlin can be cleaved enzymatically to release their C-terminal fragments, which bear two C2 domains [92].
Annexins
Annexins (annexin A1–A13) bind to membranes exposing negatively-charged phospholipids in a Ca2+-dependent manner [96, 97]. At resting cytosolic [Ca2+], annexins are diffusely localized in the cytosol. Upon cytosolic [Ca2+] increase, annexins translocate to the PM and organellar membranes [98]. Annexin 1, 2, and 5 have been associated with endosomal functions [99–103]. Annexins’ fusogenic properties and their lipid preferences make them ideal mediators of resealing. In muscle cells, annexin A1 and A2 have been shown to promote the formation of lipid patches by way of their interactions with dysferlin. In zebrafish skeletal muscle, there is sequential accumulation of dysferlin, annexin A6, A2, and A1 at injury sites [104]. In human placenta, annexin A5 has been reported to play a role in the repair of human trophoblast membranes [105].
ALG-2
ALG-2 is a Ca2+-binding protein that contains five serially-repetitive EF-hands, forming a penta-EF-hand moeity [106]. ALG-2 binds to annexin A7/A11, Sec31A, ESCRT proteins, and ALG-2-interacting protein X (ALIX) in a Ca2+-dependent manner [106–111]. Luminal Ca2+ release from secretory organelles activates ALG-2, regulating vesicle transport in the secretory pathway [112].
ALG-2 is reported to be essential for resealing in the “macrovesicle shedding” model [10, 13] (see Fig. 1). Injury-triggered Ca2+ increases lead to ALG-2-regulated assembly of an ESCRT III-ALIX-Vps4 complex at PM injury sites, resulting in cleavage and shedding of damaged membranes [13]. Interestingly, ALG-2 is a Ca2+-dependent interactor of TRPML1 [113], raising the possibility that TRPML1 may also be involved shedding-mediated membrane repair.
Calpains
Calpains are Ca2+-dependent cysteine proteases that contain a “C2-like” domain near their N-terminal catalytic regions [114]. Mutations of muscle specific calpain-3 are associated with limb girdle muscular dystrophy type 2A [115]. Resealing of transected axons in cultured neurons has been shown to involve calpains [116, 117]. Furthermore, studies in various mammalian cell types have also revealed roles for the ubiquitously-expressed calpains-1 and -2 in PM resealing [118, 119]. Interestingly, a direct link between calpain and dysferlin was reported recently, suggesting that Ca2+ sensors may interact with one another in PM resealing [92].
Ca2+-regulated ion channels
Anactamin (ANO) proteins are the best supported candidates for the once identity-elusive calcium-activated chloride channels [120–125]. Anoctamin 5 (ANO5) is highly expressed in skeletal and cardiac muscle cells and recessive ANO5 mutations in patients with muscular dystrophy are associated with sarcolemmal membrane lesions [124, 126]. It appears that ANO5 serves as a sensor for [Ca2+]injury surges, to which it responds by releasing chloride [1, 84]. Notably, a chloride current has been observed after membrane wounding of sea urchin embryos [127]. Given that the ER and lysosomes interact closely and conduct crosstalk with each other [39, 40] and the observation that ANO5 is localized primarily in the ER [128], it is possible that ANO5 may modulate lysosomal exocytosis for PM resealing.
Perspective and future directions
PM resealing is essential for cell survival. Exocytic, endocytic, and exososme-mediated mechanisms have been proposed as mediators of membrane repair (see Fig. 1). It is likely that the type and size of wound dictates the repair mechanisms to be triggered. Both Ca2+ and oxidants are putative damage signals. Ca2+ may regulate multiple steps in the resealing process through the recruitment of multiple Ca2+ sensors. Significant [Ca2+] increases at injury sites last for only seconds, though it takes tens of minutes to complete PM repair. Consistent with the repair timeline, Ca2+ sensor proteins accumulate continuously at damage sites for tens of minutes. Given the regional specificity of [Ca2+]injury fluxes, it is evident that the Ca2+ source of these fluxes is local, either from vesicle lumens or from the extracellular space. In the near future, we expect to see progress in the following areas:
Development of vesicle-targeted genetically-encoded Ca2+ sensors that can be used to monitor cytosolic and luminal Ca2+ changes;
Live cell imaging that enables Ca2+-dependent vesicle movement to be recorded;
Elucidation of the mechanisms by which Ca2+ release channels are activated upon PM damage;
The existence of resealing of intracellular membranes.
A better understanding of the resealing process should help inform the design of approaches to treat diseases caused by defective membrane integrity, such as muscular dystrophy and cardiomyopathy.
Acknowledgements
This work in the authors’ laboratory is supported by NIH grants (NS062792, NS091928, and AR060837 to H.X). The authors appreciate the encouragement and helpful comments from our colleagues in the Xu laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol. 2005;6(6):499–505. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- 2.Andrews NW, Almeida PE, Corrotte M. Damage control: cellular mechanisms of plasma membrane repair. Trends Cell Biol. 2014;24(12):734–742. doi: 10.1016/j.tcb.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suetsugu S, Kurisu S, Takenawa T. Dynamic shaping of cellular membranes by phospholipids and membrane-deforming proteins. Physiol Rev. 2014;94(4):1219–1248. doi: 10.1152/physrev.00040.2013. [DOI] [PubMed] [Google Scholar]
- 4.McNeil PL, et al. Patching plasma membrane disruptions with cytoplasmic membrane. J Cell Sci. 2000;113(Pt 11):1891–1902. doi: 10.1242/jcs.113.11.1891. [DOI] [PubMed] [Google Scholar]
- 5.Kagawa H, et al. Expression of prothrombinase activity and CD9 antigen on the surface of small vesicles from stimulated human endothelial cells. Thromb Res. 1995;80(6):451–460. doi: 10.1016/0049-3848(95)00200-6. [DOI] [PubMed] [Google Scholar]
- 6.Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106(2):157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 7.Idone V, et al. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol. 2008;180(5):905–914. doi: 10.1083/jcb.200708010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Idone V, Tam C, Andrews NW. Two-way traffic on the road to plasma membrane repair. Trends Cell Biol. 2008;18(11):552–559. doi: 10.1016/j.tcb.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tam C, et al. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J Cell Biol. 2010;189(6):1027–1038. doi: 10.1083/jcb.201003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jimenez AJ, et al. ESCRT machinery is required for plasma membrane repair. Science. 2014;343(6174):1247136. doi: 10.1126/science.1247136. [DOI] [PubMed] [Google Scholar]
- 11.Babiychuk EB, et al. Blebbing confers resistance against cell lysis. Cell Death Differ. 2011;18(1):80–89. doi: 10.1038/cdd.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babiychuk EB, et al. Intracellular Ca(2+) operates a switch between repair and lysis of streptolysin O-perforated cells. Cell Death Differ. 2009;16(8):1126–1134. doi: 10.1038/cdd.2009.30. [DOI] [PubMed] [Google Scholar]
- 13.Scheffer LL, et al. Mechanism of Ca(2)(+)-triggered ESCRT assembly and regulation of cell membrane repair. Nat Commun. 2014;5:5646. doi: 10.1038/ncomms6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Draeger A, Monastyrskaya K, Babiychuk EB. Plasma membrane repair and cellular damage control: the annexin survival kit. Biochem Pharmacol. 2011;81(6):703–712. doi: 10.1016/j.bcp.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 15.McNeil P. Membrane repair redux: redox of MG53. Nat Cell Biol. 2009;11(1):7–9. doi: 10.1038/ncb0109-7. [DOI] [PubMed] [Google Scholar]
- 16.Cai C, et al. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 2009;11(1):56–64. doi: 10.1038/ncb1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakrabarti S, et al. Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. J Cell Biol. 2003;162(4):543–549. doi: 10.1083/jcb.200305131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prins D, Michalak M. Organellar calcium buffers. Cold Spring Harb Perspect Biol. 2011;3(3) doi: 10.1101/cshperspect.a004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 20.Cheng X, et al. The intracellular Ca(2)(+) channel MCOLN1 is required for sarcolemma repair to prevent muscular dystrophy. Nat Med. 2014;20(10):1187–1192. doi: 10.1038/nm.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 22.Xu H, Martinoia E, Szabo I. Organellar channels and transporters. Cell Calcium. 2015;58(1):1–10. doi: 10.1016/j.ceca.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu T, et al. Kinetic studies of Ca2+ binding and Ca2+ clearance in the cytosol of adrenal chromaffin cells. Biophys J. 1997;73(1):532–545. doi: 10.1016/S0006-3495(97)78091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naraghi M, Neher E. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J Neurosci. 1997;17(18):6961–6973. doi: 10.1523/JNEUROSCI.17-18-06961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer PJ. The local Ca concentration profile in the vicinity of a Ca channel. Cell Biochem Biophys. 2001;35(1):49–61. doi: 10.1385/CBB:35:1:49. [DOI] [PubMed] [Google Scholar]
- 26.Strehler EE, Treiman M. Calcium pumps of plasma membrane and cell interior. Curr Mol Med. 2004;4(3):323–335. doi: 10.2174/1566524043360735. [DOI] [PubMed] [Google Scholar]
- 27.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82(4):893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 28.Lam AK, Galione A. The endoplasmic reticulum and junctional membrane communication during calcium signaling. Biochim Biophys Acta. 2013;1833(11):2542–2559. doi: 10.1016/j.bbamcr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Prakriya M. Store-operated Orai channels: structure and function. Curr Top Membr. 2013;71:1–32. doi: 10.1016/B978-0-12-407870-3.00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Csordas G, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174(7):915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan AJ, et al. Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem J. 2011;439(3):349–374. doi: 10.1042/BJ20110949. [DOI] [PubMed] [Google Scholar]
- 32.Lloyd-Evans E, et al. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med. 2008;14(11):1247–1255. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- 33.Zhu MX, et al. Two-pore channels for integrative Ca signaling. Commun Integr Biol. 2010;3(1):12–17. doi: 10.4161/cib.3.1.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tugba Durlu-Kandilci N, et al. TPC2 proteins mediate nicotinic acid adenine dinucleotide phosphate (NAADP)- and agonist-evoked contractions of smooth muscle. J Biol Chem. 2010;285(32):24925–24932. doi: 10.1074/jbc.M110.129833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruas M, et al. Purified TPC isoforms form NAADP receptors with distinct roles for Ca(2+) signaling and endolysosomal trafficking. Curr Biol. 2010;20(8):703–709. doi: 10.1016/j.cub.2010.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan AJ, Galione A. Two-pore channels (TPCs): current controversies. Bioessays. 2014;36(2):173–183. doi: 10.1002/bies.201300118. [DOI] [PubMed] [Google Scholar]
- 37.Davis LC, et al. NAADP activates two-pore channels on T cell cytolytic granules to stimulate exocytosis and killing. Curr Biol. 2012;22(24):2331–2337. doi: 10.1016/j.cub.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan AJ, et al. TPC: the NAADP discovery channel? Biochem Soc Trans. 2015;43(3):384–389. doi: 10.1042/BST20140300. [DOI] [PubMed] [Google Scholar]
- 39.Lee HC. Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization. J Biol Chem. 2012;287(38):31633–31640. doi: 10.1074/jbc.R112.349464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan AJ, Galione A. Fertilization and nicotinic acid adenine dinucleotide phosphate induce pH changes in acidic Ca(2+) stores in sea urchin eggs. J Biol Chem. 2007;282(52):37730–37737. doi: 10.1074/jbc.M704630200. [DOI] [PubMed] [Google Scholar]
- 41.Steinhardt RA, Bi G, Alderton JM. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science. 1994;263(5145):390–393. doi: 10.1126/science.7904084. [DOI] [PubMed] [Google Scholar]
- 42.Geeraerts MD, et al. Cytosolic free Ca2+ and proteolysis in lethal oxidative injury in endothelial cells. Am J Physiol. 1991;261(5 Pt 1):C889–C896. doi: 10.1152/ajpcell.1991.261.5.C889. [DOI] [PubMed] [Google Scholar]
- 43.Shen D, et al. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun. 2012;3:731. doi: 10.1038/ncomms1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong XP, Wang X, Xu H. TRP channels of intracellular membranes. J Neurochem. 2010;113(2):313–328. doi: 10.1111/j.1471-4159.2010.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8(8):622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 46.Luzio JP, Bright NA, Pryor PR. The role of calcium and other ions in sorting and delivery in the late endocytic pathway. Biochem Soc Trans. 2007;35(Pt 5):1088–1091. doi: 10.1042/BST0351088. [DOI] [PubMed] [Google Scholar]
- 47.Pryor PR, et al. The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J Cell Biol. 2000;149(5):1053–1062. doi: 10.1083/jcb.149.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adler EM, et al. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991;11(6):1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burgoyne RD, Clague MJ. Calcium and calmodulin in membrane fusion. Biochim Biophys Acta. 2003;1641(2–3):137–143. doi: 10.1016/s0167-4889(03)00089-2. [DOI] [PubMed] [Google Scholar]
- 50.Hai A, Spira ME. On-chip electroporation, membrane repair dynamics and transient in-cell recordings by arrays of gold mushroom-shaped microelectrodes. Lab Chip. 2012;12(16):2865–2873. doi: 10.1039/c2lc40091j. [DOI] [PubMed] [Google Scholar]
- 51.McDade JR, Michele DE. Membrane damage-induced vesicle-vesicle fusion of dysferlin-containing vesicles in muscle cells requires microtubules and kinesin. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marg A, et al. Sarcolemmal repair is a slow process and includes EHD2. Traffic. 2012;13(9):1286–1294. doi: 10.1111/j.1600-0854.2012.01386.x. [DOI] [PubMed] [Google Scholar]
- 53.Cheng X, et al. Mucolipins: Intracellular TRPML1–3 channels. FEBS Lett. 2010;584(10):2013–2021. doi: 10.1016/j.febslet.2009.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grimm C, et al. Role of TRPML and two-pore channels in endolysosomal cation homeostasis. J Pharmacol Exp Ther. 2012;342(2):236–244. doi: 10.1124/jpet.112.192880. [DOI] [PubMed] [Google Scholar]
- 55.Abe K, Puertollano R. Role of TRP channels in the regulation of the endosomal pathway. Physiology (Bethesda) 2011;26(1):14–22. doi: 10.1152/physiol.00048.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun M, et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet. 2000;9(17):2471–2478. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- 57.Bassi MT, et al. Cloning of the gene encoding a novel integral membrane protein, mucolipidin- and identification of the two major founder mutations causing mucolipidosis type IV. Am J Hum Genet. 2000;67(5):1110–1120. doi: 10.1016/s0002-9297(07)62941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bargal R, et al. Identification of the gene causing mucolipidosis type IV. Nat Genet. 2000;26(1):118–123. doi: 10.1038/79095. [DOI] [PubMed] [Google Scholar]
- 59.Dong XP, et al. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun. 2010;1:38. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang X, Li X, Xu H. Phosphoinositide isoforms determine compartment-specific ion channel activity. Proc Natl Acad Sci U S A. 2012;109(28):11384–11389. doi: 10.1073/pnas.1202194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grimm C, et al. Small molecule activators of TRPML3. Chem Biol. 2010;17(2):135–148. doi: 10.1016/j.chembiol.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang W, et al. Up-regulation of lysosomal TRPML1 channels is essential for lysosomal adaptation to nutrient starvation. Proc Natl Acad Sci U S A. 2015;112(11):E1373–E1381. doi: 10.1073/pnas.1419669112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen D, Wang X, Xu H. Pairing phosphoinositides with calcium ions in endolysosomal dynamics: phosphoinositides control the direction and specificity of membrane trafficking by regulating the activity of calcium channels in the endolysosomes. Bioessays. 2011;33(6):448–457. doi: 10.1002/bies.201000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thompson EG, et al. Lysosomal trafficking functions of mucolipin-1 in murine macrophages. BMC Cell Biol. 2007;8:54. doi: 10.1186/1471-2121-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vergarajauregui S, et al. Autophagic dysfunction in mucolipidosis type IV patients. Hum Mol Genet. 2008;17(17):2723–2737. doi: 10.1093/hmg/ddn174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dong XP, et al. Activating mutations of the TRPML1 channel revealed by proline-scanning mutagenesis. J Biol Chem. 2009;284(46):32040–32052. doi: 10.1074/jbc.M109.037184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samie M, et al. A TRP channel in the lysosome regulates large particle phagocytosis via focal exocytosis. Dev Cell. 2013;26(5):511–524. doi: 10.1016/j.devcel.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim HJ, et al. A novel mode of TRPML3 regulation by extracytosolic pH absent in the varitint-waddler phenotype. EMBO J. 2008;27(8):1197–1205. doi: 10.1038/emboj.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miao Y, et al. A TRP Channel Senses Lysosome Neutralization by Pathogens to Trigger Their Expulsion. Cell. 2015;161(6):1306–1319. doi: 10.1016/j.cell.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farah CA, et al. Autophosphorylation of the C2 domain inhibits translocation of the novel protein kinase C (nPKC) Apl II. J Neurochem. 2012;123(3):360–372. doi: 10.1111/j.1471-4159.2012.07930.x. [DOI] [PubMed] [Google Scholar]
- 71.Schiavo G, Osborne SL, Sgouros JG. Synaptotagmins: more isoforms than functions? Biochem Biophys Res Commun. 1998;248(1):1–8. doi: 10.1006/bbrc.1998.8527. [DOI] [PubMed] [Google Scholar]
- 72.Sudhof TC. Synaptotagmins: why so many? J Biol Chem. 2002;277(10):7629–7632. doi: 10.1074/jbc.R100052200. [DOI] [PubMed] [Google Scholar]
- 73.Pang ZP, Sudhof TC. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol. 2010;22(4):496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schapire AL, Valpuesta V, Botella MA. Plasma membrane repair in plants. Trends Plant Sci. 2009;14(12):645–652. doi: 10.1016/j.tplants.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 75.Schapire AL, et al. Arabidopsis synaptotagmin 1 is required for the maintenance of plasma membrane integrity and cell viability. Plant Cell. 2008;20(12):3374–3388. doi: 10.1105/tpc.108.063859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Butz S, et al. The subcellular localizations of atypical synaptotagmins III and VI. Synaptotagmin III is enriched in synapses and synaptic plasma membranes but not in synaptic vesicles. J Biol Chem. 1999;274(26):18290–18296. doi: 10.1074/jbc.274.26.18290. [DOI] [PubMed] [Google Scholar]
- 77.Sudhof TC, Rizo J. Synaptotagmins: C2-domain proteins that regulate membrane traffic. Neuron. 1996;17(3):379–388. doi: 10.1016/s0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- 78.Rao SK, et al. Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J Biol Chem. 2004;279(19):20471–20479. doi: 10.1074/jbc.M400798200. [DOI] [PubMed] [Google Scholar]
- 79.Posey AD, Jr, Demonbreun A, McNally EM. Ferlin proteins in myoblast fusion and muscle growth. Curr Top Dev Biol. 2011;96:203–230. doi: 10.1016/B978-0-12-385940-2.00008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jimenez JL, Bashir R. In silico functional and structural characterisation of ferlin proteins by mapping disease-causing mutations and evolutionary information onto three-dimensional models of their C2 domains. J Neurol Sci. 2007;260(1–2):114–123. doi: 10.1016/j.jns.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 81.Britton S, et al. The third human FER-1-like protein is highly similar to dysferlin. Genomics. 2000;68(3):313–321. doi: 10.1006/geno.2000.6290. [DOI] [PubMed] [Google Scholar]
- 82.Achanzar WE, Ward S. A nematode gene required for sperm vesicle fusion. J Cell Sci. 1997;110(Pt 9):1073–1081. doi: 10.1242/jcs.110.9.1073. [DOI] [PubMed] [Google Scholar]
- 83.Lek A, et al. Phylogenetic analysis of ferlin genes reveals ancient eukaryotic origins. BMC Evol Biol. 2010;10:231. doi: 10.1186/1471-2148-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han R, Campbell KP. Dysferlin and muscle membrane repair. Curr Opin Cell Biol. 2007;19(4):409–416. doi: 10.1016/j.ceb.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nalefski EA, Falke JJ. The C2 domain calcium-binding motif: structural and functional diversity. Protein Sci. 1996;5(12):2375–2390. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Illa I, et al. Distal anterior compartment myopathy: a dysferlin mutation causing a new muscular dystrophy phenotype. Ann Neurol. 2001;49(1):130–134. [PubMed] [Google Scholar]
- 87.Bashir R, et al. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet. 1998;20(1):37–42. doi: 10.1038/1689. [DOI] [PubMed] [Google Scholar]
- 88.Liu J, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet. 1998;20(1):31–36. doi: 10.1038/1682. [DOI] [PubMed] [Google Scholar]
- 89.Bansal D, et al. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423(6936):168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 90.Davis DB, et al. Calcium-sensitive phospholipid binding properties of normal and mutant ferlin C2 domains. J Biol Chem. 2002;277(25):22883–22888. doi: 10.1074/jbc.M201858200. [DOI] [PubMed] [Google Scholar]
- 91.Lek A, et al. Calpains, cleaved mini-dysferlinC72, and L-type channels underpin calcium-dependent muscle membrane repair. J Neurosci. 2013;33(12):5085–5094. doi: 10.1523/JNEUROSCI.3560-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Redpath GM, et al. Calpain cleavage within dysferlin exon 40a releases a synaptotagmin-like module for membrane repair. Mol Biol Cell. 2014;25(19):3037–3048. doi: 10.1091/mbc.E14-04-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doherty KR, et al. Normal myoblast fusion requires myoferlin. Development. 2005;132(24):5565–5575. doi: 10.1242/dev.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Davis DB, et al. Myoferlin, a candidate gene and potential modifier of muscular dystrophy. Hum Mol Genet. 2000;9(2):217–226. doi: 10.1093/hmg/9.2.217. [DOI] [PubMed] [Google Scholar]
- 95.Demonbreun AR, et al. Myoferlin regulation by NFAT in muscle injury, regeneration and repair. J Cell Sci. 2010;123(Pt 14):2413–2422. doi: 10.1242/jcs.065375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6(6):449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 97.Moss SE, Morgan RO. The annexins. Genome Biol. 2004;5(4):219. doi: 10.1186/gb-2004-5-4-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Draeger A, Wray S, Babiychuk EB. Domain architecture of the smooth-muscle plasma membrane: regulation by annexins. Biochem J. 2005;387(Pt 2):309–314. doi: 10.1042/BJ20041363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rambotti MG, Spreca A, Donato R. Immunocytochemical localization of annexins V and VI in human placentae of different gestational ages. Cell Mol Biol Res. 1993;39(6):579–588. [PubMed] [Google Scholar]
- 100.Diakonova M, et al. Localization of five annexins in J774 macrophages and on isolated phagosomes. J Cell Sci. 1997;110(Pt 10):1199–1213. doi: 10.1242/jcs.110.10.1199. [DOI] [PubMed] [Google Scholar]
- 101.Zobiack N, et al. The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes. Mol Biol Cell. 2003;14(12):4896–4908. doi: 10.1091/mbc.E03-06-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mayran N, Parton RG, Gruenberg J. Annexin II regulates multivesicular endosome biogenesis in the degradation pathway of animal cells. EMBO J. 2003;22(13):3242–3253. doi: 10.1093/emboj/cdg321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Merrifield CJ, et al. Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nat Cell Biol. 1999;1(1):72–74. doi: 10.1038/9048. [DOI] [PubMed] [Google Scholar]
- 104.Roostalu U, Strahle U. In vivo imaging of molecular interactions at damaged sarcolemma. Dev Cell. 2012;22(3):515–529. doi: 10.1016/j.devcel.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 105.Carmeille R, et al. Annexin-A5 promotes membrane resealing in human trophoblasts. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbamcr.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 106.Lo KW, et al. Apoptosis-linked gene product ALG-2 is a new member of the calpain small subunit subfamily of Ca2+-binding proteins. Biochemistry. 1999;38(23):7498–7508. doi: 10.1021/bi990034n. [DOI] [PubMed] [Google Scholar]
- 107.Missotten M, et al. Alix, a novel mouse protein undergoing calcium-dependent interaction with the apoptosis-linked-gene 2 (ALG-2) protein. Cell Death Differ. 1999;6(2):124–129. doi: 10.1038/sj.cdd.4400456. [DOI] [PubMed] [Google Scholar]
- 108.Katoh K, et al. The penta-EF-hand protein ALG-2 interacts directly with the ESCRT-I component TSG101, and Ca2+-dependently co-localizes to aberrant endosomes with dominant-negative AAA ATPase SKD1/Vps4B. Biochem J. 2005;391(Pt 3):677–685. doi: 10.1042/BJ20050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Satoh H, et al. ALG-2 interacts with the amino-terminal domain of annexin XI in a Ca(2+)-dependent manner. Biochem Biophys Res Commun. 2002;291(5):1166–1172. doi: 10.1006/bbrc.2002.6600. [DOI] [PubMed] [Google Scholar]
- 110.Satoh H, et al. The penta-EF-hand domain of ALG-2 interacts with amino-terminal domains of both annexin VII and annexin XI in a Ca2+-dependent manner. Biochim Biophys Acta. 2002;1600(1–2):61–67. doi: 10.1016/s1570-9639(02)00445-4. [DOI] [PubMed] [Google Scholar]
- 111.Tarabykina S, et al. ALG-2, a multifunctional calcium binding protein? Front Biosci. 2004;9:1817–1832. doi: 10.2741/1358. [DOI] [PubMed] [Google Scholar]
- 112.Helm JR, et al. Apoptosis-linked gene-2 (ALG-2)/Sec31 interactions regulate endoplasmic reticulum (ER)-to-Golgi transport: a potential effector pathway for luminal calcium. J Biol Chem. 2014;289(34):23609–23628. doi: 10.1074/jbc.M114.561829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vergarajauregui S, Martina JA, Puertollano R. Identification of the penta-EF-hand protein ALG-2 as a Ca2+-dependent interactor of mucolipin-1. J Biol Chem. 2009;284(52):36357–36366. doi: 10.1074/jbc.M109.047241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ono Y, Sorimachi H. Calpains: an elaborate proteolytic system. Biochim Biophys Acta. 2012;1824(1):224–236. doi: 10.1016/j.bbapap.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 115.Nigro V, Savarese M. Genetic basis of limb-girdle muscular dystrophies: the 2014 update. Acta Myol. 2014;33(1):1–12. [PMC free article] [PubMed] [Google Scholar]
- 116.Xie XY, Barrett JN. Membrane resealing in cultured rat septal neurons after neurite transection: evidence for enhancement by Ca(2+)-triggered protease activity and cytoskeletal disassembly. J Neurosci. 1991;11(10):3257–3267. doi: 10.1523/JNEUROSCI.11-10-03257.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Godell CM, et al. Calpain activity promotes the sealing of severed giant axons. Proc Natl Acad Sci U S A. 1997;94(9):4751–4756. doi: 10.1073/pnas.94.9.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mellgren RL, et al. Calpain is required for the rapid, calcium-dependent repair of wounded plasma membrane. J Biol Chem. 2007;282(4):2567–2575. doi: 10.1074/jbc.M604560200. [DOI] [PubMed] [Google Scholar]
- 119.Mellgren RL, et al. Calcium-dependent plasma membrane repair requires m- or mu-calpain, but not calpain-3, the proteasome, or caspases. Biochim Biophys Acta. 2009;1793(12):1886–1893. doi: 10.1016/j.bbamcr.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stohr H, et al. TMEM16B, a novel protein with calcium-dependent chloride channel activity, associates with a presynaptic protein complex in photoreceptor terminals. J Neurosci. 2009;29(21):6809–6818. doi: 10.1523/JNEUROSCI.5546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang YD, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455(7217):1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 122.Schroeder BC, et al. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134(6):1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Caputo A, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322(5901):590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 124.Hartzell HC, et al. Anoctamin/TMEM16 family members are Ca2+-activated Cl− channels. J Physiol. 2009;587(Pt 10):2127–2139. doi: 10.1113/jphysiol.2008.163709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Almaca J, et al. TMEM16 proteins produce volume-regulated chloride currents that are reduced in mice lacking TMEM16A. J Biol Chem. 2009;284(42):28571–28578. doi: 10.1074/jbc.M109.010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Galindo BE, Vacquier VD. Phylogeny of the TMEM16 protein family: some members are overexpressed in cancer. Int J Mol Med. 2005;16(5):919–924. [PubMed] [Google Scholar]
- 127.Mizuta K, et al. Molecular characterization of GDD1/TMEM16E, the gene product responsible for autosomal dominant gnathodiaphyseal dysplasia. Biochem Biophys Res Commun. 2007;357(1):126–132. doi: 10.1016/j.bbrc.2007.03.108. [DOI] [PubMed] [Google Scholar]
- 128.Duran C, et al. ANOs 3–7 in the anoctamin/Tmem16 Cl− channel family are intracellular proteins. Am J Physiol Cell Physiol. 2012;302(3):C482–C493. doi: 10.1152/ajpcell.00140.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]