Abstract
Background and Purpose
There is a paucity of effective treatment options to reduce falls in Parkinson’s disease (PD). Although a variety of rehabilitative approaches have been shown to improve balance, evidence of a reduction in falls has been mixed. Prior balance trials suggest that programs with highly challenging exercises had superior outcomes. We investigated the effects of a theoretically driven, progressive, highly challenging group exercise program on fall rate, balance, and fear of falling.
Methods
Twenty-three subjects with PD participated in this randomized cross-over trial. Subjects were randomly allocated to 3 months of active balance exercises or usual care followed by the reverse. During the active condition, subjects participated in a progressive, highly challenging group exercise program twice weekly for 90 minutes. Outcomes included a change in fall rate over the 3-month active period and differences in balance (Mini-BESTest), and fear of falling (Falls Efficacy Scale-International (FES-I)) between active and usual care conditions. Results: The effect of time on falls was significant (regression coefficient = −0.015 per day, p<0.001). The estimated rate ratio comparing incidence rates at time points one month apart was 0.632 (95% CI 0.524 to 0.763). Thus, there was an estimated 37% decline in fall rate per month (95% CI 24% to 48%). Improvements were also observed on the Mini-BESTest (p=0.037) and FES-I (p=0.059).
Discussion and Conclusions
The results of this study show that a theoretically based, highly challenging, and progressive exercise program was effective in reducing falls, improving balance, and reducing fear of falling in PD.
Keywords: Parkinson disease, falls, exercise, rehabilitation, balance, postural control
Introduction
Impaired balance is one of the cardinal signs of Parkinson’s disease (PD). Sixty-eight percent of people living with PD in the community sustain at least one fall per year which is double the fall rate reported in healthy older adults.1 Notably, 50.5% of fallers with PD reported recurrent falls (at least two) over a one-year period.2 Falls are a major cause of disability and reduced quality of life in people with PD and result in devastating injuries such as hip fractures that are significantly more common in PD than in those with other medical conditions.3,4 In addition, falls are associated with increased risk of hospital and nursing home admissions and ultimately with decreased survival rates. The economic impact related to healthcare costs is about twice as much in fallers as non-fallers with PD.5
Identifying interventions that successfully improve postural control and reduce fall rate is critical to reduce disability, improve QOL and potentially increase survival in people with PD. Although the gold standard dopaminergic pharmacological interventions are effective in reducing bradykinesia, rigidity, and tremor, these medications are not effective in ameliorating balance deficits and reducing falls in people with PD.6,7 Several randomized, controlled trials (RCTs) show that a variety of rehabilitative approaches have been effective in improving balance in persons with PD.8 A meta-analysis of fifteen randomized and quasi-randomized controlled trials of exercise and motor training interventions targeting balance in PD found significant improvement in balance in the context of walking velocity, transitioning from sitting to standing, and standing balance activities.9 Despite the evidence of improved balance, results revealed no evidence of a reduction in falling; however, only 2 trials included falls as an outcome.9
More recent clinical trials have specifically examined the effects of exercise interventions on reducing falls in persons with PD. A 10-week group strengthening and balance training program in persons with PD showed no significant difference in incidence rate for falls compared to a usual care control condition.10 A 6-month tai chi program in PD resulted in a lower incidence of falls compared with stretching but not compared to resistance training.11 An 8-week strength training program and a movement strategy training program both resulted in significantly fewer falls at 12 months compared to a life-skills education program.12 In addition, a 6-month minimally supervised, home-based balance and strengthening program plus cueing strategies to reduce freezing of gait did not significantly reduce fall rate compared to a usual care condition.13 Finally, a 4-week sensorimotor agility program was found to be of value for improving balance when delivered as individualized physical therapy, but not when delivered as a group class, or through a home exercise program. Although these recent studies provide some evidence suggesting that falls are modifiable in PD, results are mixed.
Balance exercises alone, balance combined with strengthening exercises, cueing, gait training on a treadmill, tai chi, and functional training have all been shown to improve balance control in PD.8,9,14,15 The large degree of variability in these rehabilitative approaches suggests they may share salient, common features that contribute to the greatest improvements in balance and therefore may be most likely to impact falls.8,9 The aforementioned meta-analysis suggested that higher doses of training and highly challenging balance training (i.e., exercises that specifically involved movement of the center of mass, narrowing the base of support, and minimizing the upper extremity support) had the most robust outcomes (5 studies) and may be necessary to reduce falls.9
Given the relative paucity of trials examining the effects of exercise training on falls and the suggestion that higher doses of highly challenging approaches may lead to better outcomes, the purpose of this study was to investigate the effects of a theoretically driven, highly challenging balance program on fall rate in a randomized, controlled cross-over trial. We hypothesized that the number of falls among subjects in the active treatment period would significantly decline over the course of the 3-month intervention period. In addition, we hypothesized that balance and fear of falling would significantly improve during the active treatment period compared to the inactive period in which usual care was provided.
Methods
Design Overview
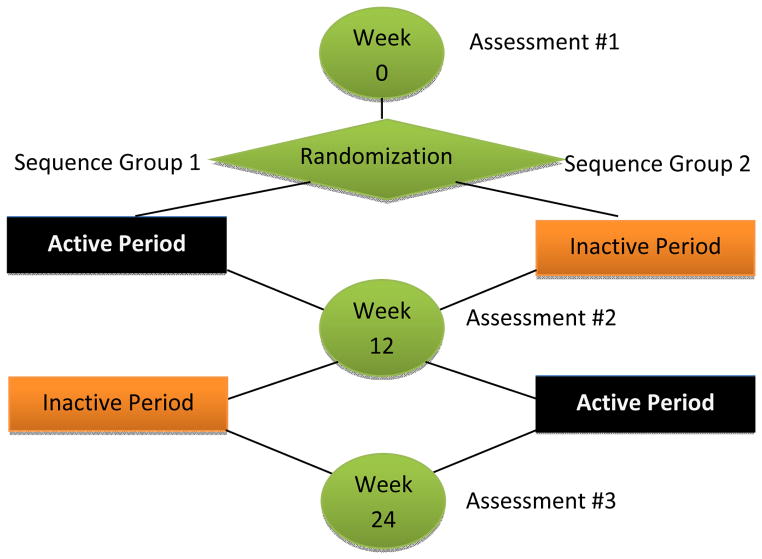
A randomized cross-over trial of a 3-month high intensity balance exercise intervention for people with PD was conducted in 2012 to 2013. Participants were recruited from XXXXXX XXXXXXX Parkinson’s Disease and Movement Disorders Center and the Center for Neurorehabilitation. Subjects participated in a baseline assessment session, followed by random allocation to 3 months of active balance exercises or 3 months of inactivity. Following this 3-month period, active subjects were switched to 3 months of usual care and subjects receiving usual care were switched to 3 months of active balance exercises. A computerized randomization schedule was generated and held by an investigator not involved in subject recruitment or assessment (TE). All subjects were re-assessed at 3 and 6 months by a physical therapist blind to participants’ group allocations (Figure 1).
Figure 1.
Study design
Participants
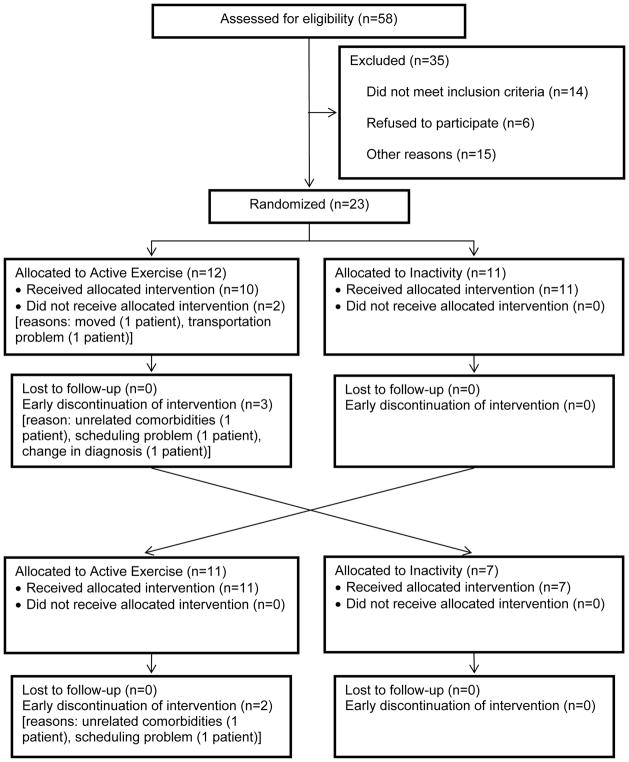
Twenty-three subjects with idiopathic PD (using UK Brain Bank Criteria) were enrolled (Figure 2). Subjects had stage 2 and 3 disease on the Hoehn and Yahr (H&Y) staging scale in the “on” medication state, were on a stable dose of PD medications for ≥ 2 weeks prior to enrollment, experienced ≥ 1 fall in the past 3 months and ≥ 2 falls in the past year, and were able to walk without physical assistance or an assistive device for at least 5 continuous minutes. Subjects were excluded if they had a diagnosis of atypical Parkinsonism, a Mini Mental Status score of <26, previous surgical management of PD, or serious co-morbidities that may interfere with ability to participate in the exercise program. Subjects were required to sign informed consent approved by the institutional review board at Boston University (ClinicalTrials.gov: NCT02302144).
Figure 2.
CONSORT diagram
Intervention
During the active condition, subjects participated in a highly challenging group exercise program focusing on improving balance and reducing falls. The exercise program was held twice weekly for 90 minute sessions over a 3-month period at the Center for Neurorehabilitation at XXXX XXXXXXXX. Three physical therapists with expertise in PD administered the group exercise program. In order to ensure adequate level of challenge across all sessions for all participants, the exercise program was conducted in the clinical setting only - without supplemental practice of the exercises at home.
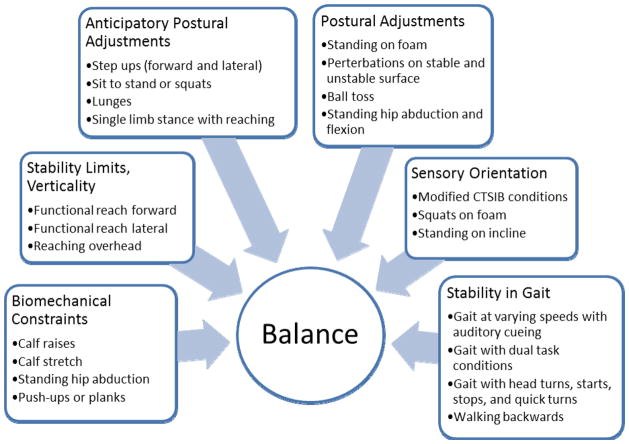
The intervention was developed using Horak’s theoretical balance framework for PD which describes 6 interacting systems contributing to balance control (Figure 3).16 Each of the exercises (i.e., strengthening, range of motion, anticipatory and reactive balance activities, altering sensory input, and gait training) was developed to address one or more of the six systems of balance control (Figure 3) and consisted of a progression ranging from less challenging to more challenging. Participants rated the challenge level of each exercise using a ten-part Likert scale (10 being the greatest level of challenge and 1 being no challenge) as it related specifically to balance control. Exercises were tailored to each individual and were progressed to increase challenge level when perceived challenge to balance was <7. Weighted vests and/or variable surface conditions were used to increase resistance and challenge level in a strengthening exercise. During the inactive condition, participants received usual care.
Figure 3.
Balance interventions corresponding to the six interacting systems contributing to balance control
Outcomes
Falls
Subjects from both groups were interviewed during the active intervention phase to collect detailed information about falls following the recommendations of the Prevention of Falls Network Europe consensus statement.17 At each session, patients were asked if they had any falls since the previous session. A fall was defined as a loss of balance where the person inadvertently came to rest on the ground or other lower level.2 The date of the fall along with the circumstances surrounding it (i.e., location, direction of fall, activity being performed, presence of environmental trigger) were recorded.
Balance
The Mini-BESTest assesses dynamic balance and contains 14 items from the original BESTest.18 Each item is scored on a 3-level ordinal scale from 0 to 2, with 2 representing no impairment in balance and 0 representing severe impairment of balance. The maximum total score is 28. The Mini-BESTest has high interrater and test-retest reliability in PD.19 The Mini-BESTest was administered by a trained physical therapist not involved in the intervention and blinded to treatment condition.
Fear of Falling
The Falls Efficacy Scale-International (FES-I) is a self-report questionnaire developed for use in elderly populations to assess fear of falling.20 A series of 16 questions assesses the respondent’s fear of falling for a range of ADLs. Each one is rated on a four-point scale from 1 (not at all concerned) to 4 (very concerned). The FES-I has been found to have extremely good internal consistency (Cronbach’s α = 0.96) and test-retest reliability (intraclass correlation coefficient = 0.96).20
Statistical Analysis
A paired t test was used to compare each outcome (FES-I and Mini-BEST) measured at the end of the active period with that measured at the end of the inactive period in the same patient.21 Thus, for each subject in sequence group 1 (active→inactive), the outcome score at the end of period 2 (inactive) was subtracted from the outcome score at the end of period 1 (active). For each subject in sequence group 2 (inactive→active), the outcome score at the end of period 1 (inactive) was subtracted from the outcome score at the end of period 2 (active). Thus, the overall treatment effect is the mean of the mean differences in outcome measurements between the active and inactive periods in sequence group 1 and sequence group 2, respectively. To assess carry-over effects, the mean of the two outcome scores measured at the end of each period (active, inactive) is calculated for each subject ([subject score period 1 + subject score period 2] / 2). The mean of these subject means of sequence group 1 is compared to that of sequence group 2 using a two-sample t test for independent samples. If there is no carry-over effect, there would be no difference in the means of the subject mean scores between the two sequence groups.
To test if the number of falls is a function of length of time in the intervention among subjects in the active treatment period, we modeled daily fall count as a linear function of time (intervention day) using generalized estimating equations (GEE) with the log link function, the negative binomial distribution, and the exchangeable correlation structure to account for correlated counts from each patient.22
Analyses were carried out using SAS v9.3 using PROC TTEST with the CROSSOVER option to account for the crossover design and PROC GENMOD to fit the negative binomial regression model for the number of falls.
Results
Twenty-three subjects with PD were enrolled and sixteen completed the study. Six subjects withdrew due to scheduling, transportation issues, or unrelated comorbidities, and one was withdrawn by the PI due to a change in diagnosis from typical to atypical PD. Sixty-three percent of participants were male, mean age was 66.7 years with a disease duration of 4.3 years (Table 1). Participants had mild-to-moderate PD (mean H&Y stage 2.5, mean motor UPDRS score 36).
TABLE 1.
Baseline characteristics (n=16)
| Variable | Mean ± SD or No. (%) |
|---|---|
| Age, yr | 66.7 ± 5.7 |
| Sex | |
| Male | 10 (62.5%) |
| Female | 6 (37.5%) |
| Disease duration, yr | 4.3 ± 3.3 |
| Hoehn and Yahr stagea | |
| 2 | 4 (25%) |
| 2.5 | 8 (50%) |
| 3 | 4 (25%) |
| MDS-UPDRS-III score | 36.0 ± 9.6 |
| FES-I | 28.3 ± 7.3 |
| Mini-BESTest | 20.9 ± 4.1 |
Hoehn and Yahr stage in the “on” state
There was no significant difference between the mean scores of the two sequence groups at baseline on FES-I and Mini-BESTest scores. Analysis of the overall treatment effect of the exercise intervention (active vs. inactive) on each outcome was performed using a paired t test with a cross-over design (Table 2). The estimated overall treatment effect on FES-I scores was −3.2 (95% confidence interval [CI] −6.4 to 0.1, p=0.059). To check for a carry-over effect, we analyzed the mean patient scores of both periods comparing the two groups using a two-sample t test for independent samples which was not significant (p=0.944). The estimated overall treatment effect on Mini-BESTest scores was 1.5 (95% CI 0.1 to 2.9, p=0.037). Though the test for the carry-over effect was only borderline significant (p=0.061), as a precaution, we analyzed the treatment effect in the first period only using a two-sample t test for independent samples. This yielded an estimated treatment effect of 4.8 (95% CI 1.3 to 8.2, p=0.010). L-dopa equivalents were not significantly different between the active and inactive conditions (p=0.54).
TABLE 2.
Summary statistics of treatment efficacy
| Sequence Group
|
Overall treatment effect Active vs. Inactive | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Active→Inactive N=7 |
Inactive→Active N=9 |
||||||||
| Active Inactive | Diffa | Meanb | Active | Inactive | Diffa | Meanb | |||
| FES-I | |||||||||
| Mean | 24.1 | 26.0 | −1.9 | 25.1 | 22.7 | 27.1 | −4.4 | 24.9 | −3.2 (p=0.059) |
| sd | 7.1 | 7.6 | 6.1 | 6.7 | 3.5 | 5.3 | 6.0 | 3.3 | |
| MiniBEST | |||||||||
| Mean | 25.0 | 24.1 | 0.9 | 24.6 | 22.3 | 20.2 | 2.1 | 21.3 | 1.5 (p=0.037) |
| sd | 2.3 | 2.1 | 1.7 | 2.0 | 4.5 | 3.7 | 3.1 | 3.9 | |
patient difference between active and inactive scores
patient mean of active and inactive scores
The number of daily falls per patient was modeled on the length of time in the active phase of the intervention (number of days) to determine if the intervention reduced falls over time (total falls observed = 150). A repeated measures negative binomial regression using the GEE approach indicated a statistically significant effect (coefficient = −0.015 per day, p<0.001). The estimated rate ratio comparing incidence rates at time points one month apart was 0.632 (95% CI 0.524 to 0.763) indicating an estimated 37% decline in the fall rate per month (95% CI 24% to 48%).
There were two adverse events (back pain, abdominal pain) during the inactive period. There were five adverse events (back pain, knee pain, abdominal pain, quadriceps pain, lightheadness) during the active period. Two of these were considered to be related to the intervention (knee pain, quadriceps pain). These occurred during one exercise session and were resolved by the subsequent session.
Discussion
The results of this study show that a theoretically based, highly challenging, and progressive exercise program was effective in reducing falls, improving balance, and reducing fear of falling in persons with PD. Although previous studies show improved balance in PD with a variety of approaches to rehabilitation, there is limited evidence of the impact of rehabilitation on reducing falls.9 Few studies have included falls as an outcome and results have been mixed among studies investigating the impact of rehabilitation on reducing falls.9–13,23,24 Studies included in a recent meta-analysis provided a relatively low dose of intervention which may have contributed to the lack of impact on fall reduction.9
Previous studies that included highly challenging balance training programs appeared to have the most robust outcomes regarding improvements in balance-related activity performance.9 Prior studies also suggest that balance training that included a strengthening component was more effective in improving balance compared to balance training alone.25–28 In addition, a recent RCT in PD also showed significant improvements in off-medication UPDRS-III scores following a 2-year progressive resistance exercise program compared to a non-progressive stretching, balance, and strengthening program suggesting the importance of progressively more challenging exercises.29 Also, prior work in the area of exercise in both animal models and humans with PD suggests the importance of goal-based motor skill training to enhance motor learning and motor control.30,31
Balance exercise programs derived from a sound theoretical framework targeting the essential postural control subsystems may also contribute to more robust improvements in balance control and a subsequent reduction in falls. Despite an incomplete understanding of postural control mechanisms underlying postural instability and falling in PD,32 multiple physiological systems are known to contribute to postural control. Horak and colleagues16,33 have identified six different balance control systems (biomechanical constraints, stability limits/verticality, anticipatory postural adjustments, postural responses, sensory orientation and stability in gait) underlying the complex skill of balancing that may be important to systematically target in exercise programs aimed at fall reduction in PD.
The exercise program in our study incorporated the most salient features from prior work (e.g., theoretically driven, highly challenging, progressive, goal oriented, balance plus strengthening exercises, high dose) which may explain the significant reduction in falls observed over 3 months. Exercises were chosen based on theoretical framework described by Horak and colleagues16,33 to address key elements of postural instability in PD while ensuring sufficient challenge across the six interacting systems. We operationalized “challenge to balance” using a ten-part Likert scale to determine when to progress subjects. At each session, subjects rated the level of difficulty of each exercise based on the level of challenge to balance. Exercises were goal oriented in that patients were given a target to achieve (i.e., reaching greater distances out of base of support). The program included both balance and strengthening exercises that were progressive (e.g., increased load added to weighted vests); 90-minute sessions were performed twice weekly over 3 months (total of 36 hours over 12 weeks) that exceeds the dosage provided in most of the previous balance trials (average 18 hours over 7 weeks).9
Our results also revealed significant improvements in balance and fear of falling. In a meta-analysis examining the effect of balance interventions on gait and balance outcomes, Hedge’s g effect sizes ranged from −0.622 to 1.271 among the 19 PD studies reviewed.9 In the present study, the Hedges’ g effect size for the mini-Bestest was 1.22 suggesting that the exercise program contributed to a large improvement in balance. With regard to the FES-I, the effect size (−0.77) suggested that fear of falling can be attenuated with a highly challenging balance exercise program.
There are several limitations to our study including a small sample size. However, despite a small sample, significant improvements were observed in fall rates, balance, and fear of falling suggesting the potential benefits of this intervention approach. Consistent with other exercise trials in PD, our results revealed a limited carry-over effect suggesting that the benefits of treatment dissipate over time – confirming the need for ongoing, sustained participation. Study subjects had mild-to-moderate PD (stage 2 and 3 on H&Y staging scale) so results may not be generalizable to more severe disease. Although frequent, in-person interviews were used to optimize ascertainment of all falls, this approach still relied on the accuracy of patient report.
Conclusions
These results show that a theoretically based, highly challenging, and progressive exercise program was effective in reducing falls, improving balance, and reducing fear of falling in persons with mild-to-moderate PD. The data suggest the potential efficacy of these aspects of training in persons with mild-to-moderate PD, but this requires further investigation.
Supplementary Material
Acknowledgments
Source of Funding:
This study was funded by the Boston Claude D. Pepper Older Americans Independence Center (NIH 5P30AG031679). Additional support was provided by the American Parkinson Disease Association (ADPA); ADPA MA Chapter.
We would like to thank Bernard Rosner for his assistance in many aspects of the study design and data analysis and Deborah DeMolles for her invaluable technical and programming support.
Footnotes
Conflicts of Interest
There were no conflicts of interest for any of the authors.
Clinical Trial Registry: ClinicalTrials.gov: NCT02302144
Contributor Information
David Sparrow, Email: david.sparrow@va.gov.
Tamara R. DeAngelis, Email: trork@bu.edu.
Kathryn Hendron, Email: khendron@bu.edu.
Cathi A. Thomas, Email: neurocat@bu.edu.
Marie Saint-Hilaire, Email: neuromsh@bu.edu.
Terry Ellis, Email: tellis@bu.edu.
References
- 1.Campbell AJ, Robertson MC, Gardner MM. Elderly people who fall: identifying and managing the causes. Br J Hosp Med. 1995;54(10):520–3. [PubMed] [Google Scholar]
- 2.Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson’s disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry. 2002;72(6):721–725. doi: 10.1136/jnnp.72.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrag A, Jahanshahi M, Quinn N. How does Parkinson’s disease affect quality of life? A comparison with quality of life in the general population. Mov Disord. 2000;15(6):1112–8. doi: 10.1002/1531-8257(200011)15:6<1112::aid-mds1008>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 4.Genever RW, Downes TW, Medcalf P. Fracture rates in Parkinson’s disease compared with age- and gender-matched controls: a retrospective cohort study. Age Ageing. 2005;34(1):21–24. doi: 10.1093/ageing/afh203. [DOI] [PubMed] [Google Scholar]
- 5.Spottke AE, Reuter M, Machat O, et al. Cost of illness and its predictors for Parkinson’s disease in Germany. Pharmacoeconomics. 2005;23(8):817–836. doi: 10.2165/00019053-200523080-00007. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm. 2007;114(10):1339–1348. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloem BR, Beckley DJ, van Dijk JG, Zwinderman AH, Remler MP, Roos RA. Influence of dopaminergic medication on automatic postural responses and balance impairment in Parkinson’s disease. Mov Disord. 1996;11(5):509–521. doi: 10.1002/mds.870110506. [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson CL, Patel S, Meek C, et al. Physiotherapy intervention in Parkinson’s disease: systematic review and meta-analysis. BMJ. 2012;345:e5004. doi: 10.1136/bmj.e5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen NE, Sherrington C, Paul SS, Canning CG. Balance and falls in Parkinson’s disease: A meta-analysis of the effect of exercise and motor training. Mov Disord. 2011;26:1605–1615. doi: 10.1002/mds.23790. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin VA, Richards SH, Henley W, Weings P, Taylor AH, Campbell J. An exercise intervention to prevent falls in people with Parkinson’s disease: a pragmatic randomized controlled trial. J Neurol Neurosurg Psychiatry. 2011;82:1232–8. doi: 10.1136/jnnp-2011-300919. [DOI] [PubMed] [Google Scholar]
- 11.Li F, Harmer P, Fitzgerald K, et al. Tai Chi and postural stability in patients with Parkinson’s disease. N Engl J Med. 2012;366:511–9. doi: 10.1056/NEJMoa1107911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris ME, Menz HB, McGinley JL, Watts JJ, Huxham FE, Murphy AT, Danoudis ME, Iansek R. A randomized controlled trial to reduce falls in people with Parkinson’s disease. Neurorehabil Neural Repair. 2015 doi: 10.1177/1545968314565511. [DOI] [PubMed] [Google Scholar]
- 13.Canning CG, Sherrington C, Lord SR, Close JC, Heritier S, Heller GZ, Howard K, Allen NE, Latt MD, Murray SM, O’Rourke SD, Paul SS, Song J, Fung V. Exercise for falls prevention in Parkinson disease: a randomized controlled trial. Neurology. 2015;84(3):304–312. doi: 10.1212/WNL.0000000000001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schenkman M, Hall DA, Barón AE, Schwartz RS, Mettler P, Kohrt WM. Exercise for people in early- or mid-stage Parkinson disease: a 16-month randomized controlled trial. Phys Ther. 2012;92(11):1395–410. doi: 10.2522/ptj.20110472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smania N, Corato E, Tinazzi M, et al. Effect of balance training on postural instability in patients with idiopathic Parkinson’s disease. Neurorehabilitation. 2010;24:826–834. doi: 10.1177/1545968310376057. [DOI] [PubMed] [Google Scholar]
- 16.Horak FB, Wrisley DM, Frank J. The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits. Phys Ther. 2009;89(5):484–98. doi: 10.2522/ptj.20080071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamb SE, Jorstad-Stein EC, Hauer K, Becker C. Development of a common outcome data set for fall injury prevention trials: The prevention of falls network Europe consensus. J Am Geriatr Soc. 2005;53:1618–1622. doi: 10.1111/j.1532-5415.2005.53455.x. [DOI] [PubMed] [Google Scholar]
- 18.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med. 2010;42:323–31. doi: 10.2340/16501977-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leddy AL, Crowner BE, Earhart GM. Utility of the Mini-BESTest, BESTest, and BESTest sections for balance assessments in individuals with Parkinson disease. J Neurol Phys Ther. 2011;35:90–97. doi: 10.1097/NPT.0b013e31821a620c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale-International (FES-I) Age Ageing. 2005;34:614–619. doi: 10.1093/ageing/afi196. [DOI] [PubMed] [Google Scholar]
- 21.Rosner B. Fundamentals of Biostatistics. 7. Boston: Brooks/Cole Publishing Co; 2011. [Google Scholar]
- 22.Robertson MC, Campbell AJ, Herbison P. Statistical analysis of efficacy in falls prevention trials. J Gerontol A Biol Sci Med Sci. 2005;60(4):530–534. doi: 10.1093/gerona/60.4.530. [DOI] [PubMed] [Google Scholar]
- 23.Protas EJ, Mitchell K, Williams A, Qureshy H, Caroline K, Lai EC. Gait and step training to reduce falls in Parkinson’s disease. NeuroRehabilitation. 2005;20:183–190. [PubMed] [Google Scholar]
- 24.Nieuwboer A, Kwakkel G, Rochester L, et al. Cueing training in the home improves gait-related mobility in Parkinson’s disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78:134–40. doi: 10.1136/jnnp.200X.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keus SH, Bloem BR, Hendriks EJ, Bredero-Cohen AB, Munneke M. Evidence-based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Mov Disord. 2007;22(4):451–60. doi: 10.1002/mds.21244. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson’s disease. Arch Phys Med Rehabil. 2003;84(8):1109–17. doi: 10.1016/s0003-9993(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 27.Corcos DM, Comella CL, Goetz CG. Tai chi for patients with Parkinson’s disease. N Engl J Med. 2012;366(18):1737–8. doi: 10.1056/NEJMc1202921. [DOI] [PubMed] [Google Scholar]
- 28.Falvo MJ, Schilling BK, Earhart GM. Parkinson’s disease and resistive exercise: rationale, review, and recommendations. Mov Disord. 2008;23(1):1–11. doi: 10.1002/mds.21690. Review. [DOI] [PubMed] [Google Scholar]
- 29.Corcos DM, Robichaud JA, David FJ, Leurgans SE, Vaillancourt DE, Poon C, Rafferty MR, Kohrt WM, Comella CL. A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov Disord. 2013;28:1230–1240. doi: 10.1002/mds.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013;12(7):716–26. doi: 10.1016/S1474-4422(13)70123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher BE, Wu AD, Salem GJ, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Arch Phys Med Rehabil. 2008;89(7):1221–9. doi: 10.1016/j.apmr.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10(8):734–44. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(Suppl 2):ii7–ii11. doi: 10.1093/ageing/afl077. Review. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.