Abstract
Deficits in N-methyl-d-aspartate receptor (NMDAR)-mediated neurotransmission may underlie dopaminergic hyperactivity in schizophrenia. Dysregulation of the GABAergic system has also been implicated. In this study we investigated a role for GABAB receptors as an intermediate step in the pathway leading from NMDAR stimulation to DA regulation. Since glycine (GLY) has been found to ameliorate treatment resistant negative symptoms in schizophrenia, we treated a group of rats with 16% GLY food for 2 weeks. DA levels in prefrontal cortex (PFC) and striatum (STR) were assessed by dual-probe microdialysis and HPLC–EC in freely moving rats. Infusion of the GABAB receptor agonists SKF97541 and baclofen into PFC and STR significantly reduced basal DA, an effect that was reversed by the antagonist, CGP52432. In PFC, GABAB agonists also reduced AMPH-induced DA release following treatment with either 1 or 5 mg/kg AMPH. Similar effects were seen following subchronic glycine treatment in the absence, but not presence of CGP52432 during 5 mg/kg AMPH treatment. In STR SKF97541 decreased only the 1 mg/kg AMPH-induced DA release. Subchronic GLY treatment in STR leads to a significant reduction in basal DA levels, but did not affect AMPH (5 mg/kg)-induced release. Our findings support a model in which NMDA/glycine-site agonists modulate DA release in part through presynaptic GABAB receptors on DA terminals, with both GABAB ligands and GLY significantly modulating AMPH-induced DA release. Both sites, therefore, may represent appropriate targets for drug development in schizophrenia and substance abuse disorders.
Keywords: NMDA receptors, Glycine, Amphetamine, GABAB receptors, Dopamine, Schizophrenia
1. Introduction
Dopaminergic dysfunction is a core component of schizophrenia, potentially related to dysfunction of local and long-range feedback mechanisms (Carlsson et al., 2001). A major challenge has been to delineate underlying neurochemical and molecular events, and to develop strategies for improved dopamine (DA) regulation. Since the introduction of the glutamatergic hypotheses of schizophrenia focusing on disturbances of N-methyl-d-aspartate (NMDA) neurotransmission (Javitt and Zukin, 1991), numerous studies have examined the neurochemical effects of NMDA receptor antagonists on cortical (Balla et al., 2003; Del Arco and Mora, 1999; Jentsch et al., 1997) and subcortical (Balla et al., 2001; Jentsch et al., 1998; Takahata and Moghaddam, 2003) regulation of brain DA systems.
The validity of the NMDA/glutamatergic approach is affirmed not only in rodent (Balla et al., 2001, 2003) and primate models (Linn et al., 1999), but importantly also in comparative in vivo neuroimaging studies of amphetamine (AMPH)-induced DA release both in schizophrenia patients (Breier et al., 1997; Laruelle et al., 1996) and healthy volunteers challenged with ketamine (Kegeles et al., 2000). A particular target of NMDA-based treatment approaches has been the GLY binding site in the NMDAR complex. High affinity GLY type 1 transporters are co-localized with NMDA receptors, keeping GLY levels low in the microvicinity of NMDA receptors and permitting physiologic regulation of NMDA receptors by GLY. Further, NMDA agonists, including glycine (GLY) and GLY transport inhibitors reverse PCP-induced potentiation of AMPH-induced DA release in prefrontal cortex (PFC) and striatum (STR), suggesting utility of this model in new treatment development in schizophrenia (Javitt et al., 2004; Sershen et al., 2008). The present study further investigates mechanisms underlying modulatory effects of NMDA agonists on brain DA systems in vivo.
DA neurons projecting to PFC and STR are regulated by corticofugal glutamatergic neurons both directly and via GABAergic interneurons (Carlsson et al., 2001; Sesack and Carr, 2002) in brain regions such as PFC, limbic system, basal ganglia and thalamus. GABA mediates its inhibitory effects through two main receptor subtypes, GABAA/C, which are fast, ionotropic receptors, and GABAB, which are slower, metabotropic receptors. GABAB receptors are broadly expressed in CNS, and are located both pre- and postsynaptically, as well as on extrasynaptic membranes of axons or dendrites. GABAB receptors, therefore, may play a critical role in regulation of brain DA systems (Del Arco and Mora, 2002; Santiago et al., 1993a; Smolders et al., 1995; Yonezawa et al., 1998). We have suggested previously that NMDA modulators mediate their effects by stimulating local GABA release within brain regions such as PFC or STR, leading to increased stimulation of GABAB receptors located on presynaptic DA terminals (Javitt et al., 2005). For the present study, we utilized in vivo microdialysis techniques to examine relative effects of GABAB ligands on both basal and AMPH-induced DA release in PFC and STR in control and in GLY-treated rats. Prior studies of GABAB effects of dopaminergic transmission have used primarily the non-selective agent baclofen. The present study utilizes the more selective GABAB agonist and antagonist, SKF97541 and CGP52432, respectively.
2. Methods and materials
2.1. Animal preparation
Studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health. Male Sprague– Dawley rats (280–320 g) bred in our animal colony were used. Rats were maintained under a 10 h/14 h dark/light cycle, and were allowed food and water ad libitum. One group of rats (180–220 g) was fed with 16% GLY enriched food exclusively for 2–3 weeks and during the experimental procedure. The mean serum GLY level in rats receiving subchronic 16% GLY diet for 2 weeks, 1236.0 ± 253.4 nmol/ml, was within the range obtained during recent clinical studies with GLY (Javitt et al., 2004). For microdialysis studies, animals were anesthetized with chloral hydrate (400 mg/kg i.p.) and mounted in a stereotaxic frame (David Kopf Instrument, USA). CMA/12 guide cannulae (CMA/Microdialysis, Sweden) were implanted on opposite site relative to bregma into medial PFC (AP: +4.1, L: +1.0, V: −1.2), and into striatum (AP: +1.0, L: −2.5, V: −6.0) according to the atlas of Paxinos and Watson (1998). The guide cannula inserted into the cortical region was angled forward 20° from normal so that it projected rostal to caudal (see Fig.1). The guide cannulae and 2 skull screws were secured in place with dental cement. Buprenorphine (0.03 mg/kg) was given i.p. in order to post-operative analgesia. Animals were allowed to recover 48–72 h before microdialysis experiment, which was carried out in awake, freely moving rats using a BAS BeeKeeper™ System (BAS Inc., USA).
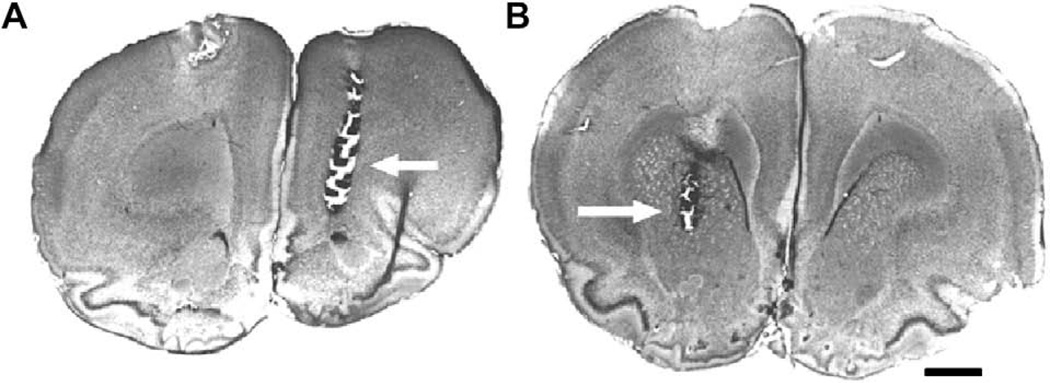
Fig. 1.
The locations of the microdialysis probes were confirmed histologically in Nissl stained sections. The locations of sampling in the PFC (A) were medial and rostral to the anterior forceps of the corpus callosum (arrow). Striatal sampling sites (B) were in the rostral caudoputamen (arrow). Scale bar = 1 mm.
2.2. Drugs
16%-Enriched GLY food was purchased from Dyets Inc. (Bethlehem, PA, USA). Amphetamine was obtained from RBI (Natick, MA, USA). Baclofen and SKF97541 GABAB agonists and CGP52432 GABAB antagonist were purchased from TOCRIS (Ellisville, MO, USA).
Stock solutions of GABAB modulators were prepared (1 mM in H2O) and kept until use at −20 °C. Before use, stock solutions were diluted with Mg2+-free Ringer to the desired concentrations.
2.3. Microdialysis procedure
On the day of the experiment CMA 12 microdialysis probes (CMA/Microdialysis, Sweden) with a molecular cutoff of 20 kDa were inserted into the guide cannulae. A 4.00 mm membrane length probe was used in PFC and 2 mm membrane length was used in STR. Probes were perfused with artificial Mg-free Ringer (in mM, NaCl 147; KCl 4; CaCl2 1.2) at a flow rate of 1.5 µl/min. After a 2-h equilibration period, three 30-min baseline samples were collected. GABAB receptor agonist, SKF97541 (1 µM) or baclofen (50 µM) or GABAB receptor antagonist, CGP52432 (50 µM) was infused into PFC and into STR through the microdialysis probes and kept till the end of the experiment for another 210 min. When CGP52432 (50 µM) was administered together with SKF97541 (1 µM) or with baclofen (50 µM), it was infused 30 min before the agonists. An additional group of animals was challenged with AMPH challenge (1 or 5 mg/kg kg s.c.) with or without pretreatment with GABAB receptor modulators after three 30-min baseline samples. The GABAB modulators were given 30 min before AMPH challenge. A total of 10 dialysate samples (300 min) were collected. GABAB receptor agonists and antagonists doses were based on prior literature (Bon and Galvan, 1996; Fedele et al., 1997; Kawahara et al., 1999; Santiago et al., 1993a,b; Westerink et al., 1998).
On the day following the experiment, rats were anesthetized with chloral hydrate (600 mg/kg i.p.), decapitated and the brains were removed and kept overnight in 10% formaldehyde. Brains were then sectioned and probe locations were verified histologically.
2.4. HPLC analysis
DA and metabolites were measured by electrochemical detection (HPLC–EC) using a BAS-480 system (BAS Inc., USA). Standards and dialysate samples were injected onto a microbore C18 column 2 × 100 mm (BAS Inc., USA). To prevent degradation, 1.5 µl of a 10% ethyl alcohol and 15 mM Na2EDTA (1:1) mixture was added to the dialysate samples and to the standards and stored frozen at −80 °C until assayed (Sershen et al., 2008). The mobile phase consisted of NaH2PO4 25 mM, sodium citrate 50 mM, disodium–EDTA 27 mM, diethylamine–HCl 10 mM, 1-octanesulfonic acid 2.2 mM, acetonitrile 1.7%, and dimethylacetamide 1.1% adjusted to pH 3.5. Flow rate was 0.4 ml/min. A dual glassy carbon electrode vs. Ag/AgCl reference electrode was used and set at 0.60 V and 0.75 V potential. Retention times for DOPAC, DA and for HVA were 4.8, 6.5, and 9.8 min and the sensitivity was between 0.2 and 20 nA.
Data were acquired on a PC using BAS-5 interface (BAS Inc., USA). Standard curves were constrsucted using 7 pts between 0.5 and 250 pg/10 µl for DA. Correlation coefficients of >0.98 were obtained for all curves. The limit of detection is 0.5 pg/10 µl for DA.
2.5. Statistical analysis
Primary dependent measures consisted of DA levels prior to and following GABAB modulators and AMPH administration. Data were analyzed initially using repeated-measures ANOVA with Geisser-Greenhouse correction; with between-subject measure of drug condition (control, GABAB agonist/antagonist, and subchronic GLY) and within-subject measure of time (i.e., fraction) following GABAB drugs infusion and AMPH administration. Significant group × time interaction effects were further analyzed using separate between-group ANOVAs for mean DA levels within early (0–90 min) and late (150–210 min) intervals following AMPH administration. Intervals are based upon prior studies investigating GLY effects on AMPH-induced DA release (Balla et al., 2001, 2003; Javitt et al., 2003). Significant overall between-condition effects were followed up by using pairwise post hoc t-tests. A Levene’s Test for Equality of Variances was used to determine whether a homogeneity- or heterogeneity of variance model should be used for each test. Two-tailed statistics with an α level of significance of p < 0.05 were used throughout. Effect sizes for pair-wise comparisons were calculated by dividing the mean difference by the pooled variance of the values, and were expressed in std. dev (d) units. Effect sizes were interpreted according to the convention of Cohen (1988), with cutoffs of 0.2, 0.5 and 0.8 for small, medium and large effect-sizes, respectively. Data in the text are mean ± SEM.
3. Results
3.1. GABAB ligands on the basal release of DA in prefrontal cortex and in striatum
The effects of the GABAB agonists SKF97541 (1 µM) and baclofen (50 µM) and antagonist CGP52432 (50 µM) on extracellular levels of DA in PFC and in STR were assessed using local infusion through the microdialysis probe (Fig. 2A, B and Table 1).
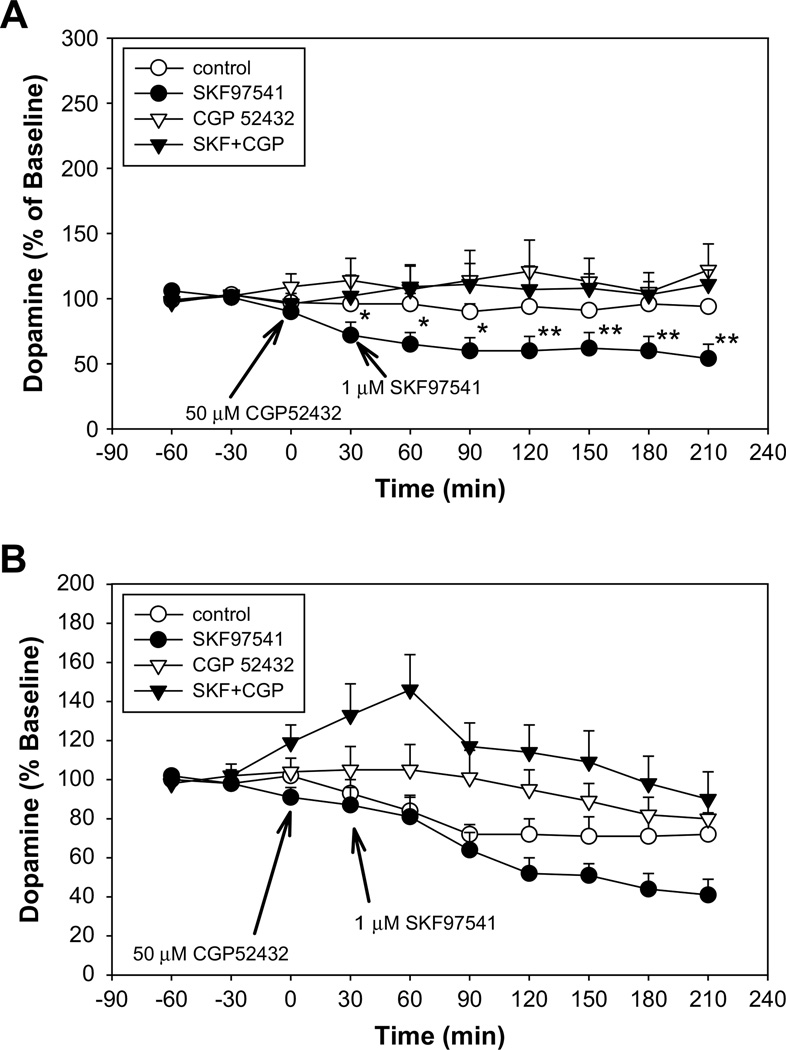
Fig. 2.
Shows the effect of the GABAB agonist SKF97541 on extracellular dopamine levels in the presence and absence of the antagonist CGP52432 following local administration into prefrontal cortex (A) or striatum (B) at indicated doses. (A) Baseline DA levels for control group 1.92 ± 0.41 pg/10 µl (n = 6), for the SKF97541 group 1.95 ± 0.56 pg/10 µl (n = 10), for the CGP52432 group 1.55 ± 0.35 pg/10 µl (n = 9) and for the SKF97541 + CGP52432 group 1.56 ± 0.37 pg/10 µl (n = 6). (B) Baseline DA levels for control group 10.30 ± 2.15 pg/10 µl (n = 8), for the SKF97541 group 10.63 ± 1.43 pg/10 µl (n = 8), for the CGP52432 group 8.06 ± 1.13 pg/10 µl (n = 8) and for the SKF97541 + CGP52432 group 7.35 ± 1.71 pg/10 µl (n = 5).
Table 1.
Effects of GABAB receptor agonist baclofen, on the extracellular level of DA in PFC and in STR in the absence or presence of the GABAB antagonist CGP52432.
| Treatment | Prefrontal cortex | Striatum |
|---|---|---|
| Dopamine (% of baseline) | ||
| Control | 94 ± 3 (n = 6) | 71 ± 10 (n = 7) |
| Baclofen (50 µM) | 53 ± 6* (n = 6) | 67 ± 13 (n = 7) |
| CGP52432 (50 µM) | 113 ± 17# (n = 9) | 84 ± 9 (n = 8) |
| Baclofen + CGP | 155 ± 37 (n = 6) | 98 ± 20 (n = 6) |
Data are expressed as a percent of baseline values and showing the mean value of the (150–210 min) time period after baclofen and/or CGP52432 administration (*p = 0.00, #p = 0.16 relative to control).
In PFC both GABAB agonists SKF97541 and baclofen reduced the extracellular level of DA, which was prevented by prior treatment with CGP52432 (Fig. 2A and Table 1). This was manifest across conditions as a significant main effect of GABAB modulators. (F5,37 = 3.66, p = 0.01). There was also a highly significant condition × time interaction (F35,259 = 2.65, p < 0.01). Follow-up ANOVAs were conducted independently over 2 time frames – 0–90 and 150–210 min following drug administration – to further evaluate the condition × time interaction. Effects of GABAB modulators were more robust during the late (150–210 min) (F5,37 = 4.71, p < 0.01) than early (0–90 min) (F5,37 = 2.48, p = 0.05) time period. Post hoc t-test showed significant between-group differences in both the SKF97541 (p = 0.01) and baclofen (p < 0.01) conditions relative to control, but not in the CGP52432 (p = 0.39) group, CGP52432 + SKF97541 (p = 0.24) or CGP52432 + baclofen (p = 0.16) groups.
In STR, modulation of GABAB receptors by SKF97541, baclofen or CGP52432 significantly modulated the basal levels of DA (F5,36 = 3.16, p = 0.02), with no observed condition × time interaction (F35,252 = 0.97, p = 0.49) (Fig. 2B and Table 1). Further no significant between-group differences in DA levels were observed by GABAB agonists following drug administration at either the early (0–90 min) or late (150–210 min) post-drug interval, although SKF97541 had a tendency to reduce the basal DA release during the 150–210 min period. A significant increase was detected at the early time points (0–90 min) after drug administration, when CGP52432 was administered together with SKF97541 (p < 0.01) or with baclofen (p = 0.03).
3.2. GABAB agonists on AMPH-induced DA release in prefrontal cortex and in striatum
The effects of GABAB receptor agonists SKF97541 (1 µM) and baclofen (50 µM) were tested on AMPH-induced DA release in PFC and in STR (Fig. 3A–D).
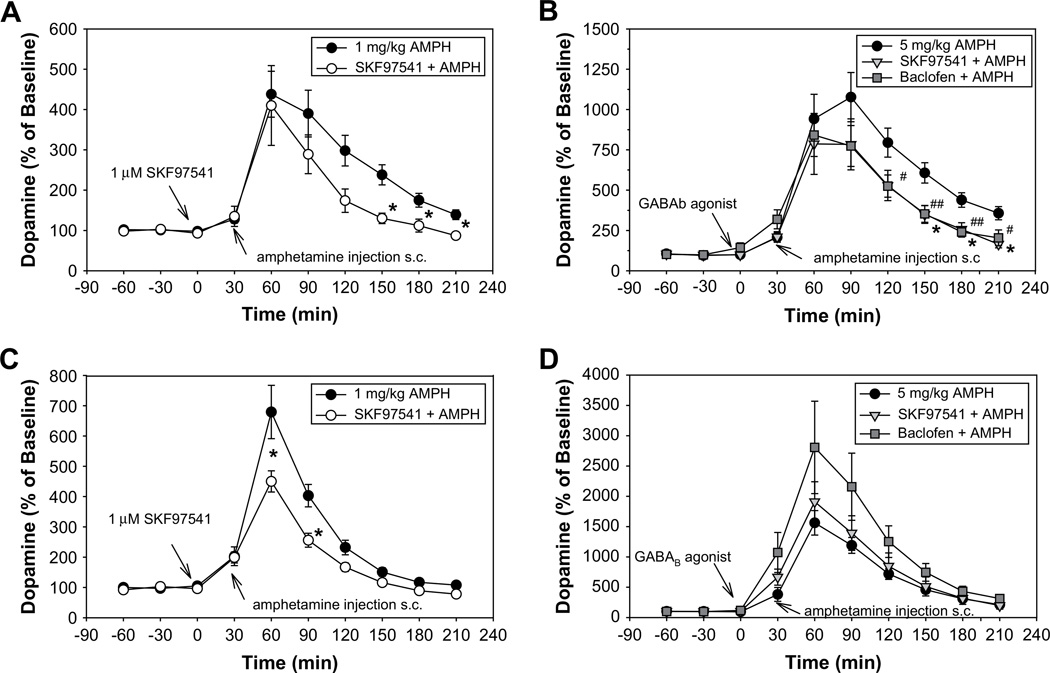
Fig. 3.
Represent the effect of local administration of the GABAB agonists on AMPH-induced DA release (1 and 5 mg/kg) in PFC (A, B) and in STR (C, D) at indicated doses. (A) Baseline DA levels for control group getting 1 mg/kg AMPH injection 1.33 ± 0.19 pg/10 µl (n = 10), for SKF97541+1AMPH group 1.22 ± 0.12 pg/10 µl (n = 5). (B) Baseline DA levels for group getting 5 mg/kg AMPH injection 1.17 ± 0.19 pg/10 µl (n = 12), for SKF97541+5AMPH group 1.49 ± 0.41 pg/10 µl (n = 7) and for the Baclofen+5AMPH group 1.34 ± 0.24 pg/10 (n = 7). (C) Following baseline DA levels were detected: in control group getting 1 mg/kg AMPH injection 11.70 ± 2.00 pg/10 µl (n = 15) and for SKF97541 +1AMPH group 13.52 ± 1.27 pg/ 10 µl (n = 9). (D) Baseline DA levels for control group getting 5 mg/kg AMPH injection 13.13 ± 1.12 pg/10 µl (n = 14), for SKF97541 +5AMPH group 10.90 ± 1.20 pg/10 µl (n = 7) and for the Baclofen + 5AMPH group 10.50 ± 1.99 pg/10 µl (n = 9).
In PFC in control animals, AMPH induced a dose-dependent elevation in DA levels that peaked at 60 min following 1 mg/kg administration (Fig. 3A) and 90 min following 5 mg/kg administration (Fig. 3B). DA levels returned to close to baseline by 210 min after the 1 mg/kg dose, but remained elevated to 357 ± 38% after the 5 mg/kg dose. Local administration of SKF97541, significantly reduced the AMPH-induced DA release in both conditions at the 150–210 min postinjection interval (1 mg/kg F2,15 = 5.64, p = 0.02; 5 mg/kg F5,53 = 2.65, p = 0.03), although the repeated-measures ANOVA revealed a significant difference across drug conditions (F5,48 = 3.86, p = 0.01) and a significant condition × time interaction (F35,336 = 2.38, p = 0.02) only at the 5 mg/kg AMPH dose. A similar effect was observed with baclofen at the 5 mg/kg AMPH group (Fig. 3B).
Post-hoc t-test revealed significant, large effect-size (d > 0.8), between-group differences throughout the 150–210 min post-treatment interval in both AMPH conditions by GABAB agonists (SKF91541 + 1AMPH vs. 1AMPH p = 0.01, d = 1.44, SKF97541+5AMPH vs. 5AMPHp= 0.01, d= 1.66, Baclofen + 5AMPH vs. 5AMPH p = 0.01, d = 1.33) (Fig. 3A, B).
In STR AMPH treatment induced dose-dependent increases in DA levels that peaked at 60 min after drug administration for both the 1 and 5 mg/kg doses (Fig. 3C, D). DA levels returned to baseline by 210 min following the 1 mg/kg dose, but remained elevated to 212 ± 29% following the 5 mg/kg dose. SKF97541 and baclofen significantly attenuated the AMPH-induced DA release at both doses as reflected by a significant effect of drug condition (1 mg/kg F2,28 = 4.77, p = 0.02 and 5 mg/kg F5,45 = 3.85, p = 0.01). A significant condition × interaction was also observed but only at the higher dose of AMPH (F35,315 = 3.07, p < 0.01). A follow-up ANOVA at the higher dose revealed a significant change in AMPH-induced DA level only at early (0–90 min) time points after drug administration (F5,49 = 2.99, p = 0.02). Post hoc t-test showed that neither SKF97541, nor baclofen modulated AMPH-induced DA release significantly at either time interval following 5 mg/kg AMPH treatment, but that SKF97541 significantly reduced AMPH-induced DA release following 1 mg/kg AMPH in STR during both early (0– 90 min) and late (150–210 min) time intervals (1AMPH vs. SKF97541 + 1AMPH p = 0.01, d = 0.95 and p = 0.03, d = 0.72) (Fig. 3C, D), with moderate and large effect-sizes, respectively.
3.3. NMDA/GABAB interaction
To determine whether GABAB receptors are involved in mediating the effect of NMDARs on regulating the DA levels in PFC and in STR, a group of animals was pretreated subchronically with GLY for 2 weeks. Because of large dosing requirements, GLY was administered using specially modified (16% by weight GLY) diet. This treatment produces plasma GLY levels similar to those observed in clinical trials.
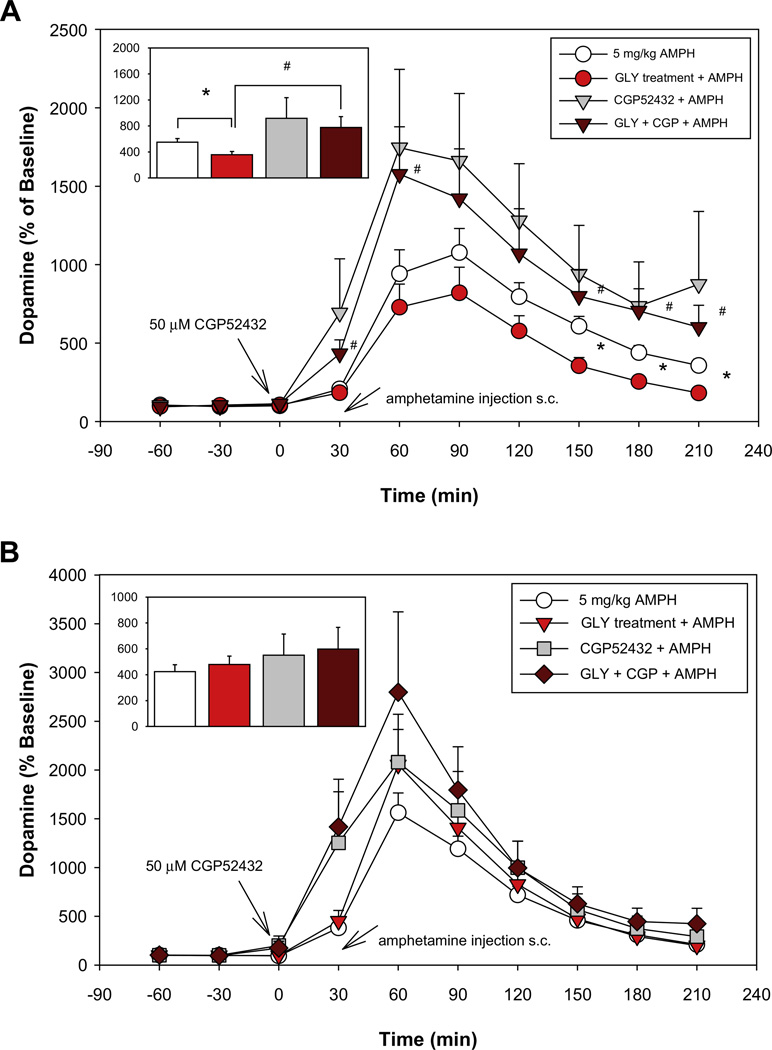
In PFC chronic GLY treatment significantly reduced the AMPH (5 mg/kg)-induced DA release (F5,48 = 3.86, p = 0.01) overall, with follow-up ANOVA showing a significant treatment × condition interaction (F35,336 = 2.38, p = 0.02). Post-hoc t-test during the late (150–210 min) time interval showed a significant, large effect-size reduction in AMPH-induced DA release during this interval (5AMPH vs. GLY + 5AMPH p = 0.01, d = 1.20) (Fig. 4A). In PFC, GLY treatment did not significantly modulate basal DA levels (Table 2). Intra-PFC application of CGP52432 (50 µM) through the micro-dialysis probe did not affect AMPH-induced DA release in the control group (5AMPH vs. CGP52432 + 5AMPH p = 0.31), but significantly attenuated the degree of reduction induced by GLY in the GLY-treated group (5AMPH vs. GLY + CGP + 5AMPH p = 0.12) (Fig. 4A).
Fig. 4.
Effect of subchronic administration of GLY on AMPH-induced DA (5 mg/kg) DA release in PFC (A) or STR (B) in the absence or presence of the GABAB antagonist CGP52432 infused locally through the microdialysis probe. Inset: each bar represents a mean ± SEM value (percent of baseline) of the 150–210 min time period after AMPH administration. (A) Baseline DA values for control group receiving 5 mg/kg AMPH 1.17 ± 0.19 pg/10 µl (n = 12), for CGP52432 + 5AMPH group 0.96 ± 0.18 pg/10 µl (n = 11), for the GLY-treated group receiving 5 mg/kg AMPH injection 1.08 ± 0.21 pg/10 µl (n = 14) and for GLY-treated group receiving CGP52432 + 5AMPH 0.94 ± 0.21 pg/10 µl (n = 6). (B) Baseline DA values for control group receiving 5 mg/kg AMPH 13.13 ± 1.12 pg/10 µl (n = 14), for CGP52432 + 5AMPH group 11.06 ± 2.60 pg/10 µl (n = 9), for the GLY-treated group receiving 5 mg/kg AMPH injection 8.11 ± 1.29 pg/10 µl (n = 10) and for GLY-treated group receiving CGP52432 + 5AMPH 6.10 ± 1.03 pg/10 µl (n = 6).
Table 2.
Effects of glycine (16% by diet) on the basal levels of dopamine in PFC and STR (pg/10 µl).
| Treatment | Prefrontal cortex | Striatum |
|---|---|---|
| Dopamine (pg/10 µl) | ||
| Control | 1.39 ± 0.21 (n = 17) | 12.60 ± 1.15 (n = 20) |
| Glycine treatment | 1.10 ± 0.19 (n = 23) | 7.91 ± 0.94* (n = 17) |
Table shows the average value of the first 2 basal samples in control and in GLY treated animals.
Data are mean ± SEM (*p = 0.01 relative to control).
In STR subchronic dietary GLY administration did not significantly modulate AMPH (5 mg/kg)-induced DA release (Fig. 4B), but significantly reduced the basal extracellular level of DA (p = 0.01, d = 1.03) (Table 2). Neither GLY nor CGP52432 induced significant modulation of AMPH-induced DA release relative to control when given alone. However, combined CGG52432 and GLY significantly stimulated AMPH-induced DA release relative to control during the early, but not late, time period (5AMPH vs. GLY + CGP + 5AMPH p = 0.03, d = 1.1) (Fig. 4B).
4. Discussion
This study investigated the effects of GABAB ligands on basal and AMPH-induced DA release in both PFC and STR, as well as the relative role of GABAB receptors in mediating the inhibitory effect of NMDAR modulation by oral administration of GLY. We compared responses of balcofen with a more potent and selective agonist (SKF97541) and antagonist (CGP52432) of the GABAB receptor and characterized the effect of these drugs on both basal and AMPH-induced DA release in both control and GLY-treatment condition. A primary reason for investigating AMPH-induced DA release in rodents is that AMPH-induced synaptic DA concentration has been shown to be elevated in schizophrenia using in vivo imaging, with inferred percentage increases following AMPH administration of between 400 and 1500% (Breier et al., 1997; Laruelle et al., 1996). AMPH-induced DA release in rodents may therefore model this phenomenon.
Our data indicate, that both GABAB agonists employed, baclofen and SKF97541, significantly reduced both basal and AMPH-induced DA release in the PFC (see Fig. 2A, 3A, Table 1). Chronic GLY treatment, in contrast, did not modulate the basal DA levels in PFC (Table 2), but did significantly reduce AMPH-induced DA release (see Fig. 4A). Our results are consonant with previous observations that NMDA receptor agonists increase the extracellular levels of GABA and glutamate (GLU) (Del Arco and Mora, 1999, 2002) and reduce the extracellular levels of DA (Feenstra et al., 1995) in PFC, while NMDA antagonists (PCP, MK-801) reduce GABA (Yonezawa et al., 1998) and increase DA levels (Yonezawa et al., 1998). In PFC, NMDA receptors exert a tonic inhibitory control over basal dopamine release (Takahata and Moghaddam, 1998), supporting the hypothesis that GLU may regulate the DA release in PFC via NMDA receptors on GABAergic interneuron. An ultrastructural study by DeBiasi et al. also supports the localization of NMDAR to GABA interneurons (DeBiasi et al., 1996). In the presence of CGP52432, GLY did not significantly alter AMPH-induced DA release (see Fig. 4A), supporting the concept that in PFC NMDAR attenuates DA release through modulation of local GABAergic feedback mechanisms.
In STR, GABAB ligands and GLY showed a differential pattern of action than that observed in PFC. In particular, no significant effect of GABAB ligands was observed on basal DA levels, relative to controls, although this was influenced by a gradual reduction of STR DA levels over the course of the microdialysis even in the control condition. By contrast chronic GLY treatment significantly reduced basal DA levels in STR, whereas no similar pattern was observed in PFC. This finding may reflect a true difference between regions, although the much lower basal DA levels in PFC may have made detection of GLY-related changes more difficult.
In STR, NMDA receptors exert a dual facilitatory–inhibitory influence on basal dopaminergic function, consistent with dual preand post-synaptic localizations (Cheramy et al., 1998; Krebs et al., 1993). This effect can be local or through the corticostriatal pathway where the net effect of glutamate on extracellular level of DA is probably excitatory (Karreman and Moghaddam, 1996). Other studies indicate that activation of NMDA receptor increases striatal DA release in vivo and in vitro (Javitt et al., 2000; Segovia et al.,1997) and increases the extracellular level of GABA (Hernandez et al., 2003; Javitt et al., 2005; Krebs et al., 1993) demonstrating the physiological relevance of a feedback mechanism through NMDA receptors located on intrinsic GABAergic interneurons. Our findings are consistent with the net inhibitory effect of NMDA receptors on tonic STR DA release, based upon observed effects of GLY in STR, so that treatment with an NMDA agonist may produce limited net effect in the absence of perturbation of baseline NMDA function.
In STR, neither GABAB ligands nor subchronic GLY treatment significantly affected the degree of AMPH-induced DA release following 5 mg/kg treatment, but SKF97541 significantly reduced release following 1 mg/kg AMPH. As with differences in effects on basal DA levels by GLY, this may reflect different neural regulation mechanisms controlling DA release via GABAB receptors in the two brain regions.
In STR, GABAB receptors are also located on presynaptic nigrostriatal terminals (Boyes and Bolam, 2003), where they would be expected to produce net inhibitory effect on DA release; confirmed by the reduction of the extracellular level of DA induced by the lower 1 mg/kg dose of AMPH (Fig. 3C). However, receptors are also located on glutamatergic terminals and intrinsic local GABAergic inhibitory neurons (Lacey et al., 2005; Nisenbaum et al., 1993; Santiago et al., 1993a), at which site they would be expected to reduce local feedback inhibition onto DA terminals and thus disinhibit DA release. This may account for the absence of a net effect of either GABAB ligands or GLY on high AMPH-induced DA release, and may reflect the offsetting effects of local and long-loop feedback mechanisms, which would be present in in vivo studies but absent in studies of isolated STR (Javitt et al., 2005).
The regional differences in effects of GABAB ligands that we observed in the present study are consistent with prior investigations of GABA/DA interactions in PFC and STR. For example, Del Arco and Mora (Del Arco and Mora, 2002; Segovia et al., 1997) found that GLU facilitates GABAergic transmission in PFC via both NMDA and AMPA receptors, with reduced DA neurotransmission being mediated primarily through NMDA receptors (Del Arco and Mora, 1999). Similarly, Santiago et al. (1993a) observed, significant reduction of basal DA release in PFC and substantia nigra (SN) by baclofen, but no significant effect in the STR. As in the present study, the inhibitory effect of gamma-vinyl GABA, a GABA-transaminase inhibitor (GVG) over PCP-induced DA release were more pronounced in PFC than in subcortical regions (Schiffer et al., 2001).
Finally, in clinical studies, compounds that stimulate the NMDA/ glycine site, including GLY, d-serine, d-alanine and the partial agonist d-cycloserine, as well as the prototypic glycine transport inhibitor sarcosine, have been found to produce improvement in persistent symptoms of schizophrenia when administered together with antipsychotic drugs (Javitt, 2006). A converging body of evidence implicates dysregulation of the GABA system in the pathology of schizophrenia (Carlsson et al., 2001; Hashimoto et al., 2008). Assuming reduced GABAergic tone in schizophrenia, the present findings would predict beneficial effects of both GABAB and NMDA/glycine-site agonists.
In summary this study supports prior research indicating that PFC and STR DA projection systems may be modulated by subchronically administered GLY, operating at least in part through presynaptic GABAB receptors. Thus, a potential mechanism whereby GLY-site agonists may influence DA release in PFC and STR occurs via modulation of local and/or long-range feedback loops. At present, no psychotherapeutic agents are available that selectively target central presynaptic GABAB receptors. Given the importance of AMPH-induced DA release both as a model for DA dysfunction in schizophrenia and mechanism underlying psychotomimetic effects of AMPH and other DAergic agents, present findings suggest that GABAB receptors are a potential site of therapeutic intervention in psychotic and drug abuse disorders.
Acknowledgements
This work was supported by grants R01 DA03383 and R37 MH49334 to DCJ. DCJ holds intellectual property rights for use of NMDA agonists in treatment of schizophrenia.
References
- Balla A, Koneru R, Smiley J, Sershen H, Javitt DC. Continuous phencyclidine treatment induces schizophrenia-like hyperreactivity of striatal dopamine release. Neuropsychopharmacology. 2001;25:157–164. doi: 10.1016/S0893-133X(01)00230-5. [DOI] [PubMed] [Google Scholar]
- Balla A, Sershen H, Serra M, Koneru R, Javitt DC. Subchronic continuous phencyclidine administration potentiates amphetamine-induced frontal cortex dopamine release. Neuropsychopharmacology. 2003;28:34–44. doi: 10.1038/sj.npp.1300019. [DOI] [PubMed] [Google Scholar]
- Bon C, Galvan M. Electrophysiological actions of GABAB agonists and antagonists in rat dorso-lateral septal neurones in vitro. Br. J. Pharmacol. 1996;118:961–967. doi: 10.1111/j.1476-5381.1996.tb15493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes J, Bolam JP. The subcellular localization of GABA(B) receptor subunits in the rat substantia nigra. Eur. J. Neurosci. 2003;18:3279–3293. doi: 10.1111/j.1460-9568.2003.03076.x. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc. Natl. Acad. Sci. U. S. A. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu. Rev. Pharmacol. Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- Cheramy A, L’hirondel M, Godeheu G, Artaud F, Glowinski J. Direct and indirect presynaptic control of dopamine release by excitatory amino acids. Amino Acids. 1998;14:63–68. doi: 10.1007/BF01345244. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. second ed. Hillsdale, NJ.: Lawrence Erlbaum Assoc; 1988. [Google Scholar]
- DeBiasi S, Minelli A, Melone M, Conti F. Presynaptic NMDA receptors in the neocortex are both auto- and heteroreceptors. Neuroreport. 1996;7:2773–2776. doi: 10.1097/00001756-199611040-00073. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Effects of endogenous glutamate on extracellular concentrations of GABA, dopamine, and dopamine metabolites in the prefrontal cortex of the freely moving rat: involvement of NMDA and AMPA/KA receptors. Neurochem. Res. 1999;24:1027–1035. doi: 10.1023/a:1021056826829. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. NMDA and AMPA/kainate glutamatergic agonists increase the extracellular concentrations of GABA in the prefrontal cortex of the freely moving rat: modulation by endogenous dopamine. Brain Res. Bull. 2002;57:623–630. doi: 10.1016/s0361-9230(01)00758-4. [DOI] [PubMed] [Google Scholar]
- Fedele E, Varnier G, Raiteri M. In vivo microdialysis study of GABA(A) and GABA(B) receptors modulating the glutamate receptor/NO/cyclic GMP pathway in the rat hippocampus. Neuropharmacology. 1997;36:1405–1415. doi: 10.1016/s0028-3908(97)00113-5. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, van der WW, Botterblom MH. Concentration-dependent dual action of locally applied N-methyl-d-aspartate on extracellular dopamine in the rat prefrontal cortex in vivo. Neurosci. Lett. 1995;201:175–178. doi: 10.1016/0304-3940(95)12164-1. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez LF, Segovia G, Mora F. Effects of activation of NMDA and AMPA glutamate receptors on the extracellular concentrations of dopamine, acetylcholine, and GABA in striatum of the awake rat: a microdialysis study. Neurochem. Res. 2003;28:1819–1827. doi: 10.1023/a:1026115607216. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Is the glycine site half saturated or half unsaturated? Effects of glutamatergic drugs in schizophreniapatients. Curr. Opin. Psychiatry. 2006;19:151–157. doi: 10.1097/01.yco.0000214340.14131.bd. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Sershen H, Hashim A, Lajtha A. Inhibition of striatal dopamine release by glycine and glycyldodecylamide. Brain Res. Bull. 2000;52:213–216. doi: 10.1016/s0361-9230(00)00258-6. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Balla A, Burch S, Suckow R, Xie S, Sershen H. Reversal of phencyclidine-induced dopaminergic dysregulation by N-methyl-d-aspartate receptor/glycine-site agonists. Neuropsychopharmacology. 2004;29:300–307. doi: 10.1038/sj.npp.1300313. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Hashim A, Sershen H. Modulation of striatal dopamine release by glycine transport inhibitors. Neuropsychopharmacology. 2005;30:649–656. doi: 10.1038/sj.npp.1300589. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Le D, Youngren KD, Roth RH. Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology. 1997;17:92–99. doi: 10.1016/S0893-133X(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR, Roth RH. Subchronic phencyclidine administration increases mesolimbic dopaminergic system responsivity and augments stress-and psychostimulant-induced hyperlocomotion. Neuropsychopharmacology. 1998;19:105–113. doi: 10.1016/S0893-133X(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J. Neurochem. 1996;66:589–598. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Kawahara H, Westerink BH. Tonic regulation of the activity of noradrenergic neurons in the locus coeruleus of the conscious rat studied by dual-probe microdialysis. Brain Res. 1999;823:42–48. doi: 10.1016/s0006-8993(99)01062-8. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Zea-Ponce Y, Rodenhiser-Hill J, Mann JJ, Van Heertum RL, Cooper TB, Carlsson A, Laruelle M. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol. Psychiatry. 2000;48:627–640. doi: 10.1016/s0006-3223(00)00976-8. [DOI] [PubMed] [Google Scholar]
- Krebs MO, Kemel ML, Gauchy C, Desban M, Glowinski J. Local GABAergic regulation of the N-methyl-d-aspartate-evoked release of dopamine is more prominent in striosomes than in matrix of the rat striatum. Neuroscience. 1993;57:249–260. doi: 10.1016/0306-4522(93)90060-s. [DOI] [PubMed] [Google Scholar]
- Lacey CJ, Boyes J, Gerlach O, Chen L, Magill PJ, Bolam JP. GABA(B) receptors at glutamatergic synapses in the rat striatum. Neuroscience. 2005;136:1083–1095. doi: 10.1016/j.neuroscience.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn GS, O’Keeffe RT, Schroeder CE, Lifshitz K, Javitt DC. Behavioral effects of chronic phencyclidine in monkeys. Neuroreport. 1999;10:2789–2793. doi: 10.1097/00001756-199909090-00017. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Berger TW, Grace AA. Depression of glutamatergic and GABAergic synaptic responses in striatal spiny neurons by stimulation of presynaptic GABAB receptors. Synapse. 1993;14:221–242. doi: 10.1002/syn.890140306. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. fourth ed. San Diego: Academic Press Inc; 1998. [Google Scholar]
- Santiago M, Machado A, Cano J. In vivo release of dopamine from rat striatum, substantia nigra and prefrontal cortex: differential modulation by baclofen. Br. J. Pharmacol. 1993a;109:814–818. doi: 10.1111/j.1476-5381.1993.tb13647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago M, Machado A, Cano J. Regulation of the prefrontal cortical dopamine release by GABAA and GABAB receptor agonists and antagonists. Brain Res. 1993b;630:28–31. doi: 10.1016/0006-8993(93)90638-4. [DOI] [PubMed] [Google Scholar]
- Schiffer WK, Gerasimov M, Hofmann L, Marsteller D, Ashby CR, Brodie JD, Alexoff DL, Dewey SL. Gamma vinyl-GABA differentially modulates NMDA antagonist-induced increases in mesocortical versus mesolimbic DA transmission. Neuropsychopharmacology. 2001;25:704–712. doi: 10.1016/S0893-133X(01)00268-8. [DOI] [PubMed] [Google Scholar]
- Segovia G, Del Arco A, Mora F. Endogenous glutamate increases extracellular concentrations of dopamine, GABA, and taurine through NMDA and AMPA/kainate receptors in striatum of the freely moving rat: a microdialysis study. J. Neurochem. 1997;69:1476–1483. doi: 10.1046/j.1471-4159.1997.69041476.x. [DOI] [PubMed] [Google Scholar]
- Sershen H, Balla A, Aspromonte JM, Xie S, Cooper TB, Javitt DC. Characterization of interactions between phencyclidine and amphetamine in rodent prefrontal cortex and striatum: implications in NMDA/glycine-site-mediated dopaminergic dysregulation and dopamine transporter function. Neurochem. Int. 2008;52:119–129. doi: 10.1016/j.neuint.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB. Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiol. Behav. 2002;77:513–517. doi: 10.1016/s0031-9384(02)00931-9. [DOI] [PubMed] [Google Scholar]
- Smolders I, De Klippel N, Sarre S, Ebinger G, Michotte Y. Tonic GABA-ergic modulation of striatal dopamine release studied by in vivo microdialysis in the freely moving rat. Eur. J. Pharmacol. 1995;284:83–91. doi: 10.1016/0014-2999(95)00369-v. [DOI] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B. Glutamatergic regulation of basal and stimulus-activated dopamine release in the prefrontal cortex. J. Neurochem. 1998;71:1443–1449. doi: 10.1046/j.1471-4159.1998.71041443.x. [DOI] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B. Activation of glutamate neurotransmission in the prefrontal cortex sustains the motoric and dopaminergic effects of phencyclidine. Neuropsychopharmacology. 2003;28:1117–1124. doi: 10.1038/sj.npp.1300127. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Enrico P, Feimann J, De Vries JB. The pharmacology of mesocortical dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and prefrontal cortex of the rat brain. J. Pharmacol. Exp. Ther. 1998;285:143–154. [PubMed] [Google Scholar]
- Yonezawa Y, Kuroki T, Kawahara T, Tashiro N, Uchimura H. Involvement of gamma-aminobutyric acid neurotransmission in phencyclidine-induced dopamine release in the medial prefrontal cortex. Eur. J. Pharmacol. 1998;341:45–56. doi: 10.1016/s0014-2999(97)01435-0. [DOI] [PubMed] [Google Scholar]