Abstract
This study was designed to assess whether functional magnetic resonance imaging (fMRI) following antidepressant administration (pharmaco-fMRI) is sufficiently sensitive to detect differences in patterns of activation between enantiomers of the same compound. Healthy adult males (n = 11) participated in a randomized, double-blind, cross-over trial with three medication periods during which they received citalopram (racemic mixture), escitalopram (S-citalopram alone), or placebo for 2 weeks. All participants had high expression serotonin transporter genotypes. An fMRI scan that included passive viewing of overt and covert affective faces and affective words was performed after each medication period. Activation in response to overt faces was greater following escitalopram than following citalopram in the right insula, thalamus, and putamen when the faces were compared with a fixation stimulus. For the rapid covert presentation, a greater response was observed in the left middle temporal gyrus in the happy versus fearful contrast following escitalopram than following citalopram. Thus, the combination of genomics and fMRI was successful in discriminating between two very similar drugs. However, the pattern of activation observed suggests that further studies are indicated to understand how to optimally combine the two techniques.
Keywords: Functional magnetic resonance imaging, Pharmaco-fMRI, Antidepressant, Imaging genetics, Affective stimulus
1. Introduction
Recently, there has been increased interest in the use of brain imaging to study drug effects in the central nervous system (CNS). While some authors have suggested that measuring receptor occupancy may be most useful when evaluating engagement with a specific molecular target (Cole et al., 2012), it is an expensive and technologically complex undertaking to develop the appropriate ligand on a timeline that informs the drug development program. A surrogate for receptor occupancy is to study the effects of drugs on circuitry relevant to the disease targeted by the drug candidate. Functional magnetic resonance imaging (fMRI) is a technique that focuses on changes in regional perfusion in response to perturbations of the CNS. Pharmaco-fMRI has been used by multiple investigators to examine the effects of selective serotonin reuptake inhibitors (SSRIs) on the mood circuitry in healthy volunteers (Anderson et al., 2007; Arce et al., 2008; Simmons et al., 2009; Windischberger et al., 2010). This technique has also been used to investigate the role of serotonin in attention (Wingen et al., 2008). Magnetic resonance approaches have also been incorporated into clinical trials to determine the effects of treatments on brain function in clinical populations (Frodl et al., 2011; Arnone et al., 2012). Although the techniques have been shown to be sufficiently sensitive to detect drug effects on brain circuitry, the limits of the sensitivity of the method have not been defined. Accordingly, this study was designed to assess whether fMRI is sufficiently sensitive to detect the differences between a racemate (citalopram) with both agonist and antagonist properties versus one of the constituent enantiomers with only agonist properties.
Citalopram is a racemic mixture of R-citalopram and S-citalopram, with S-citalopram responsible for the clinical activity of the compound. Preclinical evidence suggests that R-citalopram may actually serve as an antagonist, reducing the efficiency of S-citalopram (Sanchez et al., 2004). Escitalopram consists only of the S-enantiomer. We hypothesized that if R-citalopram indeed functions as an antagonist, then administering equal doses of S-citalopram with and without R-citalopram should result in differences in the degree to which the two drugs affect the activity of the mood circuitry in response to affective stimuli. Specifically, we expected a greater attenuation of activation with the S-enantiomer.
The circuitry involved in mood regulation consists of a dorsal neocortical component and a ventral limbic component, both of which connect with the rostral anterior cingulate cortex (Mayberg, 1997). Depression is associated with decreased activity in the dorsal stream and increased activity in the ventral stream, an effect that is reversed by antidepressant treatment. However, the specific regions of activation in response to antidepressant medications have varied across studies. Multiple brain regions are involved in processing of emotional faces. The fusiform gyrus has a specialized role in face processing (Kanwisher et al., 1997). Masked or covert presentation of happy and fearful faces has been shown to activate occipital and parietal regions as well as regions such as the cingulate gyrus, lingual gyrus, and insula (Pine et al., 2001). Multiple investigators have also observed amygdala activation in response to fearful faces that is attenuated by escitalopram or citalopram (Anderson et al., 2007). Based on these earlier studies and this model of affective illness, we predicted that administration of escitalopram and citalopram would lead to increased activation in dorsal cortical regions such as the dorsolateral prefrontal cortex and attenuated activation in limbic regions including the insula, subgenual cingulate cortex, and caudate.
To reduce the variability in the amount of the molecular target available, a serotonin transporter genotype that predicts the expression of this protein was used to exclude individuals likely to have lower amounts of the serotonin transporter protein. A polymorphism in the promoter region of the serotonin transporter (SLC6A4) gene has been shown to affect the amount of the transporter protein in living and postmortem brain (Heinz et al., 1998; Little et al., 1998) and to affect clinical response to antidepressants (Porcelli et al., 2012). The short allele (S), which produces less protein, has been associated with increased risk of depression as well as poorer response to antidepressant treatment, particularly in individuals who have experienced stressful life events (Caspi et al., 2003; Smits et al., 2004), although subsequent meta-analyses have had mixed results (Risch et al., 2009; Karg et al., 2011). This allele is also associated with greater amygdala activation in response to fearful faces in fMRI studies (Hariri et al., 2002). In a further refinement, it is now recognized that a single nucleotide polymorphism within the long allele (L) results in an Lg allele that functionally resembles the S allele and an La allele that is high in its level of expression (Hu et al., 2006).
We hypothesized that fMRI has adequate sensitivity to discriminate between the effects of citalopram and escitalopram in the mood circuitry of healthy adults. To test this hypothesis we administered racemic citalopram, escitalopram, and placebo in a randomized cross-over design to healthy volunteers with genotypes associated with medium or high SLC6A4 expression.
2. Methods
The protocol was approved by the Institutional Review Boards at St. Elizabeth’s Medical Center and McLean Hospital and conducted according to the Declaration of Helsinki. The trial is registered in ClinicalTrials.gov under accession number NCT00825825.
2.1. Participants
Healthy male volunteers, ages 21–50, with no history of psychiatric disorders or significant medical conditions (including hypertension) were recruited by advertisement. Of the 85 candidates who came to the clinic for screenings, 24 were scanned at least once. All subjects provided written informed consent. All subjects were non-smokers and had normal visual acuity either uncorrected or corrected with contact lenses. Subjects with a serotonin transporter genotype of SS or SLg were excluded. All subjects provided information on their educational background and employment during their screening visit. Enrolled participants included individuals who were unemployed and had limited education. However, the majority of the completers were current undergraduate or graduate students or middle class professionals, including some who worked as researchers themselves.
2.2. Study procedures
All subjects were evaluated for Axis I and II psychopathology by a board-certified psychiatrist (MEH) and the absence of Axis I psychiatric disorders was confirmed using the Structured Clinical Interview for DSM-IV (SCID-I) (First et al., 2002). Screening procedures included urine toxicology and cotinine screens and a visual acuity test. At each medication visit and scanning visit, the clinician-rated Quick Inventory of Depressive Symptomatology (QIDS) (Rush et al., 2003) and the Young Mania Rating Scale (YMRS) (Young et al., 1978) were administered. The Discontinuation Emergent Signs and Symptoms (DESS) scale (Rosenbaum et al., 1998) was administered approximately 2 weeks after stopping medication for each medication period.
2.3. Medication protocol
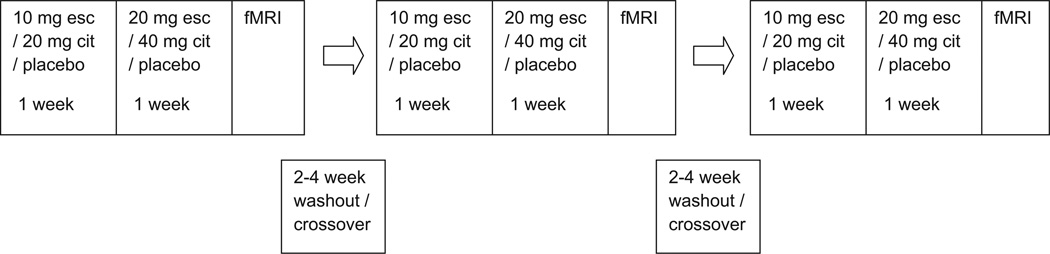
Subjects participated in three medication periods of 14 days in a randomized, double-blind, crossover design. The medications were escitalopram (10 mg during week 1 and 20 mg during week 2), citalopram (20 mg during week 1 and 40 mg during week 2), and matched placebo. The dosing scheme was selected to provide equivalent doses of S-citalopram in the presence or absence of R-citalopram. In cases where an MRI was delayed, the number of dosing days was increased up to two additional days to ensure that drug levels were maintained. Medication periods were separated by a washout period of 2–4 weeks.
2.4. BOLD fMRI data acquisition
Each medication period was followed by an MRI session at the Neuroimaging Center at McLean Hospital. Scans were performed on a 3 T Siemens Trio MR imaging system (Siemens AG, Erlangen, Germany). A 3-plane scout scan (conventional FLASH sequence with isotropic voxels of 2.8 mm) was acquired and used for prescription of the fMRI image stack: gradient echo EPI, TR/TE = 3000/30 ms, flip angle = 90, 224 × 224 mm FOV, 41 3.5-mm interleaved axial slices starting from the spinal cord covering the entire brain, no gap, AP readout, 64 × 64 pixel, full k-space acquisition, no SENSE acceleration; pulse sequence-enhanced version of the Siemens epibold, yielding isotropic 3.5 mm voxels. The BOLD scan comprised 567 acquired images, with an additional two images (6 sec) at the beginning that were not acquired, in order to ensure steady-state magnetization. Automatic second order shimming was performed over the fMRI imaging volume prior to acquisition. A conventional T1 scan was performed on the same functional prescription; therefore it had identical susceptibility distortion (“matched-warped”; identical geometry to the fMRI scans except for 256 × 256 pixels; (Rohan et al., 2001)). A standard T1 weighted MP-RAGE3D scan (FOV = 256 × 256 × 170 mm, 256 × 256 × 128, flip angle = 12, TR/TE/TI = 2100/2.74/1100 ms) also was collected. All subjects had one clinical-quality anatomical scan evaluated by a radiologist to ensure the absence of structural brain abnormalities.
2.5. Stimulus presentations
Subjects passively viewed affective faces (happy and fearful) in overt, covert, and rapid covert presentations using the masking procedure previously described by Whalen et al. (1998). Faces were selected from the MacArthur Foundation NimStim set (Tottenham et al., 2009). A fourth stimulus set included words with positive and negative affective valences. The overt faces scan comprised 120 volumes. Half of the volumes were fixation blanks, 25% showed a happy face for 1 s followed by fixation for 2 s, and the remaining 25% showed a fearful face for 1 s followed by fixation for 2 s. Stimuli were randomized and counterbalanced across the session in an event-related design, identical for all subjects. The same paradigm was used for the positive and negative words. In the covert presentation, the happy or fearful face was displayed for 30 ms, followed by the corresponding neutral face for 160 ms and then fixation for the remaining time in the volume. The neutral fixation stimulus consisted of a black asterisk on a gray background. The rapid covert presentation included 60 volumes presented in a randomized, counterbalanced block design, identical for all subjects, divided into 12 blocks of five volumes each. Each block included a single emotion (happy or fearful). Each volume contained six faces, consisting of the emotional face for 30 ms, corresponding neutral face for 160 ms, and fixation for 264 ms, with a pause of 276 ms before the next volume.
2.6. BOLD fMRI analysis: preprocessing
Analyses were performed using the fMRI Expert Analysis Tool (FEAT) version 5.98 in FSL (FMRIB Analysis Group, Oxford University, UK, http://www.fmrib.ox.ac.uk/fsl), using default settings except as noted. An in-house despiking filter was applied to all functional data sets. If maximum absolute motion reported by MCFLIRT (Jenkinson et al., 2002) exceeded 3.5 mm (the voxel dimension), that run was discarded. Slice timing correction was performed. Spatial and temporal filtering parameters were 5 mm full width half maximum (FWHM), and 22.5 s (rapid covert images) or 9.0 s (all other runs), respectively.
2.7. BOLD fMRI analysis: individual statistical analysis
Regressors of interest were as follows: happy and fearful for the overt and covert scans, happy – fearful (single regressor) for the rapid covert scan, and positive and negative for the word scans. Motion estimates were included as nuisance regressors. Regressors of interest were subjected to a linear filter modeling the hemodynamic response function, and to the same temporal filter that was applied to the data. Regressors varied in a counterbalanced manner during the experiment, identical over all subjects and visits. The results of this analysis were discarded except for the residuals. A principal component analysis was performed on the 5000 voxels in the residuals that had the largest variance. The first eight components were retained and used as additional nuisance regressors (without hemodynamic or other temporal filtering) in a new general linear model, using the same pre-processed functional data (Madsen and Lund, 2006). Linear combinations of these parameter estimates yielded quantities of interest. Contrasts for the overt and covert conditions included happy–fearful faces, fearful–happy faces, as well as all faces regardless of emotion, while in the rapid covert condition only the happy–fearful and fearful–happy contrasts were possible. For the words, the contrasts were positive–negative, negative–positive, and any word regardless of valence.
2.8. BOLD fMRI analysis: registration and group analysis
Functional results were aligned with the matched T1-weighted scan (six degrees of freedom (DOF)), in turn with the MP-RAGE scan (12 DOF), and finally with the Montreal Neurological Institute (MNI) 152 standard brain using FMRIB’s Nonlinear Image Registration Tool (FNIRT), at 2 mm isotropic resolution. Subjects were included only if valid data existed for all three drug conditions. Single scan results were combined in a mixed effects repeated measures model, with a dummy regressor for each subject, set to unity for that subject and zero otherwise. Contrasts were generated for all six signed comparisons among the three drug conditions. For data quality assurance purposes, activation and deactivation due to placebo only were also calculated, for comparison with the literature. Voxels were thresholded to p < 0.01 (uncorrected), and clusters to p < 0.05, familywise error (FWE) corrected for multiple comparisons over the whole brain.
2.9. Serotonin transporter genotyping
Genomic deoxyribonucleic acid (DNA) was extracted using the QIAamp DNA mini kit (Qiagen, Valencia, CA). The linked promoter polymorphism in the serotonin transporter (SLC6A4) was genotyped for long and short alleles and the La/Lg polymorphism using a two-stage polymerase chain reaction with fluorogenic allelic discrimination probes as described previously (Hu et al., 2006). The first stage used fluorogenic probes to discriminate between the 182 base pair L amplicon and the 138 base pair S amplicon. The second stage used probes labeled with VIC and FAM to discriminate between the La and Lg alleles.
2.10. Plasma analysis of drug and metabolite levels
Blood samples were collected in tubes containing ethylenediaminetetraacetic acid (EDTA) immediately after each scan and plasma was isolated by centrifugation at 2500 × g. Levels of R-citalopram, S-citalopram, R-demethylcitalopram, S-demethylcitalopram, R-didemethylcitalopram, and S-didemethylcitalopram were quantified in the analytical laboratories at Forest Research Institute (Farmingdale, NY). Quantification employed an optimized high performance liquid chromatography and mass spectrometry procedure using [2H4] citalopram, [2H4] demethylcitalopram, and [2H4] didemethylcitalopram internal standard solutions. The assay has a validated concentration range of 1 ng/mL to 150 ng/mL for all analytes, with 1 ng/mL as the lower limit of quantification.
3. Results
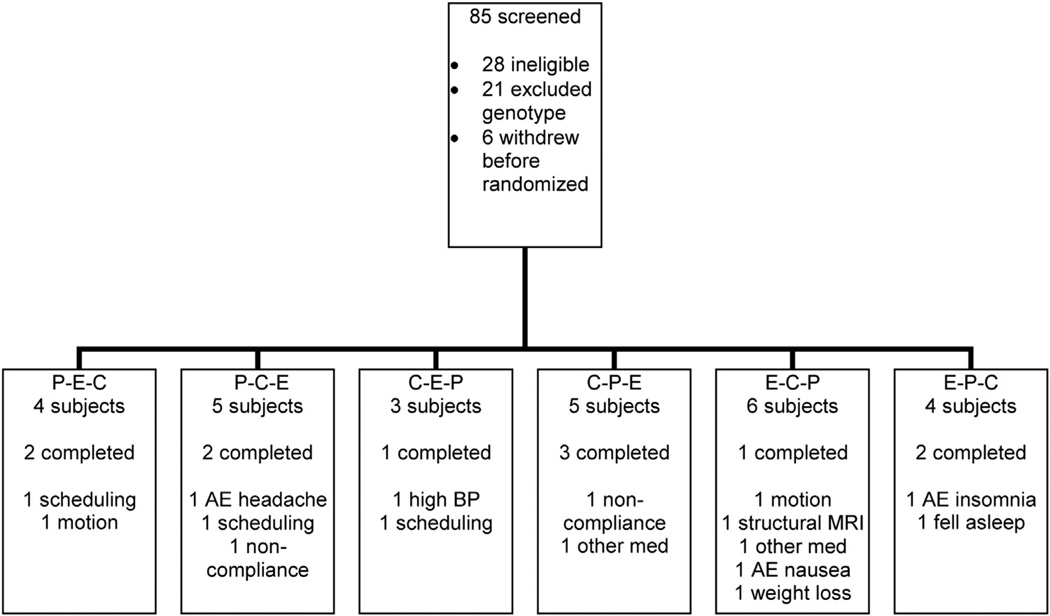
Fig. 1 describes the flow of participants through the trial, including individuals who withdrew or had unusable or incomplete data. Unlike most studies with a similar design, 21 of the 85 subjects who were screened were eliminated due to serotonin transporter genotype. The final cohort included in the analysis consisted of 11 subjects who ranged in age from 21 to 42 years (mean 30.1 ± 6.8). The analysis for the words stimulus condition included 10 subjects and the covert faces included nine subjects. Of these, there were eight with the SLa genotype, two with the LaLa genotype, and one with the LaLg genotype. A diagram of the experimental design is shown in Fig. 2.
Fig. 1.
Flow chart of participant enrollment and randomization. P = placebo, C = citalopram, E = escitalopram and letters indicate order of medication periods. AE = adverse event, BP = blood pressure.
Fig. 2.
Illustration of experimental design. Medication periods were organized in a randomized, crossover design with a washout period between each medication period.
Although the medications were generally well tolerated, three subjects withdrew due to adverse events. One subject experienced nausea that required prescription anti-nausea medication, one experienced headaches, and the third withdrew due to insomnia. Two subjects complained of significant drowsiness but remained in the trial. No subjects reported discontinuation effects that are sometimes observed with abrupt discontinuation of SSRIs. Scores on the QIDS were below 5, which are considered to indicate an absence of depressive symptomatology. Likewise, YMRS scores were at or near zero at all visits for all of the subjects, indicating an absence of treatment-emergent manic symptoms during the study. No discontinuation symptoms emerged, based on the DESS performed after each washout period. Of the 24 subjects who completed at least one medication period, three were likely non-compliant based on plasma drug levels. Analyses were performed both including and excluding the subjects without detectable plasma metabolite levels, with similar results.
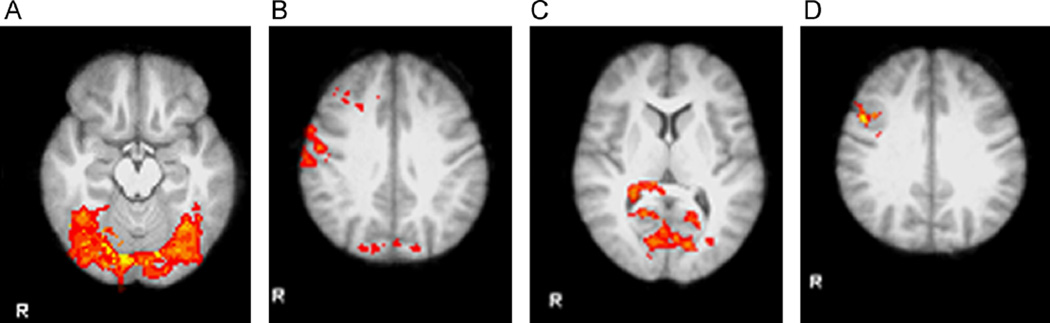
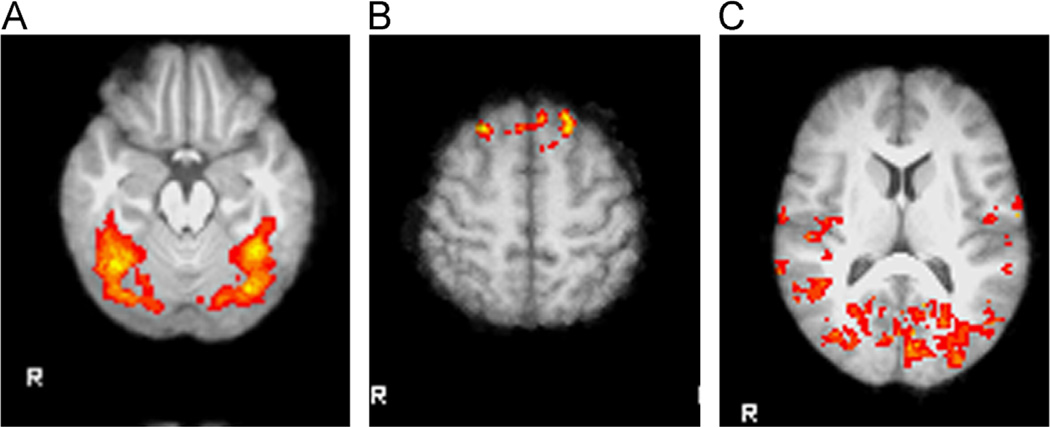
Activation in the placebo condition was examined to determine which regions were activated in response to the stimuli in the absence of medication for both the overt (Fig. 3) and covert and rapid covert (Fig. 4) paradigms. Specific areas with coordinates are listed in Table 1. In the overt presentation condition, large clusters in sensory regions including visual and somatosensory cortex showed decreased activation following placebo (Table 2).
Fig. 3.
Activation in response to affective faces shown with overt presentation in placebo condition illustrates task effects. (A) Widespread activation was observed in response to faces, compared with fixation stimulus. Regions of activation of at least 50 voxels included the bilateral occipital pole, bilateral occipital fusiform gyrus, bilateral lingual gyrus, right inferior temporal gyrus, and temporal occipital fusiform cortex. (B) Activation was less in response to faces than in response to fixation stimulus in the bilateral middle frontal cortex, right precentral and postcentral gyrus, and right precuneous and cuneous. (C) Regions where activation was greater in response to happy faces than in response to fearful faces included the bilateral lingual gyrus, right intracalcarine cortex, and right supracalcarine cortex. (D) The only region where activation was less in response to happy faces than in response to fearful faces was the right precentral gyrus.
Fig. 4.
Activation in response to affective faces shown with covert or rapid covert presentation in placebo condition illustrates task effects. (A) Covert faces activated the bilateral occipital and occipital fusiform cortex, right middle and inferior temporal gyrus, bilateral lingual gyrus, and bilateral occipital pole, in comparison with a fixation stimulus. (B) Covert happy faces activated the bilateral superior frontal cortex, in comparison with fearful faces. (C) Happy faces presented in a rapid covert paradigm produced widespread activation in occipital and temporal regions in comparison with fearful faces.
Table 1.
Regions showing increased activation following placebo in response to affective faces.
| Stimulus | Cluster size | Z-max | P | x (mm) | y (mm) | z (mm) | Brain region |
|---|---|---|---|---|---|---|---|
| Covert happy-fearful | 97 | 3.27 | 0.00908 | −6 | 34 | 54 | Left superior frontal gyrus |
| Covert happy-fearful | 23 | 3.25 | N/A | 26 | 28 | 56 | Right superior frontal gyrus |
| Overt fearful-happy | 365 | 4.15 | 0.0377 | 50 | 8 | 30 | Right precentral gyrus |
| Overt happy-fearful | 1789 | 4.12 | 8.23 × 10−9 | 12 | −74 | −18 | Lingual gyrus, occipital fusiform gyrus |
| Overt face-fixation | 7964 | 5.29 | 9.88 × 10−34 | 18 | −84 | −14 | Occipital fusiform gyrus, lingual gyrus |
Table 2.
Regions showing deactivation in response to face stimuli in overt unmasked presentation following placebo.
| Cluster size | Z-max | x (mm) | y (mm) | z (mm) | Region |
|---|---|---|---|---|---|
| 1076 | 3.97 | 6 | −74 | 24 | Brodmann area 18 |
| 920 | 3.94 | 56 | −10 | 36 | Primary somatosensory cortex |
| 794 | 3.92 | 20 | 16 | 58 | Premotor cortex |
| 268 | 3.27 | −34 | 4 | 44 | Middle frontal gyrus |
| 267 | 3.27 | −32 | −52 | 60 | Superior parietal lobule |
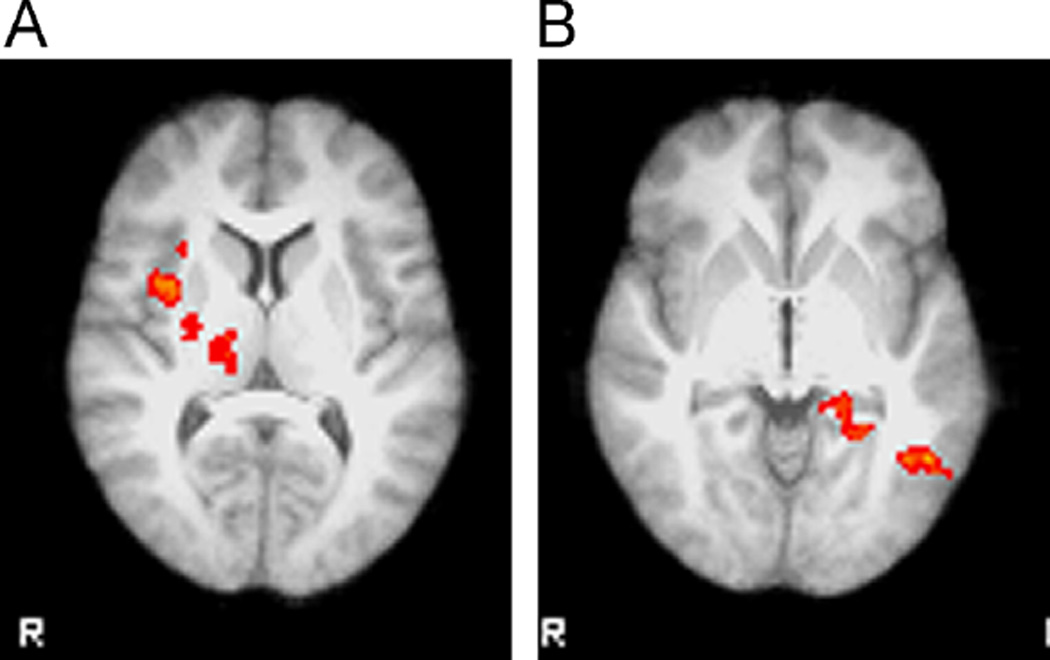
Medication effects were observed for all stimulus conditions and in many cases did not depend on the emotion being displayed. Much of the activation occurred in regions responsible for visual processing (Table 3). The exception was the rapid covert presentation where there were significant differences between citalopram and placebo in the happy–fearful contrast that extended across a large region of the cortex (Table 4). Differences between citalopram and placebo and between escitalopram and placebo were observed in a similar set of regions (Fig. 5). In all cases where there was a difference between escitalopram and citalopram, the activation was greater with escitalopram (Fig. 6). Activation in response to overt faces was greater following escitalopram than following citalopram in the right insular cortex, a region involved in self-perception, as well as the thalamus and putamen. Drug effects were more pronounced with the rapid covert presentation than with the covert presentation. In the rapid covert presentation, the contrast between happy and fearful faces elicited a greater response following escitalopram in the left middle temporal gyrus, consistent with the role of this region in recognizing and interpreting information about faces and perceiving visual stimuli. In addition, the contrast between words and fixation resulted in differential activation in the right lateral occipital cortex following citalopram (Fig. 7). Although the reported values are not corrected for multiple testing, almost all significant differences would remain significant if corrected for the six comparisons.
Table 3.
Regions showing drug effects in response to affective stimuli.
| Drug condition | Stimulus | Cluster size | P | x (mm) | y (mm) | z (mm) | Brain region |
|---|---|---|---|---|---|---|---|
| E vs. C | Overt face-fixation | 735 | 1.62 × 10−5 | 34 | 4 | 6 | Left insular cortex |
| C vs. P | Covert face-fixation | 658 | 9.36 × 10−6 | 48 | −66 | −24 | Left occipital fusiform gyrus |
| C vs. P | Covert face-fixation | 253 | 0.0279 | 20 | −68 | 48 | Left lateral occipital cortex |
| E vs. P | Covert face-fixation | 396 | 0.00124 | 32 | −86 | −10 | Left inferior lateral occipital cortex |
| E vs. C | Rapid covert happy-fearful | 478 | 0.000704 | −50 | −58 | −4 | Left middle temporal gyrus |
| P vs. C | Any word-fixation | 619 | 6.33 × 10−5 | 18 | −64 | −2 | Left lingual gyrus, left superior lateral occipital cortex |
| E vs. C | Any word-fixation | 289 | 0.0241 | 38 | −60 | −24 | Left primary visual cortex |
P = placebo, C = citalopram, E = escitalopram. The drug listed first showed greater activation than the contrasting drug condition. Coordinates reflect the center of activation based on the Montreal Neurological Institute atlas.
Table 4.
Cluster of 2455 voxels showing reduced activation following citalopram compared with placebo in happy-fearful contrast (P = 1.26 × 10−14).
| Cluster size (voxels) | z-max | X (mm) | y (mm) | z (mm) | Region |
|---|---|---|---|---|---|
| 2455 | 4.26 | −16 | −76 | −10 | Left lingual gyrus, occipital fusiform gyrus |
| 1108 | 3.72 | 18 | −78 | 20 | Right cuneal cortex, visual cortex |
| 368 | 4.28 | 22 | −58 | 42 | Right lateral occipital cortex, superior division |
| 317 | 4.24 | −28 | 28 | 8 | Left insula, left inferior fronto-occipital fasciculus |
| 285 | 4.14 | 50 | 18 | −6 | Right inferior frontal gyrus |
| 260 | 4.21 | −18 | 30 | 36 | Left superior frontal gyrus |
Fig. 5.
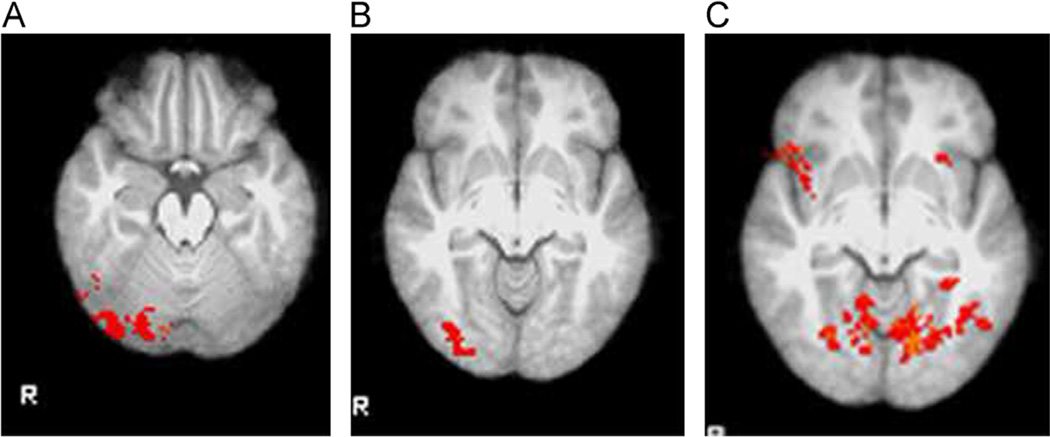
Citalopram and escitalopram are associated with similar responses to covert faces in comparison with placebo, whereas rapid covert faces produced a distinct response. Notably, the number of voxels and regions activated in the rapid covert condition was much greater than in the covert condition. (A) Covert faces activated the right occipital fusiform gyrus following 2 weeks of citalopram. (B) Covert faces activated a region that included the right lateral occipital cortex following 2 weeks of escitalopram. (C) Rapid covert faces (happy in comparison with fearful) resulted in less activation than in the placebo condition following 2 weeks of citalopram in a large region encompassing the bilateral lingual gyrus, bilateral occipital cortex and occipital fusiform gyrus, and left frontal orbital cortex.
Fig. 6.
Differential effects of citalopram and escitalopram were observed in the overt and rapid covert presentation paradigms. (A) Overt faces produced greater activation in the right insula, right thalamus, and right putamen following escitalopram than following citalopram. (B) In the rapid covert paradigm there was a drug by affective valence effect in the happy vs. fearful contrast, where activation was greater following escitalopram than following citalopram in temporal and occipital regions as well as the brainstem.
Fig. 7.
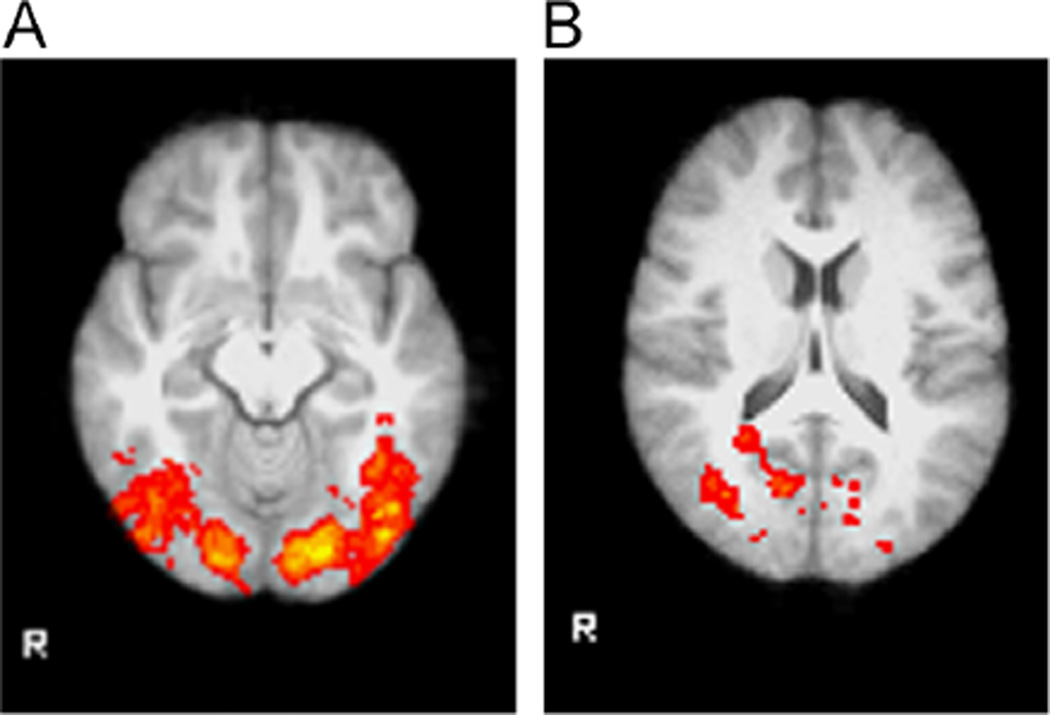
Passive viewing of affective words led to extensive activation of temporal and occipital regions, which was attenuated by citalopram but not escitalopram. (A) In the placebo condition, the word viewing task activated bilateral temporal, occipital, and fusiform regions similar to those activated by affective faces. (B) Activation was decreased in the right lateral occipital cortex following 2 weeks of citalopram.
R-citalopram and its metabolites were not detectable following 2 weeks of escitalopram. In approximately half of the subjects, the S-didemethyl metabolite was below the limit of detection (1 ng/mL). Table 5 shows the mean (± standard deviation) of each enantiomer or metabolite for the 11 subjects included in the final analysis. Levels of S-citalopram and its metabolites were not significantly different between the two medications. Notably, the ratio of plasma levels of R-citalopram to S-citalopram in the citalopram condition was 1.86, despite the 1:1 mixture of the enantiomers in the compound.
Table 5.
Levels of R-citalopram and S-citalopram and associated metabolites following two weeks of escitalopram or racemic citalopram.
| Metabolite | Escitalopram (ng/mL) | Citalopram (ng/mL) |
|---|---|---|
| R-Citalopram | N/A | 46.68 ± 13.16 |
| S-Citalopram | 23.92 ± 9.13 | 25.03 ± 9.25 |
| R-Demethyl Citalopram | N/A | 15.80 ± 6.83 |
| S-Demethyl Citalopram | 11.19 ± 4.97 | 12.16 ± 3.95 |
| R-Didemethyl Citalopram | N/A | 2.90 ± 1.27 |
| S-Didemethyl Citalopram | 1.91 ± 0.54 | 1.79 ± 0.32 |
4. Discussion
The primary finding of this study is that pharmaco-fMRI is sufficiently sensitive to detect differential effects of racemic citalopram and S-citalopram in healthy volunteers with SLC6A4 genotypes associated with medium to high levels of the serotonin transporter protein in cellular models. Several regions showed changes in activity with the different drug conditions and activation paradigms. There were no contrasts where citalopram produced a greater degree of activation than escitalopram and the plasma levels of the S-enantiomer did not significantly differ between the escitalopram and citalopram groups. Taken together, these observations support the preclinical findings that R-citalopram functions as an antagonist at SLC6A4 and suggests that the any differences between the two drug conditions likely reflect the influence of R-citalopram.
The limited number of regions showing differences between the escitalopram and citalopram conditions suggests they are not a reflection of global changes in blood flow. In the rapid covert presentation, the contrast between happy and fearful faces elicited a greater response to escitalopram than citalopram in the left middle temporal gyrus, with a greater response to happy faces. This region plays a role in sensory processing pathways (Mesulam, 1998). By more effectively modulating regions involved both in self-perception and perception of sensory input, escitalopram may have a slight advantage over citalopram in regulating depressive symptoms that involve perception of the self. Accordingly, a previous fMRI study reported greater response to positive stimuli than negative pictures and words in the left middle temporal gyrus (Kensinger and Schacter, 2006).
Although the amygdala is a region that has reproducibly been shown to change when an individual is responding to fearful faces, other regions including the pulvinar, anterior cingulate, and anterior insula have also been implicated in processing of fearful faces (Morris et al., 1998). Windischberger and colleagues independently conducted a study with a very similar design to this study but with minor differences in the stimulus paradigm, medication dosing, and sex composition of the sample, using image acquisition parameters optimized to detect a difference in the amygdala (Windischberger et al., 2010). They observed medication effects in the amygdala, parahippocampal gyrus, fusiform gyrus, and medial frontal gyrus. Notably, they also observed medication effects in the left middle temporal gyrus, a region where we also found a differential response between escitalopram and citalopram to faces with affective valence. In their study, activation was lower in the bilateral medial frontal gyrus with escitalopram than citalopram. In contrast, we did not observe any areas where activation was greater with citalopram than escitalopram. The discrepancies may reflect the genetic differences between the samples and/or their use of an active facial expression discrimination task compared with the passive viewing approach employed in the present study.
Tasks involving affective stimuli appear to activate brain regions targeted by antidepressants depending on the emotional valence and whether processing of the emotion is explicit or implicit (Phillips et al., 2004; Fusar-Poli et al., 2009). Notably, the response of the amygdala and insula to fearful and disgusted faces respectively that was observed in an overt presentation was not detected when faces were shown in a covert presentation. Individuals with major depressive disorder demonstrate an increased amygdala response to masked emotional faces that is attenuated following successful antidepressant treatment (Sheline et al., 2001). Although the hypothesis was that medication effects would be observed in regions that comprise the mood circuitry (Mayberg, 1997), the observed differences in activation were actually more widespread across the cortex. This finding may reflect the differential response of the cortex in healthy volunteers compared to the response in affectively ill individuals.
One of the unique features of this study was selection of subjects based on serotonin transporter genotype at an SLC6A4 promoter polymorphism that alters in vitro expression (Lesch et al., 1996; Hu et al., 2006) and in vivo expression (Heinz et al., 1998; Little et al., 1998) of this gene. The genotype for this polymorphism affects the abundance of the serotonin transporter protein, which is the substrate for SSRIs. The long (L) allele and specifically the La allele is associated with increased transcription of the transporter and higher levels of serotonin reuptake (Lesch et al., 1996; Hu et al., 2006). Within the S and L alleles, which differ in number of copies of a repeat sequence, an additional single nucleotide polymorphism results in a tri-allelic genotype where long alleles together with a guanine nucleotide (Lg) are functionally similar to the short allele (S), both being expressed at lower levels, and long alleles with an adenine nucleotide (La) are expressed at high levels (Hu et al., 2006). Hariri and colleagues (2002) found that the response of the amygdala to fearful stimuli varies as a function of genotype, with an increased response for those with one or more S alleles. A meta-analysis by Munafo and colleagues (2008) suggested that 10% of the variability in amygdala activation can be attributed to genotype at this locus. Therefore, by excluding individuals with the SS genotype and SLg genotype (which is functionally equivalent to SS), the cohort was restricted to individuals with greater levels of transporter protein. Thus, the absence of change in the BOLD signal in the amygdala may reflect the fact that the study excluded subjects with the genotype that has been reported to have the greatest amygdala response, in addition to, or instead of, not optimizing the scanning parameters for studying this region. This highlights the potential power and pitfalls of combining these techniques.
The major limitation of the study was the small sample size, which may account for the lack of amygdala activation and other differences between this and other studies. However, other similar studies have included as few as eight subjects (Bigos et al., 2008) or 12 subjects (Anderson et al., 2007). The ability of other groups to detect amygdala activation with small samples suggests that other factors such as the genetic make-up and degree of susceptibility artifact that is encountered at higher field strengths may have contributed to the results reported here.
Another limitation of this study was the passive viewing of the stimuli with no task to monitor the subjects’ attention and alertness. Some subjects reported feeling drowsy in the scanner, which likely affected functional activity. Performance of a task engages the brain differently from passively viewing stimuli, as does motivated versus unmotivated passive viewing, and may account for differences in the pattern of activation observed here in comparison to other studies (Windischberger et al., 2010; Phan et al., 2002; Skelly and Decety, 2012). Moreover, the stimuli were restricted to happy and fearful faces and could be extended in future studies to examine response to sad or angry faces or other affective stimuli.
It is also possible that the responsiveness of neural circuitry differs between healthy volunteers and affectively ill individuals. Multiple studies have shown that currently depressed subjects exhibit a greater amygdala response to sad faces (Arnone et al., 2012; Victor et al., 2010) and fearful faces (Godlewska et al., 2012) than healthy controls before antidepressant treatment, whereas healthy controls showed a greater response to happy faces. Given that responses to affective faces are exaggerated in depressed patients, it is not surprising that the pattern and degree of brain activation differs between ill individuals and healthy volunteers. However, the finding that the effects of antidepressant medications on the pattern of activation in brain regions associated with mood symptoms can be detected by pharmaco-fMRI supports the utility of these methods in the earlier phases of drug development. Further studies are warranted to understand the most effective way to combine these techniques to aid drug development.
Future exploration in this area should utilize a multimodal imaging approach that includes examining the effects of serotonergic antidepressants on functional connectivity using resting fMRI. In particular, a set of regions referred to as the default mode network, shows altered connectivity in depression and other psychiatric disorders (Greicius et al., 2007). The default mode network includes the posterior cingulate cortex, ventral anterior cingulate cortex, orbitofrontal cortex, and inferior parietal cortex (Greicius et al., 2003). Understanding how similar compounds such as escitalopram and racemic citalopram uniquely affect both activation in response to stimuli and resting state connectivity will provide a more comprehensive understanding of the mechanistic similarities and differences between the drugs.
Acknowledgments
The study was funded by Forest Research Institute as an Investigator-Initiated Trial awarded to MEH. Escitalopram was supplied by Forest and metabolite assays were performed in their analytical laboratory. We thank Hanady Gouta, Athari Alyazidi, and Sophia Shrand for assistance with subject screenings; Ayu Tesfaye for investigational pharmacy services; Cherisse Ferrier for serotonin transporter genotyping; and Bonnie Adams for performing magnetic resonance scans.
Disclosures
MEH received research funding from Forest Research Institute to conduct the present study; has received research grants from Sunovion, Shire, Bracco Diagnostics, Ridge Diagnostics, Eli Lilly, and Pfizer; and has received consulting fees as an advisor to Ridge Diagnostics. TLL has received research funding from Pfizer. PFR has received consulting fees for Kyowa Hakko, Novartis, Repligen, and Roche; has received research funding from GlaxoSmithKline and Roche; and is also an inventor on patent applications assigned to McLean Hospital and the University of Utah.
Footnotes
This study was presented at the 2012 NCDEU meeting in Phoenix, AZ.
References
- Anderson IM, Del-Ben CM, Mckie S, Richardson P, Williams SR, Elliott R, Deakin JF. Citalopram modulation of neuronal responses to aversive face emotions: a functional MRI study. Neuroreport. 2007;18:1351–1355. doi: 10.1097/WNR.0b013e3282742115. [DOI] [PubMed] [Google Scholar]
- Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology (Berlin) 2008;196:661–672. doi: 10.1007/s00213-007-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, McKie S, Elliott R, Thomas EJ, Downey D, Juhasz G, Williams SR, Deakin JF, Anderson IM. Increased amygdala responses to sad but not fearful faces in major depression: relation to mood state and pharmacological treatment. American Journal of Psychiatry. 2012;169:841–850. doi: 10.1176/appi.ajp.2012.11121774. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology. 2008;33:3221–3225. doi: 10.1038/npp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cole PE, Schwarz AJ, Schmidt ME. Applications of imaging biomarkers in the early clinical development of central nervous system therapeutic agents. Clinical Pharmacology and Therapeutics. 2012;91:315–320. doi: 10.1038/clpt.2011.286. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) Washington, DC: American Psychiatric Association; 2002. Nov, [Google Scholar]
- Frodl T, Scheuerecker J, Schoepf V, Linn J, Koutsouleris N, Bokde AL, Hampel H, Moller HJ, Bruckmann H, Wiesmann M, Meisenzahl E. Different effects of mirtazapine and venlafaxine on brain activation: an open randomized controlled fMRI study. Journal of Clinical Psychiatry. 2011;72:448–457. doi: 10.4088/JCP.09m05393blu. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Godlewska BR, Norbury R, Selvaraj S, Cowen PJ, Harmer CJ. Short-term SSRI treatment normalises amygdala hyperactivity in depressed patients. Psychological Medicine. 2012;42:2609–2617. doi: 10.1017/S0033291712000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Heinz A, Higley JD, Gorey JG, Saunders RC, Jones DW, Hommer D, Zajicek K, Suomi SJ, Lesch KP, Weinberger DR, Linnoila M. In vivo association between alcohol intoxication, aggression, and serotonin transporter availability in nonhuman primates. American Journal of Psychiatry. 1998;155:1023–1028. doi: 10.1176/ajp.155.8.1023. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. American Journal of Human Genetics. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of General Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Processing emotional pictures and words: effects of valence and arousal. Cognitive, Affective, and Behavioral Neuroscience. 2006;6:110–126. doi: 10.3758/cabn.6.2.110. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Little KY, McLaughlin DP, Zhang L, McFinton PR, Dalack GW, Cook EH, Jr, Cassin BJ, Watson SJ. Brain dopamine transporter messenger RNA and binding sites in cocaine users: a postmortem study. Archives of General Psychiatry. 1998;55:793–799. doi: 10.1001/archpsyc.55.9.793. [DOI] [PubMed] [Google Scholar]
- Madsen K, Lund T. Filtering fMRI data by unsupervised modeling of physiological noise artifacts. Twelfth Annual Meeting of the Organization for Human Brain Mapping. 2006 [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. Journal of Neuropsychiatry and Clinical Neuroscience. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Part 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(Part 1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biological Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams LM, Heining M, Herba CM, Russell T, Andrew C, Bullmore ET, Brammer MJ, Williams SC, Morgan M, Young AW, Gray JA. Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage. 2004;21:1484–1496. doi: 10.1016/j.neuroimage.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Pine DS, Grun J, Zarahn E, Fyer A, Koda V, Li W, Szeszko PR, Ardekani B, Bilder RM. Cortical brain regions engaged by masked emotional faces in adolescents and adults: an fMRI study. Emotion. 2001;1:137–147. doi: 10.1037/1528-3542.1.2.137. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Fabbri C, Serretti A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. European Neuropsychopharmacology. 2012;22:239–258. doi: 10.1016/j.euroneuro.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. Journal of American Medical Association (JAMA) 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan M, Killgore W, Eskesen J, Renshaw PF, Yurgelun-Todd HD. Match-Warped EPI Anatomic Images and the Amygdala: Imaging in Hard Places. International Society for Magnetic Resonance and Medicine, Ninth Scientific Meeting and Exhibition. 2001 [Google Scholar]
- Rosenbaum JF, Fava M, Hoog SL, Ascroft RC, Krebs WB. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biological Psychiatry. 1998;44:77–87. doi: 10.1016/s0006-3223(98)00126-7. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Bogeso KP, Ebert B, Reines EH, Braestrup C. Escitalopram versus citalopram: the surprising role of the R-enantiomer. Psychopharmacology (Berlin) 2004;174:163–176. doi: 10.1007/s00213-004-1865-z. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Simmons AN, Arce E, Lovero KL, Stein MB, Paulus MP. Subchronic SSRI administration reduces insula response during affective anticipation in healthy volunteers. International Journal of Neuropsychopharmacology. 2009;12:1009–1020. doi: 10.1017/S1461145709990149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly LR, Decety J. Passive and motivated perception of emotional faces: qualitative and quantitative changes in the face processing network. PLoS One. 2012;7:e40371. doi: 10.1371/journal.pone.0040371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits KM, Smits LJ, Schouten JS, Stelma FF, Nelemans P, Prins MH. Influence of SERTPR and STin2 in the serotonin transporter gene on the effect of selective serotonin reuptake inhibitors in depression: a systematic review. Molecular Psychiatry. 2004;9:433–441. doi: 10.1038/sj.mp.4001488. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Archives of General Psychiatry. 2010;67:1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windischberger C, Lanzenberger R, Holik A, Spindelegger C, Stein P, Moser U, Gerstl F, Fink M, Moser E, Kasper S. Area-specific modulation of neural activation comparing escitalopram and citalopram revealed by pharmaco-fMRI: a randomized cross-over study. Neuroimage. 2010;49:1161–1170. doi: 10.1016/j.neuroimage.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Wingen M, Kuypers KP, van de Ven V, Formisano E, Ramaekers JG. Sustained attention and serotonin: a pharmaco-fMRI study. Human Psychopharmacology. 2008;23:221–230. doi: 10.1002/hup.923. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]