Abstract
Objective
Cortical structural and functional anomalies have been found to associate with language impairments in both schizophrenia patients and genetic high risk individuals for developing schizophrenia. However, subcortical structures that contribute to language processing haven't been well studied in this population, and thus became the main objective of this study.
Method
We examined structural MRI data from 20 patients with schizophrenia, 21 individuals at genetic high risk, and 48 controls. Surface shape and volume differences of 6 subcortical structures that are involved in language processing, including nuclei pallidum, putamen, caudate, amygdala, thalamus, and hippocampus from both hemispheres, were compared between groups. Performance scores of language-associated cognitive tests were obtained to identify relationships of subcortical structures to language-related behaviors.
Results
Significantly reduced volumes of both the left and right side caudate nuclei, thalami and right side amygdala were shown in patients when compared with controls. Very interestingly, the high risk group demonstrated significantly increased correlations between volumes of left side pallidum nucleus and bilateral thalami and language-related cognitive test scores when compared to controls.
Conclusions
This study furthers our understanding of subcortical structural alterations in schizophrenia and high risk individuals, and suggests the contribution of subcortical structures to the language impairments that may serve as an early sign for impending development of schizophrenia.
Keywords: Structural MRI, Schizophrenia, Genetic high-risk, Subcortical structures, Language processing
1. Introduction
Schizophrenia is a complicated and debilitating mental disorder characterized by altered behaviors, emotions, perceptions, and cognition. Impaired language function is one of the central features of this severe disorder. The anatomical circuit for language processing is a highly distributed multi-level bidirectional network involving multiple cortical and subcortical structures. In this circuit, bilateral superior temporal gyri and occipital cortex serve as auditory and visual input centers (McNealy et al., 2006). The supramarginal gyrus is responsible for phonological encoding in word production, and the angular gyrus for memory of visual word forms (Poeppel and Hickok, 2004). These two sites interface with widely distributed conceptual knowledge systems located primarily at the junction of the left temporal, occipital, and parietal lobes, i.e. inferior temporal region (Wernicke’s area), fusiform and lingual gyri. The sound or visual-based system transfers the input information to the conceptual knowledge systems, and then reaches the frontal motor systems (Broca’s area) via an auditory-motor interface system in the inferior parietal lobe. Besides these key cortical sites, subcortical structures are frequently involved in the language processing pathways. Particularly, the thalamus, which has extensive white matter connections to cerebral cortices and the striatum, plays the unique role in filtering the visual and/or auditory input information and passing needed information to the conceptual knowledge systems; whereas the nuclei of striatum, including caudate, putamen and pallidum, connect with thalamus and widespread cortical regions and involve in cognitive control of motor patterns during language processing (McFarland and Haber, 2002a). In addition, the hippocampus and amygdala work together for memory formation that is critical in language processing (Mcdonald and White, 2013). While extensive studies have reported structural and functional anomalies in cortical regions that contribute to aberrant language processing in patients with schizophrenia (Zhang et al., 2014;Catani et al., 2011), the role of the subcortical structures within these circuits that contribute to language impairment in this disorder is not yet well known.
Schizophrenia has significant and complex genetic heritability (Modinos et al., 2013). Early disturbances in brain pathways can later progress to chronic schizophrenia, indicating the benefits of studying individuals at high genetic risk, for evidence of developmental changes in brain structures that contribute to the onset of the disorder (DeLisi et al., 2002). Our previous neuroimaging and clinical studies have specifically demonstrated functional and structural alterations of cortical areas involved in the language-processing pathways in the genetic high-risk subjects for developing schizophrenia (Li et al., 2007a;Li et al., 2012a;Li et al., 2012b). While these previous studies have consistently highlighted that cortical gray matter and white matter structural and functional abnormalities significantly contribute to the disorganized language observed within schizophrenia and present early before onset of the disorder, there is less information regarding how the subcortical structures, which are essential components of the complete language circuit, may also be implicated in the atypical language processes in patients with schizophrenia or even early at the high-risk stage.
Very recently, there has been more evidence to support the existence of subcortical structural defects in people at high risk for developing schizophrenia. One study of genetic high risk relatives of schizophrenia patients revealed alterations in the hippocampus, pallidum, and putamen (Dougherty et al., 2012). Disrupted normal pattern of asymmetry has been identified in the amygdala-hippocampus connectivity, striatum, and thalamus of patients with schizophrenia and their high risk siblings, compared to control subjects (Qiu et al., 2009). The current study further explores subcortical abnormalities in individuals with schizophrenia and those at genetic high risk, to clarify their potential roles within the cortical-subcortical-cortical circuits that contribute to disrupted language processing, which is associated with the auditory hallucinations and thought disorder that are hallmark features of this diagnosis. A total of 6 subcortical regions of interest (ROIs) were investigated in individuals with schizophrenia, individuals at genetic high risk, and healthy controls, including the left and right side nuclei of caudate, pallidum, putamen, amygdala, hippocampus, and thalamus. Selection of these subcortical regions was based on their theoretical roles in the language processing circuits that have been reviewed earlier in this section. Volumetric and vertex-based shape analyses were performed. Associations between these structural measures and language-related behavioral measures were conducted. We hypothesized that both subjects with schizophrenia and those at high risk would have distinct subcortical structural defects that underlie language processing defects, as compared to healthy controls.
2. Methods
2.1. Subjects
Data from a total of 89 subjects were included in this study. These data were from 3 independent clinical groups (20 patients with schizophrenia, 21 individuals at genetic high risk for developing schizophrenia, and 48 healthy controls). Each patient was interviewed using the Diagnostic Interview for Genetic Studies (DIGS) (Nurnberger, Jr. et al., 1994) and was diagnosed with DSM-IV criteria. Evaluations were made based on interviews, information collected from family members, and medical records, as appropriate. Individuals in the control group did not have personal or family history of any psychotic disorder, psychiatric hospitalization, or suicide in any first or second-degree relatives, and were not found to have any evidence of a psychotic illness (schizophrenia, bipolar disorder or psychosis not otherwise specified) upon evaluation. Subjects in the high-risk group were 16–30 years old, which is within the peak age range of risk for developing schizophrenia (Li et al., 2007b) and originated from families in which at least one first-degree relative (parent or sibling) had a diagnosis of schizophrenia or schizoaffective disorder. Although none of the high risk group subjects had a history of psychosis, 4 had a history of at least one episode of major depression. Controls were age and sex matched to high-risk subjects. Neither the normal controls nor the high-risk participants were ever treated with medication for psychotic symptoms. All patients with schizophrenia were taking conventional or atypical antipsychotic medications.
Individuals with schizophrenia and those at high-risk were recruited by advertisements in newspapers and newsletters distributed by the National Alliance on Mental Illness (NAMI), or from families who had previously participated in past genetic studies of schizophrenia (DeLisi et al., 2002). Control subjects were independently solicited from the community by public advertisements. This study received Institutional Review Board Approval for human subject research at the Nathan S. Kline Institute for Psychiatric Research, a New York State Institution, and at New York University Langone School of Medicine, where this study was performed. Every participant provided written informed consent after a careful explanation of the study and its procedures.
2.2. Language-related cognitive tests
To characterize the general and language-related cognitive capacity of each subject, information was aggregated from a series of cognitive tests: 1) Full-scale, Verbal, and Performance IQ scores (FSIQ, VIQ, and PIQ, respectively) from the age-appropriate Wechsler test were used to measure general cognitive ability (Wechsler, 2004); 2) Verbal Comprehension Index (WAIS-VCI) was used to assess verbal capacity; 3) Peabody Picture Vocabulary Test, 3rd Edition (PPVT-II) measured receptive language capacity (Dunn and Dunn, 1997); 4) Wide Range Achievement Test, 3rd Edition (WRAT)-reading subtest evaluated word decoding and academic skills (Wilkinson GS, 1993); 5) Boston Naming Test (BNT) detected difficulties in word retrieval (Kaplan et al., 1983); 6) Woodcock Johnson Tests of Achievement, 3rd Edition (WJTA) evaluated reading comprehension (Woodcock et al., 2000); and 7) California Verbal Learning Test (CVLT) determined verbal memory capacity (Delis et al., 1987). Any subject with an IQ less than 85 was excluded from the study. The Edinburgh Handedness Inventory was used to test handedness (Oldfield, 1971). Group comparisons of demographic, medical, and language-related behavioral characteristics are shown in Table 1.
Table 1.
Subject demographics analyzed by one-way ANOVA.
| NC (n = 48) | HR (n = 21) | SCH (n = 20) | d.f. |
P Value |
|
|---|---|---|---|---|---|
| Age (mean ± SD) | 22.0 ± 5.1 | 21.1 ± 5.5 | 37.7 ± 10.3 | 2 | 0.000 |
| Age (range) | 16–32 | 16–30 | 20–55 | ||
| Male/female (Chi-sq.) | 24/24 | 7/14 | 13/7 | 2 | 0.176 |
| Left/right handed | 3/45 | 3/18 | 2/18 | - | - |
| Education (years) | 14.8 ± 3.0 | 12.6 ± 2.8 | 14.5 ± 2.2 | 2 | 0.634 |
| Mother’s education | 13.8 ± 3.5 | 14.5 ± 2.5 | 12.5 ± 2.2 | 2 | 0.695 |
| Father’s education | 15.1 ± 2.1 | 15.0 ± 2.2 | 14.5 ± 3.3 | 2 | 0.942 |
| WAIS-VCI | 113.2 ± 17.4 | 108.1 ± 14.6 | 105.5 ± 14.3 | 2 | 0.882 |
| PPVT-III | 102.5 ± 12.9 | 101.3 ± 12.2 | 102.5 ± 16.6 | 2 | 0.873 |
| WRAT | 104.1 ± 13.1 | 105.9 ± 9.1 | 106.6 ± 11.9 | 2 | 0.631 |
| WJTA | 102.4 ± 14.2 | 104.5 ± 11.1 | 103.5 ± 13.3 | 2 | 0.670 |
| BNT | 103.6 ± 15.7 | 103.1 ± 16.6 | 101.5 ± 12.1 | 2 | 0.714 |
| CVLT | 104.7 ± 12.9 | 103.9 ± 12.5 | 105.7 ± 18.7 | 2 | 0.760 |
NC: normal control group; HR: high-risk group; SCH: schizophrenia group; d.f.: degrees of freedom; WAIS-VCI: Wechsler Adult Intelligence Scale – Verbal Comprehension Index; PPVT-III: Peabody Picture Vocabulary Test, Third Edition; WRAT: Wide Range Achievement Test; WJTA: Woodcock Johnson Tests of Achievement, Third Edition; BNT: Boston Naming Test; CVLT: California Verbal Learning Test.
2.3. MRI data acquisition and processing in individuals
The MRI data were acquired on a 1.5T Siemens Vision scanner (Erlangen Germany). The high-resolution 3D T1-weighted magnetization prepared rapid gradient echo (MPRAGE) sequence was performed with the following parameters: TR=11.6 s; TE=4.9 ms; flip angle=15°; FOV=256×256×56 mm3; voxel size=1.0×1.0×1.0 mm3. We also acquired T2-weighted FLAIR data for possible spatial corrections. T1-weighted data were initially skull-stripped using the Brain Extraction Tool (BET) (Smith et al., 2002). Any remained non-brain tissues were then manually removed. The FSL/FIRST tool, a model-based segmentation/registration tool, was used to analyze subcortical structural shapes and volumes (Patenaude et al., 2008). The models used in FSL/FIRST were constructed from manually segmented images provided by the Center for Morphometric Analysis (CMA), MGH, Boston. Manual labels were parameterized as surface meshes and displayed as a point distribution model. Deformable surfaces were used to automatically parameterize the mesh labels and were constrained to preserve vertex correspondence across training data. Normalized intensities along the surface were sampled and modeled. The shape and appearance model was based on multivariate Gaussian assumptions, which is a generalization of the one-dimensional (univariate) normal distribution to higher dimensions. During the subcortical structure shape modeling aided by the FIRST training sample, a new observation is assumed to follow the multivariate Gaussian model and derive the distribution of the mean and precision given the previously observed training data. A marginalisation over the mean and precision is performed to obtain a distribution for the new observation given the training data. Shape was then expressed as a mean with modes of variation (principal components). Based on learned models, FIRST searches linear combinations of shape modes for the most probable shape given the observed intensities in T1-weighted images. For each subject, the left and right lateral caudate, thalamus, hippocampus, putamen, pallidum, and amygdala were segmented. Three-dimensional coordinates of the corresponding vertices of each segmented subcortical region were constructed to represent local shapes. The results of each step in the FSL/FIRST procedure were visually inspected.
The volumes of the above-mentioned subcortical regions were calculated for each subject and each hemisphere. To reduce head size related variability between subjects, individual volumes were multiplied by a normalization factor obtained from the corresponding T1-weighted image using the SIENAX tool (Smith et al., 2002).
2.4. Statistical Analyses
One-way analysis of variance (ANOVA) and post hoc t-tests were performed to determine any demographic, clinical, or language-related cognitive differences between the three groups.
For shape analysis, an F-statistic was used to compare the vertex measures of each subcortical region among the three groups. The volume of each subcortical region was compared among the three groups using ANOVA. The group differences in volumetric lateralization of these subcortical structures were assessed by a General Linear Model, with controls, high-risk, and patients as the groups, and the volume of the region at each hemisphere (left vs. right) as repeated measures. The laterality index, LI = (L – R)/(|L|+|R|), was calculated for each ROI, with L and R representing volume of the ROI in the left and right hemispheres.
Linear regression analysis was utilized to assess possible relationships between the volume of each subcortical region and the language-related cognitive measures among the three groups.
For each group level statistic test throughout the study, a significance level of p < 0.05 was used, potential confounding from age, FSIQ, and gender were controlled by adding them as covariates, multiple comparisons were corrected by using the Bonferroni correction with α=0.05.
3. Results
As per Table 1, the age of patients with schizophrenia was significantly older than that of the high-risk subjects and normal controls (p = 0.001). There were no significant between-group differences in other demographic items or in the behavioral scores for any language-related cognitive tests.
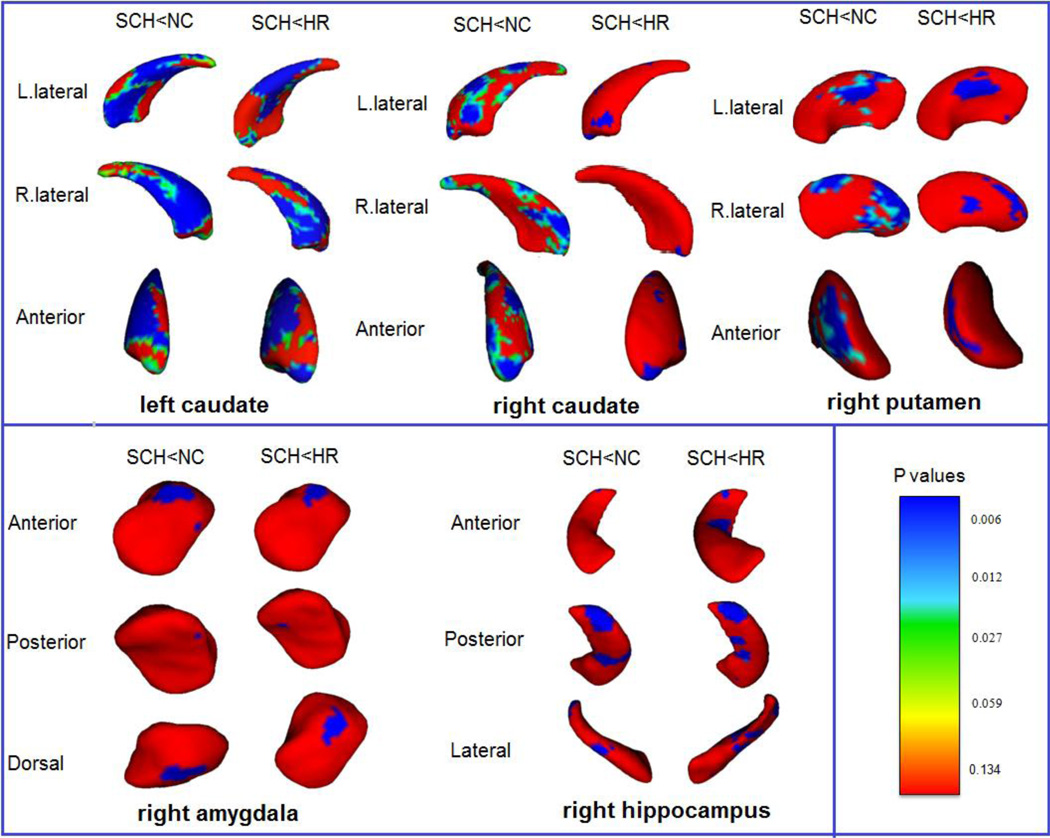
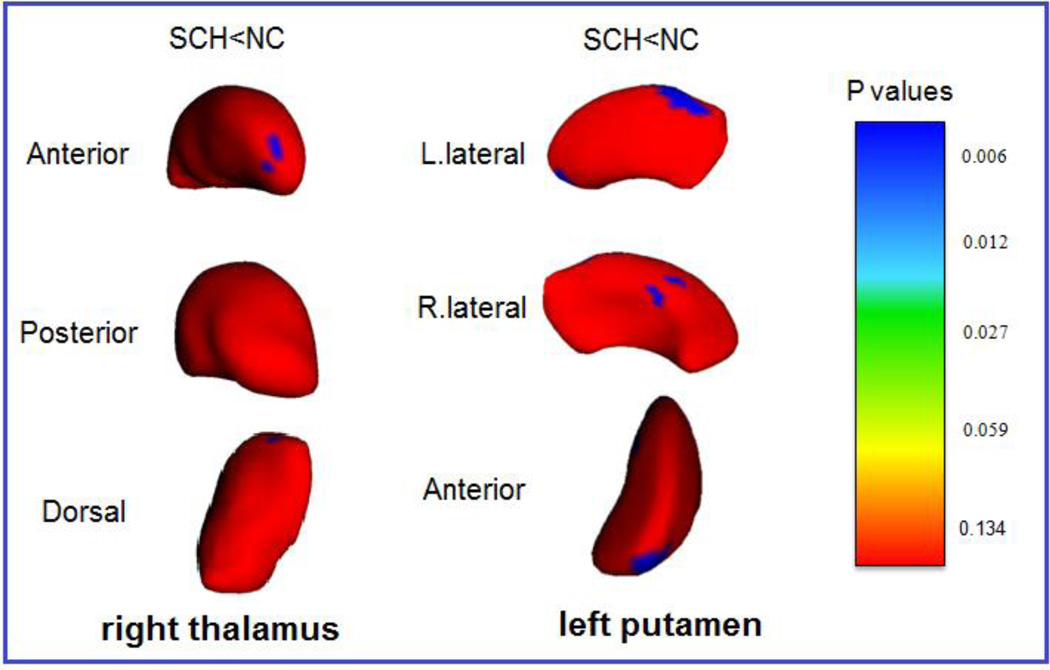
Analyses of the vertex-based surface shapes of the subcortical structures showed that significant regional surface shape atrophy in the both sides caudate nuclei, right amygdala, right hippocampus, and right putamen existed in the patient group when compared with the high-risk and control groups (Figure 1). Furthermore, significant regional shape atrophy in the right thalamus and left putamen were also detected in the patient group when comparing to the controls, but not to the high-risk (Figure 2). There were no significant differences between normal control and high-risk participants in the surface shapes of any subcortical structures.
Figure 1.
Statistical maps show significant differences in local atrophy of subcortical regions between SCH and both NC and HR groups. Left caudate nucleus, right caudate nucleus, right amygdala, right hippocampus, and right putamen are shown for both between-group comparisons (SCH vs. NC and SCH vs. HR). FDR corrected figures are shown. SCH: schizophrenia group; NC: normal control group; HR: high-risk group; L: left; R: right. The color-coded bar gives p values, with p = 0.134 (F = 2), p = 0.059 (F = 2.75), p = 0.027 (F = 3.5), p= 0.012 (F = 4.25), and p = 0.006 (F = 5) (degrees of freedom: 3, 32).
Figure 2.
Statistical maps composed using vertex-based comparison show subcortical regions with only differences between the schizophrenia group and normal control group. Local atrophy of right thalamus and left putamen are shown on FDR corrected figures. L: left; R: right. The color-coded bar gives p values, with p = 0.134 (F = 2), p = 0.059 (F = 2.75), p = 0.027 (F = 3.5), p= 0.012 (F = 4.25), and p = 0.006 (F = 5) (degrees of freedom: 3, 32).
Significant between-group differences in volumes of the subcortical structures were demonstrated across the three diagnostic groups (Table 2). Significant volume reductions on both sides of the caudate nuclei and thalami, and right amygdala of the schizophrenia group were shown when compared with the controls. Significant volume reductions on both sides of the caudate nuclei also existed in patients when compared with the high-risk group. Table 2 also shows that the mean volumes of both sides of the thalami of the high-risk subjects were lower than that of the controls, and also participants with schizophrenia. However, the variation (shown as SD) in these values were very large. This result suggested that at least a portion of the high-risk subjects had significantly smaller thalamic volumes when compared with the controls and patients. In addition, it is important to note that there were no significant differences found in the volumetric laterality indices of any subcortical volume among the three groups.
Table 2.
Group comparisons of the volumes (mm3) of subcortical structures.
| Subcortical Structure |
SCH Volume |
HR Volume |
NC Volume | F |
P Value |
Post-Hoc t- test (P Value) |
|---|---|---|---|---|---|---|
| L. Caudate | 2037.2 ± 350.8 | 2277.3 ± 432.8 | 2369.0 ± 274.2 | 8.93 | 0.0003 | SCH < NC (0.000) SCH < HR (0.007) |
| R. Caudate | 2114.9 ± 296.5 | 2247.7 ± 435.7 | 2357.8 ± 365.3 | 6.27 | 0.003 | SCH < NC (0.001) SCH < HR (0.020) |
| R. Amygdala | 548.4 ± 193.1 | 607.2 ± 192.5 | 613.0 ± 172.7 | 4.05 | 0.021 | SCH < NC (0.011) |
| L. Thalamus | 5375.5 ± 658.8 | 5292.9 ± 747.4 | 5497.7 ± 395.5 | 3.40 | 0.039 | SCH < NC (0.030) |
| R. Thalamus | 5167.6 ± 646.3 | 5045.7 ± 708.0 | 5310.1 ± 425.5 | 3.48 | 0.036 | SCH < NC (0.032) |
This table only shows volumes of the subcortical regions with significant between-group differences. Volumes are given in mean (mm3) ± SD. P-values < 0.05 were considered significant. R: right; L: left; NC: normal control group; HR: high-risk group; SCH: schizophrenia group.
The relationships between the subcortical structure volumes and the cognitive measures showed interesting group-specific patterns (Table 3). First, the associations of volumes of left caudate, left pallidum, left thalamus, and both sides putamen to the WAIS-VCI measure (the verbal comprehension index of WAIS-III) showed significant between-group differences. In the control group, the volumes of these structures showed negative correlations with the WAIS-VCI measures, whereas the high-risk and patient groups showed progressively increased positive correlations. Second, the patient group showed significant positive associations between volumes of left pallidum and both sides thalami and the WRAT-Reading, which measured the expressive language and academic skills, whereas the high-risk and control groups showed negative associations of these measures. Most interestingly, volumes of both sides pallidum, right thalamus and right putamen showed significantly higher positive associations with the CVLT metric (testing verbal memory capacity) in the high-risk group than in control or patient groups.
Table 3.
Group comparisons of the associations of volumes (mm3) of subcortical structures to the performance measures for language-related cognition.
| Slope (β) | NC | HR | SCH |
F Statistic |
P Value |
Post hoc t tests (P-Value) |
|---|---|---|---|---|---|---|
| Region | ||||||
| WAIS-VCI | ||||||
| L. Caudata | −18.9 | 99.2 | 59.5 | 4.52 | 0.01 | NC<HR (0.003) |
| L. Pallidum | 1.57 | 9.93 | 30.2 | 3.18 | 0.048 | NC<SCH (0.013) HR<SCH (0.014) |
| L. Putamen | −7.18 | 23.2 | 110.3 | 4.64 | 0.014 | NC<SCH (0.013) |
| L. Thalamus | −20.4 | 108.4 | 182.2 | 5.00 | 0.01 | NC<HR (0.011) NC<SCH (0.007) |
| R. Putamen | −13.7 | 5.38 | 103.3 | 4.85 | 0.01 | NC<SCH (0.003) HR<SCH (0.001) |
| WRAT | ||||||
| L. Pallidum | −0.20 | −3.10 | 9.27 | 4.61 | 0.01 | NC<SCH (0.020) HR<SCH (0.010) |
| L. Thalamus | −11.07 | −6.44 | 53.7 | 4.81 | 0.01 | NC<SCH (0.001) HR<SCH (0.002) |
| R. Thalamus | −6.86 | −15.1 | 47.9 | 3.91 | 0.03 | NC<SCH (0.009) HR<SCH (0.010) |
| CVLT | ||||||
| L. Pallidum | 5.11 | 62.57 | −20.2 | 3.45 | 0.04 | NC<HR (0.010) |
| L. Thalamus | 37.0 | 32.9 | −17.3 | 3.74 | 0.03 | NC>SCH (0.03) |
| R. Pallidum | −15.1 | 79.3 | −18.9 | 5.10 | 0.009 | NC<HR (0.005) HR>SCH (0.008) |
| R. Putamen | −54.7 | 252.8 | −35.5 | 3.90 | 0.03 | NC<HR (0.014) HR>SCH (0.020) |
| R. Thalamus | 29.9 | 320.9 | −16.9 | 3.71 | 0.03 | NC<HR (0.032) HR>SCH (0.006) |
β indicates the slope of the linear regression analysis of the subcortical structure volumes plotted against the language-associated fMRI and behavioral measures. NC: normal control group; HR: high-risk group; SCH: schizophrenia group. P< 0.05 were considered significant. WAIS-VCI: Wechsler Adult Intelligence Scale – Verbal Comprehension Index; WRAT: Wide Range Achievement Test; CVLT: California Verbal Learning Test.
4. Discussion
The current study assessed the regional shape deformations and volume changes of 6 (bilateral) subcortical structures that are essentially involved in the brain pathways for language processing, and evaluated their associations with the language-related behavioral performance scores, in individuals with schizophrenia and those at genetic high-risk for developing the disorder. The results indicated structural anomalies of these subcortical structures and aberrant patterns of their associations with the language-related performances in patients and the high-risk individual when compared to controls, which might underlie language impairments in schizophrenia that can be indicated early before onset of the disorder.
The thalamus is an integral subcortical part of a large-scale network mediating attentional and cognitive information and operates as a filter for incoming sensory information and directs it to the correct cortical areas, by coordinating activities in cortical areas (McFarland and Haber, 2002b). Because of this central role, the thalamus is involved in all aspects of language processing. Previous research in patients with schizophrenia have identified reduced thalamic volumes (Buchmann et al., 2014), metabolic and glutamatergic abnormalities in thalamus (Clinton and Meador-Woodruff, 2004), and impaired thalamo-cortical neural networks (Csernansky and Cronenwett, 2008), which all likely contribute to the auditory verbal hallucinations and thought disorder symptoms of schizophrenia (Pinault, 2011).
In the current study, we found that compared to the controls, the patients with schizophrenia exhibited focal surface atrophy in the anterodorsal areas of right thalamus and significantly reduced volumes of both the left and right thalami. They also demonstrated significant positive correlations between the thalamic volumes and the cognitive measures representing verbal comprehension (WAIS-VCI) and word-decoding (WRAT). Although the thalamic surface shape parameterization analyses did not demonstrate group-level differences between the high-risk and control groups, the volumes of both sides of the thalami in the high-risk group showed a trend of reduction when compared to the age-matched controls. In addition, the association between the volume of left thalamus and the measure of verbal comprehension in the high-risk group showed a similar pattern with the patient group, which was significantly higher than that in the normal controls. Very interestingly, the high-risk group showed a unique pattern of significant positive correlations between volume of right side thalamus and the measure of verbal memory (CVLT), compared to patients and controls.
Together with existing findings in patients with schizophrenia, our findings suggest that structural aberrations in the thalamus exist and relate to language function in those with the genotype, and may underlie onset of schizophrenia.
Besides the thalamus, striatum is the other important subcortical component of the cortico-striato-thalamo-cortical (CSTC) loops, which is involved in integrating and modulating a diverse range of neurological stimuli, making them integral for language generation (Crosson, 1985). Caudate and putamen nuclei together comprise the neostriatum (dorsal striatum), which is the gateway to the basal ganglia and receives white matter fibers from all portions of the cerebral cortex and the intralaminar nuclei of the thalamus, and thus are involved in nearly all sensory and cognitive information processing. Along with the striatum, the pallidum is a major component of the basal ganglia that is involved in regulating movements occurring on the subconscious level (Obler et al., 2010). Recent neuroimaging studies in patients with schizophrenia have frequently reported structural and functional abnormalities in these subcortical nuclei, such as abnormal volumes and gray matter intensity (Stip et al., 2008), and abnormal cortical-pallidum and cortical-striato functional connectivity (Bracht et al., 2013), etc. A previous study in patients with schizophrenia also has demonstrated correlations between reduced putamen volume and poorer verbal learning, working memory, and executive function, which are all essential mechanisms for language processing (Hartberg et al., 2011). Consistent with the existing findings, our study showed significantly reduced volumes and extensively altered regional surface shapes in both the left and right caudate nuclei in patients with schizophrenia compared to both people at high-risk and normal controls. The right putamen surfaces of patients with schizophrenia also demonstrated relatively diffuse atrophy compared to the controls and high-risk subjects, while the left putamen exhibited focal areas of anterolateral atrophy. In addition, the patients with schizophrenia showed significantly increased positive associations between the volumes of left caudate and bilateral putamen and verbal comprehension (measured by WAIS-VCI), when compared to the control and high-risk groups. It is important to note that the high-risk group did not significantly differ from the controls in volume and surface shape analyses of the caudate, putamen, and pallidum nuclei in both hemispheres. However, significantly stronger positive structural-behavioral associations were demonstrated in the high-risk individuals when correlating the volume of left caudate and verbal comprehension capacity, as well as both sides of the pallidum and the right putamen volumes with verbal memory capacity.
These findings demonstrate the volumetric and morphometric changes in the caudate, putamen, and pallidum nuclei, and their relationship to language function in patients with schizophrenia. The exceptionally strong associations between the volumes of these structures and the language performance in the high-risk group may implicate the early involvement and significant impact of these subcortical structures on the language processing impairments and onset of the disorder.
The hippocampus and amygdala are main centers of the limbic system. These two subcortical regions are intimately linked in structure and function, and both are imperative for proper memory, attention, and recall of language processing (Isenberg et al., 1999). The hippocampus is commonly cited as vital for declarative memory, which includes recollection of vocabulary and lexicons (Tamminga et al., 2010). Recent evidence suggests that the amygdala also has a role mediating language acquisition (Ortiz-Mantilla et al., 2010). Volumetric reductions of both hippocampus and amygdala have been reported in individuals with schizophrenia, associated with visual hallucinations (Ford et al., 2014). The current study also found structural atrophy of the right hippocampus and amygdala in patients with schizophrenia when compared to high-risk and control subjects. In the comparisons between the high-risk group and the controls, the hippocampus and amygdala did not show significant betweengroup differences. In addition, there were no significant between-group differences when assessing the associations between these two subcortical structures and the language cognition capacities among the three groups. These results may suggest that although the hippocampus and amygdala are two affected subcortical structures in progress of chronic schizophrenia, they do not significantly contribute to the language impairment of the disorder, neither significantly involve prior to the onset of the disorder.
We acknowledge several limitations of the present study. First, the group with schizophrenia was significantly older than the control and high-risk groups, because schizophrenia typically manifests in early adulthood (DeLisi et al., 2002). Age was thus controlled in all group-level analyses to reduce its potential impact on results. A very recent neuroimaging study had assessed age effect on subcortical structures in a large sample of healthy adults aged from 19 to 85 years old. The results showed that although age-related volume reduction exist in several subcortical structures in a linear regression analysis of the whole sample, further non-linear analyses showed that samples after 60 years old significantly contribute to the age-related reduction, while ages of 20s to 40s did not show such relationship (Goodro et al., 2012). In our study, we do not have any subject over 60 years old. Most of the subjects were in their 20s and 30s. Second, each group included a few left-handed subjects. However, this is unlikely to significantly affect results because handedness-associated cortical lateralization is more substantial than subcortical lateralization (RIKLAN and LEVITA, 1965). Third, all of the 20 subjects in the patient group were taking medications. There is a potential concern about the medication impact on brain subcortical structure integrity. It would be valuable to look at the relationship between medication dosage and volume of the brain subcortical structures in the patient group. However, we found that although all the patients had been treated, different conventional or atypical antipsychotic medications were used. It was thus impossible to run a statistically powerful enough analysis in each subgroup. In addition, longitudinal analyses of a larger cohort of high-risk and schizophrenia individuals is needed to verify whether the structural alterations of these subcortical regions can indicate progression of the disorder.
As a summary, this study is the first of its kind to demonstrate anomalies in the subcortical structures that are integral to cortico-subcortical-cortical circuits involved in language processing in individuals with schizophrenia, and those at high-genetic risk. Our findings significantly expand the existing literature which has primarily investigated the cortical structures related to language. Our findings collectively suggest that individuals with schizophrenia demonstrate differences within the thalamus, basal ganglia, hippocampus, and amygdala as compared to the other groups, and also evidence differences in the associations between these structures and standardized indices of language processing. Although there was a trend, the high-risk group did not show significant regional shape deformations and volume reductions of the six subcortical structures when compared to the controls. However, the aberrantly higher associations of the volumes of thalamus, caudate and pallidum nuclei with the verbal comprehension and memory capacities in the high-risk group may indicate their early involvement in language impairments that may contribute to the onset of schizophrenia.
Acknowledgements
This project was partially supported by a grant from NIMH, R21 MH071720.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests
All authors reported no actual and potential conflict of interests.
Contributors
Dr. Li designed the neuroimaging study, managed the literature searches, and wrote the first draft of the manuscript.
Dr. Black contributed on data analysis and manuscript writing.
Drs. Xia and Zhan processed the neuroimaging data and statistical analyses.
Dr. Bertisch administered the neurocognitive assessments.
Dr. Branch designed the MRI scan protocols.
Dr. Delisi provided the general hypotheses and administered the clinical interviews.
All authors contributed to and have approved the final manuscript
Reference List
- Bracht T, Schnell S, Federspiel A, Razavi N, Horn H, Strik W, Wiest R, Dierks T, Muller TJ, Walther S. Altered cortico-basal ganglia motor pathways reflect reduced volitional motor activity in schizophrenia. Schizophr Res. 2013;143:269–276. doi: 10.1016/j.schres.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Buchmann A, Dentico D, Peterson MJ, Riedner BA, Sarasso S, Massimini M, Tononi G, Ferrarelli F. Reduced mediodorsal thalamic volume and prefrontal cortical spindle activity in schizophrenia. Neuroimage. 2014 doi: 10.1016/j.neuroimage.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Craig MC, Forkel SJ, Kanaan R, Picchioni M, Toulopoulou T, Shergill S, Williams S, Murphy DG, McGuire P. Altered integrity of perisylvian language pathways in schizophrenia: relationship to auditory hallucinations. Biological Psychiatry. 2011;70:1143–1150. doi: 10.1016/j.biopsych.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Clinton SM, Meador-Woodruff JH. Abnormalities of the NMDA Receptor and Associated Intracellular Molecules in the Thalamus in Schizophrenia and Bipolar Disorder. Neuropsychopharmacology. 2004;29:1353–1362. doi: 10.1038/sj.npp.1300451. [DOI] [PubMed] [Google Scholar]
- Crosson B. Subcortical functions in language: a working model. Brain and Language. 1985;25:257–292. doi: 10.1016/0093-934x(85)90085-9. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Cronenwett WJ. Neural networks in schizophrenia. American Journal of Psychiatry. 2008;165:937–939. doi: 10.1176/appi.ajp.2008.08050700. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan DT, Ober BA. California Verbal Learning Test, Research. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- DeLisi LE, Shaw SH, Crow TJ, Shields G, Smith AB, Larach VW, Wellman N, Loftus J, Nanthakumar B, Razi K, Stewart J, Comazzi M, Vita A, Heffner T, Sherrington R. A genome-wide scan for linkage to chromosomal regions in 382 sibling pairs with schizophrenia or schizoaffective disorder. American Journal of Psychiatry. 2002;159:803–812. doi: 10.1176/appi.ajp.159.5.803. [DOI] [PubMed] [Google Scholar]
- Dougherty MK, Gu H, Bizzell J, Ramsey S, Gerig G, Perkins DO, Belger A. Differences in subcortical structures in young adolescents at familial risk for schizophrenia: a preliminary study. Psychiatry Research. 2012;204:68–74. doi: 10.1016/j.pscychresns.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn M, Dunn M. Peabody Picture Vocabulary Test. 3rd edition. American Guidance Service; 1997. [Google Scholar]
- Ford JM, Palzes VA, Roach BJ, Potkin SG, van Erp TG, Turner JA, Mueller BA, Calhoun VD, Voyvodic J, Belger A, Bustillo J, Vaidya JG, Preda A, McEwen SC, Mathalon DH. Visual Hallucinations Are Associated With Hyperconnectivity Between the Amygdala and Visual Cortex in People With a Diagnosis of Schizophrenia. Schizophrenia Bulletin. 2014 doi: 10.1093/schbul/sbu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodro M, Sameti M, Patenaude B, Fein G. Age effect on subcortical structures in healthy adults. Psychiatry Res. 2012;203:38–45. doi: 10.1016/j.pscychresns.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartberg CB, Sundet K, Rimol LM, Haukvik UK, Lange EH, Nesvag R, Melle I, Andreassen OA, Agartz I. Subcortical brain volumes relate to neurocognition in schizophrenia and bipolar disorder and healthy controls. Progress in Neuropsychopharmacology and Biological Psychiatry. 2011;35:1122–1130. doi: 10.1016/j.pnpbp.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Isenberg N, Silbersweig D, Engelien A, Emmerich S, Malavade K, Beattie B, Leon AC, Stern E. Linguistic threat activates the human amygdala. Proc Natl Acad Sci U S A. 1999;96:10456–10459. doi: 10.1073/pnas.96.18.10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EL, GOODGLASS H, WEINTRAUB S. Boston Naming Test. Second Edition. San Antonio, TX: The Psychological Corporation; 1983. [Google Scholar]
- Li X, Alapati V, Jackson C, Xia S, Bertisch HC, Branch CA, DeLisi LE. Structural abnormalities in language circuits in genetic high-risk subjects and schizophrenia patients. Psychiatry Res. 2012a Mar 31;201(3):182–189. doi: 10.1016/j.pscychresns.2011.07.017. 2012 Epub 2012 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Branch CA, Ardekani BA, Bertisch H, Hicks C, DeLisi LE. fMRI study of language activation in schizophrenia, schizoaffective disorder and in individuals genetically at high risk. Schizophr Res. 2007a;96:14–24. doi: 10.1016/j.schres.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Branch CA, Bertisch HC, Brown K, Szulc KU, Ardekani BA, DeLisi LE. An fMRI study of language processing in people at high genetic risk for schizophrenia. Schizophr Res. 2007b;91:62–72. doi: 10.1016/j.schres.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xia S, Bertisch HC, Branch CA, DeLisi LE. Unique topology of language processing brain network: A systems-level biomarker of schizophrenia. Schizophr Res. 2012b Nov;141(2–3):128–136. doi: 10.1016/j.schres.2012.07.026. 2012 Epub 2012 Aug 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald RJ, White NM. A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behavioral Neuroscience. 2013;127:835–853. doi: 10.1037/a0034883. [DOI] [PubMed] [Google Scholar]
- McFarland NR, Haber SN, et al. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. Journal of Neuroscience. 2002a;22:8117–8132. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. Journal of Neuroscience. 2002b;22:8117–8132. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNealy K, Mazziotta JC, Dapretto M. Cracking the language code: neural mechanisms underlying speech parsing. Journal of Neuroscience. 2006;26:7629–7639. doi: 10.1523/JNEUROSCI.5501-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G, Iyegbe C, Prata D, Rivera M, Kempton MJ, Valmaggia LR, Sham PC, van OJ, McGuire P. Molecular genetic gene-environment studies using candidate genes in schizophrenia: a systematic review. Schizophr Res. 2013;150:356–365. doi: 10.1016/j.schres.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of General Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863-4. [DOI] [PubMed] [Google Scholar]
- Obler LK, Rykhlevskaia E, Schnyer D, Clark-Cotton MR, Spiro AI, Hyun J, Kim DS, Goral M, Albert ML. Bilateral brain regions associated with naming in older adults. Brain Lang. 2010 Jun;113(3):113–123. doi: 10.1016/j.bandl.2010.03.001. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ortiz-Mantilla S, Choe MS, Flax J, Grant PE, Benasich AA. Associations between the size of the amygdala in infancy and language abilities during the preschool years in normally developing children. Neuroimage. 2010;49:2791–2799. doi: 10.1016/j.neuroimage.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith S, Kennedy D. FIRST-FMRIB's integrated registration and segmentation tool. Human Brain Mapping Conference. 2008 [Google Scholar]
- Pinault D. Dysfunctional thalamus-related networks in schizophrenia. Schizophrenia Bulletin. 2011;37:238–243. doi: 10.1093/schbul/sbq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppel D, Hickok G. Towards a new functional anatomy of language. Cognition. 2004 May-Jun;92(1–2):1–12. doi: 10.1016/j.cognition.2003.11.001. 2004. [DOI] [PubMed] [Google Scholar]
- Qiu A, Wang L, Younes L, Harms MP, Ratnanather JT, Miller MI, Csernansky JG. Neuroanatomical asymmetry patterns in individuals with schizophrenia and their nonpsychotic siblings. Neuroimage. 2009;47:1221–1229. doi: 10.1016/j.neuroimage.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIKLAN M, LEVITA E. LATERALITY OF SUBCORTICAL INVOLVEMENT AND PSYCHOLOGICAL FUNCTIONS. Psychological Bulletin. 1965;64:217–224. doi: 10.1037/h0022295. [DOI] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Stip E, Mancini-Marie A, Fahim C, Bentaleb LA, Letourneau G, Potvin S. Decrease in basal ganglia grey matter density associated with atypical antipsychotic treatment in schizophrenia patients. Schizophr Res. 2008;103:319–321. doi: 10.1016/j.schres.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. American Journal of Psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Third Edition. San Antonio, TX: The Psychological Corporation; 2004. [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test. 3rd edition. Wilmington, DE: Wide Range, Inc; 1993. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson. Third Editio. Itasca, IL: Riverside Publishing; 2000. [Google Scholar]
- Zhang R, Wei Q, Kang Z, Zalesky A, Li M, Xu Y, Li L, Wang J, Zheng L, Wang B, Zhao J, Zhang J, Huang R. Disrupted brain anatomical connectivity in medication-naive patients with first-episode schizophrenia. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0706-z. [DOI] [PubMed] [Google Scholar]