Abstract
Fluorescent biomaterials have attracted significant research efforts in the past decades. Herein, we report a new series of biodegradable, fluorescence imaging-enabled copolymers, biodegradable photoluminescent poly(lactide-co-glycolide) (BPLP-co-PLGA). Photoluminescence characterization shows that BPLP-co-PLGA solutions, films and nanoparticles all exhibit strong, tunable and stable photoluminescence. By adjusting the molar ratios of L-lactide (LA)/glycolide (GA) and (LA+GA)/BPLP, full degradation of BPLP-co-PLGA can be achieved in 8 to 16 weeks. The fluorescence decay behavior of BPLP-co-PLGA can be used for non-invasive monitoring of material degradation. In vitro cytotoxicity and in vivo foreign body response evaluations demonstrate that BPLP-co-PLGA exhibits similar biocompatibility to poly(lactide-co-glycolide) (PLGA). The imaging-enabled BPLP-co-PLGA was fabricated into porous scaffolds whose degradation can be monitored through non-invasive imaging and nanoparticles that show theranostic potential demonstrated by fluorescent cellular labeling, imaging and sustained 5-fluorouracil delivery. The development of inherently fluorescent PLGA copolymers is expected to impact the use of already widely accepted PLGA polymers for applications where fluorescent properties are highly desired but limited by the conventional use of cytotoxic quantum dots and photobleaching organic dyes.
Keywords: biodegradable, photoluminescence, bioimaging, tissue engineering, drug delivery, PLGA
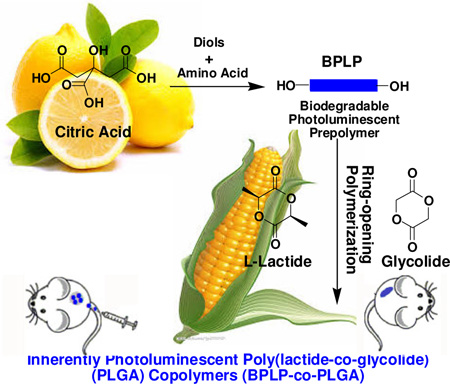
Graphical abstract
Development of Photoluminescent Poly(lactide-co-glycolide) Copolymers with Tunable Biodegradability: BPLP-co-PLGA was developed by ring-opening polymerization of L-lactide and glycolide using citrate-based biodegradable photoluminescent prepolymer (BPLP) as a macromolecular initiator. The combination of BPLP and widely-used PLGA is not only a route to the creation of totally biodegradable photoluminescent PLGA, but also an opportunity to demonstrate the production scalability and commercial applications of our innovative polymer, BPLP.
1. Introduction
Emerging biomedical technologies rely on the development of advanced biomaterials, including the development of medical materials with improved functionality and the application of these biomaterials to new fields [1–3]. During the past decades, as emergent biomaterials, biodegradable polymers have received extensive research and development efforts for biomedical applications, such as tissue engineering, regenerative medicine and drug delivery [4–8]. Recently, the development of biodegradable polymers with imaging capabilities, especially fluorescence imaging, to analyze biomolecules, track biological processes, and visualize diseases and therapeutic efficacy, has become increasingly desirable [9, 10]. In particular, non-invasive evaluation of biodegradable materials, real-time monitoring of tissue engineering scaffolds and targeted drug delivery are emerging fields that demand biodegradable photoluminescent polymers. To date, tremendous effort has been made to mix or conjugate biodegradable polymers with semiconducting quantum dots or organic dyes to create photoluminescent biodegradable materials [11–14]. However, the inevitable photobleaching and low dye-to-polymer labeling ratios of organic dyes and the innate toxicity from the heavy metal of quantum dots prevent their practical use in vivo [15, 16]. Green fluorescent protein (GFP) is an alternative that shows intrinsic photoluminescence, but it is still capable of causing cellular toxicity due to over-expression and protein aggregation. There are also few reports on combining GFP with biodegradable polymers [17, 18]. Thus, the development of biocompatible and highly photo-stable biodegradable, photoluminescent polymers for imaging-aided tissue engineering and drug delivery still remains a significant challenge.
Recently, our lab has made a progress in the development of biodegradable photoluminescent polymer (BPLP) with inherent photoluminescence. BPLPs have tunable fluorescence with high quantum yields (up to 62.33%) and superior photo-stability, making BPLPs promising materials for a wide range of biomedical applications [19]. BPLP is a fully degradable and cyto-compatible aliphatic oligomer with low-molecular weight (~1200 Da) which can be thermally crosslinked into biodegradable elastomers. However, the tensile strength of crosslinked BPLP is not high enough for many tissue engineering applications. In addition, BPLP nanoparticles tend to aggregate in physiological conditions due to their sticky nature, caused by its low molecular weight, which limits the application of BPLP nanoparticles as theranostic probes [20]. A practical approach to address the above issues is to incorporate BPLP into traditional or commercialized biodegradable polymers to achieve higher molecular weight, improved mechanical strength, and favorable processability. Our efforts have led to the development of urethane-doped BPLP (UBPLP) and BPLP-co-poly(L-lactide) (BPLP-PLLA) [20, 21]. However, the synthesis of BPLP-PLLA used an enzyme-catalyzed polymerization process, which cannot be applied in large-scale industrial production. Additionally, the application of PLLA polymers is limited by their rigidity, brittleness and slow degradation. To expand the application of these fluorescent polymers in soft tissue engineering and drug delivery and explore the possibility of their large-scale industrial production, we incorporated BPLP into the relatively fast-biodegradable polymers, poly(lactide-co-glycolide) (PLGA), using an industrially used coordination polymerization process. PLGA has been widely accepted as the gold standard material for many drug delivery and tissue engineering devices approved by the U.S. Food and Drug Administration (FDA) due to its favorable biocompatibility, relatively fast biodegradation rate, and considerable mechanical strength [22–26]. PLGA can be conveniently synthesized through a ring-opening polymerization of cyclic dimers of lactic acid and glycolic acid, lactide (LA) and glycolide (GA), initiated by hydroxyl group-containing compounds [27–29]. Varying the molar ratio of LA/GA and monomer/initiator results in tailored mechanical performance and degradation profiles that supporting controlled drug release and tissue regeneration. By controlling the BPLP synthesis procedure, excessive hydroxyl groups, which can initialize PLGA polymerization, remain present at the chain ends of BPLP [19, 21, 30]. In this study, a new family of biodegradability tunable, inherently photoluminescent BPLP-poly(lactide-co-glycolide) (BPLP-co-PLGA) copolymers were synthesized. Chemical, thermal, mechanical, and optical properties, as well as the degradation and biocompatibility of the resulting BPLP-co-PLGA copolymers have been comprehensively examined. BPLP-co-PLGA copolymers present several remarkable features: (i) inherent photoluminescence inherited from BPLP [19, 31]; (ii) excellent biocompatibility; (iii) Tunable thermal and mechanical properties, degradation rates and luminescence by varying compositions of BPLP, LA and GA during copolymer synthesis; and (iv) good processability for the fabrication of films, micro-/nano-particles and porous scaffolds. The strategy of combining BPLP with PLGA to produce inherently photoluminescent PLGA copolymers may open new avenues in numerous biomedical applications for which both biodegradable materials and optical imaging are highly desired.
2. Experimental Section
2.1. Synthesis of BPLP-co-PLGA copolymers
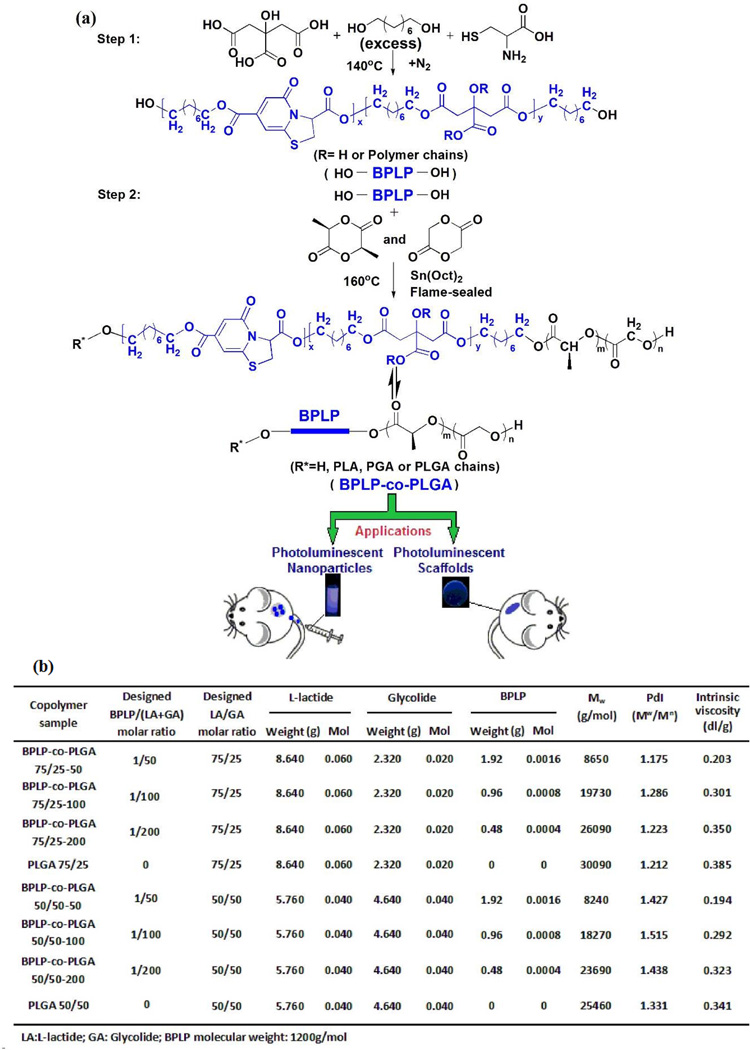
BPLP-co-PLGA copolymers were synthesized in two steps (Figure 1a). Step one involves the synthesis of a hydroxyl-terminated BPLP prepolymer as described in our previous work [19]. Representatively, citric acid, 1, 8-octanediol and L-cysteine with the molar ratio of 1:1.1:0.2 were added into a three-necked round bottom flask and then heated to 160°C under a constant flow of nitrogen till all reactants melted. The temperature of the reaction was subsequently lowered to 140°C and the solution was allowed to react for another ~1 h. The reaction was stopped before the stirring bar stopped stirring completely by adding 1, 4-dioxane to dissolve the obtained prepolymer. The obtained prepolymer was purified by drop-wise precipitation of prepolymer solution in 1, 4-dioxane into deionized water to remove unreacted monomers. The purified prepolymer was collected and lyophilized in a Freezone 6 Freeze Dryer (Labconco, Kansas City, MO) for 24h to obtain BPLP prepolymer, BPLP-Cys. The fluorophore structure of BPLP-Cys was recently verified as a fused ring structure ((5-oxo-3,5-dihydro-thiazolopyridine-3,7-dicarboxylic acid, TPA) (Fig. 1a) [31].
Fig. 1.
(a) Synthesis of BPLP-co-PLGA. Step 1: Synthesis of BPLP via polycondensation; Step 2: Synthesis of BPLP-co-PLGA via a ring-opening polymerization using BPLP as a macromolecular initiator. (b) Feeding ratios, molecular weights (Mw), polydispersity indexes (PDI), and intrinsic viscosities ([η]) of PLGA and BPLP-co-PLGA copolymers synthesized in this study.
In the second step, the BPLP prepolymer was used as a macro-initiator to react with L-lactide and glycolide via a ring-opening polymerization catalyzed by stannous 2-ethylhexanoate (Sn(OCt)2) to obtain BPLP-co-PLGA copolymers. Briefly, L-lactide and glycolide at various molar ratios were added to an oven-dried reaction tube with various amounts of BPLP prepolymers. Then, Sn(OCt)2 (0.1% by weight of L-lactide and glycolide mixture) was added as a solution in dry dichloromethane. The dichloromethane was evaporated in vacuum over a period of 1 hour. The tube was vacuumed and purged with nitrogen three times, and then flame-sealed and subsequently immersed in a 160°C oil bath for 48 hrs. After 48 hours, the reaction product was cooled to ambient temperature. The obtained solid materials were dissolved in chloroform and precipitated three times in an excess of pure ethanol to remove unreacted raw materials. Finally, BPLP-co-PLGA copolymers were recovered by vacuum filtration and dried in vacuum at room temperature for at least 1 week.
2.2. Fabrication of BPLP-co-PLGA copolymers films, scaffolds and nanoparticles
BPLP-co-PLGA films were prepared by casting their dichloromethane solution into poly (tetrafluoroethylene) (PTFE) dishes followed by evaporation [32]. BPLP-co-PLGA scaffolds were fabricated by a convenient salt-leaching method. BPLP-co-PLGA copolymers were dissolved in 1, 4-dioxane, followed by the addition of sieved salt with a desired size. The solvent was evaporated and the salt was leached out by DI water, and the samples were freeze-dried for 24 hours to obtain BPLP-co-PLGA scaffolds. BPLP-co-PLGA nanoparticles and 5-fluorouracil (5-Fu) loaded BPLP-co-PLGA nanoparticles were prepared using single emulsion (O/W) method and double emulsion (W/O/W) method, respectively. For BPLP-co-PLGA nanoparticle preparation, 100 mg of the BPLP-co-PLGA copolymers were dissolved in 5 mL of chloroform. The polymeric solution was then added dropwise to 40 mL of the PVA aqueous solution (5%, w/v) and ultrasonicated simultaneously to produce an oil-in-water (O/W) emulsion. The O/W emulsion was stirred for 24h in a chemical hood to evaporate chloroform. The resultant nanoparticles were collected by centrifugation and washed with distilled water three times followed by freeze-drying to obtain a fine powder of BPLP-co-PLGA nanoparticles. For 5-Fu loaded BPLP-co-PLGA nanoparticle preparation, 100mg of the BPLP-co-PLGA copolymers were dissolved in 5mL of chloroform. 2mL 5-Fu aqueous solution (5mg/mL) was slowly added into BPLP-co-PLGA organic solution and ultrasonicated simultaneously. The primary water-in-oil (W/O) emulsion was formed and was slowly added into 40mL of the PVA aqueous solution (5%, w/v) by ultrasonicating homogenization to produce the water-in-oil-in-water (W/O/W) emulsion. The W/O/W emulsion was stirred for 24 hours in a chemical hood to evaporate chloroform. The resultant nanoparticles were collected by centrifugation and washed with deionized (DI) water three times followed by freeze-drying to give the 5-Fu loaded BPLP-co-PLGA nanoparticles.
2.3. Polymer general characterization
Molecular weights and molecular weight distributions of BPLP-co-PLGA and PLGA copolymers were measured using a Shimadzu HPLC system equipped with a Phenomenex Phenogel 5µm 10E3 SEC column, a Wyatt miniDAWN light scattering detector and an OptiLab RI detector. PLGA and BPLP-co-PLGA copolymers were dissolved in chloroform (chloroform was used as the mobile phase as well). For each experiment, the injection volume was 100 µL, while a flow rate of 1 mL/min and 40 °C column temperature was used. 30 KDa monodisperse polystyrene served as the standard for calibration of both detectors. Intrinsic viscosities of copolymers were also measured following the steps as described below: 50mg of PLGA or BPLP-co-PLGA copolymer was dissolved in 10mL chloroform to make a polymer solution, which was then filtered into an Ubbelohde viscometer (size 1, Paragon Scientific Ltd., UK) using a 0.22 µm PTFE syringe filter. The filled viscometer was equilibrated in a 30°C water bath for 15 min. The flux time of pure chloroform and the polymer solution between two marks was recorded. Three measurements were performed for each sample, and the variation of flux time was limited within 0.3 s. The intrinsic viscosity ([η]) was calculated using the following Equation (1):
| Equation (1) |
Where ηr is the relative viscosity (the flux time ratio of the polymer solution to the pure chloroform) and C is the polymer concentration in units of grams per deciliter. 1H-NMR spectra were obtained in chloroform-d at 300 MHz using a Bruker DPX-300 FT-NMR spectrometer. Attenuated total reflectance-Fourier transform infrared (ATR-FTIR) characterization was performed on a Bruker Vertex 70 FTIR spectrometer with a Pike Miracle Single-Bounce diamond crystal plate accessory at room temperature. FTIR spectra were recorded over a wavelength range of 4000–500 cm−1. Mechanical tests were conducted with an Instron 5966 machine fitted with a 500N load cell. The copolymer films cut into rectangle shaped specimens were loaded and pulled at a rate of 500 mm min−1, and elongated to failure. The Young’s modulus was calculated by measuring the gradient from 0 to 10% elongation of the stress-strain curve. Six specimens per sample were tested and averaged. The thermal properties of copolymers were characterized by differential scanning calorimetry (DSC, −50°C~150°C) (TA Instruments Q2000) and thermal gravimetric analysis (TGA, 20°C~600°C) (TA instruments 2050 TGA) at a heating rate of 10°C/min under nitrogen atmosphere. The water-in-air contact angles of copolymer films were measured at room temperature by a Rame-Hart goniometer and imaging system (Rame-Hart Model 295) within 10 s after dropping. Four independent measurements at different sites were averaged. The change of water-in-air contact angle with time was also monitored from 0 to 75 minutes after water was dropped on the surface of the films. The morphologies of films and scaffolds were observed by scanning electron microscope (SEM) (Hitachi 3500 N, EPIC). The morphology of nanoparticles was observed by transmission electron microscopy (TEM, Philips EM 420). The particle size and size distribution of nanoparticles were measured by dynamic light scattering (DLS) (Malvern Zetasizer).
2.4. Photoluminescent properties
All photoluminescence spectra were acquired on HORIBA Scientific Fluoromax-4 spectrofluorometer. All copolymers were dissolved in chloroform to test photoluminescence unless otherwise noted. Both the excitation and the emission slit widths were set at 1.5nm. The quantum yields of copolymers were measured by the Williams’ method [33]. Anthracene (quantum yield =27% in ethanol) was used as the standard. The extinction coefficient (ε, mol−1•L•cm−1) of BPLP-co-PLGA was calculated according to the Beer-Lambert law, A = εCL. Here C and L are the concentration (mol/L) and length (cm) of the dye solution in a UV-vis cuvette respectively. The brightness and photostability are two important intrinsic photophysical properties of fluorescent materials. The brightness intensities of BPLP-co-PLGA can be calculated by the extinction coefficient times the quantum yield. Photostability was measured by continuously illuminating copolymer solutions with excitation light at 365 nm and measuring the resulting emission at 430 nm for 3 hours. Photoluminescence stability of rhodamine B was tested with its maximum excitation at 540 nm and maximum emission at 625 nm for the same 3 hours in aqueous solution. For nanoparticle photoluminescence characterization, the copolymer nanoparticles were dispersed in DI water and tested using the same method used for copolymer solutions. All the experiments were carried out in triplicate.
2.5. In vitro degradation
In vitro degradation was conducted with 50 mg of copolymer film (with thickness around 0.15–0.30 mm) placed in a tube containing 10mL of phosphate buffered saline (PBS, pH=7.4) and incubated at 37°C. PBS was replaced every two weeks until compete degradation. At each time point, the samples were taken out and thoroughly washed with deionized water 3 times, lyophilized for 1 week, each sample was weighed and the degradation was calculated by mass loss. Six parallel tests were averaged at each time point and the results are presented as means ± standard deviation. The effect of degradation on photoluminescence was also monitored by testing the photoluminescence of degrading copolymers as well as the supernatant PBS.
2.6. In vitro cytotoxicity of copolymer films and degradation products
Cytotoxicity of BPLP-co-PLGA films was quantitatively assessed by Methylthiazoletetrazolium (MTT) assay against human mesenchymal stem cells (hMSCs, Lonza Walkersville Inc, US). PLGA 75/25 was used as a control. All testing films were cut into circular discs (7 mm in diameter) to fit the inner diameter of 96-well plates. The films were sterilized in 70% ethanol for 3 hours followed by another 30 min exposure to UV light. Subsequently, 200 µL of a cell suspension (5×104 cells/mL) in complete Dulbecco’s modified eagle’s medium (DMEM, with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin streptomycin) was added to each well in a 96-well plate with disk-shaped specimens on the bottom. The culture plates were maintained in an incubator at 37 °C, 5% CO2 and 95% relative humidity. MTT assay analysis was performed after 24 hours of culture as per the manufacturer’s protocol. Absorbance was analyzed with an Infinite 200 microplate reader (Tecan Group Ltd., Switzerland) at 570 nm. At the same time, the cell morphology of hMSCs on copolymer films after 1 day culture was observed by scanning electron microscopy (SEM) after cell fixing, gradient dehydration by ethanol solutions with increasing concentrations (from 50%, 70%, to 90% and finally 100%), and freeze-drying.
The cytotoxicity of BPLP-co-PLGA degradation products was also evaluated against hMSCs. Equal weight (0.25 g) of PLGA and BPLP-co-PLGA copolymers were fully degraded in 5 mL of 1 M NaOH solution and the resultant solutions were adjusted to pH 7.4 with 0.5M HCl solution and then were diluted to 1×, 10× and 100× concentrations (1× was the pH adjusted solution of degradation products with no dilution; 10× and 100× means 10 times and 100 times dilution of 1× solution, respectively) using PBS (pH 7.4). All the above solutions were passed through a sterilized 0.22 µm filter prior to use for cell culture. To each well of a 96-well cell culture plate, 200 µL of hMSC cell solution in complete DMEM with a density of 5×104 cells/mL was added. On the second day, 20 µL of degradation products with 1×, 10× and 100× concentrations were added and cells were incubated for another 24 hours followed by MTT assay analysis. The cell viabilities of hMSCs in media containing polymer degradation solutions were normalized to that of cells cultured in normal media. To assess the effect of polymer degradation products on cell proliferation, Live/Dead staining assay (Life Technologies Inc., US) was conducted on hMSCs cultured in 10× degradation products 1 day, 3 days and 7 days post cell seeding and the addition of 10× degradation products to complete DMEM medium. Cell culture media containing 200 µL of complete DMEM and 20 µL of 10× polymer degradation products was changed every other day.
2.7. Cellular uptake and fluorescence labeling of nanoparticles
Cellular uptake of the nanoparticles was also examined in vitro. hMSCs were seeded onto sterile cover slips at a density of 5,000 cells/mL. Cells were allowed to attach and grow for 24 hours before uptake studies were performed. The cover slips were washed with PBS and transferred into a Petri dish. After 4 hours incubation with BPLP-co-PLGA50/50-50 nanoparticle solution (500 µg/mL), the medium was aspirated and the cells were washed three times with PBS to remove free nanoparticles. The cells were fixed with 4.0% paraformaldehyde solution for 2 hours. After fixing, the cover slips were mounted on glass slides and imaged under a Leica DMLP fluorescence microscope (Leica Microsystems, Bannockbum, IL) equipped with a Nikon E500 Camera (8.4V, 0.9A, Nikon Corp., Japan).
2.8. In vivo foreign body response
To evaluate the in vivo host response, PLGA75/25 and BPLP-co-PLGA 75/25-100 disks (0.5–0.75 mm thickness and 8 mm diameter) were placed subcutaneously in 6-month-old female Sprague Dawley rats (Harlan Sprague Dawley, Inc., Indianapolis, IN) under deep isoflurane-O2 general anesthesia. Animal experimentation was performed according to protocols approved by the Institutional Animal Care and Use Committee at the Pennsylvania State University. Rats were sacrificed with excess CO2, and the copolymers with surrounding tissues were harvested and fixed by soaking in 10% formalin for 2 days. The samples were processed on an automated tissue processor, embedded in paraffin wax, and sectioned into 4 µm sections. Six slides from different areas of the explants were stained with hematoxylin and eosin staining (H & E staining) [34]. The cross-sections were examined using a Leica DMLP microscope (Leica Microsystems Inc., Bannockbum, IL) fitted with a Nikon E500 CCD camera (Nikon Corp., Japan). For quantitative analysis, all the cells in a 200 × 200 µm2 region of the skin-side tissue near the implants from 400× images of H & E staining were counted. For one sample, at least 8 different square regions from different specimens were analyzed and the numbers were averaged.
2.9. Characterization of 5-fluorouracil (5-Fu) loaded nanoparticles
5-Fu calibration curve was established as follows: 10 mg of 5-Fu was dissolved in a 50 mL volumetric flask to obtain a final concentration of 0.2 mg/mL stock solution. The standard stock solution was diluted with water to obtain a series of standard solutions with various concentrations (0.1~100 µg/mL). The 5-Fu standard solutions were filtered through a 0.40 µm membrane before analysis. A Shimadzu high-performance liquid chromatography (HPLC) system with UV detector was used for the analysis. A Phenomenex Kinex C18 column (4.6×150 mm, 5 µm) was used for separation. The column temperature was maintained at 25°C. The standards and samples were determined using a mobile phase consisting of methanol and water (10:90, v/v) at a flow rate of 1.0 mL/min. The injection volume was 20 µL. The peak of 5-Fu was detected at the wavelength of 265 nm.
Measurement of 5-Fu encapsulation efficiency and loading content was conducted as follows: 5-Fu loaded nanoparticles solutions were ultra-speed centrifuged (25,000g for 30 min at 4°C) twice, and each time the supernatant was collected. A Shimadzu UFLC system equipped with UV detector was used to test the 5-Fu absorbance in the supernatant as described above. The 5-Fu content in the supernatant was calculated based on the 5-Fu calibration curve. The encapsulation efficiency (EE%) and loading content (LC) of 5-Fu were calculated using Equation (2) and Equation (3), respectively:
| Equation (2) |
| Equation (3) |
Where Wtotal and Wfree are the total weight of initial 5-Fu and the weight of 5-Fu detected in supernatant after centrifugation, respectively. WNP is the weight of copolymer in nanoparticle solutions.
In vitro drug release from 5-Fu loaded nanoparticles was studied in triplicate using a dialysis method with phosphate buffered saline (PBS, pH=7.4) as the release medium. 5-Fu loaded PLGA 75/25 nanoparticles were chosen as control. Briefly, 50 mg of 5-Fu loaded nanoparticle powders was dispersed in 5.0 mL PBS (pH=7.4) and placed into a dialysis membrane bag (with a molecular cut-off of 6.1 Kda, Spectra/Por® 6.1 kD dialysis membrane pre-wetted RC tubing; Spectrum, New Brunswick, NJ), tied and then incubated in a capped centrifuge tube containing 45 mL of PBS (pH=7.4). The centrifuge tube was kept at 37°C in a water bath on an orbital shaker that was under shaking at 120 rpm. At appropriate intervals, 2 mL release medium was extracted and 2 mL fresh PBS solution was replenished into the centrifuge tube right after sample withdrawal. The amount of 5-Fu in the release medium was analyzed by HPLC as described above. All measurements were performed in triplicate and the values were averaged.
3. Results and Discussion
BPLP-co-PLGA copolymers were synthesized in two steps (Fig. 1a). First, a hydroxyl-terminated BPLP prepolymer was synthesized according to our previously published methods [19]. Then the hydroxyl-terminated BPLP (BPLP-Cys) prepolymers were used as macro-initiators to react with L-lactide and glycolide via a ring-opening polymerization catalyzed by stannous 2-ethylhexanoate in a flame sealed reactor under vacuum to give BPLP-co-PLGA. The obtained BPLP-co-PLGA copolymers exhibited inherent photoluminescence from BPLP [19], which enabled visualization by fluorescence imaging. Various BPLP-co-PLGA copolymers were synthesized with different molar feeding ratios of L-lactide to glycolide (LA/GA as 75:25 and 50:50) and different molor ratios of BPLP to total L-lactide and glycolide (BPLP/(LA+GA) of 1:50, 1:100, and 1:200). Pure PLGA copolymers with the same L-lactide to glycolide molar feeding ratios were also synthesized as controls. The resulting polymers with different feeding ratios, molecular weights (Mw), polydispersity indexes (Mw/Mn) and intrinsic viscosities ([η]) are summarized in Fig. 1b. The average molecular weight (Mw) was 8650, 19730, 26090 and 30090 g/mol for BPLP-co-PLGA 75/25-50 (LA/GA=72/25, BPLP/(LA+GA)=50), BPLP-co-PLGA 75/25-100, BPLP-co-PLGA 75/25-200 and PLGA 75/25, respectively. The average molecular weight (Mw) was 8240, 18270, 23690 and 25460 g/mol for BPLP-co-PLGA 50/50-50, BPLP-co-PLGA 50/50-100, BPLP-co-PLGA 50/50-200 and PLGA 50/50, respectively. The molecular weights of the copolymers correlated well with BPLP/(LA+GA) ratios: the molecular weight decreased with the increase of the ratio of BPLP/(LA+GA). BPLP also acted as a molecular weight controller in the synthesis of BPLP-co-PLGA copolymers. It was found that the LA/GA molar ratio also impacted the molecular weight of the synthesized copolymers. With the introduction of the same molar ratio of BPLP, molecular weight decreased with the increase of the molar percentage of glycolide in (LA+GA) (Fig. S1). The relationship between the intrinsic viscosity of the synthesized copolymers and the molar ratio of BPLP/(LA+GA) is illustrated in Fig. S2. The impact of the molar ratio of BPLP/(LA+GA) on the intrinsic viscosity was similar to that on the molecular weight, which verified that control of molecular weight could be achieved by adjusting the amount of BPLP in the synthesis of BPLP-co-PLGA copolymers.
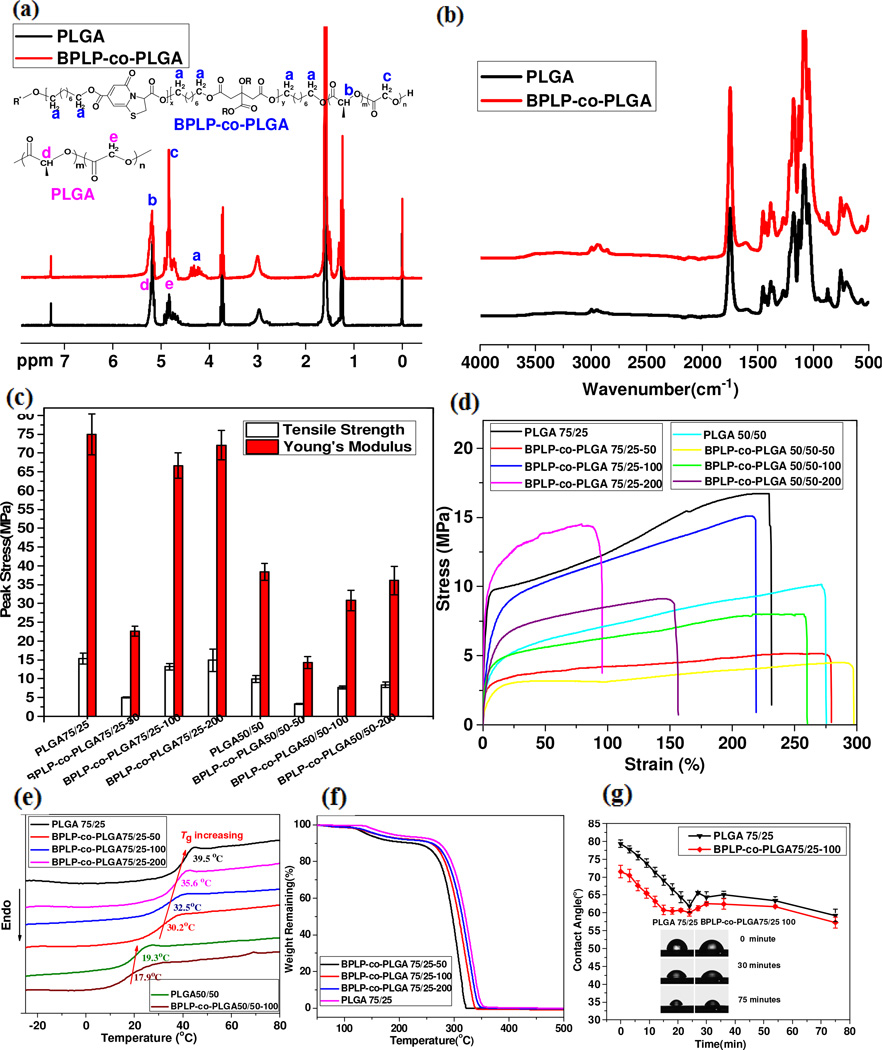
1H-NMR spectra of PLGA and BPLP-co-PLGA verified the successful incorporation of BPLP into the PLGA copolymer matrix (Fig. 2a), as indicated by the appearance of signals located between 4.0 and 4.30 ppm (a), which were assigned to the protons in -CH2O- from 1, 8-octanediol in BPLP. The signals at ~5.2 ppm (b, d) and ~4.6–4.9 ppm (c, e) were attributed to the protons from lactic acid -CH- and the glycolic acid -CH2- respectively, randomly distributed in the copolymer backbone. The ratios of the signal intensity of the characteristic proton peaks from related chemical compositions of PLGA 75/25 and various BPLP-co-PLGA copolymers were calculated (Fig. S3a–e, Supporting Information). The resulting compositions of the synthesized copolymers were well correlated to the feeding ratios between BPLP, LA and GA (Fig. S3f, Supporting Information). The actual composition of BPLP-co-PLGA copolymers could be well controlled by varying the feeding ratios of raw materials, including BPLP (as an initiator) and monomers. It could be deduced that when the number of protons from -CH2O- in 1,-8-octanediol of BPLP (α in Fig. S3f) was around 20 (meaning that the polymerization degree of BPLP was around 5, which is in accordance with our previous molecular weight characterization [19]), the determined compositions of BPLP/(LA+GA) were 1/199.52, 1/100.5, 1/49.0 and 1/104.98 for BPLP-co-PLGA 75/25-200, BPLP-co-PLGA 75/25-100, BPLP-co-PLGA 75/25-50 and BPLP-co-PLGA 50/50-100, respectively, which matched the designed compositions of 1/200, 1/100, 1/50, and 1/100 very well. Similar ATR-FTIR spectrums of BPLP-co-PLGA and PLGA (with characteristic absorption of ester groups at 1745 cm−1) further confirmed their similar chemical structure, given the fact that BPLP only took up a small portion in BPLP-co-PLGA copolymer (Fig. 2b).
Fig. 2.
Chemical, mechanical and thermodynamic properties and wettability of BPLP-co-PLGA. 1H-NMR (a) and ATR-FTIR (b) spectra, Tensile strength and Young’s modulus (c), strain-stress curves (d), differential scanning calorimetry (DSC, e) and thermal gravity analysis (TGA, f) curves of representative PLGA and BPLP-co-PLGA. Water-in-air contact angle vs. time curves (g) of representative PLGA and BPLP-co-PLGA copolymers films.
The thermal properties of representative PLGA and BPLP-co-PLGA copolymers were determined by differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA). The glass transition temperatures (Tgs) of copolymers were indicated by the DSC curves (Fig. 2e). The Tg values of PLGA 75/25, BPLP-co-PLGA 75/25-200, BPLP-co-PLGA 75/25-100 and BPLP-co-PLGA 75/25-50 were 39.5, 35.6, 32.5 and 30.2°C, respectively. Clearly, the Tg value decreased with an increase of the incorporation ratio of the sticky and soft BPLP chain (Fig. S4, Supporting Information). The Tgs of PLGA 50/50 and BPLP-co-PLGA 50/50-100 were 19.3 and 17.9°C, respectively, also displaying a similar Tg decreasing tendency. It was also found that Tg values of PLGA and BPLP-co-PLGA increased with increasing ratios of LA/GA in the copolymers. Higher LA percentage brought more rigid L-methyl side groups, which would produce a stronger steric hindrance effect, reducing the flexibility of the copolymer chain, thus increasing the Tgs of the copolymers. Moreover, the Tgs of BPLP-co-PLGA 75/25-200 and BPLP-co-PLGA 75/25-100 copolymers were 35.6 and 32.5°C, respectively, just below body temperature (37°C). Thus, these copolymers would stay in a viscoelastic state when applied in vivo. A viscoelastic copolymer matrix can have a higher permeability to water compared with rigid polymers, which may lead to improved hydration, degradation and drug release rate. TGA curves of BPLP-co-PLGA 75/25-50, BPLP-co-PLGA 75/25-100, BPLP-co-PLGA 75/25-200 and PLGA75/25 copolymers are presented (Fig. 2f) and the related analysis results are also summarized (Table S2, Supporting Information). The onset mass loss temperatures for BPLP-co-PLGA 75/25-50, BPLP-co-PLGA 75/25-100 and BPLP-co-PLGA 75/25-200 copolymers were all about 100°C, while the start of thermal decomposition for PLGA 75/25 copolymers occurred at around 140°C, which demonstrates that the incorporation of BPLP lowered the thermal stability of the copolymers. The temperatures at 5%, 10% and 100% mass loss further confirmed that increased BPLP incorporation lowers the thermal stability of the copolymers.
The mechanical properties of PLGA and BPLP-co-PLGA copolymer films were investigated through tensile testing. The results are shown in Fig. 2c (tensile strength and Young’s modulus values) and Fig. 2d (stress-strain curves). The detailed mechanical properties of PLGA and BPLP-co-PLGA copolymer films are also summarized and listed in Table S1 (Supporting Information). The tensile strength and the Young’s modulus of BPLP-co-PLGA 75/25 series copolymer films were 14.955±2.992 MPa and 72.083±3.859 MPa, 13.185±0.894 MPa and 66.643±3.359 MPa, and 5.078±0.112 MPa and 22.663±1.282 MPa for 1/200, 1/100 and 1/50 feeding ratios of BPLP/(LA+GA), respectively. The tensile strength and the Young’s modulus greatly decreased when BPLP/(LA+GA) increased from 1/100 to 1/50. PLGA films exhibited a slightly higher but comparable tensile strength and Young’s Modulus (15.347±1.403 MPa and 74.949±5.429 MPa) compared to their BPLP-co-PLGA counterparts. Similar phenomena could be seen in the BPLP-co-PLGA 50/50 series copolymer films. However, the tensile strength and Young’s modulus of BPLP-co-PLGA 75/25 series were much higher than those of corresponding BPLP-co-PLGA 50/50 series due to the higher content of LA as hard segments. All BPLP-co-PLGA copolymers exhibited the characteristics of plastic polymers similar to PLGA copolymers, although there are no obvious yield points in their stress-strain curves (in contrast to PLGA). A decrease of the BPLP/(LA+GA) ratio resulted in a decrease of elongation at break. When the ratio of BPLP/(LA+GA) decreased from 1/100 to 1/200, the elongation at break of BPLP-co-PLGA 75/25 series and BPLP-co-PLGA 50/50 series dramatically decreased from 219.37±24.45% to 85.80±8.98% and 260.43±29.78% to 156.74±23.93%, respectively. BPLP-co-PLGA films with BPLP/(LA+GA) of 1/100 and their PLGA counterparts showed comparable elongations at break and similar stress-strain curves.
The equilibrium water-in-air contact angles vs. time curves of PLGA 75/25 and BPLP-co-PLGA 75/25-100 copolymer films were plotted to analyze the surface properties (Fig. 2g). It was shown that water droplets had slightly lower contact angles on BPLP-co-PLGA 75/25-100 film than on PLGA75/25 film. The initial water contact angle of BPLP-co-PLGA 75/25-100 film was about 71.5° followed by a quick decrease through the first 30 minutes and then an almost equilibrium contact angle of about 60° was observed, a trend which was comparable with the water contact angle of PLGA75/25 film. A decrease of water droplet volumes occurred during the 75 minute test, which is attributed to water adsorption in polymer films and also some water evaporation. The contact angle results confirm that BPLP-co-PLGA 75/25-100 exhibits better wettability than PLGA 75/25, which may be favorable for cell adhesion, drug encapsulation and release properties [32].
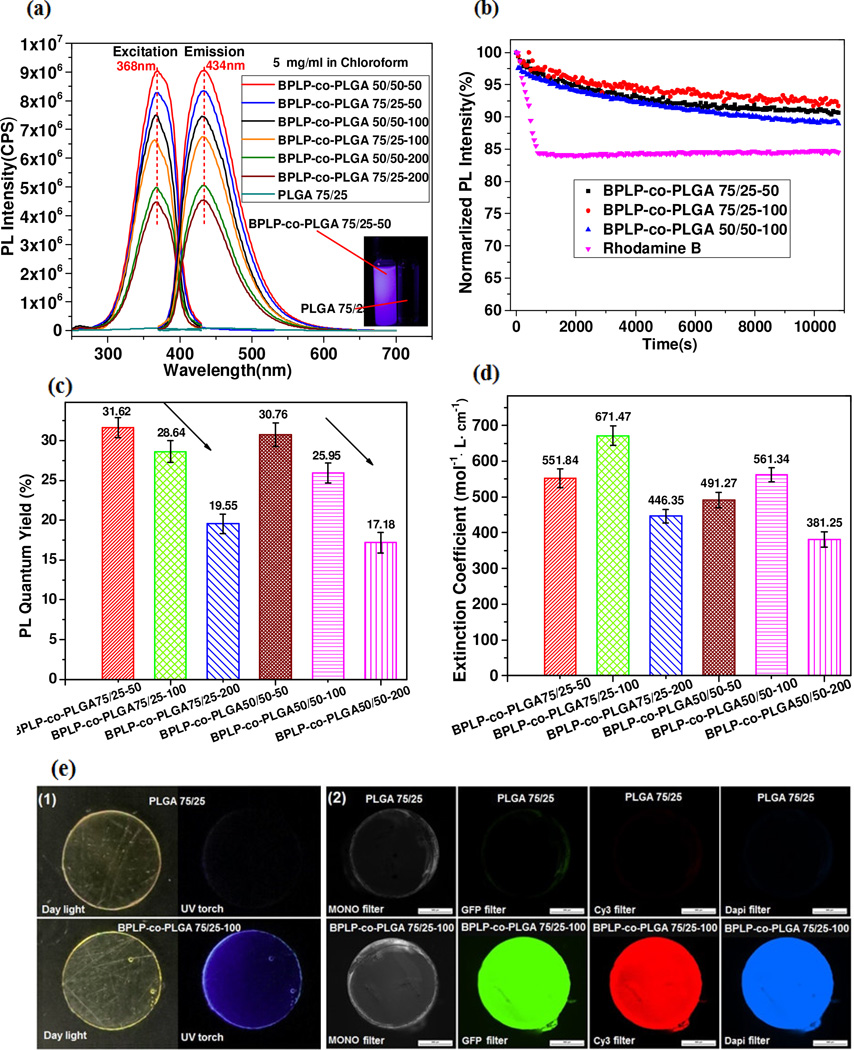
The photoluminescence of the obtained PLGA and BPLP-co-PLGA copolymers was evaluated and the excitation and emission spectra are depicted (Fig. 3a). Obviously, PLGA itself has no photoluminescence, while all BPLP-co-PLGA copolymers showed significant photoluminescence and possess the same maximum excitation (368 nm) and emission (434 nm) wavelengths as BPLP. Since BPLP is the fluorophore, emission intensity of BPLP-co-PLGA copolymers increased with the increase of the molar ratio of BPLP to (LA+GA) in BPLP-co-PLGA copolymers (Fig. S5, Supporting Information). Moreover, BPLP-co-PLGA copolymers possessed much better photostability than commercial organic dyes such as Rhodamine B (Fig. 3b) under continuous ultraviolet (UV) light illumination. BPLP-co-PLGA copolymers also displayed high quantum yields and extinction coefficients, comparable to other polymeric photoluminescent materials, such as poly(amido amine)s [35, 36], which further demonstrated the strong photoluminescence of BPLP-co-PLGA copolymers (Fig. 3c, d). BPLP-co-PLGA 75/25-100 films showed strong fluorescence emission under UV light and fluorescence microscope with GFP, Cy3 and DAPI filters (Fig. 3e). BPLP-PLGA copolymers have brightness intensities in the range of 6550~19230, which are comparable to that of some frequently used small molecular organic dyes including Alexa Fluor 430 (8800) and even DAPI (15660) [37]. These photoluminescent properties make BPLP-co-PLGA copolymers promising biomaterials for applications such as tissue engineering and drug delivery where fluorescent properties are desired.
Fig. 3.
Photoluminescent (PL) properties of BPLP-co-PLGA solutions, films. (a) Photoluminescent excitation and emission spectra of BPLP-co-PLGA copolymers solutions (5 mg/mL in chloroform) (insert, optical imaging of BPLP-co-PLGA 75/25-100 solution under UV light), PLGA 75/25 was used as control. (b) Photoluminescence stability evaluation of representative BPLP-co-PLGA copolymers and Rhodamine B. (c) and (d) Quantum yields and extinction coefficients of BPLP-co-PLGA copolymers. (e) Imaging effect of PLGA 75/25 and BPLP-co-PLGA 75/25-100 films (1) under white or UV light, (2) under fluorescent microscope with mono filter, GFP filter, Cy3 filter and DAPI filter.
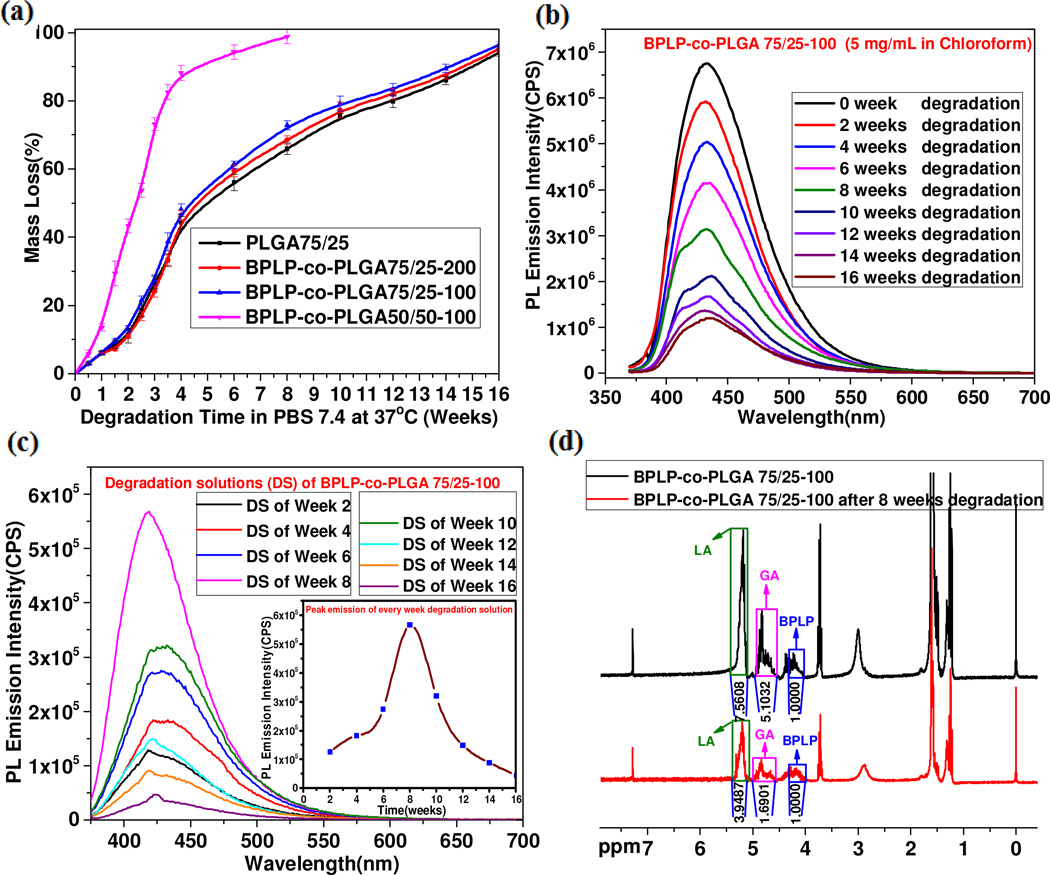
Degradation study in PBS for representative PLGA and BPLP-co-PLGA copolymers was conducted and the corresponding degradation profiles are shown in Fig. 4a. It was observed that BPLP-co-PLGA 75/25-100, BPLP-co-PLGA 75/25-200 and PLGA 75/25 underwent a very similar degradation process and the increased incorporation of BPLP slightly accelerated the degradation rate of BPLP-co-PLGA copolymers due to the slight increase in hydrophilicity. The fact that BPLP-co-PLGA 50/50-100 degraded much faster than BPLP-co-PLGA 75/25-100 showed that the increase of hydrophilic GA content greatly increased the degradation rate of BPLP-co-PLGA copolymers. The degradation rate could be easily adjusted by varying the feeding composition ratio to meet the application requirements. The photoluminescence emission intensity of BPLP-co-PLGA 75/25-100 copolymer decreased with increasing degradation time and the emission peaks always showed up at the same wavelength of 434 nm during degradation (Fig. 4b). Noteworthy, it was also found that the photoluminescence emission peak intensity retaining percentage remained higher than the mass remaining percentage of BPLP-co-PLGA 75/25-100 copolymer in the process of degradation. The photoluminescence intensity retained was about 17.63% even when the mass remaining was about 3.62% at the degradation time of 16 weeks (Fig. S6, Supporting Information). The photoluminescence emission intensity of PBS solutions in the process of BPLP-co-PLGA 75/25-100 degradation was tracked weekly to investigate the degradation rate as a function of time of BPLP in BPLP-co-PLGA 75/25-100 (Fig. 4c). It could be seen that the fastest degradation rate for BPLP in BPLP-co-PLGA 75/25-100 should occur around the time of the 8th week. The comparison between the 1H-NMR spectra of original BPLP-co-PLGA 75/25-100 copolymer and BPLP-co-PLGA 75/25-100 after 6 weeks degradation showed significant changes in the molar ratios of LA to GA and BPLP to (LA + GA) (Fig. 4d). After 6 weeks degradation of BPLP-co-PLGA 75/25-100, the ratio of signal intensity for BPLP/LA/GA changed from the original 1/7.5608/5.1032 to 1/3.9487/1.6901. The actual molar ratios of LA to GA and BPLP to (LA+GA) increased from 74.76/25.23 to 82.37/17.63 and from (1/α)/5.056 to (1/α)/2.397, respectively (Table S3, Supporting Information, α means number of protons from -CH2O- in 1,-8-octanediol of BPLP). The results demonstrated that in the copolymer chain of BPLP-co-PLGA 75/25-100, the GA segment degraded fastest and the BPLP segment degraded slowest. The degradation behavior of BPLP-co-PLGA indicates that strong and stable photoluminescence as an inherent characteristic will be preserved in almost the entire lifetime of BPLP-co-PLGA till complete degradation, which is different from our previous studies on BPLP-co-PLLA from which the percentage of fluorescence signal loss of BPLP-co-PLLA20 closely matches the percentage of in vitro weight loss of polymer due to the relatively similar degradation rate of BPLP and PLLA [21]. The above results suggest that the correlation between fluorescence signal loss and material’s degradation can be controlled by selecting the degradation rates of non-fluorescent sections of the co-polymers. Anyhow, understating the relationships of fluorescence signal decay and material degradation is a key to enabling non-invasive material visualization and degradation tracking through fluorescence imaging.
Fig. 4.
In vitro degradation of BPLP-co-PLGA. (a) Weight loss in PBS (pH 7.4) of BPLP-co-PLGA and PLGA 75/25 copolymers at 37°C. (b) Photoluminescent emission spectra of BPLP-co-PLGA 75/25-100 copolymers (5 mg/mL in chloroform) at various times degradation in PBS. (c) Photoluminescence emission spectra of degradation solutions (DS) of BPLP-co-PLGA 75/25-100 (insert: emission intensity vs. degradation time). (d) 1H-NMR spectra of original and degrading (6 weeks in PBS) BPLP-co-PLGA 75/25-100.
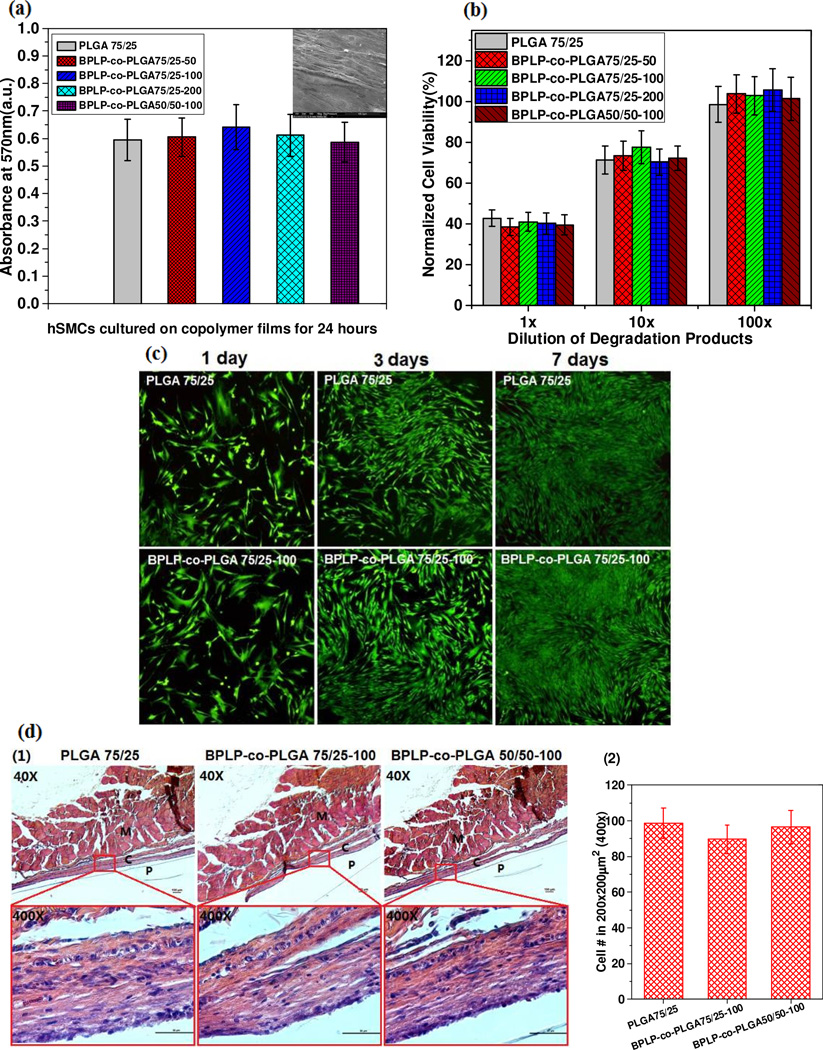
In vitro cell cytotoxicity of BPLP-co-PLGA films and degradation products, bio-imaging effect by cellular uptake of BPLP-co-PLGA nanoparticles and the in vivo foreign body response to BPLP-co-PLGA implants were evaluated (Fig. 5). The comparable cell viability on all films by MTT assay and the stretched morphology of cells on BPLP-co-PLGA75/25-100 film by SEM indicate that BPLP-co-PLGA supported human mesenchymal stem cells (hMSCs) adhesion and proliferation and exhibited a cytotoxicity in vitro similar to that of PLGA (Fig. 5a). The normalized cell viabilities of hSMCs cultured in the presence of PLGA or BPLP-co-PLGA degradation products were all around 40%, 75% and 100% for 1×, 10× and 100× dilutions, respectively (Fig. 5b). The results illustrate that both PLGA and BPLP-co-PLGA degradation products had a dose-dependent cytotoxicity comparable to one another. The Live/Dead assay images of hSMCs cultured with 10× dilutions of PLGA and BPLP-co-PLGA degradation products both display a robust growth trend from day 1 to day 7, which further confirmed the biocompatibility of BPLP-co-PLGA (Fig. 5c). In order to study the in vivo foreign body response, representative BPLP-co-PLGA copolymer films and PLGA films (as controls) were implanted in vivo to assess the cell behavior of tissue surrounding the implants using H&E staining. All samples implanted for 1 month produced a thin fibrous capsule between the copolymer film and muscle indicating minimal inflammatory reactions. The cell density around all implants was also similar and BPLP-co-PLGA implant samples did not elicit more severe foreign body reactions than the PLGA implant sample (Fig. 5d). The results of cytotoxicity and in vivo biocompatibility studies strongly demonstrated that incorporating BPLPs into PLGAs does not compromise the cyto/tissue-compatibility of the materials, thus meriting BPLP-PLGAs for future biomedical applications.
Fig. 5.
In vitro cytotoxicity and in vivo foreign body responses of BPLP-co-PLGA. (a) MTT assay (570 nm) against human mesenchymal stem cells (hMSCs) cultured on copolymer films for 24 hours (insert, SEM image of hMSCs cultured for 24 hours on BPLP-co-PLGA 75/25-100 film). (b) Normalized cell viability of hMSCs cultured with the presence of different dilutions of BPLP-co-PLGA degradation products, PLGA 75/25 was used as control. (c) Live/Dead assay images of hMSCs cultured with 10× diluted degradation products of PLGA 75/25 and BPLP-co-PLGA 75/25-100 for 1, 3 and 7 days. (d) Foreign body response evaluations: (1) Representative H&E staining images of surrounding tissues of BPLP-co-PLGA and PLGA copolymer films after 1 month of implantation (P: polymer, C: fibrous capsule, M: muscle); (2) Cell numbers in a 200 × 200 µm2 square region (from 400× images of H & E staining) of the skin-side tissue near the implants.
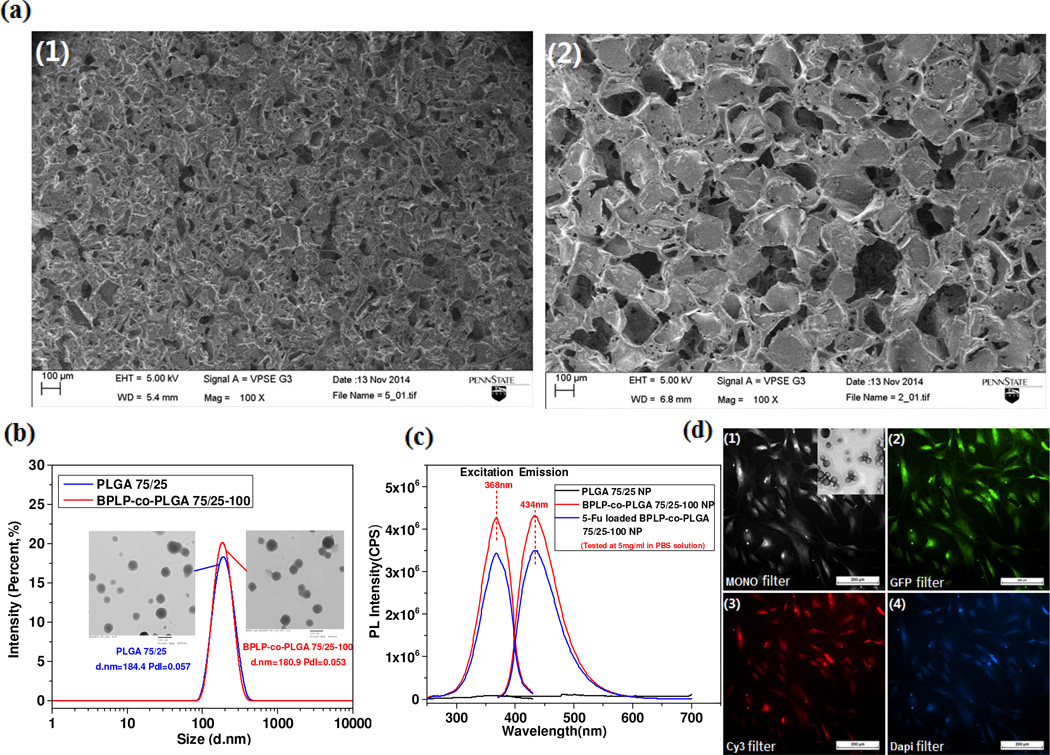
To verify the application potential of our BPLP-co-PLGA copolymers in tissue engineering and drug delivery, BPLP-co-PLGA porous scaffolds and nanoparticles were fabricated. BPLP-co-PLGA porous scaffolds with controlled pore sizes (here, 50–125 µm or 250–425 µm) and porosity and high interconnectivity were fabricated via a conventional salt-leaching method. The SEM images of the obtained scaffolds are shown in Fig. 6a. BPLP-co-PLGA nanoparticles were fabricated using a convenient oil in water (O/W) emulsion evaporation method, and the size, size distribution, and morphology of so-obtained nanoparticles were investigated (Fig. 6b). The average size of PLGA 75/25 and BPLP-co-PLGA 75/25-100 nanoparticles measured by dynamic light scattering (DLS) were 184.4 and 180.9 nm, respectively, and both of them were dispersed very evenly with polydispersity indexs (PDIs) of 0.057 and 0.053 respectively. The above results were also confirmed by TEM observation (insert images in Fig. 6b). BPLP-co-PLGA 75/25-100 nanoparticles in phosphate buffered saline (PBS, pH=7.4) solution still exhibited strong and stable photoluminescent performance (Fig. 6c). Cellular uptake of the BPLP-co-PLGA nanoparticles was conducted. The fluorescent images of BPLP-co-PLGA 75/25-50 nanoparticles (average size about 110 nm) up-taken by hMSCs were also recorded, confirming the cell labeling ability of the nanoparticles (Fig. 6d).
Fig. 6.
Scaffold and nanoparticle fabrication of BPLP-co-PLGA. (a) SEM images of BPLP-co-PLGA 75/25-100 scaffolds with pore sizes of 50-100 µm (1), and 200-250 µm (2). (b) The particle sizes and distributions of representative PLGA and BPLP-co-PLGA nanoparticle dispersions (insert: TEM images of corresponding nanoparticles). (c) Excitation and emission spectra of BPLP-co-PLGA 75/25-100 nanoparticles, 5-Fu loaded BPLP-co-PLGA 75/25-100 nanoparticles and control PLGA 75/25 nanoparticles with a concentration of 5 mg/mL in PBS solution. (d) BPLP-co-PLGA 75/25-50 nanoparticle (500µg/mL)-uptaken hMSCs observed under fluorescence microscope with (1) Monochrome filter (insert, TEM image of BPLP-co-PLGA 75/25-50 nanoparticles, particle size: 110nm), (2) GFP filter, (3) Cy3 filter, and (4) DAPI filter.
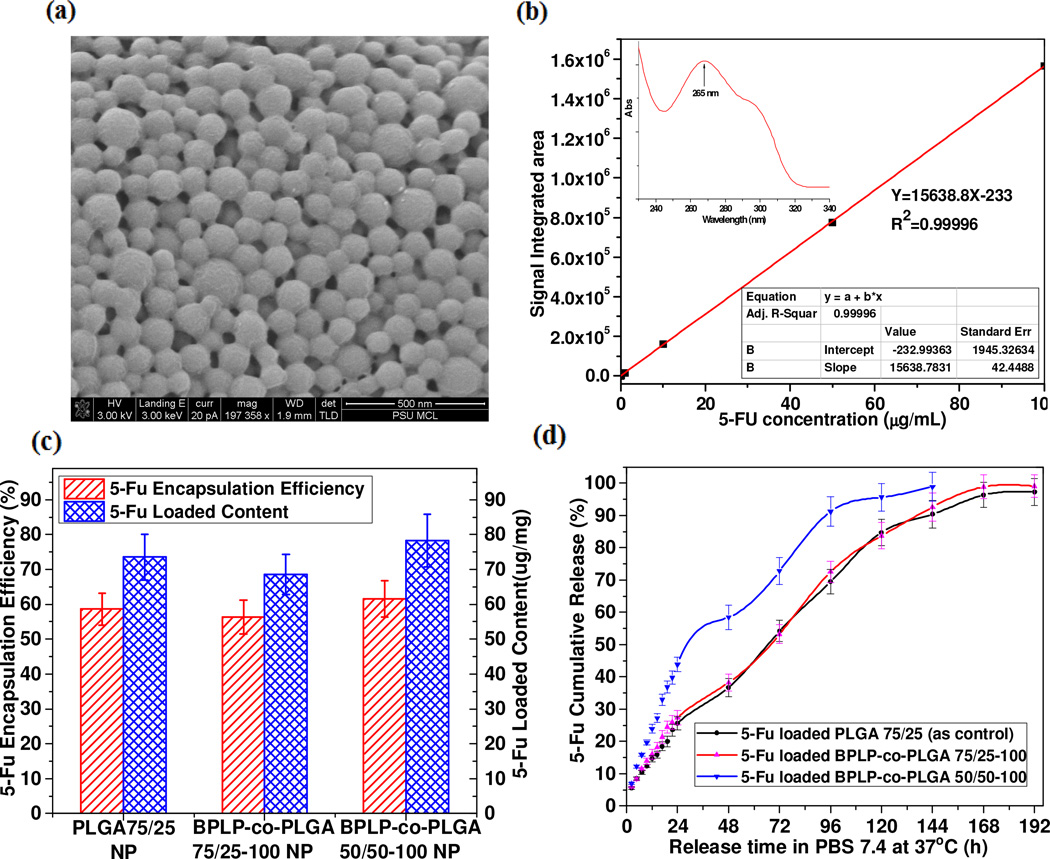
To validate the theranostic potential of BPLP-co-PLGA, 5-fluorouracil (5-Fu), an anti-tumor agent, was selected as a model drug to evaluate the drug encapsulation and release capability of BPLP-co-PLGA nanoparticles. 5-Fu loaded BPLP-co-PLGA 75/25-100 nanoparticles still exhibited strong photoluminescence when was dispersed in PBS as shown in Fig. 6c. The SEM images of 5-Fu loaded BPLP-co-PLGA 75/25-100 nanoparticles confirmed that these particles displayed relatively regular spherical morphology with a consistent surface and an even size distribution of 100-150 nm which avoided a burst release after encapsulation (Fig. 7a). The encapsulation efficiency and load content of representative PLGA and BPLP-co-PLGA nanoparticles against 5-Fu (Fig. 7c) were determined using a 5-Fu standard curve obtained by HPLC (Fig. 7b). The results revealed that higher molar content of GA in BPLP-co-PLGA resulted in higher encapsulation efficiency and load content due to an improvement in hydrophilicity, which led to more encapsulation of water soluble 5-Fu (Fig. 7c). The release profiles of 5-Fu from 5-Fu loaded PLGA as control and 5-Fu loaded BPLP-co-PLGA nanoparticles in PBS at 37°C were studied (Fig. 7d). 5-Fu encapsulated within PLGA and BPLP-co-PLGA nanoparticles experienced a relatively sustained release during the following 6–8 days. The release profiles illustrate that although all 5-Fu loaded PLGA and BPLP-co-PLGA nanoparticles exhibited a burst release in the first 24 hours, they then underwent a slow continuous release in the following days. 5-Fu loaded PLGA 75/25 nanoparticles and 5-Fu loaded BPLP-co-PLGA 75/25-100 nanoparticles exhibited very similar release profiles. While the released amount and rate of 5-Fu from BPLP-co-PLGA 50/50-100 nanoparticles was much faster than that from BPLP-co-PLGA 75/25-100 nanoparticles, which could be explained by the higher percentage of hydrophilic GA in in BPLP-co-PLGA 50/50-100 than in BPLP-co-PLGA 75/25-100 leading to faster hydration, faster biodegradation and lower glass transition temperature. The in vitro drug loading and release studies demonstrated that BPLP-co-PLGA could be used as multifunctional nano-reservoir systems to control on-demand drug release.
Fig. 7.
Drug loading and release study of BPLP-co-PLGA nanoparticles. (a) SEM image of 5-fluorouracil (5-Fu) loaded BPLP-co-PLGA 75/25-100 nanoparticles. (b) Standard curve of 5-Fu obtained by high-performance liquid chromatography (HPLC) (insert, UV-vis curve of 5-Fu). (c) 5-Fu encapsulation efficiency and loaded contents in PLGA and BPLP-co-PLGA nanoparticles. (d) Complete release profiles of 5-Fu from 5-Fu loaded PLGA nanoparticles (as control) and 5-Fu loaded BPLP-co-PLGA nanoparticles in PBS (pH 7.4) at 37 °C.
4. Conclusion
We have synthesized and characterized a novel series of biodegradable photoluminescent polymer-poly(lactide-co-glycolide) (BPLP-co-PLGA) copolymers. BPLP-co-PLGAs possess inherent photoluminescence from BPLP and favorable mechanical properties, cyto-/tissue-compatibility and tailored biodegradability from PLGA. These copolymers can be easily fabricated into various morphologies such as films, nanoparticles and porous scaffolds while enabling fluorescence imaging capability. BPLP-co-PLGA is a new class of biodegradable photoluminescent polymers with potential in a wide range of biomedical applications where fluorescence labeling and imaging are becoming enabling tools such as tissue engineering and cancer drug delivery and imaging.
Supplementary Material
Acknowledgements
This work was supported in part by National Institutes of Health Awards (to J.Y., NIBIB EB012575, NCI CA182670, NHLBI HL118498), the International Science & Technology Cooperation Program of Guangzhou, China (to J.H., 2012J5100043) and a project supported by Key Laboratory of New Lithium-ion Battery and Mesoporous Materials of Shenzhen, China (to J.H., 20120213).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maia FR, Bidarra SJ, Granja PL, Barrias CC. Functionalization of biomaterials with small osteoinductive moieties. Acta Biomater. 2013;9:8773–8789. doi: 10.1016/j.actbio.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Huebsch N, Mooney DJ. Inspiration and application in the evolution of biomaterials. Nat. 2009;462:426–432. doi: 10.1038/nature08601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32:762–798. [Google Scholar]
- 4.Guo J, Xie Z, Tran RT, Xie D, Jin D, Bai X, et al. Click chemistry plays a dual role in biodegradable polymer design. Adv Mater. 2014;26:1906–1911. doi: 10.1002/adma.201305162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Q, Jiang L, Shi R, Zhang L. Synthesis, preparation, in vitro degradation, and application of novel degradable bioelastomers—A review. Prog Polym Sci. 2012;37:715–765. [Google Scholar]
- 6.Xie D, Guo J, Mehdizadeh MR, Tran RT, Chen R, Sun D, et al. Development of injectable citrate-based bioadhesive bone implants. J Mater Chem B. 2015;3:387–398. doi: 10.1039/C4TB01498G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren K, He C, Xiao C, Li G, Chen X. Injectable glycopolypeptide hydrogels as biomimetic scaffolds for cartilage tissue engineering. Biomaterials. 2015;51:238–249. doi: 10.1016/j.biomaterials.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Giannitelli SM, Mozetic P, Trombetta M, Rainer A. Combined additive manufacturing approaches in tissue engineering. Acta Biomater. 2015;24:1–11. doi: 10.1016/j.actbio.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 9.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, et al. Quantum dots for live cells in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escobedo JO, Rusin O, Lim S, Strongin RM. NIR dyes for bioimaging applications. Curr Opin Chem Biol. 2010;14:64–70. doi: 10.1016/j.cbpa.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 12.Maria SM, Prukner C, Sheikh Z, Müller FA, Komarova SV, Barralet JE. Characterization of biomimetic calcium phosphate labeled with fluorescent dextran for quantification of osteoclastic activity. Acta Biomater. 2015;20:140–146. doi: 10.1016/j.actbio.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Liu J, Li C, Zhang J, Liu J, Dong A, et al. Real-time and non-invasive fluorescence tracking of in vivo degradation of the thermosensitive PEGlyated polyester hydrogel. J Mater Chem B. 2014;2:4185. doi: 10.1039/c4tb00275j. [DOI] [PubMed] [Google Scholar]
- 14.Artzi N, Oliva N, Puron C, Shitreet S, Artzi S, bon Ramos A, et al. In vivo and in vitro tracking of erosion in biodegradable materials using non-invasive fluorescence imaging. Nat Mater. 2011;10:704–709. doi: 10.1038/nmat3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaiswal JK, Mattoussi H, Mauro JM, Simon SM. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat biotechnol. 2003;21:47–51. doi: 10.1038/nbt767. [DOI] [PubMed] [Google Scholar]
- 16.Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian AM. Biological applications of quantum dots. Biomaterials. 2007;28:4717–4732. doi: 10.1016/j.biomaterials.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Yanushevich YG, Staroverov DB, Savitsky AP, Fradkov AF, Gurskaya NG, Bulina ME, et al. A strategy for the generation of non-aggregating mutants of anthozoa fluorescent proteins. FEBS Lett. 2002;511:11–14. doi: 10.1016/s0014-5793(01)03263-x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Yang J. Design strategies for fluorescent biodegradable polymeric biomaterials. J Mater Chem B. 2013;1:132–148. doi: 10.1039/C2TB00071G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Zhang Y, Gautam S, Liu L, Dey J, Chen W, et al. Development of aliphatic biodegradable photoluminescent polymers. Proc Natl Acad USA. 2009;106:10086–10091. doi: 10.1073/pnas.0900004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Tran RT, Qattan IS, Tsai YT, Tang L, Liu C, et al. Fluorescence imaging enabled urethane-doped citrate-based biodegradable elastomers. Biomaterials. 2013;34:4048–4056. doi: 10.1016/j.biomaterials.2013.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Z, Zhang Y, Liu L, Weng H, Mason RP, Tang L, et al. Development of intrinsically photoluminescent and photostable polylactones. Adv Mater. 2014;26:4491–4496. doi: 10.1002/adma.201306070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makadia HK, Siegel SJ. Poly(lactic-co-glycolic ccid) (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–2490. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 24.Cai Q, Shi G, Bei J, Wang S. Enzymatic degradation behavior and mechanism of poly(lactide-co-glycolide) foams by trypsin. Biomaterials. 2003;24:629–638. doi: 10.1016/s0142-9612(02)00377-0. [DOI] [PubMed] [Google Scholar]
- 25.Allison SD. Effect of structural relaxation on the preparation and drug release behavior of poly(lactic-co-glycolic)acid microparticle drug delivery systems. J Pharm Sci. 2008;97:2022–2035. doi: 10.1002/jps.21124. [DOI] [PubMed] [Google Scholar]
- 26.Borden M, Attawia M, Khan Y, Laurencin CT. Tissue engineered microsphere-based matrices for bone repair: design and evaluation. Biomaterials. 2002;23:551–559. doi: 10.1016/s0142-9612(01)00137-5. [DOI] [PubMed] [Google Scholar]
- 27.Bendix D. Chemical synthesis of polylactide and its copolymers for medical applications. Polym Degrad Stab. 1998;59:129–135. [Google Scholar]
- 28.Deasy PB, Finan M, Meegan MJ. Preparation and characterization of lactic/glycolic acid polymers and copolymers. J Microencapsul. 1989;6:369–378. doi: 10.3109/02652048909019919. [DOI] [PubMed] [Google Scholar]
- 29.Jerome C, Lecomte P. Recent advances in the synthesis of aliphatic polyesters by ring-opening polymerization. Adv Drug Deliv Rev. 2008;60:1056–1076. doi: 10.1016/j.addr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Ntziachristos V. Fluorescence molecular imaging. Annu Rev Biomed Eng. 2006;8:1–33. doi: 10.1146/annurev.bioeng.8.061505.095831. [DOI] [PubMed] [Google Scholar]
- 31.Kasprzyk W, Bednarz S, Bogdal D. Luminescence phenomena of biodegradable photoluminescent poly(diol citrates) Chem Comm. 2013;49:6445–6447. doi: 10.1039/c3cc42661k. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Motlagh D, Allen JB, Webb AR, Kibbe MR, Aalami O, et al. Modulating expanded polytetrafluoroethylene vascular graft host response via citric acid-based biodegradable elastomers. Adv Mater. 2006;18:1493–1498. [Google Scholar]
- 33.Williams ATR, Winfield SA, Miller JN. Relative fluorescence quantum yield using a computer-controlled luminescence spectrometer. Analyst. 1983;108:1067–1071. [Google Scholar]
- 34.Zhou J, Tsai YT, Weng H, Baker DW, Tang L. Real time monitoring of biomaterial-mediated inflammatory responses via macrophage-targeting NIR nanoprobes. Biomaterials. 2011;32:9383–9390. doi: 10.1016/j.biomaterials.2011.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D, Imae T, Miki M. Fluorescence emission from PAMAM and PPI dendrimers. J Colloid Interface Sci. 2007;306:222–227. doi: 10.1016/j.jcis.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 36.Yang W, Pan CY. Synthesis and fluorescent properties of biodegradable hyperbranched poly(amido amine)s. Macromol Rapid Comm. 2009;30:2096–2101. doi: 10.1002/marc.200900482. [DOI] [PubMed] [Google Scholar]
- 37.Data from online. “ http://www.cincinnatichildrens.org/assets/0/78/1067/2481/2525/2527/d420beff-63a9-41d8-a18c-bbadea6693ac.pdf”. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.