Abstract
Adipose tissue is composed of many functionally and developmentally distinct cell types, the metabolic core of which is the adipocyte. The classification of “adipocyte” encompasses three primary types – white, brown, and beige – with distinct origins, anatomic distributions, and homeostatic functions. The ability of adipocytes to store and release lipids, respond to insulin, and perform their endocrine functions (via secretion of adipokines) is heavily influenced by the immune system. Various cell populations of the innate and adaptive arms of the immune system can resist or exacerbate the development of the chronic, low-grade inflammation associated with obesity and metabolic dysfunction. Here, we discuss these interactions, with a focus on their consequences for adipocyte and adipose tissue function in the setting of chronic overnutrition. In addition, we will review the effects of diet composition on adipose tissue inflammation and recent evidence suggesting that diet-driven disruption of the gut microbiota can trigger pathologic inflammation of adipose tissue.
Keywords: Adipocyte, immunocyte, obesity, hyperplasia, hypertrophy
1. Introduction
Adipose tissue (AT) has multiple roles in orchestrating systemic adaptation to changes in nutrient availability. For a long time, it was considered almost exclusively as an energy storage depot that responded to energy deficits by catabolizing its lipid droplets to provide fatty acids as a fuel source for other tissues. The last several decades of obesity research have revealed additional roles for, and complexity of, adipose tissue. Most notably, it (1) acts as an endocrine organ that not only receives input from other metabolic tissues (brain, muscle, liver), but transmits soluble signals in the form of “adipokines” that act locally and systemically to regulate nutrient balance, and (2) is infiltrated by, and crosstalks extensively with, cells of the innate and adaptive arms of the immune system. Both adipokine secretion and adipocyte-immunocyte interactions can become dysregulated during weight gain and subsequent obesity. Here, we review developments in our understanding of how fundamental adipocyte behaviors are influenced by immunocytes, and how these interactions are modulated by changes in energy balance (primarily chronic weight gain). We will also discuss the influence of the gut microbiome on metabolic inflammation and point out questions that have arisen from the exciting intersection of immunity and metabolism.
2. Immunocyte influences on metabolic functions of adipocytes
2.1 Adipocyte cell populations and anatomic distribution of adipose tissue
AT is composed of adipocytes and the “stromal vascular fraction” (SVF) - a heterogeneous mixture of mesenchymal, endothelial, and hematopoietic cell types. Adipocytes themselves are not monolithic, but rather consist of subsets with distinct developmental origins and metabolic functions. Classically, adipocytes were divided into white and brown subtypes. White adipocytes store lipid as triglycerides within unilocular droplets - a lipogenic pathway that is responsive to various stimuli, most notably insulin [1]. During times of energy deficit, other signaling pathways stimulate lipolysis of these triglyceride stores and release of free fatty acids (FFA) into the circulation. Notably, FFA released by white adipocytes can be utilized by brown adipocytes to fuel heat production via mitochondrial uncoupling, the major metabolic function of brown adipose tissue (BAT) [2]. Brown adipocytes store lipid in small, multilocular droplets that are quickly catabolized for fuel when the tissue is stimulated. Excitingly, the brown/white paradigm has been revised by the discovery of beige (also known as brite) adipocytes dispersed within WAT depots [3,4]. When rodents are exposed to cold temperatures, or, notably, after prolonged high fat diet (HFD) feeding, beige adipocytes can dissipate heat in a manner similar to classical brown adipocytes [5]. Importantly, despite their lipogenic abilities, white, brown and beige adipocytes likely have distinct lineage ancestries [6,7] (Table 1).
Table 1.
| Adipocyte Type | Primary Function(s) | Lipid droplet morphology | Depot types/locations (studied most frequently in mice) | Lineage relationship |
|---|---|---|---|---|
| White | Lipid storage | Unilocular | Subcutaneous (Inguinal) Visceral (Epididymal) |
Controversial, can derive from mesenchymal stem cells |
| Beige/brite | Lipid storage and mitochondrial uncoupling | Multilocular | Scattered within white adipose tissue depots Subcutaneous > visceral (Inguinal) |
Closely related to smooth muscle? |
| Brown | Mitochondrial uncoupling | Multilocular | Interscapular Neck (Interscapular through adulthood) | Closely related to skeletal muscle |
White and brown were the only known adipocyte types prior to the recent discovery of beige (also known as brite (brown-in-white)), which can function more like a white adipocyte or more like a brown adipocyte, depending on environmental stimuli. Cold temperatures and β-adrenergic signaling are two strong stimuli of the mitochondrial-uncoupling, energy-dissipating activity of both beige and brown adipocytes. The interscapular BAT depot of humans is most prominent in infants, while adults have uncoupling-competent adipocyte clusters in the neck region. Whether these depots are true brown, true beige, or some mix of the two, remains to be determined. Each adipocyte type appears to have a distinct progenitor, and one recent report suggests that beige adipocytes may be closely related to smooth muscle (7). However, the precise developmental ancestry of each lineage is still unclear and is a matter of intense investigation (6,9).
The white-beige-brown adipocyte continuum informs our understanding of the anatomical distribution and functional distinctions of mammalian AT depots. White adipose tissue (WAT) is classified as subcutaneous or visceral. Subcutaneous depots are found throughout the body underneath the skin, and humans have prominent, elastic depots in the abdomen and legs. Major sites of human visceral adipose tissue (VAT) are the abdominal mesenteric and omental depots [8]. The omental fat pad comprises a much larger fraction of total body fat in humans than in rodents, though the rodent epididymal depot in males (which is often sampled as the representative visceral WAT depot) may be functionally equivalent to human omental fat [8]. Other VAT depots studied in rodents include the mesenteric and retroperitoneal. BAT is located in rodent and human interscapular regions perinatally but regresses and is not found there in human adults. Rather, we possess BAT-like thermogenic cells in the neck and supraclavicular regions [9]. Functionally, AT depots differ in progenitor proliferation rates, lipogenic and lipolytic capacity, and adipokine secretion profiles [10]. The tendency of visceral depots to become more inflamed in the setting of diet-induced obesity (DIO) is especially germane to the topics covered in this review.
2.2 Immunocyte populations infiltrating white adipose tissue
Infiltration of AT by macrophages was discovered in 2003, and for several years studies of this myeloid cell population dominated the field of immunometabolism [11,12]. Twelve years later, the roll call of immunocyte populations found as resident within, or diet-driven to, AT depots reads like a census of almost all known myeloid and lymphoid subsets [13]. A useful framework for discussing these populations is to distinguish cell types associated with maintaining metabolic health in the lean state and in early stages of DIO from those believed to initiate and/or exacerbate the chronic inflammation that contributes to adipocyte dysfunction in obesity.
In lean humans and rodents, AT macrophages may promote tissue remodeling and temper inflammation by secreting anti-inflammatory cytokines [14]. Eosinophils and Type 2 innate lymphoid cells (ILCs) are thought to have a similar role, especially given their ability to produce the Type 2 cytokines IL-4 and IL-13 that sustain anti-inflammatory macrophages [15,16]. Importantly, gain- and loss-of-function studies have shown that Type 2 ILCs and eosinophils limit weight gain during HFD feeding and also promote glucose tolerance and insulin sensitivity, likely due in part to their effects on body weight [15,16]. Macrophages, mast cells and neutrophils are three pro-inflammatory populations that accumulate in AT during DIO, with neutrophil influx observed after only a few days of HFD feeding [17,18]. Contrary to results for the anti-inflammatory cell populations, genetic and/or pharmacological inhibition of mast cells and neutrophils improves metabolic indices, with the latter doing so independently of differences in body weight vis-à-vis wild-type control mice [17,18]. In many tissues, macrophages can adopt a spectrum of phenotypes, and AT is likely no exception. An early model proposed that the anti-inflammatory macrophages in the WAT of lean individuals undergo a “phenotypic switch” to a pro-inflammatory phenotype closely related to classically, LPS and IFNγ-activated, M1 macrophages, during DIO [19]. Several studies have refined this model [20,21], including the recent description of “metabolically activated” macrophages that accumulate in AT during DIO and can be induced by a cocktail of metabolic stimuli (insulin, glucose and the saturated fatty acid palmitate). This population secretes pro-inflammatory cytokines but is distinguished from classical M1 macrophages by expression of genes regulating lipid metabolism [22].
B and T lymphocyte subsets can similarly be segregated based on their associations with limiting or exacerbating the pro-inflammatory tone of AT. A population of regulatory B cells, notable for their constitutive production of IL-10, is abundant in the AT of lean mice. B cell-derived IL-10 restrains the HFD-induced accumulation of pro-inflammatory macrophages and CD8+ T cells in VAT [23]. The AT-resident B cell population in lean mice may be heterogeneous, as a second, distinct population of IL-10 producing anti-inflammatory B cells has been found in VAT [24]. Prior to the description of AT-resident regulatory B cells, a population of CD4+Foxp3+ regulatory T cells (Tregs) was discovered in the visceral, and (to a lesser extent) subcutaneous, AT of lean individuals. The fractional representation of Tregs within the CD4+ T cell compartment is far higher in VAT than in lymphoid tissues, and their numbers decline specifically in VAT with diet-induced or genetic obesity [25–27]. Importantly, systemic and AT-specific ablation of Tregs exacerbates diet-induced AT inflammation and metabolic dysfunction [25,28,29]. VAT Treg maintenance in AT may be supported by a resident population of invariant natural killer T (iNKT) cells that produces IL-2. These anti-inflammatory iNKT cells also produce IL-10 and may act in concert with VAT Tregs and Bregs to maintain metabolic homeostasis [30].. On the pro-inflammatory side are IFNγ-producing CD4+ T cells that resemble classic Th1 cells and IFNγ-producing CD8+ T cells. Genetic ablation or antibody-mediated depletion of CD8+ T cells ameliorates AT inflammation during DIO [31,32]. B cells also have a pro-inflammatory, metabolically deleterious role in AT inflammation, as shown by DIO studies in B cell-deficient mice [33,34].
2.3 Effects of immunocytes on adipocyte functions
While we will try to highlight the diverse cell types known to change with DIO, since this review is focused on how adipocyte integrity is influenced by immunocytes, we will likely be disproportionately featuring macrophages, since the most is known about their direct effects on adipocyte differentiation and functionality. Major themes from these studies include the ability of immunocyte-derived cytokines to interfere with insulin receptor signaling, and to activate intracellular stress pathways that cause adipocyte dysfunction. Additionally, immunocytes can affect white and beige adipocyte differentiation.
2.3.1 Insulin signaling
In adipocytes, insulin signaling promotes glucose and fatty acid uptake and lipogenesis and suppresses lipolysis [35]. The cytokines TNFα, IL1-β, and IFNγ all interfere with insulin receptor signaling in adipocytes [36,37]. In the decades since its discovery as a major mediator of diet-induced inflammation [38], many groups have shown that TNFα is disruptive to insulin-stimulated: glucose uptake, proteolytic activation of lipogenic gene expression, and induction of adipogenesis (reviewed in [39]). More recent studies have demonstrated similar deleterious effects of IFNγ and IL-17 on adipocytes [40,41]. These data suggest that any activated immunocyte capable of secreting these cytokines could affect adipocyte function, yet few reports demonstrate a direct role for specific cell subsets in enhancing or blocking insulin signaling in adipocytes. Notably, it has been shown that macrophages can secrete a soluble form of the fatty acid binding protein FABP4 (commonly known as aP2), and that macrophage-derived aP2 can worsen systemic insulin resistance by, in part, suppressing insulin-stimulated AKT phosphorylation and glucose uptake by adipocytes [42]. Conversely, it is likely that IL-10 (which is secreted by multiple anti-inflammatory immunocyte populations) promotes insulin signaling in adipocytes, via its ability to directly repress their synthesis of inflammatory cytokines [25].
2.3.2 Adipogenesis and beiging
The anti-adipogenic effects of TNFα signaling have been known for many years. Two other pro-inflammatory cytokines, IFNγ and IL-17, have a similar effect, with all three likely suppressing the core transcriptional cascade required for pre-adipocyte differentiation [35,41,43]. More recently, the Type 2 cytokines IL-4 and IL-33 have been found to promote the differentiation of beige adipocytes [44,45]. In rodents, signaling through IL4Rα is required for maximal preadipocyte proliferation early in life and injection of IL-4 complexes is sufficient to stimulate the proliferation of beige preadipocytes [44]. Similarly, systemic injection of IL-33 is sufficient to induce beiging in a manner dependent on ILC2s [44,45]. Whether these IL-33-induced ILC2s primarily beige via secretion of the opioid-like peptide methionine-enkephalin [45], or via activation of an IL-4R signaling axis in preadipocytes [44], is unclear.
2.3.3 Lipolysis
Pro-inflammatory cytokines can promote lipolysis via suppression of the insulin signaling pathway. In addition, certain pro-inflammatory cytokines can induce lipolysis in adipocytes independently of insulin. This group is composed of the usual suspects: TNFα, IL-1α and IL-1β, as well as another IL-1 superfamily member, IL-18 [46]. Interestingly, and perhaps paradoxically, cytokine signaling pathways associated with resistance to DIO and dampening of AT inflammation, such as IL-4, can also induce lipolysis [47]. Given recent findings on the preference of fatty acids (FA) as a fuel source for certain anti-inflammatory immunocytes, including Tregs and anti-inflammatory macrophages [48,49], it is interesting to consider whether these AT-resident immunocytes may secrete lipolytic cytokines to increase the local concentration of FA for use as fuel substrates.
2.3.4 Crosstalk via adipokines
In addition to their primary role in lipid storage and release, adipocytes secrete “adipokines,” which can regulate local and systemic metabolic pathways. Hundreds of adipokines have been described [50], and we will limit discussion to those shown to have an effect on, or be affected by, immunocyte populations found in AT. A broad classification of adipokines is their division into “pro” and “anti”-inflammatory sub-groups, and though this schema is likely oversimplified, it is nonetheless useful as a framework for discussion of the salient features of each molecule. Leptin is an adipokine that exerts its most profound actions via CNS regulation of feeding behavior, where it promotes satiety and prevents weight gain [51]. Leptin can also directly signal through its receptor expressed on immunocytes, where it induces expression of TNFα and IL-6 by monocytes, chemokines by macrophages, and Th1 cytokines from polarized CD4+ T cells [52–54]. Hence, leptin has been classified as a pro-inflammatory adipokine. In combination with TCR triggering, leptin supports proliferation of activated T cells, likely through its up-regulation of the glucose transporter Glut1 [53]. Resistin is an especially interesting pro-inflammatory adipokine because of its species-specific regulation, being produced by adipocytes in rodents but predominantly by macrophages in humans [55]. Similar to leptin, its role in exacerbating AT inflammation is likely due, at least in part, to its ability to stimulate production of IL-6 and TNFα from macrophages [56,57].
Adiponectin is the most intensively studied anti-inflammatory adipokine. It is secreted almost exclusively by adipocytes and its levels in plasma are strongly correlated with insulin sensitivity and glucose tolerance [58]. Rodent gain- and loss-of-function models show that adiponectin levels are inversely correlated with the degree of AT inflammation [59]. It can directly interfere with inflammatory cytokine production in macrophages and can induce expression of the anti-inflammatory cytokine IL-10 [60,61]. In adipocytes, signaling via TNFα or IL-6 can inhibit production of adiponectin [62]. On the whole, far more metabolically detrimental, rather than beneficial, adipokines have been identified and thus, far less is known about interactions between other anti-inflammatory adipokines and AT immunocytes.
3. Changes in energy balance – role of immunocytes in adipose tissue elasticity
Immunocytes dynamically circulate through lymphoid and parenchymal tissues – and AT is no exception. Importantly, diet-induced immunocyte turnover and trafficking occur in the context of a tissue bed that is expanding or contracting in response to chronic alterations in energy balance.
3.1 Hypertrophy and fibrosis
Adipose tissue is remarkable for its elasticity. Once formed in an anatomic site, an AT depot can expand by enlargement of pre-existing adipocytes (hypertrophy) and/or proliferation and differentiation of new adipocytes (hyperplasia). The contribution of each process to DIO is an area of intense and ongoing debate [8]. The association of excessive hypertrophy with cell stress and adipocyte dysfunction fuels this debate, as do reports of depot-specific preferences for hypertrophy or hyperplasia. During DIO, initial hypertrophic expansion likely limits ectopic lipid deposition in other tissues and delays the onset of adipocyte dysfunction. However, hypertrophy is opposed by the fibrosis that can occur in AT as a maladaptive response to HFD feeding, especially in human VAT [63,64]. The fibrotic characteristics of fat in obese humans can be recapitulated by feeding a HFD to mice of the fibrosis-prone C3H strain. This model showed that enhanced fibrosis is characterized by limited adipocyte hypertrophy in eWAT, suppression of lipogenic and lipolytic gene expression, adipocyte necrosis, and accumulation of macrophages and mast cells. Notably, this response requires expression of TLR4 on bone-marrow-derived cells, suggesting that resident immunocytes promote HFD-induced AT fibrosis [65]. Interestingly, in humans, mast cells preferentially accumulate in visually distinct fibrotic bundles within visceral and subcutaneous depots of individuals with Type 2 diabetes [66], and their abundance is positively correlated with fibrosis, macrophage accumulation and endothelial cell inflammation.
3.2 Hyperplasia
Several studies support a role for macrophages in mediating HFD-induced adipocyte hyperplasia [67,68]. After 8–12 weeks of HFD-feeding in rodents, the wave of adipogenesis that occurs in VAT is preceded by significant adipocyte cell death and formation of crown-like structures of macrophages that likely phagocytose dead adipocytes prior to their replacement with newly differentiated, smaller adipocytes [69]. Experiments in a different adipocyte hyperplastic model, that of remodeling due to chronic β– adrenergic stimulation, showed that macrophages recruited to foci of dying adipocytes also secrete osteopontin, which promotes recruitment, proliferation and differentiation of adipocyte progenitors [70]. An attractive idea is that macrophages play a similar role in HFD-induced hyperplasia, and while the pattern of crown-like structure formation is similar in the two models [70], further experiments will be needed to determine whether similar macrophage-driven adipogenesis occurs during DIO.
3.3 Adipose tissue contraction
Compared with diet-induced AT expansion, less is known about the role of immunocytes in AT shrinkage during weight loss. In one interesting report, Kosteli and colleagues showed that when HFD-fed obese mice were calorie-restricted by 30%, macrophages infiltrated WAT depots for the first week, where they accumulated intracellular lipid droplets but, unlike classic “foamy” lipid-laden macrophages in atherosclerotic lesions, these lipid-laden macrophages in AT did not become pro-inflammatory. Rather, they functioned to suppress further adipocyte lipolysis [71]. This study shed light on earlier observations of macrophage recruitment to AT during weight loss induced by chronic β-adrenergic signaling [72]. Whether the macrophages merely act as a buffer for released lipid species, or whether they have additional roles in remodeling the underlying tissue scaffolding during adipocyte hypotrophy, remains to be determined.
4. Changes in energy sources – influence of diet composition on adipocyte-immunocyte interactions
4.1 Saturated fatty acids
The progression of obesity and its metabolic sequelae are likely affected not only by consuming more calories than one burns, but also by the nutritional content of those calories. The effects of dietary fats have received the most attention with respect to their general pro- vs. anti-inflammatory effects in AT. Fatty acids are classified into short, medium and long-chain based on the number of carbons in their aliphatic tails, and they can be saturated or unsaturated. Modern diets, especially in America, are rich in medium and long-chain saturated fatty acids (SFA) derived from vegetable oils, meat and dairy products, and are abundant in fast foods and processed foods. In humans, SFA are more obesigenic than unsaturated FA and diets rich in SFA are positively correlated with metabolic syndrome [73].
Mechanistically, SFA are implicated in the initiating events of pathologic, diet-induced AT inflammation due to their reported ability to act as ligands for the pathogen-sensing toll-like receptors (particularly TLRs 2 and 4). Two abundant nutritional SFAs, palmitate and oleate, were shown to signal through TLR4 in macrophages in vitro, though this finding is disputed owing to the technical challenge inherent in finding reagents free of the primary TLR2/4 ligand, LPS [74–76]. Nonetheless, in rodents, very long-term feeding of a diet enriched in SFAs causes greater weight gain in TLR4-deficient than in TLR4-sufficient mice. Despite the increased weight gain, TLR4-null mice are relatively glucose-tolerant and insulin-sensitive [74]. An alternative and intriguing hypothesis to ligation of TLR4 by SFAs centers on obesity-associated dysregulation of the gut microbiota, which can result in elevated systemic LPS that is sufficient to trigger TLR signaling in AT [77].
4.2 Polyunsaturated fatty acids
In contrast to the deleterious effects of diets rich in SFA, diets enriched for polyunsaturated fatty acids (PUFAs) may prevent or ameliorate multiple aspects of metabolic syndrome. N-3 PUFAs, specifically docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) of marine origin, are the best studied in the context of DIO. Both compounds are anti-obesigenic, which needs to be considered as a primary mechanism for their anti-inflammatory effects in AT [78,79]. However, supplementation of a HFD with EPA can be metabolically beneficial independent of any effect on adiposity. Rather, compared with a HFD alone, one supplemented with EPA results in higher levels of beneficial adipokines, decreases infiltration of AT by pro-inflammatory immunocytes and lowers levels of pro-inflammatory cytokines. These effects are likely mediated in part through EPA’s ability to directly suppress lipogenesis and increase FAO in adipocytes. Notably, if EPA is used therapeutically, after 6 weeks of HFD feeding, it still improves glucose tolerance [80].
Direct effects of n-3 PUFA on immunocytes have also been reported, including the discovery that n-3 PUFA are ligands for a G-protein-coupled receptor (GPR), GPR120, expressed on macrophages. Triggering of GPR120 by n-3 PUFA suppresses NFκB and JNK signaling in macrophages and, importantly, this signaling is required for the robust insulin-sensitizing effects of n-3 PUFA in vivo [81]. Since this initial discovery, humans with loss-of-function mutations in GPR120 have been identified and they are at increased risk for obesity and metabolic syndrome [82]. In the lymphocyte compartment, supplementation of rodent diets with fish oil ameliorates the pro-inflammatory effects of CD8+ T cells on cultured adipocytes, suppressing NFκB activity and lowering their production of pro-inflammatory cytokines [83].
5. Role of the intestinal microbiota in obesity-associated inflammation of adipose tissue
Microbial symbionts colonizing the human gastrointestinal tract are implicated in regulating several aspects of energy balance, including satiety, energy expenditure, fat storage and extraction of calories from the diet [84,85]. Especially salient to AT biology is the observation that obesity is associated with a less diverse biota in both humans and rodents [73,86]. Though the gut microbiota likely affects AT inflammation indirectly, through primary effects on body weight, accumulating evidence suggests that the microbiota influences AT inflammation independently of its role in regulating fat mass.
Several lines of in vivo evidence implicate gut-microbiota-derived pathogen-associated molecular patterns (PAMPs) as the proximal inflammatory triggers of diet-induced AT inflammation. It has been reported that obese humans and HFD-fed rodents have circulating LPS levels that are two-to-three fold higher than that of their lean counterparts, a phenomenon termed “metabolic endotoxemia” (however, these LPS levels are still 10–50-fold below those measured during a bacterial infection [87,88]. The ability of gut-derived LPS to influence AT inflammation has been studied in germ-free (GF) mice mono-colonized with either wild-type E. coli or with a strain expressing a less immunogenic variant of LPS. Interestingly, LPS immunogenicity is not required for increased fat mass in GF mice, but is required for macrophage accumulation in AT [89], suggesting that microbiota-derived LPS could potentiate immunocyte infiltration into AT. Similarly, a recent report implicates the gut microbiota in the AT inflammation that results from consumption of a diet high in saturated, rather than in unsaturated, fat. When GF mice were fed a lard diet, they were largely spared the impressive infiltration of AT by macrophages seen in conventionally raised mice fed the same diet. Importantly, the inflammatory effects of the SFA-rich diet required both TLR signaling and the gut microbiota, and cecal transplant of biota from mice fed a PUFA-rich diet to those fed an SFA-rich diet ameliorated SFA-induced adipose tissue inflammation [90]. Taken together, these data suggest that the gut microbiota significantly influences diet-induced inflammation of AT.
6. Conclusions and Perspectives
Although chronic, low-grade systemic inflammation affects other primary metabolic tissues, such as liver and muscle, AT is disproportionally infiltrated by immunocytes, in both the lean and obese states. While these immunocyte populations clearly have a role in policing their own, via the production of immunomodulatory cytokines and chemokines that act to maintain the proper balance of pro- and anti-inflammatory subsets, modulation of adipocyte function by the immune system is an emerging theme of immunometabolism research (Figure 1). Pro-inflammatory cytokines interfere with insulin signaling in adipocytes, causing dysfunction of lipogenic and lipolytic pathways. Recent studies have uncovered the critical role of multiple immunocyte populations in supporting the function of brown and beige AT depots in energy dissipation. Immunocytes also modulate the hyperphagic and hyperplastic expansion of adipocytes in response to HFD feeding, and they can respond to weight-loss induced lipolysis. Importantly, AT inflammation is influenced by compositional changes in dietary fats, and intriguing new data suggest that this pathway works via the gut microbiota. The results highlighted here raise many questions, such as - how do the profiles of resident immunocyte populations shape the responses of distinct AT depots to nutrient overload, particularly with respect to the insulin sensitivity of adipocytes, and their mechanisms of expansion? Do different types of dietary fats have cell-type-specific effects on AT immunocytes, and if so, can these pathways be triggered by small molecules? Lastly, are there mechanisms, in addition to modulating serum LPS levels, whereby the gut microbiota influence the composition and activation states of AT immunocyte populations? Answering these and other questions should advance our understanding of, and hopefully facilitate our ability to successfully manipulate, adipocyte and immunocyte populations for the treatment of obesity and its associated metabolic dysfunctions.
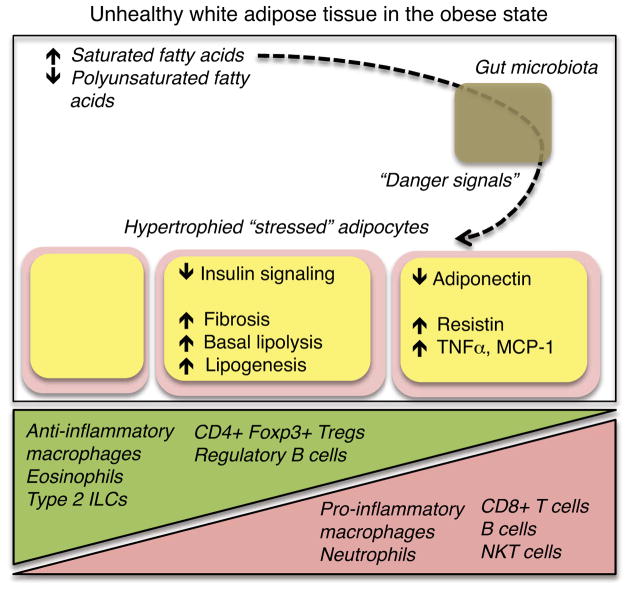
Figure 1. Inflammatory cascades that contribute to adipocyte dysfunction.
Diets high in saturated fatty acids, when consumed in a state of chronic caloric excess, contribute to adipocyte dysfunction. While healthy adipocytes are insulin-sensitive and have low levels of basal lipolysis, adipocytes that reach their lipid-storage limit and/or are exposed to chronically elevated levels of pro-inflammatory cytokines may exhibit the dysfunctions listed above. Chronic overnutrition also disrupts the balance of immunocyte populations present in adipose tissue. A possible contributing pathway involves diet-induced alterations in gut symbionts that, through yet unclear mechanisms, triggers pathologic adipose tissue inflammation.
Highlights.
Adipose tissue is composed of adipocytes, stromal cells and an expanding panoply of immunocytes
Adipocyte-immunocyte interactions are influenced by changes in energy balance
Adipocyte-immunocyte interactions are also affected by diet composition
The gut microbiota has direct and indirect effects on adiposity and adipose tissue inflammation
Acknowledgments
This work was funded by NIH grant RO1DK092541 and the JPB Foundation to DM. JRD was supported by an American Diabetes Association postdoctoral fellowship.
Abbreviations
- AT
Adipose tissue
- SVF
Stromal vascular fraction
- WAT
White adipose tissue
- BAT
Brown adipose tissue
- DIO
Diet-induced obesity
- FFA
Free fatty acid
- Treg
Regulatory T cell
- ILC
Innate lymphoid cell
- SFA
Saturated fatty acids
- PUFA
Polyunsaturated fatty acids
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 2010;11(4):268–272. doi: 10.1016/j.cmet.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res. 2007;48(1):41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res. 2012;53(4):619–629. doi: 10.1194/jlr.M018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 7.Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, et al. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014;19(5):810–820. doi: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17(5):644–656. doi: 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith U. Abdominal obesity: a marker of ectopic fat accumulation. J Clin Invest. 2015;125(5):1790–1792. doi: 10.1172/JCI81507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17(6):851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odegaard JI, Chawla A. Type 2 responses at the interface between immunity and fat metabolism. Curr Opin Immunol. 2015;36:67–72. doi: 10.1016/j.coi.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210(3):535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332(6026):243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15(8):940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49(9):1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31(9):1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 21.Xu X, Grijalva A, Skowronski A, van EM, Serlie MJ, Ferrante AW., Jr Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18(6):816–830. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20(4):614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura S, Manabe I, Takaki S, Nagasaki M, Otsu M, Yamashita H, et al. Adipose natural regulatory B cells negatively control adipose tissue inflammation. Cell Metab. 2013;18:759–766. doi: 10.1016/j.cmet.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Wu L, Parekh VV, Hsiao J, Kitamura D, Van KL. Spleen supports a pool of innate-like B cells in white adipose tissue that protects against obesity-associated insulin resistance. Proc Natl Acad Sci U S A. 2014;111(43):E4638–E4647. doi: 10.1073/pnas.1324052111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15(8):921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deiuliis J, Shah Z, Shah N, Needleman B, Mikami D, Narula V, et al. Visceral adipose inflammation in obesity is associated with critical alterations in T regulatory cell numbers. PLoS ONE. 2011;6(1):e16376. doi: 10.1371/journal.pone.0016376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eller K, Kirsch A, Wolf AM, Sopper S, Tagwerker A, Stanzl U, et al. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60(11):2954–2962. doi: 10.2337/db11-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486(7404):549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C, et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T(reg) cells and macrophages in adipose tissue. Nat Immunol. 2015;16(1):85–95. doi: 10.1038/ni.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103(5):467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 33.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17(5):610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A. 2013;110(13):5133–5138. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czech MP, Tencerova M, Pedersen DJ, Aouadi M. Insulin signalling mechanisms for triacylglycerol storage. Diabetologia. 2013;56(5):949–964. doi: 10.1007/s00125-013-2869-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389(6651):610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 37.Xu H, Hirosumi J, Uysal KT, Guler AD, Hotamisligil GS. Exclusive action of transmembrane TNF alpha in adipose tissue leads to reduced adipose mass and local but not systemic insulin resistance. Endocrinology. 2002;143(4):1502–1511. doi: 10.1210/endo.143.4.8715. [DOI] [PubMed] [Google Scholar]
- 38.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 39.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 40.Duffaut C, Zakaroff-Girard A, Bourlier V, Decaunes P, Maumus M, Chiotasso P, et al. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol. 2009;29(10):1608–1614. doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- 41.McGillicuddy FC, Chiquoine EH, Hinkle CC, Kim RJ, Shah R, Roche HM, et al. Interferon gamma attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway. J Biol Chem. 2009;284(46):31936–31944. doi: 10.1074/jbc.M109.061655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furuhashi M, Fucho R, Gorgun CZ, Tuncman G, Cao H, Hotamisligil GS. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Invest. 2008;118(7):2640–2650. doi: 10.1172/JCI34750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuniga LA, Shen WJ, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185(11):6947–6959. doi: 10.4049/jimmunol.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160(1–2):74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519(7542):242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grant RW, Stephens JM. Fat in flames: influence of cytokines and pattern recognition receptors on adipocyte lipolysis. Am J Physiol Endocrinol Metab. 2015;309(3):E205–E213. doi: 10.1152/ajpendo.00053.2015. [DOI] [PubMed] [Google Scholar]
- 47.Tsao CH, Shiau MY, Chuang PH, Chang YH, Hwang J. Interleukin-4 regulates lipid metabolism by inhibiting adipogenesis and promoting lipolysis. J Lipid Res. 2014;55(3):385–397. doi: 10.1194/jlr.M041392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186(6):3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4(1):13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fasshauer M, Bluher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015;36(7):461–470. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 51.Friedman J. 20 years of leptin: leptin at 20: an overview. J Endocrinol. 2014;223(1):T1–T8. doi: 10.1530/JOE-14-0405. [DOI] [PubMed] [Google Scholar]
- 52.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12(1):57–65. [PubMed] [Google Scholar]
- 53.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394(6696):897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 54.Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999;194(1):6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz DR, Lazar MA. Human resistin: found in translation from mouse to man. Trends Endocrinol Metab. 2011;22(7):259–265. doi: 10.1016/j.tem.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174(9):5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 57.Kaser S, Kaser A, Sandhofer A, Ebenbichler CF, Tilg H, Patsch JR. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem Biophys Res Commun. 2003;309(2):286–290. doi: 10.1016/j.bbrc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8(7):731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 60.Yamaguchi N, Argueta JG, Masuhiro Y, Kagishita M, Nonaka K, Saito T, et al. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005;579(30):6821–6826. doi: 10.1016/j.febslet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 61.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109(17):2046–2049. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 62.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96(9):939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 63.Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59(11):2817–2825. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121(6):2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vila IK, Badin PM, Marques MA, Monbrun L, Lefort C, Mir L, et al. Immune cell Toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell Rep. 2014;7(4):1116–1129. doi: 10.1016/j.celrep.2014.03.062. [DOI] [PubMed] [Google Scholar]
- 66.Divoux A, Moutel S, Poitou C, Lacasa D, Veyrie N, Aissat A, et al. Mast cells in human adipose tissue: link with morbid obesity, inflammatory status, and diabetes. J Clin Endocrinol Metab. 2012;97(9):E1677–E1685. doi: 10.1210/jc.2012-1532. [DOI] [PubMed] [Google Scholar]
- 67.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19(10):1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim SM, Lun M, Wang M, Senyo SE, Guillermier C, Patwari P, et al. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab. 2014;20(6):1049–1058. doi: 10.1016/j.cmet.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strissel KJ, Stancheva Z, MIYOSHI H, Perfield JW, DeFuria J, Jick Z, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56(12):2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 70.Lee YH, Petkova AP, Granneman JG. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 2013;18(3):355–367. doi: 10.1016/j.cmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120(10):3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mottillo EP, Shen XJ, Granneman JG. Role of hormone-sensitive lipase in beta-adrenergic remodeling of white adipose tissue. Am J Physiol Endocrinol Metab. 2007;293(5):E1188–E1197. doi: 10.1152/ajpendo.00051.2007. [DOI] [PubMed] [Google Scholar]
- 73.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3(4):213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity (Silver Spring) 2008;16(6):1248–1255. doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- 76.Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler Thromb Vasc Biol. 2009;29(11):1944–1949. doi: 10.1161/ATVBAHA.109.194050. [DOI] [PubMed] [Google Scholar]
- 77.Jin C, Flavell RA. Innate sensors of pathogen and stress: linking inflammation to obesity. J Allergy Clin Immunol. 2013;132(2):287–294. doi: 10.1016/j.jaci.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 78.Ruzickova J, Rossmeisl M, Prazak T, Flachs P, Sponarova J, Veck M, et al. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids. 2004;39(12):1177–1185. doi: 10.1007/s11745-004-1345-9. [DOI] [PubMed] [Google Scholar]
- 79.Siriwardhana N, Kalupahana NS, Cekanova M, Lemieux M, Greer B, Moustaid-Moussa N. Modulation of adipose tissue inflammation by bioactive food compounds. J Nutr Biochem. 2013;24(4):613–623. doi: 10.1016/j.jnutbio.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 80.Kalupahana NS, Claycombe K, Newman SJ, Stewart T, Siriwardhana N, Matthan N, et al. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr. 2010;140(11):1915–1922. doi: 10.3945/jn.110.125732. [DOI] [PubMed] [Google Scholar]
- 81.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483(7389):350–354. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- 83.Monk JM, Liddle DM, De Boer AA, Brown MJ, Power KA, Ma DW, et al. Fish-oil-derived n-3 PUFAs reduce inflammatory and chemotactic adipokine-mediated cross-talk between co-cultured murine splenic CD8+ T cells and adipocytes. J Nutr. 2015;145(4):829–838. doi: 10.3945/jn.114.205443. [DOI] [PubMed] [Google Scholar]
- 84.Khan MT, Nieuwdorp M, Backhed F. Microbial modulation of insulin sensitivity. Cell Metab. 2014;20(5):753–760. doi: 10.1016/j.cmet.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 85.Rosenbaum M, Knight R, Leibel RL. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metab. 2015;26(9):493–501. doi: 10.1016/j.tem.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Amar J, Serino M, Lange C, Chabo C, Iacovoni J, Mondot S, et al. Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept. Diabetologia. 2011;54(12):3055–3061. doi: 10.1007/s00125-011-2329-8. [DOI] [PubMed] [Google Scholar]
- 88.Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermudez-Humaran LG, et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med. 2011;3(9):559–572. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caesar R, Reigstad CS, Backhed HK, Reinhardt C, Ketonen M, Lunden GO, et al. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut. 2012;61(12):1701–1707. doi: 10.1136/gutjnl-2011-301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Backhed F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015;22(4):658–668. doi: 10.1016/j.cmet.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]