Abstract
Background
The purpose of this study is to assess the rates of perioperative medication errors (MEs) and adverse drug events (ADEs) as percentages of medication administrations, evaluate their root causes, and formulate targeted solutions to prevent them.
Methods
In this prospective observational study, anesthesia-trained study staff (anesthesiologists/nurse anesthetists) observed randomly selected operations at a 1,046 bed tertiary care academic medical center to identify MEs and ADEs over eight months. Retrospective chart abstraction was performed to flag events that were missed by observation. All events subsequently underwent review by two independent reviewers. Primary outcomes were the incidence of MEs and ADEs.
Results
A total of 277 operations were observed with 3,671 medication administrations of which 193 (5.3%, 95% CI 4.5 to 6.0) involved a ME and/or ADE. Of these, 153 (79.3%) were preventable and 40 (20.7%) were non-preventable. The events included 153 (79.3%) errors and 91 (47.2%) ADEs. While 32 (20.9%) of the errors had little potential for harm, 51 (33.3%) led to an observed ADE and an additional 70 (45.8%) had the potential for patient harm. Of the 153 errors, 99 (64.7%) were serious, 51 (33.3%) were significant and 3 (2.0%) were life-threatening.
Conclusions
One in twenty perioperative medication administrations included an ME and/or ADE. More than one third of the MEs led to observed ADEs, and the remaining two thirds had the potential for harm. These rates are markedly higher than those reported by retrospective surveys. Specific solutions exist which have the potential to decrease the incidence of perioperative MEs.
Introduction
Medication administration in the perioperative setting presents particular patient safety challenges compared to other hospital settings.1 Unlike in the inpatient hospital ward setting, perioperative medication administration today often bypasses standard safety checks, such as electronic physician order entry with decision support, pharmacy approval of specific drugs prior to administration, and multiple nursing checks at the time of medication administration. Furthermore, the high-stress, time-sensitive nature of operating room care may lead to both higher rates of medication errors (MEs) and errors of high severity.
Perioperative syringe swaps, ampoule swaps, and wrong dose errors can all cause serious harm.2 In fact, the most frequently cited critical incidents in anesthesia are drug administration errors.3 However, the literature on perioperative ME rates is sparse and contains largely self-reported data,4-7 consisting of either spontaneous self-reports of errors5, 7 or facilitated incident reporting of whether or not an error occurred.1, 6 The validity and reliability of studies based on self-reporting of MEs in other patient care areas has been called into question.8-10 For example, in a study of 2,557 doses of medications administered on hospital wards, Flynn and colleagues found 456 medication errors by direct observation, 34 by chart review and only one by self-report.8 Without valid and reliable assessments of perioperative errors and their root causes, proposed solutions may be less effective, more costly and subject to considerable resistance to implementation, and their impact cannot be accurately measured.
Reductions in MEs in other patient care areas, including inpatient units and outpatient clinics, have occurred because error rates were measured, errors were categorized in order to determine their root causes and potential for harm, solutions were designed and implemented, and error rates were then systematically re-measured to show a reduction. In addition, typically the costs of the solutions were assessed to justify their widespread adoption. This process has occurred with solutions such as computerized physician order entry systems,11, 12 bar code scanning systems for medication administration in hospital pharmacies,13 and outpatient electronic prescribing systems.14, 15 Perioperative areas are among the only remaining patient care areas that have not had rigorous assessments of MEs to guide proposed solutions. Thus, it is not surprising that there have been few specific improvements to perioperative medication errors since they were originally flagged as a problem in 1978.16
The aim of this epidemiologic study was to assess the rates, types, severity and preventability of MEs and potential adverse drug events (ADEs) in the perioperative setting, from initiation of anesthesia care to handover of patient care in the recovery room or intensive care unit.
Materials and Methods
Data for the study were collected during a seven month period from November 2013 to June 2014. We obtained approval from the Partners Human Research Office (Boston MA, protocol #2012P000833).
Study Site
This study was conducted in the perioperative area at a 1,046 bed tertiary care academic medical center that performs over 40,000 operations annually in 64 operating rooms excluding off-site anesthetizing locations. The anesthesia providers use an electronic anesthesia information management system (MetaVision, iMDSoft, MA) to document patient demographic information, vital signs, medications administered and perioperative events. The hospital also recently introduced a bar code-assisted syringe labeling system (Safe Label System, Codonics, OH). Providers scan the manufacturer-issued bar code on each medication vial, and the system prints a color syringe label containing, at a minimum: drug name, strength, quantity, diluent and diluent volume, expiration date and time, and the provider's initials. The system also provides audio and visual readback of drug name and concentration, and clinical alerts for recalled and expired vials.
Definitions
The perioperative medication administration process starts when a medication is requested or obtained from the anesthesia cart and ends with appropriate monitoring after the medication has reached the patient. The stages in this process are shown in Table 1, and any of these stages may involve one or more errors. A medication error is defined as failure to complete a required action in the medication administration process, or the use of an incorrect plan or action to achieve a patient care aim.17 An adverse drug event is defined as patient harm or injury due to a medical intervention related to a drug, regardless of whether an error in the medication process occurs.11
Table 1.
Stages of Medication Administration
| Term | Definition |
|---|---|
| Requesting | Prescriber requests medication from pharmacy or from medication dispensing system; this step may be bypassed when provider obtains a medication directly from anesthesia cart |
| Dispensing | Pharmacist dispenses a medication directly to the provider, or provider withdraws medication from dispensing system |
| Preparing | Medication is prepared by provider (e.g., drawn from vial, placed into a labeled syringe, diluted, etc.) |
| Administration | Medication reaches the patient either by self-administration or administration via an anesthesia provider. |
| Documenting | The medication and dose are documented in the anesthesia information management system |
| Monitoring | Following vital signs or relevant labs after medication administration (e.g., checking glucose after insulin administration) |
In order to adapt these definitions for the perioperative setting, we built on a previously described framework used to identify and classify MEs in inpatient and outpatient settings.14;17 Using this ME detection framework, we assessed various ME scenarios with consultation from clinical and ME experts to make the necessary iterative revisions to ensure that all elements of the framework were mutually exclusive and collectively exhaustive in the perioperative setting. We then created an observer training manual based on the error definitions outlined in the framework. A full list of definitions with examples is shown in Table 2 and their associated severity levels are defined in Table 3.
Table 2.
Event Definitions
| Term | Definition | Examples |
|---|---|---|
| Medication Error (ME) | Failure to complete a required action, or the use of a wrong plan to achieve an aim; may involve any of the stages of medication administration (Table 1) regardless of whether an injury occurred or the potential for injury was present. | • Patient given a dose of medication which was not intended. • Significant hypotension (mean arterial pressure < 55mmHg) that is not treated. |
| Error with no Potential for Harm | Violates strict standards but has essentially no potential for patient harm. | • Not including provider initials on a syringe label. |
| Error with Little Potential for Harm | A medication error that has little possibility of causing injury. | • Propofol infusion increased from 50 to 150 micrograms/kilogram/minute but not documented. |
| Error with Potential for an Adverse Drug Event (ADE) | A medication error that has the possibility of causing injury. | • A patient with history of upper gastrointestinal bleed given a nonsteroidal anti-inflammatory drug with no resultant bleeding. |
| Error with an ADE | An injury due to a medical intervention related to a drug that resulted from an error in the medication process. | • A patient with positive cocaine toxicology screen receives beta blockers and has severe hypertension. • Administering penicillin to a patient with a penicillin allergy who subsequently develops a rash. • A patient who develops mean arterial pressure < 55 mmHg after 4mg/kg propofol bolus. |
| ADE without Error | An injury due to a medical intervention related to a drug with no error in the medication process. | • An allergic reaction in a patient not previously known to be allergic to that particular medication. • A patient with a history of postoperative nausea and vomiting (PONV) who is given a combination of antiemetics perioperatively and subsequently develops PONV. • A patient who develops mean arterial pressure < 55 mmHg after a standard dose of propofol. |
| Ameliorable ADE | An ADE whose severity could have been substantially reduced if different actions had been taken. | • A patient with continuing PONV who did not receive antiemetics within 30 minutes • A patient with >4/10 pain on emergence that is not treated until after arriving in the recovery room |
Table 3.
Severity of Medication Error or Adverse Drug Event
| Term | Definition | Examples |
|---|---|---|
| Life-threatening | The event has the potential to cause symptoms that if not treated, would put the patient at risk of death. | • More than three consecutive premature ventricular contractions. • Patient with a prior anaphylactic reaction to penicillin who is given penicillin or cefazolin. |
| Serious | The event has the potential to cause symptoms that are associated with a serious level of harm that is not high enough to be life-threatening. | • Failing to administer antibiotics before incision in a person requiring antibiotics. • Patient given insulin without subsequently checking blood glucose levels. |
| Significant | The event has the potential to cause symptoms that while harmful to the patient pose little or no threat to the patient's function. | • Blood glucose levels not checked in a patient with diabetes. |
Study Participants
All 237 anesthesia care providers (excluding study staff) were eligible to participate. The providers included 81 (34.2%) anesthesiologists, 53 (22.4%) certified registered nurse anesthetists (CRNAs), and 103 (43.5%) house staff. We held an informational session in conjunction with department-wide grand rounds to describe the study purpose and provide an opportunity for anesthesia providers ask questions. We subsequently sent a consent email to all anesthesia providers, providing them with the option to opt-out of participation at any time during the study period.
Observers
Four fully-trained, practicing clinician observers (three anesthesiologists and one nurse anesthetist) independently observed medication administration by anesthesia providers during routine patient care without intervention, in order to detect MEs and/or ADEs per our error detection framework. Observer training prior to participation included: Thorough review of the observer training manual, which includes ME and ADE definitions and examples; at least one three-hour formal training session led by a ME expert to review error terminology and classification, including scenario-based case discussions; and collecting data simultaneously with an experienced, trained observer for a minimum of ten cases, with an emphasis on techniques used to minimize the effect of the observer on the observed (Hawthorne effect)10 such as minimizing interaction with participants, remaining outside of the participants’ immediate workspace.
Data Collection
We randomly selected operating rooms for data collection, excluding pediatric, cardiac surgery and off-site locations due to unique medication administration considerations in these areas. A two-pronged approach was used to capture suspected medication errors and/or adverse drug events: direct observation and chart review.
The primary method of data collection was continuous direct observation, originally described by Barker and colleagues, who demonstrated that with properly trained observers, there is negligible if any Hawthorne effect.10, 18 Observations began when the anesthesia provider assumed care for the patient, and ended when the patient arrived in the recovery room or intensive care unit. Using paper data collection forms, observers documented in real time all medications administered as well as any MEs and/or ADEs observed. They recorded the event type (ME and/or ADE), error type, time of event, provider type, and other comments (free text) associated with the event. If an ADE occurred in conjunction with a ME, the observer completed the Naranjo Algorithm19 to determine the likelihood that the ADE was related to the ME. All field observations were entered into our study database by a clinical research coordinator.
The second data collection method was guided chart abstraction from our anesthesia information management system by trained anesthesiologists. For all directly observed patient care encounters, we also queried our anesthesia information management system database for cases of drug dosages and/or vital signs during the observational period that were outside of our defined acceptable range, some of which are outlined in Table 2. These cases were put forward for further review to determine whether a ME or ADE was present. Duplicate events (detected by both chart review and observation) were deleted.
Event Classification
The study team, including observers, met weekly to review and discuss events, further assess and reclassify them as needed. All events identified during this data collection phase subsequently underwent review by two independent members of the adjudication committee, which was comprised of board-certified anesthesiologists and/or ME experts. Events not deemed to be MEs and/or ADEs were excluded. For example, moderate hypotension (with mean arterial pressure > 55 mmHg) in a patient without cardiac risks factors after receiving a standard dose of propofol (<3.5 milligrams per kilogram) was excluded. Mean arterial pressure less than 55 mmHg was classified as an adverse event.20 The committee judged ADE and potential ADE severity on a four-point Likert scale (significant, serious, life-threatening and fatal), and preventability on a four-point Likert scale (definitely preventable, probably preventable, probably not preventable and definitely not preventability), with the scale collapsed to two categories (probably preventable or probably not preventable) prior to analysis. The committee also assigned each ME type to a prevention strategy which, in their judgment, has potential to reduce the likelihood of the ME and/or associated ADE. Rater disagreements were resolved by consensus through discussion between the two raters.
Statistical Analysis
We present the results as the number and rate of MEs and ADEs per 100 medication administrations as well as the percentage of medication administrations with at least one error. Based on medication error rates in other patient care areas, we expected 10% of medication administrations to involve at least one error.1, 15, 16, 21 Sample size estimation was performed using the binomial distribution to ensure that the width of the 95% confidence interval for the number of medication administrations involving at least one error was approximately +/−1.5%. With approximately 1,380 medication administrations and an expected rate of 10%, the 95% confidence interval for the number of medication administrations having at least one error was approximately 3%. Due to the large number of expected cases with zero errors, the association between error rate and demographic/clinical characteristics was assessed using the Zero-Inflated Poisson Regression, and we considered that a single medication administration could involve multiple errors. The inter-rater reliability between adjudication committee members for incident classification, severity, and preventability was assessed using Cohen's kappa statistic. All analyses were performed using SAS(R) version 9.3 (Cary, NC) and statistical significance was defined as p<0.05.
Our primary outcomes were the incidence of MEs and ADEs in the perioperative setting. Secondary outcomes were MEs and ADEs by patient characteristics, specifically age, sex, race, American Society of Anesthesiologists (ASA) physical status score,22 body mass index (BMI), procedure type, procedure duration and number of medication administrations.
Results
Data were collected during an eight month period from November 2013 to June 2014. Of 237 eligible anesthesia care providers, 11 opted out: Seven attending anesthesiologists, two CRNAs, and two house staff. Thus, our eligible study population consisted of 74 (32.7%) attending anesthesiologists, 51 (22.6%) CRNAs and 101 (44.7%) house staff. Over 105 observation days, four anesthesia-trained observers observed 277 operations on 275 patients, with a total of 3,671 medication administrations (Table 4) by 24 (8.7%) attending anesthesiologists, 160 (57.8%) CRNAs and 93 (33.6%) house staff. Of the 277 operations observed, 124 (44.8%) included one or more ME and/or ADE. A total of 227 (82.0%) operations required general anesthesia and 37 (13.4%) involved sedation only. There was no significant difference between event rates for general anesthesia (227 operations, 3,297 medication administrations, 5.3% event rate) versus sedation (50 operations, 374 medication administrations, 4.6% event rate, p=0.52).
Table 4.
Patient and Procedure Characteristics
| Total Patients (N = 275)a | Medication Administrations (N = 3,671)b | Events (N=193)c | Medication Errors (N = 153)c | Adverse Drug Events (N = 91)c | |
|---|---|---|---|---|---|
| Age – Years | p = 0.59 | p = 0.63 | p = 0.69 | ||
| Mean: 55.73; Range: 20-94 | |||||
| 18-30 | 15 (5.5%) | 163 (4.4%) | 9 (5.5%) | 8 (4.9%) | 3 (1.8%) |
| 30-50 | 86 (31.3%) | 1132 (30.8%) | 63 (5.6%) | 49 (4.3%) | 26 (2.3%) |
| 50-65 | 90 (32.7%) | 1294 (35.2%) | 57 (4.4%) | 45 (3.5%) | 30 (2.3%) |
| 65+ | 84 (30.6%) | 1082 (29.5%) | 64 (5.9%) | 51 (4.7%) | 32 (3.0%) |
| Sex | p = 0.95 | p = 0.79 | p = 0.38 | ||
| Female | 165 (60.0%) | 2209 (60.2%) | 116 (5.3%) | 93 (4.2%) | 58 (2.6%) |
| Male | 110 (40.0%) | 1462 (39.8%) | 77 (5.3%) | 60 (4.1%) | 33 (2.3%) |
| Race or Ethnic Group | p = 0.01 | p = 0.03 | p = 0.002 | ||
| Caucasian | 232 (84.4%) | 3103 (84.5%) | 168 (5.4%) | 132 (4.3%) | 79 (2.5%) |
| Asian | 9 (3.3%) | 107 (2.9%) | 4 (3.7%) | 3 (3.7%) | 0 (0.0%) |
| Hispanic | 8 (2.9%) | 128 (3.5%) | 9 (7.0%) | 6 (4.7%) | 6 (4.7%) |
| Black | 3 (1.1%) | 51 (1.4%) | 4 (7.8%) | 3 (5.9%) | 3 (5.9%) |
| Not Recorded | 12 (4.4%) | 149 (4.1%) | 8 (5.4%) | 8 (5.4%) | 3 (2.0%) |
| Other | 11 (4.0%) | 133 (3.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| ASA Scored | p = 0.29 | p = 0.56 | p = 0.15 | ||
| 1 | 25 (9.1%) | 309 (8.4%) | 10 (3.2%) | 9 (2.9%) | 3 (1.0%) |
| 2 | 171 (62.2%) | 2329 (63.4%) | 128 (5.5%) | 95 (4.1%) | 68 (2.9%) |
| 3 | 77 (28.0%) | 1019 (27.8%) | 54 (5.3%) | 48 (4.7%) | 20 (2.0%) |
| 4 | 2 (0.7%) | 14 (0.4%) | 1 (7.1%) | 1 (7.1%) | 0 (0.0%) |
| Body Mass Index (BMI) | p = 0.38 | p = 0.61 | p = 0.12 | ||
| Mean: 28.43; Range: 15.5-57.7 | |||||
| Normal: 18-24.9 | 95 (34.6%) | 1195 (32.6%) | 59 (4.9%) | 46 (3.8%) | 27 (2.3%) |
| Overweight: 25-29.9 | 95 (34.6%) | 1328 (36.2%) | 65 (4.9%) | 55 (4.1%) | 27 (2.0%) |
| Obese: 30+ | 85 (30.9%) | 1148 (31.3%) | 69 (6.0%) | 52 (4.5%) | 37 (3.2%) |
| Procedure Type | p = 0.27 | p = 0.47 | p = n.c.e | ||
| Orthopedic | 51 (18.6%) | 641 (17.5%) | 35 (5.5%) | 25 (3.9%) | 19 (3.0%) |
| Gynecological | 46 (16.7%) | 629 (17.1%) | 29 (4.6%) | 25 (4.0%) | 9 (1.4%) |
| Urology | 39 (14.2%) | 526 (14.3%) | 20 (3.8%) | 16 (3.0%) | 10 (1.9%) |
| General | 38 (13.8%) | 561 (15.4%) | 43 (7.7%) | 31 (5.5%) | 23 (4.1%) |
| Breast | 24 (8.7%) | 258 (7.0%) | 15 (5.8%) | 13 (5.0%) | 7 (2.7%) |
| Thyroid/parathyroid | 16 (5.8%) | 236 (6.4%) | 12 (5.1%) | 9 (3.8%) | 8 (3.4%) |
| Thoracic | 14 (5.1%) | 205 (5.6%) | 12 (5.9%) | 10 (4.9%) | 7 (3.4%) |
| Plastic | 13 (4.7%) | 171 (4.7%) | 7 (4.1%) | 5 (2.9%) | 4 (2.3%) |
| Interventional radiology | 12 (4.4%) | 123 (3.4%) | 3 (2.4%) | 3 (2.4%) | 0 (0.0%) |
| Neurosurgery | 9 (3.3%) | 160 (4.4%) | 6 (3.8%) | 6 (3.8%) | 1 (0.6%) |
| Vascular | 6 (2.2%) | 59 (1.6%) | 4 (6.8%) | 3 (5.1%) | 1 (1.7%) |
| Other | 7 (2.5%) | 102 (2.7%) | 7 (16.1%) | 7 (16.1%) | 2 (4.9%) |
| Duration of Procedure | p = 0.0004 | p = 0.0006 | p = 0.04 | ||
| Mean:2.4 hr; Range: 0.3-10.5hr | |||||
| <1 hour | 64 (23.27%) | 601 (16.4%) | 20 (3.3%) | 18 (3.0%) | 6 (1.0%) |
| 1-3 hours | 134 (48.73%) | 1732 (47.2%) | 95 (5.5%) | 72 (4.2%) | 48 (2.8%) |
| 3-6 hours | 63 (22.91%) | 1093 (29.8%) | 58 (5.3%) | 45 (4.1%) | 28 (2.6%) |
| 6+ hours | 14 (5.09%) | 245 (6.7%) | 20 (8.2%) | 18 (7.3%) | 9 (3.7%) |
| Medication Administrations | p = 0.02 | p = 0.11 | p = 0.002 | ||
| Mean: 13.31; Range: 2-28 | |||||
| 12 or fewer | 127 (46.2%) | 1116 (30.4%) | 61 (5.5%) | 54 (4.8%) | 20 (1.8%) |
| 13 or more | 148 (53.8%) | 2555 (69.6%) | 132 (5.2%) | 99 (3.9%) | 71 (2.8%) |
Percentages calculated with denominator of 275 patients
Percentages calculated with denominator of 3,671 medication administrations
Percentages calculated with denominator of total medication administrations in corresponding category
American Society of Anesthesiologists Physical Status Score20
n.c. = non-convergence
A total of 211 medication errors and/or ADEs were detected, of which 172 (81.5%) were directly observed and 39 (18.5%) were discovered through targeted review of the anesthesia records from operations that we observed. Of the 211 errors, 14 (6.6%) were excluded by the adjudication committee and 4 (1.9%) were determined to have no potential for harm, leaving a final sample of 193 events (5.3%, 95% CI: 4.5 to 6.0) associated with 187 unique medication administrations (5.1%, 95% CI: 4.4 to 5.8). Inter-rater reliability between adjudication committee members for event classification was good (Kappa=0.97, 4 cases resolved by consensus).
Errors and Adverse Events
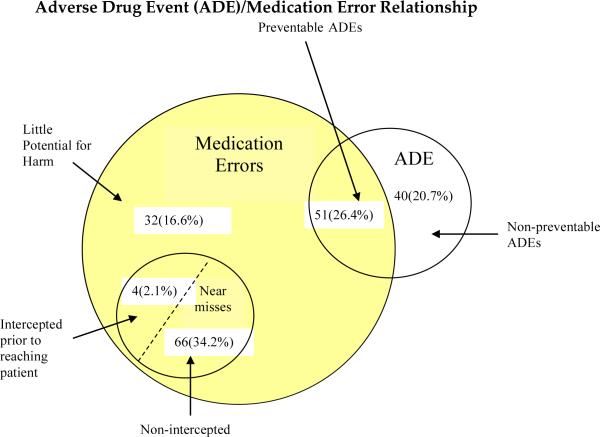
The 193 events detected included 153 (79.3%) MEs and 91 (47.2%) ADEs. A single event can involve both an error and an ADE (Figure 1). Of these events, 40 (20.7%) were ADEs that did not involve a ME, 51 (26.4%) were MEs that led to an observed ADE, 70 (36.3%) were MEs with the potential for an ADE, and 32 (16.6%) were MEs with little potential for harm (Figure 1). Of the 70 MEs with the potential for an ADE, four (5.7%) were intercepted. A total of 153 (79.3%) events were deemed preventable and 40 (20.7%) were deemed non-preventable. Inter-rater reliability for preventability classification was good (Kappa=0.98, 1 case resolved by consensus). Of the 193 events, 104 (53.9%) occurred within 20 minutes of the induction period. None of the observed or potential ADEs were fatal, three (1.6%) were life threatening, 133 (68.9%) were serious and 57 (29.5%) were significant. Inter-rater reliability for severity classification was good (Kappa=0.85, 12 cases resolved by consensus). Of the 51 medication errors that led to an ADE, the most prevalent error types were inappropriate medication doses (N=24; 47.1%) and omitted medications/failure to act (N=16; 31.4%). Using the Naranjo algorithm, 28 (54.9%) of the observed ADEs with error were probably due to the error, 22 (43.1%) were possibly due to the error, and 1(2.0%) was doubtfully due to the error.
Figure 1.
The 193 events detected included 153 (79.3%) medication errors (MEs) and 91 (47.2%) adverse drug events (ADEs). A single event can involve both an error and an ADE. Of these events, 40 (20.7%) were ADEs that did not involve a ME, 51 (26.4%) were MEs that led to an observed ADE, 70 (36.3%) were MEs with the potential for an ADE (four intercepted and 66 non-intercepted), and 32 (16.6%) were MEs with little potential for harm.
Of the 153 medication errors recorded, 51 (33.3%) led to an observed ADE and an additional 102 (66.7%) errors were associated with a potential ADE. The most common overall error type was labeling error (N=37; 24.2%), followed by wrong dose error (N=35; 22.9%) and omitted medication/failure to act (N=27; 17.6%). Of the 153 errors recorded (Table 5), 117 (76.5%) were associated with a specific medication administration and 36 (23.5%) were associated with other factors such as a delay or failure to treat an adverse event, or an error in monitoring. Medications most frequently associated with errors were propofol 30 (25.6%), phenylephrhine 12 (10.3%) and fentanyl 11 (9.4%). No significant difference existed between the event rates of house staff (N=68 events, 5.1% event rate), nurse anesthetists (N=111 events, 5.5% event rate) and attending anesthesiologists (N=14 events, 4.5% event rate, p=0.79).
Table 5.
Types of Medication Errors and Examples of Associated Potential Adverse Drug Events (ADEs)
| Error Type | n(%) | Error Example | Potential ADE Example |
|---|---|---|---|
| Labeling Error | 37(24.2%) | No Phenylephrine label. | Wrong dose or drug error. |
| Wrong Dose | 35(22.9%) | 1 mg Remifentanil bolus for 86 kg patient. | Bradycardia and hypotension. |
| Omitted Medication/Failure to Act | 27(17.6%) | No redosing of Cefazolin during all day case. | Surgical site infection. |
| Documentation Error | 26(17.0%) | Intubation not documented. Potential failure to recognize difficult airway on subsequent anesthetic. | Airway trauma or hypoxia during unexpected difficult intubation. |
| Monitoring Error | 10(6.5%) | No blood pressure check prior to induction. | Blood pressure > 200mmHg on first check after induction. |
| Wrong Medication | 9(5.9%) | CRNA obtained vial from Ondansetron slot in omnicell, put needle into vial to draw up drug, and then noticed it was Phenylephrine. | Life-threatening hypertension. |
| Wrong Timing | 5(3.3%) | 7 minute delay in administration of Ephedrine in the setting of hypotension. | Organ hypoperfusion with mean arterial pressure < 55 mmHg. |
| Inadvertent Bolus | 2(1.3%) | Phenylephrine infusion connected distal to antibiotic bolus site. | Hypertension due to inadvertent phenylephrine bolus with antibiotic. |
| Other | 2(1.3%) | Syringe of Hydromorphone left unattended on anesthesia machine before case. | Narcotic diversion/theft. |
| Total | 153(100.0%) |
Patient characteristics as well as event rates by patient characteristic are shown in Table 4. No association exists between ME and/or ADE rates and patient age, sex, American Society of Anesthesiologists (ASA) physical status score,22 body mass index (BMI) or procedure type. Longer procedures, especially those greater than six hours, had higher total event rates (p < 0.0001), ME rates (p < 0.0001) and ADE rates (p=0.004) than shorter procedures. Also, procedures with 13 or more medication administrations had higher event rates (p=0.02) and ADE rates (p=0.002) than those with 12 or fewer medication administrations. Finally, event rates (p=0.01), ME rates (p=0.03) and ADE rates (p=0.02) varied by patient race.
Contributing Factors and Solutions
We identified several strategies which, in our judgment, can be mapped to particular ME types in order to reduce the likelihood of ME and/or ADE. These strategies include both technology-based interventions and process-based interventions. Examples of technology-based interventions include: point-of-care bar code-assisted anesthesia documentation systems, which have the potential to eliminate 17.0% of MEs and 25.5% of potential ADEs; specific drug decision support, 28.8% of MEs, 13.7% of potential ADEs and 58.8% of ADEs; and alerts, 52.9% of MEs, 32.4% of potential ADEs and 94.1% of ADEs. An individual error can be prevented by multiple solutions.
Process-based interventions included changing the timing of documentation, which had the potential to eliminate 35.3% of MEs, 21.6% of potential ADEs and 62.8% of ADEs; reducing opportunities for workarounds, 24.2% of MEs and 36.3% of potential ADEs; connecting infusions to the most proximal IV port, 1.3% of MEs and 2.0% of potential ADEs; and rigorous vendor selection with strong training, which could work synergistically with the other interventions to reduce MEs and ADEs.
Discussion
We found that approximately one in twenty perioperative medication administrations and every second operation resulted in a ME and/or ADE. More than one third of these errors led to observed patient harm, and the remaining two thirds had the potential for patient harm. More than two-thirds of the harm or potential harm was classified as serious. Thus, there is substantial potential for medication-related harm, and a number of opportunities to improve safety in the peri-operative setting. Longer procedures, especially those greater than six hours in duration, had higher event, ME and ADE rates than those less than one hour. Also, procedures with 13 or more medication administrations had higher event and ADE rates than those with 12 or fewer medication administrations. Further research is required to assess whether this is related to fatigue and lapses in vigilance over longer time periods and more medication administrations.
The pre-existing literature on perioperative ME rates is sparse and often uses self-reports as a primary data source.4-7 In one study of anesthesiologists, the reported drug administration error rate was one per 133 anesthetics.4 In another survey study, 85% of anesthesiologists reported at least one drug error or near miss during their careers.5 These error rates are markedly lower than the rates that we found, which may be due to provider reluctance to self-report errors, or failure of providers to recognize errors they have made.
Self-reporting results in missing the vast majority of medication errors in most settings, and should not be expected to reliably assess ME rates.23 High-fidelity simulation has also been used to assess medication errors, with a reported error rate of approximately 10%.24 While measures were taken to make errors more likely in this setting, artificially raising the error rate, the simulation setting itself may also inherently lead to different error rates than found in an actual clinical practice setting. Merry and colleagues present the only previous investigation of perioperative errors that used direct observation as a method for data collection.1 In five operating rooms in a tertiary academic center in New Zealand, they found a perioperative medication error rate of 11.6% in a study group that used conventional non-electronic methods for anesthetic record keeping. However, they did not assess the errors’ potential for harm as this was a before and after study designed only to assess the impact of a specific anesthesia information management system on ME rates. Notably, this is also the only study to measure perioperative medication errors as a percentage of medications administered, which has been the standard denominator used to measure medication errors in other areas.14-16, 21 Previous studies in the perioperative setting had used the number of anesthetics administered as the denominator,4, 5, 7 which lacks the benefit of explicitly negative administrations.
In our Contributing Factors and Solutions Section, we identified several strategies to minimize perioperative MEs and/or ADEs, including technology-based interventions and process-based interventions. Examples of technology-based interventions include bar code-assisted syringe labeling systems, point-of-care bar code-assisted anesthesia documentation systems, specific drug decision support, and alerts.
Bar code-assisted syringe labeling systems have the potential to eliminate labeling errors. Despite the recently introduced bar code-assisted syringe labeling system at the study site, 37(24.2%) events involved a labeling error. These occurred when the provider did not use the labeling system either because it was not installed in that particular location or there was a workaround available to circumvent its use. This is addressed with the process-based interventions.
Point-of-care bar code-assisted anesthesia documentation systems allow the syringe label to be scanned immediately prior to drug administration, and automatically populate the anesthesia record with the medication and/or dose administered. These have the potential to reduce the incidence of documentation errors.
Specific drug decision support, including features such as dose calculators and maximum dose checking, has the capacity to reduce the incidence of wrong dose and wrong drug errors.
Alerts that are thoughtfully implemented into an electronic anesthesia information management system in a tiered manner to minimize cognitive overload can decrease the incidence of omitted medication/failure to act errors, and monitoring errors. For example, reminders to redose antibiotics or record a blood pressure after ten minutes without a reading have the potential to eliminate many of these errors.
Process-based interventions include determining optimal timing for documentation, reducing opportunities for workarounds, connecting infusions to the most proximal IV port, rigorous vendor selection and strong training.
Timing of documentation is critical in taking full advantage of decision support. In our study, most practitioners documented medications after they were administered. If syringe labels were scanned via a point-of-care bar code-assisted documentation system immediately prior to administration, the system could provide decision support such as dose calculators, maximum dose checks, allergy warnings and other alerts, eliminating many of the wrong dose errors. Even without comprehensive decision support, Merry and colleagues have shown that a system allowing syringe labels to be scanned immediately prior to administration with visual and auditory medication verification reduced perioperative MEs by 21%.1
Reducing the opportunity for workarounds is a key step in ensuring proper use of systems to reduce errors. For example, when a bar code-assisted syringe labeling system is installed and providers are fully trained on its use, manual sticker labels may be removed from the immediate workspace so that the easiest option is for providers to use the bar code-assisted, fully compliant labels. In the event of a bar code scanning system failure, appropriate manual backup labels should be readily available in a nearby location, such as the anesthesia workroom. We found that in most instances where the labeling system was not used, manual sticker labels were available and the provider used those instead.
Connecting infusions to the most proximal IV port, and ideally through a dedicated carrier line, may minimize the potential for inadvertent boluses of intravenous infusion. Boluses given through an infusion carrier line have the potential to inadvertently deliver a significant amount of infusion drug along with the intended bolus. While we observed cases where this led to significant hemodynamic changes, these are lessened when the infusion is connected to the most proximal IV port as the volume of infusion drug in the carrier IV line is minimized.
Rigorous vendor selection, with strong training, should eliminate vendors that are unwilling to commit to iteratively revise and improve a technology based on user feedback. Long-term, on-site training that covers all shifts is also important to minimize workflow disruptions.
This study has several limitations. First, due to the Hawthorne effect, the observed anesthesia providers may have altered their behavior during the observations. Barker and colleagues have shown that with proper observer training, the Hawthorne effect is negligible.10 If there were some residual Hawthorne effect present during our study, it would have artificially decreased our event rate, suggesting that the actual event rate is likely higher than we have reported. Second, our primary method of data collection was direct observation, which may not capture all events that occurred. We did undertake a corresponding chart abstraction to capture additional events that may have been missed by observation. However, our results may still underrepresent the actual number of events. Third, our study setting was a large tertiary care academic institution, where anesthesia is administered by residents, fellows, CRNAs and attending anesthesiologists, and our findings may not be generalizable to nonteaching hospitals. Fourth, our sample may not have been large enough to detect small differences in event rates by patient characteristic such as ASA score, BMI and procedure type. While we did find that event rates varied by patient race, this result may not be robust or representative of large populations as the proportion of minority patients in our sample was very small. Detecting differences in event rates by patient characteristic was not a primary aim of our study, and future research can be designed to assess whether patient characteristics affect rates of MEs and ADEs. Fifth, while we assumed that each medication administration was an independent event, this assumption was not directly assessed. We indirectly assessed the independence of each medication administration by examining the association between event rates and procedure type (length and complexity), provider type and number of medication administrations in the procedure, and found no indication of strong dependence between medication administrations. Finally, our center has an electronic anesthesia information management system and a bar code-assisted syringe labeling system, both of which may reduce the frequency of medication errors and/or ADEs.25 Thus, our findings may not be generalizable to centers without these tools.
In summary, we found that approximately one in twenty perioperative medication administrations, and every second operation, resulted in a ME and/or ADE. More than one third of these errors led to observed patient harm, and the remaining two thirds had the potential for patient harm. These rates are markedly higher than those reported by existing retrospective surveys. Future analyses should target the creation and implementation of process- and technology-based solutions that may address the root causes of the errors in order to reduce their incidence.
Acknowledgment
We would like to thank Dr. Jeffrey Cooper (Ph.D., Massachusetts General Hospital, Boston MA) and Dr. Robert Peterfreund (M.D., Ph.D., Massachusetts General Hospital, Boston MA) for their contributions to the study design. We would also like to thank Ms. Kwun Yee Trudy Poon (M.S., Massachusetts General Hospital, Boston MA) for her contributions to statistical analysis. Finally, we would like to thank Dr. Alireza Jafari (M.D., Shahid Beheshti University of Medical Sciences, Tehran Iran) for his assistance with the data collection process.
Funding sources: This work was supported by grants from the Doctors Company Foundation (Napa, CA) and the National Institute of General Medical Sciences (Bethesda, MD) of the National Institutes of Health (Award Number T32GM007592). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Doctors Company Foundation or the National Institutes of Health. Neither the Doctors Company Foundation nor the National Institutes of Health had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Competing interests: Dr. Bates is a coinventor on Patent No. 6029138 held by Brigham and Women's Hospital on the use of decision support software for radiology medical management, licensed to the Medicalis Corporation. He holds a minority equity position in the privately held company Medicalis. He serves on the board of SEA Medical, which makes technologies that can identify medications in solution. He receives equity and cash compensation from QPID, Inc, a company focused on intelligence systems for electronic health records.
Dr. Nanji, Dr. Patel, Ms. S. Shaikh and Ms. D. Seger have no competing interests.
References
- 1.Merry AF, Webster CS, Hannam J, Mitchell SJ, Henderson R, Reid P, Edwards KE, Jardim A, Pak N, Cooper J, Hopley L, Frampton C, Short TG. Multimodal system designed to reduce errors in recording and administration of drugs in anaesthesia: prospective randomised clinical evaluation. BMJ. 2011;343:d5543. doi: 10.1136/bmj.d5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fasting S, Gisvold SE. Adverse drug errors in anesthesia, and the impact of coloured syringe labels. Can J Anaesth. 2000;47(11):1060–7. doi: 10.1007/BF03027956. [DOI] [PubMed] [Google Scholar]
- 3.Cooper JB, Newbower RS, Kitz RJ. An analysis of major errors and equipment failures in anesthesia management: considerations for prevention and detection. Anesthesiology. 1984;60(1):34–42. doi: 10.1097/00000542-198401000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Webster CS, Merry AF, Larsson L, McGrath KA, Weller J. The frequency and nature of drug administration error during anaesthesia. Anaesth Intensive Care. 2001;29(5):494–500. doi: 10.1177/0310057X0102900508. [DOI] [PubMed] [Google Scholar]
- 5.Orser BA, Chen RJ, Yee DA. Medication errors in anesthetic practice: a survey of 687 practitioners. Can J Anaesth. 2001;48(2):139–46. doi: 10.1007/BF03019726. [DOI] [PubMed] [Google Scholar]
- 6.Llewellyn RL, Gordon PC, Wheatcroft D, Lines D, Reed A, Butt AD, Lundgren AC, James MF. Drug administration errors: a prospective survey from three South African teaching hospitals. Anaesth Intensive Care. 2009;37(1):93–8. doi: 10.1177/0310057X0903700105. [DOI] [PubMed] [Google Scholar]
- 7.Shridhar Iyer U, Fah KK, Chong CK, Macachor J, Chia N. Survey of medication errors among anaesthetists in Singapore. Anaesth Intensive Care. 2011;39(6):1151–2. [PubMed] [Google Scholar]
- 8.Flynn E, Barker K, Pepper G, Bates D, Mikeal R. Comparison of methods for detecting medication errors in 36 hospitals and skilled-nursing facilities. Am J Health Syst Pharm. 2002;59(5):436–46. doi: 10.1093/ajhp/59.5.436. [DOI] [PubMed] [Google Scholar]
- 9.Barker KN, McConnell WE. The problems of detecting medication errors in hospitals. Am J Health Syst Pharm. 1962;19:360–69. [Google Scholar]
- 10.Barker KN, Flynn EA, Pepper GA. Observation method of detecting medication errors. Am J Health Syst Pharm. 2002;59(23):2314–6. doi: 10.1093/ajhp/59.23.2314. [DOI] [PubMed] [Google Scholar]
- 11.Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, Laffel G, Sweitzer BJ, Shea BF, Hallisey R, Vander Vliet M, Nemeskal AR, Leape LL, Hojnowski-Diaz RN, Petrycki S, Cotugno M, Patterson H, Hickey M, Kleefield S, Cooper J, Kinneally E, Demonaco MS, Clapp MD, Gallivan T, Ives J, Porter K, Thompson BT, Hackman JR, Edmondson A. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29–34. [PubMed] [Google Scholar]
- 12.Bates DW, Leape LL, Cullen DJ, Laird N, Petersen LA, Teich JM, Burdick E, Hickey M, Kleefield S, Shea B, Vander Vliet M, Seger DL. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280(15):1311–6. doi: 10.1001/jama.280.15.1311. [DOI] [PubMed] [Google Scholar]
- 13.Poon EG, Cina JL, Churchill W, Patel N, Featherstone E, Rothschild JM, Keohane CA, Whittemore AD, Bates DW, Gandhi TK. Medication dispensing errors and potential adverse drug events before and after implementing bar code technology in the pharmacy. Ann Intern Med. 2006;145(6):426–34. doi: 10.7326/0003-4819-145-6-200609190-00006. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi TK, Weingart SN, Seger AC, Borus J, Burdick E, Poon EG, Leape LL, Bates DW. Outpatient prescribing errors and the impact of computerized prescribing. J Gen Intern Med. 2005;20(9):837–41. doi: 10.1111/j.1525-1497.2005.0194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi TK, Weingart SN, Borus J, Seger AC, Peterson J, Burdick E, Seger DL, Shu K, Federico F, Leape LL, Bates DW. Adverse drug events in ambulatory care. N Engl J Med. 2003;348(16):1556–64. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 16.Cooper JB, Newbower RS, Long CD, McPeek B. Preventable anesthesia mishaps: a study of human factors. Anesthesiology. 1978;49(6):399–406. doi: 10.1097/00000542-197812000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Rothschild JM, Landrigan CP, Cronin JW, Kaushal R, Lockley SW, Burdick E, Stone PH, Lilly CM, Katz JT, Czeisler CA, Bates DW. The Critical Care Safety Study: The incidence and nature of adverse events and serious medical errors in intensive care. Crit Care Med. 2005;33(8):1694–700. doi: 10.1097/01.ccm.0000171609.91035.bd. [DOI] [PubMed] [Google Scholar]
- 18.Allan EL, Barker KN. Fundamentals of medication error research. Am J Hosp Pharm. 1990;47(3):555–71. [PubMed] [Google Scholar]
- 19.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 20.Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, Cywinski J, Thabane L, Sessler DI. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119(3):507–15. doi: 10.1097/ALN.0b013e3182a10e26. [DOI] [PubMed] [Google Scholar]
- 21.Nanji KC, Rothschild JM, Salzberg C, Keohane CA, Zigmont K, Devita J, Gandhi TK, Dalal AK, Bates DW, Poon EG. Errors associated with outpatient computerized prescribing systems. J Am Med Inform Assoc. 2011;18(6):767–73. doi: 10.1136/amiajnl-2011-000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Society of Anesthesiologists ASA Physical Status Classification. doi: 10.1177/0310057X0203000516. http://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system. October 15, 2014. [DOI] [PubMed]
- 23.Cullen DJ, Bates DW, Small SD, Cooper JB, Nemeskal AR, Leape LL. The incident reporting system does not detect adverse drug events: a problem for quality improvement. Jt Comm J Qual Improv. 1995;21(10):541–8. doi: 10.1016/s1070-3241(16)30180-8. [DOI] [PubMed] [Google Scholar]
- 24.Merry AF, Weller JM, Robinson BJ, Warman GR, Davies E, Shaw J, Cheeseman JF, Wilson LF. A simulation design for research evaluating safety innovations in anaesthesia*. Anaesthesia. 2008;63(12):1349–57. doi: 10.1111/j.1365-2044.2008.05638.x. [DOI] [PubMed] [Google Scholar]
- 25.Jelacic S, Bowdle A, Nair BG, Kusulos D, Bower L, Togashi K. A System for Anesthesia Drug Administration Using Barcode Technology: The Codonics Safe Label System and Smart Anesthesia Manager. Anesth Analg. 2015;121:410–21. doi: 10.1213/ANE.0000000000000256. [DOI] [PubMed] [Google Scholar]