Abstract
Abstract
Two new diterpenoids, salyunnanins I and J (1 and 2), together with ten analogues, were isolated from the roots of Salvia yunnanensis. The structures of the new isolates, possessing different neo-clerodane and seco-abietane diterpenoid skeletons respectively, were elucidated on the basis of comprehensive spectroscopic data. All of the compounds were tested for the inhibitory activities against six human tumor lines in vitro, and several ones showed moderate cytotoxic activities.
Graphical Abstract
Electronic supplementary material
The online version of this article (doi:10.1007/s13659-015-0080-4) contains supplementary material, which is available to authorized users.
Keywords: Salvia yunnanensis, Diterpenoid, Cytotoxicity
Introduction
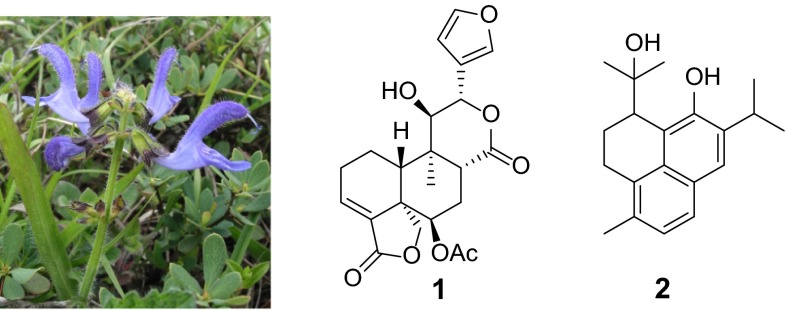
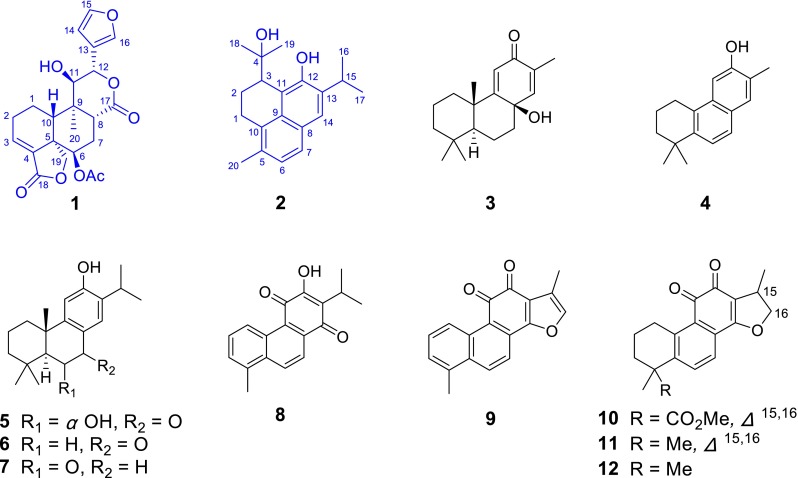
The genus of Salvia is a large pool of diterpenoids with structural diversity and biological properties [1–3]. Many diterpenoids with interesting bioactivities, such as tanshinone IIA, salvicine, neotanshinlactone, and salvinorin A, have been reported from this genus [4–6]. Salvia yunnanensis is a traditional Chinese herb used as the surrogate of S. miltiorrhiza (Danshen) for the treatment of various cardiovascular diseases [7]. Many bioactive abietane diterpenoids, especially a series of abietane type diterpene alkaloids, have been reported from this plant [8–10]. In a continuation of our research work on the diterpenoids from Salvia species, we examined the constituents of S. yunnanensis collected in Juhuacun traditional medicine market in the Yunnan province. As a result, two new diterpenoids, salyunnanins I and J (1 and 2), together with ten known analogues (3–12), were isolated (Fig. 1). It’s noteworthy that the structures of the new isolates were elucidated to possess neo-clerodane and seco-abietane diterpenoid skeletons respectively. All of the compounds were tested for the inhibitory activities against six human tumor lines in vitro, and several ones showed moderate cytotoxic activities. Herein, we report the isolation, structural elucidation, and the biological evaluation of all the obtained isolates.
Fig. 1.
Structures of 1–12 isolated from Salvia yunnanensis
Results and Discussion
The acetone extract of the roots of S. yunnanensis was subjected to silica gel column chromatography eluting with petroleum ether-EtOAc to obtain seven fractions (I-VII), which were further purified by silica gel, Sephadex LH-20, and HPLC to afford two new diterpenoids (salyunnanins I and J, 1 and 2) and ten known analogues: normiltioane (3) [11]; 5,6,7,8-tetrahydro-2,8,8-trimethyl-3-phenanthrenol (4) [12]; 6α-hydroxysugiol (5) [13]; sugiol (6) [14] 6-oxo-ferruginol (7) [15]; danshenxinkun B (8) [16]; tanshinone I (9) [17]; methyltanshinonate (10) [18]; tanshinone IIA (11) [19] and crypotanshinone (12) [20].
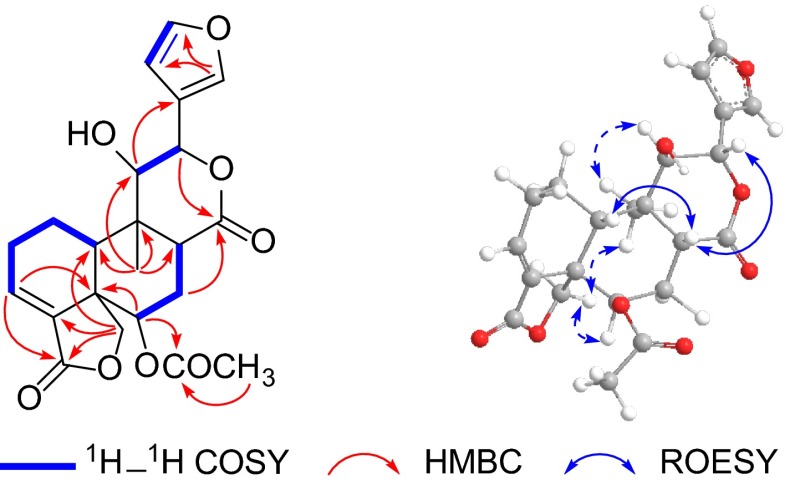
Salyunnanin I (1) was obtained as white powder. Its molecular formula C22H24O8 was established by its 13C NMR and HR-EIMS data ([M]+m/z 416.1467, calcd 416.1471). The IR spectrum exhibited absorption bonds due to hydroxyl (3425 cm−1) and lactone groups (1776 and 1747 cm−1). The 13C NMR and DEPT spectroscopic data (Table 1) exhibited 22 carbon signals assignable to two methyls (δC 15.4 and 21.0), four methylenes (including an oxygenated one at δC 71.5), nine methines (including four olefinic and three oxygenated ones at δC 69.6, 75.8 and 78.4), and seven quaternary carbons (including two olefinic ones and three lactonic carbonyls at δC 167.9, 170.0, and 173.5). Careful analysis of these 13C NMR data indicated that the characteristic signals for a neo-clerodane diterpenoid of two quaternary carbons (δC 49.9, C-5; 40.7, C-9), a methine (δC 39.6, C-10), an oxygenated methylene (δC 71.5, C-19), and a substituted furan ring (δC 125.5, C-13; 109.8, C-14; 144.8, C-15; 141.1, C-16) were all presented. These evidence, conjugated with some same type of diterpenoid were previously isolated in our laboratory [21], suggested that compound 1 could be a neo-clerodane diterpenoid. The 1H and 13C NMR data (Table 1) of 1 were very similar to those of dugesin E [21], except that the methylene carbon at δC 43.4 (C-11) in dugesin E was absent, while a down-field methine at δC 75.8 was instead present in 1. The difference implied that compound 1 was a C-11 oxygenated product of dugesin E. This assumption was supported by the HR-EIMS data, and further supported by the correlation from δH 0.80 (Me-20) to δC 75.8 (C-11) in the HMBC spectrum. Other partial planar structure of 1 was identical to those of dugesin E by detailed analysis of 1H–1H COSY and HMBC spectra (Fig. 2).
Table 1.
13C (125 MHz) NMR spectral data (δ in ppm and J in Hz) of 1 and 2
| No. | 1 a | 2 b | ||
|---|---|---|---|---|
| δ C type | δ H, mult. | δ C type | δ H, mult. | |
| 1 | 20.9 CH2 | 1.91, brd (12.8) | 23.4 CH2 | 2.89, dd (16.9, 5.6) |
| 1.28, m | 2.77, m | |||
| 2 | 27.6 CH2 | 2.32, m | 24.3 CH2 | 2.33, m |
| 2.23, m | 1.92, m | |||
| 3 | 139.9 CH | 6.79, dd (7.9, 2.0) | 42.6 CH | 3.50, brd (5.8) |
| 4 | 136.0 C | 78.9 C | ||
| 5 | 49.9 C | 131.2 C | ||
| 6 | 69.6 CH | 5.24, brd (5.3) | 125.6 CH | 7.09, d (8.3) |
| 7 | 24.8 CH2 | 2.28, m | 125.4 CH | 7.51, d (8.3) |
| 2.10, m | ||||
| 8 | 39.0 CH | 3.20, dd (12.4, 4.9) | 127.6 C | |
| 9 | 40.7 C | 129.7 C | ||
| 10 | 39.6 CH | 2.90, m | 129.9 C | |
| 11 | 75.8 CH | 3.90, t (6.2) | 117.3 C | |
| 12 | 78.4 CH | 5.19, d (6.2) | 150.6 C | |
| 13 | 125.5 C | 137.6 C | ||
| 14 | 109.8 CH | 6.58, brs | 123.5 CH | 7.49, s |
| 15 | 144.8 CH | 7.69, brs | 27.7 CH | 3.45, sept (6.8) |
| 16 | 141.1 CH | 7.60, brs | 23.1 CH3 | 1.27, d (6.8) |
| 17 | 173.5 C | 22.5 CH3 | 1.35, d (6.8) | |
| 18 | 167.9 C | 29.6 CH3 | 1.56, s | |
| 19 | 71.5 CH2 | 4.44, d (8.9) | 28.4 CH3 | 0.93, s |
| 4.18, d (8.9) | ||||
| 20 | 15.4 CH3 | 0.80, s | 19.7 C | 2.34, s |
| COMe | 170.0 C | |||
| COMe | 21.0 CH3 | 1.99, s | ||
aRecorded in acetone-d 6
bRecorded in CDCl3
Fig. 2.
Key HMBC, 1H–1H COSY, and NOESY correlations of 1
In the NOESY spectrum, the NOE correlations of Me-20 with H2-19, of H-19a (δH 4.44) with H-6, of H-10 with H-8, and of H-8 with H-12 indicated that the relative configurations of C-5, C-6, C-8, C-9, C-10, and C-12 of 1 were the same as dugesin E. In addition, the strong NOE contact between Me-20 and H-11 defined the α-orientation of H-11 (Fig. 2). Hence, the structure of 1 was elucidated and named salyunnanin I.
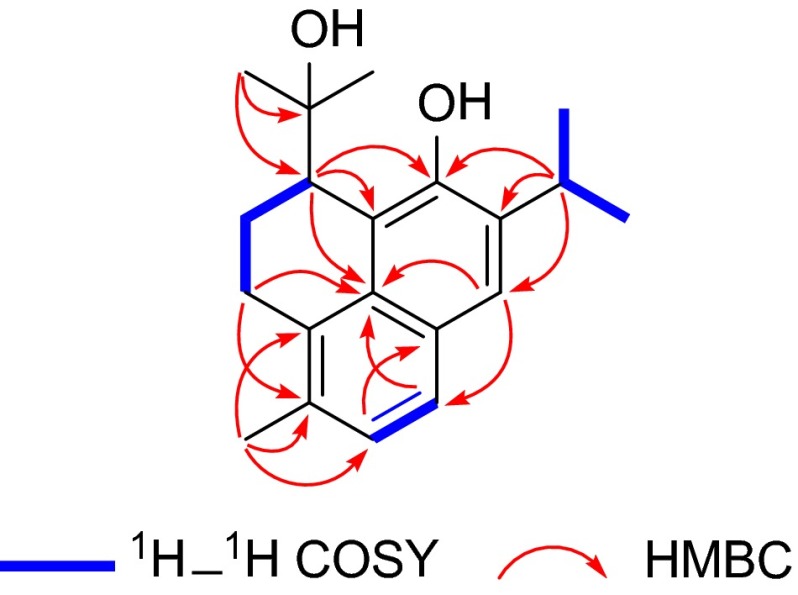
Salyunnanin J (2) was assigned the molecular formula C20H26O2 by its 13C NMR and HR-ESIMS (m/z 321.1835, [M + Na]+) data, 18 mass units more than that of (3-rac)-4,12-Epoxy-3,11-cyclo-4,5-seco-20(10 → 5)-abeo-abieta-5(10),6,8,11,13-pentaene, a cyclization product of aethiopionone [22]. Comparing of the NMR spectroscopic data of 2 (Table 1) with those of the cyclization product, about 14.7 and 1.7 ppm up-field shifts for C-4 (δC 78.9) and C-12 (δC 150.6), respectively, were found in 2, which indicated that 2 was a hydrolytic derivative of the known compound. The HR-ESIMS data (m/z 321.1835 [M + Na]+, calcd for 321.1830) supported this assumption. Furthermore, the correlations of H-1 (δH 2.89, 2.77)/H-2 (δH 2.33, 1.92)/H-3 (δH 3.50) and H-6 (δH 7.09)/H-7 (δH 7.51) in the 1H–1H COSY spectrum, together with the correlations from H-1 to C-5 (δC 131.2) and C-9 (δC 129.7), from H-3 to C-9, C-11 (δC 117.3), and C-12 (δC 150.6), from H-15 (δH 3.45) to C-12, C-13 (δC 137.6), and C-14 (δC 123.5), and from Me-20 (δH 2.34) to C-5, C-6 (δC 125.6), and C-10 (δC 129.9) in the HMBC spectrum further confirmed the structure of 2 (Fig. 3).
Fig. 3.
Key HMBC and 1H–1H COSY correlations of 2
Salvia (including 700–1050 species) is the biggest genus in the medicinal important Labiatae family and widely distributed in the world [23, 24]. These plants are also a rich source of various diterpenoids, especially the clerodane and abietane diterpenoids with diverse skeletons. Structurally, the clerodane diterpeoids are typical bicyclic diterpenoids, while abietane are tricyclic diterpenoids. Accordingly, their biogenetic pathways are totally different with each other. Detailed investigation of the literatures revealed an interesting relationship between the diterpenoids constituents and its taxonomy of Salvia plants: the clerodane diterpenoids are usually reported from plants distributed in America, while abietane and related diterpenoids are commonly reported from plants in Asia. Up to now, these two types of diterpenoids have only been reported from a few Salvia plants, such as S. cardiophylia and S. lavanduloides, simultaneously [25, 26].
All of the compounds were tested for their cytotoxic effects against six human cancer cell lines (Hela, NCI-H460, PC3, KB-3-1, MCF-7, and K562) using a previously described MTT method [27]. As shown in Table 2, compounds 5, 6, and 11 exhibited moderate activities.
Table 2.
Cytotoxcities of the isolates on six cancer cell lines with IC50 values (μM)
| Compounda | HeLa | KB-3-1 | NCI-H 460 | PC3 | MCF-7 | K562 |
|---|---|---|---|---|---|---|
| 5 | 7.7 | 7.7 | 7.4 | 6.0 | 8.7 | 5.0 |
| 6 | 10.9 | 9.1 | 8.9 | 10.5 | 11.4 | 6.7 |
| 7 | >20 | >20 | 17.2 | >20 | >20 | 15.9 |
| 9 | 16.7 | 12.8 | >20 | 5.2 | >20 | 6.1 |
| 11 | 10.1 | 9.2 | >20 | 8.9 | >20 | 5.5 |
| 12 | 17.1 | 10.3 | >20 | >20 | >20 | 13.6 |
| Paclitaxelb | 0.01 | 0.003 | 0.004 | 0.007 | 0.21 | 0.005 |
aOther selected ones not listed in the table were inactive (IC50 > 20 μM) for all cell lines
bPaclitaxel was used as positive controls
In conclusion, totally twelve diterpenoids, including two new ones (salyunnanins I and J), were characterized from S. yunnanensis in this study. Eleven of the isolates were abietane diterpenoids, while salyunnanin I (1) was elucidated to be a neo-clerodane diterpenoid. In the bioassay of these isolates, several compounds exhibited moderate cytotoxic activities.
Experimental
General Experimental Procedures
Optical rotations were measured on a Jasco P-1020 polarimeter. UV spectra were detected on a Shimadzu UV-2401PC spectrometer. IR spectra were determined on a Bruker FT-IR Tensor-27 infrared spectrophotometer with KBr disks. 1D and 2D NMR spectra were recorded on DRX-500 spectrometers using TMS as an internal standard. Unless otherwise specified, chemical shifts (δ) were expressed in ppm with reference to the solvent signals. ESIMS and HR-EIMS analysis were carried out on Waters Xevo TQS and Waters AutoSpec Premier P776 mass spectrometers, respectively. Semi-preparative HPLC was performed on an Agile 1100 HPLC with a Zorbax SB-C18 (9.4 × 250 mm) column. Silica gel (100–200 and 200–300 mesh, Qingdao Marine Chemical Co., Ltd., PR China), and Amphichroic RP-18 gel (40–63 μm, Merck, Darmstadt, Germany) and MCI gel (75–150 μm, Mitsubishi Chemical Corporation, Tokyo, Japan) were used for column chromatography.
Plant Material
The roots of S. yunnanensis were collected in Kunming, Yunnan Province, PR China, in October 2010. The plant was identified by Dr. En-De Liu, Kunming Institute of Botany, Kunming, PR China. A voucher specimen was deposited at the Kunming Institute of Botany with identification number 201010S01.
Extraction and Isolation
The air-dried powdered material (20 kg) was extracted with acetone (3 × 50 L × 24 h) at room temperature and the solution was evaporated in vacuum to give a crude extract (1.2 kg). The extract was subjected to column chromatography over silica gel, eluting with CHCl3–EtOAc to afford seven fractions (I–VII). Fraction II (311 g) was further chromatographed on silica gel (eluting with a gradient of EtOAc in petroleum ether) to yield seven subfractions (A–G). Subfraction B (54 g) was separated over an MCI-gel column (MeOH–H2O from 3:2 to 10:0) to obtain five fractions (Fr. B1–B5). Fr. B1 (12.5 g) was then chromatographed on a silica gel column eluted with petroleum ether–Chloroform (from 10:1 to 0:10), to yield four fractions (Fr. B1a–B1d). Fr. B1a (3.3 g) was repeatedly subjected to silica gel columns eluted with petroleum ether–EtOAc (from 500:1 to 2:1), and was then further purified by semi-preparative HPLC (MeOH–H2O, 85:15) to afford compounds 1 (6 mg) and 8 (4 mg). Fr. B2 (22 g) was isolated over an MCI gel column (MeOH–H2O from 75:25 to 100:0) to provide 2 (15 mg) and 3 (3 mg). Similarly, compounds 4 (35 mg), 5 (10 mg), 6 (18 mg), and 7 (10 mg) from subfraction C (32 g), and 9 (5 mg), 10 (430 mg), 11 (12 g), 12 (5 g) from subfraction D (120 g) were obtained.
Cytotoxicity Assays
The following human tumor cell lines were used: HeLa, NCI-H460, PC3, KB-3-1, MCF-7, and K562, which were kindly provided by Prof. David WF Fong of Hong Kong Baptist University. All cells were cultured in RPMI-1640 or DMEM medium [Gibco, Invitrogen (Shanghai), China] supplemented with 10 % fetal bovine serum [Gibco, Invitrogen (NY), USA] at 37 °C in a humidified atmosphere with 5 % CO2. Cell viability was assessed by conducting colorimetric measurements of the amount of insoluble formazan formed in living cells based on the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma, St. Louis, MO, USA) [28]. Briefly, 100 μL of adherent cells (HeLa, NCI-H460, PC3, KB-3-1, MCF-7) was seeded into each well of a 96-well cell culture plate and allowed to adhere for 24 h before test compound addition, while suspended cells (K562) were seeded just before test compound addition, both with an initial density of 1 × 105 cells/mL in 100 μL of medium. Each tumor cell line was exposed to the test compound at various concentrations in triplicate for 48 h, with paclitaxel (Sigma) as positive control. After the incubation, 10 μL of MTT (5 mg/mL in PBS) was added to each well, and the incubation continued for 4 h at 37 °C. After removing the medium, 100 μL per well of DMSO was added to dissolve the residue and the optical density was measured at 492 nm in a 96-well microtiter plate reader (Bio-Rad 680). The IC50 value of each compound was calculated by Reed and Muench’s method [27].
Salyunnanin J (1)
White powder; [α]21D −95.7 (c 0.22, MeOH); UV (MeOH) λmax (log ε): 208 (4.10), 249 (3.24) nm; IR (KBr) νmax 3425, 1776, 1747, 1374, 1241, 1189, 1169, 1024, 1003 cm−1; 1H and 13C NMR data, see Table 1; negative ESIMS m/z 451 [M + Cl]−; HR-EIMS m/z 416.1467 [M]+ (calcd for C22H24O8, 416.1471).
Salyunnanin K (2)
Yellow powder; [α]16D −12.6 (c 0.10, MeOH); UV (MeOH) λmax (log ε): 195 (4.22), 237 (4.69), 288 (3.68), 332 (3.36) nm; IR (KBr) νmax 3431, 2963, 2923, 2855, 1626, 1461, 1417, 1249, 1231 cm−1; 1H and 13C NMR data, see Table 1; EIMS m/z 298 [M]+; HR-ESIMS m/z 321.1835 [M + Na]+ (calcd for C20H26O2Na, 321.1830).
Electronic supplementary material
Acknowledgments
The work was financially supported by the National Natural Sciences Foundation of China (81373291), the National Science and Technology Support Program of China (2013BAI11B02), and the foundation from Chinese Academy of Sciences to Dr G. Xu.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Fan Xia and Chun-Yan Wu have contributed equally to this work.
Contributor Information
Xian Li, Email: xianlikm@163.com.
Gang Xu, Phone: +86 871 65217971, Email: xugang008@mail.kib.ac.cn.
References
- 1.Rodriguez-Hahn L, Esquivel B, Sanchez AA, Cardenas J, Tovar OG, Soriano-Garcia M, Toscano A. J. Org. Chem. 1988;53:3933–3936. doi: 10.1021/jo00252a010. [DOI] [Google Scholar]
- 2.Chang HM, Cheng KP, Choang TF, Chow HF, Chui KY, Hon PM, Tan FWL, Yang Y, Zhong ZP. J. Org. Chem. 1990;55:3537–3543. doi: 10.1021/jo00298a029. [DOI] [Google Scholar]
- 3.Pan ZH, Xu G, Zhao QS. Guang Xi Zhi Wu. 2010;30:781–790. [Google Scholar]
- 4.Xue M, Shi YB, Chui Y, Zhang B, Luo YJ, Zhou ZT, Xia WJ, Zhao RC, Wang HQ. Nat. Prod. Res. Dev. 2000;12:27–32. [Google Scholar]
- 5.Meng LH, Zhang JS, Ding J. J. Biochem. Pharm. 2001;62:733–741. doi: 10.1016/S0006-2952(01)00732-8. [DOI] [PubMed] [Google Scholar]
- 6.Wang XH, Bastow KF, Sun CM, Lin YL, Yu HJ, Don MJ, Wu TS, Nakamura S, Lee KH. J. Med. Chem. 2004;47:5816–5819. doi: 10.1021/jm040112r. [DOI] [PubMed] [Google Scholar]
- 7.Kunming Institute of Botany, Chinese Academy of Sciences . Flora Yunnannica. Beijing: Science Press; 1997. pp. 656–689. [Google Scholar]
- 8.Don MJ, Shen CC, Lin YL, Syu WJ, Ding YH, Sun CM. J. Nat. Prod. 2005;68:1066–1070. doi: 10.1021/np0500934. [DOI] [PubMed] [Google Scholar]
- 9.Lin FW, Damu AG, Wu TS. J. Nat. Prod. 2006;69:93–96. doi: 10.1021/np050368f. [DOI] [PubMed] [Google Scholar]
- 10.Xu G, Peng LY, Lu L, Weng ZY, Zhao Y, Li XL, Zhao QS, Sun HD. Planta Med. 2006;72:84–86. doi: 10.1055/s-2005-873184. [DOI] [PubMed] [Google Scholar]
- 11.Zhang DW, Liu X, Xie D, Chen R, Tao XY, Zou JH, Dai J. Chem. Pharm. Bull. 2013;61:576–580. doi: 10.1248/cpb.c12-00987. [DOI] [PubMed] [Google Scholar]
- 12.Chang HM, Chui KY, Tan FWL, Yang Y, Zhong ZP, Lee CM, Sham HL, Wong HNC. J. Med. Chem. 1991;34:1675–1692. doi: 10.1021/jm00109a022. [DOI] [PubMed] [Google Scholar]
- 13.Chyu CF, Chiang YM, Lin HC, Kuo YH. Tetrahedron Lett. 2004;45:641–643. doi: 10.1016/j.tetlet.2003.07.008. [DOI] [Google Scholar]
- 14.Wenkert E, De Paiva Campillo J, McChesney JD, Watts DJ. Phytochemistry. 1974;13:2545–2549. doi: 10.1016/S0031-9422(00)86934-4. [DOI] [Google Scholar]
- 15.Kuo YH, Yu MT. J. Nat. Prod. 1997;60:648–650. doi: 10.1021/np960389x. [DOI] [Google Scholar]
- 16.Nagy G, Gunther G, Mathe I, Blunden G, Yang MH, Crabb TA. Phytochemistry. 1999;52:1105–1109. doi: 10.1016/S0031-9422(99)00343-X. [DOI] [Google Scholar]
- 17.Shi YR, Zaesung N, Sung HK, Jong WA. Planta Med. 1997;63:44–46. doi: 10.1055/s-2006-957601. [DOI] [PubMed] [Google Scholar]
- 18.Kakisawa H, Hayashi T, Okazaki I, Ohashi M. Tetrahedron Lett. 1968;28:3231–3234. doi: 10.1016/S0040-4039(00)89532-5. [DOI] [Google Scholar]
- 19.Chen WS, Jia XM, Zhang WD, Lou ZY, Qiao CZ. Acta Pharm. Sin. 2003;5:354–357. [PubMed] [Google Scholar]
- 20.Gonzalez AG, Aguiar ZE, Grillo TA, Luis JG. Phytochemistry. 1992;31:1691–1695. doi: 10.1016/0031-9422(92)83130-Q. [DOI] [Google Scholar]
- 21.Xu G, Zhao F, Yang XW, Zhou J, Yang LX, Shen XL, Hu YJ, Zhao QS. Nat. Prod. Bioprospect. 2011;1:81–86. doi: 10.1007/s13659-011-0016-6. [DOI] [Google Scholar]
- 22.Sexmero Cuadrado MJ, De la Torre MC, Lin LZ, Cordell GA, Rodriguez B, Perales A. J. Org. Chem. 1992;57:4722–4728. doi: 10.1021/jo00043a034. [DOI] [Google Scholar]
- 23.Wu YB, Ni ZY, Shi QW, Dong M, Kiyota H, Gu YC, Cong B. Chem. Rev. 2012;112:5967–6026. doi: 10.1021/cr200058f. [DOI] [PubMed] [Google Scholar]
- 24.Dong YZ, Morris Natschke SL, Lee KH. Nat. Prod. Rep. 2011;28:529–542. doi: 10.1039/c0np00035c. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez AG, Herrera JR, Luis JG, Ravelo AG, Ferro EA. Phytochemistry. 1988;27:1540–1541. doi: 10.1016/0031-9422(88)80236-X. [DOI] [Google Scholar]
- 26.Maldonard E, De los Angeles Flores M, Salazar B, Ortega A. Phytochemistry. 1994;37:1480–1482. doi: 10.1016/S0031-9422(00)90438-2. [DOI] [Google Scholar]
- 27.Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 28.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A. J. Nat. Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.