Abstract
Purpose
Foxo3 protein is required in the oocyte nucleus for the maintenance of primordial follicles in a dormant state. PI3K/AKT-dependent phosphorylation of Foxo3 leads to its relocalization to the cytoplasm and subsequent follicular activation. However, the nature of the upstream signals controlling Foxo3 activity and subcellular localization remains unknown. We aimed to study the in vitro effects of Kit ligand (stem cell factor) on the subcellular localization of Foxo3 in primordial follicles within the postnatal mouse ovary.
Methods
This was an in vitro study using explants of intact neonatal mouse ovaries. The study was performed in laboratory animal facility and basic science research laboratory at a University Hospital. The animals used for this study were FVB mice. Neonatal FVB mice ovaries at postnatal day 7 (PD7) were harvested and incubated in culture medium (DMEM) at 37 °C and 5 % CO2 for 60–90 min with (n = 3) or without (n = 3) Kit ligand at 150 ng/mL (8 nM). Similar experimental conditions were used to establish a dose–response curve for the effects of Kit ligand and assess the effects of imatinib (small molecule inhibitor of the Kit receptor). Immunofluorescence was used to identify the subcellular location of Foxo3 in oocytes. Proportions of cytoplasmic versus nuclear Foxo3 in primordial follicles were determined.
Results
Kit ligand treatment increased the cytoplasmic localization of Foxo3 from 40 % in the untreated ovaries to 74 % in the treated group (p = 0.007 in paired samples and p = 0.03 in unpaired samples). Furthermore, this effect was reversible with imatinib (p = 0.005). A dose–response curve for Kit ligand treatment showed that maximum effect was seen at 150 ng/mL.
Conclusion
Kit ligand treatment in vitro increases the proportion of cytoplasmic Foxo3 in primordial follicles at PD7, lending support to the idea that Kit receptor/ligand controls Foxo3 activity in the context of primordial follicle activation
Keywords: Primordial follicle, Foxo3, Kit, Kit ligand, Stem cell factor
Introduction
Mammalian females are born with a finite number of oocytes, and the initial size of this oocyte pool is one of the major determinants of female reproductive lifespan. Subsequently, through the process of folliculogenesis, these primary oocytes become invested in a single layer of granulosa cells, giving rise to a limited number of primordial follicles. The timing of this follicular formation is species-specific, occurring at about 20 weeks of gestation in humans and approximately 2–4 days postnatally in mice [1, 2].
Oocytes remain in these primordial follicles in a quiescent state until growth is triggered in individual follicles through a highly regulated and yet poorly understood process termed primordial follicle activation (PFA) [3]. From a histological point of view, the hallmark of PFA is an increase in the size of the oocyte in primordial follicle and conversion of its encasing single layer of granulosa cells from flat to cuboidal [4]. In addition to the initial number of oocytes, the rate of PFA is another crucial factor that determines the length of female reproductive life. Once PFA initiates, a follicle will either complete its development into fully mature stages and subsequently undergo ovulation or, more commonly, undergo atresia at some interim stages during their development before completing the maturation process [5]. The initial stages of follicular development, including PFA, are gonadotropin-independent [6, 7] and presumably occur under the influence of a cross talk between the oocyte and its surrounding somatic cells, most notably granulosa cells [8, 9]. Several transcription factors, cytokines, growth factors, and other intermediary proteins have been implicated in this process, and experiments with transgenic animal models support these hypotheses. Some of the better-studied factors with a role in PFA include the forkhead transcription factor Foxo3 [10–13], components of the phosphatidylinositol 3-kinase (PI3K) pathway such as PTEN [14], TSC1 [15], and TSC2 [16], and members of the transforming growth factor beta (TGFβ) superfamily such as AMH, GDF-9 and BMP-15 [17], and p27 [18]; however, downstream and upstream signaling of these molecules is not well characterized.
Among these factors, Foxo3 was the first to be identified as a major suppressor of PFA with critical importance for maintenance of primordial follicles in a dormant state in the mouse [11, 19]. Foxo transcription factors, including Foxo3, are major effectors of the PI3K pathway and are downregulated upon increased activity of the PI3K pathway signaling [20–22].
Activation of PI3K-Akt pathway in oocytes triggers phosphorylation of Foxo3 and its subsequent intracellular translocation from the oocyte nucleus into the cytoplasm [23]. This nucleo-cytoplasmic shuttling of Foxo3, followed by its degradation in the cytoplasm, results in lifting of its inhibitory effects on PFA and consequently results in primordial follicle activation [23]. Activation of the PI3K-Akt pathway can be achieved through various extracellular signals that act via cell surface receptors, most notably receptor tyrosine kinases (RTKs), G-protein-coupled receptors (GPCRs), and small GTPases [24–27]. However, in the context of PFA, the identity of the specific cell surface receptor and its cognate ligand that activates PI3K–Akt pathway and subsequently leads to the phosphorylation and inhibition of Foxo3, is not known.
Several different cell surface receptors have been suggested as candidates for transduction of the presumptive external signal(s) for “awakening” of the oocytes in primordial follicles [28]. Among these candidates, due to various lines of evidence, signaling through Kit and Kit ligand [a.k.a. stem cell factor (SCF)] is of particular interest [29, 30].
First of all, the paramount importance of Kit/Kit ligand signaling for the process of primordial germ cell proliferation and migration to the undifferentiated gonads makes it plausible that it would have a continued role in subsequent stages of gamete maturation and development [31, 32]. Furthermore, the pattern of expression for the components of this signaling system, with Kit being expressed as a cell surface receptor on oocyte membranes and Kit ligand being expressed by granulosa cells in the immediate vicinity of the oocyte, perfectly fits the model in which the cross talk between the oocyte and its surrounding granulosa cells is the major triggering signal for primordial follicle activation [30] .
On an experimental level, previous studies have shown that Kit ligand exerts a stimulatory effect on the activation of primordial follicles in vitro and promotes their transition into more advanced stage follicles [33]. In addition, inhibition of Kit signaling in vivo through injection of ACK2, a murine antibody blocking Kit actions, results in slowing of follicular growth and maturation [34].
However, some studies have argued against a critical role for Kit signaling in primordial follicle activation. Using a genetically engineered Kit construct in which the tyrosine residue at position 719 is replaced with phenylalanine (KitY719F), resulting in the inability of Kit to interact with the SH2 domain of the P85 regulatory subunit of PI3K and consequently eliminating any signaling through PI3K pathway [35], it has been shown that females homozygous for KitY719F have no major defect in PFA [36, 37]. The observed phenotype in these mutant mice was a slowed transition rate from primary follicles to secondary follicles, as well as an increase in the number of morphologically abnormal follicles [36, 37]. However, the possibility of signaling through pathways other than PI3K, or the existence of compensatory mechanisms, could not be ruled out.
Considering the above clues for the possible role of Kit signaling in PFA, we sought to investigate the hypothesis that Kit ligand stimulation through Kit receptors on oocyte membrane in primordial follicles leads to increased activity of intra-oocyte PI3K pathway with subsequent phosphorylation of Foxo3, resulting in its translocation from the oocyte nucleus into the oocyte cytoplasm.
Our experiments demonstrated that stimulation of ovarian explants by Kit ligand resulted in a rapid shift in subcellular localization of Foxo3 from the nucleus into the cytoplasm in mouse primordial follicles. The effects of Kit ligand on Foxo3 subcellular localization were dose-dependent and subject to inhibition with imatinib (Gleevec®), a kinase inhibitor with activity against Kit and closely related kinases. Taken together, the above results suggest that Kit ligand treatment in vitro increases the proportion of cytoplasmic Foxo3 in primordial follicles, supporting the hypothesis that Kit signaling operates upstream of Foxo3 activity in the context of PFA.
Materials and methods
Mouse husbandry
This study was approved by the Institutional Animal Care and Use Committee (IACUC) at University of Texas Southwestern Medical Center in Dallas. All experiments were performed on FVB mice. Animals were fed on standard chow ad libitum and were kept in microisolator cages in rooms with standard light cycling (12-h light/dark cycles). One FVB stud male and up to three young adult females were placed in the breeding cages and inspected every morning. The day of birth was termed postnatal day 1 (PD1).
Organ culture
Ovaries were carefully dissected and immediately transferred to 24-well tissue culture plate with Dulbecco’s modified Eagle’s medium (DMEM) media and incubated at 37 °C with 5 % CO2 for 60–90 min. Recombinant mouse Kit ligand (R&D Systems catalog# Q78ED8) was reconstituted in phosphate-buffered saline (PBS) and added at varying concentrations to the culture media for the experimental samples. Samples in the control group were cultured in DMEM only, without any Kit ligand supplementation. Imatinib (Selleckchem catalog# S2475) was reconstituted in DMSO and added to the culture media for Kit inhibition assays at a concentration of 3 μM. In experiments where imatinib was used, it was simultaneously added to the culture media with Kit ligand. PI3K pathway inhibition was achieved with addition of LY294002 (Cell Signaling catalog# 9901) at a concentration of 25 μM.
Tissue processing, immunohistochemistry, and immunofluorescence
After 60–90-min incubation at 37 ° C with 5 % CO2, ovaries were immediately transferred to 10 % formalin and kept overnight at 4 °C for fixation and then processed and embedded in paraffin for histological evaluation. Paraffin blocks were sectioned at 5 μm and subsequently mounted on slides. After drying overnight, slides were then subjected to deparaffinization with xylene and subsequent rehydration with ethanol series. Antigen retrieval was performed through boiling in sodium citrate 10 mM solution for 10 min, followed by 20-min cooling period at room temperature. Blocking was performed with 3 % bovine serum albumin (BSA) for 1 h. The antibody used to detect Foxo3 was a rabbit polyclonal antibody (Santa Cruz Biotechnology catalog# SC-11351) raised against amino acids 329–472 of Foxo3 of human origin with proven reactivity with Foxo3 of mouse, rat, and human origin [13]; the antibody was used at a concentration of 1:200 in 2 % BSA/PBS, and slides were incubated with antibody for 1 h in room temperature. After washing with PBS, slides were incubated with secondary antibody (Alexa Fluor 555 goat anti-rabbit antibody, Invitrogen A-21429) at a concentration of 1:1000 in 2 % BSA/PBS, at room temperature for 1 h. After a second wash with PBS, nuclei were stained with DAPI (Pierce Protein Research Products catalog# 46290) at 1:10,000 dilution in PBS. Vectashield was used to cover the slides, and an Olympus BX51 microscope equipped for epifluorescence was used to obtain the images.
Histomorphometry and follicle counts
For the purpose of this study, subcellular location of Foxo3 was assigned to either cytoplasmic or nuclear. In cases where Foxo3 signal was detectable in both nucleus and cytoplasm, whichever compartment had a stronger signal was scored as the location of Foxo3. The signal from DAPI staining was used to differentiate between an equatorial cut through the nucleus and cytoplasm versus a tangential cut through the cytoplasm, and only those follicles with the visible nucleus in the plane of section were counted for statistical analysis. A total of three ovaries from three mice (one ovary per mouse) were sectioned for each control and experimental groups. Three sections per ovary were used for counting follicles, and for each section, all follicles with a detectable Foxo3 signal were counted and allocated to either nuclear or cytoplasmic groups. Inter-observer and intra-observer concordance was confirmed to be above 90 %.
Statistical analysis
Relative proportions of nuclear versus cytoplasmic staining were determined using Image J software. A negative binomial regression model, ANOVA, and t tests were used to determine statistical significance.
Results
Foxo3 undergoes a change in subcellular location during folliculogenesis
As previously shown, Foxo3 is almost exclusively cytoplasmic at PD1. Although follicle assembly (i.e., individualization) is almost complete by postnatal day 3 (PD3), the process of Foxo3 translocation into the nucleus continued until PD16, again as previously reported. At PD16, Foxo3 was only detected in the oocyte nucleus. To determine the optimal time point during this translocation process for experiments to elucidate the potential role of Kit ligand in driving Foxo3 into the cytoplasm, we replicated our previous results by quantification of Foxo3 location at different time points. Although in this particular series of experiments, Foxo3 was noted to be 17 % cytoplasmic and 82 % nuclear on PD7 (Fig. 1), an estimated average of all our experiments noted that on PD7, Foxo3 was relatively equally distributed between the nucleus (on average 60 %) and cytoplasm (on average 40 %), and hence, we decided to use this time point for our subsequent experiments.
Fig. 1.
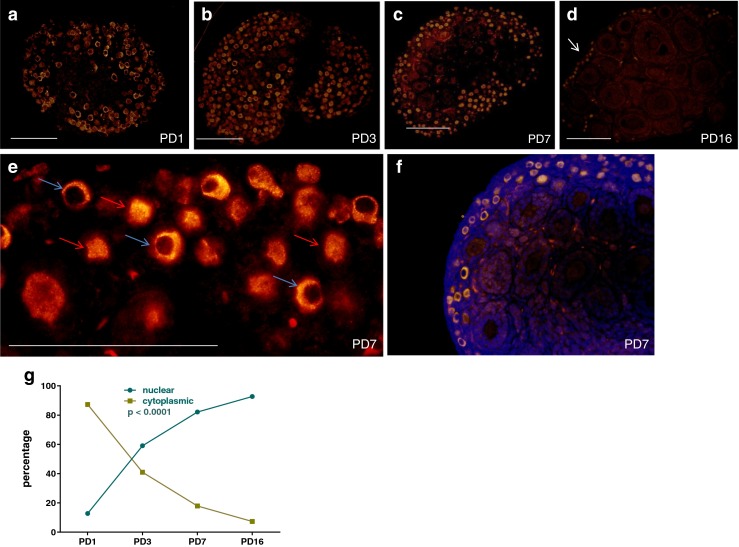
Relocalization of oocyte Foxo3 during normal postnatal development. a–d Representative sections of ovaries with IF staining for Foxo3. Scale bar = 200 μ. In panel (d), arrow indicates primordial follicles in the ovarian cortex. e Representative section showing cytoplasmic localization (blue arrow) and nuclear localization (red arrow). Scale bar = 100 μm. f Representative section counterstained with DAPI to confirm nuclear versus cytoplasmic localization. g The graph shows the change in subcellular location of Foxo3 from PD1 to PD16, showing an increase in nuclear location along with a corresponding decrease in cytoplasmic location (p value based on ANOVA test)
Nucleocytoplasmic shuttling of Foxo3 is subject to rapid modulation by a PI3K pathway inhibitor
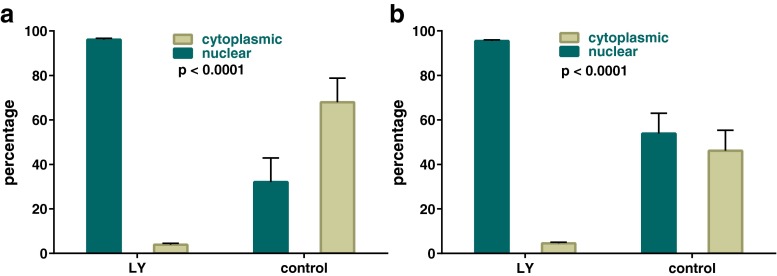
Next, we confirmed the responsiveness of our in vitro experimental model to study the effects of the PI3K pathway inhibitor LY294002 and demonstrated that a relatively short incubation period of 60–90 min in culture was adequate to effect near-complete nuclear sequestration of Foxo3 in oocytes. This was seen both in ovaries harvested at PD3 and PD7 (Fig. 2a, b).
Fig. 2.
Effect of pharmacologic PI3K inhibition on Foxo3 oocyte localization at PD3-7. Graph demonstrates responsiveness of the in vitro experimental model to effects of PI3K pathway inhibitor. a PD3 ovaries. b PD7 ovaries. Error bars = SEM (p values based on t test)
Incubation of explanted ovaries with Kit ligand results in a rapid change in subcellular location of Foxo3 from the nucleus into the cytoplasm
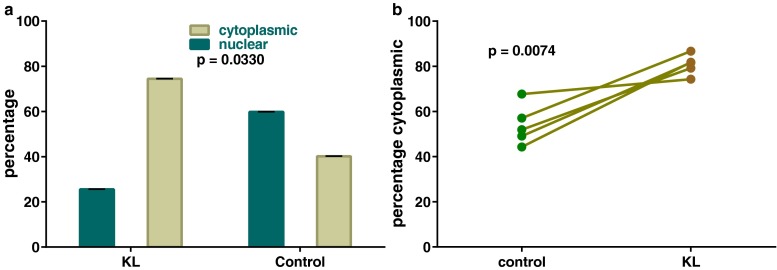
Once the reproducibility of our prior findings and the responsiveness and sensitivity of the experimental model were established, the effect of Kit ligand on Foxo3 localization was studied. Based on our hypothesis that Kit signaling results in a change in subcellular location of Foxo3 from the nucleus and into the cytoplasm, we determined that PD7 was the best time point to study this hypothesis. The suitability of PD7 is due to the fact that at this time point, Foxo3 was nuclear in 60 % of oocytes and therefore its translocation to the cytoplasm would be easily detectable. We found that incubation of explanted ovaries at PD7 with Kit ligand at 150 ng/mL for 60–90 min resulted in a decrease in proportion of oocytes with nuclear Foxo3 as compared to controls (59 vs. 25 %, p = 0.03), along with a reciprocal increase in proportion of oocytes with cytoplasmic Foxo3 (Fig. 3a). We then repeated the experiment with matched pairs where ovaries of the same animal were compared with each other, i.e., one ovary was treated with only the vehicle and the other ovary was treated with Kit ligand. This experiment was designed to minimize the potential confounding effects of variations in development, as animals from the same litter can exhibit some variation in developmental hallmarks. With this matched experimental design, the effects of Kit ligand in increasing the proportion of cytoplasmic Foxo3 were again documented and noted to be statistically significant with a P value of 0.007 (Fig. 3b).
Fig. 3.
Effect of Kit ligand on Foxo3 oocyte localization. a Incubation of explanted ovaries at PD7 with kit ligand (KL) at 150 ng/mL for 60–90 min resulted in decreased proportion of oocytes with nuclear Foxo3 as compared to controls (59 vs. 25 %, p = 0.03, based on t test), along with a reciprocal increase in proportion of oocytes with cytoplasmic Foxo3. Error bars = SEM. b Matched pair experimental design with additional samples again showed changes in subcellular localization that were statistically significant (p value based on two-tailed paired t test)
The effects of Kit ligand on Foxo3 localization are dose-dependent and subject to inhibition with imatinib
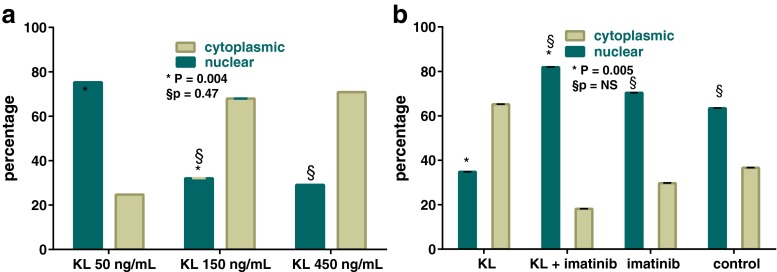
Next, in order to further support our experimental findings on the effects of Kit signaling on Foxo3 subcellular location, we replicated the experiments with varying concentrations of Kit ligand and established a dose–response relationship where the effects of Kit ligand reached a maximum at 150 ng/mL (50 vs. 150 ng/mL, p = 0.004; 150 vs. 450 ng/mL, p = 0.47) (Fig. 4a). Moreover, we observed that the effects of Kit ligand were subject to inhibition with Kit inhibitor, imatinib (Kit ligand vs. Kit ligand + imatinib, p = 0.005) (Fig. 4b).
Fig. 4.
Dose–response effects of Kit ligand on Foxo3 localization. a Dose–response curve for the effects of Kit ligand (KL) on Foxo3 subcellular localization. b Effects of Kit ligand are subject to inhibition with kit inhibitor, imatinib (KL vs. KL+ imatinib, p = 0.005; NS, non-significant). Error bars = SEM (p values based on t test)
Discussion
Our experiments demonstrate that treatment of mouse ovaries in vitro with recombinant Kit ligand results in a rapid and dose-dependent translocation of Foxo3 from the oocyte nucleus into the cytoplasm. This observation adds to the compendium of available experimental evidence supporting a role for Kit signaling in PFA. Based on this study and in line with previous findings [36, 38, 39], it seems likely that at least one of the downstream effectors of Kit signaling in the context of PFA is Foxo3. Conversely, it seems likely to conclude that Kit signaling is at least one of the upstream regulators of Foxo3. Previous studies have shown that interruption of Kit signaling specifically through PI3K pathway results in abnormal nuclear retention of Foxo3 in primary oocytes [36], and this study is consistent with this data.
On the other hand, the existing evidence does not appear to be conclusive in its support for an exclusive and indispensable role for Kit signaling for PFA. Abrogation of Kit signaling, at least through PI3K pathway, does not have a major effect on PFA. These seemingly conflicting results could be reconciled by noting several issues: First, it is possible that Kit signaling works through multiple pathways, one of which is PI3K pathway-dependent. Another possible explanation would be that, like many other biological systems, there is a possibility for redundancy in the required signaling to effect and advance PFA. In other words, Kit might regulate PFA, but it may do so through a multitude of downstream pathways regulated by Kit (e.g., SRC, JAK/STAT, and MAPK) acting in concert. Mutations that limit signaling through only one of these pathways (such as KitY719F) might well be compensated for by these alternative pathways [37, 40, 41]. Future studies would likely benefit from novel Kit alleles designed for studies of PFA, such as conditional activating alleles active in the germline, or floxed alleles for conditional knockout of Kit in primordial oocytes. Second, it is also worth noting that soluble and membrane-bound Kit ligands may have differing effects on follicular development with regard to functions such as anti-apoptosis, proliferation, growth, and PFA [42–44]. In vitro experiments have all used the soluble form of Kit ligand, and therefore, little is known about the role of membrane-bound isoform of Kit ligand in the ovary.
Gaining a thorough and systematic understanding of the molecular mechanisms underlying PFA may someday prove helpful in formulating effective strategies for interventions aimed at in vitro maturation of follicles and future use of cryopreserved ovarian tissue for fertility preservation among cancer patients. Furthermore, these studies may help to elucidate the pathophysiology of at least some cases of premature ovarian insufficiency and will provide an opportunity for development of more accurate diagnostic modalities and hopefully more effective preventive and/or treatment measures.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by NICHD R01 HD048690 grant to D.H.C.
Footnotes
Capsule
In the context of primordial follicle activation, Kit signaling increases the proportion of cytoplasmic Foxo3 in primordial follicles at PD7.
Contributor Information
M. Max Ezzati, Email: ezzatim@pamf.org.
Diego H. Castrillon, Phone: (214) 648 - 4032, Email: Diego.Castrillon@UTSouthwestern.edu
References
- 1.Pepling ME. Follicular assembly: mechanisms of action. Reproduction. 2012;143(2):139–49. doi: 10.1530/REP-11-0299. [DOI] [PubMed] [Google Scholar]
- 2.Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/S0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 3.Reddy P, Zheng W, Liu K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol Metab. 2010;21(2):96–103. doi: 10.1016/j.tem.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Lintern-Moore S, Moore GP. The initiation of follicle and oocyte growth in the mouse ovary. Biol Reprod. 1979;20(4):773–8. doi: 10.1095/biolreprod20.4.773. [DOI] [PubMed] [Google Scholar]
- 5.Peters H, Byskov AG, Himelstein-Braw R, Faber M. Follicular growth: the basic event in the mouse and human ovary. J Reprod Fertil. 1975;45(3):559–66. doi: 10.1530/jrf.0.0450559. [DOI] [PubMed] [Google Scholar]
- 6.Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54(1):197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 7.Peters H, Byskov AG, Lintern-Moore S, Faber M, Andersen M. The effect of gonadotrophin on follicle growth initiation in the neonatal mouse ovary. J Reprod Fertil. 1973;35(1):139–41. doi: 10.1530/jrf.0.0350139. [DOI] [PubMed] [Google Scholar]
- 8.Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296(5576):2178–80. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- 9.Albertini DF, Barrett SL. Oocyte-somatic cell communication. Reprod Suppl. 2003;61:49–54. [PubMed] [Google Scholar]
- 10.John GB, Shirley LJ, Gallardo TD, Castrillon DH. Specificity of the requirement for Foxo3 in primordial follicle activation. Reproduction. 2007;133(5):855–63. doi: 10.1530/REP-06-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301(5630):215–8. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 12.Gallardo TD, John GB, Shirley L, Contreras CM, Akbay EA, Haynie JM, et al. Genomewide discovery and classification of candidate ovarian fertility genes in the mouse. Genetics. 2007;177(1):179–94. doi: 10.1534/genetics.107.074823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarnawa ED, Baker MD, Aloisio GM, Carr BR, Castrillon DH. Gonadal expression of Foxo1, but not Foxo3, is conserved in diverse mammalian species. Biol Reprod. 2013;88(4):103. doi: 10.1095/biolreprod.112.105791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319(5863):611–3. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- 15.Adhikari D, Zheng W, Shen Y, Gorre N, Hamalainen T, Cooney AJ, et al. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2010;19(3):397–410. doi: 10.1093/hmg/ddp483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adhikari D, Flohr G, Gorre N, Shen Y, Yang H, Lundin E, et al. Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol Hum Reprod. 2009;15(12):765–70. doi: 10.1093/molehr/gap092. [DOI] [PubMed] [Google Scholar]
- 17.Pangas SA. Regulation of the ovarian reserve by members of the transforming growth factor beta family. Mol Reprod Dev. 2012;79(10):666–79. doi: 10.1002/mrd.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajareddy S, Reddy P, Du C, Liu L, Jagarlamudi K, Tang W, et al. p27kip1 (cyclin-dependent kinase inhibitor 1B) controls ovarian development by suppressing follicle endowment and activation and promoting follicle atresia in mice. Mol Endocrinol. 2007;21(9):2189–202. doi: 10.1210/me.2007-0172. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan SD, Castrillon DH. Insights into primary ovarian insufficiency through genetically engineered mouse models. Semin Reprod Med. 2011;29(4):283–98. doi: 10.1055/s-0031-1280914. [DOI] [PubMed] [Google Scholar]
- 20.Uhlenhaut NH, Treier M. Forkhead transcription factors in ovarian function. Reproduction. 2011;142(4):489–95. doi: 10.1530/REP-11-0092. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Wang Y, Zhu WG. Applications of post-translational modifications of FoxO family proteins in biological functions. J Mol Cell Biol. 2011;3(5):276–82. doi: 10.1093/jmcb/mjr013. [DOI] [PubMed] [Google Scholar]
- 22.Christian M, Lam EW, Wilson MS, Brosens JJ. FOXO transcription factors and their role in disorders of the female reproductive tract. Curr Drug Targets. 2011;12(9):1291–302. doi: 10.2174/138945011796150253. [DOI] [PubMed] [Google Scholar]
- 23.John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321(1):197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27(41):5527–41. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 25.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 26.Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer. 2015;15(1):7–24. doi: 10.1038/nrc3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker MD, Ezzati M, Aloisio GM, Tarnawa ED, Cuevas I, Nakada Y, et al. The small GTPase Rheb is required for spermatogenesis but not oogenesis. Reproduction. 2014;147(5):615–25. doi: 10.1530/REP-13-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaughlin EA, McIver SC. Awakening the oocyte: controlling primordial follicle development. Reproduction. 2009;137(1):1–11. doi: 10.1530/REP-08-0118. [DOI] [PubMed] [Google Scholar]
- 29.Merkwitz C, Lochhead P, Tsikolia N, Koch D, Sygnecka K, Sakurai M, et al. Expression of KIT in the ovary, and the role of somatic precursor cells. Prog Histochem Cytochem. 2011;46(3):131–84. doi: 10.1016/j.proghi.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Thomas FH, Vanderhyden BC. Oocyte-granulosa cell interactions during mouse follicular development: regulation of kit ligand expression and its role in oocyte growth. Reprod Biol Endocrinol. 2006;4:19. doi: 10.1186/1477-7827-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutt KJ, McLaughlin EA, Holland MK. KIT/KIT ligand in mammalian oogenesis and folliculogenesis: roles in rabbit and murine ovarian follicle activation and oocyte growth. Biol Reprod. 2006;75(3):421–33. doi: 10.1095/biolreprod.106.051516. [DOI] [PubMed] [Google Scholar]
- 32.Hutt KJ, McLaughlin EA, Holland MK. Kit ligand and c-Kit have diverse roles during mammalian oogenesis and folliculogenesis. Mol Hum Reprod. 2006;12(2):61–9. doi: 10.1093/molehr/gal010. [DOI] [PubMed] [Google Scholar]
- 33.Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140(9):4262–71. doi: 10.1210/endo.140.9.6994. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida H, Takakura N, Kataoka H, Kunisada T, Okamura H, Nishikawa SI. Stepwise requirement of c-kit tyrosine kinase in mouse ovarian follicle development. Dev Biol. 1997;184(1):122–37. doi: 10.1006/dbio.1997.8503. [DOI] [PubMed] [Google Scholar]
- 35.Serve H, Hsu YC, Besmer P. Tyrosine residue 719 of the c-kit receptor is essential for binding of the P85 subunit of phosphatidylinositol (PI) 3-kinase and for c-kit-associated PI 3-kinase activity in COS-1 cells. J Biol Chem. 1994;269(8):6026–30. [PubMed] [Google Scholar]
- 36.John GB, Shidler MJ, Besmer P, Castrillon DH. Kit signaling via PI3K promotes ovarian follicle maturation but is dispensable for primordial follicle activation. Dev Biol. 2009;331(2):292–9. doi: 10.1016/j.ydbio.2009.05.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kissel H, Timokhina I, Hardy MP, Rothschild G, Tajima Y, Soares V, et al. Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J. 2000;19(6):1312–26. doi: 10.1093/emboj/19.6.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Risal S, Gorre N, Busayavalasa K, Li X, Shen Y, et al. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol. 2014;24(21):2501–8. doi: 10.1016/j.cub.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 39.Reddy P, Shen L, Ren C, Boman K, Lundin E, Ottander U, et al. Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev Biol. 2005;281(2):160–70. doi: 10.1016/j.ydbio.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411(6835):355–65. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 41.Liu K. Stem cell factor (SCF)-kit mediated phosphatidylinositol 3 (PI3) kinase signaling during mammalian oocyte growth and early follicular development. Front Biosci. 2006;11:126–35. doi: 10.2741/1785. [DOI] [PubMed] [Google Scholar]
- 42.Wehrle-Haller B, Weston JA. Soluble and cell-bound forms of steel factor activity play distinct roles in melanocyte precursor dispersal and survival on the lateral neural crest migration pathway. Development. 1995;121(3):731–42. doi: 10.1242/dev.121.3.731. [DOI] [PubMed] [Google Scholar]
- 43.Tajima Y, Moore MA, Soares V, Ono M, Kissel H, Besmer P. Consequences of exclusive expression in vivo of Kit-ligand lacking the major proteolytic cleavage site. Proc Natl Acad Sci U S A. 1998;95(20):11903–8. doi: 10.1073/pnas.95.20.11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ismail RS, Dube M, Vanderhyden BC. Hormonally regulated expression and alternative splicing of kit ligand may regulate kit-induced inhibition of meiosis in rat oocytes. Dev Biol. 1997;184(2):333–42. doi: 10.1006/dbio.1997.8531. [DOI] [PubMed] [Google Scholar]