Summary
Fibrodysplasia ossificans progressiva (FOP) is a rare disease characterized by progressive ossification of soft tissues, for which there is no effective treatment. Mutations in the bone morphogenetic protein (BMP) type I receptor activin receptor-like kinase 2 (ACVR1/ALK2) are the main cause of FOP. We generated human induced pluripotent stem cells (hiPSCs) from FOP patients with the ALK2 R206H mutation. The mutant ALK2 gene changed differentiation efficiencies of hiPSCs into FOP bone-forming progenitors: endothelial cells (ECs) and pericytes. ECs from FOP hiPSCs showed reduced expression of vascular endothelial growth factor receptor 2 and could transform into mesenchymal cells through endothelial-mesenchymal transition. Increased mineralization of pericytes from FOP hiPSCs could be partly inhibited by the ALK2 kinase inhibitor LDN-212854. Thus, differentiated FOP hiPSCs recapitulate some aspects of the disease phenotype in vitro, and they could be instrumental in further elucidating underlying mechanisms of FOP and development of therapeutic drug candidates.
Highlights
-
•
A pluripotent cell model of FOP was established
-
•
The generation and maintenance of FOP hiPSC-derived ECs were impaired
-
•
FOP hiPSC-derived pericytes demonstrated increased osteoblast differentiation
-
•
LDN-212854 partly blocks osteoblast differentiation of pericytes from FOP hiPSCs
In this article, ten Dijke and colleagues established hiPSCs from urine-derived cells of fibrodysplasia ossificans progressiva (FOP) patients. The mutant FOP gene ALK2 impaired the differentiation of hiPSC-derived endothelial cells and enhanced the differentiation of hiPSC-derived pericytes to osteoblasts. These hiPSCs will be a valuable resource for further mechanistic research and new drug identification for FOP treatment.
Introduction
Fibrodysplasia ossificans progressiva (FOP) is an autosomal dominant genetic disorder in which acute inflammation may trigger the formation of a second skeleton of heterotopic bone. Classic FOP is caused by gain-of-function mutation (617G > A; R206H) in the activin receptor-like kinase 2 (ACVR1/ALK2) gene, encoding the bone morphogenetic protein (BMP) type I receptor (Shore et al., 2006). Enhanced BMP signaling in patients with the ALK2 R206H mutation has been attributed to loss of inhibitory activity of the ALK2-interacting protein FK506-binding protein-12 (FKBP12) (Chaikuad et al., 2012, van Dinther et al., 2010). Previous publications indicated that Tie2+ endothelial cells (ECs) and mesenchymal cells (MCs) contributed as progenitor cells to the episodic heterotopic ossification (HO) in FOP (Medici et al., 2010, Wosczyna et al., 2012). Other cells like circulating osteogenic precursors, skeletal myoblasts, and vascular smooth muscle cells also were found in FOP lesions and may contribute to HO in FOP (Hegyi et al., 2003, Lounev et al., 2009, Suda et al., 2009).
Despite recent advances in understanding of the disease (Hatsell et al., 2015), to date there is no cure or even treatment for HO in FOP. A comprehensive understanding of the molecular mechanisms underlying abnormal behavior of bone-forming progenitor cells in FOP could be one approach toward effective treatment for HO in FOP, and to other more prevalent situations with HO that, for example, may occur after traumatic accidents or deep tissue burns. The traditional way of obtaining human biopsy tissues from FOP patients is limited as physical and surgical injury can induce HO. New protocols to produce well-characterized FOP bone-forming progenitor cells for research and therapeutic drug screening are needed. The ability to generate human induced pluripotent stem cells (hiPSCs) (Takahashi et al., 2007) from adult tissues provides new opportunities for research on FOP. If derived from patients with genetic disease, hiPSCs allow production of large numbers of diseased target cells for basic research and drug development since they are immortal and pluripotent (Sterneckert et al., 2014).
In this study, we generated FOP hiPSCs from kidney cells isolated from urine by episomal vectors. The expression of pluripotent markers and ability to form derivatives of the three germ layers were comparable in FOP and control hiPSCs. However, the mutation in ALK2 reduced the efficiency of differentiation of hiPSCs into ECs and affected the phenotypes of ECs and pericytes. The hiPSC-derived ECs (hiPSC-ECs) from FOP patients exhibited reduced expression of vascular endothelial growth factor receptor 2 (VEGFR2) and could be transformed into MCs through endothelial-mesenchymal transition (EndMT). The hiPSC-derived pericytes (hiPSC-pericytes) from the FOP group showed increased ability to mineralize compared with the control. Our experiments demonstrated that disease-relevant cells differentiated from FOP hiPSCs possessed phenotypes reminiscent of the FOP disease.
Results
Generation of FOP hiPSCs from Urine Cells
We used a rapid and non-invasive procedure to isolate kidney cells in urine from FOP patients (Xue et al., 2013). The cells were isolated from 50–100 ml middle stream of the micturition from two male FOP patients (Dutch and Chinese, F2 and F3) diagnosed with the classic R206H mutation and two healthy male donors (Dutch and Chinese, C2 and C3) (Figure S1B).
A schematic representation of hiPSC generation is shown in Figure S1A. In summary, cultured cells from urine were electroporated with episomal vectors containing OCT4, SOX2, KLF4, and the pCEP4-miR-302-367 cluster (containing miR-302b, c, a, d, and miR-367) (Xue et al., 2013). Transfected urine cells were maintained in serum-free mTesR1 medium supplemented with a cocktail of small molecule inhibitors to promote reprogramming: CHIR99021, PD0325901, A83-01, and thiazovivin (Wang et al., 2013). Small colonies of cells appeared that progressively adopted a human embryonic stem cell (hESC)-like morphology. Selected hiPSCs were picked manually and expanded at day 20; hiPSCs maintained their hESC-like morphology with prominent nuclei and little cytoplasm and stained positive for alkaline phosphatase (ALP) (Figure S1B).
The hiPSCs from one healthy donor (C3) and from two FOP patients (F2 and F3) were characterized; the other control hiPSC line (UE017C1) was obtained from the Guangzhou Stem Cell Bank produced by the same method and was characterized previously (Xue et al., 2013). The presence of classical mutation in the ALK2 gene was confirmed in FOP hiPSCs (Figure S1C). FOP and control hiPSC karyotypes were checked before passage 10 and these were normal (Figure S1D). The loss of exogenous reprogramming factors and episomal backbones was demonstrated by genomic PCR that specifically amplifies exogenous factors (Figure S1E). The quantitative real-time PCR analysis revealed that, compared to urine cells, FOP hiPSCs had upregulated expression of endogenous hESC transcriptional genes (endogenous OCT4, endogenous SOX2, NANOG, and REX1), and they had expression levels comparable with established H1 hESCs (Figure S1F). Immunofluorescence microscopy showed expression of pluripotency-associated antigens OCT4, SSEA-4, TRA-1-60, and TRA-1-81 (Figure S1G). In addition, hiPSCs formed teratomas in mice in vivo, confirming the pluripotency of FOP iPSCs (Figure S1H). Therefore, hiPSCs from FOP patients corresponded phenotypically and functionally to hESCs.
Impaired EC Differentiation of FOP hiPSCs
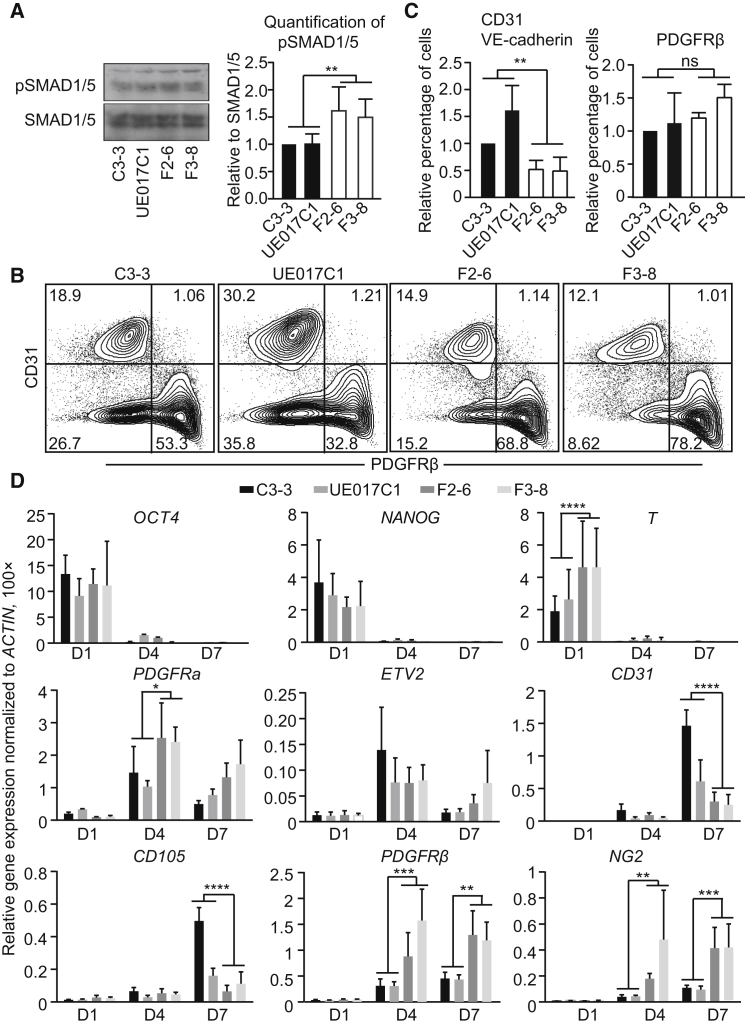
A slight elevation of pSMAD1/5 signaling in FOP hiPSCs compared to control hiPSCs was observed when these cells were cultured for 24 hr in medium with low serum concentrations, but not in the undifferentiated FOP iPSCs (Figures 1A and S1I). We differentiated hiPSCs into ECs and pericytes to examine how minor changes in ALK2 R206H/SMAD signaling influenced the fate of two possible progenitor cells of FOP, i.e., ECs and pericytes. On days 10–12 of differentiation, flow cytometry (fluorescence-activated cell sorting [FACS]) analysis showed that two cell populations formed as follows: CD31+ ECs and platelet-derived growth factor receptor (PDGFR) β+ pericytes (Figure 1B). The generation of CD31+/VE-cadherin+ cells was significantly impaired in FOP hiPSCs compared with controls, while general mesoderm induction was slightly enhanced in FOP hiPSCs (Figures 1B and 1C).
Figure 1.
Differentiation of FOP hiPSCs into ECs and Pericytes
(A) Total SMAD1/5 and phospho-SMAD1/5 (pSMAD1/5) level in control hiPSCs (C3-3 and UE017C1) and FOP hiPSCs (F2-6 and F3-8). Note that the antibodies used here also may recognize SMAD8 and pSMAD8 bands.
(B) FACS analysis of EC marker (CD31) or pericyte marker (PDGFRβ) expression at differentiation days 10–12 is shown.
(C) Quantification of the FACS analysis data for relative percentage of CD31 and VE-cadherin double-positive ECs and PDGFRβ-positive pericytes. All values were adjusted to the control colony C3-3, which is defined as 1.
(D) Relative gene expression at different time points during the differentiation. ACTIN was used to normalize gene expression.
Data are presented as mean and SD from three independent experiments in (A), (C), and (D).
To verify the FACS data, we examined the expression of early mesoderm and EC-specific genes (Figure 1D). Differentiation resulted in efficient downregulation of pluripotent markers (OCT4 and NANOG) in the control and FOP hiPSCs. Primitive streak/mesoderm lineage markers (T and PDGFRα) were upregulated in FOP hiPSCs on day 4 of differentiation, which may due to the positive effect of the BMP-signaling pathway on mesoderm formation. The induction of the early endothelial transcription factor (ETV2) on day 4 was similar between the two groups. However, consistent with the FACS data, we observed that endothelial-specific genes (CD31 and CD105) were downregulated in FOP hiPSCs on day 7 of differentiation. Early pericyte markers PDGFRβ and NG2 proteoglycan were expressed more abundantly in differentiating FOP hiPSCs. Overall, we observed that the EC differentiation efficiency was impaired while general mesoderm differentiation was enhanced in FOP hiPSCs; this difference may due to the elevated level of ALK2 R206H/SMAD signaling in FOP compared to control hiPSCs.
Characterization of FOP hiPSC-ECs
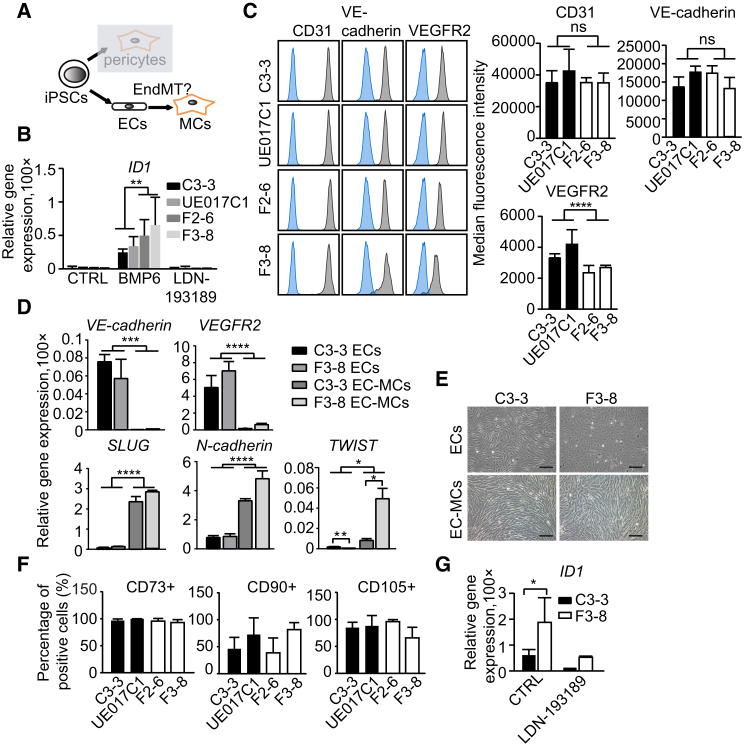
Differentiated cell populations were divided into CD31+ ECs and CD31− cells by CD31 antibody-coupled magnetic bead sorting (Figure 2A). The expression levels of BMP type I receptors ALK1 and ALK2 were not different in control versus FOP CD31+ cells (Figure S2A). Sorted FOP hiPSC-ECs were more sensitive to low concentrations of BMP6 (5 ng/ml) and the activated BMP signaling could be inhibited by BMP type I receptor kinase inhibitor LDN-193189 (Yu et al., 2008; Figure 2B). FOP hiPSC-ECs exhibited poor viability and increased expression of senescence-associated β-galactosidase expression compared to controls (Figures S2B and S2C). As the VEGF-signaling pathway is known to regulate survival and proliferation of ECs through VEGFR2 (Kelly and Hirschi, 2009), we found that the expression of VEGFR2 was lower in FOP hiPSC-ECs compared with the control by FACS analysis, while the expression levels of other EC genes (CD31 and VE-cadherin) were unchanged (Figure 2C). The lower expression of VEGFR2 may be responsible for the failure of FOP hiPSC-ECs to propagate in vitro, and this may be related to the activated BMP/SMAD signaling in these cells (Figure 2B).
Figure 2.
ECs Derived from hiPSCs Undergo EndMT
(A) Schematic representation shows EC and pericyte derivation from hiPSCs.
(B) Analysis of relative expression of ID1 in hiPSC-ECs is shown.
(C) (Left) Representative FACS analysis for EC markers in hiPSC-ECs is shown. Blue, unstained cells; gray, antibody as indicated. (Right) Median fluorescence intensity of EC markers expression in hiPSC-ECs is shown.
(D) Relative gene expression analysis of EC and EndMT markers in ECs and EC-MCs is shown.
(E) Bright-field images of ECs and EC-MCs in the healthy donor (C3-3) and FOP patient (F3-8) are shown. Scale bar, 100 μm.
(F) Percentages of CD73-, CD90-, and CD105-positive cells in EC-MCs. Representative FACS plots of CD73, CD90, and CD105 are shown in Figure S2E.
(G) Relative expression of ID1 in EC-MCs is shown.
Data are presented as mean and SD from three independent experiments in (B), (C), (D), (F), and (G). The quantitative real-time PCR results in (B), (D), and (G) were normalized to ACTIN. See also Figure S2.
Moreover, we observed that ECs cultured in EGM-2 medium with 2% serum showed increased expression of EndMT markers (SLUG, N-cadherin, and TWIST; Figure 2D) and lost the expression of endothelial-specific markers confirmed by quantitative real-time PCR (VE-cadherin and VEGFR2; Figure 2D) and FACS analysis (CD31, VE-cadherin, and VEGFR2; Figure S2D). ECs turned to mesenchymal-like cells (EC-MCs) in EGM2 medium and the expression of mesenchymal markers CD73, CD90, and CD105 also were induced (Figures 2E, 2F, and S2E). BMP signaling also was activated at an elevated level in FOP EC-MCs, as demonstrated by increased ID1 expression (Figure 2G), a direct BMP/SMAD target gene (Korchynskyi and ten Dijke, 2002). Therefore, increased BMP signaling in FOP hiPSC-ECs correlates with impaired EC viability, increased senescence, and increased EndMT.
Activated BMP Signaling in FOP hiPSC-Pericytes
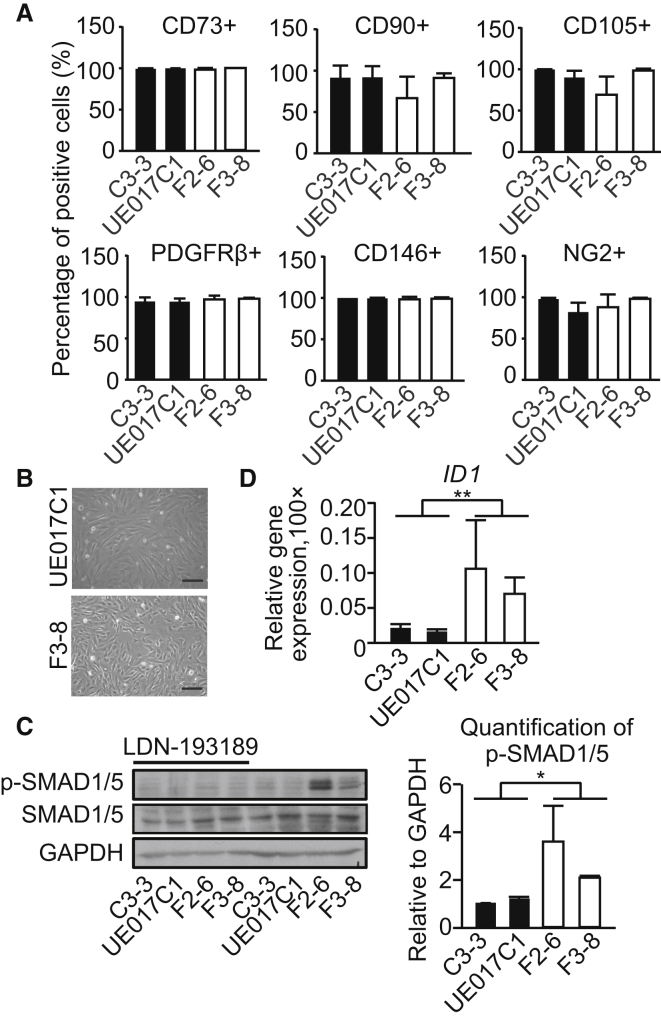
The selected CD31− cells were cultured in DMEM with 10% fetal bovine serum (FBS) or DMEM with 10% FBS supplemented with PDGF-BB and transforming growth factor (TGF) β3 for 1 day. The homogenous population expressed pericytes and MC markers (CD73, CD90, CD105, PDGFRβ, CD146, and NG2; Figures 3A, 3B, and S3A). SMAD1/5 phosphorylation was increased in the FOP group in low-serum conditions, and this could be inhibited by LDN-193189 (Figure 3C). The mRNA expression level of ALK2 was not different in control and FOP groups (Figure S3B). The SMAD1/5 downstream target gene ID1 also was significantly upregulated in the FOP group (Figure 3D). Thus, FOP hiPSC-pericytes exhibited elevated SMAD1/5 levels.
Figure 3.
Activated BMP Signaling in FOP hiPSC-Pericytes
(A) Percentages of CD73-, CD90-, CD105-, PDGFRβ-, CD146-, and NG2-positive cells in hiPSC-pericytes. Representative FACS plots of CD73, CD90, CD105, PDGFRβ, CD146, and NG2 are shown in Figure S3A.
(B) Bright-field images of pericytes from healthy donor (UE017C1) and FOP patient (F3-8) are shown. Scale bar, 100 μm.
(C) Western blot results showing SMAD1/5 phosphorylation in the hiPSC-pericytes. GAPDH was used as the loading control.
(D) Relative expression of ID1. All experiments were normalized to ACTIN.
Data are presented as mean and SD from three independent experiments in (C) and (D); the average of four independent experiments is shown in (A). See also Figure S3.
FOP hiPSC-Pericyte Mineralization as an Assay to Identify New ALK2 Inhibitors
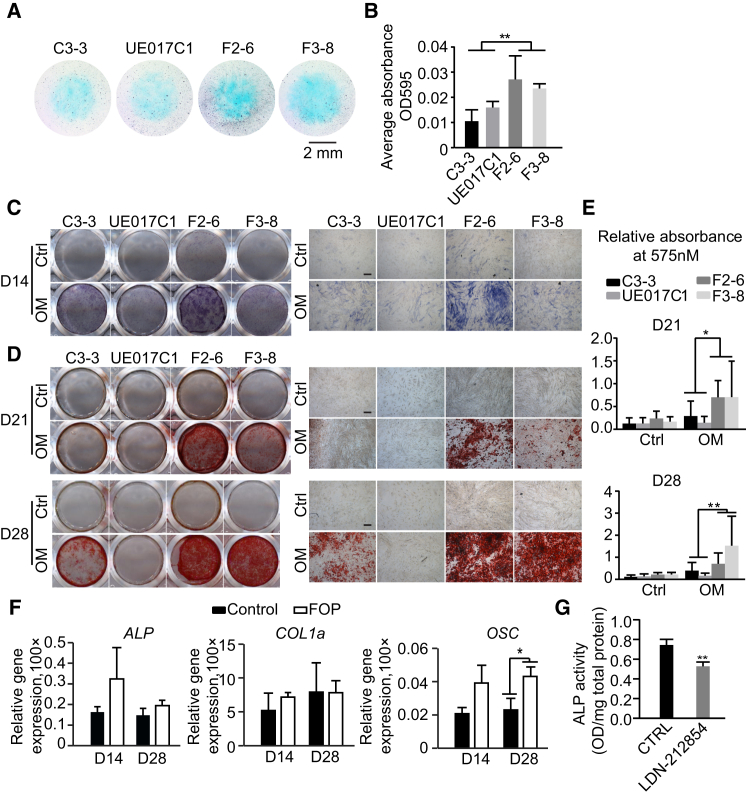
Heterotopic bone formation in FOP follows the progressive heterotopic endochondral ossification in soft connective tissues, a process in which condensing mesenchymal progenitor cells differentiate into chondrocytes and are eventually mineralized into osteoblasts and bone (Mackie et al., 2011). We examined the effect of ALK2 R206H mutation on chondrogenic differentiation by differentiating hiPSC-pericytes in the two-dimensional micromass culture system without the addition of growth factors. The hiPSC-pericytes were stained with Alcian blue for early differentiation and extracellular matrix production, such as glycosaminoglycans (GAGs) and sulfated GAGs. After 3 days of chondrocytic differentiation, Alcian blue staining indicated that FOP hiPSC-pericytes have more GAG expression than control hiPSC-pericytes (Figures 4A and 4B).
Figure 4.
Increased Mineralization of FOP hiPSC-Pericytes
(A) Representative Alcian blue staining of the micromass for 3 days chondrogenic differentiation is shown. Scale bar, 2 mm.
(B) Alcian blue staining of micromasses was quantified by stain extraction and absorbance reading at 595 nm.
(C) ALP staining was performed 14 days (D14) after maintaining in α-MEM with 10% FBS (Ctrl) and osteogenic medium (OM). Results are shown as representative scanned images (left) and images taken at 4× magnification (right). Scale bar, 200 μm.
(D) The mineralization was visualized by 2% alizarin red S staining on day 21 (D21) and day 28 (D28). Results are shown as representative scanned images (left) and images taken at 4× magnification (right). Scale bar, 200 μm.
(E) Alizarin red S staining on D21 and D28 was quantified by stain extraction and absorbance reading at 570 nm.
(F) Relative gene expression analysis of ALP, COL1α, and OSC on day 14 and day 28 of osteoblast differentiation. All experiments were normalized to ACTIN. Control, the average of the results from C3-3 and UE017C1; FOP, the average of the results from F2-6 and F3-8.
(G) ALP assay on day 7 of F3-8 after maintaining in OM pretreated with LDN-212854 is shown.
Data are presented as mean and SD from four times independent experiments in (E) and three times in (B) and (G).
We further assessed the mineralization capability of FOP hiPSC-pericytes when grown in osteogenic medium. The osteoblast differentiation of pericytes was measured by determining ALP activity, an early marker of osteoblast differentiation. Histochemical staining revealed that ALP activity in the FOP group was enhanced (Figure 4C); F2-6 hiPSC-pericytes showed higher pSMAD1/5 signaling compared to F3-8 (Figures 3C and 3D), which may explain the stronger ALP activity in F2-6. Furthermore, we analyzed osteoblast differentiation by alizarin red S staining to detect calcium deposition. On day 21 and day 28 of differentiation, we detected higher mineralization in the FOP group (Figures 4D and 4E). Consistent with the ALP activity and alizarin red S staining, quantitative real-time PCR analysis confirmed that expression of osteoblast markers ALP and OSTEOCALCIN (OSC) (Figure 4F) was increased in the FOP group, but we did not detect altered expression of COLLAGEN type I alpha 1 (COL1α) in FOP and control cells (Figure 4F). Together these data indicate that FOP hiPSC-pericytes were prone to mineralization, which may be due to activated BMP/SMAD signal in these cells. After pretreating pericytes with LDN-212854, a BMP type I receptor kinase inhibitor that is more selective for ALK2 compared with other BMP type I receptors (Mohedas et al., 2013), we observed that the ALP activity of FOP hiPSC-pericytes was partly inhibited (Figure 4G).
Discussion
Our results demonstrated that hiPSCs could be used as an in vitro disease model for FOP. The hiPSC-ECs and hiPSC-pericytes can be applied to investigate the molecular mechanisms underlying the pathology of FOP, as well as to identify new therapeutic drugs.
We have shown that FOP hiPSCs could be established from cells in urine using non-integrating episomal vectors. BMP signaling contributes to the initial stage of iPSC reprogramming in mouse (Samavarchi-Tehrani et al., 2010), but induces differentiation of hESCs (Xu et al., 2002). Consistent with prior research (Matsumoto et al., 2013), our FOP hiPSCs were generated without the addition of exogenous BMP inhibitors during the reprogramming process. We did not observe activated SMAD1/5 signaling in FOP hiPSCs maintained in hESC medium mTeSR1 unless these cells were placed in differentiation culture conditions. This may explain why FOP hiPSCs could be maintained and passaged in defined medium. Besides, we observed heterogeneous BMP/SMAD signaling and mineralization in FOP hiPSCs and derivative cells, indicating that intrinsic genetic and/or epigenetic features of donor cells may influence properties of hiPSCs and the progeny. To eliminate the heterogeneity, the isogenic correction FOP hiPSCs can help to facilitate more stringent screening for effects arising from clonal variations in hiPSCs (Matsumoto et al., 2015).
We found that the generation and maintenance of ECs from FOP hiPSCs were impaired. One explanation is the elevated BMP signaling in FOP hiPSC-ECs, which resulted in downregulation of VEGFR2 expression that mediated VEGF-induced proliferation and survival of ECs; this may have caused the decrease in EC viability. Inhibition of BMP receptor kinase activity by LDN-212854 during the vascular specification stage (from day 3 of hiPSC differentiation) did not rescue the impaired FOP hiPSC-EC phenotypes (unpublished data). BMPs are indispensable for the formation of mesoderm where ECs originate, but they may function as a context-dependent regulator in vascular morphogenesis (Kim et al., 2014). In addition, a recent publication indicated activin A signals through the mutant ALK2 R206H to stimulate HO in FOP conditional-on knockin mice (Hatsell et al., 2015). Of note, in our EC differentiation protocol, we used activin A and BMP4, both of which were shown to induce SMAD1/5 signaling through mutant ALK2 R206H (Hatsell et al., 2015). These ligands may thus combine with the SMAD1/5 signal to contribute to the FOP hiPSC-EC phenotypes that we observed. The other explanation of low EC yields is the increased EndoMT in ALK2-mutated ECs. Consistent with a previous publication (Medici et al., 2010), FOP EC-MCs showed higher expression of EndMT markers (N-cadherin and TWIST), which might be due to the interaction of mutant ALK2 R206H and VEGF signaling in these cells. Further investigation of the crosstalk between BMP signaling and VEGF signaling might contribute to the better understanding of the EndMT mechanism in FOP lesions and also help in identifying new drug-treatable targets to prevent HO in FOP patients.
Lastly, we demonstrated that the mutant ALK2 R206H contributed to the increased mineralization of FOP hiPSC-pericytes and, as such, is a useful human in vitro disease model for identifying and evaluating the bioactivity of ALK2 inhibitors. More evidence indicates that MCs are the major contributors of HO (Wosczyna et al., 2012), while ECs indirectly contribute to osteogenic differentiation by acting in a paracrine manner via crosstalk between ECs and MCs (Bidarra et al., 2011, Lin et al., 2014). Even though Matsumoto et al. partly exhibited FOP phenotypes by directly differentiating hiPSCs into osteoblast cells (Matsumoto et al., 2013), they provided little evidence of how the specific cell types contributed to the increased mineralization. To further clarify the mineralization capacity of FOP hiPSCs in vitro, we differentiated hiPSCs into pericytes. ALP activity of FOP hiPSC-pericytes can be inhibited by pretreating with BMP inhibitor LDN-212854. As the ALP assay has been used as a high-throughput screening (HTS) readout for screening regulators in osteogenic differentiation (Alves et al., 2011), our platform could be used for drug screening and further verifying the bioactivity of ALK2 inhibitors in the future.
Even though in this study we only studied ECs and pericytes, the two most well-known HO progenitor cells in FOP, it is possible other unidentified cells types also may be involved in the HO process in FOP. Due to the pluripotent characterization of hiPSCs, FOP hiPSCs can be differentiated into other (unknown) progenitor cells to discover the underlying molecular mechanisms of HO in the future.
Experimental Procedures
Primary human cells were obtained with informed consent. Experiments involving human subjects were approved by Institutional Review Board (IRB) GIBH-IRB02-2009002 at Guangzhou Institutes of Biomedicine and Health (GIBH) and 12/467 (2013 January) at VU University Medical Center. The animal research was approved by the IRB at GIBH (2010012). The generation and differentiation of hiPSCs were described previously (Orlova et al., 2014, Xue et al., 2013). Differences between the control group and the FOP group were evaluated by t test or one-way ANOVA with Tukey’s multiple comparison tests (ns, not significant; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001). For further information, see the Supplemental Experimental Procedures.
Author Contributions
J.C., V.V.O., X.C., G.P., and P.t.D. planned the experiments. E.M.W.E. and K.Z. provided patients materials. J.C. and V.V.O. performed research and acquired data. J.C. analyzed data and wrote the paper. D.P., C.L.M., G.P., and P.t.D. supervised the project.
Acknowledgments
We would like to acknowledge Keyu Lai for performing karyotype of hiPSCs, Jian Zhang for performing teratoma injection, Dr. Ke Ding and Dr. Paul Yu for providing reagents, Dr. Amaya García de Vinuesa for helpful suggestions on chondrogenic differentiation, Dr. Gerard Pals for help with urine collection from Dutch patients, and Francijna E.van den Hil for help with the preparation of EC differentiation and EC sorting. This work was supported by the LeDucq Foundation, the China Exchange Programme (CEP) of the Royal Netherlands Academy of Arts and Sciences (KNAW), and the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement 602423: Plurimes.
Published: November 25, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures and three figures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.10.020.
Supplemental Information
References
- Alves H., Dechering K., Van Blitterswijk C., De Boer J. High-throughput assay for the identification of compounds regulating osteogenic differentiation of human mesenchymal stromal cells. PLoS ONE. 2011;6:e26678. doi: 10.1371/journal.pone.0026678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidarra S.J., Barrias C.C., Barbosa M.A., Soares R., Amédée J., Granja P.L. Phenotypic and proliferative modulation of human mesenchymal stem cells via crosstalk with endothelial cells. Stem Cell Res. 2011;7:186–197. doi: 10.1016/j.scr.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Chaikuad A., Alfano I., Kerr G., Sanvitale C.E., Boergermann J.H., Triffitt J.T., von Delft F., Knapp S., Knaus P., Bullock A.N. Structure of the bone morphogenetic protein receptor ALK2 and implications for fibrodysplasia ossificans progressiva. J. Biol. Chem. 2012;287:36990–36998. doi: 10.1074/jbc.M112.365932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsell S.J., Idone V., Wolken D.M., Huang L., Kim H.J., Wang L., Wen X., Nannuru K.C., Jimenez J., Xie L. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci. Transl. Med. 2015;7:303ra137. doi: 10.1126/scitranslmed.aac4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegyi L., Gannon F.H., Glaser D.L., Shore E.M., Kaplan F.S., Shanahan C.M. Stromal cells of fibrodysplasia ossificans progressiva lesions express smooth muscle lineage markers and the osteogenic transcription factor Runx2/Cbfa-1: clues to a vascular origin of heterotopic ossification? J. Pathol. 2003;201:141–148. doi: 10.1002/path.1413. [DOI] [PubMed] [Google Scholar]
- Kelly M.A., Hirschi K.K. Signaling hierarchy regulating human endothelial cell development. Arterioscler. Thromb. Vasc. Biol. 2009;29:718–724. doi: 10.1161/ATVBAHA.109.184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.D., Lee H.W., Jin S.W. Diversity is in my veins: role of bone morphogenetic protein signaling during venous morphogenesis in zebrafish illustrates the heterogeneity within endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2014;34:1838–1845. doi: 10.1161/ATVBAHA.114.303219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchynskyi O., ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J. Biol. Chem. 2002;277:4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- Lin R.Z., Moreno-Luna R., Li D., Jaminet S.C., Greene A.K., Melero-Martin J.M. Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proc. Natl. Acad. Sci. USA. 2014;111:10137–10142. doi: 10.1073/pnas.1405388111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounev V.Y., Ramachandran R., Wosczyna M.N., Yamamoto M., Maidment A.D., Shore E.M., Glaser D.L., Goldhamer D.J., Kaplan F.S. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J. Bone Joint Surg. Am. 2009;91:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie E.J., Tatarczuch L., Mirams M. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J. Endocrinol. 2011;211:109–121. doi: 10.1530/JOE-11-0048. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Hayashi Y., Schlieve C.R., Ikeya M., Kim H., Nguyen T.D., Sami S., Baba S., Barruet E., Nasu A. Induced pluripotent stem cells from patients with human fibrodysplasia ossificans progressiva show increased mineralization and cartilage formation. Orphanet J. Rare Dis. 2013;8:190. doi: 10.1186/1750-1172-8-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Ikeya M., Hino K., Horigome K., Fukuta M., Watanabe M., Nagata S., Yamamoto T., Otsuka T., Toguchida J. New Protocol to Optimize iPS Cells for Genome Analysis of Fibrodysplasia Ossificans Progressiva. Stem Cells. 2015;33:1730–1742. doi: 10.1002/stem.1981. [DOI] [PubMed] [Google Scholar]
- Medici D., Shore E.M., Lounev V.Y., Kaplan F.S., Kalluri R., Olsen B.R. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat. Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohedas A.H., Xing X., Armstrong K.A., Bullock A.N., Cuny G.D., Yu P.B. Development of an ALK2-biased BMP type I receptor kinase inhibitor. ACS Chem. Biol. 2013;8:1291–1302. doi: 10.1021/cb300655w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova V.V., Drabsch Y., Freund C., Petrus-Reurer S., van den Hil F.E., Muenthaisong S., Dijke P.T., Mummery C.L. Functionality of endothelial cells and pericytes from human pluripotent stem cells demonstrated in cultured vascular plexus and zebrafish xenografts. Arterioscler. Thromb. Vasc. Biol. 2014;34:177–186. doi: 10.1161/ATVBAHA.113.302598. [DOI] [PubMed] [Google Scholar]
- Samavarchi-Tehrani P., Golipour A., David L., Sung H.K., Beyer T.A., Datti A., Woltjen K., Nagy A., Wrana J.L. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell. 2010;7:64–77. doi: 10.1016/j.stem.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Shore E.M., Xu M., Feldman G.J., Fenstermacher D.A., Cho T.J., Choi I.H., Connor J.M., Delai P., Glaser D.L., LeMerrer M. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- Sterneckert J.L., Reinhardt P., Schöler H.R. Investigating human disease using stem cell models. Nat. Rev. Genet. 2014;15:625–639. doi: 10.1038/nrg3764. [DOI] [PubMed] [Google Scholar]
- Suda R.K., Billings P.C., Egan K.P., Kim J.H., McCarrick-Walmsley R., Glaser D.L., Porter D.L., Shore E.M., Pignolo R.J. Circulating osteogenic precursor cells in heterotopic bone formation. Stem Cells. 2009;27:2209–2219. doi: 10.1002/stem.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- van Dinther M., Visser N., de Gorter D.J., Doorn J., Goumans M.J., de Boer J., ten Dijke P. ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation. J. Bone Miner. Res. 2010;25:1208–1215. doi: 10.1359/jbmr.091110. [DOI] [PubMed] [Google Scholar]
- Wang L., Wang L., Huang W., Su H., Xue Y., Su Z., Liao B., Wang H., Bao X., Qin D. Generation of integration-free neural progenitor cells from cells in human urine. Nat. Methods. 2013;10:84–89. doi: 10.1038/nmeth.2283. [DOI] [PubMed] [Google Scholar]
- Wosczyna M.N., Biswas A.A., Cogswell C.A., Goldhamer D.J. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J. Bone Miner. Res. 2012;27:1004–1017. doi: 10.1002/jbmr.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R.H., Chen X., Li D.S., Li R., Addicks G.C., Glennon C., Zwaka T.P., Thomson J.A. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat. Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- Xue Y., Cai X., Wang L., Liao B., Zhang H., Shan Y., Chen Q., Zhou T., Li X., Hou J. Generating a non-integrating human induced pluripotent stem cell bank from urine-derived cells. PLoS ONE. 2013;8:e70573. doi: 10.1371/journal.pone.0070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P.B., Deng D.Y., Lai C.S., Hong C.C., Cuny G.D., Bouxsein M.L., Hong D.W., McManus P.M., Katagiri T., Sachidanandan C. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat. Med. 2008;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.