Abstract
Glycan structures are synthesized by a series of reactions conducted by glycosylation-related (GR) proteins such as glycosyltransferases, glycan-modifying enzymes, and nucleotide-sugar transporters. For example, the common core region of glycosaminoglycans (GAGs) is sequentially synthesized by peptide-O-xylosyltransferase, β1,4-galactosyltransferase I, β1,3-galactosyltransferase II, and β1,3-glucuronyltransferase. This raises the possibility that functional impairment of GR proteins involved in synthesis of the same glycan might result in the same phenotypic abnormality. To examine this possibility, comprehensive silencing of genes encoding GR and proteoglycan core proteins was conducted in Drosophila. Drosophila GR candidate genes (125) were classified into five functional groups for synthesis of GAGs, N-linked, O-linked, Notch-related, and unknown glycans. Spatiotemporally regulated silencing caused a range of malformed phenotypes that fell into three types: extra veins, thick veins, and depigmentation. The clustered phenotypes reflected the biosynthetic pathways of GAGs, Fringe-dependent glycan on Notch, and glycans placed at or near nonreducing ends (herein termed terminal domains of glycans). Based on the phenotypic clustering, CG33145 was predicted to be involved in formation of terminal domains. Our further analysis showed that CG33145 exhibited galactosyltransferase activity in synthesis of terminal N-linked glycans. Phenotypic clustering, therefore, has potential for the functional prediction of novel GR genes.
Introduction
A wide variety of glycans play important roles in a diverse range of biological processes, such as organ development (Haltiwanger & Lowe 2004), lymphocyte homing (Carlow et al. 2009), and cancer invasion (Isaji et al. 2010), by regulating protein–protein, lipid–protein, and cell–cell interactions. Glycans are synthesized by sequential reactions conducted by glycosylation-related (GR) proteins such as glycosyltransferases, glycan-modifying enzymes, and nucleotide-sugar transporters (Nishihara 2007; Yamamoto-Hino et al. 2012). Accordingly, different glycan structures are synthesized by different sets of GR proteins. Thus, it is likely that mutation of GR genes involved in synthesis of the same glycans will result in the same phenotype. For example, glycosaminoglycans (GAG) are sequentially synthesized by peptide-O-xylosyltransferase, β1,4-galactosyltransferase I, β1,3-galactosyltransferase II, and β1,3-glucuronyltransferase (Nishihara 2010; Mikami & Kitagawa 2013). Mutations of these GAG synthesizing enzymes principally impair the same developmental pathways, namely those regulated by decapentaplegic, wingless, hedgehog, and fibroblast growth factor in Drosophila (Haltiwanger & Lowe 2004; Nishihara 2010; Yamamoto-Hino et al. 2012). However, because complete sets of GR gene mutants are not available in metazoa, no comprehensive examination has yet been undertaken to determine whether impairment of GR genes involved in synthesis of the same glycans results in the same phenotypes.
It is possible to silence almost all the genes in Drosophila and Caenorhabditis elegans by RNA interference (RNAi) (Yamamoto-Hino & Goto 2013). In particular, spatiotemporally regulated gene silencing is possible in Drosophila when it is implemented using the Gal4/upstream activation sequence (UAS) system (Brand & Perrimon 1993). In this system, the yeast Gal4 transcription factor binds to the UAS and activates expression of the downstream gene; theoretically, the gene downstream of the UAS is not expressed in the absence of Gal4. Consequently, a genetic cross between UAS- and Gal4-fly strains will induce expression of the gene downstream of the UAS. By placing genes expressing hairpin RNAs downstream of a UAS, RNAi is readily induced by genetic crossing. In addition, there are a large number of Gal4 strains in which the Gal4 gene is conditionally expressed, such as in a specific tissue, at a particular developmental stage, or under specific temperature conditions (Hayashi et al. 2002). Therefore, spatiotemporal patterns and levels of expression of hairpin RNAs can be controlled by the Gal4 strains and temperature conditions used.
In this study, we determined 120 Drosophila GR genes and five core proteins by sequence similarity searches and literature mining. Of these GR genes, 72 were silenced in the whole body. Silencing of 56 of these genes resulted in lethality before eclosion. Thus, it was not possible to assess phenotypic clustering of essential GR genes when genes were silenced in the whole organism. To overcome this difficulty, spatiotemporally regulated gene silencing was carried out using several Gal4 driver strains. The induced phenotypes were linked to the biosynthetic pathways of GAGs, Fringe-dependent glycan on Notch, and terminal domains of glycans. Based on this phenotypic clustering, the functionally unknown gene CG33145 was predicted to be involved in the synthesis of terminal domains. Our biochemical analysis provided direct evidence that CG33145 functioned as a novel galactosyltransferase in terminal N-linked glycan synthesis. In summary, phenotypic clustering in this study proved useful for functional prediction of novel GR genes.
Results
Drosophila GR genes
Drosophila GR genes (67) were identified through similarity to human glycosylation genes using the human GlycoGene DataBase (http://jcggdb.jp/rcmg/ggdb/). The Drosophila GR gene set comprised 54 glycosyltransferases, seven glycan-modifying enzymes, and six nucleotide-sugar transporters. In addition, we manually identified Drosophila genes encoding 44 glycosyltransferases, eight glycan-modifying enzymes, one sugar-nucleotide transporter, and five core proteins from literature searches. In total, 98 glycosyltransferases, fifteen glycan-modifying enzymes, seven sugar-nucleotide transporters, and five core protein genes were identified (Table1). Based on biochemical activities that were directly measured or predicted from homologous mammalian genes, 108 of these 125 GR proteins could be assigned to the following categories: formation of sugar linkages, modification of glycans, or core proteins (Fig.1, Table1).
Table 1.
Drosophila GR genes
| Family of proteins/protein name | Protein/gene name | CG No. | References | Glycan structure | Mammalian orthologue |
|---|---|---|---|---|---|
| Glycosyltransferase | |||||
| N-acetylgalactosaminyltransferase | |||||
| UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase | pgant1/GalNAc-T1 | CG8182 | Ten Hagen et al. 2003 | Mucin-type O-glycan | |
| pgant2 | CG3254 | Ten Hagen et al. 2003 | Mucin-type O-glycan | GALNT2 | |
| pgant3 | CG4445 | Ten Hagen et al. 2003 | Mucin-type O-glycan | ||
| pgant4 | CG31956 | Ten Hagen et al. 2003 | Mucin-type O-glycan | ||
| pgant5 | CG31651 | Ten Hagen et al. 2003 | Mucin-type O-glycan | GALNT5 | |
| pgant6 | CG2103 | Ten Hagen et al. 2003 | Mucin-type O-glycan | GALNT1 | |
| pgant7/GalNAc-T2 | CG6394 | Schwientek et al. 2002; Ten Hagen et al. 2003 | Mucin-type O-glycan | GALNT7 | |
| pgant8 | CG7297 | Ten Hagen et al. 2003 | Mucin-type O-glycan | ||
| pgant35A | CG7480 | Schwientek et al. 2002; Ten Hagen et al. 2003 | Mucin-type O-glycan | GALNT11 | |
| dppGalNAcT9 | CG30463 | ND | Mucin-type O-glycan | GALNT3 | |
| dppGalNAcT10 | CG10000 | ND | Mucin-type O-glycan | ||
| CG31776 | ND | Mucin-type O-glycan | |||
| dppGalNAcT11 | CG7304 | ND | Mucin-type O-glycan | ||
| dppGalNAcT12 | CG7579 | ND | Mucin-type O-glycan | ||
| α1,4-N-acetylgalactosaminyltransferase | α4GT1 | CG17223 | Mucha et al. 2004 | Glycolipid | A4GALT |
| α4GT2 | CG5878 | Chen et al. 2007 | Glycolipid | ||
| β1,4-N-acetylgalactosaminyltransferase | β4GalNAcTA | CG8536 | Haines & Irvine 2005; Chen et al. 2007; Sasaki et al. 2007 | Glycolipid, N-glycan | B4GALT2 |
| β4GalNAcTB | CG14517 | Haines & Irvine 2005; Chen et al. 2007 | Glycolipid | B4GALT3 | |
| N-acetylglucosaminyltransferase | |||||
| UDP-GlcNAc:polypeptide O-β-N-acetylglucosaminyltransferase | dO-GnT/Sxc | CG10392 | Sinclair et al. 2009* | O-GlcNAc | OGT |
| α3-D-mannoside-β1,2-N-acetylglucosaminyltransferase | dMGAT1/Mgat1 | CG13431 | Sarkar & Schachter 2001; Ichimiya et al. 2004 | N-glycan | MGAT1 |
| α6-D-mannoside-β1,2-N-acetylglucosaminyltransferase | dMGAT2/Mgat2 | CG7921 | Ichimiya et al. 2004 | N-glycan | MGAT2 |
| β4-D-mannoside-β1,4-N-acetylglucosaminyltransferase | dMGAT3 | CG31849 | ND | N-glycan | MGAT3 |
| α3-D-mannoside-β1,4-N-acetylglucosaminyltransferase | dMGAT4-1 | CG9384 | ND | N-glycan | MGAT4A |
| dMGAT4-2 | CG17173 | ND | N-glycan | MGAT4B | |
| i-β1,3-N-acetylglucosaminyltransferase | diβ3GnT1 | CG3253 | ND | Unknown | |
| diβ3GnT2 | CG9171 | ND | Unknown | ||
| diβ3GnT3 | CG15483 | ND | Unknown | ||
| diβ3GnT4 | CG11149 | ND | Unknown | ||
| diβ3GnT5 | CG9996 | ND | Unknown | ||
| diβ3GnT6 | CG11388 | ND | Unknown | ||
| β1,3-N-acetylglucosaminyltransferase | Brn | CG4934 | Muller et al. 2002 | Glycolipid | |
| Fng | CG10580 | Bruckner et al. 2000; Moloney et al. 2000 | Notch O-glycan | RFNG | |
| β1,3-N-acetylglucosaminyltransferase or | dβ3GnT or GalT1 | CG33145 | this study | N-glycan | |
| β1,3-galactosyltransferase* | dβ3GnT or GalT2 | CG11357 | ND | Unknown | |
| dβ3GnT or GalT3 | CG3038 | ND | Unknown | ||
| dβ3GnT or GalT4 | CG8668 | ND | Unknown | ||
| dβ3GnT or GalT5 | CG8673 | ND | Unknown | ||
| Dolichyl phosphate N-acetylglucosaminyltransferase | dAlg14 | CG6308 | ND | N-glycan | ALG14 |
| dAlg7 | CG5287 | ND | N-glycan | DPAGT1/ALG7 | |
| dAlg13 | CG14512 | ND | N-glycan | GLT28D1/ALG13 | |
| Chondroitin synthase | |||||
| Chondroitin synthase | dCHSY | CG9220 | ND | GAG (CS) | CHSY1 |
| Chondroitin polymerization factor | dCHPF | CG43313 | ND | GAG (CS) | CHPF |
| Chondroitin N-acetylgalactosaminyltransferase | dCSGalNAcT1 | CG12913 | ND | GAG (CS) | ChGn |
| Chitin synthase | Chitin Syn1/Kkv | CG2666 | ND | Chitin | |
| Chitin Syn2 | CG7464 | ND | Chitin | ||
| Fucosyltransferase | |||||
| α1,3/1,4-fucosyltransferase or | FucTA | CG6869 | Fabini et al. 2001 | N-glycan | |
| α1,3-fucosyltransferase* | FucTB | CG4435 | ND | Unknown | FUT1 |
| FucTD | CG9169 | ND | Unknown | ||
| FucTC | CG40305 | ND | Unknown | ||
| α1,6-fucosyltransferase | dα6Fut/FucT6 | CG2448 | Paschinger et al. 2005 | N-glycan | FUT8 |
| Protein O-fucosyltransferase | OFut1 | CG12366 | Okajima & Irvine 2002 | Notch | POFUT1 |
| OFut2 | CG14789 | Luo et al. 2006 | Thrombospondin | POFUT2 | |
| Galactosyltransferase | |||||
| GAGβ1,4-galactosy-ltransferase I | dGAGβ4GalTI/β4GalT7 | CG11780 | Nakamura et al. 2002; Vadaie et al. 2002; Takemae et al. 2003 | GAG (common) | B4GALT7 |
| GAGβ1,3-galactosyltransferase II | dGAGβ3GalTII | CG8734 | Ueyama et al. 2008 | GAG (common) | B3GALT6 |
| core1β1,3-galactosyltransferase | dC1GalT1/C1GalTA | CG9520 | Muller et al. 2005; Yoshida et al. 2008* | Mucin-type O-glycan | C1GALT1 |
| dC1GalT2 | CG8708 | Muller et al. 2005 | Mucin-type O-glycan | ||
| dC1GalT3 | CG18558 | ND | Mucin-type O-glycan | ||
| dC1GalT4 | CG2975 | Muller et al. 2005 | Mucin-type O-glycan | ||
| dC1GalT5/Tgy | CG7440 | ND | Mucin-type O-glycan | ||
| dC1GalT6 | CG34056 | Muller et al. 2005 | Mucin-type O-glycan | ||
| CG34057 | Muller et al. 2005 | Mucin-type O-glycan | |||
| dC1GalT7 | CG3119 | ND | Mucin-type O-glycan | ||
| dC1GalT8 | CG2983 | ND | Mucin-type O-glycan | ||
| dC1GalT9 | CG9109 | ND | Mucin-type O-glycan | ||
| Glucosyltransferase | |||||
| Dolichyl phosphate glucosyltransferase | dAlg5/Wol | CG7870 | ND | N-glycan | ALG5 |
| Dolichyl pyrophosphate glucosyltransferase | dAlg6/Gny | CG5091 | ND | N-glycan | ALG6 |
| dAlg8 | CG4542 | ND | N-glycan | ALG8 | |
| dAlg10 | CG32076 | ND | N-glycan | ALG10 | |
| Glucosylceramide synthase | dGlcCerT/GlcT-1 | CG6437 | Kohyama-Koganeya et al. 2004 | Glycolipid | UGCG |
| Protein O-glucosyltransferase | Rumi | CG31152 | Acar et al. 2008 | Notch | |
| Ugt | CG6850 | Parker et al. 1995 | N-glycan | UGCGL1 | |
| Glucuronyltransferase | |||||
| GAG glucuronyltransferase I | dGlcAT-I | CG32775 | Kim et al. 2003 | GAG (common) | B3GAT1 |
| β1,3-glucuronyltransferase | dGlcAT-BSI/GlcAT-S | CG3881 | Kim et al. 2003 | GAG (common), other glycan ? | |
| dGlcAT-BSII/GlcAT-P | CG6207 | Kim et al. 2003 | GAG (common), other glycan ? | ||
| CG30438 | ND | glucuronidation | CGT | ||
| Hereditary multiple exostoses (EXT) protein | dExt1/Ttv | CG10117 | ND | GAG (HS) | EXT1 |
| dExt2/Sotv | CG8433 | ND | GAG (HS) | ||
| dExt3/Botv | CG15110 | Kim et al. 2002 | GAG (HS) | EXTL3 | |
| Mannosyltransferase | |||||
| β1,4-mannosyltransferase | β1,4ManT/Egh | CG9659 | Wandall et al. 2003 | Glycolipid | |
| Dolichyl pyrophosphate mannosyltransferase | dAlg1 | CG18012 | ND | N-glycan | ALG1 |
| dAlg2 | CG1291 | ND | N-glycan | ALG2 | |
| dAlg11 | CG11306 | ND | N-glycan | ALG11 | |
| dAlg3/l(2)not | CG4084 | ND | N-glycan | ALG3 | |
| dAlg9 | CG11851 | ND | N-glycan | ALG9 | |
| dAlg12 | CG8412 | ND | N-glycan | ALG12 | |
| dDPM | CG10166 | ND | N-glycan | DPM1 | |
| Protein O-mannosyltransferase | dPomt1/Rt | CG6097 | Ichimiya et al. 2004 | Dystroglycan | POMT1 |
| dPomt2/Tw | CG12311 | Ichimiya et al. 2004 | Dystroglycan | POMT2 | |
| Sialyltransferase | |||||
| Galactoside α2,6-sialyltransferase | dST6Gal I | CG4871 | Koles et al. 2004 | N-glycan | ST6GAL2 |
| Xylosyltransferase | |||||
| Peptide-O-xylosyltransferase | dXylT/Oxt | CG32300 | Wilson 2002 | GAG (common) | XYLT1 |
| Oligosaccharyltransferase | |||||
| Oligosaccharyltransferase | OST | CG33303 | N-glycan | ||
| CG9022 | N-glycan | ||||
| CG7830 | N-glycan | ||||
| CG6370 | N-glycan | ||||
| CG13393 | N-glycan | ||||
| STT | CG1518 | N-glycan | |||
| STT | CG7748 | N-glycan | |||
| Fukutin-related protein | CG15651 | ND | Dystroglycan | FKRP | |
| Sulfotransferase | |||||
| Chondroitin 4-O-sulfotransferase | dC4ST | CG31743 | ND | GAG (CS) | CHST13 |
| N-acetylgalactosamine-4-O-sulfotransferase | d4ST1 | CG14024 | ND | GAG (CS), N-glycan ? | CHST11 |
| d4ST2 | CG13937 | ND | GAG (CS), N-glycan ? | ||
| N-acetylgalactosamine/N-acetylglucosamine/galactose | d6ST1 | CG31637 | ND | GAG (CS), N-glycan ? | |
| 6-O-sulfotransferase | d6ST2 | CG9550 | ND | GAG (CS), N-glycan ? | |
| Heparan sufate sulfotransferase | Pipe | CG9614 | Zhu et al. 2005*; Xu et al. 2007 | GAG (HS) | |
| Heparan N-deacetylase/N-sulfotransferase | Sfl | CG8339 | ND | GAG (HS) | NDST2 |
| Heparan sulfate 2-O-sulfotransferase | HS2ST | CG10234 | Kamimura et al. 2006*; Xu et al. 2007 | GAG (HS) | HS2ST1 |
| Heparan sulfate 6-O-sulfotransferase | dHS6ST | CG4451 | Kamimura et al. 2001 | GAG (HS) | HS6ST1 |
| Heparan sulfate d-glucosaminyl 3-O-sulfotransferase | dHS3OSTA | CG33147 | ND | GAG (HS) | HS3ST5 |
| dHS3OSTB | CG7890 | Kamimura et al. 2004 | GAG (HS) | HS3ST3A1 | |
| C5 epimerase | Heparan sulfate C5-epimerase | CG3194 | ND | GAG (HS) | |
| Sugar-nucleotide transporter | |||||
| GDP-Fuc transporter (Golgi) | Gfr/Nac | CG9620 | Luhn et al. 2004; Ishikawa et al. 2005; Geisler et al. 2012* | ||
| GDP-Fuc/UDP-GlcNAc/UDP-Xyl transporter (ER) | Efr | CG3774 | Ishikawa et al. 2010 | SLC35B4 | |
| UDP-Gal/UDP-GalNAc transporter | Csat | CG2675 | Segawa et al. 2002 | SLC35A2 | |
| UDP-sugar transporter | Frc | CG3874 | Goto et al. 2001; Selva et al. 2001 | Notch, GAG | SLC35D1 |
| Sugar-nucleotide transporter | Meigo | CG5802 | ND | SLC35B1 | |
| PAPS transporter | Sll | CG7623 | Kamiyama et al. 2003; Luders et al. 2003 | GAG | SLC35B2 |
| dPAPST2 | CG7853 | Goda et al. 2006 | GAG | SLC35B3 | |
| Core protein | |||||
| Glypican | Dally | CG4974 | GAG (HS) | ||
| Glypican | Dlp | CG32146 | GAG (HS) | ||
| Dystroglycan | αDystroglycan | CG18250 | O-Man | ||
| Syndecan | dSdc | CG10497 | GAG (HS) | ||
| Perlecan | dPerlecan/Trol | CG33950 | GAG (HS) | ||
| Glycosidase | |||||
| α-mannosidase I | α-Man-I | CG42275 | ND | N-Glycan | |
| α-Man-II | CG18802 | Cao et al. 2011 | N-Glycan | ||
| β-N-acetylglucosaminidase | Fdl | CG8824 | Leonard et al. 2006 | N-Glycan | |
Determined by mutant phenotype.
ND: not determined.
Figure 1.
Drosophila GR genes assigned to linkage formation and modification of N-linked glycan (A), glycosaminoglycans (B), mucin-type glycans (C), Notch-related glycans (D), Dystroglycan-related glycan (E), and arthro-series of glycolipid (F). Core proteins are also assigned (B, D, and E).
There are structural variants of N-linked glycans. Aoki and colleagues determined the number of N-linked glycan variants in Drosophila embryo using mass spectrometry (Aoki et al. 2007). The authors detected GlcNAc structures that were synthesized by Mgat1, Mgat2, and Mgat4, and also observed extended forms such as Galβ-3GlcNAc and SAα2-6Galβ-3GlcNAc. However, no terminal GlcNAc structures synthesized by Mgat3, Mgat5, or Mgat6 were detected. Accordingly, sequence comparisons showed the absence of Mgat5 and Mgat6 in Drosophila, and expression of Mgat3 was very low (Flybase). In addition, a small amount of N-linked glycans was capped by LacdiNAc (GalNAc-GlcNAc) or GlcA in the Drosophila embryo (Aoki & Tiemeyer 2010). LacdiNAc was also found in arthro-series glycosphingolipids in embryo. LacdiNAc structures on glycoproteins and glycosphingolipids were synthesized by Drosophila β4GalNAcTA (Sasaki et al. 2007).
Gene silencing in the whole Drosophila body
To examine the phenotypes caused by silencing of GR genes, we established RNAi-inducible fly strains for 72 Drosophila GR genes. RNAi could not be established for the remaining 53 genes. The established UAS-IR strains bore transgenes containing IR sequences of the target genes under the control of the UAS. First, we calculated off-target probability scores (OTPS) for each UAS-IR strain using the dsCheck website (http://dscheck.rnai.jp/, Table2). Our previous research showed that UAS-IR strains with OTPS <3 were most likely to silence on-target genes (Yamamoto-Hino et al. 2010). Therefore, UAS-IR strains with OTPS >2 were not analyzed further.
Table 2.
Off-target probability score (OTPS) and phenotypes caused by whole-body gene silencing
| Family of proteins/protein name | Protein/gene name | CG No. | OTPS | Act5C |
|---|---|---|---|---|
| Glycosyltransferase | ||||
| N-acetylgalactosaminyltransferase | ||||
| UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase | pgant1/GalNAc-T1 | CG8182 | 1 | Lethal |
| pgant2 | CG3254 | 0 | Lethal | |
| pgant3 | CG4445 | 2 | Viable | |
| pgant4 | CG31956 | 0 | Lethal | |
| pgant5 | CG31651 | 0 | Lethal | |
| pgant6 | CG2103 | 0 | Lethal | |
| pgant7/GalNAc-T2 | CG6394 | 1 | Lethal | |
| pgant8 | CG7297 | 0 | Lethal | |
| pgant35A | CG7480 | 0 | Lethal | |
| dppGalNAcT9 | CG30463 | 2 | Lethal | |
| dppGalNAcT10 | CG10000 | 1 | N.T. | |
| CG31776 | no line | N.T. | ||
| dppGalNAcT11 | CG7304 | no line | N.T. | |
| dppGalNAcT12 | CG7579 | 1 | N.T. | |
| α1,4-N-acetylgalactosaminyltransferase | α4GT1 | CG17223 | 0 | Viable |
| α4GT2 | CG5878 | 0 | N.T. | |
| β1,4-N-acetylgalactosaminyltransferase | β4GalNAcTA | CG8536 | 0 | N.T. |
| β4GalNAcTB | CG14517 | 1 | Viable | |
| N-acetylglucosaminyltransferase | ||||
| UDP-GlcNAc:polypeptide O-β-N-acetylglucosaminyltransferase | dO-GnT/Sxc | CG10392 | 0 | Lethal |
| α3-D-mannoside-β1,2-N-acetylglucosaminyltransferase | dMGAT1/Mgat1 | CG13431 | 2 | Lethal |
| α6-D-mannoside-β1,2-N-acetylglucosaminyltransferase | dMGAT2/Mgat2 | CG7921 | 1 | Lethal |
| β4-D-mannoside-β1,4-N-acetylglucosaminyltransferase | dMGAT3 | CG31849 | 1 | Lethal |
| α3-D-mannoside-β1,4-N-acetylglucosaminyltransferase | dMGAT4-1 | CG9384 | 2 | Viable |
| dMGAT4-2 | CG17173 | 0 | Lethal | |
| i-β1,3-N-acetylglucosaminyltransferase | diβ3GnT1 | CG3253 | 1 | Viable |
| diβ3GnT2 | CG9171 | 38 | N.T. | |
| diβ3GnT3 | CG15483 | 0 | Viable | |
| diβ3GnT4 | CG11149 | 0 | Lethal | |
| diβ3GnT5 | CG9996 | 0 | Lethal | |
| diβ3GnT6 | CG11388 | 0 | Lethal | |
| β1,3-N-acetylglucosaminyltransferase | Brn | CG4934 | 0 | Lethal |
| Fng | CG10580 | 0 | Lethal | |
| β1,3-N-acetylglucosaminyltransferase or | dβ3GnT or GalT1 | CG33145 | 0 | Lethal |
| β1,3-galactosyltransferase | dβ3GnT or GalT2 | CG11357 | 2 | Lethal |
| dβ3GnT or GalT3 | CG3038 | 0 | Viable | |
| dβ3GnT or GalT4 | CG8668 | 0 | Lethal | |
| dβ3GnT or GalT5 | CG8673 | 11 | N.T. | |
| Dolichyl phosphate N-acetylglucosaminyltransferase | dAlg14 | CG6308 | no line | N.t. |
| dAlg7 | CG5287 | 0 | Lethal | |
| dAlg13 | CG14512 | 0 | Lethal | |
| Chondroitin synthase | ||||
| Chondroitin synthase | dCHSY | CG9220 | 2 | n.t. |
| Chondroitin polymerization factor | dCHPF | CG43313 | 0 | Lethal |
| Chondroitin N-acetylgalactosaminyltransferase | dCSGalNAcT1 | CG12913 | 2 | Viable |
| Chitin synthase | Chitin Syn1/Kkv | CG2666 | 0 | Lethal |
| Chitin Syn2 | CG7464 | 0 | Lethal | |
| Fucosyltransferase | ||||
| α1,3/1,4-fucosyltransferase or | FucTA | CG6869 | 6 | N.T. |
| α1,3-fucosyltransferase | FucTB | CG4435 | 0 | Lethal |
| FucTD | CG9169 | 1 | Lethal | |
| FucTC | CG40305 | no line | N.T. | |
| α1,6-fucosyltransferase | dα6Fut/FucT6 | CG2448 | 1 | Lethal |
| Protein O-fucosyltransferase | OFut1 | CG12366 | 0 | Lethal |
| OFut2 | CG14789 | 0 | Viable | |
| Galactosyltransferase | ||||
| GAGβ1,4-galactosyltransferase I | dGAGβ4GalTI/β4GalT7 | CG11780 | 0 | Lethal |
| GAGβ1,3-galactosyltransferase II | dGAGβ3GalTII | CG8734 | 1 | Lethal |
| core1β1,3-galactosyltransferase | dC1GalT1/C1GalTA | CG9520 | 0 | Viable |
| dC1GalT2 | CG8708 | 1 | Lethal | |
| dC1GalT3 | CG18558 | 0 | N.T. | |
| dC1GalT4 | CG2975 | 8 | N.T. | |
| dC1GalT5/Tgy | CG7440 | 0 | Lethal | |
| dC1GalT6 | CG34056 | 8 | N.T. | |
| CG34057 | 8 | N.T. | ||
| dC1GalT7 | CG3119 | 2 | N.T. | |
| dC1GalT8 | CG2983 | 2 | Viable | |
| dC1GalT9 | CG9109 | 1 | Lethal | |
| Glucosyltransferase | ||||
| Dolichyl phosphate glucosyltransferase | dAlg5/Wol | CG7870 | 2 | N.T. |
| Dolichyl pyrophosphate glucosyltransferase | dAlg6/Gny | CG5091 | 0 | N.T. |
| dAlg8 | CG4542 | 2 | N.T. | |
| dAlg10 | CG32076 | 1 | N.T. | |
| Glucosylceramide synthase | dGlcCerT/GlcT-1 | CG6437 | 1 | Lethal |
| Protein O-glucosyltransferase | Rumi | CG31152 | no line | N.T. |
| Ugt | CG6850 | no line | N.T. | |
| Glucuronyltransferase | ||||
| GAG glucuronyltransferase I | dGlcAT-I | CG32775 | 0 | Lethal |
| β1,3-glucuronyltransferase | dGlcAT-BSI/GlcAT-S | CG3881 | 0 | Viable |
| dGlcAT-BSII/GlcAT-P | CG6207 | 24 | N.T. | |
| CG30438 | 0 | Viable | ||
| Hereditary multiple exostoses (EXT) protein | dExt1/Ttv | CG10117 | 0 | Lethal |
| dExt2/Sotv | CG8433 | 0 | Lethal | |
| dExt3/Botv | CG15110 | ? | Lethal | |
| Mannosyltransferase | ||||
| β1,4-mannosyltransferase | β1,4ManT/Egh | CG9659 | 0 | N.T. |
| Dolichyl pyrophosphate mannosyltransferase | dAlg1 | CG18012 | 0 | Lethal |
| dAlg2 | CG1291 | 2 | N.T. | |
| dAlg11 | CG11306 | 0 | N.T. | |
| dAlg3/l(2)not | CG4084 | 0 | N.T. | |
| dAlg9 | CG11851 | no line | N.T. | |
| dAlg12 | CG8412 | 0 | N.T. | |
| dDPM | CG10166 | 0 | N.T. | |
| Protein O-mannosyltransferase | dPomt1/Rt | CG6097 | 0 | Lethal |
| dPomt2/Tw | CG12311 | 0 | Lethal | |
| Sialyltransferase | ||||
| Galactoside α2,6-sialyltransferase | dST6Gal I | CG4871 | 3 | N.T. |
| Xylosyltransferase | ||||
| Peptide-O-xylosyltransferase | dXylT/Oxt | CG32300 | 0 | Lethal |
| Oligosaccharyltransferase | ||||
| Oligosaccharyltransferase | OST | CG33303 | 0 | N.T |
| CG9022 | no line | N.T | ||
| CG7830 | 0 | N.T | ||
| CG6370 | no line | N.T | ||
| CG13393 | no line | N.T | ||
| STT | CG1518 | 0 | N.T | |
| STT | CG7748 | no line | N.T | |
| Fukutin-related protein | CG15651 | 0 | Lethal | |
| Sulfotransferase | ||||
| Chondroitin 4-O-sulfotransferase | dC4ST | CG31743 | 0 | Viable |
| N-acetylgalactosamine-4-O-sulfotransferase | d4ST1 | CG14024 | 6 | N.T. |
| d4ST2 | CG13937 | 0 | Viable | |
| N-acetylgalactosamine/N-acetylglucosamine/galactose | d6ST1 | CG31637 | 0 | N.T. |
| 6-O-sulfotransferase | d6ST2 | CG9550 | 0 | Lethal |
| Heparan sufate sulfotransferase | Pipe | CG9614 | 1 | Lethal |
| Heparan N-deacetylase/N-sulfotransferase | Sfl | CG8339 | 1 | N.T. |
| Heparan sulfate 2-O-sulfotransferase | HS2ST | CG10234 | 0 | Viable |
| Heparan sulfate 6-O-sulfotransferase | dHS6ST | CG4451 | 0 | N.T. |
| Heparan sulfate d-glucosaminyl 3-O-sulfotransferase | dHS3OSTA | CG33147 | 2 | N.T. |
| dHS3OSTB | CG7890 | 3 | N.T. | |
| C5 epimerase | Heparan sulfate C5-epimerase | CG3194 | 0 | lethal |
| Sugar-nucleotide transporter | ||||
| GDP-Fuc transporter (Golgi) | Gfr/Nac | CG9620 | 0 | N.T. |
| GDP-Fuc/UDP-GlcNAc/UDP-Xyl transporter (ER) | Efr | CG3774 | no line | N.T. |
| UDP-Gal/UDP-GalNAc transporter | Csat | CG2675 | 0 | Lethal |
| UDP-sugar transporter | Frc | CG3874 | 2 | Lethal |
| Sugar-nucleotide transporter | Meigo | CG5802 | 0 | Lethal |
| PAPS transporter | Sll | CG7623 | 0 | Lethal |
| dPAPST2 | CG7853 | 2 | Lethal | |
| Core protein | ||||
| Glypican | Dally | CG4974 | 4 | N.T. |
| Glypican | Dlp | CG32146 | 2 | Lethal |
| Dystroglycan | αDystroglycan | CG18250 | 1 | N.T. |
| Syndecan | dSdc | CG10497 | 3 | N.T. |
| Perlecan | dPerlecan/Trol | CG33950 | 0 | N.T. |
| Glycosidase | ||||
| α-mannosidase I | α-Man-I | CG42275 | no line | N.T. |
| α-Man-II | CG18802 | 0 | Lethal | |
| β-N-acetylglucosaminidase | Fdl | CG8824 | no line | N.T. |
no line: no UAS-IR line; N.T.: not tested.
Next, we examined whether RNAi-mediated gene silencing reduced the amounts of corresponding mRNA and glycan in Drosophila. Peptide-O-xylosyltransferase (XylT, CG17772) is required for the formation of the common core region of GAGs such as heparan sulfate (HS) GAG and chondroitin sulfate (CS) GAG, whereas hereditary multiple exostoses protein 3 (DExt3, CG15110) participates in the extension of HS but not CS. Expression of XylT and DExt3 was silenced in whole larval bodies using Act5C-Gal4. XylT and DExt3 mRNA in the silenced larvae were reduced to 15–30% of control levels (Act5C-Gal4) (Fig.2). GAG fractions were extracted from the silenced larvae, treated with heparitinase, and subjected to HPLC for detailed analyses of GAGs. Silencing of XylT resulted in the reduction in both HS and CS, whereas DExt3 silencing caused the specific reduction in HS (Fig.2). These results clearly showed that the RNAi-mediated silencing in the present study resulted in specific reduction in GAGs as well as the mRNA expression levels of each glycosyltransferase.
Figure 2.
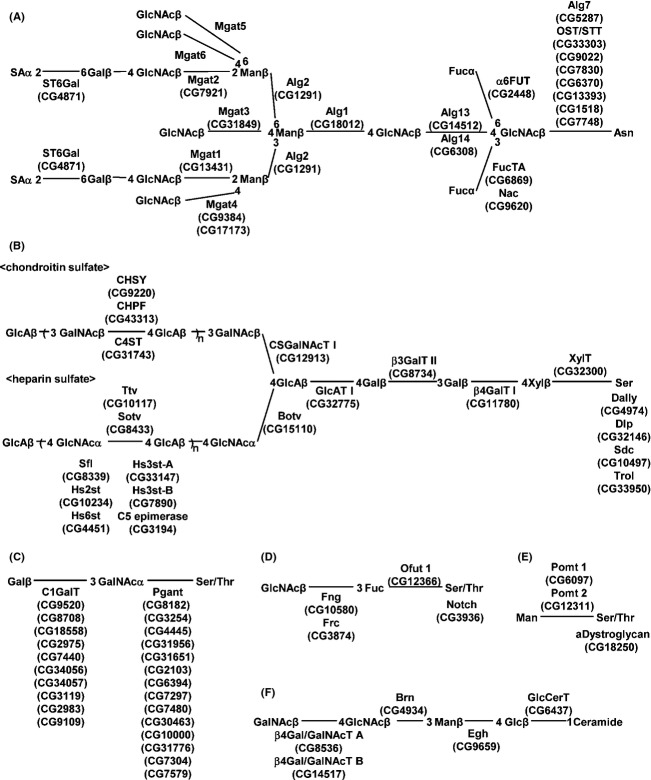
Reduction in mRNA and GAG levels by silencing of CG15110 (Dext3) and CG32300 (dXylT). (A, B) The mRNA levels of CG15110 and CG32300 in Act5C-GAL4/UAS-IR-CG15110 (A), Act5C-GAL4/UAS-IR-CG17772 (B), and Actin5C-GAL4/+ (as control in A and B) were quantified by real-time PCR. (C–E) Typical chromatograms of GAG-derived oligosaccharides in the third instar larvae of Actin5C-GAL4/+ (C), Act5C-GAL4/UAS-IR-CG15110 (D), and Act5C-GAL4/UAS-IR-CG17772 (E). HS, chromatograms of unsaturated disaccharides from heparan sulfate. CS, chromatograms of unsaturated disaccharides from low-sulfated chondroitin 4-sulfate. Peaks: 1, ΔUA-GlcNAc; 2, ΔUA-GlcNS; 3, ΔUA-GlcNAc6S; 4, ΔUA-GlcNS6S; 5, ΔUA2S-GlcNS; 6, ΔUA2S-GlcNS6S; 7, ΔDi-0S; and 8, ΔDi-4S. The peak heights in each chromatogram reflect the amount of oligosaccharides and can be compared between different genotypes.
The Act5C-Gal4 driver strain was crossed to 72 UAS-IR strains to induce gene silencing in whole bodies during all developmental stages. Progeny from 56 of the crosses (78%) died before developing into third instar larvae, suggesting that these genes were essential for development (Table2). As it was difficult to classify these GR genes from lethality alone, we next carried out spatiotemporally regulated gene silencing using several Gal4 driver strains.
Gene silencing in a spatiotemporally regulated manner
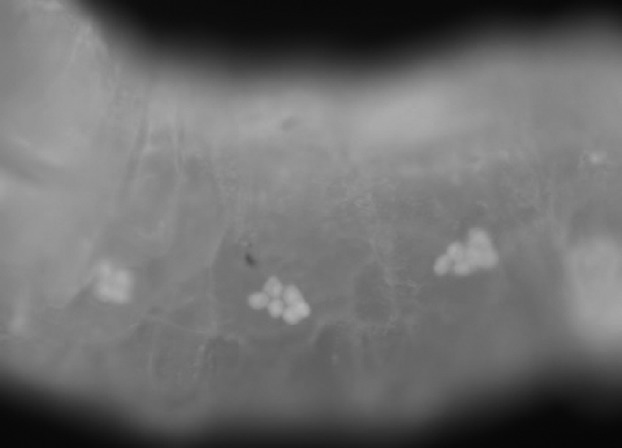
For spatiotemporal RNAi, MS1096/A9-Gal4, scalloped (sd)-Gal4, patched (ptc)-Gal4, and engrailed (en)-Gal4 driver strains were used to induce gene silencing in wing disks, and 69B-Gal4 was used for expression in larval histoblasts and wing disks (Fig.3). Of the 72 strains tested, 20 showed abnormalities in adult wings and abdomens. In wings, extra or thick veins were formed by gene silencing using MS1096/A9-Gal4, scalloped (sd)-Gal4, patched (ptc)-Gal4, and engrailed (en)-Gal4 drivers (Figs4,5, Table3). By contrast, gene silencing using 69B-Gal4 caused abdominal depigmentation (Fig.6, Table3). Formation of extra and thick veins was mainly observed by silencing of genes involved in synthesis of GAGs and Fringe-dependent glycans on Notch, respectively (Figs4,5, Table3). These phenotypes corresponded with those observed for mutant strains (Panin et al. 1997; Goto et al. 2001; Selva et al. 2001; Nybakken & Perrimon 2002). By contrast, abdominal depigmentation has not been observed previously. Depigmentation was caused by silencing of dα6fut/fucT6, gfr/nac, Csat, and CG33145 (Fig.6, Table3). Dα6Fut/FucT6 adds a fucose moiety to the core region of N-linked glycans via α1,6-linkage (Paschinger et al. 2005), whereas Gfr/Nac transports GDP-fucose to the Golgi lumen for fucose addition, including α1,3-fucosylation of the core N-linked glycans (Ishikawa et al. 2010; Geisler et al. 2012). As Gal and GalNAc are often added at or near nonreducing ends of glycans, Csat, a UDP-Gal/UDP-GalNAc transporter (Segawa et al. 2002), may be involved in terminal glycosylation. Therefore, the depigmentation group is possibly involved in synthesis of glycans at or near nonreducing ends, namely terminal domains. We therefore next examined whether CG33145 participated in terminal glycosylation.
Figure 3.
69B-Gal4 expression in larval histoblasts. The late third instar larva of 69B-Gal4/UAS-GFP expressed GFP in histoblasts.
Figure 4.
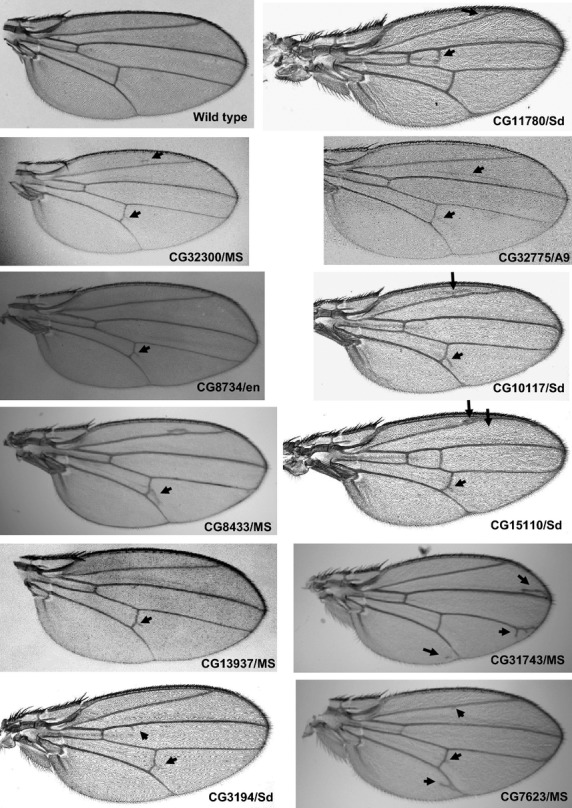
Adult wing phenotypes caused by silencing of GAG genes. Extra veins are indicated by arrows. The combination of UAS-IR and Gal4 strains is indicated under each panel.
Figure 5.
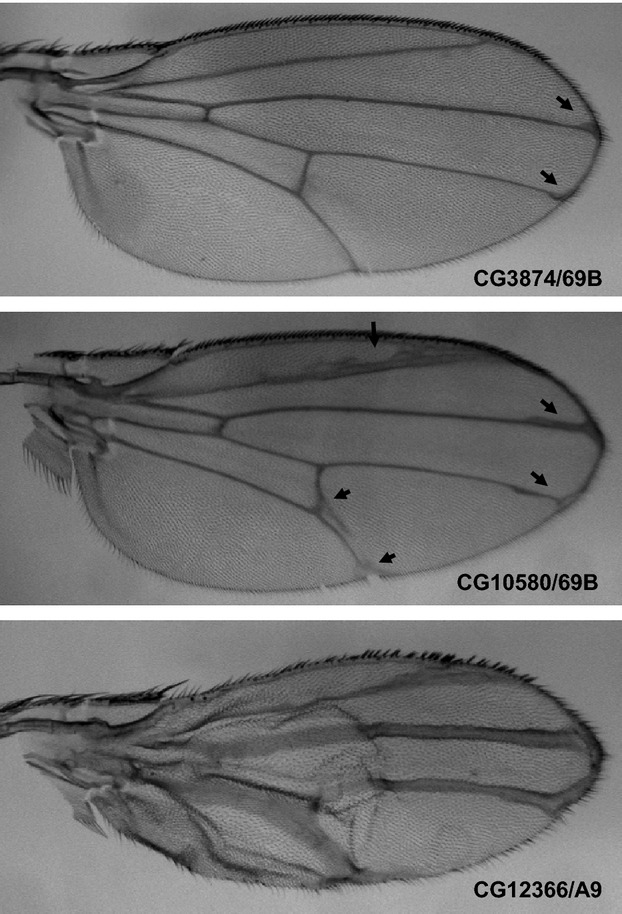
Adult wing phenotypes caused by silencing of Notch glycosylation genes. Thick veins are indicated by arrows. The combination of UAS-IR and Gal4 strains is indicated in each panel.
Table 3.
Phenotypes caused by spatiotemporally regulated gene silencing
| Family of proteins/protein name | Protein/gene name | CG No. | Gal4 driver line | Glycans | ||||
|---|---|---|---|---|---|---|---|---|
| MS1096/A9 | SD | en | ptc | 69B | ||||
| N-acetylgalactosamine-4-O-sulfotransferase | d4ST2 | CG13937 | Extra vein | GAGs | ||||
| GAG glucuronyltransferase I | dGlcAT-I | CG32775 | Extra vein1 | |||||
| peptide-O-xylosyltransferase | dXylT/Oxt | CG32300 | Extra vein | Extra vein | ||||
| GAGβ1,4-galactosyltransferase I | dGAGβ4GalTI/β4GalT7 | CG11780 | Extra vein | Extra vein | ||||
| C5 epimerase | Heparan sulfate C5-epimerase | CG3194 | Extra vein | Extra vein | Extra vein2 | |||
| Hereditary multiple exostoses (EXT) protein | dExt1/Ttv | CG10117 | Extra vein3 | Extra vein | ||||
| Hereditary multiple exostoses (EXT) protein | dExt2/Stv | CG8433 | Extra vein | Extra vein | Extra vein | |||
| Hereditary multiple exostoses (EXT) protein | dExt3/Botv | CG15110 | Extra vein | acv deletion | ||||
| β1,3-glucuronyltransferase | dGlcAT-BSII/GlcAT-P | CG6207 | Extra vein | |||||
| GAGβ1,3-galactosyltransferase II | dGAGβ3GalTII | CG8734 | Extra vein | |||||
| PAPS transporter | Sll | CG7623 | Extra vein | |||||
| Chondroitin 4-O-sulfotransferase | dC4ST | CG31743 | Extra vein | |||||
| Syndecan | dSdc | CG10497 | Thick vein | |||||
| Protein O-fucosyltransferase | OFut1 | CG12366 | Thick vein4 | Notch | ||||
| β1,3-N-acetylglucosaminyltransferase | Fng | CG10580 | Thick vein | Thick vein | Thick vein5 | |||
| UDP-sugar transporter | Frc | CG3874 | Thick vein | |||||
| α1,6-fucosyltransferase | dα6Fut/FucT6 | CG2448 | Thick vein | Depigmentation6 | Terminal | |||
| GDP-Fuc transporter (Golgi) | Gfr/Nac | CG9620 | Depigmentation7 | |||||
| UDP-Gal/UDP-GalNAc transporter | Csat | CG2675 | Depigmentation8 | |||||
| β1,3-N-acetylglucosaminyltransferase or β1,3-galactosyltransferase | dβ3GnT or GalT1 | CG33145 | Depigmentation9 | |||||
| Glucosylceramide synthase | dGlcCerT/GlcT-1 | CG6437 | Thick vein | Glycolipid | ||||
| UDP-GlcNAc:polypeptide O-β-N-acetylglucosaminyltransferase | dO-GnT/Sxc | CG10392 | Thick vein | pcv deletion | O-GlcNAc | |||
Number of abnormal wings/number of tested wings = 40/76 (1), 46/46 (2), 70/70 (3), 64/64 (4) and 8/8 (5).
Number of depigmented males/number of tested males = 66/79 (6), 5/15 (7), 45/175 (8) and 9/69 (9).
Figure 6.
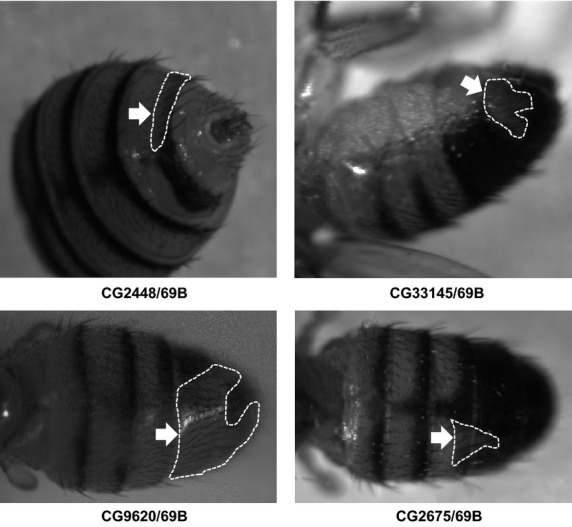
Adult abdominal phenotypes caused by silencing of N-glycan genes. Depigmented regions are indicated by arrows and surrounded by dotted lines. The combination of UAS-IR and Gal4 strains is indicated under each panel.
CG33145 has β1,3-galactosyltransferase activity for terminal N-glycans
As CG33145 has high sequence similarity to the members of human β1,3-N-acetylglucosaminyltransferase family (60–64%) and those of human β1,3-galactosyltransferase family (61–68%), we searched for glycan structures, including GlcNAc or Gal moiety, via β1,3-linkage in insects including Drosophila. Galβ1,3GalNAc was found in a complex-type N-linked glycan on royal jelly glycoproteins of honeybee: Galβ1,3GalNAcβ1,4GlcNAcβ1,2Manα1,6(Galβ1,3GalNAcβ1,4GlcNAcβ1,2Manα1,3)Manβ1,4GlcNAcβ1,4GlcNAc (E5, Fig.7A) (Kimura et al. 2006, 2007). Thus, we examined whether CG33145 added Gal to GalNAcβ1,4GlcNAcβ1,2Manα1,6(GalNAcβ1,4GlcNAcβ1,2Manα1,3)Manβ1,4GlcNAcβ1,4GlcNAc (E2, Fig.7A) via β1,3-linkage. CG33145 protein was expressed in Sf9 cells, and the β1,3galactosyltransferase activity was assessed (Fig.7B,C). An in vitro assay showed that CG33145 protein transferred the Gal moiety to E2 and produced the products Galβ1,3GalNAcβ1,4GlcNAcβ1,2Manα1,6(GalNAcβ1,4GlcNAcβ1,2Manα1,3)Manβ1,4GlcNAcβ1,4GlcNAc or GalNAcβ1,4GlcNAcβ1,2Manα1,6(Galβ1,3GalNAcβ1,4GlcNAcβ1,2Manα1,3)Manβ1,4GlcNAcβ1,4GlcNAc (E4), and Galβ1,3GalNAcβ1,4GlcNAcβ1,2Manα1,6(Galβ1,3GalNAcβ1,4GlcNAcβ1,2Manα1,3)Manβ1,4GlcNAcβ1,4GlcNAc (E5). Digestion of the fractionated E5 product by β1,3galactosidase produced E2 and E4 (Fig.7D), confirming that the linkage between Gal and GalNAc was a β1,3-linkage. These data clearly show that CG33145 protein is a novel β1,3galactosyltransferase of N-glycan.
Figure 7.
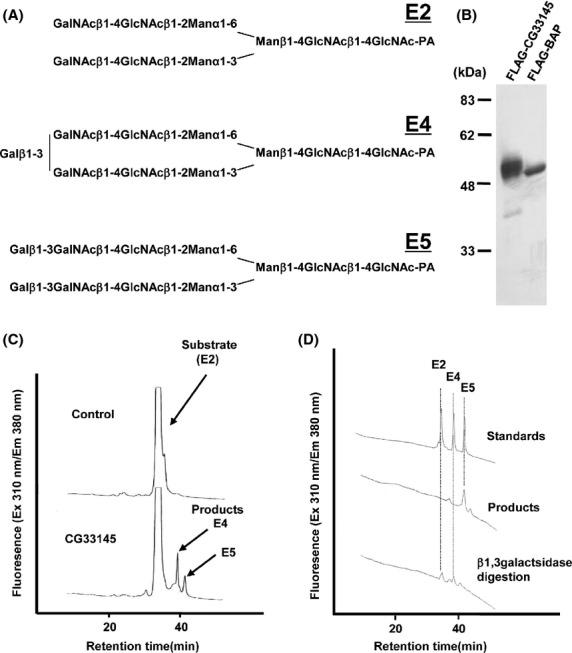
Identification of CG33145 as a novel β1,3galactosyltransferase of N-glycosylation. (A) The structures of an acceptor substrate E2 and its Gal extended forms, E4 and E5, which have one and two terminal Gal moieties, respectively. (B) FLAG-CG33145-PB and FLAG-BAP expressed in insect cells were purified and detected by anti-FLAG antibody. (C) Products of the CG33145-mediated reaction were analyzed by HPLC. CG33145 produced E4 and E5. (D) β1,3galactosidase treatment of reaction product E5. The E5 product peak shifted to peaks corresponding to E4 and E2 after β1,3galactosidase treatment.
Discussion
A wide variety of glycans are involved in diverse biological processes. To date, more than 200 genes in the human genome have been identified as GR candidates. However, biological and biochemical functions of the gene products remain to be studied in detail. In this study, large-scale RNAi silencing was used with Drosophila GR genes. Silencing of genes involved in synthesis of the same glycan resulted in the same phenotypes. Phenotypic clustering was used to identify galactosyltransferase terminal N-glycosylation activity in the previously uncharacterized protein CG33145. This suggests that phenotypic clustering is potentially valuable for the identification of specific glycans synthesized by genes of interest.
Using sequence comparisons, we identified 132 GR gene candidates in the Drosophila genome. Of these, the biochemical and biological functions of 50 genes remain to be studied in detail. However, it is difficult to determine the biochemical properties of GR proteins without predictive information because appropriate substrates and conditions are needed for biochemical assays. For example, a sialyltransferase that adds a sialic acid to a nonreducing end of N-glycans requires both CMP-sialic acid and a part of N-glycans for its biochemical assay. Pgant, a peptidyl-N-acetyl-galactosaminyltransferase that transfers GalNAc to mucin-type proteins, needs both UDP-GalNAc and appropriate peptides. Therefore, to determine the biochemical property of a novel gene, it is advantageous to predict what type of glycosylation is involved. In the present study, we examined the utility of phenotypic clustering for glycosylation prediction.
Silencing of GR genes using several Gal4 drivers resulted in various phenotypes such as formation of extra and thick veins and abdominal depigmentation; however, RNAi abnormalities were less severe than those resulting from classical mutations such as deletion, point mutation, or transposon-insertion. For example, silencing of fringe (fng) and fringe-connection (frc), which play an essential role in Notch glycosylation, produced a thick vein phenotype that was milder than the deleted margin phenotype of their null mutations. This may be due to low efficiency of gene silencing by RNAi and/or unusual persistence of GR proteins. Maternally provided Frc protein and/or mRNA was sufficient for a strong frc mutant to survive to the late third larval stage (Goto et al. 2001).
Knockdown phenotypes also depend on the RNAi library. Mummery-Widmer et al. identified CG12366 (Ofut1), but neither CG10580 (fng) nor CG3874 (frc), as a Notch regulator using Vienna RNAi library (Mummery-Widmer et al. 2009). The reason may be that the different lengths of dsRNAs between Vienna and NIG RNAi libraries. Long dsRNAs (500 bp) in NIG silence target gene expression more effectively than short ones (approximately 300 bp) in Vienna.
RNAi and conventional mutation phenotypes were similar, albeit with milder phenotypes observed with silencing. For example, knockdown of genes involved in GAG synthesis and Notch glycosylation resulted in formation of extra and thick veins, respectively. These phenotypes were also reported in strains with mutations in the corresponding genes. By contrast, the abdominal depigmentation phenotype produced upon knockdown of genes involved in synthesis of terminal domains of glycans has not been observed previously.
Sequence similarity and phenotype-based gene clustering in the present study suggested that CG33145 had a β1,3galactosyltransferase activity in N-glycan synthesis. Biochemical analysis confirmed that the CG33145 protein had β1,3galactosyltransferase activity for N-glycosylation. These results suggest that phenotype-based clustering can be indicative of molecular function. Similarly, Csat (CG2675), which also exhibited the abdominal pigmentation phenotype, may contribute to the synthesis of N-glycan.
The N-glycan gene cluster did not include glycosyltransferases involved in the production of core regions of N-linked glycans. It is possible that core regions of N-linked glycans are essential for protein folding and quality control and that deletion of whole N-linked glycan structures may cause lethal defects. By contrast, nonreducing ends of N-linked glycans play more specific roles such as regulation of ligand-receptor interactions, protein complex formation, and protein trafficking. Thus, defects of the nonreducing ends of N-linked glycans might result in specific, less severe phenotypes such as depigmentation.
In mice, branch positioning near nonreducing ends of N-glycans is required for proper trafficking of Glucose transporter 2, which is essential for glucose-stimulated insulin secretion (Ohtsubo et al. 2005). In Drosophila, the same branch structure and the insulin pathway were shown to be involved in cuticle pigmentation (Shakhmantsir et al. 2014). Therefore, abdominal depigmentation may be caused by impaired trafficking of membrane and/or secretory proteins in the insulin pathway.
Biological functions of some glycans are conserved between Drosophila and humans. For example, POMT1 and POMT2, which transfer a mannose to Dystroglycan via an O-type linkage, are mutated in Walker–Warburg syndrome, a type of muscular dystrophy (Akasaka-Manya et al. 2004; van Reeuwijk et al. 2005). rotated abdomen and twisted, Drosophila mutants of POMT1 (CG6097) and POMT2 (CG12311), respectively, which mediate O-linked mannosylation, also exhibit muscle defects in adults, suggesting a conserved biological function of the O-mannosyl glycan (Martin-Blanco & Garcia-Bellido 1996; Ichimiya et al. 2004; Ueyama et al. 2010). These mutants exhibited the behavioral abnormalities, the shortened lifespan and ultrastructural defects of muscles, as seen in human patients, also indicating that Drosophila POMT mutants are models for human muscular dystrophy. Then enhanced apoptosis was found in muscle progenitor cells of these mutants and provided new insight into the mechanism of WWS development, namely increased numbers of apoptotic myoblasts causing muscle disorganization (Ueyama et al. 2010). Therefore, phenotypic information obtained in Drosophila may shed light on glycan functions in other organisms, including humans.
Experimental procedures
Generation of RNAi fly lines
A 500-bp-long cDNA fragment of the N-terminal region of the ORF of each target gene was amplified by PCR. The fragment was inserted as an inverted repeat (IR) into a modified pUAST transformation vector, pUAST-R57 (GenBank accession: AB233207), which possessed an IR formation site consisting of paired KpnI-CpoI and XbaI-SfiI restriction sites. To enhance the RNAi effect (Kalidas & Smith 2002), pUAST-R57 carries a 282-bp-long genome fragment containing introns 5 and 6 of the Drosophila Ret oncogene between the two IR fragments. The IR was constructed in a head-to-head orientation using a combination of tag sequences in the PCR primers and restriction sites in the vector. Transformation of Drosophila embryos was carried out according to Spradling (Spradling 1986) in the w1118 fly backgrounds. Each line was mated with several of the GAL4 driver lines: Act5C-GAL4 (Bloomington Drosophila Stock Center), GMR-GAL4 (Freeman 1996), ey-GAL4 (Bloomington Drosophila Stock Center), dpp-GAL4 (Staehling-Hampton et al. 1994), en-GAL4 (Johnson et al. 1995), pnr-GAL4 (Heitzler et al. 1996), ptc-GAL4 (Speicher et al. 1994), sd-GAL4 (Milan et al. 1997), A9-GAL4 (Sun & Artavanis-Tsakonas 1997), 29BD-GAL4 (Nakayama et al. 1997), 69B-GAL4 (Brand & Perrimon 1993), and MS1096-GAL4 (Capdevila & Guerrero 1994). F1 progeny were raised at 28°C, and their phenotypes were analyzed. F1 progeny of w1118 crossed with each of the GAL4 driver lines were used as a control, for example, Act5C-GAL4/+, GMR-GAL4/+, etc.
Quantitative analysis of mRNA by real-time PCR
Total RNA was extracted from Act5C-GAL4/UAS-IR-CG4351, CG15110, CG17772, and Act5C-GAL4/+ third instar larvae. First-strand cDNA was synthesized using a SuperScript II first-strand synthesis kit (Invitrogen) according to the manufacturer’s instructions. Quantitation of CG4351, CG15110, and CG17772 mRNA expression was carried out by real-time PCR using the following primers: forward, 5′-ccacgacgtgatcgctttct-3′ (CG4351), 5′-ggagtgcgcggaaatgg-3′ (CG15110), and 5′-gaaatctgcggcggattcta-3′ (CG17772); and reverse, 5′-cagtccctgcggatgtaagag-3′ (CG4351), 5′-tgtttgggcctcagttcacctt-3′ (CG15110), and 5′-agtggtggccgccagtt-3′ (CG17772). The probe, which consisted of 5′-tagtcgggattatgtccaggctcgca-3′ (CG4351), 5′-ccgcccgaaggaaatacctgcttaccta-3′ (CG15110), or 5′-ccatgaacatatacgagaccggaatagccaa-3′ (CG17772), was labeled at the 5′-end with the reporter dye 3FAM and at the 3′-end with the quencher dye TAMRA (Applied Biosystems, Foster City, CA). Real-time PCR was carried out using a TaqMan Universal PCR Master Mix (Applied Biosystems). Relative amounts of CG4351, CG15110, and CG17772 mRNAs were normalized against ribosomal protein L32 (RpL32) mRNA levels from the same cDNA.
Determination of the amount of chondroitin sulfate and heparan sulfate in Drosophila
GAGs were prepared from approximately 20 mg of lyophilized flies. Unsaturated disaccharides were produced by enzymatic digestion and analyzed by fluorometric postcolumn high-performance liquid chromatography, as described previously (Toyoda et al. 2000).
Expression and purification of CG33145 protein
The putative catalytic domain of candidate CG33145 protein (amino acids 92 to 466, CG33145-PB) was cloned using DGC clone RE52041, expressed in insect cells as a secreted protein fused with a FLAG peptide, and purified using Anti-FLAG M1 Affinity gel (Sigma), as described previously (Ueyama et al. 2008).
Galactosyltransferase activity assay
β1,3galactosyltransferase activity was assessed. The acceptor substrate E2 and standards, E4 and E5, were prepared as described previously (Kimura et al. 2006, 2007). Enzymatic reactions, product detection, and product confirmation were also carried out as noted previously (Kimura et al. 2006, 2007).
Acknowledgments
We thank H. Hamamoto, Y. Omae, K. Ohtsu, and W. Awano for technical assistance. This work was supported by the Core Research for Evolutional Science and Technology (CREST) program of the Japan Science and Technology Agency (JST), and MEXT/JSPS KAKENHI.
References
- Acar M, Jafar-Nejad H, Takeuchi H, Rajan A, Ibrani D, Rana NA, Pan H, Haltiwanger RS. Bellen HJ. Rumi is a CAP10 domain glycosyltransferase that modifies Notch and is required for Notch signaling. Cell. 2008;132:247–258. doi: 10.1016/j.cell.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasaka-Manya K, Manya H. Endo T. Mutations of the POMT1 gene found in patients with Walker-Warburg syndrome lead to a defect of protein O-mannosylation. Biochem. Biophys. Res. Commun. 2004;325:75–79. doi: 10.1016/j.bbrc.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Aoki K, Perlman M, Lim JM, Cantu R, Wells L. Tiemeyer M. Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo. J. Biol. Chem. 2007;282:9127–9142. doi: 10.1074/jbc.M606711200. [DOI] [PubMed] [Google Scholar]
- Aoki K. Tiemeyer M. The glycomics of glycan glucuronylation in Drosophila melanogaster. Methods Enzymol. 2010;480:297–321. doi: 10.1016/S0076-6879(10)80014-X. [DOI] [PubMed] [Google Scholar]
- Brand AH. Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Bruckner K, Perez L, Clausen H. Cohen S. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature. 2000;406:411–415. doi: 10.1038/35019075. [DOI] [PubMed] [Google Scholar]
- Cao J, Li Y, Xia W, Reddig K, Hu W, Xie W, Li HS. Han J. A Drosophila metallophosphoesterase mediates deglycosylation of rhodopsin. EMBO J. 2011;30:3701–3713. doi: 10.1038/emboj.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J. Guerrero I. Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J. 1994;13:4459–4468. doi: 10.1002/j.1460-2075.1994.tb06768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlow DA, Gossens K, Naus S, Veerman KM, Seo W. Ziltener HJ. PSGL-1 function in immunity and steady state homeostasis. Immunol. Rev. 2009;230:75–96. doi: 10.1111/j.1600-065X.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- Chen YW, Pedersen JW, Wandall HH, Levery SB, Pizette S, Clausen H. Cohen SM. Glycosphingolipids with extended sugar chain have specialized functions in development and behavior of Drosophila. Dev. Biol. 2007;306:736–749. doi: 10.1016/j.ydbio.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Fabini G, Freilinger A, Altmann F. Wilson IB. Identification of core alpha 1,3-fucosylated glycans and cloning of the requisite fucosyltransferase cDNA from Drosophila melanogaster. Potential basis of the neural anti-horseradish peroxidase epitope. J. Biol. Chem. 2001;276:28058–28067. doi: 10.1074/jbc.M100573200. [DOI] [PubMed] [Google Scholar]
- Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- Geisler C, Kotu V, Sharrow M, Rendic D, Poltl G, Tiemeyer M, Wilson IB. Jarvis DL. The Drosophila neurally altered carbohydrate mutant has a defective Golgi GDP-fucose transporter. J. Biol. Chem. 2012;287:29599–29609. doi: 10.1074/jbc.M112.379313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda E, Kamiyama S, Uno T, Yoshida H, Ueyama M, Kinoshita-Toyoda A, Toyoda H, Ueda R. Nishihara S. Identification and characterization of a novel Drosophila 3′-phosphoadenosine 5′-phosphosulfate transporter. J. Biol. Chem. 2006;281:28508–28517. doi: 10.1074/jbc.M605045200. [DOI] [PubMed] [Google Scholar]
- Goto S, Taniguchi M, Muraoka M, Toyoda H, Sado Y, Kawakita M. Hayashi S. UDP-sugar transporter implicated in glycosylation and processing of Notch. Nat. Cell Biol. 2001;3:816–822. doi: 10.1038/ncb0901-816. [DOI] [PubMed] [Google Scholar]
- Haines N. Irvine KD. Functional analysis of Drosophila beta1,4-N-acetlygalactosaminyltransferases. Glycobiology. 2005;15:335–346. doi: 10.1093/glycob/cwi017. [DOI] [PubMed] [Google Scholar]
- Haltiwanger RS. Lowe JB. Role of glycosylation in development. Annu. Rev. Biochem. 2004;73:491–537. doi: 10.1146/annurev.biochem.73.011303.074043. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Ito K, Sado Y, Taniguchi M, Akimoto A, Takeuchi H, Aigaki T, Matsuzaki F, Nakagoshi H, Tanimura T, Ueda R, Uemura T, Yoshihara M. Goto S. GETDB, a database compiling expression patterns and molecular locations of a collection of Gal4 enhancer traps. Genesis. 2002;34:58–61. doi: 10.1002/gene.10137. [DOI] [PubMed] [Google Scholar]
- Heitzler P, Haenlin M, Ramain P, Calleja M. Simpson P. A genetic analysis of pannier, a gene necessary for viability of dorsal tissues and bristle positioning in Drosophila. Genetics. 1996;143:1271–1286. doi: 10.1093/genetics/143.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimiya T, Manya H, Ohmae Y, Yoshida H, Takahashi K, Ueda R, Endo T. Nishihara S. The twisted abdomen phenotype of Drosophila POMT1 and POMT2 mutants coincides with their heterophilic protein O-mannosyltransferase activity. J. Biol. Chem. 2004;279:42638–42647. doi: 10.1074/jbc.M404900200. [DOI] [PubMed] [Google Scholar]
- Isaji T, Kariya Y, Xu Q, Fukuda T, Taniguchi N. Gu J. Functional roles of the bisecting GlcNAc in integrin-mediated cell adhesion. Methods Enzymol. 2010;480:445–459. doi: 10.1016/S0076-6879(10)80019-9. [DOI] [PubMed] [Google Scholar]
- Ishikawa HO, Ayukawa T, Nakayama M, Higashi S, Kamiyama S, Nishihara S, Aoki K, Ishida N, Sanai Y. Matsuno K. Two pathways for importing GDP-fucose into the endoplasmic reticulum lumen function redundantly in the O-fucosylation of Notch in Drosophila. J. Biol. Chem. 2010;285:4122–4129. doi: 10.1074/jbc.M109.016964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa HO, Higashi S, Ayukawa T, Sasamura T, Kitagawa M, Harigaya K, Aoki K, Ishida N, Sanai Y. Matsuno K. Notch deficiency implicated in the pathogenesis of congenital disorder of glycosylation IIc. Proc. Natl Acad. Sci. USA. 2005;102:18532–18537. doi: 10.1073/pnas.0504115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RL, Grenier JK. Scott MP. patched overexpression alters wing disk size and pattern: transcriptional and post-transcriptional effects on hedgehog targets. Development. 1995;121:4161–4170. doi: 10.1242/dev.121.12.4161. [DOI] [PubMed] [Google Scholar]
- Kalidas S. Smith DP. Novel genomic cDNA hybrids produce effective RNA interference in adult Drosophila. Neuron. 2002;33:177–184. doi: 10.1016/s0896-6273(02)00560-3. [DOI] [PubMed] [Google Scholar]
- Kamimura K, Fujise M, Villa F, Izumi S, Habuchi H, Kimata K. Nakato H. Drosophila heparan sulfate 6-O-sulfotransferase (dHS6ST) gene. Structure, expression, and function in the formation of the tracheal system. J. Biol. Chem. 2001;276:17014–17021. doi: 10.1074/jbc.M011354200. [DOI] [PubMed] [Google Scholar]
- Kamimura K, Koyama T, Habuchi H, Ueda R, Masu M, Kimata K. Nakato H. Specific and flexible roles of heparan sulfate modifications in Drosophila FGF signaling. J. Cell Biol. 2006;174:773–778. doi: 10.1083/jcb.200603129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura K, Rhodes JM, Ueda R, McNeely M, Shukla D, Kimata K, Spear PG, Shworak NW. Nakato H. Regulation of Notch signaling by Drosophila heparan sulfate 3-O sulfotransferase. J. Cell Biol. 2004;166:1069–1079. doi: 10.1083/jcb.200403077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama S, Suda T, Ueda R, Suzuki M, Okubo R, Kikuchi N, Chiba Y, Goto S, Toyoda H, Saigo K, Watanabe M, Narimatsu H, Jigami Y. Nishihara S. Molecular cloning and identification of 3′-phosphoadenosine 5′-phosphosulfate transporter. J. Biol. Chem. 2003;278:25958–25963. doi: 10.1074/jbc.M302439200. [DOI] [PubMed] [Google Scholar]
- Kim BT, Kitagawa H, Tamura Ji J, Kusche-Gullberg M, Lindahl U. Sugahara K. Demonstration of a novel gene DEXT3 of Drosophila melanogaster as the essential N-acetylglucosamine transferase in the heparan sulfate biosynthesis: chain initiation and elongation. J. Biol. Chem. 2002;277:13659–13665. doi: 10.1074/jbc.M111630200. [DOI] [PubMed] [Google Scholar]
- Kim BT, Tsuchida K, Lincecum J, Kitagawa H, Bernfield M. Sugahara K. Identification and characterization of three Drosophila melanogaster glucuronyltransferases responsible for the synthesis of the conserved glycosaminoglycan-protein linkage region of proteoglycans. Two novel homologs exhibit broad specificity toward oligosaccharides from proteoglycans, glycoproteins, and glycosphingolipids. J. Biol. Chem. 2003;278:9116–9124. doi: 10.1074/jbc.M209344200. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Sakamura S, Ushijima T, Hama Y, Kajiura H, Fujiyama K, Okihara K, Hashimoto K, Sugimoto H. Yamada H. Evidence for new beta1-3 galactosyltransferase activity involved in biosynthesis of unusual N-glycan harboring T-antigen in Apis mellifera. Biosci. Biotechnol. Biochem. 2007;71:1111–1114. doi: 10.1271/bbb.70081. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Ushijima T, Maeda M, Hama Y, Kimura M, Okihara K, Sugimoto H. Yamada H. Tumor antigen occurs in N-glycan of royal jelly glycoproteins: honeybee cells synthesize T-antigen unit in N-glycan moiety. Biosci. Biotechnol. Biochem. 2006;70:2583–2587. doi: 10.1271/bbb.60331. [DOI] [PubMed] [Google Scholar]
- Kohyama-Koganeya A, Sasamura T, Oshima E, Suzuki E, Nishihara S, Ueda R. Hirabayashi Y. Drosophila glucosylceramide synthase: a negative regulator of cell death mediated by proapoptotic factors. J. Biol. Chem. 2004;279:35995–36002. doi: 10.1074/jbc.M400444200. [DOI] [PubMed] [Google Scholar]
- Koles K, Irvine KD. Panin VM. Functional characterization of Drosophila sialyltransferase. J. Biol. Chem. 2004;279:4346–4357. doi: 10.1074/jbc.M309912200. [DOI] [PubMed] [Google Scholar]
- Leonard R, Rendic D, Rabouille C, Wilson IB, Preat T. Altmann F. The Drosophila fused lobes gene encodes an N-acetylglucosaminidase involved in N-glycan processing. J. Biol. Chem. 2006;281:4867–4875. doi: 10.1074/jbc.M511023200. [DOI] [PubMed] [Google Scholar]
- Luders F, Segawa H, Stein D, Selva EM, Perrimon N, Turco SJ. Hacker U. Slalom encodes an adenosine 3′-phosphate 5′-phosphosulfate transporter essential for development in Drosophila. EMBO J. 2003;22:3635–3644. doi: 10.1093/emboj/cdg345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhn K, Laskowska A, Pielage J, Klambt C, Ipe U, Vestweber D. Wild MK. Identification and molecular cloning of a functional GDP-fucose transporter in Drosophila melanogaster. Exp. Cell Res. 2004;301:242–250. doi: 10.1016/j.yexcr.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Luo Y, Koles K, Vorndam W, Haltiwanger RS. Panin VM. Protein O-fucosyltransferase 2 adds O-fucose to thrombospondin type 1 repeats. J. Biol. Chem. 2006;281:9393–9399. doi: 10.1074/jbc.M511975200. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E. Garcia-Bellido A. Mutations in the rotated abdomen locus affect muscle development and reveal an intrinsic asymmetry in Drosophila. Proc. Natl Acad. Sci. USA. 1996;93:6048–6052. doi: 10.1073/pnas.93.12.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami T. Kitagawa H. Biosynthesis and function of chondroitin sulfate. Biochim. Biophys. Acta. 2013;1830:4719–4733. doi: 10.1016/j.bbagen.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Milan M, Campuzano S. Garcia-Bellido A. Developmental parameters of cell death in the wing disc of Drosophila. Proc. Natl Acad. Sci. USA. 1997;94:5691–5696. doi: 10.1073/pnas.94.11.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney DJ, Panin VM, Johnston SH, Chen J, Shao L, Wilson R, Wang Y, Stanley P, Irvine KD, Haltiwanger RS. Vogt TF. Fringe is a glycosyltransferase that modifies Notch. Nature. 2000;406:369–375. doi: 10.1038/35019000. [DOI] [PubMed] [Google Scholar]
- Mucha J, Domlatil J, Lochnit G, Rendic D, Paschinger K, Hinterkorner G, Hofinger A, Kosma P. Wilson IB. The Drosophila melanogaster homologue of the human histo-blood group Pk gene encodes a glycolipid-modifying alpha1,4-N-acetylgalactosaminyltransferase. Biochem. J. 2004;382:67–74. doi: 10.1042/BJ20040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller R, Altmann F, Zhou D. Hennet T. The Drosophila melanogaster brainiac protein is a glycolipid-specific beta 1,3N-acetylglucosaminyltransferase. J. Biol. Chem. 2002;277:32417–32420. doi: 10.1074/jbc.C200381200. [DOI] [PubMed] [Google Scholar]
- Muller R, Hulsmeier AJ, Altmann F, Ten Hagen K, Tiemeyer M. Hennet T. Characterization of mucin-type core-1 beta1-3 galactosyltransferase homologous enzymes in Drosophila melanogaster. FEBS J. 2005;272:4295–4305. doi: 10.1111/j.1742-4658.2005.04838.x. [DOI] [PubMed] [Google Scholar]
- Mummery-Widmer JL, Yamazaki M, Stoeger T, Novatchkova M, Bhalerao S, Chen D, Dietzl G, Dickson BJ. Knoblich JA. Genome-wide analysis of Notch signalling in Drosophila by transgenic RNAi. Nature. 2009;458:987–992. doi: 10.1038/nature07936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Haines N, Chen J, Okajima T, Furukawa K, Urano T, Stanley P. Irvine KD. Identification of a Drosophila gene encoding xylosylprotein beta4-galactosyltransferase that is essential for the synthesis of glycosaminoglycans and for morphogenesis. J. Biol. Chem. 2002;277:46280–46288. doi: 10.1074/jbc.M203873200. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Kaiser K. Aigaki T. Ectopic expression of sex-peptide in a variety of tissues in Drosophila females using the P[GAL4] enhancer-trap system. Mol. Gen. Genet. 1997;254:449–455. doi: 10.1007/s004380050438. [DOI] [PubMed] [Google Scholar]
- Nishihara S. 4.05 - Drosophila development, RNAi, and glycobiology. In: Kamerling H, editor. Comprehensive Glycoscience. Oxford: Elsevier; 2007. pp. 49–79. [Google Scholar]
- Nishihara S. Glycosyltransferases and transporters that contribute to proteoglycan synthesis in Drosophila: identification and functional analyses using the heritable and inducible RNAi system. Methods Enzymol. 2010;480:323–351. doi: 10.1016/S0076-6879(10)80015-1. [DOI] [PubMed] [Google Scholar]
- Nybakken K. Perrimon N. Heparan sulfate proteoglycan modulation of developmental signaling in Drosophila. Biochim. Biophys. Acta. 2002;1573:280–291. doi: 10.1016/s0304-4165(02)00395-1. [DOI] [PubMed] [Google Scholar]
- Ohtsubo K, Takamatsu S, Minowa MT, Yoshida A, Takeuchi M. Marth JD. Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell. 2005;123:1307–1321. doi: 10.1016/j.cell.2005.09.041. [DOI] [PubMed] [Google Scholar]
- Okajima T. Irvine KD. Regulation of notch signaling by o-linked fucose. Cell. 2002;111:893–904. doi: 10.1016/s0092-8674(02)01114-5. [DOI] [PubMed] [Google Scholar]
- Panin VM, Papayannopoulos V, Wilson R. Irvine KD. Fringe modulates Notch-ligand interactions. Nature. 1997;387:908–912. doi: 10.1038/43191. [DOI] [PubMed] [Google Scholar]
- Parker CG, Fessler LI, Nelson RE. Fessler JH. Drosophila UDP-glucose:glycoprotein glucosyltransferase: sequence and characterization of an enzyme that distinguishes between denatured and native proteins. EMBO J. 1995;14:1294–1303. doi: 10.1002/j.1460-2075.1995.tb07115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschinger K, Staudacher E, Stemmer U, Fabini G. Wilson IB. Fucosyltransferase substrate specificity and the order of fucosylation in invertebrates. Glycobiology. 2005;15:463–474. doi: 10.1093/glycob/cwi028. [DOI] [PubMed] [Google Scholar]
- van Reeuwijk J, Janssen M, van den Elzen C, et al. POMT2 mutations cause alpha-dystroglycan hypoglycosylation and Walker-Warburg syndrome. J. Med. Genet. 2005;42:907–912. doi: 10.1136/jmg.2005.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar M. Schachter H. Cloning and expression of Drosophila melanogaster UDP-GlcNAc:alpha-3-D-mannoside beta1,2-N-acetylglucosaminyltransferase I. Biol. Chem. 2001;382:209–217. doi: 10.1515/BC.2001.028. [DOI] [PubMed] [Google Scholar]
- Sasaki N, Yoshida H, Fuwa TJ, Kinoshita-Toyoda A, Toyoda H, Hirabayashi Y, Ishida H, Ueda R. Nishihara S. Drosophila beta 1,4-N-acetylgalactosaminyltransferase-A synthesizes the LacdiNAc structures on several glycoproteins and glycosphingolipids. Biochem. Biophys. Res. Commun. 2007;354:522–527. doi: 10.1016/j.bbrc.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Schwientek T, Bennett EP, Flores C, et al. Functional conservation of subfamilies of putative UDP-N-acetylgalactosamine:polypeptide N-acetylgalactosaminyltransferases in Drosophila Caenorhabditis elegans, and mammals. One subfamily composed of l(2)35Aa is essential in Drosophila. J. Biol. Chem. 2002;277:22623–22638. doi: 10.1074/jbc.M202684200. [DOI] [PubMed] [Google Scholar]
- Segawa H, Kawakita M. Ishida N. Human and Drosophila UDP-galactose transporters transport UDP-N-acetylgalactosamine in addition to UDP-galactose. Eur. J. Biochem. 2002;269:128–138. doi: 10.1046/j.0014-2956.2001.02632.x. [DOI] [PubMed] [Google Scholar]
- Selva EM, Hong K, Baeg GH, Beverley SM, Turco SJ, Perrimon N. Hacker U. Dual role of the fringe connection gene in both heparan sulphate and fringe-dependent signalling events. Nat. Cell Biol. 2001;3:809–815. doi: 10.1038/ncb0901-809. [DOI] [PubMed] [Google Scholar]
- Shakhmantsir I, Massad NL. Kennell JA. Regulation of cuticle pigmentation in Drosophila by the nutrient sensing insulin and TOR signaling pathways. Dev. Dyn. 2014;243:393–401. doi: 10.1002/dvdy.24080. [DOI] [PubMed] [Google Scholar]
- Sinclair DA, Syrzycka M, Macauley MS, Rastgardani T, Komljenovic I, Vocadlo DJ, Brock HW. Honda BM. Drosophila O-GlcNAc transferase (OGT) is encoded by the Polycomb group (PcG) gene, super sex combs (sxc) Proc. Natl Acad. Sci. USA. 2009;106:13427–13432. doi: 10.1073/pnas.0904638106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speicher SA, Thomas U, Hinz U. Knust E. The Serrate locus of Drosophila and its role in morphogenesis of the wing imaginal discs: control of cell proliferation. Development. 1994;120:535–544. doi: 10.1242/dev.120.3.535. [DOI] [PubMed] [Google Scholar]
- Spradling AC. P element-mediated transformation. In: Roberts DB, editor. Drosophila: A Practical Approach. Oxford: IRL Press; 1986. pp. 175–197. [Google Scholar]
- Staehling-Hampton K, Jackson PD, Clark MJ, Brand AH. Hoffmann FM. Specificity of bone morphogenetic protein-related factors: cell fate and gene expression changes in Drosophila embryos induced by decapentaplegic but not 60A. Cell Growth Differ. 1994;5:585–593. [PubMed] [Google Scholar]
- Sun X. Artavanis-Tsakonas S. Secreted forms of DELTA and SERRATE define antagonists of Notch signaling in Drosophila. Development. 1997;124:3439–3448. doi: 10.1242/dev.124.17.3439. [DOI] [PubMed] [Google Scholar]
- Takemae H, Ueda R, Okubo R, Nakato H, Izumi S, Saigo K. Nishihara S. Proteoglycan UDP-galactose:beta-xylose beta 1,4-galactosyltransferase I is essential for viability in Drosophila melanogaster. J. Biol. Chem. 2003;278:15571–15578. doi: 10.1074/jbc.M301123200. [DOI] [PubMed] [Google Scholar]
- Ten Hagen KG, Tran DT, Gerken TA, Stein DS. Zhang Z. Functional characterization and expression analysis of members of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family from Drosophila melanogaster. J. Biol. Chem. 2003;278:35039–35048. doi: 10.1074/jbc.M303836200. [DOI] [PubMed] [Google Scholar]
- Toyoda H, Kinoshita-Toyoda A, Fox B. Selleck SB. Structural analysis of glycosaminoglycans in animals bearing mutations in sugarless, sulfateless, and tout-velu. Drosophila homologues of vertebrate genes encoding glycosaminoglycan biosynthetic enzymes. J. Biol. Chem. 2000;275:21856–21861. doi: 10.1074/jbc.M003540200. [DOI] [PubMed] [Google Scholar]
- Ueyama M, Akimoto Y, Ichimiya T, Ueda R, Kawakami H, Aigaki T. Nishihara S. Increased apoptosis of myoblasts in Drosophila model for the Walker-Warburg syndrome. PLoS ONE. 2010;5:e11557. doi: 10.1371/journal.pone.0011557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama M, Takemae H, Ohmae Y, Yoshida H, Toyoda H, Ueda R. Nishihara S. Functional analysis of proteoglycan galactosyltransferase II RNA interference mutant flies. J. Biol. Chem. 2008;283:6076–6084. doi: 10.1074/jbc.M709189200. [DOI] [PubMed] [Google Scholar]
- Vadaie N, Hulinsky RS. Jarvis DL. Identification and characterization of a Drosophila melanogaster ortholog of human beta1,4-galactosyltransferase VII. Glycobiology. 2002;12:589–597. doi: 10.1093/glycob/cwf074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandall HH, Pedersen JW, Park C, Levery SB, Pizette S, Cohen SM, Schwientek T. Clausen H. Drosophila egghead encodes a beta 1,4-mannosyltransferase predicted to form the immediate precursor glycosphingolipid substrate for brainiac. J. Biol. Chem. 2003;278:1411–1414. doi: 10.1074/jbc.C200619200. [DOI] [PubMed] [Google Scholar]
- Wilson IB. Functional characterization of Drosophila melanogaster peptide O-xylosyltransferase, the key enzyme for proteoglycan chain initiation and member of the core 2/I N-acetylglucosaminyltransferase family. J. Biol. Chem. 2002;277:21207–21212. doi: 10.1074/jbc.M201634200. [DOI] [PubMed] [Google Scholar]
- Xu D, Song D, Pedersen LC. Liu J. Mutational study of heparan sulfate 2-O-sulfotransferase and chondroitin sulfate 2-O-sulfotransferase. J. Biol. Chem. 2007;282:8356–8367. doi: 10.1074/jbc.M608062200. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Hino M. Goto S. In vivo RNAi-based screens: studies in model organisms. Genes. 2013;4:646–665. doi: 10.3390/genes4040646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto-Hino M, Kanie Y, Awano W, Aoki-Kinoshita KF, Yano H, Nishihara S, Okano H, Ueda R, Kanie O. Goto S. Identification of genes required for neural-specific glycosylation using functional genomics. PLoS Genet. 2010;6:e1001254. doi: 10.1371/journal.pgen.1001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto-Hino M, Okano H, Kanie O. Goto S. Structure, Function and Formation of Glycans in Drosophila. In: Mora Montes HM, editor; Glycans: Biochemistry, Characterization and Applications. Hauppauge, NY: Nova Science Publishers, Inc; 2012. pp. 165–188. [Google Scholar]
- Yoshida H, Fuwa TJ, Arima M, Hamamoto H, Sasaki N, Ichimiya T, Osawa K, Ueda R. Nishihara S. Identification of the Drosophila core 1 beta1,3-galactosyltransferase gene that synthesizes T antigen in the embryonic central nervous system and hemocytes. Glycobiology. 2008;18:1094–1104. doi: 10.1093/glycob/cwn094. [DOI] [PubMed] [Google Scholar]
- Zhu X, Sen J, Stevens L, Goltz JS. Stein D. Drosophila pipe protein activity in the ovary and the embryonic salivary gland does not require heparan sulfate glycosaminoglycans. Development. 2005;132:3813–3822. doi: 10.1242/dev.01962. [DOI] [PubMed] [Google Scholar]