Abstract
Articular cartilage injuries and degenerative joint diseases are responsible for progressive pain and disability in millions of people worldwide, yet there is currently no treatment available to restore full joint functionality. As the tissue functions under mechanical load, an understanding of the physiologic or pathologic effects of biomechanical factors on cartilage physiology is of particular interest. Here we highlight studies that have measured cartilage deformation at scales ranging from the macroscale to the microscale, as well as the responses of the resident cartilage cells, chondrocytes, to mechanical loading using in vitro and in vivo approaches. From these studies, it is clear that there exists a complex interplay between mechanical, inflammatory, and biochemical factors that can either support or inhibit cartilage matrix homeostasis under normal or pathologic conditions. Understanding these interactions is an important step toward developing tissue engineering approaches and therapeutic interventions for cartilage pathologies.
Keywords: Chondrocyte, Osteoarthritis, Mechanotransduction, Loading, Strain, Deformation, Magnetic Resonance Imaging, Atomic Force Microscopy, Pericellular Matrix, Extracellular Matrix, Inflammation, Interleukin-1, Proinflammatory Cytokines, Animal Models, Growth Factors
1: Introduction
Articular cartilage serves a critical mechanical role in diarthrodial joints by providing a smooth, lubricated surface that allows joint articulation while minimizing wear. It also acts to support and distribute forces across the joint during activities of daily living. Under normal physiologic conditions, the cells in cartilage, chondrocytes, synthesize and maintain crucial extracellular matrix (ECM) components that confer the functional properties of cartilage [1]. However, under pathologic conditions, such as osteoarthritis (OA), chondrocytes exhibit an imbalance of anabolic and catabolic activities that are characterized by degenerative changes in the cartilage matrix and other joint tissues, including the subchondral bone and synovium [2, 3].
Far more than a disease of simple “wear and tear”, OA represents a family of diseases that involve active processes by which cartilage and the surrounding tissues respond pathologically to environmental factors, particularly mechanical loading. Physical activity in healthy, asymptomatic adults reduces the risk of cartilage thinning, cartilage defects, and bone marrow lesions [4], demonstrating the protective role that joint loading can play in a physiologic biochemical and biomechanical environment. On the other hand, biomechanical risk factors for OA, such as obesity, trauma, and joint destabilization [5], illustrate the central role that mechanical factors in an altered biochemical and/or biomechanical setting can have in OA development and progression [6]. In this regard, understanding the interplay between cartilage loading and other biological factors within the joint can provide insight into the factors that influence OA disease progression, and may help identify targets for therapeutic intervention. The goal of this review is to examine the interactions of these biomechanical and biological factors in cartilage and their effects on chondrocytes to help inform our understanding of cartilage diseases, such as OA.
2: Cartilage Loading and Deformation in Health and Disease
2.1 Cartilage Tissue Level Deformation
In healthy cartilage, the unique composition and complex structure of the ECM confers specific mechanical properties that allow the tissue to withstand a lifetime of cyclic loading deformation [1]. Cartilage ECM is primarily composed of water, negatively-charged proteoglycans, and fibrillar and non-fibrillar collagens. During cartilage loading, water in this highly hydrated tissue is gradually squeezed out of the tissue, causing direct tissue, cellular, and nuclear strains [7]. Concomitantly, mechanical loading of cartilage generates secondary biophysical signals such as hydrostatic and osmotic pressures, and their importance in cartilage mechanobiology will be discussed in later sections.
Deformation caused by mechanical loading is typically reported as strain, defined in one dimension as the change in thickness divided by the original thickness. The measurement of cartilage strains under in vivo loading conditions has been challenging; however, recent advances in imaging, such as magnetic resonance imaging (MRI), alone or in combination with high-speed dual-fluoroscopy, have allowed measurements of cartilage deformation during or after various activities (Figure 1A) [8–6]. These studies show that cartilage strains are dependent on both the anatomic location within the joint, as well as the specific activity undertaken. For example, a relatively short bout of running (20 minutes) leads to transient cartilage strains of approximately 20% in the weight bearing regions of the femur, while in the tibia, strains of up to 30% are observed following activity [11]. With walking, peak strains in the tibiofemoral contact area range from 7 to 23%, and tend to result in higher strains on the medial side of the joint [15]. Due to the biphasic (solid/fluid) nature of cartilage, the tissue exhibits significant viscoelastic behavior and takes time to recover its original height after loading. Thus, the repeated loading of the tissue over the course of a day, without time to recover to its original height, results in decreased cartilage thickness from morning to evening. This strain accumulates through the course of the day and recovers overnight. Diurnal cartilage strains vary significantly with location in the knee, ranging from 11% (medial tibia) to no significant diurnal strain in certain locations, such as the femoral groove [9].
Figure 1.
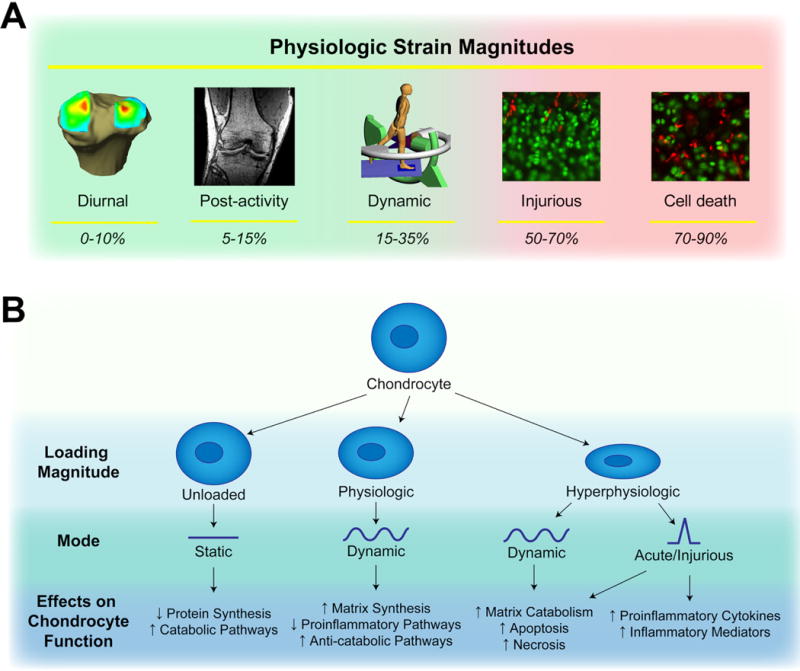
A. Physiologic strain magnitudes measured in articular cartilage. During normal activities, diurnal strains range from 0–10% [9, 10], post activity strains range from 5–15% [12–14, 102], and dynamic strains during activity range from 15–35% [15, 16]. At higher nominal strain magnitudes (50–70%), mechanical compression can cause injury [35–39, 41], eventually inducing cell death via necrosis and apoptosis at strains of the highest levels (70–90%) [40, 47, 48]. B. Effects of different loading conditions on chondrocyte function. Static loading decreases cartilage metabolic activity [46], physiologic levels of dynamic loading can be anabolic or anti-inflammatory [42, 45, 85, 91, 95, 96], while hyperphysiologic levels of dynamic loading and injurious loading can induce catabolic or pro-inflammatory response [41, 49, 50, 91].
Cartilage strains also vary significantly with injury and disease, and these altered loading patterns may impact subsequent OA progression. In individuals with a high body mass index (BMI), a known risk factor for OA, the diurnal cartilage strain in the medial tibia increases significantly from 3% to 5% [10]. In another example, full weight bearing in the ankles of patients suffering from chronic lateral ankle instability increases cartilage strain by 8% when compared to the uninjured ankle [8]. Furthermore, the location of the peak strain also moves anteriorly and laterally, which corresponds to the location where patients with lateral ankle instability tend to develop OA. Similarly, loss of the anterior cruciate ligament (ACL), another risk factor for OA development, also coincides with increased knee strains upon weight bearing [16]. On the other hand, deep knee bends cause less strain in knee cartilage of older individuals (ages 50–78) than in young individuals (ages 20–30), indicating that age can have an effect on cartilage deformation [12]. These examples illustrate the importance of cartilage loading patterns on maintaining cartilage health, and underscore cartilage loading as a critical factor toward understanding cartilage pathology.
2.2 The Micromechanical Environment of Chondrocytes
As the tissue is compressed, the hierarchical structure of articular cartilage results in a complex and non-uniform deformation field at the tissue and cell levels, which in turn may influence the responses of chondrocytes to joint loading [17–19]. In particular the chondron, which encompasses both the chondrocyte and its pericellular matrix (PCM), shows variable deformation in different zones of the tissue when cartilage is subjected to macroscale compression [20]. In the superficial zone, where the ECM modulus is the lowest, cells within the chondron are shielded from ECM strains as compared to those in the deeper zones, where the PCM serves to amplify strain magnitudes relative to those in the ECM. This finding indicates that the PCM plays a role in regulating cellular strains throughout the tissue depth to provide a more uniform environment for the chondrocytes, perhaps protecting the cells from injurious strain [20–22]. In this context, the PCM has emerged as a potential transducer of mechanical signals in cartilage, showing an ability to either amplify or attenuate local mechanical strains, as well as to convert tissue deformation to physicochemical [23] or biochemical changes [24] in the chondrocyte microenvironment.
The ability of the PCM to perform these functions is tied to its unique structure and biochemical composition, which impart specific biomechanical and physicochemical properties. The mechanical properties of the PCM have been measured using a variety of techniques, including micropipette aspiration [25], atomic force microscopy (AFM) [26, 27], and computational models [28]. Together, these techniques confirm that the chondrocyte PCM has an intermediate modulus between the modulus of the cell and the surrounding ECM. More recent advances in AFM techniques have allowed for spatial mapping in situ of PCM and local ECM properties, and have revealed that the cartilage PCM is isotropic, and its modulus is constant throughout the depth of the tissue [29]. Interestingly, these findings are in stark contrast with local ECM properties, which show distinct anisotropy and zonal variations in elastic modulus [29].
More recent investigations in specific PCM molecular constituents via combined immunofluorescence staining and AFM probing of PCM properties indicate that the biomechanical properties of the PCM is heavily influenced by its biochemical makeup [27, 30]. Of particular interest is the finding that the PCM shows high resistance to enzymatic degradation [31], which may help protect the cell from tissue breakdown in an inflammatory environment, such as that observed in OA. It is known, however, that the chondrocyte PCM is enlarged and less stiff in OA cartilage, as compared to healthy cartilage [32]. Thus, despite some innate resistance to degradation, the micromechanical environment of the chondrocyte can be affected in OA. The precise cause of these changes remains under investigation, but the increased synthesis of matrix macromolecules as well as degradative enzymes (i.e., matrix metalloproteases (MMPs), aggrecanases, elastase, etc.) in the OA joint play a role in this phenomenon. A potential early event may be the disruption of the PCM by the serine protease, high temperature requirement A1 (HTRA1), which can be induced by TGF-β1 upon biomechanical stress [33].
3: Mechanical Loading at the Chondrocyte Level
3.1 Effects of Loading on Chondrocytes
Chondrocytes respond to mechanical load, as a means of regulating growth, cellular differentiation, and metabolism in the cartilage ECM, throughout development and maturation. However, while mechanical loading of chondrocytes is an important stimulator of matrix synthesis, certain types of loading can provoke pathologic responses. This contrast between the protective versus pathologic response of chondrocytes to mechanical loading is well documented in studies of physiologic loading and cartilage injury (Figure 1B) [34–41]. Cartilage responds to physiologic magnitudes of dynamic compression (~10–20%) with enhanced synthesis of ECM molecules, including proteoglycans, collagens, and cartilage oligomeric matrix protein (COMP) [42–45]. Importantly, the responses of chondrocytes to mechanical loading are highly dependent on parameters such as frequency, strain-rate, loading history, and loading amplitude. For example, super-physiologic magnitudes of loading (>20%) fail to enhance matrix production [42], while static or very low frequency loading [46] inhibits matrix synthesis. The damaging effects of high magnitude, high strain-rate impact loading are likely a combination of direct cellular damage, such as chondrocyte apoptosis and necrosis [47, 48], as well as a shift of chondrocyte-mediated matrix metabolism towards catabolism [41, 49, 50]. Interestingly, the surviving cell population after impact loading lacks a biosynthetic response to dynamic, physiologic levels of loading that uninjured explants normally exhibit [50], suggesting that sustained alteration of chondrocyte mechanotransduction occurs following injury, even when the tissue is returned to an apparently normal biomechanical setting.
In the cartilage ECM, highly negatively charged sulfated proteoglycans attract counterions to maintain electroneutrality, which in turn creates an osmotic differential with the synovial fluid. This osmotic gradient confers a swelling pressure to the proteoglycans to expand, but their expansion is restrained by the collagen network. As the tissue is compressed, water is gradually exuded from the tissue. However, during the initial stages of cartilage loading, the low permeability of the tissue leads to fluid pressurization, exposing the chondrocytes to increases in hydrostatic pressure. With prolonged and/or higher magnitude loads, exudation of interstitial water and ions leads to not only direct deformation, but also to the generation of numerous indirect biophysical signals, such as streaming potentials, fluid flow, and changes in local pH and osmolarity [51]. These phenomena are largely due to the compaction of the entangled proteoglycans increasing the fixed charge density, thus increasing the concentration of dissolved ions within the tissue. With removal of loading, water is reabsorbed, and the biophysical environment returns to its initial state, leading to dynamic changes in these parameters with dynamic loading regimes.
The effects and significance of these dynamic biophysical signals have constituted the subject of a number of recent investigations. For example, chondrocytes are known to increase ECM production in response to dynamic hydrostatic pressure [52, 53] and dynamic osmotic changes [54], similar to their response to mechanical loading. Though it is unclear whether chondrocytes in loaded tissue in vivo are responding to deformation of the tissue and cells or to these secondary biophysical effects, in vitro experiments that deliver individual mechanical or biophysical signals suggest that each of these parameters contributes to mechanical regulation of cartilage homeostasis. Furthermore, defining the mechanisms of mechanotransduction should be highly useful in addressing this question.
3.2 Chondrocyte Mechanotransduction
A number of key transduction mechanisms have been identified that facilitate the mechanically-driven enhancement of cartilage ECM biosynthesis and functional properties, including mechanosensitive ion channels [55, 56] and signaling through integrins [57] and primary cilia [58]. Transient receptor potential vanilloid 4 (TRPV4) is an osmo-mechanosensitive ion channel highly expressed in articular chondrocytes [55]. TRPV4-mediated Ca2+ signaling in response to mechanical loading plays a primary role in the enhanced matrix biosynthesis and decreased expression of catabolic and proinflammatory genes in chondrocytes after moderate, dynamic loading [54]. Specifically inhibiting TRPV4 prevents loading-mediated increases in matrix synthesis, and activating TRPV4 in the absence of loading increases matrix synthesis in a manner analogous to loading [54]. As a ubiquitous second-messenger, mechanically-induced Ca2+ signaling is an especially attractive regulator of mechanotransduction, as it is known to regulate multiple signaling pathways, including nuclear factor of activated T lymphocytes (NFAT), protein kinase C, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), c-Jun N-terminal kinase 1 (JNK1), and cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB) [59]. In superficial zone chondrocytes, the mechanically-driven enhancement of Prg4 (lubricin) expression, appears to involve a number of signaling pathways that also involve intracellular Ca2+ signaling, including ATP/P2X7 and PKA/CREB [60]. Additional studies have further proposed TGF-β/Smad, Erk1/2, p38, and ciliary signaling [55, 61], as well as integrin/FAK signaling [62–64] in mediating the responses of chondrocytes to loading.
Though less is known about the transduction of mechanical load in pathologic settings, these processes appear to involve the enhancement of catabolic effectors, such as MMPs and aggrecanases [65, 66], and recent studies suggest the involvement of ciliary signaling [67] and histone modification [68]. Further mechanistic studies in the setting of injurious loading, or joint impact followed by physiologic loading, will reveal clinically relevant information for the treatment of patients following destabilizing and traumatic joint injuries.
3.3 Animal Models of Cartilage Loading
Animal models of joint loading provide additional data supporting the role of mechanical factors in cartilage physiology, as well as pathology. The two most common animal models of altered joint loading are ACL transection (ACLT), which causes increased joint laxity [69], and meniscal injury or meniscectomy, which alters cartilage contact pressure distributions [70]. Both of these models lead to degenerative joint changes similar to human joint injury [71, 72], and have been effective in identifying a number of disease-modifying enzymes, including ADAMTS5 [73], MMP-13 [74], and PAR-2 [75]. Furthermore, knockout mouse models have shed light on the interaction between mechanical loading and arthritis by examining OA development in mice lacking critical elements in mechanotransduction pathways. For example, mice lacking TRPV4 develop OA earlier than wild type mice [76], and mice lacking type VI collagen, a key structural element of the PCM, have softer PCMs and develop OA in the hip earlier than wild type mice [25]. Mice lacking primary cilia also show signs of OA development [77]. Altered joint loading by ACLT or meniscal injury combined with knockout mouse models will likely play an integral role in unraveling the complicated interaction of pathologic loading and cartilage homeostasis.
4: Interaction of Cartilage Loading and Biochemical Factors
4.1 Biomechanics and Inflammation
Injury and arthritic degradation affect both the biomechanical as well as the biochemical environments of cartilage. Following joint injury and in OA joints, inflammatory cytokines are upregulated [78], with median synovial fluid concentrations of IL-1α and IL-1β rising to 43 pg/mL and 109 pg/mL, respectively, in mild OA joints, and 288 pg/mL and 122 pg/mL in moderate OA joints [79]. While OA is known to inhibit secretion of the active form of IL-1β in articular cartilage [80], inflammatory cytokines are known to be produced by other tissues in the joint, such as the synovium [81] and infrapatellar fat pad [82]. Treatment of articular cartilage explants with physiological concentrations of IL-1 (which are far lower than most in vitro studies) increases Ca2+ signaling, MMP activity, sulfated glycosaminoglycan (S-GAG) degradation, release of the proinflammatory mediator nitric oxide (NO), and leads to decreases in the mechanical properties of healthy cartilage [79]. The pleiotropic and concurrent effects of catabolic mediators on the biochemical and biomechanical properties of the cartilage environment highlight the interrelationship that exists between biomechanical factors and inflammatory factors in the joint [83, 84].
Generally, physiologic magnitudes of mechanical loading suppress the proinflammatory and catabolic effects of IL-1, while injurious magnitudes of loading activate proinflammatory and catabolic pathways leading to cartilage degradation. Dynamic compression of articular cartilage explants at physiologic magnitudes blocks IL-1 induced increases in the mRNA levels of the degradative enzymes ADAMTS-4, ADAMTS-5, MMP-1, and MMP-3 [85] and aggrecan breakdown [86], and increases TIMP-3 expression, suggesting a net decrease in MMP activity under these conditions [85]. Dynamic 15% compression of agarose-embedded primary chondrocytes also decreases IL-1 mediated production of the proinflammatory mediators NO and prostaglandin E2 (PGE2) and increases matrix biosynthesis rates [87]. Additional studies using the agarose-embedded chondrocyte model system in the presence of IL-1 and inhibitors of the MAPK signaling pathways have shown that dynamic compression increases chondrocyte proliferation and proteoglycan synthesis, suggesting the potential therapeutic benefit of biophysical and/or pharmacologic interventions to block IL-1 induced cartilage degradation [88]. Chondrocytes in two-dimensional bioreactor systems also respond to cyclic tensile strain with a reduction in IL-1-induced catabolic activity (nitric oxide synthase 2 (NOS2), cyclooxygenase 2 (COX2), and MMP-1 mRNA and protein levels) and a loading-mediated enhancement of chondrosupportive gene expression (TIMP-2, type II collagen, and aggrecan mRNA levels) and proteoglycan synthesis [89].
Individually, dynamic strain and IL-1 induce similar signaling cascades, such as ERK1/2 phosphorylation [90]. The differential effects of these two stimuli, however, may lie upstream of ERK1/2 phosphorylation. For example, IL-1 activates B-Raf kinase activity, while dynamic strain causes the activation of c-Raf kinase activity, and furthermore, causes inhibition of IL-1 induced B-Raf activation. Perhaps these unique signaling phenomena may explain the differential processing of mechanical signals in the presence of inflammation. ERK1/2 activation also occurs three-times faster in response to mechanical signals than IL-1. Therefore, perhaps by upstream activation of kinases in response to dynamic strain, initiates a feedback loop to suppress the signaling cascades activated by IL-1 [90].
Experimental and theoretical modeling studies reveal that inflammation is differentially regulated at low (10%) and high (30%) magnitudes of dynamic compressive strain [91]. In the presence of IL-1 at low magnitudes, NOS2 transcription is suppressed and this correlates with attenuation of the NF-κB signaling pathway, which activates transcription of proinflammatory genes. At high magnitudes of dynamic compressive strain, NOS2 expression is activated, promoting a proinflammatory environment with pathologic loading. Furthermore, static compression of cartilage explants at 50% strain in the presence of IL-1 receptor antagonist (IL 1ra) increases proteoglycan synthesis and upregulates IL-1 and NOS2 transcription [92]. These findings further suggest that injurious magnitudes of loading activate proinflammatory mediators and ultimately catabolic pathways that lead to cartilage degradation. Furthermore, a recent study has shown that immobilization can prevent degenerative changes in a mouse model of joint injury by decreasing mechanically-induced protease expression, further demonstrating the important role of mechanical loading [93]. While the complex signaling and regulatory cascades between biomechanical factors and inflammatory mediators in cartilage are slowly being elucidated, the effects of a variety of other biochemical factors, such as anabolic growth factors, and mechanical loading on chondrocytes must also be considered in order to identify potential targets for OA therapy.
4.2 Effects of Load and Growth Factors on Chondrocytes
Mechanical stimulation of cartilage also exhibits complex interactions with anabolic factors and processes. Many studies have shown that incubation with growth factors and mechanical stimulation of either cartilage explants or isolated chondrocytes can cause additive or synergistic effects on matrix synthesis and organization [24, 94–98]. For example, dynamic loading enhances the effects of insulin-like growth factor-I (IGF-I) on proteoglycan and collagen synthesis in articular cartilage explants in a synergistic manner [95]. This synergy is also observed when chondrocyte-seeded agarose is exposed to dynamic loading in combination with IGF-I or transforming growth factor beta-1 (TGF-β1) [96]. Static compression, on the other hand, significantly diminishes the anabolic effect of IGF-I [97].
Hydrostatic pressure also interacts strongly with anabolic growth factors. For example, the combination of TGF-β1 and hydrostatic pressure cause additive effects on aggregate and Young’s modulus of self-assembled cartilage tissue and synergistic effects on collagen content [98]. Rather than simply integrating the external cues of soluble anabolic factors, chondrocytes appear to also modulate the endogenous production and signaling of these anabolic pathways when exposed to biomechanical cues [54, 99]. Furthermore, this two-way interaction between biomechanical cues and growth factor signaling provides a potential mechanism for how growth factor signaling is both altered by and influences OA progression [100].
A complete understanding of how mechanical stimulation interacts with growth factors has yet to be achieved, but each new investigation illuminates the potential mechanisms of these interactions. For example, the PCM surrounding each chondrocyte functions not only as a mechanical transducer, but also serves to sequester and retain growth factors in the microenvironment of the chondrocyte. In fact, the pericellular component perlecan is uniquely able to sequester basic fibroblast growth factor (bFGF) [94], which is essential for signal transduction during articular cartilage loading [24]. However, aberrant mechanical stimulation, such as that observed in OA joints, is also associated with increases in TGF-β1 which can trigger production of HTRA1, a protease that degrades PCM components [33]. Therefore, interactions between articular cartilage loading and the biochemical factors surrounding chondrocytes can have a significant effect on chondrocyte metabolism. Interactions, such as these, that show promise in producing cartilage matrix components may be used to inhibit OA progression and/or regenerate cartilage.
5: Conclusions
The lack of therapeutic interventions following cartilage injury or disease and the important mechanical function of the tissue has prompted numerous studies investigating the influence of loading on cartilage homeostasis and metabolism. These studies have revealed the complex biochemical and biomechanical hierarchy of articular cartilage and the important roles of loading, inflammation, and growth factors on chondrocyte signaling pathways. It is clear that inflammatory mediators, such as IL-1, play a significant role in modulating the response of chondrocytes to mechanical load, and that depending on the mode, magnitude, duration of application, and combination with growth factors, mechanical loading can have either beneficial or detrimental effects on the tissue.
Given the strong links between inflammation, mechanical load, and cartilage homeostasis, targeting receptors of inflammatory mediators and chondrocyte mechanotransduction machinery, such as the TRPV4 ion channel and primary cilia, may be direct ways of controlling the response of chondrocytes to pathologic loading or disease [101]. While more work needs to be done to understand chondrocyte signaling in pathologic conditions, the present data support biomechanics and mechanobiology of articular cartilage as crucial regulators of cartilage health and disease.
Acknowledgments
Supported in part by the Arthritis Foundation and NIH grants AR48182, AR48852, AG15768, AR50245, AG46927, and AG40868.
References
- 1.Mow VC, Proctor CS, Kelly MA. Biomechanics of Articular Cartilage. In: Nordin M, Frankel V, editors. Basic Biomechanics of the Muskuloskeletal system. 2nd. Lea and Febiger; Philadelphia: 1989. [Google Scholar]
- 2.Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis & Rheumatism. 2000;43(9):1916–1926. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 3.Loeser RF, Goldring SR, Scanzello CR, et al. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Racunica TL, Teichtahl AJ, Wang Y, et al. Effect of physical activity on articular knee joint structures in community-based adults. Arthritis Rheum. 2007;57(7):1261–8. doi: 10.1002/art.22990. [DOI] [PubMed] [Google Scholar]
- 5.Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006;20(1):3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25(6):815–23. doi: 10.1016/j.berh.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mow VC, Bachrach NM, Setton LA, et al. Stress, Strain, Pressure and Flow Fields in Articular Cartilage and Chondrocytes. In: Mow VC, et al., editors. Cell Mechanics and Cellular Engineering. Springer; New York: 1994. pp. 345–379. [Google Scholar]
- 8.Bischof JE, Spritzer CE, Caputo AM, et al. In vivo cartilage contact strains in patients with lateral ankle instability. J Biomech. 2010;43(13):2561–6. doi: 10.1016/j.jbiomech.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman JL, Widmyer MR, Leddy HA, et al. Diurnal variations in articular cartilage thickness and strain in the human knee. J Biomech. 2013;46(3):541–7. doi: 10.1016/j.jbiomech.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widmyer MR, Utturkar GM, Leddy HA, et al. High body mass index is associated with increased diurnal strains in the articular cartilage of the knee. Arthritis Rheum. 2013;65(10):2615–22. doi: 10.1002/art.38062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosher TJ, Smith HE, Collins C, et al. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology. 2005;234(1):245–9. doi: 10.1148/radiol.2341040041. [DOI] [PubMed] [Google Scholar]
- 12.Eckstein F, Hudelmaier M, Putz R. The effects of exercise on human articular cartilage. J Anat. 2006;208(4):491–512. doi: 10.1111/j.1469-7580.2006.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckstein F, Tieschky M, Faber S, et al. Functional analysis of articular cartilage deformation, recovery, and fluid flow following dynamic exercise in vivo. Anat Embryol (Berl) 1999;200(4):419–24. doi: 10.1007/s004290050291. [DOI] [PubMed] [Google Scholar]
- 14.Eckstein F, Tieschky M, Faber SC, et al. Effect of physical exercise on cartilage volume and thickness in vivo: MR imaging study. Radiology. 1998;207(1):243–8. doi: 10.1148/radiology.207.1.9530322. [DOI] [PubMed] [Google Scholar]
- 15.Liu F, Kozanek M, Hosseini A, et al. In vivo tibiofemoral cartilage deformation during the stance phase of gait. J Biomech. 2010;43(4):658–65. doi: 10.1016/j.jbiomech.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van de Velde SK, Bingham JT, Hosseini A, et al. Increased tibiofemoral cartilage contact deformation in patients with anterior cruciate ligament deficiency. Arthritis Rheum. 2009;60(12):3693–702. doi: 10.1002/art.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae WC, Lewis CW, Levenston ME, et al. Indentation testing of human articular cartilage: effects of probe tip geometry and indentation depth on intra-tissue strain. J Biomech. 2006;39(6):1039–47. doi: 10.1016/j.jbiomech.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Chen AC, Bae WC, Schinagl RM, et al. Depth- and strain-dependent mechanical and electromechanical properties of full-thickness bovine articular cartilage in confined compression. J Biomech. 2001;34(1):1–12. doi: 10.1016/s0021-9290(00)00170-6. [DOI] [PubMed] [Google Scholar]
- 19.Schinagl RM, Ting MK, Price JH, et al. Video microscopy to quantitate the inhomogeneous equilibrium strain within articular cartilage during confined compression. Ann Biomed Eng. 1996;24(4):500–12. doi: 10.1007/BF02648112. [DOI] [PubMed] [Google Scholar]
- 20.Choi JB, Youn I, Cao L, et al. Zonal changes in the three-dimensional morphology of the chondron under compression: the relationship among cellular, pericellular, and extracellular deformation in articular cartilage. J Biomech. 2007;40(12):2596–603. doi: 10.1016/j.jbiomech.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poole CA, Ayad S, Schofield JR. Chondrons from articular cartilage: I. Immunolocalization of type VI collagen in the pericellular capsule of isolated canine tibial chondrons. J Cell Sci. 1988;90(Pt 4):635–43. doi: 10.1242/jcs.90.4.635. [DOI] [PubMed] [Google Scholar]
- 22.Poole CA, Flint MH, Beaumont BW. Chondrons in cartilage: ultrastructural analysis of the pericellular microenvironment in adult human articular cartilages. J Orthop Res. 1987;5(4):509–22. doi: 10.1002/jor.1100050406. [DOI] [PubMed] [Google Scholar]
- 23.Haider MA, Schugart RC, Setton LA, et al. A mechano-chemical model for the passive swelling response of an isolated chondron under osmotic loading. Biomech Model Mechanobiol. 2006;5(2–3):160–71. doi: 10.1007/s10237-006-0026-1. [DOI] [PubMed] [Google Scholar]
- 24.Vincent TL, Hermansson MA, Hansen UN, et al. Basic fibroblast growth factor mediates transduction of mechanical signals when articular cartilage is loaded. Arthritis Rheum. 2004;50(2):526–33. doi: 10.1002/art.20047. [DOI] [PubMed] [Google Scholar]
- 25.Alexopoulos LG, Youn I, Bonaldo P, et al. Developmental and osteoarthritic changes in Col6a1-knockout mice: biomechanics of type VI collagen in the cartilage pericellular matrix. Arthritis Rheum. 2009;60(3):771–9. doi: 10.1002/art.24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darling EM, Wilusz RE, Bolognesi MP, et al. Spatial mapping of the biomechanical properties of the pericellular matrix of articular cartilage measured in situ via atomic force microscopy. Biophys J. 2010;98(12):2848–56. doi: 10.1016/j.bpj.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilusz RE, Defrate LE, Guilak F. Immunofluorescence-guided atomic force microscopy to measure the micromechanical properties of the pericellular matrix of porcine articular cartilage. J R Soc Interface. 2012;9(76):2997–3007. doi: 10.1098/rsif.2012.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim E, Guilak F, Haider MA. An axisymmetric boundary element model for determination of articular cartilage pericellular matrix properties in situ via inverse analysis of chondron deformation. J Biomech Eng. 2010;132(3):031011. doi: 10.1115/1.4000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLeod MA, Wilusz RE, Guilak F. Depth-dependent anisotropy of the micromechanical properties of the extracellular and pericellular matrices of articular cartilage evaluated via atomic force microscopy. J Biomech. 2013;46(3):586–92. doi: 10.1016/j.jbiomech.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilusz RE, Defrate LE, Guilak F. A biomechanical role for perlecan in the pericellular matrix of articular cartilage. Matrix Biol. 2012;31(6):320–7. doi: 10.1016/j.matbio.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilusz RE, Guilak F. High resistance of the mechanical properties of the chondrocyte pericellular matrix to proteoglycan digestion by chondroitinase, aggrecanase, or hyaluronidase. J Mech Behav Biomed Mater. 2013 doi: 10.1016/j.jmbbm.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilusz RE, Zauscher S, Guilak F. Micromechanical mapping of early osteoarthritic changes in the pericellular matrix of human articular cartilage. Osteoarthritis Cartilage. 2013 doi: 10.1016/j.joca.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu L, Golshirazian I, Asbury BJ, et al. Induction of high temperature requirement A1, a serine protease, by TGF-beta1 in articular chondrocytes of mouse models of OA. Histology and Histopathology. 2014;29(5):609–618. doi: 10.14670/HH-29.10.609. [DOI] [PubMed] [Google Scholar]
- 34.Guilak F, Hung CT. Physical Regulation of Cartilage Metabolism. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechanobiology. 3rd. Lippincott, Williams & Wilkins; Philedalphia, PA: 2004. pp. 259–300. [Google Scholar]
- 35.Patwari P, Cheng DM, Cole AA, et al. Analysis of the relationship between peak stress and proteoglycan loss following injurious compression of human post-mortem knee and ankle cartilage. Biomech Model Mechanobiol. 2007;6(1–2):83–9. doi: 10.1007/s10237-006-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patwari P, Gaschen V, James IE, et al. Ultrastructural quantification of cell death after injurious compression of bovine calf articular cartilage. Osteoarthritis Cartilage. 2004;12(3):245–52. doi: 10.1016/j.joca.2003.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurz B, Lemke A, Kehn M, et al. Influence of tissue maturation and antioxidants on the apoptotic response of articular cartilage after injurious compression. Arthritis Rheum. 2004;50(1):123–30. doi: 10.1002/art.11438. [DOI] [PubMed] [Google Scholar]
- 38.Patwari P, Cook MN, DiMicco MA, et al. Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum. 2003;48(5):1292–301. doi: 10.1002/art.10892. [DOI] [PubMed] [Google Scholar]
- 39.Kurz B, Jin M, Patwari P, et al. Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J Orthop Res. 2001;19(6):1140–6. doi: 10.1016/S0736-0266(01)00033-X. [DOI] [PubMed] [Google Scholar]
- 40.Loening AM, I, James E, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381(2):205–12. doi: 10.1006/abbi.2000.1988. [DOI] [PubMed] [Google Scholar]
- 41.Quinn TM, Grodzinsky AJ, Hunziker EB, et al. Effects of injurious compression on matrix turnover around individual cells in calf articular cartilage explants. J Orthop Res. 1998;16(4):490–9. doi: 10.1002/jor.1100160415. [DOI] [PubMed] [Google Scholar]
- 42.Wong M, Siegrist M, Cao X. Cyclic compression of articular cartilage explants is associated with progressive consolidation and altered expression pattern of extracellular matrix proteins. Matrix Biology. 1999;18(4):391–9. doi: 10.1016/s0945-053x(99)00029-3. [DOI] [PubMed] [Google Scholar]
- 43.Buschmann MD, Kim YJ, Wong M, et al. Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow. Arch Biochem Biophys. 1999;366(1):1–7. doi: 10.1006/abbi.1999.1197. [DOI] [PubMed] [Google Scholar]
- 44.Mauck RL, Soltz MA, Wang CC, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122(3):252–60. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 45.Ng KW, Mauck RL, Wang CC, et al. Duty Cycle of Deformational Loading Influences the Growth of Engineered Articular Cartilage. Cell Mol Bioeng. 2009;2(3):386–394. doi: 10.1007/s12195-009-0070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guilak F, Meyer BC, Ratcliffe A, et al. The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthritis Cartilage. 1994;2(2):91–101. doi: 10.1016/s1063-4584(05)80059-7. [DOI] [PubMed] [Google Scholar]
- 47.Stolberg-Stolberg JA, Furman BD, Garrigues NW, et al. Effects of cartilage impact with and without fracture on chondrocyte viability and the release of inflammatory markers. J Orthop Res. 2013;31(8):1283–92. doi: 10.1002/jor.22348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Natoli RM, Scott CC, Athanasiou KA. Temporal effects of impact on articular cartilage cell death, gene expression, matrix biochemistry, and biomechanics. Ann Biomed Eng. 2008;36(5):780–92. doi: 10.1007/s10439-008-9472-5. [DOI] [PubMed] [Google Scholar]
- 49.Chan PS, Schlueter AE, Coussens PM, et al. Gene expression profile of mechanically impacted bovine articular cartilage explants. J Orthop Res. 2005;23(5):1146–51. doi: 10.1016/j.orthres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 50.Ashwell MS, Gonda MG, Gray K, et al. Changes in chondrocyte gene expression following in vitro impaction of porcine articular cartilage in an impact injury model. J Orthop Res. 2013;31(3):385–91. doi: 10.1002/jor.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guilak F, V, Mow C. The mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions in articular cartilage. J Biomech. 2000;33(12):1663–73. [PubMed] [Google Scholar]
- 52.Smith RL, Lin J, Trindade MC, et al. Time-dependent effects of intermittent hydrostatic pressure on articular chondrocyte type II collagen and aggrecan mRNA expression. Journal of Rehabilitation Research and Development. 2000;37(2):153–61. [PubMed] [Google Scholar]
- 53.Smith RL, Rusk SF, Ellison BE, et al. In vitro stimulation of articular chondrocyte mRNA and extracellular matrix synthesis by hydrostatic pressure. J Orthop Res. 1996;14(1):53–60. doi: 10.1002/jor.1100140110. [DOI] [PubMed] [Google Scholar]
- 54**.O’Conor CJ, Leddy HA, Benefield HC, et al. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci U S A. 2014;111(4):1316–21. doi: 10.1073/pnas.1319569111. This study identifies TRPV4-mediated intracellular Ca2+ signaling as a key mechanosensitive pathway in articular chondrocytes and its involvement in regulating chondrocyte ECM biosynthesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phan MN, Leddy HA, Votta BJ, et al. Functional Characterization of TRPV4 as an Osmotically Sensitive Ion Channel in Porcine Articular Chondrocytes. Arthritis Rheum. 2009;60(10):3028–3037. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mobasheri A, Barrett-Jolley R, Carter SD, et al. Functional Roles of Mechanosensitive Ion Channels, ss1 Integrins and Kinase Cascades in Chondrocyte Mechanotransduction. In: Kamkin A, Kiseleva I, editors. Mechanosensitivity in Cells and Tissues. Moscow: 2005. [PubMed] [Google Scholar]
- 57.Millward-Sadler SJ, Salter DM. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann Biomed Eng. 2004;32(3):435–46. doi: 10.1023/b:abme.0000017538.72511.48. [DOI] [PubMed] [Google Scholar]
- 58*.Wann AKT, Zuo N, Haycraft CJ, et al. Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. Faseb Journal. 2012;26(4):1663–1671. doi: 10.1096/fj.11-193649. This work demonstrates a possible link between primary cilia in chondrocytes and ATP reception, and further establishes the primary cilia as a mechanotransductive element. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dolmetsch RE, Lewis RS, Goodnow CC, et al. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386(6627):855–8. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 60**.Ogawa H, Kozhemyakina E, Hung HH, et al. Mechanical motion promotes expression of Prg4 in articular cartilage via multiple CREB-dependent, fluid flow shear stress-induced signaling pathways. Genes and Development. 2014;28(2):127–39. doi: 10.1101/gad.231969.113. This study identifies specific signaling pathways involved in the mechanical regulation of Prg4 expression in articular chondrocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61*.Bougault C, Aubert-Foucher E, Paumier A, et al. Dynamic compression of chondrocyte-agarose constructs reveals new candidate mechanosensitive genes. PLoS One. 2012;7(5):e36964. doi: 10.1371/journal.pone.0036964. Chondrocytes are shown to be highly responsive to mechanical stimulation, upregulating and downregulating a diverse array of genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chowdhury TT, Appleby RN, Salter DM, et al. Integrin-mediated mechanotransduction in IL-1 beta stimulated chondrocytes. Biomech Model Mechanobiol. 2006;5(2–3):192–201. doi: 10.1007/s10237-006-0032-3. [DOI] [PubMed] [Google Scholar]
- 63.Chai DH, Arner EC, Griggs DW, et al. Alphav and beta1 integrins regulate dynamic compression-induced proteoglycan synthesis in 3D gel culture by distinct complementary pathways. Osteoarthritis Cartilage. 2010;18(2):249–56. doi: 10.1016/j.joca.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang W, Ren K, Liu F, et al. Periodic mechanical stress stimulates the FAK mitogenic signal in rat chondrocytes through ERK1/2 activity. Cell Physiol Biochem. 2013;32(4):915–30. doi: 10.1159/000354495. [DOI] [PubMed] [Google Scholar]
- 65.Fitzgerald JB, Jin M, Dean D, et al. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J Biol Chem. 2004;279(19):19502–11. doi: 10.1074/jbc.M400437200. [DOI] [PubMed] [Google Scholar]
- 66.Lin PM, Chen CT, Torzilli PA. Increased stromelysin-1 (MMP-3), proteoglycan degradation (3B3- and 7D4) and collagen damage in cyclically load-injured articular cartilage. Osteoarthritis Cartilage. 2004;12(6):485–96. doi: 10.1016/j.joca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 67.Thompson CL, Chapple JP, Knight MM. Primary cilia disassembly down-regulates mechanosensitive hedgehog signalling: a feedback mechanism controlling ADAMTS-5 expression in chondrocytes. Osteoarthritis Cartilage. 2014;22(3):490–8. doi: 10.1016/j.joca.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saito T, Nishida K, Furumatsu T, et al. Histone deacetylase inhibitors suppress mechanical stress-induced expression of RUNX-2 and ADAMTS-5 through the inhibition of the MAPK signaling pathway in cultured human chondrocytes. Osteoarthritis Cartilage. 2013;21(1):165–74. doi: 10.1016/j.joca.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 69.Lopez MJ, Kunz D, Vanderby R, Jr, et al. A comparison of joint stability between anterior cruciate intact and deficient knees: a new canine model of anterior cruciate ligament disruption. J Orthop Res. 2003;21(2):224–30. doi: 10.1016/S0736-0266(02)00132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arunakul M, Tochigi Y, Goetz JE, et al. Replication of chronic abnormal cartilage loading by medial meniscus destabilization for modeling osteoarthritis in the rabbit knee in vivo. J Orthop Res. 2013;31(10):1555–60. doi: 10.1002/jor.22393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15(9):1061–9. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 72.Kuroki K, Cook CR, Cook JL. Subchondral bone changes in three different canine models of osteoarthritis. Osteoarthritis Cartilage. 2011;19(9):1142–9. doi: 10.1016/j.joca.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 73.Glasson SS, Askew R, Sheppard B, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–8. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 74.Little CB, Barai A, Burkhardt D, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60(12):3723–33. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amiable N, Martel-Pelletier J, Lussier B, et al. Proteinase-activated receptor-2 gene disruption limits the effect of osteoarthritis on cartilage in mice: a novel target in joint degradation. J Rheumatol. 2011;38(5):911–20. doi: 10.3899/jrheum.100710. [DOI] [PubMed] [Google Scholar]
- 76.Clark AL, Votta BJ, Kumar S, et al. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis Rheum. 2010;62(10):2973–83. doi: 10.1002/art.27624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77*.Chang CF, Ramaswamy G, Serra R. Depletion of primary cilia in articular chondrocytes results in reduced Gli3 repressor to activator ratio, increased Hedgehog signaling, and symptoms of early osteoarthritis. Osteoarthritis Cartilage. 2012;20(2):152–61. doi: 10.1016/j.joca.2011.11.009. This study shows that loss of the chondrocyte primary cilia, a putative mechanotransducer in cartilage, leads to osteoarthritic changes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lotz M. Cytokines in cartilage injury and repair. Clinical Orthopaedics & Related Research 2001. 391 Suppl(391 Suppl):S108–15. doi: 10.1097/00003086-200110001-00011. [DOI] [PubMed] [Google Scholar]
- 79.McNulty AL, Rothfusz NE, Leddy HA, et al. Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. J Orthop Res. 2013;31(7):1039–45. doi: 10.1002/jor.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bougault C, Gosset M, Houard X, et al. Stress-Induced Cartilage Degradation Does Not Depend on the NLRP3 Inflammasome in Human Osteoarthritis and Mouse Models. Arthritis and Rheumatism. 2012;64(12):3972–3981. doi: 10.1002/art.34678. [DOI] [PubMed] [Google Scholar]
- 81.Jovanovic D, Pelletier JP, Alaaeddine N, et al. Effect of IL-13 on cytokines, cytokine receptors and inhibitors on human osteoarthritis synovium and synovial fibroblasts. Osteoarthritis Cartilage. 1998;6(1):40–9. doi: 10.1053/joca.1997.0091. [DOI] [PubMed] [Google Scholar]
- 82.Ushiyama T, Chano T, Inoue K, et al. Cytokine production in the infrapatellar fat pad: another source of cytokines in knee synovial fluids. Ann Rheum Dis. 2003;62(2):108–12. doi: 10.1136/ard.62.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deschner J, Hofman CR, Piesco NP, et al. Signal transduction by mechanical strain in chondrocytes. Curr Opin Clin Nutr Metab Care. 2003;6(3):289–93. doi: 10.1097/01.mco.0000068964.34812.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guilak F, Fermor B, Keefe FJ, et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004423:17–26. doi: 10.1097/01.blo.0000131233.83640.91. [DOI] [PubMed] [Google Scholar]
- 85*.Torzilli PA, Bhargava M, Chen CT. Mechanical Loading of Articular Cartilage Reduces IL-1-Induced Enzyme Expression. Cartilage. 2011;2(4):364–373. doi: 10.1177/1947603511407484. This study shows that applying mechanical load in combination with IL-1 can reduce the mRNA expression of matrix degrading enzymes that would normally be upregulated in response to IL-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Torzilli PA, Bhargava M, Park S, et al. Mechanical load inhibits IL-1 induced matrix degradation in articular cartilage. Osteoarthritis Cartilage. 2009 doi: 10.1016/j.joca.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chowdhury TT, Bader DL, Lee DA. Dynamic compression inhibits the synthesis of nitric oxide and PGE(2) by IL-1beta-stimulated chondrocytes cultured in agarose constructs. Biochem Biophys Res Commun. 2001;285(5):1168–74. doi: 10.1006/bbrc.2001.5311. [DOI] [PubMed] [Google Scholar]
- 88.Chowdhury TT, Salter DM, Bader DL, et al. Signal transduction pathways involving p38 MAPK, JNK, NFkappaB and AP-1 influences the response of chondrocytes cultured in agarose constructs to IL-1beta and dynamic compression. Inflamm Res. 2008;57(7):306–13. doi: 10.1007/s00011-007-7126-y. [DOI] [PubMed] [Google Scholar]
- 89.Xu Z, Buckley MJ, Evans CH, et al. Cyclic tensile strain acts as an antagonist of IL-1 beta actions in chondrocytes. J Immunol. 2000;165(1):453–60. doi: 10.4049/jimmunol.165.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90**.Perera PM, Wypasek E, Madhavan S, et al. Mechanical signals control SOX-9, VEGF, and c-Myc expression and cell proliferation during inflammation via integrin-linked kinase, B-Raf, and ERK1/2-dependent signaling in articular chondrocytes. Arthritis Res Ther. 2010;12(3):R106. doi: 10.1186/ar3039. This study revealed a mechanism of differential kinase activation upstream of ERK1/2 phosphorylation that may regulate the effects of inflammation during mechanical loading in chondrocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nam J, Aguda BD, Rath B, et al. Biomechanical thresholds regulate inflammation through the NF-kappaB pathway: experiments and modeling. PLoS One. 2009;4(4):e5262. doi: 10.1371/journal.pone.0005262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Murata M, Bonassar LJ, Wright M, et al. A role for the interleukin-1 receptor in the pathway linking static mechanical compression to decreased proteoglycan synthesis in surface articular cartilage. Arch Biochem Biophys. 2003;413(2):229–35. doi: 10.1016/s0003-9861(03)00129-2. [DOI] [PubMed] [Google Scholar]
- 93**.Burleigh A, Chanalaris A, Gardiner MD, et al. Joint immobilization prevents murine osteoarthritis and reveals the highly mechanosensitive nature of protease expression in vivo. Arthritis Rheum. 2012;64(7):2278–88. doi: 10.1002/art.34420. This study shows a strong link between OA progression and mechanosensitivity in a mouse model. [DOI] [PubMed] [Google Scholar]
- 94.Aviezer D, Hecht D, Safran M, et al. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 1994;79(6):1005–13. doi: 10.1016/0092-8674(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 95.Bonassar LJ, Grodzinsky AJ, Frank EH, et al. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J Orthop Res. 2001;19(1):11–7. doi: 10.1016/S0736-0266(00)00004-8. [DOI] [PubMed] [Google Scholar]
- 96.Mauck RL, Nicoll SB, Seyhan SL, et al. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9(4):597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 97.Bonassar LJ, Grodzinsky AJ, Srinivasan A, et al. Mechanical and physicochemical regulation of the action of insulin-like growth factor-I on articular cartilage. Arch Biochem Biophys. 2000;379(1):57–63. doi: 10.1006/abbi.2000.1820. [DOI] [PubMed] [Google Scholar]
- 98.Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS One. 2008;3(6):e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allen JL, Cooke ME, Alliston T. ECM stiffness primes the TGFbeta pathway to promote chondrocyte differentiation. Molecular Biology of the Cell. 2012;23(18):3731–42. doi: 10.1091/mbc.E12-03-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van der Kraan PM. Age-related alterations in TGF beta signaling as a causal factor of cartilage degeneration in osteoarthritis. Biomed Mater Eng. 2014;24:75–80. doi: 10.3233/BME-140976. [DOI] [PubMed] [Google Scholar]
- 101.Vincent TL. Targeting mechanotransduction pathways in osteoarthritis: a focus on the pericellular matrix. Curr Opin Pharmacol. 2013;13(3):449–54. doi: 10.1016/j.coph.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 102.Sutter EG, Widmyer MR, Utturkar GM, et al. In vivo measurement of localized tibiofemoral cartilage strains in response to dynamic activity. American Journal of Sports Medicine in revision. doi: 10.1177/0363546514559821. [DOI] [PMC free article] [PubMed] [Google Scholar]


