Abstract
Background
Human genetic factors influence the outcome of pegylated interferon and ribavirin hepatitis C therapy. We explored the role of IL28B, APOH and ITPA SNPs on the outcomes of triple therapy including telaprevir or boceprevir in patients with compensated cirrhosis chronically infected with HCV-1.
Patients and Methods
A total of 256 HCV-1 Caucasian treatment-experienced patients with compensated cirrhosis from the ANRS CO20-CUPIC cohort were genotyped for a total of 10 candidate SNPs in IL28B (rs12979860 and rs368234815), APOH (rs8178822, rs12944940, rs10048158, rs52797880, rs1801689 and rs1801690) and ITPA (rs1127354 and rs7270101). We tested the association of IL28B and APOH SNPs with sustained virological response and of ITPA SNPs with anemia related phenotypes by means of logistic regression assuming an additive genetic model.
Results
None of the six APOH SNPs were associated with sustained virological response. The favorable alleles of the IL28B SNPs rs12979860 and rs368234815 were associated with sustained virological response (rs12979860: OR = 2.35[1.50–3.70], P = 2x10-4). Refined analysis showed that the effect of IL28B SNPs on sustained virological response was restricted to prior PegIFN/RBV relapse (OR = 3.80[1.82–8.92], P = 8x10-4). We also confirmed the association between ITPA low activity alleles and protection against early hemoglobin decline in triple therapy (P = 2x10-5).
Conclusion
Our results suggest that the screening of rs12979860 may remain interesting for decision making in prior relapse HCV-1 Caucasian patients with compensated cirrhosis eligible for a telaprevir- or boceprevir-based therapy.
Introduction
HCV infection is a major public health issue with ~80 million people chronically infected worldwide [1]. Up to 2011, standard of care treatment was based on pegylated interferon and ribavirin (PegIFN/RBV) which leads to viral clearance in ~50% of the patients [2]. Well-established baseline predictors of sustained virological response (SVR) to PegIFN/RBV include viral load, HCV genotype, age, ethnicity, body weight, insulin resistance, steatosis, fibrosis stage, and IL28B single nucleotide polymorphism (SNP) rs12979860 [2–5]. A dinucleotide frameshift variant (rs368234815) creating a novel gene encoding IFN-λ-4, in strong linkage disequilibrium (LD) with rs12979860, was recently identified as a stronger predictor than rs12979860 of treatment-induced clearance [6]. One of the most common side effects of ribavirin therapy is anemia that mainly appears at the beginning of treatment. Two variants, rs1127354 and rs7270101, from the inosine triphosphate (ITPA) gene, encoding a protein that hydrolyses inosine triphosphate, are independent predictors of RBV-induced anemia [7].
Since 2011, first generation direct acting antiviral drugs (DAAs) targeting the HCV NS3/4A protease, such as telaprevir (TVR) and boceprevir (BOC), are available in several countries. These drugs combined with a PegIFN/RBV backbone significantly improved the SVR as compared to PegIFN/RBV alone in both treatment-naive and previous treatment-failure patients with chronic HCV genotype 1 (HCV-1) infection [8,9]. However, side effects such as anemia are more frequent, particularly in patients with cirrhosis [10]. More recently, new IFN-free therapies with second generation DAAs have emerged and provide SVR rates over 90% [11]. However, these therapies are still very expensive and not yet widely used in real life settings. Therefore, triple therapy combining PegIFN/RBV with first generation protease inhibitors (PIs) remains the standard of care for HCV-1 infected patients in most countries. In the present study we aimed to explore the role of IL28B, APOH and ITPA SNPs on the outcomes of triple therapy including telaprevir or boceprevir in patients with compensated cirrhosis chronically infected with HCV-1.
Patients and Methods
Study population
The ANRS CO20-CUPIC (Compassionate Use of Protease Inhibitors in viral C Cirrhosis) study is a French multicenter cohort study that enrolled 660 HCV genotype 1 (HCV-1) treatment-experienced cirrhotic patients to assess safety and efficacy of triple therapy with TVR or BOC for difficult to treat patients in real-life settings [10,12]. Briefly, patients with compensated cirrhosis chronically infected with HCV-1, and who failed a prior course of IFN alone or IFN/RBV started a triple combination therapy including PegIFN/RBV and TVR or BOC for a total course of 48 weeks [10]. The choice between TVR and BOC was at the investigator’s discretion. Results showed a substantial benefit of triple therapy in difficult to treat patients with SVR rates of 43–52% but with an increased frequency and severity of side effects [12]. Interestingly, a recent study conducted in 189 patients from the CUPIC cohort identified baseline levels of apolipoprotein H (apoH), encoded by APOH gene, as a surrogate marker for SVR to triple therapy [13]. APOH polymorphisms have previously been associated with triglyceride levels, which itself is an independent correlate of HCV clearance [14].
Written informed consent was obtained from each patient before enrolment. The study was conducted in accordance with the Declaration of Helsinki and French law for biomedical research and was approved by the “Ile de France IX” Ethics Committee (Créteil, France).
Outcomes and statistical analysis
In the present study we took advantage of the well characterized CUPIC cohort study to assess the role of candidate SNPs in IL28B, APOH and ITPA on efficacy and safety of TVR- or BOC-based triple therapy. Only Caucasian patients who gave their consent for genetic testing were included (n = 256). Efficacy was assessed by SVR, defined as an undetectable HCV-RNA level 12 weeks after the end of therapy. For safety analysis, we focused on anemia and first considered a broad definition of clinically relevant anemia corresponding to patients with grade 2, 3 or 4 anemia (i.e. Hb<9.5g.dl-1) and/or blood transfusion and/or use of erythropoietin (EPO) occurring during the 48 weeks of treatment. We also focused on early significant hemoglobin decline, defined as a decrease of hemoglobin level of at least 3g.dl-1 between baseline and week 4 as proposed in [7]. For early significant hemoglobin decline analysis, patients for whom EPO therapy (N = 22) or RBV dose reduction (N = 4) was instituted before week 4 were excluded and 209 patients were included in this analysis.
For the SVR binary phenotype, all statistical analyses were conducted by means of logistic regression. Association with IL28B and APOH SNPs was tested by assuming an additive genetic model (i.e. the coding of the genotype represents the number of reference alleles 0, 1 or 2). We performed both univariate and multivariate analysis, including covariates previously identified as independent predictors of SVR in the CUPIC cohort (i.e.: prior treatment response, lead-in phase, platelet count and HCV-1 subtypes) [12]. Interaction between the IL28B SNP rs12979860 and binary covariates such as prior response to treatment (non-response versus relapse including breakthrough) and treatment group (TVR versus BOC), was modeled in the logistic regression framework by adding an interaction multiplicative term between the two main effects, e.g. IL28B SNP and the prior response to treatment.
The logistic regression framework was also used for the statistical analyses of the anemia related binary phenotypes (i.e. clinically relevant anemia and early significant hemoglobin decline). We performed both univariate and multivariate analysis, including ITPA SNPs (assuming an additive model), and other predictors of anemia (i.e.: age, sex, lead-in phase, hemoglobin at baseline, albumin at baseline <35g/L). To measure the joint effect of the two ITPA SNPs on anemia we considered a combined variable which estimates the severity of ITPA deficiency from rs1127354 and rs7270101 genotypes, as previously done in [7]. The severity of ITPA deficiency was defined as follows (S4 Table): Full ITPA activity (100%) was considered for rs1127354 C/C and rs7270101 A/A genotypes combination; 60% ITPA activity was considered for rs1127354 C/C and rs7270101 A/C genotypes combination; 30% ITPA activity was considered for rs1127354 C/C and rs7270101 C/C genotypes combination or rs1127354 A/C and rs7270101 A/A genotypes combination; and very low ITPA activity (0%) was considered for combined heterozygosity or rs1127354 A/A and rs7270101 A/A genotype combination [15]. Predicted ITPA activity was then considered as a quantitative covariate with four possible values (0; 0.3; 0.6; 1) in our logistic regression model. For the early hemoglobin decline phenotype, interaction between ITPA activity and the binary lead-in covariate was model in the logistic regression framework by adding a multiplicative interaction term between the two main effects, i.e. ITPA activity and lead-in.
All analyses were performed using the R software version 3.1.2 (http://cran.r-project), and p-values lower than 0.05 were considered as significant.
Genotyping
We genotyped 10 SNPs (S1 Fig) using TaqMan SNP genotyping assays (Applied Biosystems Inc., Foster City, CA) a total of 10 SNPs, two ITPA SNPs (rs1127354 and rs7270101), two IL28B variants (rs12979860 and rs368234815) and six APOH SNPs (rs8178822 [16,17], rs12944940, rs10048158 [18], rs52797880, rs1801689 [16,19] and rs1801690 [16,20]) selected based on their potential impact on apoH plasma levels via a search on NCBI Pubmed and regulomeDB (score≥2b, http://regulomedb.org/).
Results
Patient characteristics
A total of 256 Caucasian patients were genotyped, 162 receiving TVR and 94 receiving BOC with comparable baseline characteristics (S1 Table). A total of 172 (67%) individuals were men. The mean (Standard deviation, SD) age at inclusion was 58.1y (9.8y). Prior treatment response was null in 31 (12%), partial in 108 (42%), breakthrough in 10 (4%) and relapse in 92 (36%) patients. As previously proposed [21,22], null and partial responders were grouped in a “prior non-response” category, and breakthrough and relapse were grouped in a “prior relapse” category for further analyses. Eighty six individuals (33%) were infected with genotype 1a, 147 (57%) with 1b and 21 (8%) with 1c. The mean (SD) hemoglobin level at baseline was 14.6g.dl-1 (1.7g.dl-1), and the mean (SD) platelet count at baseline was 150,000mm-3 (66,000mm-3). As expected by the protocol, patients treated with BOC were more likely to receive 4 weeks lead-in with PegIFN/RBV (95.7% vs 26.5%, P<0.001).
Association of SVR status with APOH and IL28B SNPs
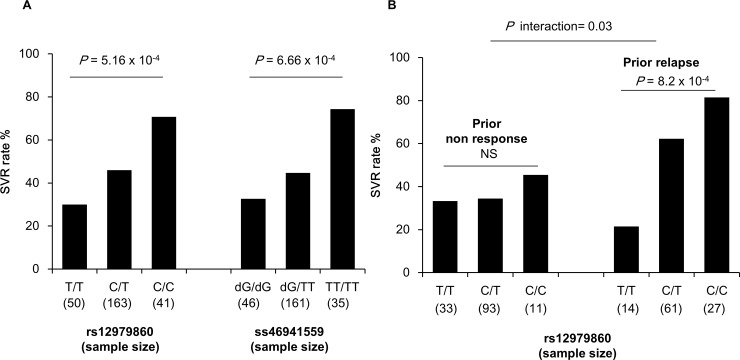
SVR was achieved for 119 (46.5%) patients and no significant difference (P = 0.48) was observed between the two treatment groups. Results of univariate analysis of IL28B and APOH SNPs are presented in Table 1. All APOH SNPs were in Hardy Weinberg equilibrium (HWE), and some of them were in LD (S1 Fig). No significant association was observed between APOH SNPs and SVR. As previously observed in Caucasian individuals, IL28B variants, rs12979860 and rs368234815, were in almost complete LD (r² = 0.94; S1 Fig). As the results for both SNPs were very similar and the call rate of rs12979860 was slightly higher than that of rs368234815, results are presented only for rs12979860. As expected by the selection bias of the sample including only treatment-experienced patients, rs12979860 was not in HWE (P = 9.9x10-6) due to an enrichment of unfavorable allele T for clearance (52% in CUPIC patients vs 32% in the 1000 genomes European population (www.ensembl.org). Despite this skewed distribution, the favorable allele C of rs12979860 was significantly associated with SVR to triple therapy in univariate analysis (P = 2x10-4) with an Odds ratio (OR) of achieving SVR per increase of one copy of the favorable allele C (i.e. ORC/CvsC/T or ORC/TvsT/T) of 2.35 (95% confidence interval: 1.50–3.70) (Table 1 and Fig 1A). The effect of rs12979860 genotype on SVR did not differ significantly between the two treatment groups (Pinteraction = 0.3).
Table 1. Effects of IL28B and APOH variants on SVR and of IPTA variants on anemia in univariate analysis.
| SNP | chr:position | Closest gene (variant type) | Reference/ alternative (aaf*) | n | call rate | HWE p-value | OR (95%CI) | P-value |
|---|---|---|---|---|---|---|---|---|
| SVR phenotype | ||||||||
| rs12979860 | 19:39248147 | IL28B (intron) | C/T (0.52) | 254 | 99.2 | 9.9 10−6 | 2.35 [1.50–3.70] | 2.0x10-4 |
| rs368234815 | 19:39248514 | IL28B (splice) | TT/dG (0.52) | 242 | 94.5 | 3.8 10−7 | 2.35 [1.46–3.79] | 4.7x10-4 |
| rs1801690 | 17:66212167 | APOH (missense) | C/G (0.07) | 251 | 98.0 | 0.62 | 1.47 [0.73–2.96] | 0.28 |
| rs1801689 | 17:66214462 | APOH (missense) | A/C (0.04) | 251 | 98.0 | 1 | 0.65 [0.26–1.61] | 0.35 |
| rs52797880 | 17:66220736 | APOH (missense) | A/G (0.08) | 242 | 94.5 | 1 | 1.40 [0.71–2.74] | 0.33 |
| rs8178822 | 17:66229411 | APOH (5’UTR) | G/T (0.08) | 253 | 98.8 | 1 | 1.31 [0.67–2.57] | 0.43 |
| rs12944940 | 17:66235598 | APOH (intron) | T/C (0.21) | 252 | 98.4 | 0.33 | 1.01 [0.64–1.57] | 0.98 |
| rs10048158 | 17:66240200 | APOH (intron) | C/T (0.47) | 251 | 98.0 | 0.42 | 0.96 [0.68–1.36] | 0.84 |
| Clinically relevant anemia | ||||||||
| rs1127354 | 20:3213196 | ITPA (missense) | C/A (0.06) | 255 | 99.6 | 1 | 1.36 [0.65–2.84] | 0.42 |
| rs7270101 | 20:3213247 | ITPA (intron) | A/C (0.13) | 255 | 99.6 | 0.83 | 1.31 [0.78–2.19] | 0.31 |
| Early Hb decline | ||||||||
| rs1127354 | 20:3213196 | ITPA (missense) | C/A (0.06) | 209 | 99.6 | 1 | 4.20 [1.38–12.8] | 0.01 |
| rs7270101 | 20:3213247 | ITPA (intron) | A/C (0.13) | 209 | 99.6 | 0.83 | 2.27 [1.20–4.29] | 0.01 |
* aaf, alternative allele frequency.
Fig 1. Rate of SVR by genotypes for IL28B SNPs.
(A) SVR rates by genotypes for rs12979860 (left panel) and rs368234815 (right panel). (B) SVR rates by genotypes for rs12979860 according to prior treatment response.
We performed further multivariate analysis including covariates previously identified as independent predictors of SVR (i.e. prior treatment response, no lead-in phase, platelet count≥100,000mm-3 and HCV-1b subtype [12]) (S2 Table), and showed that rs12979860 was independently associated with SVR (OR = 2.05[1.24–3.48], p = 5.9x10-3). The best predictor of SVR in the multivariate analysis was previous relapse to PegIFN/RBV (OR = 2.69[1.50–4.88], p = 9.6x10-4). Hence, we further explored the combined effect of rs12979860 and prior treatment response on SVR, and found a significant interaction (p = 0.03) between these two factors. As shown in Fig 1B, the effect of rs12979860 on SVR was observed only in prior relapse (P = 8.2x10-4) with a stronger OR of 3.80[1.82–8.92], while this effect is no more significant (P = 0.6) in prior non-responders with an OR of 1.20[0.63–2.33]. Multivariate analysis restricted to previous relapse patients (S2 Table) showed that rs1279860 has the most significant effect on SVR among other known predictors (P = 4.4x10-4) with an OR of 5.01[2.16–13.3]. Overall, our results suggest that the effect of rs12979860 on triple therapy-induced clearance in treatment-experienced patients is restricted to those who experienced prior PegIFN/RBV relapse.
Association of anemia with ITPA SNPs
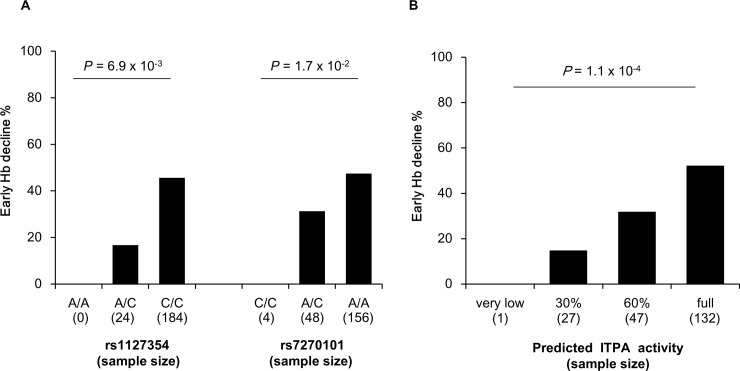
Clinically relevant anemia and early significant Hb decline were observed in 155 (60.5%) and 89 (42.6%) patients, respectively. Both ITPA SNPs were in HWE (Table 1), and were not in LD (S1 Fig). The frequencies of major alleles of rs1127354 (C) and rs7270101 (A), associated with normal ITPA activity, were 0.94 and 0.87, respectively. In univariate analysis, clinically relevant anemia was associated neither with rs1127354 (p = 0.42), nor with rs7270101 (p = 0.31). In contrast, early significant Hb decline was significantly associated with rs1127354 (ORC/CvsC/A = ORC/AvsA/A = 4.20[1.38–12.8], p = 0.01), and with rs7270101 (ORA/AvsA/C = ORA/CvsC/C = 2.27[1.20–4.29], P = 0.01) (Table 1 and Fig 2A). In multivariate analysis including other predictors of anemia (i.e. age in years, sex, lead-in, Hb at baseline and albumin at baseline [10,12]), rs1127654 and rs7270101 major alleles were strongly and independently associated with early significant Hb decline (rs1127354, OR = 7.83[2.64–29.2], P = 6.0x10-4; rs7270101, OR = 3.28[1.65–6.95], P = 1.2x10-3).
Fig 2. Rate of early hemoglobin decline by genotypes for rs1127354 and rs7270101 (A) and by predicted ITPA activity (B).
Severity of ITPA deficiency was defined as in Fellay et al [7] considering rs1127354 C and rs7270101 A equivalent low activity variants.
We further explored the joint effect of rs1127354 and rs7270101 by considering as a quantitative covariate the severity of ITPA deficiency estimated from the genotypes at rs1127354 and rs7270101, as shown in S4 Table [7]. Consistent with the SNP effects, clinically relevant anemia was not associated (p = 0.12) with ITPA activity while there was a strong effect of ITPA activity (P = 1.1x10-4) on the rate of early Hb decline ranging from 15% for 30% activity to 52.3% for full activity (Fig 2B). Early significant Hb decline was defined at week 4 of therapy and was the consequence of either PegIFN/RBV alone for patients having had a lead-in or PegIFN/RBV plus TVR or BOC for individuals who started triple therapy without a lead-in. As already shown [8,9], the absence of lead-in (and consequently the triple therapy) was significantly associated (P = 6.4x10-3 in multivariate analysis) with early Hb decline (S3 Table). However, refined analysis showed that the effect of ITPA activity on early Hb decline did not differ significantly (Pinteraction = 0.68) between the groups of patients with or without lead-in, with similar OR values observed in these two groups of 2.45[1.28–5.47] and 2.99[1.62–5.97], respectively. These results indicate that PIs increase the risk of early Hb decline, but do not have a significant influence on the relationship between early Hb decline and ITPA activity.
Discussion
Triple therapy using BOC or TVR remains the reference treatment for chronically HCV-1 infected patients in a large number of countries, and is of major importance for patients with cirrhosis who are at risk to develop severe complications as liver failure or hepatocellular carcinoma [23]. However, the overall chance of success with triple therapy is around 50% in those cirrhotic patients and the risk of adverse effects remains high [12]. Therefore, providing information that could help to the prediction of achieving SVR for cirrhotic patients under triple therapy is of major interest [23]. In this study, the unique CUPIC cohort of well characterized treatment experienced cirrhotic patients allowed us refining the association between IL28B SNPs and SVR to triple therapy, and between ITPA SNPs and anemia. IL28B genotype is the strongest predictor of SVR to the standard PegIFN/RBV therapy in patients chronically infected with HCV-1. In treatment-naïve HCV-1 patients receiving TVR or BOC in combination with PegIFN/RBV, the association between IL28B genotype and SVR remains clinically relevant [21,23,24]. We report here that IL28B alleles favorable for clearance were associated with a twofold increase of SVR rate in a cohort of treatment-experienced patients of Caucasian origin, chronically infected by HCV-1, with compensated cirrhosis and receiving either TVR or BOC in triple therapy. Refined analysis showed that the effect of IL28B SNPs was restricted to individuals who previously relapsed (i.e. breakthrough or relapse) to PegIFN alone or PegIFN/RBV therapy, with a stronger effect of these SNPs on SVR in this population. This result suggests that TVR and BOC may potentiate IL28B-dependent clearance transiently induced by PegIFN/RBV therapy, and that IL28B-independent mechanisms are involved in the non-response to PegIFN/RBV therapy.
Several studies including both treatment-naïve and treatment experienced HCV patients receiving TVR- or BOC-based therapy have also consistently identified IL28B genotype as a predictor of SVR independent of treatment history [25–28]. However, results of the few studies focusing only on treatment-experienced patients were less conclusive. In a Japanese cohort of 103 treatment-experienced patients, mono-infected with HCV-1b and receiving TVR, the IL28B variant, rs8099917, was an independent predictor of SVR [29]. In the RESPOND-2 study of BOC-based therapy in treatment-experienced patients (N = 207), rs12979860 C/C genotype was predictive of a good interferon response at week 4 but only a non-statistically significant trend was observed with SVR [21]. In the REALIZE study of TVR-based therapy in 422 treatment-experienced patients, SVR rates were slightly higher among patients with rs12979860 C/C genotype compared with C/T and T/T genotypes but the difference was not statistically significant [22]. In these two latter studies, patients included variable proportions of prior responders (slightly higher in the RESPOND-2 than in the REALIZE study), and a rather low proportion of cirrhotic patients (<30%). Further studies are needed to identify the factors, like prior response, ethnic origin, liver fibrosis status, or HCV-1 genotype, which could explain the differences observed in the strength of association between SVR and IL28B genotype in treatment-experienced patients receiving either TVR or BOC.
No significant association was observed between APOH SNPs and SVR. One explanation could be that the impact of each individual variant on apoH levels is not large enough to further impact on SVR. Moreover, our study was underpowered to detect an association signal between SVR and variant with minor allele frequency below 0.1, which is the case for four of the six tested APOH SNPs. As an example, the power to detect an association between SVR and the missense SNP rs1801690 was 55% at the 0.05 type I error level for an additive OR of 2. Finally, despite a careful literature search and public database screening, we may have missed some variants impacting on apoH levels that are not identified yet.
Anemia is a well-established adverse event with PegIFN/RBV treatment of chronic HCV infection and the addition of PIs, such as TVR and BOC, has significantly increased its incidence [8,9]. Our data are consistent with this observation as the patients without lead-in had a higher rate of early Hb decline. The role of ITPA polymorphisms on early Hb decline and/or early anemia was identified by GWAS [7] and further replicated in different ethnic groups treated both by PegIFN/RBV [30–35], and by a triple combination therapy with TVR [31,36]. Here, we confirmed the protective effect of low ITPA activity variants on early Hb decline in treatment-experienced patients having received pegIFN/RBV alone or triple therapy combination for 4 weeks. We could also show that the relationship between ITPA SNPs and early Hb decline was not influenced by the presence of PIs, indicating that the effect of PIs on Hb decline is probably independent of ITPA activity. In our study, ITPA deficiency did not protect against clinically relevant anemia, which has a broader definition based on both Hb decline and anemia management during the whole period, a finding consistent with the previous study of Aghemo et al. [36].
Overall, our results suggest that the screening of rs12979860 remains interesting for decision making in Caucasian difficult-to-treat HCV-1 patients (in particular if they presented a prior PegIFN/RBV relapse) with compensated cirrhosis eligible for a PI-based triple therapy. In those patients, the genotyping of ITPA SNPs are very useful to predict the development of early severe Hb decline.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We are grateful to all the study participants. We thank Jean-Laurent Casanova, all members of both branches of the laboratory of Human Genetics of Infectious Diseases, and Estelle Mottez from INSERM UMS20. We thank the INSERM-ANRS CO20 CUPIC Study Group, led by Christophe Hézode ( christophe.hezode@hmn.aphp.fr) and Hélène Fontaine (helene.fontaine@cch.aphp.fr):
Thierry Poynard, Service d’Hépato-gastro-entérologie, Hôpital La Pitié Salpêtrière, Paris, France; Valérie Canva, Service des Maladies de l’appareil Digestif, Centre Hospitalier Régional Universitaire de Lille–Hôpital Huriez, Lille, France; Patrice Cacoub, Service de Médecine Interne, Hôpital La Pitié Salpêtrière, Paris, France; Didier Samuel, Service d’Hépatologie, Hôpital Paul Brousse, Villejuif, France; Patrick Marcellin, Service d’Hépatologie, Hôpital Beaujon, Clichy-la Garenne, France; Laurent Alric, Pôle Digestif–Service de Médecine Interne, Centre Hospitalier Universitaire de Purpan, Toulouse, France; Victor De Lédinghen, Service d’Hépato-gastro-entérologie, Hôpital Haut-Lévêque, Pessac, France; Marc Bourlière, Service d’Hépato-gastroentérologie, fondation Hôpital Saint Joseph, Marseille, France; Jean-Pierre Zarski, Clinique Universitaire d’Hépato-gastroentérologie, Centre Hospitalier Régional Universitaire Saint Antoine, Grenoble, France; Jean-Jacques Raabe, Unité de Recherche Clinique, Centre Hospitalier de Metz,/Thionville–Hôpital Mercy, Metz, France; Sophie Métivier, pôle Digestif, Gastro-entérologie-Hépatologie, Centre hospitalier universitaire Purpan, Toulouse, France; Lawrence Serfaty, Service d’Hépatologie, Hôpital Saint-Antoine, AP-HP, Paris, France; Ghassan Riachi, Service d’Hépato-gastro-entérologie et nutrition, Centre Hospitalier Universitaire de Rouen, Rouen, France; Armand Abergel, Pôle Digestif, Centre Hospitalier Estaing, Clermont Ferrand, France; Véronique Loustaud-Ratti, Service d’Hépatologie et de Gastroentérologie, Centre Hospitalier Universitaire Dupuyten, Limoges, France; Albert Tran, Pôle Digestif, Centre hospitalier Universitaire de Nice, Nice, France; Xavier Causse, Service d’Hépatologie et de Gastroentérologie et oncologie digestive, Centre Hospitalier Régional La Source, Orléans, France; Dominique Guyader, Service des Maladies du Foie, Centre Hospitalier Universitaire de Pontchaillou, Rennes, France; Pierre-Henri Bernard, Service d’Hépatologie et de Gastroentérologie, Hôpital Saint André, Bordeaux, France; Pierre Attali, Service d’Hépatologie et de gastroentérologie, Hôpital de Bicêtre, AP-HP, Le Kremlin Bicêtre, France; Vincent Di-Martino, Service d’Hépatologie, Centre hospitalier de Jean Minjoz, Besançon, France; Paul Calès, Service d’Hépatologie et Gastroentérologie, Centre Hospitalier Universitaire, Angers, France; Véronique Grando-Lemaire, Service d’Hépatologie et de Gastroentérologie, Hôpital Jean Verdier, AP-HP, Bondy, France; Isabelle Rosa, Service d’Hépatologie et de Gastroentérologie, Centre hospitalier Intercommunal de Créteil, Créteil, France; Danièle Botta-Friedland, Service d’Hépatologie et Gastroentérologie, Hôpital de la Conception, Marseille, France; Thong Dao, Service d’Hépatologie et Gastroentérologie, Centre Hospitalier Universitaire, Caen, France; Damien Lucidarme, Service d’Hépatologie et Gastroentérologie, Centre Hospitalier de l’institut Catholique Lillois, Lille, France; Patrick Hillon, Service d’Hépatologie et Gastroentérologie, Centre Hospitalier Universitaire de Dijon, Dijon, France; Cyrille Feray, Service d’Hépatologie et Gastroentérologie, Hôpital Hôtel Dieu, Nantes, France; Thierry Fontanges, Service d’Hépatologie et Gastroentérologie, Hôpital P Oudot, Bourgoin-Jallieu, France; Jean-Didier Grange, Service d’Hépato-gastro-entérologie, Hôpital Tenon, Paris, France; Gilles Gatineau-Sailliant, Centre Hospitalier, Meaux, France; Eric Poncin, Service d’Hépatologie et Gastroentérologie, Centre Hospitalier de Dax, Dax, France; Jean-Pierre Arpurt, Service d’Hépatologie et Gastroentérologie, Centre Hospitalier, Avignon, France; Yannick Bacq, Service d’Hépatologie et Gastroentérologie, Centre Hospitalier Universitaire Trousseau, Tours, France; Patrick Delasalle, Service d’Hépatologie et Gastroentérologie, Clinique du Palais, Grasse, France; Denis Ouzan, Service d’Hépatologie et Gastroentérologie, Institut Arnaud Tzanck, Saint-Laurent du Var, France; Jean-Baptiste Nousbaum, Service d’Hépatologie et Gastroentérologie, Centre Hospitalier Universitaire de la Cavale Blanche, Brest, France; Christine Sylvain, Service d’Hépatologie et Gastroentérologie, Centre Hospitalier; Universitaire, Poitiers, France; Didier Ribard, Service d’Hépatologie et Gastroentérologie, Centre Hospitalier Universitaire Caremeau, Nîmes, France; Philippe Renard, Centre Hospitalier Victor Dupouy, Argenteuil, France; Caroline Lascoux-Combe, Service de Maladies Infectieuses, Hôpital Saint-Louis, APHP, Paris, France; Stéphanie de Montigny-Lenhardt, Centre Hospitalier Edmond Garcin, Aubagne, France; Christophe Pilette, Centre Hospitalier, Le Mans, France; Jacques Denis, Centre Hospitalier Sud Francilien, Corbeil-Essonnes, France; Thierry Allègre, Service d’Hématologie-Oncologie Médecine Interne, centre Hospitalier du Pays d’Aix, Aix en Provence, France; Matthieu Schnee, service d’Hépato-gastro-entérologie, Centre Hospitalier Départemental Les Oudairies, aA Roche Sur Yon, France; Gaëtan Franck, Cabinet de GastroEntéroHépatologie- Appareil Digestif, La Rochelle, France; Jean-Marc Combis, Clinique Ambroise Paré, Toulouse, France; Pierre Bedossa, Service d’Anatomo-Pathologie, Hôpital Beaujon, Clichy, France; Jean-Michel Pawlotsky, Centre National de Référence pour les Hépatites Virales B, C et delta, Service de Virologie, Hôpital Henri Mondor, AP-HP, Créteil, France; Marianne L’Henaff, Association de patients, ARCAT, TRT-5, Paris France; Michelle Sizorn, Association de patients, SOS Hépatites, Paris France; Ventzislava Petrov-Sanchez, Inserm-ANRS, Service Hépatites, Paris, France; Olivier Chazouillères, Service d’Hépatologie, Hôpital Saint-Antoine, AP-HP, Paris, France; Jean Dubuisson, Unité de Virologie, INSERM U1019, CNRS UMR8204, Lille, France; Francesco Negro, Hôpital Cantonal, Genève, Suisse; Georges-Philippe Pageaux, Hôpital Saint Eloi, Montpellier, France; Valérie Paradis, Service d’anatomopathologie, Hôpital Beaujon, AP-HP, Clichy, France; Bruno Spire, Santé Publique, Sciences Humaines et Sociales, INSERM, Marseille, France; Anne-Marie Taburet, Pharmacologie, Hôpital Bicêtre, AP-HP, Le Kremlin-Bicêtre, France; Jean-Claude Trinchet, Service d’Hépatologie, Hôpital Jean Verdier, AP-HP, Bondy, France; Yazdan Yazdanpanah, Economie de la Santé, Hôpital Bichat, AP-HP, Paris, France; Céline Dorival-Mouly, INSERM UMR-S 1136 Université Pierre et Marie Curie Paris 6, Paris, France; Cécilie Dufour, INSERM UMR-S 1136, Université Pierre et Marie Curie Paris 6, Paris, France; Céline Fréhaut, INSERM UMR-S 1136, Université Pierre et Marie Curie Paris 6, Paris, France; Aurélie Lesel, INSERM UMR-S 1136, Université Pierre et Marie Curie Paris 6, Paris, France; Nathalie Zahraa, INSERM UMR-S 1136, Université Pierre et Marie Curie Paris 6, Paris, France; Marion Pirot, INSERM UMR-S 1136, Université Pierre et Marie Curie Paris 6, Paris, France; Yoann Barthe, INSERM UMR-S 1136, Université Pierre et Marie Curie Paris 6, Paris, France; Frédéric Chau, INSERM UMR-S 1136, Université Pierre et Marie Curie Paris 6, Paris, France.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by INSERM-ANRS (France REcherche Nord & sud Sida-HIV Hépatites-FRENSH, Grant n° 2010-A01273-36, http://www.anrs.fr), Institut National de la Santé et de la Recherche Médicale (INSERM, http://www.inserm.fr), University Paris Descartes (http://www.parisdescartes.fr), the French National Research Agency (ANR, http://www.agence-nationale-recherche.fr) under the ‟Investments for the future” program (grant n°ANR-10-IAHU-01), and in part by the Association Française pour l'Etude du Foie (AFEF, http://www.afef.asso.fr). FA is the recipient of a fellowship from Fondation pour la Recherche Médicale (FRM, Grant n° FDM20140630671, http://www.frm.org). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014. November;61(1, Supplement):S45–57. [DOI] [PubMed] [Google Scholar]
- 2. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002. September 26;347(13):975–82. [DOI] [PubMed] [Google Scholar]
- 3. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001. September 22;358(9286):958–65. [DOI] [PubMed] [Google Scholar]
- 4. Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009. September 17;461(7262):399–401. 10.1038/nature08309 [DOI] [PubMed] [Google Scholar]
- 5. Casanova J-L, Abel L. The genetic theory of infectious diseases: a brief history and selected illustrations. Annu Rev Genomics Hum Genet. 2013;14:215–43. 10.1146/annurev-genom-091212-153448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prokunina-Olsson L, Muchmore B, Tang W, Pfeiffer RM, Park H, Dickensheets H, et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet. 2013. February;45(2):164–71. 10.1038/ng.2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fellay J, Thompson AJ, Ge D, Gumbs CE, Urban TJ, Shianna KV, et al. ITPA gene variants protect against anaemia in patients treated for chronic hepatitis C. Nature. 2010. March 18;464(7287):405–8. 10.1038/nature08825 [DOI] [PubMed] [Google Scholar]
- 8. Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011. March 31;364(13):1207–17. 10.1056/NEJMoa1009482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for Retreatment of HCV Infection. N Engl J Med. 2011. June 23;364(25):2417–28. 10.1056/NEJMoa1013086 [DOI] [PubMed] [Google Scholar]
- 10. Hézode C, Fontaine H, Dorival C, Larrey D, Zoulim F, Canva V, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC)—NCT01514890. J Hepatol. 2013. September;59(3):434–41. 10.1016/j.jhep.2013.04.035 [DOI] [PubMed] [Google Scholar]
- 11. Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourlière M, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014. April 24;370(17):1604–14. 10.1056/NEJMoa1401561 [DOI] [PubMed] [Google Scholar]
- 12. Hézode C, Fontaine H, Dorival C, Zoulim F, Larrey D, Canva V, et al. Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology. 2014. July;147(1):132–42.e4. 10.1053/j.gastro.2014.03.051 [DOI] [PubMed] [Google Scholar]
- 13. Sultanik P, Mallet V, Lagaye S, Casrouge A, Dorival C, Barthe Y, et al. Plasma apolipoprotein H limits HCV replication and associates with response to NS3 protease inhibitors-based therapy. Liver Int. 2015;n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 14. Cassader M, Ruiu G, Gambino R, Guzzon F, Pagano A, Veglia F, et al. Influence of apolipoprotein H polymorphism on levels of triglycerides. Atherosclerosis. 1994. September 30;110(1):45–51. [DOI] [PubMed] [Google Scholar]
- 15. Shipkova M, Lorenz K, Oellerich M, Wieland E, Ahsen N von. Measurement of erythrocyte inosine triphosphate pyrophosphohydrolase (ITPA) activity by HPLC and correlation of ITPA genotype-phenotype in a Caucasian population. Clin Chem. 2006. February;52(2):240–7. [DOI] [PubMed] [Google Scholar]
- 16. Mehdi H, Manzi S, Desai P, Chen Q, Nestlerode C, Bontempo F, et al. A functional polymorphism at the transcriptional initiation site in beta2-glycoprotein I (apolipoprotein H) associated with reduced gene expression and lower plasma levels of beta2-glycoprotein I. Eur J Biochem FEBS. 2003. January;270(2):230–8. [DOI] [PubMed] [Google Scholar]
- 17. Suresh S, Demirci FYK, Jacobs E, Kao AH, Rhew EY, Sanghera DK, et al. Apolipoprotein H promoter polymorphisms in relation to lupus and lupus-related phenotypes. J Rheumatol. 2009. February;36(2):315–22. 10.3899/jrheum.080482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Athanasiadis G, Sabater-Lleal M, Buil A, Souto JC, Borrell M, Lathrop M, et al. Genetic determinants of plasma β₂-glycoprotein I levels: a genome-wide association study in extended pedigrees from Spain. J Thromb Haemost JTH. 2013. March;11(3):521–8. 10.1111/jth.12120 [DOI] [PubMed] [Google Scholar]
- 19. Leduc MS, Shimmin LC, Klos KLE, Hanis C, Boerwinkle E, Hixson JE. Comprehensive evaluation of apolipoprotein H gene (APOH) variation identifies novel associations with measures of lipid metabolism in GENOA. J Lipid Res. 2008. December;49(12):2648–56. 10.1194/jlr.M800155-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mehdi H, Aston CE, Sanghera DK, Hamman RF, Kamboh MI. Genetic variation in the apolipoprotein H (beta2-glycoprotein I) gene affects plasma apolipoprotein H concentrations. Hum Genet. 1999. August;105(1–2):63–71. [DOI] [PubMed] [Google Scholar]
- 21. Poordad F, Bronowicki J-P, Gordon SC, Zeuzem S, Jacobson IM, Sulkowski MS, et al. Factors that predict response of patients with hepatitis C virus infection to boceprevir. Gastroenterology. 2012. September;143(3):608–18.e1–5. 10.1053/j.gastro.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 22. Pol S, Aerssens J, Zeuzem S, Andreone P, Lawitz EJ, Roberts S, et al. Limited impact of IL28B genotype on response rates in telaprevir-treated patients with prior treatment failure. J Hepatol. 2013. May;58(5):883–9. 10.1016/j.jhep.2012.12.023 [DOI] [PubMed] [Google Scholar]
- 23. Jacobson IM, Catlett I, Marcellin P, Bzowej NH, Muir AJ, Adda N, et al. 1369 TELAPREVIR SUBSTANTIALLY IMPROVED SVR RATES ACROSS ALL IL28B GENOTYPES IN THE ADVANCE TRIAL. J Hepatol. 2011. March 1;54:S542–3. [Google Scholar]
- 24. Holmes JA, Desmond PV, Thompson AJ. Does IL28B genotyping still have a role in the era of direct-acting antiviral therapy for chronic hepatitis C infection? J Viral Hepat. 2012. October;19(10):677–84. 10.1111/jvh.12003 [DOI] [PubMed] [Google Scholar]
- 25. Chayama K, Hayes CN, Abe H, Miki D, Ochi H, Karino Y, et al. IL28B but not ITPA polymorphism is predictive of response to pegylated interferon, ribavirin, and telaprevir triple therapy in patients with genotype 1 hepatitis C. J Infect Dis. 2011. July 1;204(1):84–93. 10.1093/infdis/jir210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akuta N, Suzuki F, Fukushima T, Kawamura Y, Sezaki H, Suzuki Y, et al. Prediction of treatment efficacy and telaprevir-resistant variants after triple therapy in patients infected with hepatitis C virus genotype 1. J Clin Microbiol. 2013. September;51(9):2862–8. 10.1128/JCM.01129-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsubota A, Shimada N, Atsukawa M, Abe H, Kato K, Ika M, et al. Impact of IL28B polymorphisms on 24-week telaprevir-based combination therapy for Asian chronic hepatitis C patients with hepatitis C virus genotype 1b. J Gastroenterol Hepatol. 2014. January;29(1):144–50. 10.1111/jgh.12402 [DOI] [PubMed] [Google Scholar]
- 28. Sterling RK, Kuo A, Rustgi VK, Sulkowski MS, Stewart TG, Fenkel JM, et al. Virological outcomes and treatment algorithms utilisation in observational study of patients with chronic hepatitis C treated with boceprevir or telaprevir. Aliment Pharmacol Ther. 2015. April;41(7):671–85. 10.1111/apt.13095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shimada N, Tsubota A, Atsukawa M, Abe H, Ide T, Takaguchi K, et al. A 48-week telaprevir-based triple combination therapy improves sustained virological response rate in previous non-responders to peginterferon and ribavirin with genotype 1b chronic hepatitis C: A multicenter study. Hepatol Res Off J Jpn Soc Hepatol. 2014. March 10; [DOI] [PubMed] [Google Scholar]
- 30. Thompson AJ, Fellay J, Patel K, Tillmann HL, Naggie S, Ge D, et al. Variants in the ITPA gene protect against ribavirin-induced hemolytic anemia and decrease the need for ribavirin dose reduction. Gastroenterology. 2010. October;139(4):1181–9. 10.1053/j.gastro.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suzuki F, Suzuki Y, Akuta N, Sezaki H, Hirakawa M, Kawamura Y, et al. Influence of ITPA polymorphisms on decreases of hemoglobin during treatment with pegylated interferon, ribavirin, and telaprevir. Hepatol Baltim Md. 2011. February;53(2):415–21. [DOI] [PubMed] [Google Scholar]
- 32. Eskesen AN, Melum E, Moghaddam A, Bjøro K, Verbaan H, Ring-Larsen H, et al. Genetic variants at the ITPA locus protect against ribavirin-induced hemolytic anemia and dose reduction in an HCV G2/G3 cohort. Eur J Gastroenterol Hepatol. 2012. August;24(8):890–6. 10.1097/MEG.0b013e3283546efd [DOI] [PubMed] [Google Scholar]
- 33. Rau M, Stickel F, Russmann S, Manser CN, Becker PP, Weisskopf M, et al. Impact of genetic SLC28 transporter and ITPA variants on ribavirin serum level, hemoglobin drop and therapeutic response in patients with HCV infection. J Hepatol. 2013. April;58(4):669–75. 10.1016/j.jhep.2012.11.027 [DOI] [PubMed] [Google Scholar]
- 34. Ahmed WH, Furusyo N, Zaky S, Eldin AS, Aboalam H, Ogawa E, et al. Pre-treatment role of inosine triphosphate pyrophosphatase polymorphism for predicting anemia in Egyptian hepatitis C virus patients. World J Gastroenterol WJG. 2013. March 7;19(9):1387–95. 10.3748/wjg.v19.i9.1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clark PJ, Aghemo A, Degasperi E, Galmozzi E, Urban TJ, Vock DM, et al. Inosine triphosphatase deficiency helps predict anaemia, anaemia management and response in chronic hepatitis C therapy. J Viral Hepat. 2013. December;20(12):858–66. 10.1111/jvh.12113 [DOI] [PubMed] [Google Scholar]
- 36. Aghemo A, Grassi E, Rumi MG, Ambrosio R D’, Galmozzi E, Degasperi E, et al. Limited utility of ITPA deficiency to predict early anemia in HCV patients with advanced fibrosis receiving Telaprevir. PloS One. 2014;9(4):e95881 10.1371/journal.pone.0095881 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.