Abstract
Single-copy rrn strains facilitate genetic ribosomal studies in Escherichia coli. Consecutive markerless deletion of rrn operons resulted in slower growth upon inactivation of the fourth copy, which was reversed by supplying transfer RNA genes encoded in rrn operons in trans. Removal of the sixth, penultimate rrn copy led to a reduced growth rate due to limited rrn gene dosage. Whole-genome sequencing of variants of single-copy rrn strains revealed duplications of large stretches of genomic DNA. The combination of selective pressure, resulting from the decreased growth rate, and the six identical remaining scar sequences, facilitating homologous recombination events, presumably leads to elevated genomic instability.
Keywords: rrl, rrs, duplication, genomic instability, ribosome
The majority of antibiotic classes currently in clinical use act by inhibiting ribosome function. Their utility is threatened by the emergence of microbial resistance, mainly due to compound efflux (Thaker et al. 2010), covalent modification of the ribosome (Goossens 2009), or alteration of the antibiotic (Zhanel et al. 2012). The binding interactions of the antibiotics with the ribosome are established predominantly with its RNA components (Poehlsgaard and Douthwaite 2005), which in Escherichia coli are encoded by seven virtually identical rrn operons: rrnA through rrnH (rrnF was renamed as rrnG) (supporting information, Figure S1A). This sevenfold redundancy limits the introduction of resistance caused by binding site mutations, mainly due to the recessive nature of resistance mutations that are suppressed by the presence of six wild-type alleles that lead to rapid cell death (Orelle et al. 2013). In addition, the multiplicity of wild-type alleles will tend to revert the mutant allele due to rapid gene conversion. This extremely low-resistance frequency is from a clinical perspective a very attractive feature for an antibiotic, and as a consequence the ribosome has remained an attractive antibacterial target despite decades of macrolide and aminoglycoside usage. From a drug-discovery perspective, however, this redundancy makes definition of a structural foundation guiding iterative chemistry programs challenging.
One recent example is negamycin, a natural compound that mediates its antibacterial activity by inhibition of translation (Mizuno et al. 1970) and for which as many as 10 different ribosomal-binding sites have been described (Schroeder et al. 2007; Olivier et al. 2014; Polikanov et al. 2014a). The location of a single binding site through which the compound mediated its activity, required for structure-based design of improved analogs, remained elusive for 40 years. Negamycin-resistant mutants of wild-type E. coli have been isolated, but the mutations affected transport of the compound into the cell rather than binding to the ribosome (McKinney et al. 2015). To obtain genetic evidence for negamycin’s functional binding site, helix 34 of the small subunit, the redundancy of wild-type E. coli ultimately needed to be circumvented by the use of markerless single rrn strains (Olivier et al. 2014; Polikanov et al. 2014a). Although this tool has found use in various seminal scientific advances of ribosomal biology (Bollenbach et al. 2009; Orelle et al. 2013; Polikanov et al. 2014b; Orelle et al. 2015), its construction was mentioned in brief (Bollenbach et al. 2009). Here we describe the construction in detail, showing that sequential deletion of four rrn genes leads to a reduced growth rate due to limiting expression of transfer RNAs (tRNAs), and that after deletion of the sixth copy the cellular ribosomal content becomes growth-limiting. Furthermore, although colony morphology suggested stability of the strains (Bollenbach et al. 2009), whole-genome analyses revealed elevated genomic instability caused by homologous recombination between the scars remaining upon rrn deletion.
Materials and Methods
Supplementary Information
File S1 contains a list of strains (Table S1), a detailed rrn deletion strategy (Figure S1, Figure S2, and Figure S3) and a map of pK4-16 (Figure S5). Additional whole-genome sequencing data are provided in Figure S4.
Data availability
E. coli strains SQ37, SQ88, SQ2203, SQ110, and SQ171, used for genomic sequencing, were obtained from the Coli Genetic Strain Center at Yale University. Complete nucleotide sequences of these strains have been deposited in Genbank under accession numbers CP011320, CP011321, CP011322, CP011323, and CP011324, respectively.
Results and Discussion
A set of single rrn deletion strains was constructed using E. coli MG1655 (Blattner et al. 1997). Seven strains, each with a single deletion spanning one of the rrn operons, were made using the recombineering method (Figure S1 and Table S1) (Datsenko and Wanner 2000). Upon resolving the kanamycin-resistance cassette from one operon, leaving a 85-bp insertion (“scar”), we introduced a new kanamycin-resistance cassette into another rrn operon, which was subsequently resolved, and so on (Figure S2 and Figure S3A). Deletions of the rrn operons were confirmed by both polymerase chain reaction (data not shown) and Southern blots (Figure S3B). However, all rrn operons encode the RNA components for the small and large ribosomal unit (rrs and rrl genes, respectively) that are interspersed with a number of different tRNA genes that would have prevented removal of more than five operons (Figure S1B). Therefore, plasmid ptRNA67 (Zaporojets et al. 2003) was introduced to provide tRNA genes of Ala-1B, Ile-1, Trp, Asp-1, Thr-1, and Glu-2 in trans (Asai et al. 1999).
At high growth rates, as much as 70% of E. coli’s resources are devoted to the translation machinery and protein synthesis (Russell and Cook 1995). The ribosome forms the core of the translation machinery and the growth rate is proportional to both cellular ribosome content and specific peptide elongation rate (Dennis et al. 2004). Feedback regulation is one mechanism of ribosomal RNA regulation in E. coli. E. coli strains with a limited number of rrn operons maintain ribosome content by increased transcription of the remaining operons; conversely, the presence of additional rrn copies does not change ribosome content (Gyorfy et al. 2015).
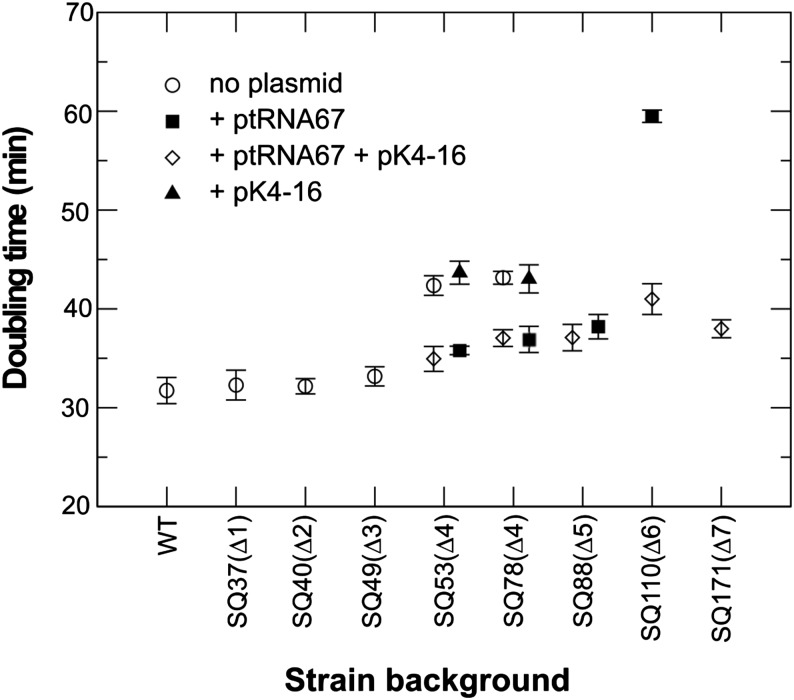
Sequential deletion of three rrn operons did not significantly alter the doubling time of strains when grown in Luria Broth at 37°, but the additional deletion of a fourth operon resulted in an increase from 33 to 43 min (30%) [rrnGBA vs. rrnGADE P = 0.0003, rrnGBA vs. rrnGBAD P = 0.0018 (Figure 1)]. Deletion of the fifth operon, rrnH, required the addition of tRNA genes on a plasmid since this deletion would remove all copies of the tRNA-Ile and tRNA-Ala genes encoded within the rrn operon spacers (Figure S1B). Despite the removal of the fifth operon, the growth rate of the ΔrrnGADEH ptRNA67 strain was significantly higher than that of the ΔrrnGADE strain, with doubling time of 38 and 43 min respectively (P = 0.0089), suggesting that the lower growth rate of the ΔrrnGADE strain was caused largely by limiting amounts of tRNA (Figure 1). This observation was confirmed by failure to increase the growth rate of the ΔrrnGADE strain upon introduction of rrn encoding plasmid pK4-16 (P = 0.87). The largest decrease in growth rate, increasing the doubling time from 38 to 60 min, was observed upon deletion of the sixth rrn copy, leaving a single chromosomal rrn operon (Figure 1). Introduction of pK4-16 restored the growth rate close to the level observed in the ΔrrnGADEH ptRNA strain (P = 0.00002).
Figure 1.
Growth rates of E. coli rrn deletion strains grown in Luria Broth medium at 37° with and without the tRNA plasmid, ptRNA67, and ribosomal RNA plasmid, pK4-16 (n ≥ 3).
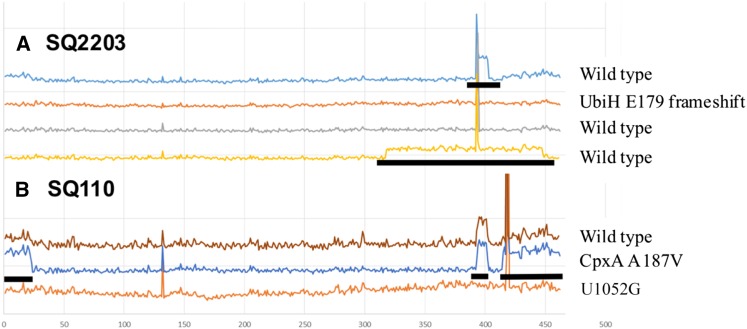
For the single rrn strains to serve as genetic tools and select for mutations in rrn, whole-genome-sequences of strains SQ37, SQ88, SQ2203, SQ110, and SQ171 were determined. Mapping of the sequence reads confirmed correct deletion of the appropriate rrn operons, and the density of sequence reads in SQ37, containing a single deletion, and SQ88, containing five deletions, was evenly distributed across the genome (Figure S4). However, in single rrn operon strains SQ2203, SQ110 and SQ171 genomic regions were found in which the density of sequence reads was doubled, reflecting DNA duplications (Figure S4). The duplications always seemed to have occurred between two scar sites. In an extension of this work, whole-genome-sequences of multiple colonies of SQ110 and SQ2203 as well as negamycin-resistant mutants derived therefrom (Olivier et al. 2014, McKinney et al. 2015) were determined, and in some of these the duplication pattern was altered (Figure 2). This observation suggests that the combination of the severe growth defect observed upon removal of the penultimate rrn operon (Figure 1) and the presence of six identical scar sequences spread through the genome promote recombination events.
Figure 2.
Relative sequence read coverage of multiple isolates of E. coli strains SQ2203 and SQ110 and negamycin-resistant mutants derived thereof (Olivier et al. 2014, McKinney et al. 2015) reveal unstable regions of genomic duplication. Regions that showed ∼2× increased relative coverage, reflecting duplication, are indicated with a black bar. (A). SQ2203 yielded two variants (blue, yellow) in additional to the predicted, single-fold coverage genome (gray). (B) All wild-type SQ110 isolates that were sequenced contained the same duplication (brown) but different arrangements were found among negamycin-resistant mutants. “Spikes” in the plots represent regions of homology of mainly transfer RNA and rrn genes/operons in resident plasmids, with genomic sequences.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant R01-GM24751 to C.L.S.
Footnotes
Literature Cited
- Asai T., Zaporojets D., Squires C., Squires C. L., 1999. An Escherichia coli strain with all chromosomal rRNA operons inactivated: complete exchange of rRNA genes between bacteria. Proc. Natl. Acad. Sci. USA 96: 1971–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R., Plunkett G., Bloch C. A., Perna N. T., Burland V., et al. , 1997. The complete genomic sequence of Escherichia coli K-12. Science 277: 1453–1474. [DOI] [PubMed] [Google Scholar]
- Bollenbach T., Quan S., Chait R., Kishony R., 2009. Nonoptimal microbial response to antibiotics underlies suppressive drug interactions. Cell 139: 707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko K. A., Wanner B. L., 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97: 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Ehrenberg M., Bremer H., 2004. Control of rRNA synthesis in Escherichia coli: a systems biology approach. Microbiol. Mol. Biol. Rev. 68: 639–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens H., 2009. Antibiotic consumption and link to resistance. Clin. Microbiol. Infect. 15(Suppl 3): 12–15. [DOI] [PubMed] [Google Scholar]
- Gyorfy Z., Draskovits G., Vernyik V., Blattner F. F., Gaal T., et al. , 2015. Engineered ribosomal RNA operon copy-number variants of E. coli reveal the evolutionary trade-offs shaping rRNA operon number. Nucleic Acids Res. 43: 1783–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney D. C., Bezdenejnih-Snyder N., Farrington K., Guo J., McLaughlin R. E., et al. , 2015. Illicit transport via the dipeptide transporter Dpp is irrelevant to efficacy of negamycin in mouse thigh models of Escherichia coli infection. ACS Infect. Dis 1: 222–230. [DOI] [PubMed] [Google Scholar]
- Mizuno S., Nitta K., Umezawa H., 1970. Mechanism of action of negamycin in Escherichia coli K12. I. Inhibition of initiation of protein synthesis. J. Antibiot. 23: 581–588. [PubMed] [Google Scholar]
- Olivier N. B., Altman R. B., Noeske J., Basarab G. S., Code E., et al. , 2014. Negamycin induces translational stalling and miscoding by binding to the small subunit head domain of the Escherichia coli ribosome. Proc. Natl. Acad. Sci. USA 111: 16274–16279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orelle C., Carlson S., Kaushal B., Almutairi M. M., Liu H., et al. , 2013. Tools for characterizing bacterial protein synthesis inhibitors. Antimicrob. Agents Chemother. 57: 5994–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orelle C., Carlson E. D., Szal T., Florin T., Jewett M. C., et al. , 2015. Protein synthesis by ribosomes with tethered subunits. Nature 524: 119–124. [DOI] [PubMed] [Google Scholar]
- Poehlsgaard J., Douthwaite S., 2005. The bacterial ribosome as a target for antibiotics. Nat. Rev. Microbiol. 3: 870–881. [DOI] [PubMed] [Google Scholar]
- Polikanov Y. S., Szal T., Jiang F., Gupta P., Matsuda R., et al. , 2014a Negamycin interferes with decoding and translocation by simultaneous interaction with rRNA and tRNA. Mol. Cell 56: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikanov Y. S., Osterman I. A., Szal T., Tashlitsky V. N., Serebryakova M. V., et al. , 2014b Amicoumacin A inhibits translation by stabilizing mRNA interaction with the ribosome. Mol. Cell 56: 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Cook G. M., 1995. Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol. Rev. 59: 48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder S. J., Blaha G., Moore P. B., 2007. Negamycin binds to the wall of the nascent chain exit tunnel of the 50S ribosomal subunit. Antimicrob. Agents Chemother. 51: 4462–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker M., Spanogiannopoulos P., Wright G. D., 2010. The tetracycline resistome. Cell. Mol. Life Sci. 67: 419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaporojets D., French S., Squires C. L., 2003. Products transcribed from rearranged rrn genes of Escherichia coli can assemble to form functional ribosomes. J. Bacteriol. 185: 6921–6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhanel G. G., Lawson C. D., Zelenitsky S., Findlay B., Schweizer F., et al. , 2012. Comparison of the next-generation aminoglycoside plazomicin to gentamicin, tobramycin and amikacin. Expert Rev. Anti Infect. Ther. 10: 459–473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
E. coli strains SQ37, SQ88, SQ2203, SQ110, and SQ171, used for genomic sequencing, were obtained from the Coli Genetic Strain Center at Yale University. Complete nucleotide sequences of these strains have been deposited in Genbank under accession numbers CP011320, CP011321, CP011322, CP011323, and CP011324, respectively.