Abstract
Bench studies have become the preferred way to evaluate the performance of airway equipment, since clinical trials are not specifically required before marketing these devices. However, it is difficult to assess the efficiency of ventilation without recording physiological data. This review analyses how efficiency of manual ventilation has been defined in recent studies, and how their results may be affected. We searched electronic databases from 2000 to April 2014. The main inclusion criterion was the analysis of performance of ventilation. Nine relevant articles were selected from 53 eligible publications. Most studies used the same parameters; tidal volume and ventilation rate. However, there were significant differences between the definitions of performance of ventilation, both in terms of criteria of judgement and methods of analysis. None of these approaches is able to provide a clear understanding of variability of ventilation during a given period. A new definition may increase the relevance of bench studies to clinical medicine, by more appropriately assessing the performance of ventilation.
Introduction
Manual ventilation has been the object of several recent publications, as it is a crucial clinical skill for assisting patients in respiratory failure and/or cardiopulmonary arrest. Indeed, it remains the preferred and easiest method of ventilation in pre-hospital situations. It is an important challenge for healthcare professionals, and many studies have highlighted the difficulty in performing it efficiently [1–5]. Inadequate ventilation may lead to adverse clinical outcomes, including barotrauma, gastric insufflation, pulmonary aspiration and tissue hypoxia [5–7].
Several devices have recently been developed with the aim of reducing these adverse effects, and this has led to a proliferation of studies evaluating airway equipment. However, as clinical trials are not specifically required to introduce this kind of device to the market, most studies have been carried out on patient simulators [8]. In these cases, physiological variables such as arterial blood gases are not available to evaluate the efficiency of ventilation. How do researchers manage to evaluate the performance of their device properly under such conditions?
The International Liaison Committee on Resuscitation (ILCOR) has established specific recommendations for manual ventilation [9–13]. Most studies have based their performance analysis in accordance with these guidelines. However, although ILCOR defines adequate tidal volumes and ventilation rates for safe practice, it does not mention how to analyse the performance of a full ventilation period reliably under bench conditions. As no real consensus has been reached [14], many studies evaluate the performance of manual ventilation devices using their own judgment parameters and methods of analysis.
This review focuses on the functional assessment of manual ventilation devices. It aims to show how ventilation performance has been defined and analysed in recent studies, and how this may affect the relevance of their results.
Methods
We searched electronic databases including Medline, Google, Clinical Trials and Cochrane from 2000 to April 2014. The key words were ‘bag valve mask’, ‘manual ventilation’ and ‘ventilation performance’, used individually and in combination. Our inclusion criteria were the analysis of performance of adult ventilation for the functional evaluation of manual ventilation devices. Articles were excluded if they were written in a language other than English or French, or if they were dealing with paediatric practice. We excluded articles that had inadequate information about judgment criteria and analysis methods for the assessment of performance of the devices studied.
We also reviewed international guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) to see how efficiency of manual ventilation was defined in them. We included the European Resuscitation Council (ERC) and the American Heart Association (AHA) guidelines published in Resuscitation and Circulation from 2000 to April 2014.
Furthermore, we carried out ventilation tests on a Laerdal® Airway Management Trainer manikin (Laerdal Medical, Stavanger, Norway) linked to an ASL5000® lung simulator (IngMar Medical, Ltd, Pittsburgh, PA, USA) to assess ventilation practices. Healthcare professionals were asked to ventilate the lungs of a simulated apnoeic patient without respiratory disease using a standard bag-valve-mask device (Ambu® Spur® II, Ambu A/S, Ballerup, Denmark). Ventilation curves obtained in this way were used to discuss our review findings.
Results
The main literature search resulted in 284 references, but only 83 abstracts were obviously referring to the performance of manual ventilation. Fifty-three articles fulfilled our main inclusion criteria, but most of them did not adequately describe their method of evaluation of performance of ventilation. Finally, only nine articles were selected (Fig.1). Of these, six were manikin-based studies and three were clinical trials [15–23]. The ventilatory variables that were recorded in bench studies were tidal volume (VT), ventilation rate (VR), peak airway pressure (Ppeak), peak inspiratory flow (PF), minute volume (Vm), gastric inflation volume (Vgastric), inspiratory time fraction (Ti/Ttot), inspiratory time (I time) and inspiratory/expiratory ratio (I/E). Clinical trials also recorded arterial oxygen saturation, mean arterial blood pressure, heart rate and end-tidal carbon dioxide. The main characteristics of these studies are presented in Table 1.
Figure 1.
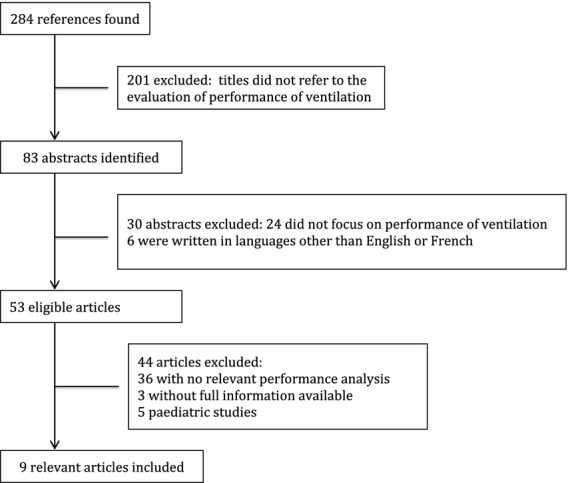
Flow chart of article identification and selection.
Table 1.
Main characteristics of the nine relevant studies dealing with performance of manual ventilation. Values are number or mean (SD)
| Study | Type | Number of participants | Recorded parameters | Ventilation duration | Analysis method | Judgment parameters | Tolerance range | Study results |
|---|---|---|---|---|---|---|---|---|
| Wagner-Berger et al. [15] | Clinical trial | 30 | SaO2, MAP, HR,ETCO2, VT, Ppeak, PF, Ti/Ttot, Vgastric | 2 min | Overall mean value* | VT | ≈500 ml | VT = 637 (123) ml |
| Noordergraaf et al. [16] | Clinical trial | 13 | ETCO2, VT, VR, Vm, Ppeak | ND | Overall mean value | ETCO2, VT, VR | 4.0–4.7 kPa 400–600 ml 12–15 bpm | ETCO2 = 4.53 (0.67) kPa VT = 712 (162) ml VR = 13 (3) bpm |
| von Goedecke et al. [17] | Clinical trial | 40 | SaO2, MAP, HR, ETCO2, VT, Vm, VR, Ppeak, PF, Ti/Ttot | 1 min | Overall mean value | SaO2, VT, VR | ≥90% ≈350 ml with 100% FiO2 10–15 bpm | 95.2 (2.9)% VT = 640 (120.9) ml VR = 15.8 (1.3) bpm |
| Busko et Blackwell [18] | Bench test | 153 | VT, Vm, Vgastric, Ppeak, I/E, VR | 1 min | Overall mean value | VT, VR | 510–595 ml (6–7 ml.kg−1) 10–12 bpm | VT = 672.2 (120.9) ml VR = 13.5 (4.8) bpm |
| Nehme et Boyle [19] | Bench test | 30 | VT, VR, Vm | 2 min | Individual mean value† | VT, VR | 480–560 ml (6–7 ml.kg−1) 8–10 bpm | 4% of optimal VT 23% of optimal VR |
| Lim et al. [20] | Bench test | 52 | VT, VR | 2 min | Individual mean value | VT, VR | 500–600 ml 8–10 bpm | 38.5% of optimal ventilation |
| Bergrath et al. [21] | Bench test | 40 | VT, I time, Ppeak | 8 min | Individual mean value | VT | 500–600 ml (6–7 ml.kg−1) | VT = 408 (164) ml 22% of optimal VT |
| Lee et al. [22] | Bench test | 30 | VT | 2 min | Breath-by-breath‡ | VT | 440–540 ml (6–7 ml.kg−1) | 26.7% of optimal VT |
| Marjanovic et al. [23] | Bench test | 50 | VT, VR, Ppeak | 3 min | Breath-by-breath | VT, VR | 400–600 ml 9–11 bpm | 25.6% of optimal VT 0% of optimal VR |
SaO2, arterial oxygen saturation; MAP, mean arterial blood pressure; HR, heart rate; ETCO2, end-tidal carbon dioxide concentration; VT, tidal volume; Ppeak, peak airway pressure; PF, peak inspiratory flow; Ti/Ttot, inspiratory time fraction; Vgastric, gastric inflation volume; VR, ventilation rate; ND, not done; Vm, minute volume; I/E, inspiratory/expiratory ratio; I time, inspiratory time.
Global mean value of each ventilatory parameter for all tests.
Mean value of each ventilatory parameter for each test.
Breath-by-breath analysis of each ventilatory parameter.
Despite the heterogeneity of the recorded variables, most studies used the same judgement parameters to define the efficiency of ventilation, namely VT and VR. However, there were significant differences between studies regarding the tolerance ranges used to define whether VT and VR were considered adequate or not. While some studies defined adequate VT from 6 ml.kg−1 to 7 ml.kg−1 [18,19,21,22], other studies used a basic range of 400–600 ml [16,23], regardless of the patient's weight. von Goedecke et al. defined an adequate VT as approximately 350 ml [17], and Bergrath et al. considered a VT lower than 200 ml to be clinically insufficient [21]. The range of what was considered an adequate ventilation rate varied from 8–10 bpm [19,20] to 12–15 bpm [20].
Moreover, three different analysis methods were used for the evaluation of performance of ventilation: an overall mean analysis of every ventilatory variable; a mean analysis of every ventilatory parameter for each single ventilation test; and a breath-by-breath analysis method. To aid the discussion of this literature review, we have reported the data of two ventilation tests from a previous study (Figs.2 and 3), in which several healthcare professionals manually ventilated a manikin connected to a test lung (ASL-5000 breathing simulator) simulating an adult patient in respiratory arrest.
Figure 2.
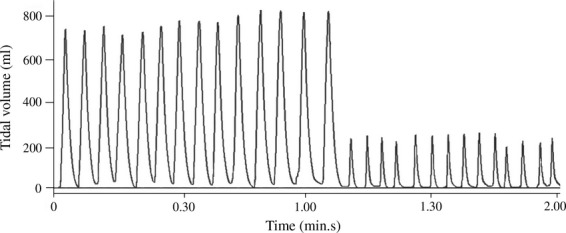
Two-minute manual ventilation window performed by a healthcare professional on an apnoeic patient model. Lung compliance and resistance were set to 70 ml.cmH2O and 5 cmH2O.l−1.s, respectively. The sudden appearance of leaks after one minute of ventilation is demonstrated.
Figure 3.
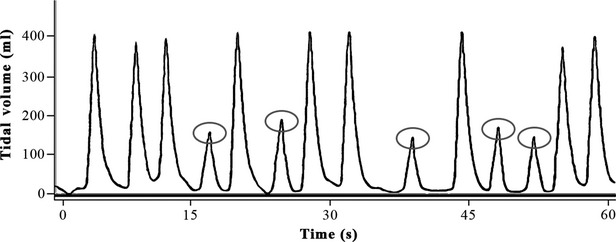
One-minute manual ventilation window performed by a healthcare professional on an apnoeic patient model. Lung compliance and resistance were set to 70 ml.cmH2O and 5 cmH2O.l−1.s, respectively. Alternating efficient and insufficient (circled) tidal volumes are shown.
Finally, we also identified different guidelines from ERC and AHA published in 2000, 2005 and 2010 (Table 2). These show that recommendations concerning adequate ventilatory variables have not evolved significantly over that period. An adequate VT is regarded as 6–7 ml.kg−1, and the VR about 8–12 breaths.min−1 (bpm). International recommendations concerning chest compressions and compression/ventilation ratio are not presented here, as this review does not focus on heart–lung interactions.
Table 2.
Evolution of international recommendations over the last decade regarding adequate ventilatory parameters when using a manual resuscitator device for an adult patient
| International recommendations | Years of publication | Recommended tidal volumes; ml.kg−1 | Recommended ventilation rates; bpm |
|---|---|---|---|
| AHA [24] | 2000 | 6–7 | ∼10 |
| ERC [25] | 2000 | 6–7 | ∼12 |
| AHA [26] | 2005 | 6–7 | 8–10 |
| ERC [27] | 2005 | 6–7 | ∼10 |
| AHA [10,12] | 2010 | 6–7 | 8–10 |
| ERC [9,11,13] | 2010 | 6–7 | 8–10 |
AHA, American Heart Association; ERC, European Resuscitation Council.
Discussion
Since 2000, AHA and ERC have reached a consensus on what tidal volume and ventilation rate should be delivered to the patient. These are 6–7 ml.kg−1 and 8–12 bpm, respectively, and may explain why VT and VR were the main variables used to define ventilation efficiency in the studies reviewed, even though many others were recorded. However, it seems difficult to define strict tolerance ranges for adequate ventilation, as these would depend on the patient's physiopathology. Clinical trials have the significant advantage of being able to correlate VT and VR with physiological data such as arterial oxygen saturation and end-tidal carbon dioxide. Noordergraaf et al. [16] defined normocapnia as their primary endpoint before evaluating VT and VR, and von Goedecke et al. [17] focused their study on maintaining adequate arterial oxygenation with the lowest VT possible. In this way, they were better able to define criteria for adequate VT and VR.
While this may help to explain why many studies do not use the same tolerance ranges for the definition of efficient ventilation, it does not tell us why bench studies do not analyse performance of ventilation using the same criteria that are clearly defined in the guidelines. It is important to note that international guidelines are mainly based on clinical trials in patients receiving mechanical ventilation. They have shown an increase in survival and a decrease in adverse outcomes when VT was set to 6–7 ml.kg−1 [28,29]. However, mechanical ventilators may not accurately deliver these pre-set tidal volumes to the patient's lungs. A recent study by Lyazidi et al. demonstrated that even modern ventilators equipped with compliance compensation algorithms were unable to deliver the intended VT accurately. A set VT of 6 ml.kg−1 actually provided patients with volumes ranging from 4 ml.kg−1 to 8 ml.kg−1, with clinically meaningful differences ranging from 1 ml.kg−1 to 2 ml.kg−1 [30]. These differences were due to compliance changes in the ventilation circuit and patient airways, and to the progressive increase in air temperature and hygrometry during the inspiration phase. The application of an inspiratory pause also has an important role, as it may affect the calculation of compensation algorithms. This study concluded that clinical trials investigating the effect of VT values on patient outcomes may have been biased by these inaccuracies. According to these observations, VT ranging from 4 ml.kg−1 to 8 ml.kg−1 may be acceptable, as long as they are precisely measured into the patient's lung. In most bench studies, VT is measured directly into the artificial lung, and thus expanding the tolerance ranges of ventilatory variables may be justified. This may have led to differing definitions of efficient ventilation in the studies reviewed.
This lack of consensus concerning the definition of successful ventilation has also been described by Boyle and Flavell [14]. Furthermore, VT and VR were always analysed separately, other than in one study [20] where they were combined and evaluated simultaneously to see if both parameters were within their tolerance range. This last strategy seems to be the most appropriate to comply with international guidelines that recommend controlling both VT and VR, and not only one of them.
Besides these various definitions of efficiency of ventilation, three different methods of analysis were also used to evaluate the performance of ventilation over a whole ventilation period. The most commonly used was the overall mean value analysis. In this approach, the average value of each ventilatory variable for all participants is analysed by combining all tests together, whatever the sample size. Data resulting from this approach can't be properly interpreted, as the averaged results do not take into account inter- and intra-individual variability in ventilatory variables. Indeed, ventilation performance may fluctuate significantly from one rescuer to another, and from one ventilation cycle to another, according to the technique they used to squeeze the bag and maintain the mask on the patient's face [31]. Inter-individual variability is likely to have the greatest impact on results of performance analysis. For instance, in a situation where half of the rescuers would provide insufficient VT (∼200 ml) and the other half excessive ones (∼800 ml), the ventilation performance would be mistakenly considered efficient according to this analysis method, as the mean VT value would be about 500 ml. Conversely, Bergrath et al. [21] showed that medical students ventilated a manikin with a mean (SD) VT of 408 (164) ml. Using this analysis method, the global ventilation performance would have been considered inefficient according to the tolerance range defined by the authors (510–595 ml), while they actually recorded optimal VT on 22% of occasions.
The second analysis method evaluates the average of every variable for each single test. Compared with the first method, it has the advantage to consider the variability among persons, but not the intra-individual variability; this latter could still result in incorrect interpretations of ventilation performance. The significance of this inter-individual variability is such that the guidelines recommend a rotation of rescuers every two minutes in order to prevent fatigue and deterioration in the quality of their care [12]. For instance, as shown in Fig.2, when the patient first receives excessive VT followed by insufficient VT, ventilation is clearly deleterious, despite the fact that the mean VT would be within the acceptable range. This kind of ventilation could be explained by a sudden change in the position of the facemask, inducing a major leak. On the other hand, the presence of a few insufficient cycles, as shown in Fig.3, does not drastically impair the efficiency of ventilation, as the patient receives at least nine efficient insufflations in the one-minute period. Despite that, the average VT would be less than 300 ml, and ventilation would be considered to be inadequate, if analysed by this method.
The third method identified in this review is a breath-by-breath analysis approach. In this technique, every single ventilation cycle is independently analysed, and the percentage of adequate and inadequate cycle for each test is then given. Although this method is more accurate as it takes into account intra- and inter-individual variability, it still does not correlate ventilatory variations with time. Indeed, time-related variability is necessary to assess the performance of ventilation over a whole time period. The importance of a chronological analysis can be easily understood when comparing Figs.2 and 3. In both cases, a high percentage of insufficient VT has been recorded, but these two ventilation tests are fundamentally different, and would not have the same impact on efficiency of ventilation. As explained previously, the first test features an entire minute of insufficient ventilation cycles that could potentially lead to hypoxia, whereas the efficiency of the second test is not really impacted by the presence of insufficient cycles because of their dispersion over time.
Our review has some limitations. First, it is difficult to make basic comparisons between studies because of the heterogeneity in design and outcome measures. They all had different objectives, carried out different experimental protocols and evaluated different ventilation devices. Second, the low number of relevant articles limits the scope of this review. We also excluded articles written in languages other than English or French.
In conclusion, this review has shown significant heterogeneity in the variables used for the evaluation of successful ventilation. Defining accepted judgment criteria and an adequate method of analysis is crucial for the interpretation of these studies, and for reaching unbiased conclusions. This is even more important for bench studies, where ventilatory data cannot be correlated with physiological measurements or patient outcomes. It would be interesting to apply these different definitions to a database to evaluate their impact on performance of ventilation. However, none of these various approaches are able to provide a clear understanding of efficiency of ventilation over a full time period. This review highlights the need to define a new standardised method, which would give a chronological view of variability in ventilation, to assess performance of manual ventilation accurately. This will allow an easier comparison of results between studies, and could also help to improve the relevance of bench studies by giving more appropriate assessments of the performance of ventilation devices.
Acknowledgments
This work was supported by an unrestricted grant from the European Commission (FEDER), the Greater Besançon Urban Area Community (CAGB), the Regional Council of Franche-Comté, and the General Council of Doubs Department.
Competing interests
No competing interests declared.
References
- 1.Cooper JA, Cooper JD, Cooper JM. Cardiopulmonary resuscitation: history, current practice, and future direction. Circulation. 2006;114:2839–49. doi: 10.1161/CIRCULATIONAHA.106.610907. [DOI] [PubMed] [Google Scholar]
- 2.Aufderheide TP. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109:1960–5. doi: 10.1161/01.CIR.0000126594.79136.61. [DOI] [PubMed] [Google Scholar]
- 3.Cho YC, Cho SW, Chung SP, Yu K, Kwon OY, Kim SW. How can a single rescuer adequately deliver tidal volume with a manual resuscitator? An improved device for delivering regular tidal volume. Emergency Medicine Journal. 2010;28:40–3. doi: 10.1136/emj.2010.099911. [DOI] [PubMed] [Google Scholar]
- 4.O'Donnell C, Davis P, Morley C. Positive pressure ventilation at neonatal resuscitation: review of equipment and international survey of practice. Acta Paediatrica. 2004;93:583–8. doi: 10.1111/j.1651-2227.2004.tb02981.x. [DOI] [PubMed] [Google Scholar]
- 5.Stone BJ, Chantler PJ, Baskett PJF. The incidence of regurgitation during cardiopulmonary resuscitation: a comparison between the bag valve mask and laryngeal mask airway. Resuscitation. 1998;38:3–6. doi: 10.1016/s0300-9572(98)00068-9. [DOI] [PubMed] [Google Scholar]
- 6.de Prost N, Ricard JD, Saumon G, Dreyfuss D. Ventilator-induced lung injury: historical perspectives and clinical implications. Annals of Intensive Care. 2011;1:28. doi: 10.1186/2110-5820-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gammon RB, Shin MS, Groves RH, Hardin JM, Hsu C, Buchalter SE. Clinical risk factors for pulmonary barotrauma: a multivariate analysis. American Journal of Respiratory and Critical Care Medicine. 1995;152:1235–40. doi: 10.1164/ajrccm.152.4.7551376. [DOI] [PubMed] [Google Scholar]
- 8.Rai MR, Popat MT. Evaluation of airway equipment: man or manikin? Anaesthesia. 2011;66:1–3. doi: 10.1111/j.1365-2044.2010.06567.x. [DOI] [PubMed] [Google Scholar]
- 9.Nolan JP, Soar J, Zideman DA, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 1. Executive summary. Resuscitation. 2010;81:1219–76. doi: 10.1016/j.resuscitation.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Berg RA, Hemphill R, Abella BS, et al. Part 5: adult basic life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:5685–705. doi: 10.1161/CIRCULATIONAHA.110.970939. [DOI] [PubMed] [Google Scholar]
- 11.Deakin CD, Nolan JP, Soar J, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 4. Adult advanced life support. Resuscitation. 2010;81:1305–52. doi: 10.1016/j.resuscitation.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Meaney PA, Bobrow BJ, Mancini ME, et al. Cardiopulmonary resuscitation quality: improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128:417–35. doi: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 13.Koster RW, Baubin MA, Bossaert LL, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 2. Adult basic life support and use of automated external defibrillators. Resuscitation. 2010;81:1277–92. doi: 10.1016/j.resuscitation.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyle M, Flavell E. Which is more effective for ventilation in the prehospital setting during cardiopulmonary resuscitation, the laryngeal mask airway or the bag-valve-mask? A review of the literature. Journal of Emergency Primary Health Care. 2012;8:1–11. [Google Scholar]
- 15.Wagner-Berger HG, Wenzel V, Voelckel WG, et al. A pilot study to evaluate the SMART BAG®: a new pressure-responsive, gas-flow limiting Bag-Valve-Mask device. Anesthesia and Analgesia. 2003;97:1686–9. doi: 10.1213/01.ANE.0000087064.29929.CE. [DOI] [PubMed] [Google Scholar]
- 16.Noordergraaf GJ, van Dun P, Kramer BP, et al. Can first responders achieve and maintain normocapnia when sequentially ventilating with a bag-valve device and two oxygen-driven resuscitators? A controlled clinical trial in 104 patients. European Journal of Anaesthesiology. 2004;21:367–72. doi: 10.1017/s0265021504005034. [DOI] [PubMed] [Google Scholar]
- 17.von Goedecke A, Wenzel V, Hörmann C, et al. Effects of face mask ventilation in apneic patients with a resuscitation ventilator in comparison with a bag-valve-mask. Journal of Emergency Medicine. 2006;30:63–7. doi: 10.1016/j.jemermed.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Busko JM, Blackwell TH. Impact of a pressure-responsive flow-limiting valve on bag-valve-mask ventilation in an airway model. Canadian Journal of Emergency Medicine. 2006;8:158–63. doi: 10.1017/s148180350001366x. [DOI] [PubMed] [Google Scholar]
- 19.Nehme Z, Boyle MJ. Smaller self-inflating bags produce greater guideline consistent ventilation in simulated cardiopulmonary resuscitation. BMC Emergency Medicine. 2009;9:1–6. doi: 10.1186/1471-227X-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim JS, Cho YC, Kwon OY, Chung SP, Yu K, Kim SW. Precise minute ventilation delivery using a bag-valve mask and audible feedback. American Journal of Emergency Medicine. 2012;30:1068–71. doi: 10.1016/j.ajem.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Bergrath S, Rossaint R, Biermann H, et al. Comparison of manually triggered ventilation and bag-valve-mask ventilation during cardiopulmonary resuscitation in a manikin model. Resuscitation. 2012;83:488–93. doi: 10.1016/j.resuscitation.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Lee HY, Jeung KW, Lee BK, et al. The performances of standard and ResMed masks during bag-valve-mask ventilation. Prehospital Emergency Care. 2013;17:235–40. doi: 10.3109/10903127.2012.729126. [DOI] [PubMed] [Google Scholar]
- 23.Marjanovic N, Le Floch S, Jaffrelot M, L'Her E. Evaluation of manual and automatic manually-triggered ventilation performance and ergonomics using a simulation model. Respiratory Care. 2013;59:735–42. doi: 10.4187/respcare.02557. [DOI] [PubMed] [Google Scholar]
- 24.American Heart Association. Part 6: advanced cardiovascular life support: section 3: adjuncts for oxygenation, ventilation, and airway control. Circulation. 2000;102:I95–I104. [PubMed] [Google Scholar]
- 25.de Latorre F, Nolan J, Robertson C, Chamberlain D, Baskett P. European Resuscitation Council Guidelines 2000 for Adult Advanced Life Support: A statement from the Advanced Life Support Working Group(1) and approved by the Executive Committee of the European Resuscitation Council. Resuscitation. 2001;48:211–21. doi: 10.1016/s0300-9572(00)00379-8. [DOI] [PubMed] [Google Scholar]
- 26.American Heart Association. Part 4: adult basic life support. Circulation. 2005;112:IV19–IV34. [Google Scholar]
- 27.Nolan JP, Deakin CD, Soar J, Böttiger BW, Smith G. European Resuscitation Council guidelines for resuscitation 2005. Resuscitation. 2005;67:539–86. doi: 10.1016/j.resuscitation.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Langhelle A, Sunde K, Wik L, Steen PA. Arterial blood-gases with 500-versus 1000-ml tidal volumes during out-of-hospital CPR. Resuscitation. 2000;45:27–33. doi: 10.1016/s0300-9572(00)00162-3. [DOI] [PubMed] [Google Scholar]
- 29.De Campos T. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. New England Journal of Medicine. 2000;342:1301–08. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 30.Lyazidi A, Thille AW, Carteaux G, Galia F, Brochard L, Richard JCM. Bench test evaluation of volume delivered by modern ICU ventilators during volume-controlled ventilation. Intensive Care Medicine. 2010;36:2074–80. doi: 10.1007/s00134-010-2044-9. [DOI] [PubMed] [Google Scholar]
- 31.Wolcke B, Schneider T, Mauer D, Dick W. Ventilation volumes with different self-inflating bags with reference to the ERC guidelines for airway management: comparison of two compression techniques. Resuscitation. 2000;47:175–8. doi: 10.1016/s0300-9572(00)00215-x. [DOI] [PubMed] [Google Scholar]


