Targeted delivery of inorganic nanoparticles, particularly of gold, may find wide utility in a number of biomedical applications due to their unique physicochemical and optoelectronic properties. [1-6] However, efficient delivery of a threshold amount of such particles is required for successful biomedical applications including imaging, detection/diagnosis and therapy. [7, 8] Efficient delivery of gold nanoparticles may be achieved by simultaneous targeting of multiple receptors on the cancer cells. [9] Hence, selection of appropriate targets is crucial for successful application of multiple/dual receptor targeted drug delivery system. Herein, we demonstrate the fabrication and characterization of gold nanoconjugates containing EGFR (epidermal receptor growth factor) and FR (folate receptor) antibodies on a single gold core, as a proof of principle study, to enhance the loading of gold nanoparticles (AuNPs) to EGFR and FR expressing cancer cells. We further demonstrate that the dual receptor targeted system (DRTS) is more efficient in delivering AuNPs to EGFR and FR expressing cancer cells than their corresponding single receptor targeting systems (SRTS). Such selective delivery of GNPs could be utilized in numerous biomedical applications such as detection, diagnosis and therapy.
The folate receptor α (FRα) and epidermal growth factor receptor (EGFR) is known to be overexpressed in a number of malignancies including ovarian cancer. [10] Farletuzumab, a monoclonal antibody to FRα is in Phase III clinical trials both alone or in combination with platinum/taxane chemotherapy for the patients experiencing recurrence. [11] Several monoclonal antibodies directed against EGFR (trastuzumab, cetuximab, pertuzumab, and panitumumab) and small molecule tyrosine kinase inhibitors (erlotinib and gefitinib) have been investigated and are currently in different phases of clinical trials in ovarian cancer. [12] Thus, FR and EGFR represent important targets for tumor-specific delivery of anticancer drugs.
Herein, we demonstrate, as a proof of principle study, fabrication and characterization of a dual receptor targeted system (DRTS) and demonstrate that it is much more efficient in delivering AuNPs to EGFR and FR expressing ovarian cancer cells Skov3-ip and OVCAR-5 than their corresponding single receptor targeting systems (SRTS). Such selective delivery of AuNPs has the potential to be used in numerous biomedical applications including detection, diagnosis and therapeutics.
EGFR and FR expression in ovarian cancer cells by western blot and confocal microscopy
The EGFR and FR expression pattern in various ovarian cancer cells lines were first determined by western blot analysis (Figure 1A). The EGFR and FR expressions follow the pattern Skov3-ip>OVCAR-5 with little to no expression in A2780 and OSE (Ovarian Surface Epithelium, normal cells). This is further substantiated by confocal microscopy after treating Skov3-ip and OVCAR-5 cells with Cy-5 labeled anti-EGFR antibody (Ab-EGFR, green) and Cy3 labeled anti-FR antibody (Ab-FR, red) at 4°C (Figure 1B and 1C, respectively). The predominant green fluorescence demonstrates higher binding of EGFR antibody supporting a higher expression of EGFR in OVCAR-5 cells. Similarly, weaker red fluorescence indicates lower reduced binding of anti-FR antibody supporting lower expression of FR. Likewise strong green and red fluorescence are observed in case of Skov3-ip cells indicating higher expression of both EGFR and FR, supporting the western blot data.
Figure 1.
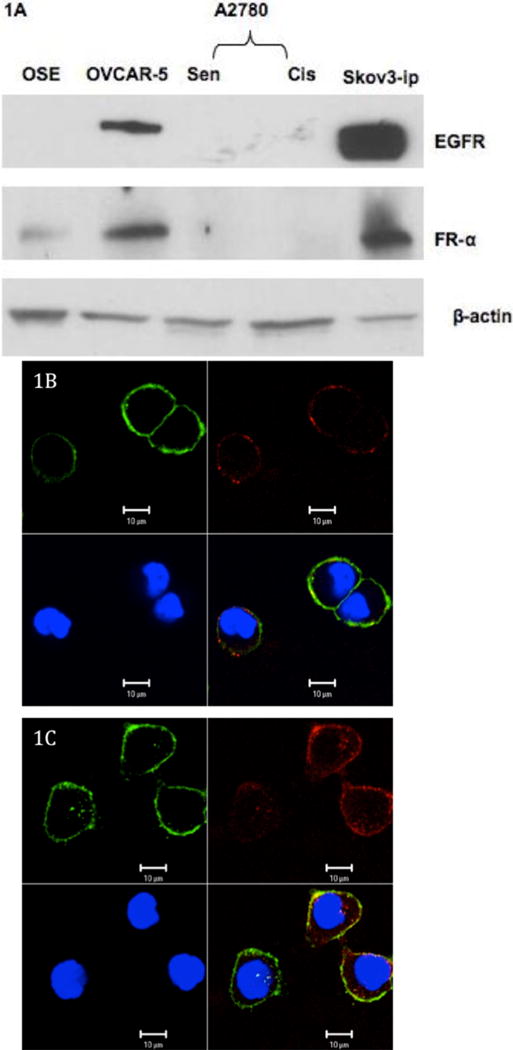
EGFR and FR expression in ovarian cancer cells; A) Western blot analysis of various ovarian cancer cells Skov3-ip, OVCAR-5, A2780 (sen= sensitive, cis = cisplatin resistant); B: OVCAR5 cells) and C: Skov3-ip cells), confocal images to demonstrate the EGFR and FR expressions in green and red fluorescence, after binding of Cy5 and Cy3 labeled anti-EGFR (green) and anti-FR (red) to OVCAR5 and Skov-ip3 cells, respectively, blue color represents the DAPI staining of the nucleus; scale bar is 10 μm.
Binding of anti-EGFR and anti-FR to GNP and the stability of the conjugates in mouse plasma
To demonstrate the ability of DRTS to effectively deliver AuNPs to ovarian cancer cells that moderately express both EGFR and FR, we selected OVCAR-5 and Skov3-ip cells. First we synthesized and characterized the gold nanoconjugates containing anti-EGFR antibody cetuximab (C225) and anti-FR antibody (Ab-FR) (methods in the experimental section). [13]
Figure 2A demonstrates the binding and stability of the nanoconjugates. It is evident from the figure 2A that ∼ 90 % of the radiolabelled 125I-C225 (C*) was bound to AuNP when incubated in the presence of equal amount of non-radiolabelled Ab-FR (F). When 125I-Ab-FR (F*) was incubated with AuNPs in the presence of non-radioiodinated C225, ∼ 70 % of Ab-FR was bound to gold. When both of the radioiodinated C225 and Ab-FR was incubated with AuNP, ∼ 80 % of the Ab were bound to AuNPs. Stability of the nanoconjugate studied in terms of the release of Ab-EGFR and Ab-FR in mouse plasma demonstrates that only 5 % of C225 was released after 24 h of incubation whereas ∼ 15 % of Ab-FR was released in the same time period, demonstrating significant stability of the gold nanoconjugates under biologically relevant condition. This is further confirmed by UV-Visible spectroscopy (Figures 2B, 2C) and transmission electron microscopy (TEM) (Figure 2D). It is evident from Figure 2B that the addition of antibodies/IgGs to AuNPs increases the absorbance of the surface plasmon resonance band from 0.18 to 0.22 suggesting the perturbation of the electrical double layer and binding of antibodies to AuNP. [14-16] Addition of 150 mM NaCl to unprotected AuNPs leads to complete aggregation of AuNPs with a significant decrease in absorbance and a larger shift in the absorption maxima (28 nm shift, Figure 2C). However, all the SRTS and DRTS conjugates are stable against salt induced aggregation in terms of minimum reduction in absorbance and shift in the absorption maxima (maximum 3 nm shift). This is further confirmed by TEM where no significant aggregation was observed (Figure 2D).
Figure 2.
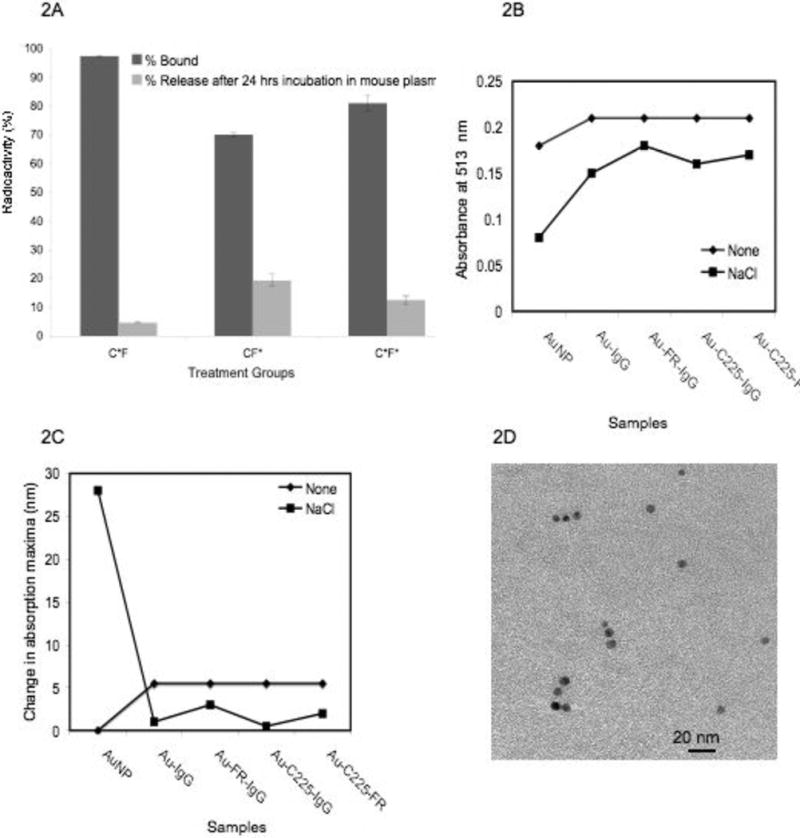
Characterization of DRTS and SRTS nanoconjugates using radioiodination, UV-Visible spectroscopy and TEM. A) Binding of anti-EGFR (C or C*) and FR (F or F*) to AuNPs and release from the nanoconjugate after incubation with mouse plasma for 24 h; * indicates 125I labeled antibody. Stability of different nanoconjugates in the presence and absence of NaCl; B) Change in absorbance of different nanoconjugates in the presence/absence of NaCl and C) Shift in absorption maxima recorded after 15 minutes incubation of the nanoconjugates in 150 mM NaCl; D) TEM image of Au-C225-FR.
Delivery of AuNPs to ovarian cancer cells via DRTS and SRTS
Next we investigated the ability of the DRTS to target EGFR and FR expressing cancer cells to efficiently deliver AuNPs and compare it with their corresponding SRTS. To maintain equal surface coverage by the antibody, IgG along with either Ab-FR or Ab-EGFR was used to generate the SRTS (Au-FR-IgG and Au-C225-IgG). Figure 3A demonstrates the uptake of the nanoconjugates, in terms of gold content, when Skov3-ip cells were treated with an equal amount of the nanoconjugates. Gold content was measured by instrumental neutron activation analysis (INAA). [17] It is clear from the figure that the DRTS is much more effective to deliver AuNPs to Skov3-ip cells, evident by the increase in gold content in dual receptor targeted Au-C225-AbFR (DRTS) vs. either single receptor targeted Au-AbFR-IgG or Au-C225-IgG (SRTS) treated cells. The figure also demonstrates some non-specific uptake of the gold when the cells are treated with non-targeted nanoconjugate, Au-IgG. Similar results were observed with OVCAR5 cells (Figure 3B). However, the difference in uptake of the nanoconjugates in OVCAR5 and Skov3-ip cells might be due to the difference in their inherent endocytic processes. [13] However, the endocytic pathways through which Skov3-ip or OVCAR-5 cells internalize antibody-receptor complex are not clearly defined. Intracellular localization of AuNPs in OVCAR-5 and Skov3-ip cells by DRTS and SRTS delivery was further demonstrated by transmission electron microscopy (TEM) (Supporting Figure S1 and S2). In OVCAR-5 and Skov3-ip cells, AuNPs in the form of DRTS and SRTS are found in double membrane bound multivesicular bodies (Supporting Figure S1 and S2, respectively). However, the uptake of non-specific Au-IgG was very minimal (data not shown).
Figure 3.
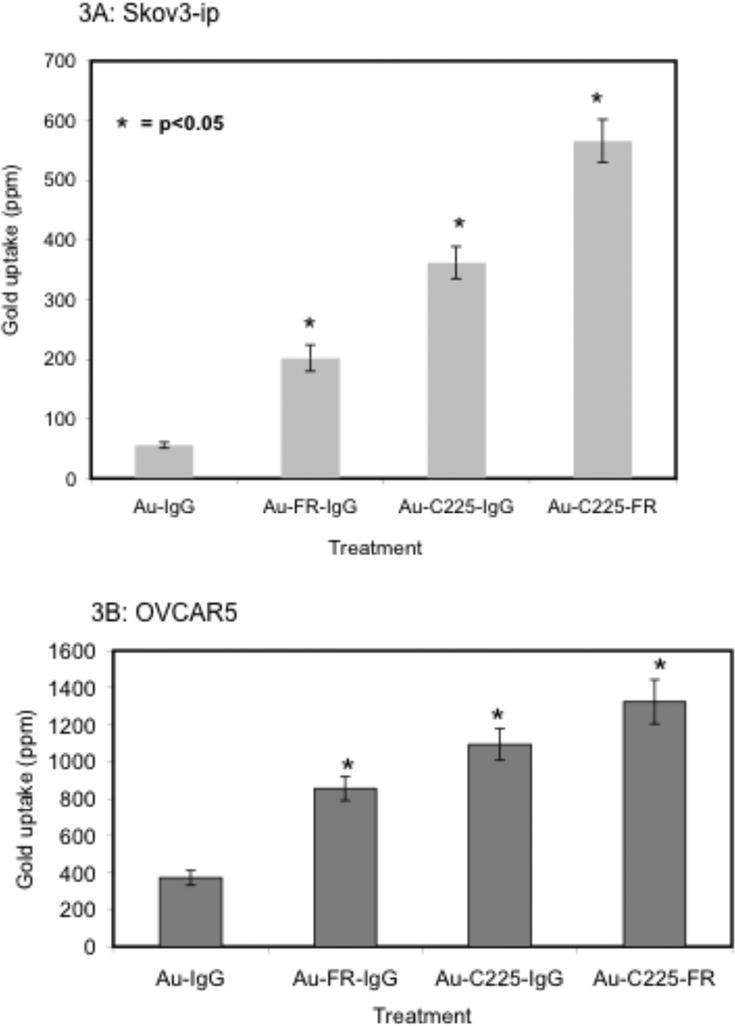
Targeted delivery of gold nanoconjugates via dual receptor targeting: 2A and 2B: Targeting efficacy of DRTS (Au-FR-C225) vs. SRTS (Au-C225-IgG and Au-FR-IgG) and non-targeted counterpart (Au-IgG) to Skov3-ip and OVCAR5 cells, respectively. Cells were treated with different nanoconjugates for 2 h. Gold content was estimated by instrumental neutron activation analysis (INAA), * indicates p<0.05
Ovarian cancer is recognized as most deadly among the gynecologic malignancies. [10] Even though patients respond to initial chemotherapy after surgical debulking, unfortunately, most patients experience a more aggressive and drug resistant relapse. [18] Targeted delivery of inorganic nanoparticles may be utilized for early detection, diagnosis and better therapeutic intervention by delivering chemotherapeutics in tumor specific manner.
As a proof-of-principle study, we demonstrate here fabrication and characterization of a dual receptor targeted system exploiting EGFR and FR as target receptors to deliver AuNPs to ovarian cancer cells having overexpression of either EGFR or FR or both. Furthermore, if the cancer cells overexpress both the receptors, ability of this DRTS to target those cancer cells will be further enhanced. As a result of this, AuNPs fabricated in a DRTS form may be delivered in a much more effective way to the cells in the heterogeneous population. [19] This form of delivery coupled with the technological advantages associated with gold nanoparticles such as hyperthermia using non-invasive RF, X-contrast behavior and as a delivery vehicle, may be utilized for the therapeutic, detection and diagnosis in cancer. [20]
Experimental Section
Reagents
Antibody was principally purchased from Cell Signaling and Santa Cruz, Cetuximab (Bristol Mayer Squib), Folate receptor antibody (Enzo life science, cat no ALX-804-439-R100), Para formaldehyde (Electron Microscopy Science, 15710), BSA (Fischer Scientific, S-5058).
Cell culture
Ovarian cancer cell lines OVCAR-5, Skov3-ip, was purchased from American Type Culture Collection, A2780 sensitive and resistant were kindly provided by Dr. Barbara Vanderhyden, University of Ottawa, Canada and cultured using DMEM and RPMI 1640 with L-glutamine (Cellgro Mediatech, Inc.) supplemented with 10 % FBS and 1% antibiotics (penicillin-streptomycin).
Synthesis of gold nanoparticles and dual nanoconjugates
Gold nanoparticles (AuNPs) were synthesized from tetrachloroauric acid by wet chemical methods using sodium borohydride as a reducing agent as previously described. [13] AuNPs thus formed was characterized using UV-Vis and TEM confirming ∼ 5 nm size spherical nanoparticles formed by this method (Data not shown). For the synthesis of dual nanoconjugates, cetuximab (2 μg /ml) and anti-folate receptor antibody (2 μg/ml) were added to the 1.0 ml of the AuNP solution and incubated at room temperature for 2 h. The solution was centrifuged at 20,000 rpm at 10 °C for 1h and the pellet was collected, measured by UV-Vis and used for the treatment.
Synthesis of conjugates containing anti-EGFR antibody labeled with Cy5 dye and anti-Folate antibody labeled with Cy3 dye
The monoclonal anti-EGFR antibody cetuximab (C225) & FR-α antibody was conjugated with monoclonal antibody labeling dye Cy5 & Cy3 respectively, according to the manufacturers protocol (GE healthcare, PA33001). [13] In brief, the antibody (2mg/mL) was diluted (1:1) with PBS buffer and then incubated with Cy3 dye for 30 minutes in the presence of coupling buffer. Free dye was separated through a gel filtration column provided in the kit. Dye labeled antibody (C225-Cy3 & FR-α) thus obtained was used to treat the cell to demonstrate the binding to the corresponding receptors and establish the receptor expression pattern.
Immunofluorescence Microscopy
Cells (2×104/well) were seeded in a 4 well chamber slides. After 24 h, each well was treated either with C225-Cy5 and anti-FR-Cy3 in a concentration of 2 μg/mL for 30 minutes at 4 °C. At the end of the experiments each well in the chamber slide was washed with cold PBS (3 times). Cells were fixed in 2 % paraformaldehyde in PBS at room temperature for 15 minutes followed by washing with PBS. It is then mounted in a mounting media containing the nuclear stain DAPI (Vectashield). Slides were then looked over in laser confocal microscopy at 63W lenses. [13]
Characterization of the nanoconjugates by radioiodine labeling
Aliquots of the antibody were labeled to a high specific activity using the chloramine-T. Briefly, 500μg of the protein was labeled with 1.0 mCi (10 μl) carrier free Iodine-125 radionuclide in the presence of 100 μg chloramine-T (100μl of 10μg/μl diluted in phosphate buffer, pH 7.4). The reaction was stopped by adding 500 μg sodium meta-bisulfite (100 μl of 50μg/μl diluted in phosphate buffer, pH 7.4). This final product was dialyzed against PBS in a 1000 MWCO dialysis membrane (Spectra/Por 7) overnight at 4 °C to obtain the purified labeled antibody. The specific activity of the protein labeling procedure was determined by TCA (trichloroacetic acid) precipitation of a small fraction of the labeled antibody and it was found to be < 99% labeled. The protein concentration in the labeled antibody stock was determined by bicinchonic acid (BCA) method.
Different amount of labeled C225 (hot) was added to GNP solution in the presence of cold anti-FR and incubated for 2 hours. Then the conjugates were centrifuged down at 45000g for 1 hour. The radioactive emission from the 10 μl of the C225-GNP conjugates before centrifugation and supernatant was measured in the Gamma Counter The difference between emission from total protein before centrifugation and free protein present in the supernatant was used to calculate the bound concentration of C225 at GNP surface. Similar experiments were performed using hot anti-FR antibody to quantify the amount of binding to GNP.
Colloidal stability of the nanoconjugates monitored by UV-Visible spectroscopy
Different amount of the antibodies (anti-EGFR, anti-FR or a mixture of both) or IgG was added to AuNP solution and incubated for 2 hours. UV-visible spectra of the final solution were recorded in a Shimadzu Spectrophotometer (UV-PC12). Once all the spectra were recorded, all the samples were subjected to aggregation test by adding 150 mM of NaCl solution. [21] After 15 minutes of incubation, UV-visible spectra of the same conjugates were recorded again.
Visualization of intracellular localization of gold nanoparticles by transmission electron microscopy (TEM)
TEM were taken in a FEI TECNAI 12 instruments operated at 100 KV after drop coating different nanoconjugates on carbon coated copper grids. The figure clearly demonstrates non-aggregated well-dispersed AuNPs in the form of Au-C225-FR throughout the grid. For intracellular localization of AuNPs, confluent cancer cells were treated with the purified nanoconjugates (2 μg/mL) for 2 h. At the end of the treatment cells were thoroughly washed with PBS, trypsinized, and washed thrice again in PBS and finally fixed in Trumps fixative and processed for TEM microscopy.
In vitro targeting of Au-C225-FR to cancer cells expressing EGFR and FR
Confluent OVCAR-5 and Skov3-ip cells in 100-mm tissue culture dishes (in triplicate) were treated with the purified nanoconjugates (2 μg/mL) for 2 h. At the end of the treatment cells were thoroughly washed with PBS, trypsinized, and washed thrice again in PBS. Finally, cells were pooled down at 1,300 rpm to obtain the pellet for the instrumental neutron activation analysis INAA) analysis as previously reported. [17] Humanized IgG was used as an isotype control, and the cells were treated with Au-IgG similarly as described above.
Western Blot Analysis
The expression of EGFR and FR was determined in untreated OSE, OVCAR-5, Skov3-ip, A2780-sensetive and A2780-resistant cells. Cells were grown in a 100 mm tissue culture dish in RPMI and DMEM medium containing serum, L-glutamine and antibiotics, respectively. Confluent cells were harvested and cell lysates were collected using NP-40 lysis buffer. Protein concentration in the cell lysates was measured using Bradford assay kit (Bio-rad). The proteins were loaded on a 10% SDS-PAGE gel for separation and transferred to a PVDF membrane. The membrane was incubated with EGFR antibody (Santa cruz, sc-03) and FR antibody (Enzo life science, cat no ALX-804-439-R100). Blot was washed thrice with TBS-Tween 20 (0.1 %) and incubated with secondary antibody conjugated with peroxidase. Membrane was stripped with stripping buffer to see the beta-actin for protein loading.
Instrumental Neutron Activation Analysis (INAA)
Samples were analyzed by INAA as previously described. [17] In brief, nanoconjugates and cell pellets were transferred and weighed into precleaned, high-density polyethylene irradiation vials and lyophilized to constant dry weight. Samples were then reconstituted with sample solution (100 μL), loaded in polyethylene transfer “rabbits” and irradiated for 90 s in a thermal flux density of ∼5×1013 ncm-2 s-1. Samples were allowed to decay for 24-48 h and counted in real-time on a high-purity germanium detector for 3600 s at a sample-to-detector distance of ∼ 5 cm. Gold mass was quantified by measuring the 411.8 keV gamma ray from β-decay of 198Au (t1/2=2.7 days), and calibrated using certified gold standard solutions as described previously.
Supplementary Material
Figure S1: TEM analysis of the OVCAR-5 cell after treatment with Au-C225-FR, Au-C225-IgG. Cell were treated with dual conjugates or mono conjugates or its isotype control Au-IG, at 37 °C for 2 h. Figure S2A, 2C and 2D represent a TEM micrographs of OVCAR-5 cells after treatment with Au-C225-FR, Au-C225-IgG and Au-FR-IgG, respectively; Figures S2B, D and F represent the high magnification image of the selected region as depicted by arrow in Figures S2A, 2C and 2E, respectively. Au-IgG was hardly seen in the cells and thus not presented.
Figure S2: TEM analysis of the Skov3-ip cells after treatment with Au-C225-FR, Au-C225-IgG and Au-FR-IgG. Cells were treated with dual conjugates or mono conjugates or its isotype control Au-IG, at 37 °C for 2 h. Figure S2A and 2C represent TEM micrographs of Skov3-ip cells after treatment with Au-C225-FR and Au-C225-IgG, respectively; Figures S2B and 2D represent the high magnification image of the selected region as depicted by arrow in Figures S2A and 2C respectively. Similar results were obtained with Au-FR-IgG (data not shown). Au-IgG was hardly seen in the cells and thus not presented.
Acknowledgments
Supported by NIH CA135011, CA136494 grants (PM).
References
- 1.Davis ME, Chen Z, Shin DM. Nat Rev Drug Discov. 2008;7:771. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 2.Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. J Phys Chem B. 2006;110:7238. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]
- 3.Kim B, Han G, Toley BJ, Kim Ck, Rotello VM, Forbes NS. Nat Nanotech. 2010;5:465. doi: 10.1038/nnano.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liz-Marzán LM. Mater Today. 2004;7:26. [Google Scholar]
- 5.Rosi NL, Mirkin CA. Chem Rev. 2005;105:1547. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 6.Whitesides GM. Nat Biotech. 2003;21:1161. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 7.Alivisatos P. Nat Biotech. 2004;22:47. doi: 10.1038/nbt927. [DOI] [PubMed] [Google Scholar]
- 8.Giljohann DA, Mirkin CA. Nature. 2009;462:461. doi: 10.1038/nature08605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arvizo R, Bhattacharya R, Mukherjee P. Exp Opin Drug Deliv. 2010;7:753. doi: 10.1517/17425241003777010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yap TA, Carden CP, Kaye SB. Nat Rev Cancer. 2009;9:167. doi: 10.1038/nrc2583. [DOI] [PubMed] [Google Scholar]
- 11.Spannuth WA, Sood AK, Coleman RL. Exp Opin Biol Ther. 2010;10:431. doi: 10.1517/14712591003592069. [DOI] [PubMed] [Google Scholar]
- 12.Mendelsohn J, Baselga J. J Clin Oncol. 2003;21:2787. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharyya S, Bhattacharya R, Curley S, McNiven MA, Mukherjee P. Proc Natl Acad Sci. 2010;107:14541. doi: 10.1073/pnas.1006507107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly KL, Coronado E, Zhao LL, Schatz GC. J Phys Chem B. 2002;107:668. [Google Scholar]
- 15.Mangeney C, Ferrage F, Aujard I, Marchi-Artzner V, Jullien L, Ouari O, Rékaï ED, Laschewsky A, Vikholm I, Sadowski JW. J Am Chem Soc. 2002;124:5811. doi: 10.1021/ja010796h. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharya R, Patra CR, Earl A, Wang S, Katarya A, Lu L, Kizhakkedathu JN, Yaszemski MJ, Greipp PR, Mukhopadhyay D, Mukherjee P. Nanomed. 2007;3:224. [Google Scholar]
- 17.Arvizo RR, Miranda OR, Thompson MA, Pabelick CM, Bhattacharya R, Robertson JD, Rotello VM, Prakash YS, Mukherjee P. Nano Lett. 2010;10:2543. doi: 10.1021/nl101140t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bristow RE, Palis BE, Chi DS, Cliby WA. Gynecol oncol. 2010;118:262. doi: 10.1016/j.ygyno.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 19.Hosta-Rigau L, Olmedo I, Arbiol J, Cruz LJ, Kogan MJ, Albericio F. Biocon Chem. 2010;21:1070. doi: 10.1021/bc1000164. [DOI] [PubMed] [Google Scholar]
- 20.Huang X, El-Sayed IH, Qian W, El-Sayed MA. J Am Chem Soc. 2006;128:2115. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 21.Patra CR, Bhattacharya R, Wang E, Katarya A, Lau JS, Dutta S, Muders M, Wang S, Buhrow SA, Safgren SL, Yaszemski MJ, Reid JM, Ames MM, Mukherjee P, Mukhopadhyay D. Cancer Res. 2008;68:1970. doi: 10.1158/0008-5472.CAN-07-6102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: TEM analysis of the OVCAR-5 cell after treatment with Au-C225-FR, Au-C225-IgG. Cell were treated with dual conjugates or mono conjugates or its isotype control Au-IG, at 37 °C for 2 h. Figure S2A, 2C and 2D represent a TEM micrographs of OVCAR-5 cells after treatment with Au-C225-FR, Au-C225-IgG and Au-FR-IgG, respectively; Figures S2B, D and F represent the high magnification image of the selected region as depicted by arrow in Figures S2A, 2C and 2E, respectively. Au-IgG was hardly seen in the cells and thus not presented.
Figure S2: TEM analysis of the Skov3-ip cells after treatment with Au-C225-FR, Au-C225-IgG and Au-FR-IgG. Cells were treated with dual conjugates or mono conjugates or its isotype control Au-IG, at 37 °C for 2 h. Figure S2A and 2C represent TEM micrographs of Skov3-ip cells after treatment with Au-C225-FR and Au-C225-IgG, respectively; Figures S2B and 2D represent the high magnification image of the selected region as depicted by arrow in Figures S2A and 2C respectively. Similar results were obtained with Au-FR-IgG (data not shown). Au-IgG was hardly seen in the cells and thus not presented.


