Abstract
The transmembrane subunit (gp41) of the HIV envelope protein complex (Env) mediates the viral fusion step of HIV entry. The membrane-proximal external region (MPER), one of the functional domains of gp41, has been the focus of a great deal of research because it is a target for neutralizing antibodies. In this study, we examined 23 amino acid residues in MPER (660-683) in both a CXCR4 co-receptor utilizing strain (HXB2) and a CCR5 utilizing strain (JRFL) by alanine scanning mutagenesis.
Despite the high degree of gp41 sequence conservation, the effects of alanine mutation in the MPER were different between the two strains. Most mutations in HXB2 had fusogenicity and protein expression levels not less than 50% of wild type in the case of cell-cell fusion. However, about thirty percent of the mutants in HXB2 showed a severe defect in fusogenicity in viral entry. Mutations in the MPER of strain JRFL had more dramatic effects than HXB2 in cell-cell fusion and viral entry. The fact that there are large differences in the effects of mutation between two strains suggests the potential for MPER interaction with non-conserved sequences such as the fusion peptide and/or other NHR domains as well as potential long-range structural effects on the conformational changes that occur with the Env complex during membrane fusion.
Introduction
The HIV-1 envelope protein (Env) is expressed as a precursor protein (gp160) and cleaved by a cellular protease into two subunits: the surface subunit (gp120) and the transmembrane subunit (gp41). The transmembrane subunit (gp41) mediates membrane fusion and is composed of several domains: the fusion peptide, the N-terminal heptad repeat (NHR), the loop region, the C-terminal heptad repeat (CHR), followed by the membrane proximal external region (MPER), and the transmembrane region (Fig 1).1
Figure 1. Alignment of gp41 amino acid sequence highlighting the MPER from the HXB2 and the JRFL strains.
Gp41 begins at the N-terminus with the fusion peptide (FP) followed by the N-terminal heptad repeat (NHR), a loop, the C-terminal heptad repeat (CHR), a membrane proximal external region (MPER), and a transmembrane domain (TM) followed by a C-terminal cytoplasmic region (C-tail). The alignment of gp41 amino acid sequence was done using CLC Main Workbench 7.5.1. The numbering is shown based on HXB2 strain and the matching residues shown as dots. The boxed area indicates the MPER region. We made 23 alanine substitutions in both strain HXB2 and strain JRFL.
MPER is located proximal to the viral lipid bilayer at the C-terminal end of the ectodomain portion of gp41. MPER is highly conserved and is essential for membrane fusion.2 A conserved tryptophan-rich motif plays an important role in Env-mediated fusion and infectivity.3 Five tryptophan residues in MPER are known to be involved in fusion-pore expansion.4
MPER has been focused on as one of the important targets in HIV vaccine development.5–7 Human antibodies, 2F5, 4E10, Z13el, and 10E8 bind to MPER and neutralize a broad range of HIV-1 strains.8–14 These broadly neutralizing antibodies are known to disrupt MPER function and membrane fusion.15, 16 The MPER sequence also makes up a portion of the only peptide entry inhibitor in the clinic, T20 (brand name-Fuzeon, generic name-enfuvirtide) as it contains 14 of the MPER amino acid residues.17–19 MPER is an important region to manipulate in attempts to improve immunogenicity and elicit neutralizing antibodies.20–25
There are issues, however, with utilizing MPER as a target. MPER is occluded by steric hindrance caused by quaternary Env packing and is exposed only transiently at a relatively late stage in the entry mechanism.26–29 Inaccessibility to MPER due to the viral membrane and structure of the Env trimer remains one of the obstacles in developing vaccines and therapeutic intervention methods targeted to this region.5
There are several crystal structures of HIV gp41, but most atomic-level structures contain only the gp41 core made up of the two helical heptad repeats and the middle loop region.30–33 The structure of full length intact gp41 with the MPER and the transmembrane region has not been solved at the atomic level. There are recent reports of smaller gp41 constructs that include the MPER sequence. One X-ray crystallographic structure reported consists of CHR and MPER constructs (residues 630-680) which form an asymmetric dimer with itself.34 Another structural study included NHR, CHR, and MPER and suggests that the MPER portion is a long, slightly bent helix and relatively flexible.35 There is a report of a crystal structure of gp41 (residues 528-680) including MPER and the fusion peptide which is located upstream of the NHR.36 This report suggests that the structure has a ~90° turn of MPER at N677.
As gp41 is a membrane protein and the viral membrane is involved in neutralization by neutralizing antibodies, it is important to consider this region in the context of the lipid environment.37 The structure of MPER in the lipid environment is not clearly understood, but there are diverse structures that have been proposed. One study suggests a metastable L-shaped structure embedded on a membrane surface.16 The L-shaped MPER structure consists of a helix and a flexible hinge followed by another helix. This L-shaped MPER structure can be disrupted by neutralizing antibodies.15 Another structural study of trimeric MPER on a detergent micelle shows a symmetrical α-helical conformation with a bend between the 2F5 and the 4E10 epitopes.38 A recent study shows that MPER can have at least two stable conformations in the lipid bilayer suggesting that the topology can be switched depending upon the physiological environment.39 Taken together, evidence suggests that flexibility in the MPER may be partially responsible for poor immunogenicity.
With this report, the study of gp41 by alanine scanning mutagenesis in the literature encompasses the NHR, CHR, loop region, and now MPER, providing insights into the role and importance of each residue in the gp41 ectodomain.40–42 Herein, we report alanine scanning studies of MPER using both CXCR4 and CCR5 utilizing strains to determine the importance of each amino acid in cell-cell fusion and viral entry. These studies help to identify critical regions for potential intervention and also regions that have the potential to be subjected to manipulations for labeling for ultrastructural studies without detrimental effects on function. This is especially important for domains such as MPER that are critical in the membrane fusion process.
Experimental Procedures
Plasmids and Mutagenesis
The envelope protein expressing plasmids which contain alanine mutations on MPER were prepared as described previously41 from pHXB2-env43 and pJRFL-env44 using the QuikChange II XL site-directed mutagenesis kit (Stratagene) and they were verified by DNA sequencing (Roswell Park Cancer Institute DNA Sequencing Laboratory). The plasmid expressing HIV Rev protein, pRev-145 and the plasmid expressing Tat protein, pCEP4-Tat46 were used in the cell-cell fusion assay. The HIV backbone plasmid, pNL4-3.HSA.R-E-47 was used in the production of HIV pseudovirus.
Cell Culture
TZM-bl48 and 293T (ATCC) cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) which contained 10% fetal bovine serum (FBS), 1% penicillin-streptomycin. Cells were incubated in 5% CO2 incubator with humidification at 37 °C.
Cell-Cell Fusion Assay
For the cell-cell fusion assay, the wild type (WT) or alanine mutants of the envelope plasmid, pHXB2-env or pJRFL-env, were transfected with pRev45 and pTat46 plasmids by a standard polyethylenimine transfection49 into 293T cells. 5 × 105 cells/well of 293T cells were prepared the day before the transfection and 3 μg of envelope plasmid, 0.8 μg of each pRev and pTat plasmid were used per well for the transfection in a 6-well plate. At 24 hours post-transfection, the cells were detached from the plate and seeded at 5 × 104 cells/well along with 5 × 104 cells/well of TZM-bl in a 96 well plate. The plate was incubated for another 24 hours at 37 °C in a 5% CO2 incubator and membrane fusion activity was measured by a luciferase assay (One-glo, Promega) according to the manufacturer’s protocol. Fusion levels were normalized to the fusion level of WT envelope protein-expressing cells.
Virus Entry Assay
HIV-1 was produced by co-transfection of 10 μg of plasmids encoding envelope protein, either WT or mutant, and 10 μg of pNL4–3.HSA.R-E-47 into 293T cells in a 100 mm dish as described previously.50 The HIV-1 p24 antigen capture enzyme-linked immunoassay (AIDS & Cancer Virus Program, National Cancer Institute at Frederick, Frederick, MD) was performed to determine p24 concentration of mutant enveloped viral particles as well as WT virus.
TZM-bl cells were seeded at 2 × 104 cells/well in a 96-well plate and virus was added at the equivalent of 100 ng of p24 per well. The virus was spinoculated at 1,000 × g for 1 hour at room temperature and then incubated at 37 °C in a 5% CO2 incubator.50, 51 At 48 hours post infection, viral entry levels were measured by a luciferase assay as described above.
Western Blot Analysis
Cellular pellets were lysed using M-PER mammalian protein extraction reagent (Thermo Scientific) containing protease inhibitor cocktail (Roche). The virus pellet was prepared by ultracentrifugation at 55,000 rpm for 1 hour using a Beckman SW55Ti rotor. The pellets were resuspended in lysis buffer [50 mM Tris (pH 7.5), 150 mM NaCl, 5 mM EDTA, 0.5% Nonidet P-40, and 0.1% SDS].40, 41
Protein was quantified using a BCA protein assay (Pierce) and SDS-PAGE was performed. After transfer, the nitrocellulose membranes were blocked with the blocking buffer (LI-COR) and then probed with mouse anti-HIV-1 gp41 monoclonal antibody (Chessie-8,52) and goat anti-HIV-1 gp120 polyclonal antibody (USBiological) simultaneously.
The secondary antibodies used in this study were IR800-conjugated donkey anti-mouse IgG (LI-COR) for gp41 and IR680-conjugated rabbit anti-goat IgG (LI-COR) for gp120. For the quantification, p24 levels were also analyzed by re-probing the membrane with anti-HIV-1 p24 monoclonal antibody.53
The membranes were scanned by the Odyssey Infrared Imaging System (LI-COR). Data for the quantification of each band were obtained from the integrated intensities (I.I) after the median background subtraction method. The I.I from each band was used to calculate the relative expression levels of proteins. For virus and virus-producing cells, the relative expression was calculated as follows: [(I.I of Mutant Env/I.I of p24)/(I.I of WT Env/I.I of p24)] X 100. In this way, the relative expression ratio of gp41, gp120, and gp160 from each mutant was obtained. For the transfected cells used for the cell-cell fusion assay, normalization was to the WT level of gp41, gp120, and gp160 respectively, as there is no expression of p24 in this case.
Results
Gp41 MPER Alanine Scanning
Two different co-receptor utilizing HIV strains were used in this study: HXB2 for CXCR4-usage and JRFL for CCR5-usage. We compared the entire amino acid sequence of gp41 from both HXB2 (NCBI GenBank accession number: K03455.1) and JRFL (NCBI GenBank accession number: U63632.1. There was about 96% identity (821 residues identical of 856) in the gp41 amino acid sequence between HXB2 and JRFL, indicating high conservation in the gp41 sequence between these two strains (Fig 1).
The MPER domain is defined as a total of 24 amino acid residues (660-683 in the HXB2 envelope sequence) based on structural studies and the HIV genome (Fig 1, boxed area). Between HXB2 and JRFL, only 2 residues are different (22 residues identical out of 24). One is residue 675 and the other is residue 678. Overall, there is 92% amino acid sequence identity between these two strains in MPER (Fig 1, the boxed area). A total of 23 amino acids out of 24 amino acids were substituted with alanine as residue 667 is already an alanine residue.
Cell-Cell Fusion and Viral Entry of gp41 MPER Mutants
To determine the effects of alanine mutagenesis on MPER, a cell-cell fusion and a viral entry assay were performed as described previously.41 For both cases, we performed three sets of experiments and obtained the average of the fusion levels, and then the fusion levels of each alanine mutant were compared to WT levels.
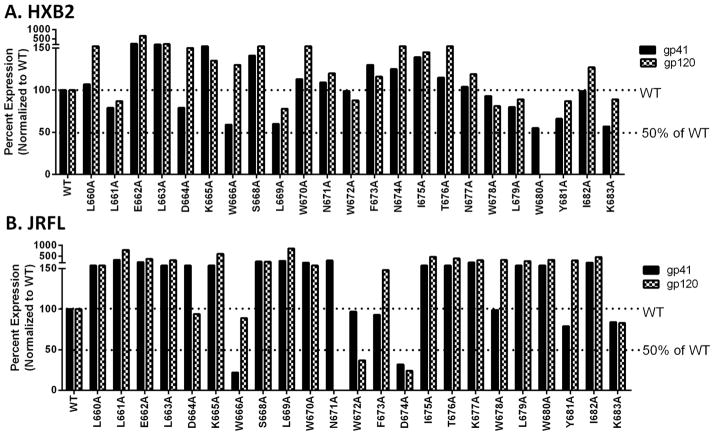
Figures 2 and 3 show activity in cell-cell fusion and viral entry in the HXB2 strain and the JRFL strain respectively. Tables 1 and 2 show the summary of these data.
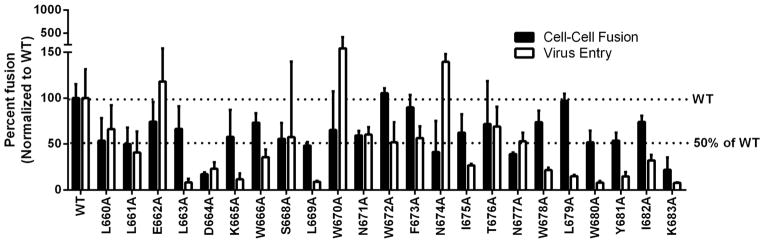
Figure 2. Effects of alanine substitution on HXB2 Env mediated cell-cell fusion and viral entry.
For cell-cell fusion (black bars), each mutant plasmid was transfected into 293T cells along with plasmids expressing the tat and rev proteins. The transfected cells were then co-incubated with the receptor cell line TZM-bl for 24 hours. Fusion activity was measured by luciferase assay. For viral entry (white bars), each mutant was transfected into 293T cells along with the HIV backbone plasmid and virus was harvested after 48 hours. The amount of virus was determined by a p24 antigen capture ELISA assay, and virus stocks with an equivalent amount of p24 protein (100 ng) were added to each well of TZM-bl cells. Viral entry levels were determined by luciferase assay at 48 hours post infection. The WT level and the 50% of WT fusion are indicated with a dotted line.
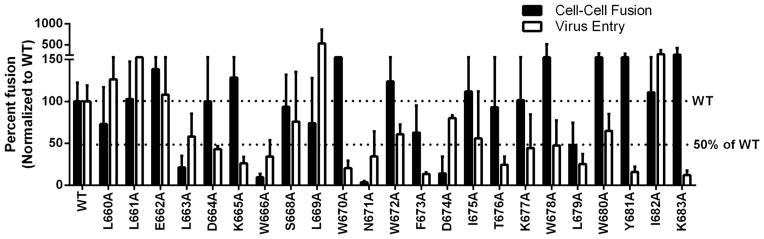
Figure 3. Effects of alanine substitution on JRFL Env mediated cell-cell fusion and viral entry.
Cell-cell fusion (black bars) and viral entry levels (white bars) were measured as described in Figure 2 using JRFL Env mutants.
Table 1.
Effect of Alanine Mutation on HXB2 Envelope Protein
| Residue | Cell-Cell Fusion | Virus Entry | ||||||
|---|---|---|---|---|---|---|---|---|
| gp120a Level | gp41b Level | Fusione Category | Effectf of alanine mutation | gp120c Level | gp41d Level | Fusione Category | Effectf of alanine mutation | |
| L660A | +++++ | ++++ | WT | - | ++++ | ++++ | WT | - |
| L661A | +++++ | ++++ | Diminished | - | ++++ | +++ | Diminished | - |
| E662A | +++++ | ++++ | WT | - | ++++ | +++++ | WT | - |
| L663A | ++++ | +++ | WT | - | ++++ | ++++ | Abolished | Severe defect |
| D664A | ++++ | ++++ | Abolished | Severe defect | ++++ | ++++ | Diminished | - |
| K665A | ++++ | ++++ | WT | - | ++++ | ++++ | Abolished | Severe defect |
| W666A | +++++ | +++++ | WT | - | ++++ | +++ | Diminished | - |
| S668A | +++++ | ++++ | Diminished | - | ++++ | ++++ | WT | - |
| L669A | +++ | ++++ | Diminished | Mild defect | +++ | +++ | Abolished | Severe defect |
| W670A | ++++ | ++++ | WT | - | ++++ | ++++ | Enhanced | Increased Env Expression |
| N671A | ++++ | +++++ | Diminished | - | ++++ | ++++ | Diminished | - |
| W672A | +++++ | +++++ | WT | - | ++++ | ++++ | WT | - |
| F673A | +++++ | +++++ | WT | - | ++++ | ++++ | Diminished | - |
| N674A | +++++ | ++++ | Diminished | - | ++++ | ++++ | Enhanced | Enhanced fusogenicity |
| I675A | ++++ | ++++ | WT | - | ++++ | ++++ | Diminished | - |
| T676A | +++++ | +++++ | WT | - | +++++ | ++++ | Diminished | - |
| N677A | ++++ | +++++ | Diminished | - | ++++ | ++++ | Diminished | - |
| W678A | +++++ | +++++ | WT | - | ++++ | ++++ | Diminished | - |
| L679A | +++++ | +++++ | WT | - | ++++ | ++++ | Abolished | Severe defect |
| W680A | +++++ | +++++ | Diminished | - | + | +++ | Abolished | Association |
| Y681A | ++++ | +++++ | Diminished | - | ++++ | +++ | Abolished | Severe defect |
| I682A | +++++ | +++++ | WT | - | ++++ | ++++ | Diminished | - |
| K683A | ++++ | +++++ | Diminished | - | ++++ | +++ | Abolished | Severe defect |
Protein (gp120 a, and gp41 b) levels were shown in the cell lysates used for cell–cell fusion and
Protein (gp120 c, and gp41 d) levels of expression shown in virus. The levels of protein expression were shown as follows: +, 0–20% of WT levels; ++, 20–50% of WT levels; +++, 50–80% of WT levels; ++++, >80% of WT levels; and +++++, >120% of WT levels.
Fusion categories were defined as follows: WT (no effect, WT level), diminished (20–80% of wild type), abolished (<20% of wild type), and enhanced ( >120% wild type).
Effect of alanine mutations were defined as follows, expression/folding (<20% of WT gp41), cleavage (<20% gp120 and gp41, WT level of gp160), association (<0.5of gp120/gp41 ratio), severe fusogenicity (>20 % of WT gp41, >0.5 gp120/gp41 ratio, < 20% of WT fusion level), mild fusogenicity (>20 % of WT gp41, >0.5 gp120/gp41 ratio, 20–80% of WT level), increased Env expression ( >120% of WT gp160 proteins and fusion level), and enhanced fusogenicity ( >120% of WT fusion, WT gp160 expression level.)
Table 2.
Effect of Alanine Mutation on JRFL Envelope Protein
| Residue | Cell-Cell Fusion | Virus Entry | ||||||
|---|---|---|---|---|---|---|---|---|
| gp120a Expression | gp41b Expression | Fusione Category | Effectf of Alanine Mutation | gp120c Level | gp41d Level | Fusione Category | Effectf of Alanine Mutation | |
| L660A | +++ | ++++ | WT | - | +++++ | +++++ | WT | - |
| L661A | +++++ | +++++ | WT | - | +++++ | +++++ | WT | - |
| E662A | +++++ | +++++ | WT | - | +++++ | +++++ | WT | - |
| L663A | +++ | +++++ | Abolished | Severe defect | +++++ | +++++ | WT | - |
| D664A | +++++ | +++++ | WT | - | ++++ | +++++ | Diminished | - |
| K665A | +++ | +++++ | WT | - | +++++ | +++++ | Diminished | Mild defect |
| W666A | +++++ | +++++ | Abolished | Severe defect | ++++ | ++ | Diminished | - |
| S668A | +++ | +++++ | WT | - | +++++ | +++++ | WT | - |
| L669A | +++++ | +++++ | WT | - | +++++ | +++++ | Enhanced | Increased Env Expression |
| W670A | +++++ | +++++ | WT | - | +++++ | +++++ | Diminished | Mild defect |
| N671A | + | +++ | Abolished | - | + | +++++ | Diminished | Expression/Association |
| W672A | ++++ | +++++ | WT | - | ++ | ++++ | WT | Association |
| F673A | +++ | +++++ | WT | +++++ | ++++ | Diminished | Mild defect | |
| D674A | +++++ | +++++ | Diminished | Mild defect | ++ | ++ | WT | - |
| I675A | ++++ | +++++ | WT | - | +++++ | +++++ | WT | - |
| T676A | ++++ | +++++ | WT | - | +++++ | +++++ | Diminished | Mild defect |
| K677A | +++++ | +++++ | WT | - | +++++ | +++++ | WT | - |
| W678A | ++ | +++++ | Enhanced | Increased Env Expression | +++++ | ++++ | WT | - |
| L679A | + | +++ | WT | - | +++++ | +++++ | Diminished | Mild defect |
| W680A | + | +++ | Enhanced | Enhanced fusogenicity | +++++ | +++++ | WT | - |
| Y681A | ++ | +++ | Enhanced | Enhanced fusogenicity | +++++ | ++++ | Diminished | Mild defect |
| I682A | + | +++ | WT | - | +++++ | +++++ | Enhanced | Increased Env Expression |
| K683A | ++++ | +++++ | Enhanced | Increased Env Expression | ++++ | ++++ | Diminished | Mild defect |
Protein (gp120 a, and gp41 b) levels were shown in the cell lysates used for cell–cell fusion and
Protein (gp120 c, and gp41 d) levels of expression shown in virus. The levels of protein expression were shown as follows: +, 0–20% of WT levels; ++, 20–50% of WT levels; +++, 50–80% of WT levels; ++++, >80% of WT levels; and +++++, >120% of WT levels.
Fusion categories were defined as follows: WT (no effect, WT level), diminished (20–80% of wild type), abolished (<20% of wild type), and enhanced ( >120% wild type).
Effect of alanine mutations were defined as follows, expression/folding (<20% of WT gp41), cleavage (<20% gp120 and gp41, WT level of gp160), association (<0.5of gp120/gp41 ratio), severe fusogenicity (>20 % of WT gp41, >0.5 gp120/gp41 ratio, < 20% of WT fusion level), mild fusogenicity (>20 % of WT gp41, >0.5 gp120/gp41 ratio, 20–80% of WT level), increased Env expression ( >120% of WT gp160 proteins and fusion level), and enhanced fusogenicity (>120% of WT fusion, WT gp160 expression level.)
For cell-cell fusion, most HXB2 alanine substituted mutants were not less than 50% of WT with the exception of D664 for which the fusion level was extremely low (Fig 2 black bars, Table 1 left panel). These data indicate that there were only modest effects if any on cell-cell fusion caused by alanine mutation in the MPER of HXB2.
However, levels differed significantly in viral entry (Fig 2 white bars, Table 1 right panel). Seven mutants (L663A, K665A, L669A, L679A, W680A, Y681A, and K683A) showed abolished fusion activity in viral entry. Five mutants were less than 50% (D664A, W666A, I675A, W678A, and I682A). Two mutants, W670A and N674A had enhanced fusion levels in viral entry. The remaining mutations were either WT level or moderately diminished. In general, alanine substitution in HXB2 MPER was more deleterious in viral entry than in cell-cell fusion.
The alanine-substituted mutants of JRFL were more variable in cell-cell fusion than were mutations in strain HXB2 (Fig 3 black bars, Table 2 left panel). One mutant, D674A had diminished cell-cell fusion activity compared to WT. There were three mutants (L663A, W666A, and N671A) that exhibited abolished activity in cell-cell fusion. Another four mutants (W678A, W680A, Y681A, and K683A) had an enhanced level of fusion. The remaining mutations were the same level as WT in cell-cell fusion.
The viral entry levels in strain JRFL varied dramatically as well (Fig 3 white bars, Table 2 right panel). Three mutants (L661A, L669A, I682A) had increased levels of viral entry. Many were decreased to below 50% of WT (K665A, W670A, F673A, T676A, L679A, Y681A, K683A). Very few mutations were near the WT level. These included L660A, E662A, and S668A. On a general level, JRFL viral entry was very sensitive to alanine scanning mutations in MPER.
Mutant Expression, Processing, and gp120-gp41 Association Effects
Alanine mutation of each residue in MPER could have effects on 1) protein expression or folding of the precursor protein gp160, trafficking of the protein to the plasma membrane via the endoplasmic reticulum and Golgi apparatus, 2) proteolytic cleavage of the precursor gp160 protein to the gp41/gp120 subunits by the cellular enzyme furin, 3) association between gp41 and gp120, or 4) a defect in fusogenicity.
To determine which of these steps the mutation affected, Western blot analysis was performed. The data were quantified and normalized as described in the experimental procedures. Tables 1 and 2 summarize the effects of alanine mutation in cell-cell fusion and viral entry for HXB2 and JRFL respectively.
HXB2 MPER Mutant Expression, Processing, and gp120-gp41 Association
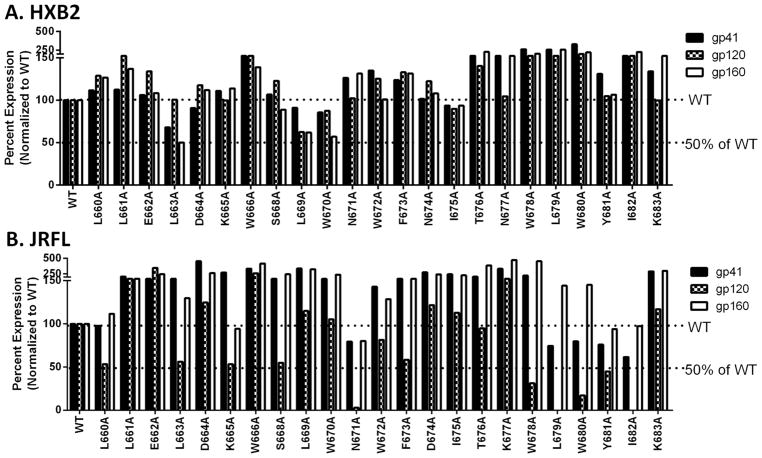
Most HXB2 mutants produced cell-cell fusion levels not less than 50% of WT levels. D664A was one of the residues most dramatically affected. Most mutants including D664A expressed all Env proteins at WT levels as seen in the cell lysate from the cell-cell fusion experiments (Fig 4A). L663A was slightly diminished but the cell-cell fusion level was not dramatically affected. L669A and W670A were diminished in protein levels, however, the cell-cell fusion effects were not dramatic. Most mutants had WT levels of cleavage, association, and fusogenicity (Fig 4A, left panel in Table 1). D664A had a fusogenicity defect as the levels of protein expression, cleavage, and association appeared to be near WT. Generally, scanning alanine mutations in MPER of HXB2 did not cause dramatic effects on cell-cell fusion at most residues.
Figure 4. Protein expression level in the cell lysates used for cell-cell fusion.
293T cells transfected with Env, Tat, and Rev expressing plasmids. The cells were lysed and the total protein concentration was quantified by BCA assay. An equal amount of protein from each transfected cell sample was loaded onto an SDS-PAGE gel followed by Western blot analysis using Chessie-8 (for gp41, and gp160) and anti-gp120 antibodies. Each membrane was scanned and band intensity was quantified. The band intensities of gp41, gp160, and gp120 were normalized to the WT level of each protein. A) Protein expression level of HXB2 Env. B) Protein expression level of JRFL Env.
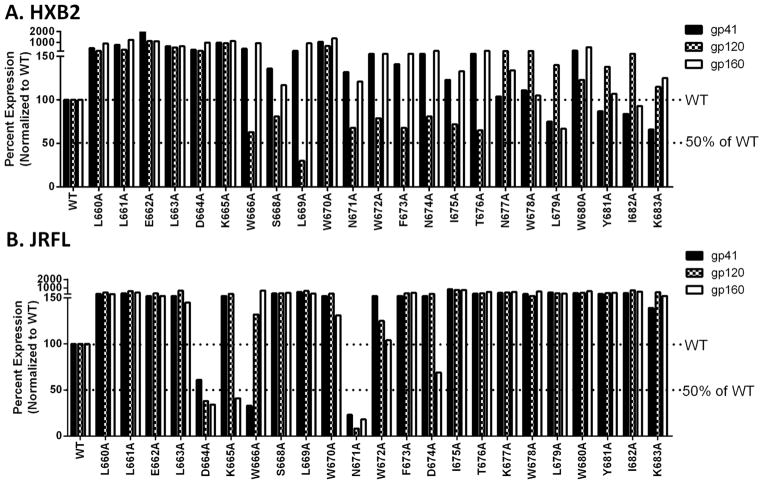
However, the results are quite different in viral entry. Most HXB2 mutants had impacts on viral entry from alanine substitution. Six of these mutants (L663A, K665A, L669A, L679A, Y681A, and K683A) showed either WT or minimally decreased Env expression in both the producer cells and in the virus samples (Fig 5A, Fig 6B, and right panel in Table 1). Some of these mutants (L663A, K665A, Y681A) also had near normal levels of cleavage and gp41/gp120 association whereas the level in viral entry was abolished indicating a defect in fusion. Alanine mutation at these residues had less impact on cell-cell fusion (Fig. 2; black bars), suggesting they play a more critical role in viral entry. L669A had a very low amount of gp120 in the producer cell lysate but the protein levels on the virus were not decreased below 50%. L679A had a lowered level of gp160 expression along with a lowered level of gp41 in the producer cell lysate, however, the levels on the virus were not dramatically decreased. K683A had lowered expression but WT levels of gp41/gp120 in the producer cells and levels in the virus not decreased below 50% of WT.
Figure 5. Protein expression level in the lysates of the virus-producing cells.
293T cells were used for producing virus. These cells were lysed and the protein concentration was quantified by BCA assay. Subsequent Western blot analysis was performed using Chessie-8 and anti-gp120 antibodies followed by band quantification as described in Figure 4. Each membrane was reprobed with anti-p24 antibodies and band intensity was quantified. Each band intensity of gp41, gp160, and gp120 was normalized to the p24 level and then normalized again to the WT level of each protein. A) Protein expression level of HXB2 virus producing cells. B) Protein expression level of JRFL virus producing cells.
Figure 6. Protein levels on the virus.
Concentrated virus with each mutant Env were lysed followed by Western blot analysis was performed as described above. A) Protein level on HXB2 virus. B) Protein level on JRFL virus.
One mutant, W680A had WT levels in the producer cell lysate, however, gp41 on the virus was diminished to approximately 50% and gp120 was undetectable. This suggests both an incorporation defect due to lowered gp41 on virus and an association defect. The ratio of the levels between gp120 and gp41 was calculated to be < 0.5, which implies that there is an association defect. This resulted in a severe disruption in viral entry in the case of W680A.
Finally, two mutants, W670A and N674A had enhanced levels of viral entry. W670A had a higher level of gp160, gp41, and gp120 in the producer cell lysate. N674A, on the other hand, had increased levels of gp160 and gp41 in the producer cell lysate but a decreased level of gp120. Despite this, both mutations (W670A and N674A) had higher levels of gp120 in the virus particles than of gp41 which was closer to the wildtype level.
JRFL MPER Mutant Expression, Processing, and gp120-gp41 Association
In contrast to strain HXB2, alanine mutation of the JRFL strain had far greater effects on cell-cell fusion and effects were seen in viral entry as well (Fig 4B, Fig 5B, Fig 6B, and Table 2). Five of the mutants (L660A, K665A, S668A, L679A, and I682A) which produced WT fusion levels in cell-cell fusion had a lower level of gp120 than gp160 (left panel in Table 2). Nevertheless, this resulted in only a minimal effect on the cell-cell fusion levels which were near WT.
Two mutants (L663A and N671A) had abolished levels of cell-cell fusion and also had low levels of gp120 compared to gp160 expression. Another mutant, W666A which had abolished levels of cell-cell fusion expressed Env protein at the WT level and had wildtype levels of gp41 and gp120. This indicates that the alanine mutation on W666 causes a fusogenicity defect.
There are also mutants (W678A, W680A, Y681A, and K683A) which had increased levels of cell-cell fusion. Interestingly, W678A, W680A, and Y681A all had an increased level of Env protein expression (gp160), a high level of gp41 but a very low level of gp120. Similarly, only K683A had an increased cell-cell fusion level along with increased levels of gp160, gp41, and gp120. The levels of gp160 and gp41 were higher than that of gp120 in this case as well.
The impact on viral entry of alanine mutation in the JRFL Env sequence was varied. Only two mutants, L669A and I682A showed enhanced levels in viral entry and increased Env expression. Each of these mutations showed dramatically higher levels of gp160, gp41 and gp120 in both the virus-producing cells and the virus samples.
Of the mutations that were decreased to 50% or below the level of WT (K665A, W670A, F673A, T676A, L679A, Y681A, K683A) many had protein levels near WT or higher (W670A, F673A, T676A, L679A, Y681A, K683A). This indicates a fusion defect. On the other hand, K665A, had dramatically lowered levels of gp160 in the producer cell lysate while all levels of gp41 and gp120 both in the producer cell lysate and in the virus sample were elevated above WT levels.
There are also two mutants (N671A and W672A) that had lowered amounts of gp120 while the viral entry levels were approximately 50%. The case of N671 is quite dramatic with gp120 levels on the virus undetectable. Taken together with the results of cell-cell fusion in which these two mutations had an impact on gp120 levels as well, there is most likely a defect in association between gp41 and gp120.
Discussion
In this study, the effects of alanine substitutions in the HIV gp41 MPER domain were extensively characterized in two different HIV-1 strains, one CCR5-utilizing (JRFL) and one CXCR4-utilizing strain (HXB2) by a side-by-side scanning mutation approach. Consistent with a previous report by our group41, HXB2 was more stable to alanine substitution in MPER as it had also been shown to be in the C-terminal heptad repeat (CHR) region when compared to JRFL.
Cell-cell fusion assays have not always correlated with the results from viral entry assays.41, 54–57, 58 This suggests that the envelope proteins function differently in these two processes. In the case of cell-cell fusion, envelope is mediating the binding and fusion between two cells of relatively similar size via fusion of the plasma membranes of the envelope-expressing and the target cells. The HIV viral particle has been observed to fuse at neutral pH without the need for endosomal internalization. 59, 60 It has also been observed to enter via an endosomal pathway. 61 In the case of fusion at the plasma membrane, as the diameter of mature HIV (approximately 110–128 nm 62) is much smaller than the receptor cell, the envelope protein complex is mediating binding and fusion between the more highly curved viral membrane and the larger receptor cell plasma membrane. However, if endocytosis is a productive route of entry, the fusion event occurs between the endosomal membrane and the viral membrane which more closely resemble each other in size and curvature.61 Further biophysical and ultrastructural studies of the two processes, cell-cell fusion vs. viral entry, are warranted in order to provide insight into the differences in the mechanistic function of the membrane fusion protein complexes. However, the results from the viral entry assays are more relevant for drug and vaccine development as the physiological role of cell-cell fusion in the host has not been conclusively shown.
HXB2 cell-cell fusion
There are several specific residues that warrant further discussion from each of the strains. In HXB2, there is only one residue that stands out as impacted in cell-cell fusion, D664A. Indeed, mutation to alanine at this residue did not significantly impact any of the protein levels. This suggests a critical role in promoting cell-cell fusion for this residue. Many of the mutants that had impacts on viral entry in the case of HXB2 had very little effect on expression, processing, incorporation, or association, which also suggests that the step impacted was membrane fusion. Gp160 levels were rarely impacted in the case of HXB2 in lysates from cell-cell fusion or in lysates from virus-producing cells indicating that expression of Env for strain HXB2 is stable to mutation.
HXB2 Viral Entry
The protein levels measured on virus-producing cells in the case of mutation HXB2 L669A suggest an association defect. The gp120 level is much lower than the gp160 and gp41 levels. However, the levels of gp120 and gp41 on the virus are not dramatically different from one another or greatly reduced. This suggests that in the case of the L669A mutant, shedding of gp120 at the cell surface may be occurring, yet the virus is able to bud with envelope complexes that have similar amounts of gp41 and gp120. In contrast, W680A seems to have both a defect in incorporation of gp41 into the virion and a defect in the gp120/gp41 association. However, the protein levels in the producer cell lysates are at or above WT level. These differences in incorporation into the virion within a distance of 11 amino acid residues suggest that the envelope complex is sensitive to changes that may be due to long-range effects upon the global structure of the large multimeric membrane complex.
W670A and N674A were both enhanced in viral entry. In the case of both W670A and N674A, there is a higher amount of gp120 protein in the virus sample than gp41. Levels in the producer cells are high for gp160, gp120, and gp41 in the case of W670A. On the other hand, the level of gp120 is less than the WT level and less than gp160 and gp41 in the producer cell line for N674A. It is interesting to note that the enhanced level of gp120 as compared to gp41 correlates with enhanced viral entry in both mutants.
JRFL cell-cell fusion
In the case of JRFL, a number of mutants with WT or enhanced fusion levels had a lowered amount of gp120 (L660A, K665A, S668A, W678A, L679A, W680A, Y681A, I682A). This suggests that gp41 can mediate membrane fusion between the plasma membranes of cells expressing envelope and cells expressing the receptors despite a lowered amount of gp120. This could be an artifact of a cell population that has been transfected to express only envelope protein and which has no budding virus particles. A gp41/gp120 association defect could result in rapid release of gp120 into the cellular milieu immediately after cleavage and presentation on the plasma membrane. This would lead to a decreased amount of gp120 detectable in the cell lysate. In the case of L663A and N671A, the abolished levels of cell-cell fusion correlated with low levels of gp120 which is to be expected. W666A has an apparent fusogenicity defect as studied in cell-cell fusion as all protein levels were wildtype or higher and yet cell-cell fusion was dramatically decreased.
JRFL viral entry
There are many mutations within the C-terminal half of this region of JRFL that have a clear defect in the fusion step of viral entry as can be concluded based upon functional levels being at or below 50% but protein levels of gp160, gp41, and gp120 at WT or greater levels (W670A, F673A, T676A, L679A, Y681A, K683A). K665A had a low level of gp160 and yet the levels of gp41/gp120 were greater than wild type. This was the case both in the virus producing cells and in the protein levels on the virus. Despite the elevated levels of gp41/gp120, entry levels were diminished. There is the possibility that gp160 in this case is cleaved more efficiently. If this is the case, then there also appears to be a fusogenicity defect. This mutant is likely causing multiple defects. N671A and W6712A also produced anomalous results. Although there seems to be a dramatic effect on the gp41/gp120 association, entry levels were around 50%.
In general the protein levels (gp120/gp41) in the virus particles in the case of strain JRFL were quite stable, however, mutations often led to defects in entry suggesting an important role in membrane fusion. There is also the possibility that long-range conformational effects could be caused by mutation that would affect gp120 binding to the receptors or that the larger global structure of the multisubunit complex could be altered. Ultrastructural studies of this complex at different stages during the entry process are warranted but technically challenging.
Correlation analysis between HXB2 and JRFL
We performed a correlation analysis between the mutants in HXB2 and JRFL using the data sets for cell-cell fusion and for viral entry (GraphPad Prism). The calculated Pearson’s r was −0.188 for the cell-cell fusion data set and −0.147 for the viral entry data set. A value of −1 represents perfect negative correlation. In both the cell-cell fusion and viral entry data sets, there was a relatively small negative correlation between the results for the two strains.
Comparison with Previously Published Alanine Substitutions in the MPER
To the best of our knowledge, this is the first report of an extensive comparison of cell-cell fusion and viral entry using alanine scanning mutagenesis of the HIV MPER for both a CCR5 and a CXCR4-utilizing strain of HIV-1. A summary of previously published results for mutation to alanine in this region is shown in Table 3. The alanine mutation at L660 in the JR2 strain had a severe defect on virus infectivity63, however, our results showed only a modest defect because of mutation to alanine at this residue. The mutation at L661 had a WT level in JR2 Env mediated viral entry,63 whereas it had a modest impact in HXB2 viral entry in our study. Residue E662 had a normal level in viral entry with the JR2 strain in agreement with our study. L663A was reported to have no impact on viral entry with the JR2 strain63, whereas in our study, this mutation was impaired in HXB2 viral entry and in JRFL cell–cell fusion. The D664A mutation was reported to have no effect in JR2 viral entry, whereas it showed a severe impact in HXB2 cell-cell fusion and a modest effect in HXB2 and JRFL viral entry in our study. The K665A mutation was reported to have no effect in JR2 viral entry63, however, it did cause a defect in both HXB2 and JRFL viral entry in our study.
Table 3.
Published Results for Alanine Substitutions Performed in the gp41 MPER Region
| Residue | Strains | Effect of Alanine mutation |
|---|---|---|
| L660A | JR2 | <30% of WT infectivity63 |
| L661A | JR2 | Similar infectivity to WT63 |
| E662A | JR2 | Similar infectivity to WT63 |
| L663A | JR2 | Similar infectivity to WT63 |
| D664A | JR2 | Similar infectivity to WT63 |
| K665A | JR2 | Similar infectivity to WT63 |
| W666A | JR2 | <10% of WT infectivity63 |
| HXB2 | Expression processing, gp120 association, and cell-cell fusion similar to WT3 | |
| S668A | JR2 (N668A) | Similar infectivity to WT63 |
| L669A | JR2 | <30% of WT infectivity63 |
| W670A | JR2 | >30% of WT infectivity63 |
| HXB2 | Expression processing, gp120 association, and cell-cell fusion similar to WT3 | |
| N671A | JR2 | Similar infectivity to WT63 |
| W672A | JR2 | <30% of WT infectivity63 |
| F673A | JR2 | Similar infectivity to WT63 |
| N674A | JR2 | Similar infectivity to WT63 |
| I675A | JR2 | <30% of WT infectivity63 |
| T676A | JR2 | >30% of WT infectivity63 |
| N677A | JR2 | Similar infectivity to WT63 |
| W678A | JR2 | >30% of WT infectivity63 |
| HXB2, NL4-3 | Expression processing, gp120 association, and cell-cell fusion similar to WT3 | |
| HXBc2 | Processing to WT level, but slightly reduced association of gp120. Cell-cell fusion similar to WT64 | |
| L679A | JR2 | <30% of WT infectivity63 |
| W680A | JR2 | >30% of WT infectivity63 |
| HXB2, NL4-3 | Expression processing, gp120 association, and cell-cell fusion similar to WT3 |
W666A also had the most severe impact on viral infectivity in the JR2 strain63, however, our results showed only a modest effect on viral entry for either strain. In the case of cell-cell fusion with the JRFL strain, this was the only residue with a severe defect. The W666A mutant was previously reported for the HXB2 strain3 and resulted in expression, processing, gp120 association, and cell-cell fusion levels similar to WT in agreement with our results.
The alanine mutation on residue 668 (S in HXB2 and JRFL, N in JR2) showed no effect on JR2 viral entry63 which is consistent with what we observed in both HXB2 and JRFL viral entry. L669A of JR2 showed a severe reduction in viral infectivity compared to infectivity of WT JR2 strain63, in agreement with our data for HXB2 viral entry. The W670A mutation in JR2 strain was reported to have a decreased level of viral entry63 in agreement with our results for JRFL viral entry.
While the N671A in JRFL in our cell-cell fusion study showed a defect in cleavage, N671A in the JR2 strain had no impact on viral entry.63 While W672A and I675A caused a severe reduction in viral entry in the JR2 strain63, we did not observe this severe impact. F673A in the JR2 strain had no impact in viral entry 63, whereas our data showed modest impact in HXB2 and JRFL viral entry. N674A in the JR2 strain also had no impact on viral entry63, however, it showed an enhanced viral entry level in HXB2. T676A in the JR2 strain63 showed diminished activity in viral entry in agreement with our study for both both HXB2 and JRFL. N677A in the JR2 strain had no impact on viral entry63 whereas our alanine mutant in HXB2 at this residue had a modest defect in viral entry. There is a report showing slightly reduced association of gp120 with gp41 of HXBc2 strain64 for the W678A mutation which we saw only in the case of JRFL cell-cell fusion. Our data showed that the L679A mutation in HXB2 produced a severe defect in viral entry in agreement with the previously reported results for JR2 Env mediated viral entry.63 The W680A mutation had a severe defect in association of gp120 with gp41 in HXB2 viral entry in our study, whereas it was reported that it had no impact on expression, processing, or gp120 association in HXB2 Env mediated cell-cell fusion in agreement with our study.3
Structural Implications
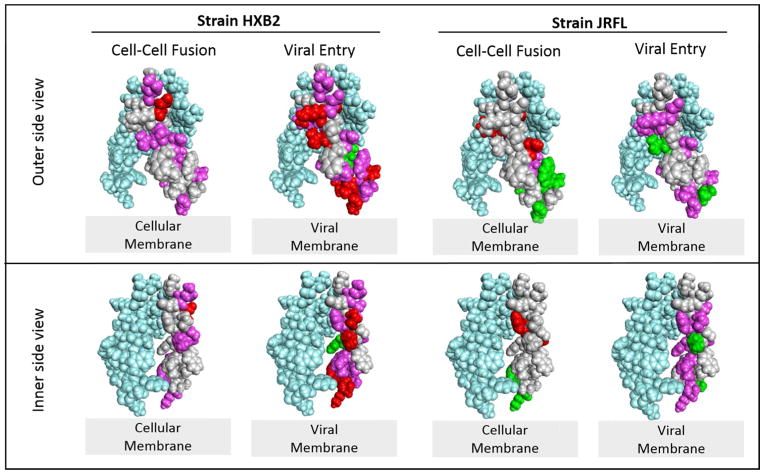
Molecular models of the MPER trimer structure are shown in Fig 7. Since there is no MPER structure available until residue K683 as well as no structure of the full length gp41 protein in the 6HB formation, we were not able to model the potential interaction of MPER with the fusion peptide region. To better understand the effects of mutation in MPER, we took the membrane-associated MPER trimer model38 and compared the locations of residues that are affected by alanine mutations.
Figure 7. Molecular models highlighting the functionality of mutations in the HIV gp41 MPER.
A membrane associated MPER trimer structure was used to diagram the residues that are either diminished or enhanced in function upon mutation to alanine 38. For simplicity, mutations are indicated only on one of the monomers (light gray) and the other two monomers making up the trimer structure are shown in light blue. The top panel is a view of the outer side of the MPER. The bottom panel is a view that is rotated to display the inner side of the MPER. Amino acid residues without highlighting (light gray) have levels 80–120% of WT. Those colored pink have levels diminished to 20–50% of the WT level. Those colored red have levels diminished to < 20% of the WT level. The residues colored green have levels increased above 120% of the WT level. The structure was rendered with Discovery Studio Visualizer 4.0 based upon the coordinates from the Protein Data Bank entry 2LP7.
Mutations that are affecting viral entry were located in the proximity of the viral membrane. Interestingly, the membrane proximal residues including L679, W680, Y681, and K683A in HXB2 were severely affected in viral entry by alanine mutations, whereas mutations in these residues either enhance or have only a modest effect in the JRFL strain. This could suggest that mutations affect the orientation of aromatic residues which can then affect stability of the whole gp41 structure leading to defects in fusogenicity. This is plausible because these residues are also known to play an important role in fusion pore expansion4 and changes in orientation may cause distinct defects in the process.
Another important observation is that mutations that affected membrane fusion were mostly on the side of MPER oriented toward the N-terminal heptad repeat/fusion peptide in the post-fusion structure.30, 32, 33, 65–69 This suggests that MPER interacts with fusion peptide and/or other NHR domain sequences and mutations on MPER may interfere with the interaction between them either in the process of folding or unfolding during the steps in the fusion mechanism.
It is very interesting as well that we observed different effects of mutation on the two HIV-1 strains despite the high degree of sequence conservation between them (Fig 1). There are only two amino acid residues that are different between these two strains in MPER. HIV gp41 has a distinct sensitivity to mutation between different strains that seems to be sequence independent but nonetheless can be affected on a functional level. This suggests the possibility that there are direct interactions or long-range structural changes affecting MPER that are caused by regions that are non-conserved within the gp41 sequence (e.g., fusion peptide).
It is also intriguing that there are significant differences in the effects of alanine mutagenesis between cell-cell fusion and cell-free viral entry. This type of difference between modes of virus transmission has been reported not only by us but also other groups. Many studies presented loss of susceptibility to entry inhibitors and neutralizing antibodies (nAbs) targeting HIV-1 Env in transmission of virus between cells while these remained effective in cell-free virus infection. For example, the nAb2 4E10 and 2F5 (targeting MPER), 17b (targeting a CD4 induced epitope), b12 (targeting CD4 binding site) were not always observed as effective at inhibiting Env mediated cell-cell transmission.54–57 A recent investigation also observed that the antibodies targeting the CD4 binding site such as CD4-IgG2, VRC01, and b12 lost their inhibitory activity easily only in the case of cell-cell virus transmission while their activity remained in viral entry.58 Taken together, these studies along with our report herein suggest that there are dramatic mechanistic differences between cell-cell fusion versus virus-cell entry that have implications in vaccine development. This body of knowledge points to the need to carefully discriminate between targets especially when considering the design of a prophylactic vaccine versus a therapeutic vaccine. Targeting viral entry will be important in the case of a prophylactic vaccine while targeting the mechanism of transmission of virus between cells will be important in the case of a potential therapeutic vaccine.
Our comparative mutagenesis study of the MPER suggests that the current discrepancies in the details of MPER structure reported based upon in vitro studies may very well be real and related to mechanistic differences between strains. For example, while one X-ray crystallography study reported an asymmetric dimer of alpha-helical MPER constructs had utilized prototype B strain HXB2 gp41 sequence34, another structural study suggesting the MPER portion with a slightly bent helix structure used subtype A isolate from Cameroon.35 Another structural study of trimeric MPER showed a symmetrical α-helical conformation with a bend between the 2F5 and the 4E10 epitopes and used B/F1 recombinant isolate 01BAB055.38 Furthermore, even with the same HXB2 gp41 MPER, different structures were suggested. For instance, a study that used HXB2 sequence showed ~90° turn of MPER at N67736 which had not been observed previously.34 On a membrane surface, HXB2 MPER showed a metastable L-shaped structure16 but another study showed MPER with at least two stable conformations in the lipid bilayer.39
MPER has been one of the most promising targets in the approach to elicit neutralizing antibodies due to the broadly neutralizing antibodies 2F5 and 4E10.5–14 However, our study suggests that there may be significant differences between virus strains in how localized regions are involved in cell-cell fusion and viral entry. The discrepancies reported for this region from in vitro structural studies if considered in the context of the dramatic differences we see between strains using mutagenesis studies in cell culture may well be real differences implying a structural plasticity in this region important for consideration in vaccine and drug design.
Another critical issue is that exposure of these vulnerable regions during the entry process is limited in timeframe. The MPER is not exposed on the native virus particle before interaction with the receptors.28 Interestingly, researchers have suggested that specific single amino acid changes in gp41 could potentially expose epitopes for neutralization by antibodies.25 If this is indeed possible, our scanning mutagenesis suggests that mutations L660A and E662A, from both strains may potentially be useful to manipulate the protein for better exposure of MPER to raise neutralizing antibodies without altering function.
Finally, the amino acid positions that are unchanged (light gray) can be seen as potential candidates for manipulation to facilitate labeling for highly desired ultrastructural studies. Many of the MPER residues were shown to be stable to alanine mutations. These are potential sites to manipulate with cutting edge labeling that will enable researchers to study the details of gp41 function.
Acknowledgments
The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: TZM-bl from Dr. John C. Kappes, Dr. Xiaoyun Wu and Tranzyme Inc48, pNL4-3.HSA.R-E- from Dr. Nathaniel Landau47, and pHXB2-env from Dr. Kathleen Page and Dr. Dan Littman43, pRev-1 from Dr. Marie-Louise Hammarskjöld and Dr. David Rekosh45, pCEP4-Tat from Dr. Lung-Ji Chang46, Chessie 8 from Dr. George Lewis52 and HIV-1 p24 Hybridoma (183-H12-5C) from Dr. Bruce Chesebro.53 We would like to thank Dr. James M. Binley (Torrey Pines Institute for Molecular Studies) for the kind gift of pCAGGS JRFL WT 160.
Funding Source Statement
Research reported herein was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R21 AI102796. This work was supported in part by the University of Rochester Center for AIDS Research National Institutes of Health Grant P30AI078498.
References
- 1.Caffrey M. HIV envelope: challenges and opportunities for development of entry inhibitors. Trends Microbiol. 2011;19:191–197. doi: 10.1016/j.tim.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salzwedel K, West JT, Hunter E. A conserved tryptophan-rich motif in the membrane-proximal region of the human immunodeficiency virus type 1 gp41 ectodomain is important for Env-mediated fusion and virus infectivity. J Virol. 1999;73:2469–2480. doi: 10.1128/jvi.73.3.2469-2480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muñoz-Barroso I, Salzwedel K, Hunter E, Blumenthal R. Role of the membrane-proximal domain in the initial stages of human immunodeficiency virus type 1 envelope glycoprotein-mediated membrane fusion. Journal of Virology. 1999;73:6089–6092. doi: 10.1128/jvi.73.7.6089-6092.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montero M, van Houten NE, Wang X, Scott JK. The membrane-proximal external region of the human immunodeficiency virus type 1 envelope: dominant site of antibody neutralization and target for vaccine design. Microbiology and molecular biology reviews : MMBR. 2008;72:54–84. doi: 10.1128/MMBR.00020-07. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlsson Hedestam GB, Fouchier RA, Phogat S, Burton DR, Sodroski J, Wyatt RT. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat Rev Microbiol. 2008;6:143–155. doi: 10.1038/nrmicro1819. [DOI] [PubMed] [Google Scholar]
- 7.Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, Wyatt RT. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5:233–236. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- 8.Nelson JD, Brunel FM, Jensen R, Crooks ET, Cardoso RM, Wang M, Hessell A, Wilson IA, Binley JM, Dawson PE, Burton DR, Zwick MB. An affinity-enhanced neutralizing antibody against the membrane-proximal external region of human immunodeficiency virus type 1 gp41 recognizes an epitope between those of 2F5 and 4E10. J Virol. 2007;81:4033–4043. doi: 10.1128/JVI.02588-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binley JM, Wrin T, Korber B, Zwick MB, Wang M, Chappey C, Stiegler G, Kunert R, Zolla-Pazner S, Katinger H, Petropoulos CJ, Burton DR. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78:13232–13252. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, Hayes D, Louder MK, Brown CR, Sapan CV, Frankel SS, Lu Y, Robb ML, Katinger H, Birx DL. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 12.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, Moore JP, Stiegler G, Katinger H, Burton DR, Parren PWHI. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. Journal of Virology. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stiegler G, Kunert R, Purtscher M, Wolbank S, Voglauer R, Steindl F, Katinger H. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 2001;17:1757–1765. doi: 10.1089/08892220152741450. [DOI] [PubMed] [Google Scholar]
- 15.Song L, Sun ZY, Coleman KE, Zwick MB, Gach JS, Wang JH, Reinherz EL, Wagner G, Kim M. Broadly neutralizing anti-HIV-1 antibodies disrupt a hinge-related function of gp41 at the membrane interface. Proc Natl Acad Sci U S A. 2009;106:9057–9062. doi: 10.1073/pnas.0901474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun ZY, Oh KJ, Kim M, Yu J, Brusic V, Song L, Qiao Z, Wang JH, Wagner G, Reinherz EL. HIV-1 broadly neutralizing antibody extracts its epitope from a kinked gp41 ectodomain region on the viral membrane. Immunity. 2008;28:52–63. doi: 10.1016/j.immuni.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Wild C, Greenwell T, Matthews T. A synthetic peptide from HIV-1 gp41 is a potent inhibitor of virus-mediated cell-cell fusion. AIDS Res Hum Retroviruses. 1993;9:1051–1053. doi: 10.1089/aid.1993.9.1051. [DOI] [PubMed] [Google Scholar]
- 18.Wild CT, Shugars DC, Greenwell TK, McDanal CB, Matthews TJ. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci U S A. 1994;91:9770–9774. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuta RA, Wild CT, Weng Y, Weiss CD. Capture of an early fusion-active conformation of HIV-1 gp41. Nat Struct Biol. 1998;5:276–279. doi: 10.1038/nsb0498-276. [DOI] [PubMed] [Google Scholar]
- 20.Kim M, Song L, Moon J, Sun ZY, Bershteyn A, Hanson M, Cain D, Goka S, Kelsoe G, Wagner G, Irvine D, Reinherz EL. Immunogenicity of membrane-bound HIV-1 gp41 membrane-proximal external region (MPER) segments is dominated by residue accessibility and modulated by stereochemistry. J Biol Chem. 2013;288:31888–31901. doi: 10.1074/jbc.M113.494609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou T, Zhu J, Yang Y, Gorman J, Ofek G, Srivatsan S, Druz A, Lees CR, Lu G, Soto C, Stuckey J, Burton DR, Koff WC, Connors M, Kwon PD. Transplanting supersites of HIV-1 vulnerability. PLoS One. 2014;9:e99881. doi: 10.1371/journal.pone.0099881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris L, Chen X, Alam M, Tomaras G, Zhang R, Marshall DJ, Chen B, Parks R, Foulger A, Jaeger F, Donathan M, Bilska M, Gray ES, Abdool Karim SS, Kepler TB, Whitesides J, Montefiori D, Moody MA, Liao HX, Haynes BF. Isolation of a human anti-HIV gp41 membrane proximal region neutralizing antibody by antigen-specific single B cell sorting. PLoS One. 2011;6:e23532. doi: 10.1371/journal.pone.0023532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim M, Sun ZY, Rand KD, Shi X, Song L, Cheng Y, Fahmy AF, Majumdar S, Ofek G, Yang Y, Kwong PD, Wang JH, Engen JR, Wagner G, Reinherz EL. Antibody mechanics on a membrane-bound HIV segment essential for GP41-targeted viral neutralization. Nat Struct Mol Biol. 2011;18:1235–1243. doi: 10.1038/nsmb.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ofek G, Guenaga FJ, Schief WR, Skinner J, Baker D, Wyatt R, Kwong PD. Elicitation of structure-specific antibodies by epitope scaffolds. Proc Natl Acad Sci U S A. 2010;107:17880–17887. doi: 10.1073/pnas.1004728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blish CA, Nguyen MA, Overbaugh J. Enhancing exposure of HIV-1 neutralization epitopes through mutations in gp41. PLoS Med. 2008;5:e9. doi: 10.1371/journal.pmed.0050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakrabarti BK, Walker LM, Guenaga JF, Ghobbeh A, Poignard P, Burton DR, Wyatt RT. Direct antibody access to the HIV-1 membrane-proximal external region positively correlates with neutralization sensitivity. J Virol. 2011;85:8217–8226. doi: 10.1128/JVI.00756-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ofek G, Tang M, Sambor A, Katinger H, Mascola JR, Wyatt R, Kwong PD. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J Virol. 2004;78:10724–10737. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimitrov AS, Jacobs A, Finnegan CM, Stiegler G, Katinger H, Blumenthal R. Exposure of the membrane-proximal external region of HIV-1 gp41 in the course of HIV-1 envelope glycoprotein-mediated fusion. Biochemistry. 2007;46:1398–1401. doi: 10.1021/bi062245f. [DOI] [PubMed] [Google Scholar]
- 29.Frey G, Peng H, Rits-Volloch S, Morelli M, Cheng Y, Chen B. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc Natl Acad Sci U S A. 2008;105:3739–3744. doi: 10.1073/pnas.0800255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan K, Liu J, Wang J, Shen S, Lu M. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc Natl Acad Sci U S A. 1997;94:12303–12308. doi: 10.1073/pnas.94.23.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malashkevich VN, Chan DC, Chutkowski CT, Kim PS. Crystal structure of the simian immunodeficiency virus (SIV) gp41 core: conserved helical interactions underlie the broad inhibitory activity of gp41 peptides. Proc Natl Acad Sci U S A. 1998;95:9134–9139. doi: 10.1073/pnas.95.16.9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 33.Chan DC, Fass D, Berger JM, Kim PS. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Deng Y, Li Q, Dey AK, Moore JP, Lu M. Role of a putative gp41 dimerization domain in human immunodeficiency virus type 1 membrane fusion. J Virol. 2010;84:201–209. doi: 10.1128/JVI.01558-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi W, Bohon J, Han DP, Habte H, Qin Y, Cho MW, Chance MR. Structural characterization of HIV gp41 with the membrane-proximal external region. J Biol Chem. 2010;285:24290–24298. doi: 10.1074/jbc.M110.111351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buzon V, Natrajan G, Schibli D, Campelo F, Kozlov MM, Weissenhorn W. Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions. PLoS Pathog. 2010;6:e1000880. doi: 10.1371/journal.ppat.1000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alam SM, Morelli M, Dennison SM, Liao HX, Zhang R, Xia SM, Rits-Volloch S, Sun L, Harrison SC, Haynes BF, Chen B. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc Natl Acad Sci U S A. 2009;106:20234–20239. doi: 10.1073/pnas.0908713106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reardon PN, Sage H, Dennison SM, Martin JW, Donald BR, Alam SM, Haynes BF, Spicer LD. Structure of an HIV-1-neutralizing antibody target, the lipid-bound gp41 envelope membrane proximal region trimer. Proc Natl Acad Sci U S A. 2014;111:1391–1396. doi: 10.1073/pnas.1309842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kyrychenko A, Freites JA, He J, Tobias DJ, Wimley WC, Ladokhin AS. Structural plasticity in the topology of the membrane-interacting domain of HIV-1 gp41. Biophys J. 2014;106:610–620. doi: 10.1016/j.bpj.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobs A, Sen J, Rong L, Caffrey M. Alanine scanning mutants of the HIV gp41 loop. J Biol Chem. 2005;280:27284–27288. doi: 10.1074/jbc.M414411200. [DOI] [PubMed] [Google Scholar]
- 41.Diaz-Aguilar B, DeWispelaere K, Yi HA, Jacobs A. Significant differences in cell–cell fusion and viral entry between strains revealed by scanning mutagenesis of the C-heptad repeat of HIV gp41. Biochemistry. 2013;52:3552–3563. doi: 10.1021/bi400201h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sen J, Yan T, Wang J, Rong L, Tao L, Caffrey M. Alanine scanning mutagenesis of HIV-1 gp41 heptad repeat 1: insight into the gp120-gp41 interaction. Biochemistry. 2010;49:5057–5065. doi: 10.1021/bi1005267. [DOI] [PubMed] [Google Scholar]
- 43.Page KA, Landau NR, Littman DR. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J Virol. 1990;64:5270–5276. doi: 10.1128/jvi.64.11.5270-5276.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulke N, Vesanen MS, Sanders RW, Zhu P, Lu M, Anselma DJ, Villa AR, Parren PW, Binley JM, Roux KH, Maddon PJ, Moore JP, Olson WC. Oligomeric and conformational properties of a proteolytically mature, disulfide-stabilized human immunodeficiency virus type 1 gp140 envelope glycoprotein. J Virol. 2002;76:7760–7776. doi: 10.1128/JVI.76.15.7760-7776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis N, Williams J, Rekosh D, Hammarskjold ML. Identification of a cis-acting element in human immunodeficiency virus type 2 (HIV-2) that is responsive to the HIV-1 rev and human T-cell leukemia virus types I and II rex proteins. J Virol. 1990;64:1690–1697. doi: 10.1128/jvi.64.4.1690-1697.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang LJ, Urlacher V, Iwakuma T, Cui Y, Zucali J. Efficacy and safety analyses of a recombinant human immunodeficiency virus type 1 derived vector system. Gene therapy. 1999;6:715–728. doi: 10.1038/sj.gt.3300895. [DOI] [PubMed] [Google Scholar]
- 47.He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ehrhardt C, Schmolke M, Matzke A, Knoblauch A, Will C, Wixler V, Ludwig S. Polyethylenimine, a cost-effective transfection reagent. Signal Transduction. 2006;6:179–184. [Google Scholar]
- 50.Yi HA, Diaz-Aguilar B, Bridon D, Quraishi O, Jacobs A. Permanent inhibition of viral entry by covalent entrapment of HIV gp41 on the virus surface. Biochemistry. 2011;50:6966–6972. doi: 10.1021/bi201014b. [DOI] [PubMed] [Google Scholar]
- 51.O’Doherty U, Swiggard WJ, Malim MH. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol. 2000;74:10074–10080. doi: 10.1128/jvi.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abacioglu YH, Fouts TR, Laman JD, Claassen E, Pincus SH, Moore JP, Roby CA, Kamin-Lewis R, Lewis GK. Epitope mapping and topology of baculovirus-expressed HIV-1 gp160 determined with a panel of murine monoclonal antibodies. AIDS Res Hum Retroviruses. 1994;10:371–381. doi: 10.1089/aid.1994.10.371. [DOI] [PubMed] [Google Scholar]
- 53.Chesebro B, Wehrly K, Nishio J, Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992;66:6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yee M, Konopka K, Balzarini J, Duzgunes N. Inhibition of HIV-1 Env-mediated cell-cell fusion by lectins, peptide T-20, and neutralizing antibodies. The open virology journal. 2011;5:44–51. doi: 10.2174/1874357901105010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Durham ND, Yewdall AW, Chen P, Lee R, Zony C, Robinson JE, Chen BK. Neutralization resistance of virological synapse-mediated HIV-1 infection is regulated by the gp41 cytoplasmic tail. Journal of Virology. 2012;86:7484–7495. doi: 10.1128/JVI.00230-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCoy L, Groppelli E, Blanchetot C, de Haard H, Verrips T, Rutten L, Weiss R, Jolly C. Neutralisation of HIV-1 cell-cell spread by human and llama antibodies. Retrovirology. 2014;11:83. doi: 10.1186/s12977-014-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beck Z, Brown BK, Matyas GR, Polonis VR, Rao M, Alving CR. Infection of human peripheral blood mononuclear cells by erythrocyte-bound HIV-1: Effects of antibodies and complement. Virology. 2011;412:441–447. doi: 10.1016/j.virol.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 58.Abela I, Berlinger L, Schanz M, Reynell L, Gunthard H, Rusert P, Trkola A. Cell-cell transmission enables HIV-1 to evade inhibition by potent CD4bs directed antibodies. PLoS Pathog. 2012;8:e1002634. doi: 10.1371/journal.ppat.1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barry S, Stein SDG, Lifson Jeffrey D, Penhallow Robert C, Bensch Klaus G, Engleman Edgar G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987;49:659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- 60.McClure MO, Marsh M, Weiss RA. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 1988;7:513–518. doi: 10.1002/j.1460-2075.1988.tb02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell. 2009;137:433–444. doi: 10.1016/j.cell.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gentile M, Adrian T, Scheidler A, Ewald M, Dianzani F, Pauli G, Gelderblom HR. Determination of the size of HIV using adenovirus type 2 as an internal length marker. Journal of virological methods. 1994;48:43–52. doi: 10.1016/0166-0934(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 63.Zwick MB, Jensen R, Church S, Wang M, Stiegler G, Kunert R, Katinger H, Burton DR. Anti-human immunodeficiency virus type 1 (HIV-1) antibodies 2F5 and 4E10 require surprisingly few crucial residues in the membrane-proximal external region of glycoprotein gp41 to neutralize HIV-1. J Virol. 2005;79:1252–1261. doi: 10.1128/JVI.79.2.1252-1261.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao J, Bergeron L, Helseth E, Thali M, Repke H, Sodroski J. Effects of amino acid changes in the extracellular domain of the human immunodeficiency virus type 1 gp41 envelope glycoprotein. J Virol. 1993;67:2747–2755. doi: 10.1128/jvi.67.5.2747-2755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu M, Blacklow SC, Kim PS. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat Struct Biol. 1995;2:1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- 66.Caffrey M, Cai M, Kaufman J, Stahl SJ, Wingfield PT, Covell DG, Gronenborn AM, Clore GM. Three-dimensional solution structure of the 44 kDa ectodomain of SIV gp41. EMBO J. 1998;17:4572–4584. doi: 10.1093/emboj/17.16.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacobs A, Simon C, Caffrey M. Thermostability of the HIV gp41 wild-type and loop mutations. Protein Pept Lett. 2006;13:477–480. doi: 10.2174/092986606776819510. [DOI] [PubMed] [Google Scholar]
- 68.Krell T, Greco F, Engel O, Dubayle J, Dubayle J, Kennel A, Charloteaux B, Brasseur R, Chevalier M, Sodoyer R, El Habib R. HIV-1 gp41 and gp160 are hyperthermostable proteins in a mesophilic environment. Characterization of gp41 mutants. Eur J Biochem. 2004;271:1566–1579. doi: 10.1111/j.1432-1033.2004.04068.x. [DOI] [PubMed] [Google Scholar]
- 69.Caffrey M. Model for the structure of the HIV gp41 ectodomain: insight into the intermolecular interactions of the gp41 loop. Biochim Biophys Acta. 2001;1536:116–122. doi: 10.1016/s0925-4439(01)00042-4. [DOI] [PubMed] [Google Scholar]