Abstract
Objectives/Hypothesis
Our laboratory has developed an in vivo rabbit model to investigate the effects of phonation on expression and turnover of the vocal fold extracellular matrix. As a logical outgrowth of this research to include phonotrauma in the present study, we investigated the hypothesis that an increase in airflow rate delivered to the glottis produces a change in glottal configuration and an increase in mean phonation intensity.
Study Design
Prospective animal study.
Methods
Six New Zealand white breeder rabbits weighing 3 to 5 kg were used in this study. A rigid endoscope and camera were used to document glottal configuration. Acoustic signals of modal and raised phonation were recorded and digitized. Two separate one-way repeated measures analysis of variance (ANOVA) tests were used to investigate within subject differences in phonation intensity and fundamental frequency between modal and raised phonation.
Results
Phonation intensity was 54.19 dB SPL (6.21 standard deviations [SD]) during modal phonation, and 60.31 dB SPL (5.68 SD) during raised phonation. Endoscopic images revealed a convergent glottis, with greater separation of the vocal folds during raised phonation. Results of ANOVA revealed a significant within subjects effect for phonation intensity (P = .011). Pairwise comparisons revealed that phonation intensity increased significantly during raised phonation, compared to modal phonation (P = .008). No differences in mean fundamental frequency were observed between phonation conditions.
Conclusions
Improved understanding of factors that control phonation output in the in vivo rabbit model will result in improved capabilities to match phonation dose across animals and provide immediate direction to future biochemical studies.
Keywords: Fundamental frequency, intensity, phonation, rabbit model, phonotrauma
INTRODUCTION
Optimal function of the vocal fold lamina propria is essential to human voice production. Prolonged phonation results in flattening of the microvilli of the squamous epithelium of the vocal fold, with eventual obliteration and desquamation of the surface epithelial cell layer.1 These microscopic changes may expose neighboring cells in the epithelium, leaving them vulnerable to the shearing forces of phonation and weakening the protective cell layer. It is likely that prolonged phonation also results in changes to the underlying extracellular matrix of the vocal fold, which may lead to dysphonia. However, there is a paucity of available data on how the lamina propria responds to phonation at the level of the extracellular matrix.
Rousseau et al. described an in vivo rabbit model to study phonation-related extracellular matrix alterations.2 In this rabbit preparation, pulsed stimulation is delivered to the cricothyroid muscles and surrounding structures to elicit vocal fold elongation and adduction, while airflow is delivered to the glottis through a cuffed endotracheal tube to produce audible phonation.3 Using this animal model, the authors’ laboratory reported alterations in matrix metalloproteinase gene expression of the normal vocal fold following prolonged phonation.2 Because vocal fold closure and phonation intensity will be important variables in future biochemical studies, the purpose of the current study was to investigate the feasibility of eliciting raised phonation in the in vivo rabbit model by increasing the airflow rate delivered to the glottis. We hypothesized that an increase in airflow rate would change the configuration of the glottis, and contribute to an increase in phonation intensity. To investigate this hypothesis, we collected endoscopic glottal images to document glottal configuration, and acoustic data to analyze phonation intensity between modal and raised phonation in an in vivo rabbit model.
MATERIALS AND METHODS
Animals
Six New Zealand white breeder rabbits weighing 3 to 5 kg were used in this study. Induction of anesthesia was achieved with ketamine, 35 mg/kg; xylazine, 5mg/kg; and acepromazine, 0.75 mg/kg administered intramuscularly. Heart rate, temperature, and oxygen saturation levels were monitored throughout the experiment to monitor the animal’s state of anesthesia and general well-being. Subsequent intramuscular injections of ketamine (17.5 mg/kg) and acepromazine (0.375 mg/kg) were provided as needed to maintain a surgical plane of anesthesia.
This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Vanderbilt University Medical Center.
Surgical Procedure
The neck was shaved and prepped for surgery from the level of the submentum down to the chest. Animals were placed in the supine position on an operating platform. The larynx and trachea were exposed by a midline incision extending from the hyoid bone to the sternal notch. The trachea was then transected just proximal to the sternum. Sutures were placed to suspend the lower portion of the trachea to the sternal fascia, providing a stable airway via a tracheostomy. A 3.5 cuffed endotracheal tube (Willy Rusch GmbH, Kernen, Germany) was then inserted into the upper portion of the bisected trachea and positioned to rest approximately 2 cm below the glottal aperture. The cuff of this endotracheal tube was inflated to seal off the trachea and deliver airflow through the glottis. Custom, stainless steel, hooked electrodes were prepared prior to each experiment. One electrode was inserted mediolaterally into the belly of each cricothyroid muscle in a direction perpendicular to the muscle fibers (approximately at a 45° angle off midline) to serve as cathodes for electrical stimulation. Two additional electrodes were then inserted into the cricothyroid membrane to serve as anodes for electrical stimulation. One electrode was placed into the membrane on each side at the intersection of a longitudinal line 1 mm lateral to midline and a transverse line 1 mm inferior to the thyroid cartilage. A flowmeter (GF-8522-1700; Gilmont Instruments, Barrington, IL) and humidifier (Conch Therm III; Hudson RCI, Temecula, CA) were used to deliver compressed humidified air heated to 37°C to the glottis.
A Grass S-88 stimulator (SA Instrumentation, Encinitas, CA) and constant current isolation unit (Grass Telefactor, Model PSIU6; Grass Technologies, West Warwick, RI) were used to provide electrical stimulation to the laryngeal apparatus. The total train duration was 10 seconds (3 seconds on; 7 seconds off).
Data Collection
A 5° 2.7-mm rigid endoscope (KARL STORZ Endoscopy-America, Inc., El Segundo, CA) and Telecam-C camera (KARL STORZ Endoscopy-America, Inc.) were used to obtain video documentation of vocal fold positioning and glottal closure during phonation. Acoustic signals were recorded using a Shure SM48 unidirectional dynamic microphone (Shure Inc., Niles, IL), placed 10 cm from the opening of the laryngoscope and digitized using the Computerized Speech Lab (CSL Model 4500, Kay-PENTAX, Lincoln Park, NJ). Three to five, 0.5 to 1.0 second samples were selected from the most stable portion of the acoustic waveform, and the CSL main program was used to extract mean phonation intensity and mean fundamental frequency.
Data Analysis
Two, one-way repeated measures analysis of variance (ANOVA) tests were used to investigate within subject differences in mean phonation intensity and mean fundamental frequency between modal and raised phonation. If the F test revealed a significant main effect, pairwise comparisons between modal and raised phonation were examined using Fisher’s least significant difference (LSD) test. For post hoc testing, a significance level of P < .025 was used to control for type I error. Analyses were performed with the use of two-tailed P values. Data were analyzed using SPSS 17.0 for Windows (SPSS, Inc., Chicago, IL).
RESULTS
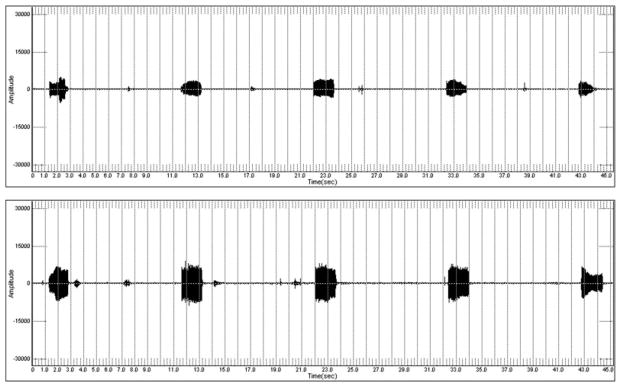
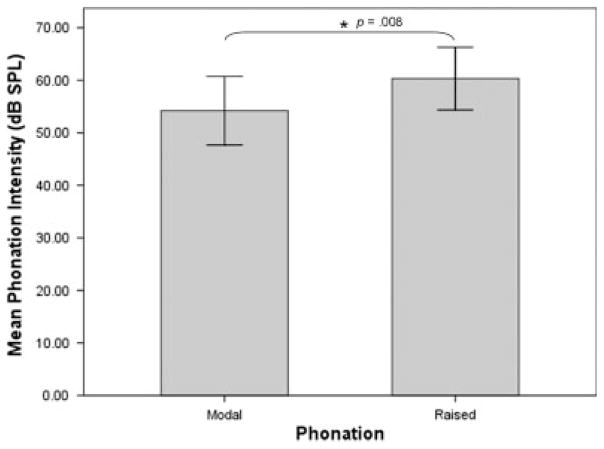
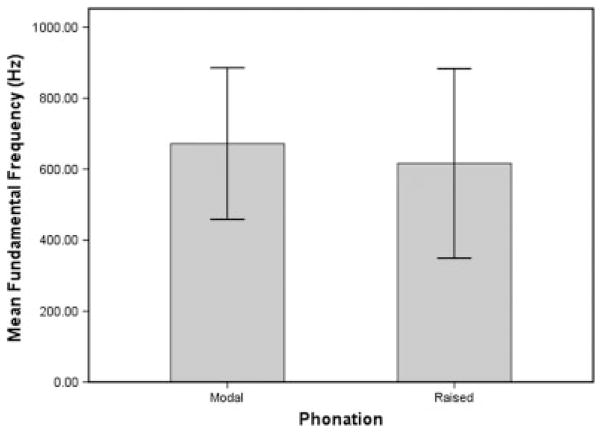
Mean phonation intensity was 54.19 dB SPL (6.21 standard deviation [SD]) during modal phonation and 60.31 dB SPL (5.68 SD) during raised phonation (Table I). Table I displays mean fundamental frequency and mean phonation intensity for modal and raised phonation. Figure 1 shows a sample CSL waveform collected during modal and raised phonation, revealing increased amplitude of the sound spectrum during raised phonation. Results of ANOVA revealed a significant within subjects effect for phonation intensity (P = .011). Pairwise comparisons using Fisher’s LSD revealed that phonation intensity increased significantly during raised phonation, compared to modal phonation (P = .008) (Fig. 2). Mean fundamental frequency was 672.02 Hz (203.00 SD) during modal phonation and 616.27 Hz (254.93 SD) during raised phonation (Fig. 3). Results of ANOVA revealed no significant within subjects differences for fundamental frequency (P > .025). Table II displays mean fundamental frequency and mean phonation intensity for all animals during modal and raised phonation.
TABLE I.
Mean Fundamental Frequency and Mean Phonation Intensity for Modal and Raised Phonation.
| Rabbit | Phonation Type | Mean F0 | Mean dB |
|---|---|---|---|
| All | Modal | 672.02 | 54.19 |
| All | Raised | 616.27 | 60.31 |
Fig. 1.
Sample waveform of modal (top) and raised (bottom) phonation, showing increased amplitude of the sound spectrum during raised phonation.
Fig. 2.
Mean phonation intensity (dB SPL) for modal and raised phonation, revealing significantly increased intensity during raised phonation (P =.008). Error bars represent 95% confidence interval of the mean.
Fig. 3.
Mean fundamental frequency (Hz) for modal and raised phonation, revealing no significant differences in fundamental frequency between phonation conditions. Error bars represent 95% confidence interval of the mean.
TABLE II.
Mean Fundamental Frequency and Mean Phonation Intensity for All Animals.
| Rabbit | Phonation Type | Mean F0 | Mean dB |
|---|---|---|---|
| 8135 | Modal | 462.76 | 50.55 |
| 8135 | Raised | 454.03 | 57.05 |
| 8171 | Modal | 519.79 | 52.52 |
| 8171 | Raised | 581.51 | 61.15 |
| 8172 | Modal | 681.79 | 64.81 |
| 8172 | Raised | 724.41 | 68.23 |
| 8173 | Modal | 891.67 | 57.09 |
| 8173 | Raised | 1060.13 | 59.15 |
| 8174 | Modal | 940.87 | 54.28 |
| 8174 | Raised | 325.37 | 62.89 |
| 8136 | Modal | 535.26 | 47.09 |
| 8136 | Raised | 552.19 | 51.73 |
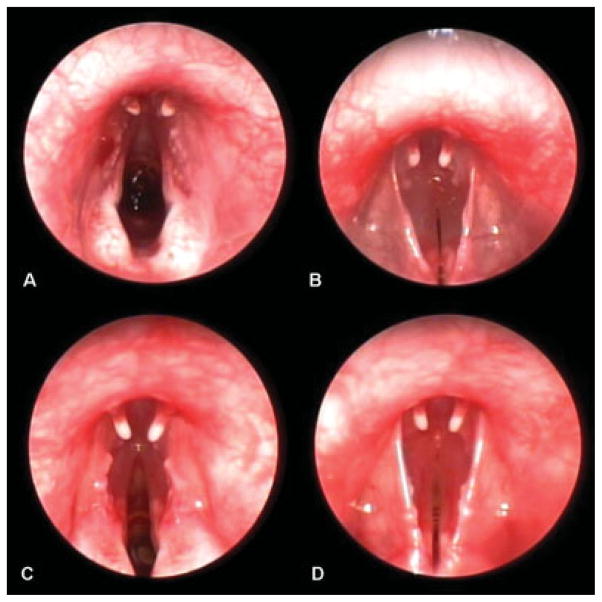
Figure 4 shows a representative endoscopic image of the vocal folds at rest and during phonation. Endoscopic images acquired during modal phonation revealed a convergent glottis, with slightly separated vocal folds (Fig. 4A, B). In contrast, endoscopic images acquired during raised phonation revealed a convergent glottis with greater separation of the vocal folds during vocalization (Fig. 4C, D).
Fig. 4.
Representative endoscopic images of vocal fold abduction and adduction, revealing a convergent glottis with slightly separated vocal folds during modal phonation (A, B) and a convergent glottis with greater separation of the vocal folds during raised phonation (C, D).
DISCUSSION
The vocal fold lamina propria is an area of connective tissue that is uniquely different from tissues found elsewhere in the body. Some histological and physiological correlations can be made to other tissues, such as skin and joints, that also undergo frequent trauma, repeated cycles of inflammation, and decrease in function secondary to poor healing and scarring. However, no other tissue in the body undergoes mechanical forces similar to the vibration of the vocal folds. Vibratory frequencies in the vocal folds during phonation range of the lamina propria has likely developed specialized properties to function under such mechanical stress.4 Because of the friction and shearing forces associated with phonation, clinicians have long suspected that the duration and intensity of voice use may be a factor in the development of vocal pathology. These suspicions have led to the widespread use of descriptors such as too long and too loud by clinicians to explain the pathophysiology of dysphonia to patients.5–8 However, data on the effects of phonation-induced trauma on alterations to the extracellular matrix and the development of vocal fold pathology are lacking.
Gray et al. investigated the effects of prolonged phonation on microscopic changes to the epithelial cell layer of the vocal fold in a canine model.1 These authors found blunting or flattening of the microvilli of the squamous epithelium, complete obliteration of the microvilli, and desquamation of the surface layers in hyperphonated canine vocal folds.1 However, data on the effects of prolonged phonation and phonation of increased intensity on changes to the underlying extracellular matrix are unavailable. Our laboratory has developed an in vivo rabbit phonation model to pursue these questions systematically.2,3 Our goal is to investigate the role of the potentially injurious mechanical forces exhibited during phonation on expression and turnover of the vocal fold lamina propria. In an earlier proof of concept study, we reported increased matrix metalloproteinase-1 messenger RNA (mRNA) levels following prolonged phonation in the normal rabbit vocal fold.2 Matrix metalloproteinase-1 is an interstitial collagenase important in the breakdown of collagen types I and III. The finding that matrix degrading enzymes are upregulated following prolonged phonation supports the idea that the vocal folds undergo dynamic changes as mechanical forces act on the tissue during vibration.4 We anticipate that further refinements in the rabbit in vivo phonation model will allow for systematic investigations into inflammation and the process of wound healing specific to the vocal folds. Ultimately, these advances may allow for the testing of clinical observations, such as too long and too loud as predictors of pathology in the model.
As an extension of the in vivo rabbit phonation model to phonotrauma, in the present study, we investigated the feasibility of eliciting raised phonation in the model for future studies aimed at characterizing responses of the vocal fold lamina propria to phonation of increased intensity. We hypothesized that an increase in airflow rate delivered to the glottis would produce a change in glottal configuration and a significant increase in phonation intensity. To investigate this hypothesis, endoscopic glottal images were acquired to document glottal configuration, and acoustic data was used to measure phonation intensity between modal and raised phonation.
Results revealed a convergent glottis, with slightly separated vocal folds during modal phonation. Phonation intensity was 54.19 dB SPL (6.21 SD). Fundamental frequency was 672.02 Hz (203.12 SD). Endoscopic results for raised phonation revealed a convergent glottis with greater separation of the vocal folds during vocalization. The change in glottal configuration was consistent with an increase in phonation intensity (60.31 dB SPL [5.68 SD]) and in the amplitude of the sound spectrum. Fundamental frequency during raised phonation was similar to that measured during modal phonation. These data provided support for the predicted hypothesis that an increase in the airflow rate delivered to the glottis would contribute to increased phonation intensity in the in vivo rabbit model. We attributed the increase in intensity to an increase in the amplitude of vibration, resulting in greater separation of the vocal folds during raised phonation. Accordingly, glottal configuration will be an important variable in future biochemical studies to determine the contributions of closure and impact during elicited phonation on changes in vocal fold mRNA expression. In this regard, phonation intensity may also provide a useful approach to monitor phonation output during evoked phonation experiments and as a predictor of changes at the level of the glottis. Another important finding was that fundamental frequency did not differ between modal and raised phonation. This was consistent with the observation that the anteroposterior length of the vocal folds did not differ between phonation types. This finding may be useful in future experiments, as it may allow laryngeal investigators to increase phonation intensity by modifying the input parameter of airflow rate without influencing vocal fold tension and the frequency of vibration. Likewise, it would be interesting in future studies to determine whether an increase in the current of stimulation delivered to the larynx results in a change in vocal fold tension and fundamental frequency. This would provide a means of modifying fundamental frequency in future experiments by changing the stimulation current input parameter. Ultimately, these refinements in the phonation model will result in improved capabilities to match phonation dose across animals in future biochemical studies.
The in vivo rabbit phonation model used in the present study provided several advantages over the rabbit phonation model used in our previous studies.2,3 First, placement of the anodes through the cricothyroid membranes rather than the superior border of the cricothyroid muscles allowed for the use of much lower currents to achieve glottal closure in the present study (0.7–2 mA), compared to our previous studies (3–4 mA).2,3 Based on the clinical use of transcutaneous injection laryngoplasty through the cricothyroid space for access to the vocal fold, we hypothesized that placement of the anodes through the cricothyroid membranes would provide for closer proximity of the anodes to the underlying adductor muscles. In doing so, adductor involvement was likely greater in the present study than in previous studies. This was confirmed from the videoendoscopic images showing graded adduction of the vocal folds towards midline with much lower stimulation currents, and phonation being elicited at stimulation levels that were two to four times lower than those used in our previous studies.
Another important aim achieved in the present study was the ability to increase phonation intensity by manipulating the airflow rate parameter. In our previous studies, no attempts to control phonation intensity were made. Instead, fixed stimulation and airflow parameters were used, which likely resulted in variable glottal configurations and phonation intensity across animals.2,3 Because the quality of elicited phonation will be an important variable in future biochemical studies, in the present study, the airflow rate parameter was adjusted to achieve increased phonation intensity between modal and raised phonation conditions. Thus, the in vivo rabbit phonation model used in the present study provided the advantage of allowing for the elicitation of characteristically modal and raised intensity phonation between phonation conditions. Interestingly, these two types of phonation will be used in future studies to determine the impact of each type of phonation on vocal fold mRNA expression, providing another useful advantage, which will allow us to better understand the cellular responses of the vocal folds to different types of phonation.
Future studies will also attempt to objectify the glottal configuration differences between modal and raised phonation. It should be possible to measure distances between the vocal folds and glottal angles more objectively. We attempted to do this in the current study. However, limitations in the video capture system we used made it difficult to precisely identify glottal boundaries, which is necessary for these more objective analyses to be meaningful.
CONCLUSION
Data on the effects of prolonged phonation and phonation of increased intensity on changes to the underlying extracellular matrix are limited. Our laboratory has developed an in vivo rabbit phonation model to investigate the role of these potentially injurious mechanical forces on expression and turnover of the vocal fold lamina propria. In the present study, we investigated the hypothesis that an increase in the airflow rate delivered to the glottis produces a change in glottal configuration and an increase in mean phonation intensity in the in vivo rabbit phonation model. Results from the present study revealed a convergent glottis, with slightly separated vocal folds during modal phonation, and a convergent glottis with greater separation of the vocal folds during raised phonation. The change in glottal configuration observed during raised phonation was consistent with an increase in phonation intensity and in the amplitude of the sound spectrum. These data will provide immediate direction to future studies aimed at investigating responses of the vocal fold lamina propria to raised phonation.
Acknowledgments
This work was supported by NIH grant R03 DC 008400 from the National Institute of Deafness and Other Communication Disorders (NIDCD).
The authors would like to thank Shan Huang, MD for technical assistance during experimental procedures, and Andrew Tritter for assistance during manuscript preparation.
Footnotes
Presented at the 130th Annual Meeting of the American Laryngological Association, Phoenix, Arizona, U.S.A., May 28–29, 2009.
BIBLIOGRAPHY
- 1.Gray SD, Titze I, Lusk RP. Electron microscopy of hyperphonated canine vocal cords. J Voice. 1987;1:109–115. [Google Scholar]
- 2.Rousseau B, Ge PJ, French LC, Zealear DL, Thibeault SL, Ossoff RH. Experimentally induced phonation increases matrix metalloproteinase-1 gene expression in normal rabbit vocal fold. Otolaryngol Head Neck Surg. 2008;138:62–68. doi: 10.1016/j.otohns.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ge PJ, French LC, Ohno T, Zealear DL, Rousseau B. Model of evoked rabbit phonation. Ann Otol Rhinol Laryngol. 2009;118:51–55. doi: 10.1177/000348940911800109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Titze IR, Hitchcock RW, Broadhead K, et al. Design and validation of a bioreactor for engineering vocal fold tissue under combined tensile and vibrational stresses. J Biomech. 2004;37:1521–1529. doi: 10.1016/j.jbiomech.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Froeschels E. Hygiene of the voice. Arch Otolaryngol. 1943;38:122–130. [Google Scholar]
- 6.Roy N, Weinrich B, Gray SD, et al. Voice amplification versus vocal hygiene instruction for teachers with voice disorders: a treatment outcomes study. J Speech Lang Hear Res. 2002;45:625–638. doi: 10.1044/1092-4388(2002/050). [DOI] [PubMed] [Google Scholar]
- 7.Roy N, Weinrich B, Gray SD, et al. An evaluation of the effects of two treatment approaches for teachers with voice disorders: a prospective randomized clinical trial. J Speech Lang Hear Res. 2001;44:286–296. doi: 10.1044/1092-4388(2001/023). [DOI] [PubMed] [Google Scholar]
- 8.Postma GN, Courey MS, Ossoff RH. The professional voice. In: Cummings CW, Flint PW, Harker LA, et al., editors. Otolaryngology—Head and Neck Surgery. 4. Philadelphia, PA: Mosby; 2005. pp. 2138–2139. [Google Scholar]