Abstract
The periodontium, consisting of gingiva, periodontal ligament (PDL), cementum, and alveolar bone, is necessary for the maintenance of tooth function. Specifically, the regenerative abilities of cementum with inserted PDL are important for the prevention of tooth loss. Periodontal ligament stem cells (PDLSCs), which are located in the connective tissue PDL between the cementum and alveolar bone, are an attractive candidate for hard tissue formation. We investigated the effects of recombinant human plasminogen activator inhibitor-1 (rhPAI-1) on cementogenic differentiation of human PDLSCs (hPDLSCs) in vitro and in vivo. Untreated and rhPAI-1-treated hPDLSCs mixed with hydroxyapatite/tricalcium phosphate (HA/TCP) and dentin matrix were transplanted subcutaneously into the dorsal surface of immunocompromised mice to assess their capacity for hard tissue formation at 8 and 10 weeks posttransplantation. rhPAI-1 accelerated mineral nodule formation and increased the mRNA expression of cementoblast-associated markers in hPDLSCs. We also observed that rhPAI-1 upregulated the levels of osterix (OSX) and cementum protein 1 (CEMP1) through Smad2/3 and p38 pathways, whereas specific inhibitors of Smad3 and p38 inhibited the enhancement of mineralization of hPDLSCs by rhPAI-1. Furthermore, transplantation of hPDLSCs with rhPAI-1 showed a great ability to promote cementogenic differentiation. Notably, rhPAI-1 induced hPDLSCs to regenerate cementum-like tissue with PDL fibers inserted into newly formed cementum-like tissue. These results suggest that rhPAI-1 may play a key role in cementogenic differentiation of hPDLSCs. rhPAI-1 with hPDLSCs may be a good candidate for future clinical applications in periodontal tissue regeneration and possibly in tooth root bioengineering.
Introduction
Periodontitis, the most widespread oral inflammatory disease, gives rise to loss of attachment, periodontal pocketing, cementum resorption, and alveolar bone resorption.1 Cementum is the outermost layer of the hard avascular connective tissue that covers and protects tooth root, and provides attachment sites for periodontal ligament (PDL) fibers to support the tooth, thus reversing tooth resorption. Therefore, regeneration of the periodontium, and especially the cementum, would be highly beneficial to periodontal tissue repair. Many researchers have previously tried to use dental mesenchymal stem cells (DMSCs) for cementum formation, but very little is known about cementoblasts responsible for cementum formation.
Human DMSCs originate from various types of dental tissues, including PDL, pulp, periapical follicle, and alveolar bone, all of which have multilineage differentiation abilities.2–4 PDL, located between the cementum and alveolar bone, is specialized connective tissue containing fibroblasts, epithelial cells, undifferentiated mesenchymal cells, bone cells, and cementum cells. It has been reported that some of these cells have the potential to generate bone and cementum. The functions of PDL are supportive, sensory, nutritive, and remodeling of the periodontium, thus PDL generation is as important as cementum formation. Our previous studies showed successful isolation and characterization of human periodontal ligament stem cells (hPDLSCs) from PDL, thus increasing their potential as candidates for therapeutic application.2 PDLSCs can differentiate into cementoblasts, osteoblasts, adipocytes, and chondrocytes.5–8 Recent studies indicate that PDLSCs have many osteoblast- and cementoblast-like properties and can differentiate into mineral-forming cells that express bone- and cementum-associated markers, such as alkaline phosphatase (ALP), bone sialoprotein (BSP), type 1 collagen (Col1), osteopontin (OPN), and osteocalcin (OCN).7,9–13 Cementum protein 1 (CEMP1) was first isolated and characterized as a human cementoblastoma-derived protein that is expressed in cementoblasts, and studies suggested that CEMP1 might play a critical role as a local regulator of cementoblast differentiation.14,15 F-spondin was recently identified as a highly expressed cementoblast-specific gene and a promoting factor for cementoblast differentiation.16 Moreover, PDLSCs showed the capacity to generate cementum with PDL in vivo.3,17 Therefore, hPDLSCs would be the most appropriate candidates in clinical trials and tissue engineering for cementum regeneration. A typical tissue engineering strategy involves cells, biomaterial scaffolds, and bioactive molecules. To enhance the therapeutic effects, growth factors and cytokines are usually introduced into the systems by direct protein delivery or viral gene delivery.
Plasminogen activator inhibitor type 1 (PAI-1) plays important roles in many physiological processes, such as fibrotic disorders, metabolic disorders, and cancer.18–21 The plasminogen/plasmin proteolytic cascade, which includes two plasminogen activators, tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA), and can be inhibited by PAI-1, also plays an important role in extracellular matrix remodeling.22 PAI-1 is expressed by bone cells and their substrates and present in bone matrix, known to be a regulator of bone remodeling.23 We have previously demonstrated that platelet-rich fibrin (PRF) promoted the differentiation of the human alveolar bone marrow stem cells and high-level expression of PAI-1 in PRF.24 Moreover, the combination of PRF and autogenous bone increased the rate of osteogenesis and the quality of the new bone.25 Taken together, these findings indicate that PAI-1 contributes to hard tissue formation, although previous studies have not clarified the role of PAI-1 in cementogenic differentiation.
In this study, we aimed to investigate the effects of recombinant human plasminogen activator inhibitor-1 (rhPAI-1) on cementogenic differentiation of hPDLSCs in vitro and in vivo and to identify the molecular mechanisms by which cementogenic differentiation occurs. We found that rhPAI-1 significantly stimulated hPDLSCs to promote cementum formation with inserted PDL fibers in a transplantation model in vivo. These results suggest that rhPAI-1 is an important factor for regulating periodontal tissue regeneration in hPDLSCs.
Materials and Methods
Primary cell culture
Human third molars were collected from three healthy young males (18–25 years old) under the protocol approved by the Institutional Review Board of the Seoul National University Dental Hospital, Seoul, South Korea (IRB No. 05004). PDL was gently separated from extracted human third molars and the separated tissues were digested in a solution of 3 mg/mL collagenase type I (Worthington Biochem, Freehold, NJ) and 4 mg/mL dispase (Boehringer, Mannheim, Germany) for 1 h at 37°C. Single-cell suspensions were obtained by passing the cells through a 40-μm strainer (Falcon BD Labware, Franklin Lakes, NJ) and were cultured in the alpha-modification of Eagle's medium (α-MEM; Gibco BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (Gibco BRL), 100 μM ascorbic acid 2-phosphate (Sigma-Aldrich, St. Louis, MO), 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Biofluids, Rockville, MD) and incubated at 37°C in 5% CO2. The medium was changed after the first 24 h and then every 3 days. The colonies were picked and cultured separately. Three colonies of hPDLSCs were randomly selected and the cellular pool of those colonies was used for in vitro proliferation and differentiation studies. All primary cells used in this study were in passage 2 or 3.
Flow cytometric analysis
To characterize the immunophenotype of hPDLSCs, the expression of mesenchymal stem cell (MSC)-associated surface markers at passage 3 was analyzed by flow cytometry as described previously.26 Briefly, cells in their third passage (1.0×106 cells) were fixed with 3.7% paraformaldehyde for 10 min and resuspended in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) (ICN Biomedicals, Aurora, OH) for 30 min for blocking. Cells were then incubated with specific antibodies for CD34, CD13, CD90, or CD146 at 4°C for 1 h, followed by incubation with fluorescence secondary antibodies at room temperature for 1 h. All antibodies were purchased from BD Biosciences (San Jose, CA). The percentage of CD13-, CD90-, and CD146-positive and CD34-negative cells was measured with a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) and the results were analyzed using CellQuest Pro software (Becton Dickinson Immunocytometry Systems).
Chondrogenic and adipogenic differentiation
For induction of chondrogenic and adipogenic differentiation, the cells were cultured in StemPro Chondrogenic and StemPro Adipogenic differentiation media (Gibco BRL), respectively, with the appropriate supplements. At week 3 postchondrogenic and postadipogenic induction, the cells were washed with PBS and fixed in 3.7% paraformaldehyde for 10 min. The cells were stained with 1% Alcian Blue (Sigma-Aldrich) and 0.3% Oil Red O dye (Sigma-Aldrich) for detection of proteoglycans and fat vacuoles as indicators of chondrogenic and adipogenic differentiation, respectively. Cells were visualized under an inverted light microscope (Olympus U-SPT; Olympus, Tokyo, Japan).
Cell proliferation/cytotoxicity assay
Cell proliferation was measured using the colorimetric 3-(4,5-dimethylthazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit (Promega, Madison, WI). Briefly, hPDLSCs (3.0×103 cells/well) were seeded in 96-well plates and cultured for 48 h. Various amounts of rhPAI-1 (Prospec-Tany TechnoGene, Rehovot, Israel) were added in 100 μL of culture media per well for final concentrations of 0, 5, 10, 20, 50, and 100 ng/mL rhPAI-1. A premixed optimized dye solution (15 μL) was added at the end of the treatment. Cells were incubated in 5% CO2 at 37°C for 4 h and then 100 μL of a solubilization/stop solution was added to the cultures to solubilize the formazan product. Each condition was prepared in triplicate, and reactions were assessed using an ELISA reader at OD 595nm (reference, 655 nm).
Alkaline phosphatase activity assay and staining
Quantitative analysis of ALP activity was performed using the p-Nitrophenyl Phosphate (pNPP) Liquid Substrate System according to the manufacturer's instructions (Sigma-Aldrich). In brief, the ALP activity in cell lysates was determined with pNPP as the substrate in an assay buffer containing 5 mM MgCl2 and 50 mM Na2CO3. Absorbance at 405 nm was measured using an ELISA reader, and the ALP activity was calculated from a standard curve. For ALP staining, cells were fixed with 10% formalin, incubated with 0.1% Triton X-100 for 5 min, and stained using the Leukocyte Alkaline Phosphatase Kit (Sigma-Aldrich) according to the manufacturer's protocol.
Induction of mineralization and Alizarin red S staining
For mineralization, the hPDLSCs were cultured in the osteogenic differentiation medium with 50 μg/mL ascorbic acid, 10 mM β-glycerophosphate, and 100 nM dexamethasone (Sigma-Aldrich) for 3 weeks. On day 21, accumulation of mineral nodules was detected by staining with 2% Alizarin red S staining at pH 4.2 (Sigma-Aldrich). For the destaining procedure to measure the calcium content, 3 mL of 10 mM sodium phosphate–10% acetylpyrimidium (pH 7.0) solution was added to each stained well and incubated at room temperature for 15 min. The destained sample was transferred to a 96-well plate and the absorbance was measured at 562 nm.
Western blot analysis
The hPDLSCs (1.0×106 cells/dish) were seeded in a 60-mm culture dish and cultured for the indicated duration. Protein concentrations of cell lysates were determined using the DC Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA). Equal amounts of protein (30 μg/lane) were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane (GE Healthcare, Buckinghamshire, United Kingdom). Primary antibodies against phospho-ERK, ERK, phospho-p38, p38, phospho-JNK, JNK, Runx2, α-tubulin (Cell Signaling Technology, Danvers, MA), osterix (OSX; Abcam, Cambridge, United Kingdom), CEMP1, F-spondin, p-Smad2/3, and Smad4 (Santa Cruz Biotechnology, Santa Cruz, CA) were used for the mechanism study. Blots were developed using horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology) and visualized using an enhanced chemiluminescence kit (GE Healthcare).
Reverse transcription–polymerase chain reaction
To evaluate gene expression levels in rhPAI-1-induced differentiated hPDLSCs, 1.0×106 cells were seeded in a 60-mm culture dish and cultured under osteogenic differentiation induction conditions. Total RNA was prepared using an RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions, and cDNA was synthesized from 2 μg of total RNA using reverse transcriptase (Superscript II Preamplification System; Invitrogen, Gaithersburg, MD). Reverse transcription–polymerase chain reaction (RT-PCR) was conducted as described previously.17 The relative expression of mRNA was compared in a histogram. The specific primer sets used for RT-PCR are listed in Table 1.
Table 1.
Primer Sequences for Reverse Transcription–Polymerase Chain Reaction
| Gene | GenBank No. | Sequences |
|---|---|---|
| OPN | J04765 | 5′-CCCACAGACCCTTCCAAGTA-3′ |
| 5′-ACACTATCACCTCGGCCATC-3′ | ||
| OCN | X53698 | 5′-GTGCAGAGTCCAGCAAAGGT-3′ |
| 5′-TCAGCCAACTCGTCACAGTC-3′ | ||
| Col1 | XM_012651 | 5′-CTGACCTTCCTGCGCCTGATGTCC-3′ |
| 5′-GTCTGGGGCACCAACGTCCAAGGG-3′ | ||
| ALP | BC090861 | 5′-GGACATGCAGTACGAGCTGA-3′ |
| 5′-GCAGTGAAGGGCTTCTTGTC-3′ | ||
| BSP | NM_004967 | 5′-CAACAGCACAGAGGVAGAAA-3′ |
| 5′-CGTACTCCCCCTCGTATTCA-3′ | ||
| Runx2 | NM_001015051 | 5′-CGCATTCCTCATCCCAGTAT-3′ |
| 5′-GACTGGCGGGGTGTAAGTAA-3′ | ||
| F-spondin | NM_006108 | 5′-TCTGGAGCAGGTGGAGAAGT-3′ |
| 5′-TGGATTTCGAAGGCATTTTC-3′ | ||
| CEMP1 | NM_001048212 | 5′-TCAAGGCAGAGGTGGGTATC-3′ |
| 5′-GGAAATGTCTCCAGGTCCAA-3′ | ||
| GAPDH | NM_002046 | 5′-ACCACAGTCCATGCCATCA-3′ |
| 5′-TCCACCACCCTGTTGCTGT-3′ |
ALP, alkaline phosphatase; BSP, bone sialoprotein; CEMP1, cementum protein 1; Col1, type 1 collagen; OPN, osteopontin; OCN, osteocalcin.
Luciferase assay
The hPDLSCs were seeded on a 24-well plate at a density of 5.0×104 cells per well. After 24 h, the cells were transfected with Lipofectamine LTX and PLUS reagent (Invitrogen) according to the manufacturer's instructions. Luciferase reporter plasmid of pGL-basic 6× OSE was kindly provided by Dr. Z.H. Lee (Seoul National University). For each transfection, 0.4 μg of luciferase reporter plasmid pGL-basic vector (control) or expression vector containing six repeats of the consensus Runx2 binding site (6× OSE) promoter was used. Transfected cells were treated with or without rhPAI-1 for 48 h and then lysed for assessment of luciferase activity using the luciferase reporter gene assay system (Promega) according to the manufacturer's instructions. The measurements were performed with a luminometer (FLUOstar OPTIMA; BMC Laboratory, Offenburg, Germany), and the experiments were conducted in triplicate.
Preparation of human-treated dentin matrix and beagle dog periodontium
Periodontal tissue, including PDL and cementum, was completely removed using a surgical bur by grinding along the root surface. The roots were cut into two pieces following the midsagittal plane and the dental pulp, odontoblast, and predentin were completely removed. Finally, the dentin was trimmed to a cylinder 5.0 mm in height and 2.0 mm in diameter. Fabrication of a human-treated dentin matrix was performed as reported previously.27
A beagle dog aged 10 months with 10 kg body weight was obtained and examined for good systemic and oral health. This study was reviewed and approved by the Institutional Animal Care and Use Committee (No. 070504-5, ilar.snu.ac.kr, IACUC) at Seoul National University (Seoul, Korea). The harvest procedure of periodontium was performed as reported previously.17
Transplantation and histological analysis
To study the effects of rhPAI-1 on cementum formation in vivo, hPDLSCs (1.0×107 cells) were mixed with 100 mg hydroxyapatite/tricalcium phosphate (HA/TCP) ceramic powder (Zimmer, Inc., Warsaw, IN) and human tooth root dentin matrix, with or without rhPAI-1 (100 μg), on a 0.5% fibrin gel. Cells were then transplanted subcutaneously into immunocompromised mice (n=6) (NIH-bg-nu/nu-xid; Harlan Sprague Dawley, Indianapolis, IN). In this transplantation model, rhPAI-1 was used to induce cementogenic differentiation in hPDLSCs with HA/TCP and human tooth root dentin matrix. For histological analysis, samples were obtained 8 and 10 weeks after transplantation and fixed in 3.7% paraformaldehyde solution for 48 h at 4°C before decalcification with 12% EDTA (pH 7.4) at 4°C. The samples were embedded in paraffin and cut serially, starting from the outermost region, in the dentin midsagittal plane. Semiserial 5-μm sections were prepared for hematoxylin and eosin (H&E) and immunohistochemical staining and examined under a light microscope (Olympus U-SPT; Olympus).
For immunohistochemistry, the deparaffinized sections were immersed in 0.6% H2O2/methanol for 20 min to quench the endogenous peroxidase activity. Sections were then preincubated with 1% BSA in PBS for 30 min and incubated overnight at 4°C with the rabbit polyclonal antibody against CEMP1 (1:200), OSX (1:100; Abcam), Col1, and OPN (1:100; Santa Cruz Biotechnology). Sections were incubated for 1 h at room temperature with the secondary antibody and reacted with the avidin–biotin–peroxidase complex (Vectastain ABC Systems; Vector Laboratories, Burlingame, CA) in PBS for 30 min. After color development with 0.05% 3,3′-diaminobenzidine tetrahydrochloride (DAB peroxidase substrate; Vector Laboratories), the sections were counterstained with hematoxylin.
PDL was identified by staining with Picrosirius red staining to reveal collagenous fibers. The Picrosirius red solution (ScyTek Laboratories, Logan, UT) was added to the deparaffinized sections to completely cover the tissue and incubated for 1 h. Sections were rinsed quickly in two changes of acetic acid solution and absolute alcohol and then counterstained with hematoxylin.
Scanning electron microscopy
Samples were fixed in 0.1 M cacodylate buffer (pH 7.3) containing 2.5% glutaraldehyde for 30 min and in 0.1 M cacodylate buffer (pH 7.4) containing 1% osmium tetroxide for 1 h. After rapid dehydration through an ethanol gradient, critical point drying, and sputter coating with gold, the PDL fibers were observed under scanning electron microscopy (SEM, S-4700; Hitachi, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using Statistical Package for Social Science 13.0 software (SPSS, Inc., Chicago, IL). Normal data with equal variance were analyzed using one-way analysis of variance (ANOVA) with a Tukey's procedure. Significance was defined as p≤0.05. Values in each graph represent mean±standard deviation. All assays were performed at least thrice and representative data are presented.
Results
Characterization of hPDLSCs
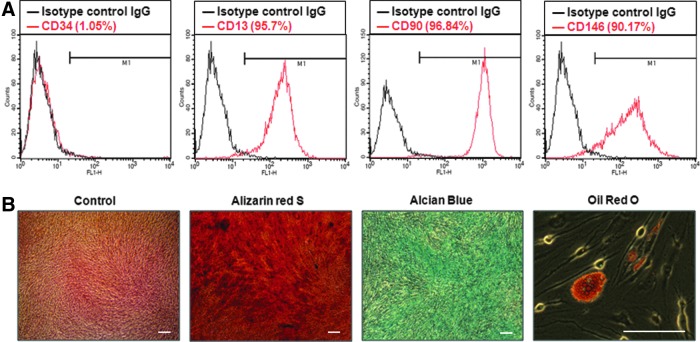
The isolated cells formed single-cell-derived colonies, and three different colonies from each of the three donors were randomly selected as hPDLSCs for studies. Flow cytometric analysis showed that ∼95.7% of hPDLSCs expressed CD13, 96.84% expressed CD90, 90.17% expressed CD146, and 1.05% expressed CD34 (Fig. 1A). The percentage of positive cells was determined by the relative intensity of antibody-binding cells. CD13, CD90, and CD146 are putative positive markers of MSCs,8,28 whereas CD34 is an MSC-negative marker that marks primitive hematopoietic progenitors and endothelial cells.29 To investigate the multilineage differentiation potential of hPDLSCs, the cells were incubated without or with osteogenic medium supplementation to induce mineralization in vitro. After 3 weeks of osteogenic induction, hPDLSCs formed extensive amounts of Alizarin red S-positive mineral deposits throughout the adherent layers. The cells were also capable of undergoing adipogenic and chondrogenic differentiation after incubation with adipogenic-inductive and chondrogenic-inductive supplements for 3 weeks. Oil Red O and Alcian Blue staining showed lipid droplet formation and Alcian Blue-positive nodules, respectively (Fig. 1B).
FIG. 1.
Characterization and multilineage differentiation of hPDLSCs. (A) MSCs markers, including CD13, CD34, CD90, and CD146, were used for fluorescence-activated cell sorting analysis of hPDLSCs. For whole-cell analysis of CD13-, CD34-, CD90-, and CD146-positive cells, the percentage of the cells positioned in the right side of the M1 gate was measured (n=3). (B) To investigate the differentiation potential of hPDLSCs, cells were induced with osteogenic, adipogenic, and chondrogenic medium for 3 weeks in vitro, respectively. hPDLSCs, human periodontal ligament stem cells; MSCs, mesenchymal stem cells. Color images available online at www.liebertpub.com/tea
Recombinant human PAI-1 induces cementogenic differentiation of hPDLSCs in vitro
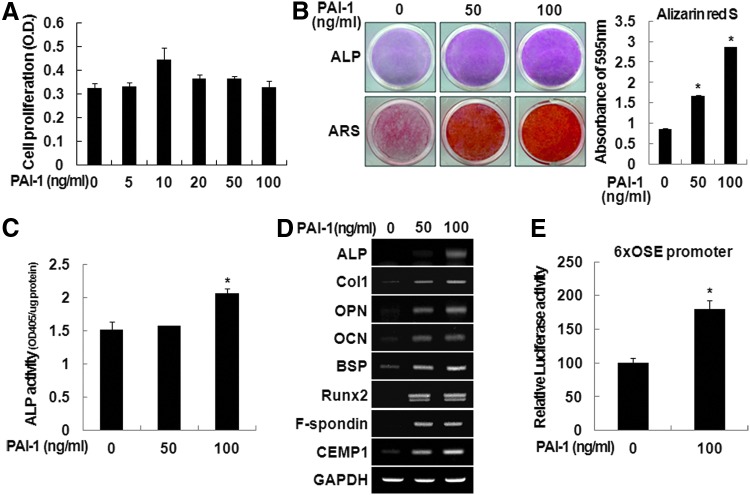
To examine the effects of rhPAI-1 on cell viability in vitro, hPDLSCs were treated with 0, 5, 10, 20, 50, or 100 ng/mL of rhPAI-1. MTT assay showed that rhPAI-1 did not have any cytotoxicity after 48 h of culture, even at high doses. Furthermore, rhPAI-1 still had no cytotoxicity in hPDLSCs after long-term culture of 1, 2, and 3 weeks (data not shown). In addition, we observed that rhPAI-1 increased proliferation of hPDLSCs at a low dose of 10 ng/mL (Fig. 2A). Next, we investigated the effects of rhPAI-1 on the differentiation of hPDLSCs in vitro. After hPDLSCs were cultured in the osteogenic differentiation medium treated without or with rhPAI-1 for 2 weeks, we observed only 50 and 100 ng/mL of rhPAI-1-induced osteogenic/cementogenic differentiation. Calcium deposition and ALP activity of hPDLSCs were significantly increased in 100 ng/mL of rhPAI-1-treated group compared to 50 ng/mL of rhPAI-1-treated group and control group (Fig. 2B, C). Higher calcium contents were observed in the rhPAI-1-treated group after a destaining procedure of Alizarin red S staining (Fig. 2B, right panel). The mRNA expression of osteogenic/cementogenic differentiation markers, such as ALP, Col1, OPN, OCN, BSP, and Runx2, increased significantly after culture with rhPAI-1 compared to untreated cells (Fig. 2D). Importantly, expression of F-spondin and CEMP1, well known as cementoblast-specific markers, was also significantly increased by rhPAI-1. To assess cementogenic differentiation ability of rhPAI-1, we measured the transcriptional activity of 6× OSE promoter. As shown in Figure 2E, rhPAI-1 treatment stimulated the transcriptional activity of 6× OSE promoter. These results suggest that rhPAI-1 can enhance the Runx2 signaling pathway to stimulate osteoblast/cementoblast-associated gene expression through Runx2 activation. Therefore, the Runx2 signaling pathway may play a critical role in cementogenic differentiation as well as osteogenic differentiation. Thus, in the osteogenic differentiation medium, rhPAI-1 could induce the differentiation of hPDLSCs into mineral-forming cells, such as cementoblasts in vitro.
FIG. 2.
Effects of rhPAI-1 on cementogenic differentiation of hPDLSCs. (A) To examine the effects of rhPAI-1 on cell proliferation of hPDLSCs in vitro, hPDLSCs were cultured for 48 h at the indicated doses of rhPAI-1 and analyzed by MTT assay. hPDLSCs were cultured in osteogenic differentiation medium without or with rhPAI-1 (50 or 100 ng/mL). (B) Alizarin red S staining was performed on day 14. The samples were destained as described in Materials and Methods section. (B) ALP staining and (C) ALP activity assay in cell lysates were performed on day 7. *p<0.01. (D) To further investigate whether rhPAI-1 induced cementogenic differentiation of hPDLSCs, cementoblast-associated genes, including ALP, Col1, OPN, OCN, BSP, Runx2, F-spondin, and CEMP1, were analyzed by reverse transcription–polymerase chain reaction. (E) The 6× OSE promoter assay was performed in hPDLSCs without or with rhPAI-1 (100 ng/mL). *p<0.01. ALP, alkaline phosphatase; BSP, bone sialoprotein; CEMP1, cementum protein 1; Col1, type 1 collagen; OCN, osteocalcin; OPN, osteopontin; rhPAI-1, recombinant human plasminogen activator inhibitor-1. Color images available online at www.liebertpub.com/tea
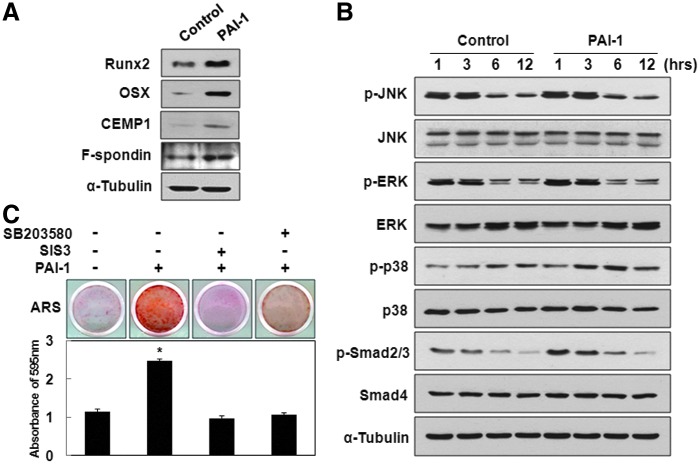
Recombinant human PAI-1-induced OSX and CEMP1 expression is mediated by p38 and Smad2/3 pathways in hPDLSCs
To inspect the key molecular mechanisms by which rhPAI-1 induces differentiation of hPDLSCs, the first targets to be examined should be among the members of transcription factors involved in osteogenesis/cementogenesis. Recent studies indicated that Runx2 and its downstream target OSX play critical roles in cementogenic differentiation, which is important for osteogenesis.30,31 Furthermore, OSX particularly controls the formation of cellular cementum.32 During in vitro cementogenic differentiation, rhPAI-1 increased Runx2 expression at 7 days and subsequently increased OSX expression at 14 days. CEMP1 and F-spondin expression also increased at 14 days of culture (Fig. 3A). The expression levels of mitogen-activated protein kinases (MAPKs) and Smads were examined as these proteins are thought to be involved in the early signaling pathways.33 Among these proteins and their phosphorylated forms, rhPAI-1 increased p-p38 activation without affecting p-JNK and p-ERK. As expected, rhPAI-1 upregulated the levels of p-Smad2/3, whereas Smad4 showed no change (Fig. 3B). To confirm whether p38 and Smad2/3 play an essential role in rhPAI-1-induced cementogenic differentiation of hPDLSCs, we investigated the effects of their specific inhibitors, SB203580 and SIS3, respectively, on differentiation induction.34,35 Compared to Alizarin red S staining positivity of rhPAI-1 group after 14 days of differentiation induction, decreased calcium nodule formation was observed in the presence of rhPAI-1 with the SB203580 and SIS3 group (Fig. 3C). The calcium contents induced by rhPAI-1 with SIS3 and SB203580 treatment were verified after destaining procedures (Fig. 3C, bottom panel). Therefore, SB203580 and SIS3 inhibited rhPAI-1-induced mineralization of hPDLSCs in vitro. Taken together, these results suggest that OSX and CEMP1 seem to play an essential role in rhPAI-1-induced cementogenic differentiation of hPDLSCs through p38 and Smad2/3.
FIG. 3.
Recombinant human PAI-1-induced OSX and CEMP1 expression was mediated by p38 and Smad2/3. (A) To inspect the molecular mechanisms of rhPAI-1-induced differentiation, hPDLSCs were cultured in osteogenic differentiation medium without or with rhPAI-1 (100 ng/mL). Whole-cell lysates were subjected to Western blot analysis with the indicated antibodies; α-tubulin served as an internal control. (B) hPDLSCs were cultured in osteogenic differentiation medium without or with rhPAI-1 (100 ng/mL) for the indicated time points. Whole-cell lysates were subjected to Western blot analysis with indicated antibodies; α-tubulin served as an internal control. (C) To confirm whether p38 and Smad2/3 contributed to rhPAI-1-induced cementogenic differentiation of hPDLSCs, SB203580 and SIS3 were used. Alizarin red S staining was performed on day 14. The samples were destained as described in Materials and Methods section. *p<0.01. OSX, osterix. Color images available online at www.liebertpub.com/tea
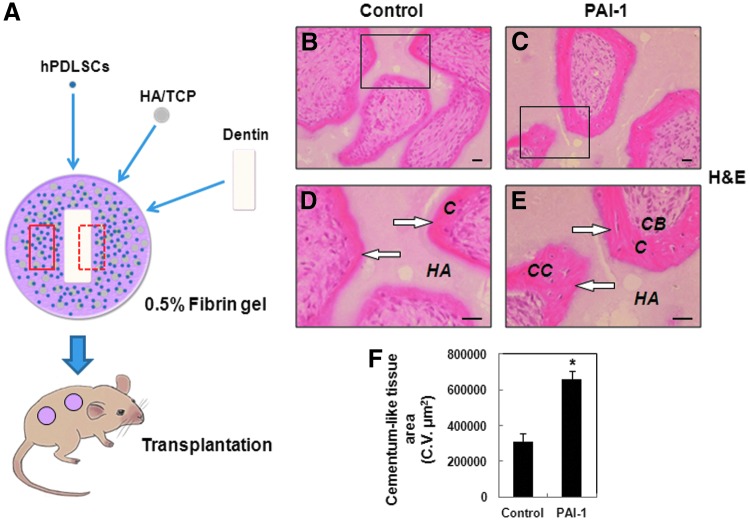
Recombinant human PAI-1 promotes newly formed cementum on the surface of HA/TCP
To assess the capacity of hPDLSCs for cementum formation in response to rhPAI-1 in vivo, we aimed to reproduce the microenvironment of human tooth root as shown in Figure 4A. The hPDLSCs were mixed with HA/TCP and dentin matrix without or with rhPAI-1 and then subcutaneously transplanted into the dorsal surface of immunocompromised mice. First, we examined the area of the red box for cementum formation. At 8 weeks after transplantation, the rhPAI-1-treated group showed significant generation of new cementum-like tissue, whereas the control group generated a limited amount of cementum-like tissue on the surface of HA/TCP (Fig. 4B, C). Furthermore, we could observe a larger number of differentiated cementoblast-like cells and cementocyte-like cells in the rhPAI-1-treated group compared to the control group (Fig. 4D, E). Histomorphometric analysis showed that cementum-like tissue area in the rhPAI-1-treated group was 2.13-fold larger than that in the control group (Fig. 4F).
FIG. 4.
Histological analysis showed the effects of rhPAI-1 on cementum formation in vivo. (A) Schematic representation of transplantation model used for periodontal tissue generation. The red box showed the surface of HA/TCP, and the black box showed the surface of dentin matrix. (B) H&E staining of generation of cementum-like tissue in control group. (C) H&E staining of generation of cementum-like tissue in rhPAI-1-treated group. (D, E) Higher magnification of the black squares in (B, C) clearly showed that much more cementum-like tissue (arrows) generated in rhPAI-1-treated group than in control group. (F) Histomorphometric analysis showed that cementum-like tissue area in the same gross area in rhPAI-1-treated group was 2.13-fold larger than that in control group. The difference in data showed statistical significance (n=6; *p<0.01). C, cementum-like tissue; CB, cementoblast-like cells; CC, cementocyte-like cells; HA/TCP, hydroxyapatite tricalcium phosphate. Scale bar=40 μm. H&E, hematoxylin and eosin. Color images available online at www.liebertpub.com/tea
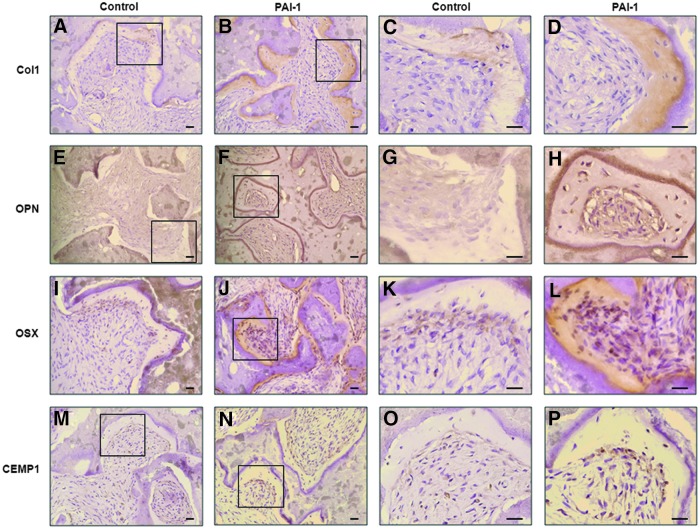
To further prove the effects of rhPAI-1 on cementoblast differentiation and cementum formation in hPDLSCs in vivo, we examined the expression of cementum matrix-associated proteins using immunohistochemical staining. Col1 is the most abundant collagenous protein of cementum matrix.36 As expected, the expression of Col1 in newly formed cementum-like tissue was much stronger in the rhPAI-1-treated group than in the control group (Fig. 5A–D). During cementum formation, cementoblasts and cementocytes have been reported to produce OPN, which plays an important role in cementogenesis.13 OPN was hardly expressed in the control group, but cementoblast-like cells and cementum-like tissue in the rhPAI-1-treated group strongly expressed OPN (Fig. 5E–H). Expression of OSX was also much stronger in the rhPAI-1-treated group than in the control group (Fig. 5I–L). Cemp1, which is regarded a specific marker of cementoblasts, was expressed more strongly in cementoblast-like cells of rhPAI-1-treated group than control group (Fig. 5M, N).
FIG. 5.
Immunohistochemical analysis showed the effects of rhPAI-1 on cementogenic differentiation of hPDLSCs in vivo. (A) Col1 expression in control group. (B) Col1 expression in rhPAI-1-treated group. (E) OPN expression in control group. (F) OPN expression in rhPAI-1-treated group. (I) OSX expression in control group. (J) OSX expression in rhPAI-1-treated group. (M) CEMP1 expression in control group. (N) CEMP1 expression in rhPAI-1-treated group. Higher magnification of the black squares was positioned (A:C, B:D, E:G, F:H, I:K, J:L, M:O, N:P). Scale bar=40 μm. Color images available online at www.liebertpub.com/tea
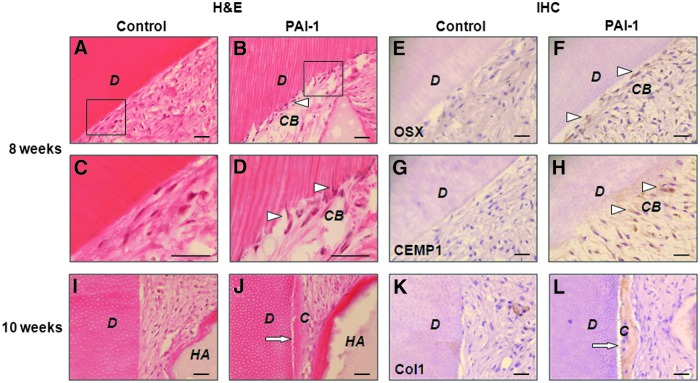
Recombinant human PAI-1 regenerates new cementum on the dentin matrix surface
To investigate the effects of rhPAI-1 on cementogenic differentiation with human tooth root dentin matrix, we next evaluated cementogenesis in the area of the red dotted box in histological analysis. At 8 weeks after transplantation, rhPAI-1 induced hPDLSCs to differentiate into cementoblast-like cells, which arranged in a line on the surface of dentin matrix, but there was little change in the control group (Fig. 6A–D, triangles). We could observe that some of the cementoblast-like cells penetrated into the dentinal tubules on the dentin matrix (Fig. 6D, triangles). At the beginning of cementoblast maturation on the dentin surface, cementoblasts extend numerous tiny cytoplasmic processes into the loosely arranged and not yet mineralized dentinal matrix to position the initially secreted collagen fibrils of the cementum matrix.37 It means that cementoblasts have the capacity to modify the cellular structure according to the surrounding tissue to make preparations for cementum formation, as we have found here. Furthermore, immunohistochemical staining showed strongly expressed OSX and CEMP1 in cementoblast-like cells of rhPAI-1-treated group, but hardly any expression in the control group (Fig. 6E–H). More importantly, at 10 weeks, rhPAI-1 significantly induced hPDLSCs to regenerate a thick layer of cementum-like tissue on the surface of dentin matrix, whereas we could not detect any such tissue in the control group (Fig. 6I, J). In addition, Col1 was strongly expressed in newly formed cementum-like tissue in the rhPAI-1-treated group compared to the control group (Fig. 6K, L). Taken together, these results suggest that rhPAI-1 markedly induces cementogenic differentiation of hPDLSCs, and the HA/TCP graft significantly improves periodontal tissue generation. Therefore, rhPAI-1 may solve the problem of attachment of the PDL fibers, which support tooth to resist masticatory load, in periodontitis.
FIG. 6.
Effects of rhPAI-1 on cementum regeneration on the dentin surface. (A) H&E staining of control group. (B) H&E staining of the differentiated cementoblast-like cells in rhPAI-1-treated group. (C, D) Higher magnification of the black squares in (A, B) clearly showed a layer of cementoblast-like cells (triangles) in rhPAI-1-treated group. (E) OSX expression in control group. (F) OSX expression in rhPAI-1-treated group. (G) CEMP1 expression in control group. (H) CEMP1 expression in rhPAI-1-treated group. The differentiated cementoblast-like cells were strongly stained positive for OSX and CEMP1. (I) H&E staining of control group. (J) H&E staining of newly formed cementum-like tissue in rhPAI-1-treated group. (K) Col1 expression in control group. (L) Col1 expression (arrows) in rhPAI-1-treated group. Col1 was strongly expressed in newly formed cementum-like tissue. D, dentin. Scale bar=40 μm. Color images available online at www.liebertpub.com/tea
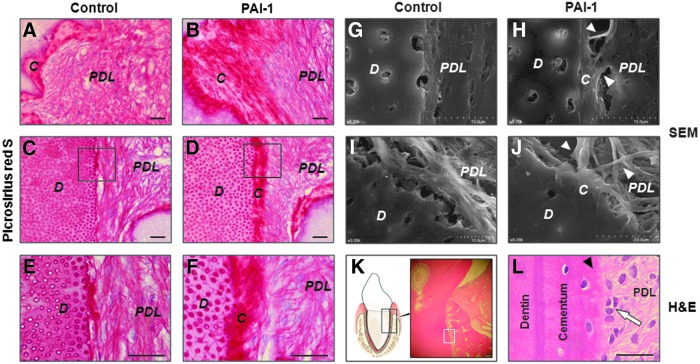
Recombinant human PAI-1 facilitates generation of PDL
PDL is the fibrous connective tissue that plays important roles in attaching tooth to the surrounding alveolar bone and absorbing heavy forces to cushion the impact.36 At 10 weeks after transplantation, we examined whether rhPAI-1 had the ability to induce hPDLSCs to generate PDL. Picrosirius red staining showed that rhPAI-1-induced PDL fibers inserted into newly formed cementum-like tissue layer on the surface of HA/TCP and dentin matrix, whereas the PDL fibers in the control group were disorganized (Fig. 7A–D). Furthermore, higher magnification images clearly showed that the PDL fibers were almost perpendicularly inserted into newly formed cementum-like tissue in the rhPAI-1-treated group, known as Sharpey's fibers (Fig. 7F). In contrast, in the control group, the PDL fibers were orientated primarily parallel to the dentin surface (Fig. 7E). To confirm the orientation of PDL fibers, we obtained high-resolution images using SEM. The rhPAI-1-treated group showed the PDL fibers inserted into newly formed cementum-like tissue on the dentin surface (Fig. 7H, J), whereas the PDL fibers were parallel to the dentin surface in the control group (Fig. 7G, I). In addition, positive control of beagle dog tooth root showed dentin, cementum, cementoblasts, PDL fibers, and Sharpey's fibers to prove the generated periodontal tissue (Fig. 7K, L).
FIG. 7.
Recombinant human PAI-1 induced generation of PDL in vivo. (A) Picrosirius red staining of PDL fibers on the HA/TCP surface in control group. (B) Picrosirius red staining of inserted PDL fibers on generated cementum-like tissue layer on the HA/TCP surface in rhPAI-1-treated group. (C) Picrosirius red staining of PDL fibers on the dentin surface in control group. (D) Picrosirius red staining of inserted PDL fibers on newly formed cementum-like tissue layer on the dentin surface in rhPAI-1-treated group. (E, F) Higher magnification of the black squares in (C, D). (G, I) SEM of PDL fibers in control group. (H, J) SEM of inserted PDL fibers in rhPAI-1-treated group. The inserted PDL fibers (white triangles) were clearly observed. (C–J) Serial sections of the same anatomical region. (K) H&E staining of positive control group. (L) Higher magnification of the white square in (K). H&E staining showed normal cementum, PDL fibers, Sharpey's fibers (black triangle), and cementoblasts (arrow). PDL, periodontal ligament. Scale bar=40 μm. SEM, scanning electron microscopy. Color images available online at www.liebertpub.com/tea
Discussion
PAI-1 is known to function as the primary physiological inhibitor of two main mammalian plasminogen activators, tPA and uPA. PAI-1 can be synthesized by various cells, including hepatocytes, adipocytes, glomerular epithelial cells, tubular epithelial cells, vascular endothelial cells, vascular smooth muscle cells, platelets, monocytes, and macrophages.38 A potential role of PAI-1 in bone remodeling was reported when osteoblastic cells from PAI-1-deficient mice were found to display a significantly enhanced potential to degrade nonmineralized bone-like matrix.39 However, the effects of PAI-1 on cementogenic differentiation in hPDLSCs have never been elucidated. Our present studies showed that rhPAI-1 stimulated cementogenic differentiation of hPDLSCs in vitro and in vivo. Above all, rhPAI-1 has a great ability to promote differentiation of hPDLSCs into cementoblast-like cells and generation of cementum-like tissue with inserted PDL.
Our in vitro results indicated that rhPAI-1 increased the mRNA expression levels of ALP, Col1, OPN, OCN, BSP, and Runx2 in hPDLSCs. These genes are known to be shared in osteoblasts and cementoblasts. Consistent with these results, the protein expression levels of Runx2 and OSX were also increased by rhPAI-1 treatment. It has been reported that Runx2 is an osteoblast-specific gene that regulates the initiation of osteogenesis.40 However, the results of this study suggest that Runx2 and OSX may participate in rhPAI-1-induced cementogenic differentiation. We also found that rhPAI-1 significantly induced CEMP1 and F-spondin expression. These data suggest that some of these transcription factors might be important for cementogenic differentiation induced by rhPAI-1.
A previous report demonstrated that transplanted hPDLSCs generated new cementum and collagen fibers in immunocompromised mice.3 However, the precise mechanisms by which hPDLSCs differentiate into cementoblasts and generate cementum when transplanted in immunocompromised mice are unclear. Based on our in vitro results, we hypothesized that hPDLSCs treated with rhPAI-1 would effectively differentiate into cementoblasts. Moreover, rhPAI-1 might have a great ability to generate cementum with inserted PDL fibers in such conditions. To test these hypotheses, histological analysis was conducted to elucidate the function of rhPAI-1 on cementogenic differentiation in hPDLSCs. Previous reports have demonstrated that xenogenic bone graft and growth factors, including transforming growth factor-beta (TGF-β), bone morphogenetic protein (BMP)-2, BMP-4, and platelet-derived growth factor (PDGF), improve periodontal tissue regeneration.41–44 MSC transplantation can supply large numbers of necessary cells and increase periodontal regenerative capacity.45,46 It also well known that MSCs can differentiate into the desired tissues, such as cementum, alveolar bone, dentin, and collagen fibers. Recently, it has been reported that CEMP1-positive progenitor cells localized in the PDL participate in the differentiation of progenitor cells for cementum formation.47 Cementum formation is critical for appropriate maturation of the periodontium, both during development and regeneration of periodontal tissue.36 Indeed, our in vivo studies showed that rhPAI-1 promoted hPDLSCs to differentiate into cementoblast-like cells and generate a large amount of cementum-like tissue, whereas the control group generated a limited amount of cementum-like tissue. Furthermore, several studies have reported that CEMP1 protein expression is localized to the cementoblast cell layer lining the cementoid surface of cementum.15,17 Immunohistochemical staining indicated that cementoblast-like cells strongly expressed CEMP1 in the rhPAI-1-treated group, whereas expression was weak in the control group. Col1 and OPN expression was much stronger in cementum-like tissue of the rhPAI-1-treated group than control group, and OSX expression was also significantly increased in the rhPAI-1-treated group compared to the control group. Therefore, OSX and CEMP1 might play an important role in cementogenesis in response to rhPAI-1. The results of the present study suggest that rhPAI-1 may promote cementoblast differentiation and cementum formation in hPDLSCs through the OSX-CEMP1 pathway. However, the mechanisms underlying rhPAI-1-induced cementogenic differentiation should be explored in future studies.
Previous studies indicated that HA/TCP had inducible effects on hard tissue formation of MSCs.48,49 Based on these results, many researchers attempted to generate hard tissues such as bone, dentin, and cementum by combining HA/TCP with MSCs. However, the clinical results for cementum formation were very poor because of the different structure and histological component between HA/TCP and tooth root dentin matrix. In this study, hPDLSCs were mixed with dentin matrix without or with rhPAI-1 and subcutaneously transplanted into the dorsal surface of immunocompromised mice. First, we demonstrated that rhPAI-1 induced hPDLSCs to differentiate into cementoblast-like cells. Most notably, the differentiated cementoblast-like cells were arranged in a line on the dentin matrix surface and penetrated into the dentinal tubules. Second, rhPAI-1 induced hPDLSCs to regenerate cementum-like tissue on the surface of dentin matrix in vivo. These results suggest that the morphology and property of differentiated cementoblast-like cells are similar to those of cementoblasts. Moreover, our data indicated that rhPAI-1-induced cementoblast-like cells showed strong expression of OSX and CEMP1 in hPDLSCs. Moreover, Col1 was significantly expressed on newly formed cementum-like tissue in the rhPAI-1-treated group, in conformity with the positive expression of Col1, OSX, and CEMP1 on cementoblasts and cementum of human tooth root (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). Taken together, these results suggest that rhPAI-1 induces cementoblast differentiation and cementum formation on the dentin matrix surface in hPDLSCs.
In periodontal disease, PDL fibers are orientated primarily parallel to the root surface as a result of cementum resorption and, are therefore, unable to absorb masticatory load and prevent tooth loss. Our initial experiments demonstrated that rhPAI-1 had a great ability to induce cementogenic differentiation in hPDLSCs. As mentioned above, PDL generation is a very important problem in periodontal tissue regeneration as well as cementum. The importance of cementum formation is emphasized by regeneration of new cementum with inserted PDL fibers on the root surface.36 In the present study, rhPAI-1-induced PDL fibers inserted into newly formed cementum-like tissue layer, whereas the PDL fibers were almost parallel to the root surface in the control group. SEM micrographs illustrated integration of PDL fibers with the mineralized matrices, including PDL inserts within the cementum.50 Accordingly, we could clearly see that the PDL fibers were inserted into the newly formed cementum-like tissue layer in the rhPAI-1-treated group using SEM compared to the control group. These results suggest that rhPAI-1 not only induces cementum formation but also generates PDL fibers that insert into cementum for supportive function. However, it is still hard to regenerate tooth root, so a dental implant is generally substituted to provide the same function as tooth root. Till now, many cases reported that dental implants induce bone resorption as a result of masticatory overload.51 This is because dental implant directly attaches to the surrounding alveolar bone in the absence of inserted PDL fibers, which normally resist forces to support tooth. In future studies, we will examine the function of rhPAI-1 and hPDLSCs on the surface of dental implants during periodontal tissue regeneration.
Conclusions
In this study, we investigated the effects of rhPAI-1 on cementogenic differentiation of hPDLSCs in vitro and in vivo. We found that rhPAI-1 induced hPDLSCs to generate cementum-like tissue with generation of PDL fibers that inserted into newly formed cementum-like tissue in vivo. rhPAI-1 might upregulate the levels of OSX and CEMP1 through Smad2/3 and p38 pathways, whereas specific inhibitors of Smad3 and p38 inhibited the enhancement of mineralization of hPDLSCs by rhPAI-1. Taken together, our data suggest that rhPAI-1 with hPDLSCs may be a good approach for future clinical applications in periodontal tissue regeneration and, furthermore, in tooth-root bioengineering.
Supplementary Material
Acknowledgment
This work was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) (No. 2011-0028922).
Disclosure Statement
No competing financial interests exist.
References
- 1.Flemmig T.F. Periodontitis. Ann Periodontol 4, 32, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Jo Y.Y., Lee H.J., Kook S.Y., et al. . Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng 13, 767, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Seo B.M., Miura M., Gronthos S., et al. . Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364, 149, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Gronthos S., Mankani M., Brahim J., Robey P.G., and Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97, 13625, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCulloch C.A., and Bordin S. Role of fibroblast subpopulations in periodontal physiology and pathology. J Periodontal Res 26, 144, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Isaka J., Ohazama A., Kobayashi M., et al. . Participation of periodontal ligament cells with regeneration of alveolar bone. J Periodontol 72, 314, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Gay I.C., Chen S., and MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res 10, 149, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Xu J., Wang W., Kapila Y., Lotz J., and Kapila S. Multiple differentiation capacity of STRO-1+/CD146+PDL mesenchymal progenitor cells. Stem Cells Dev 18, 487, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomokiyo A., Maeda H., Fujii S., Wada N., Shima K., and Akamine A. Development of a multipotent clonal human periodontal ligament cell line. Differentiation 76, 337, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Nojima N., Kobayashi M., Shionome M., Takahashi N., Suda T., and Hasegawa K. Fibroblastic cells derived from bovine periodontal ligaments have the phenotypes of osteoblasts. J Periodontal Res 25, 179, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Hunter G.K., and Goldberg H.A. Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci U S A 90, 8562, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Errico J.A., MacNeil R.L., Takata T., Berry J., Strayhorn C., and Somerman M.J. Expression of bone associated markers by tooth root lining cells, in situ and in vitro. Bone 20, 117, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Bronckers A.L., Farach-Carson M.C., Van Waveren E., and Butler W.T. Immunolocalization of osteopontin, osteocalcin, and dentin sialoprotein during dental root formation and early cementogenesis in the rat. J Bone Miner Res 9, 833, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Arzate H., Jimenez-Garcia L.F., Alvarez-Perez M.A., Landa A., Bar-Kana I., and Pitaru S. Immunolocalization of a human cementoblastoma-conditioned medium-derived protein. J Dent Res 81, 541, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Alvarez-Perez M.A., Narayanan S., Zeichner-David M., Rodriguez Carmona B., and Arzate H. Molecular cloning, expression and immunolocalization of a novel human cementum-derived protein (CP-23). Bone 38, 409, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Kitagawa M., Kudo Y., Iizuka S., et al. . Effect of F-spondin on cementoblastic differentiation of human periodontal ligament cells. Biochem Biophys Res Commun 349, 1050, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Park J.Y., Jeon S.H., and Choung P.H. Efficacy of periodontal stem cell transplantation in the treatment of advanced periodontitis. Cell Transplant 20, 271, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Liu R.M. Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis. Antioxid Redox Signal 10, 303, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaughan D.E., De Taeye B.M., and Eren M. PAI-1 antagonists: predictable indications and unconventional applications. Curr Drug Targets 8, 962, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Lundgren C.H., Brown S.L., Nordt T.K., Sobel B.E., and Fujii S. Elaboration of type-1 plasminogen activator inhibitor from adipocytes. A potential pathogenetic link between obesity and cardiovascular disease. Circulation 93, 106, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Stefansson S., McMahon G.A., Petitclerc E., and Lawrence D.A. Plasminogen activator inhibitor-1 in tumor growth, angiogenesis and vascular remodeling. Curr Pharm Des 9, 1545, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Vassalli J.D., Sappino A.P., and Belin D. The plasminogen activator/plasmin system. J Clin Invest 88, 1067, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordstrom S.M., Carleton S.M., Carson W.L., Eren M., Phillips C.L., and Vaughan D.E. Transgenic over-expression of plasminogen activator inhibitor-1 results in age-dependent and gender-specific increases in bone strength and mineralization. Bone 41, 995, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang Y.H., Jeon S.H., Park J.Y., et al. . Platelet-rich fibrin is a bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng Part A 17, 349, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Choukroun J., Diss A., Simonpieri A., et al. . Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101, 299, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Jin H., Park J.Y., Choi H., and Choung P.H. HDAC inhibitor trichostatin a promotes proliferation and odontoblast differentiation of human dental pulp stem cells. Tissue Eng Part A 19, 613, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Li R., Guo W., Yang B., et al. . Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials 32, 4525, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Parolini O., Alviano F., Bagnara G.P., et al. . Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 26, 300, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Dominici M., Le Blanc K., Mueller I., et al. . Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Hirata A., Sugahara T., and Nakamura H. Localization of runx2, osterix, and osteopontin in tooth root formation in rat molars. J Histochem Cytochem 57, 397, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakashima K., Zhou X., Kunkel G., et al. . The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108, 17, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Cao Z., Zhang H., Zhou X., et al. . Genetic evidence for the vital function of osterix in cementogenesis. J Bone Miner Res 27, 1080, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vayalil P.K., Iles K.E., Choi J., Yi A.K., Postlethwait E.M., and Liu R.M. Glutathione suppresses TGF-beta-induced PAI-1 expression by inhibiting p38 and JNK MAPK and the binding of AP-1, SP-1, and Smad to the PAI-1 promoter. Am J Physiol Lung Cell Mol Physiol 293, L1281, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arana-Argaez V.E., Delgado-Rizo V., Pizano-Martinez O.E., et al. . Inhibitors of MAPK pathway ERK1/2 or p38 prevent the IL-1{beta}-induced up-regulation of SRP72 autoantigen in Jurkat cells. J Biol Chem 285, 32824, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jinnin M., Ihn H., and Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Mol Pharmacol 69, 597, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Saygin N.E., Giannobile W.V., and Somerman M.J. Molecular and cell biology of cementum. Periodontol 2000 24, 73, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Bosshardt D.D., and Selvig K.A. Dental cementum: the dynamic tissue covering of the root. Periodontol 2000 13, 41, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Ha H., Oh E.Y., and Lee H.B. The role of plasminogen activator inhibitor 1 in renal and cardiovascular diseases. Nat Rev Nephrol 5, 203, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Daci E., Udagawa N., Martin T.J., Bouillon R., and Carmeliet G. The role of the plasminogen system in bone resorption in vitro. J Bone Miner Res 14, 946, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res 339, 189, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Camelo M., Nevins M.L., Lynch S.E., Schenk R.K., Simion M., and Nevins M. Periodontal regeneration with an autogenous bone-Bio-Oss composite graft and a Bio-Gide membrane. Int J Periodontics Restorative Dent 21, 109, 2001 [PubMed] [Google Scholar]
- 42.Chen Y.L., Chen P.K., Jeng L.B., et al. . Periodontal regeneration using ex vivo autologous stem cells engineered to express the BMP-2 gene: an alternative to alveolaplasty. Gene Ther 15, 1469, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Giannobile W.V., Finkelman R.D., and Lynch S.E. Comparison of canine and non-human primate animal models for periodontal regenerative therapy: results following a single administration of PDGF/IGF-I. J Periodontol 65, 1158, 1994 [DOI] [PubMed] [Google Scholar]
- 44.Ripamonti U., Heliotis M., van den Heever B., and Reddi A.H. Bone morphogenetic proteins induce periodontal regeneration in the baboon (Papio ursinus). J Periodontal Res 29, 439, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Hynes K., Menicanin D., Gronthos S., and Bartold P.M. Clinical utility of stem cells for periodontal regeneration. Periodontol 2000 59, 203, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Kawaguchi H., Hirachi A., Hasegawa N., et al. . Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J Periodontol 75, 1281, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Komaki M., Iwasaki K., Arzate H., Narayanan A.S., Izumi Y., and Morita I. Cementum protein 1 (CEMP1) induces a cementoblastic phenotype and reduces osteoblastic differentiation in periodontal ligament cells. J Cell Physiol 227, 649, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Ohgushi H., Okumura M., Tamai S., Shors E.C., and Caplan A.I. Marrow cell induced osteogenesis in porous hydroxyapatite and tricalcium phosphate: a comparative histomorphometric study of ectopic bone formation. J Biomed Mater Res 24, 1563, 1990 [DOI] [PubMed] [Google Scholar]
- 49.Arinzeh T.L., Tran T., McAlary J., and Daculsi G. A comparative study of biphasic calcium phosphate ceramics for human mesenchymal stem-cell-induced bone formation. Biomaterials 26, 3631, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Ho S.P., Kurylo M.P., Fong T.K., et al. . The biomechanical characteristics of the bone-periodontal ligament-cementum complex. Biomaterials 31, 6635, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isidor F. Loss of osseointegration caused by occlusal load of oral implants. A clinical and radiographic study in monkeys. Clin Oral Implants Res 7, 143, 1996 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.