Abstract
Circulating tumor cells (CTC) in blood are associated with poor survival of patients with breast, prostate, or colon cancer. We hypothesized that CTC are associated with poor survival of patients with cholangiocarcinoma (CCA). 88 patients with CCA were prospectively enrolled at Mayo Clinic Rochester between June 2010 and September 2014. The CellSearch system by Veridex was used for detection of CTC in peripheral blood. Associations between CTC, patient and tumor characteristics and survival were examined using the Cox proportional hazards model. Fifteen patients (17%) were positive for CTC ≥2 and 8 patients (9%) for CTC ≥5. CTC were associated with tumor extent. CTC ≥2 (HR, 2.5; 95%CI, 1.1–5.4; p=0.02) and CTC ≥5 (HR, 4.1; 95%CI, 1.4–10.8; p=0.01) were both independent predictors of survival. In subgroup analyses, CTC ≥2 (HR 8.2; 95%CI 1.8–57.5; p<0.01) and CTC ≥5 (HR 7.7; 95%CI 1.4–42.9; p=0.02) were both associated with shorter survival among patients with metastasis. There was a trend towards association of CTC ≥5 with shorter survival in patients with non-metastatic CCA (HR 4.3; 95%CI 1.0–13.8; p=0.06). CTC ≥2 (10.5; 95%CI 2.2–40.1; p<0.01) and CTC ≥5 (HR 10.2; 95%CI 1.5–42.3; p=0.02) were both associated with shorter survival among patients with perihilar/distal CCA. CTC ≥5 was associated with shorter survival of patients with intrahepatic CCA (HR 4.2; 95%CI 1.1–14.1; p=0.04).
Conclusion
CTC were associated with more aggressive tumor characteristics and independently associated with survival in patients with CCA. Assessment of CTC may be useful for identifying CCA patients at risk of early mortality.
Keywords: liver cancer, biliary cancer, overall survival, biomarker, CTC
Introduction
Cholangiocarcinoma (CCA) is the second most common cause of primary liver cancer. A recent population based study in the US reported an increasing incidence of intrahepatic CCA.1 Unfortunately, the prognosis of patients with CCA is dismal, as most CCAs are detected at an advanced stage for which there are no effective treatment options. With advances in molecular technologies, cancer biomarkers have been shown to have utility for early detection, prediction of treatment response and prognostic prediction in patients with various types of cancers. The development of novel cancer biomarkers for CCA may help in clinical decision making and lead to improvements in patient outcomes by facilitating early detection of cancer, prediction of the response to specific treatments, improved monitoring of patients on treatment, and better prognostication of patient outcomes, thus improving stratification for clinical trials.
As tumor cells proliferate, they promote angiogenesis and then invade into the blood stream to sites of distant metastasis. It has been estimated that approximately 1×106 tumor cells per gram of tumor tissue are released into the circulation daily.2 These circulating tumor cells (CTC) have been investigated as a prognostic indicator in various types of cancers using different CTC detection techniques.3 The CellSearch System (Janssen/Veridex; Raritan, NJ) has been tested most extensively.4 It is designed to capture cells expressing cancer specific epithelial cell adhesion molecules (EpCAM) using antibody-coated magnetic beads followed by positive identification of intact tumor cells using fluorescently labeled antibodies against cytokeratin and nuclear staining. Detection of CTC in blood has been associated with poor progression free and overall survival in patients with metastatic/non-metastatic breast cancer5–8, metastatic colon cancer,9 and bladder cancer.10 CTC were also reported to be associated with overall survival in patients with metastatic castration-resistant prostate cancer and have been shown to be detectable in patients with other metastatic cancers including ovarian and lung cancers.4,11
It has been shown that most CCA cells express EpCAM, and a pilot study of 13 patients with intrahepatic CCA reported that CTC were detectable in blood specimens from these patients.12,13 Since CTC are generally more likely to enter the bloodstream from larger tumors with a more metastatic phenotype, we tested the hypotheses that (i) CTC are detectable in blood from CCA patients by CellSearch, and (ii) the presence of CTC is independently associated with poor overall survival in patients with CCA.
Methods
Patients
This study was approved by the Mayo Clinic Institutional Review Board. Eighty-eight patients with pathologically proven CCA seen at Mayo Clinic Rochester who consented to the study were prospectively enrolled between June 2010 and September 2014. In the initial phase of the study between June 2010 and May 2012 (n=31), the goal was to confirm the hypothesis that CTC are present and detectable in the peripheral blood of patients with CCA. Therefore, consecutive patients with advanced stage CCA, which was defined as the presence of a primary tumor size >5cm, CA19-9 >1,000 U/mL, and multifocal, bilobar, or metastatic disease, were approached for consent. After confirming that CTC were detectable in the peripheral blood in patients with CCA using the CellSearch System, from May 2012 on we enrolled all CCA patients who consented to participate in the study (n=57).
Clinical information
Patient medical records were reviewed to abstract their clinical information at the time of CTC collection. This included demographic information; subtype of tumor: intrahepatic CCA (iCCA), perihilar CCA (pCCA), or distal CCA (dCCA); the extent of the tumor; and selected laboratory results. The extent of CCA was determined by cross-sectional radiographic characteristics and/or surgical pathology reports, if available. The size of the largest tumor, presence of multiple nodules, bilobar disease, loco-regional lymph node invasion, distant extra-hepatic metastasis, and the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) surgical or radiologic (if the patient did not undergo surgical resection) TNM stage (7th edition)14 were abstracted. The treatments that patients received and/or that were recommended after enrollment were reviewed. The patient vital status was updated at the study end date of December 31, 2014.
CTC analysis
Blood samples were collected after the diagnosis of CCA was made. For patients treated with curative surgical resection or OLT (N=26), blood was collected prior to surgical intervention in all patients. For patients receiving noncurative treatments/best supportive care (N=62), blood was collected before any treatment in 44 patients (71%), while on treatment in 12 patients (19%), or after active treatment while patients were only receiving best supportive care in 6 patients (10%). The CellSearch System was used for the detection and enumeration of CTC as previously described.4 Peripheral blood (8–10 mL collection, 7.5 mL used for the analysis) was collected in CellSave Tubes (Jannsen Diagnostics, Raritan NJ), shipped at room temperature, and processed within 96 hours.
Briefly, 6 ml buffer was added to 7.5 ml blood and centrifuged. After centrifugation, the tube was placed on the Autoprep machine for capturing CTC. CTC were then identified and enumerated using the CellTracks Analyzer. EpCAM antigen is specifically expressed on epithelial cells, which are not normally found in the blood of healthy individuals.4 Ferrofluid reagent containing magnetic particles coated with antibodies specific for the EpCAM antigen was used for initial capture of CTC. Further identification of captured cells was performed using immunofluorescently stained antibodies to cytokeratins 8, 18 and 19 conjugated with phycoerythrin (PE) for epithelial cell identification and the nuclear dye 4,6-diamidino-2-phenyindole (DAPI) to label the cell nucleus. Antibody specific for CD45 conjugated to allophycocyanin was used to identify leukocytes. Cells that were cytokeratin+/DAPI+/CD45- were considered positive for the CTC phenotype.
Statistical analysis
Patients were followed until December 31, 2014. To ascertain complete capture of all decedents, a proprietary information source (Accurint ®) was used to supplement death records in the medical records and the institutional registration file. Death from any cause was considered as an event in this analysis. Overall survival was estimated by the Kaplan Meier method and compared using the Log Rank test. The Cox proportional hazards model was used to identify factors affecting overall survival. Factors associated with overall survival in the univariate analysis at P<0.05 were included in the multivariate models. Given the significant association between CCA subtype and CTC positivity, CCA subtype (iCCA, pCCA or dCCA) was included in the multivariate model. Individual components of the UICC/AJCC TNM staging system (T, N, and M) were tested in the survival analysis.
The associations between detection of CTC, baseline clinical characteristics and overall survival were analyzed. Results were reported as the mean ± standard deviation or median and interquartile range for continuous variables and as percentages for categorical variables. Associations between CTC and clinical characteristics were tested by analysis of variance (ANOVA) or Kruskal-Wallis test for continuous variables and chi-square or Fisher’s exact test for categorical variables.
Statistical analyses were performed using JMP statistical software v.10 (SAS Institute, Cary, NC) and differences were considered statistically significant when the P<0.05. All P values presented are 2-sided.
Results
Clinical characteristics
The mean age of patients was 61 and 54 (61%) were male (Table 1). One or more CTC was detected in 25 (28%), two or more CTC in 15 (17%) and five or more CTC in 8 (9%) patients. There was no significant association of CTC with patient age, sex, or race. There were 41 patients with iCCA, 42 patients with pCCA, and 5 patients with dCCA. CTC ≥2 was associated with iCCA: 29% of iCCA patients had CTC ≥2, while 7% and 0% of patients with pCCA and dCCA had CTC ≥2, respectively (P=0.01). All patients with CCA occurring in the context of PSC (n= 17; 15 pCCA, 1 iCCA, and 1 dCCA) had CTC <2 (P<0.01). There was no association of CTC with cirrhosis. Baseline laboratory results (AST, ALT, bilirubin, and alkaline phosphatase) were comparable except that the mean total bilirubin level was higher in patients with CTC ≥5 compared to patients with CTC <5. The higher bilirubin levels found in patients with CTC ≥5 may be due to the larger tumor extent in these patients, which leads to biliary obstruction and cholestasis. Thus, larger CTC numbers appear to correlate with a larger tumor burden, which is associated with a higher likelihood of biliary obstruction.
Table 1.
Clinical Characteristics
| CTC ≥2 (N=15) | CTC <2 (N=73) | P | CTC ≥5 (N=8) | CTC <5 (N=80) | P | |
|---|---|---|---|---|---|---|
| Age, year, mean ± SD | 62 ± 13 | 60 ± 15 | 0.56 | 63 ± 17 | 60 ± 14 | 0.71 |
| Sex (Male), n (%) | 9 (60%) | 45 (62%) | 0.91 | 4 (50%) | 50 (63%) | 0.49 |
| Race (White), n (%) | 12 (86%) | 68 (93%) | 0.38 | 5 (71%) | 75 (94%) | 0.10 |
| Tumor type | 0.01 | 0.22 | ||||
| iCCA, n (%) | 12 (80%) | 29 (40%) | 6 (75%) | 35 (44%) | ||
| pCCA, n (%) | 3 (20%) | 39 (53%) | 2 (25%) | 40 (50%) | ||
| dCCA, n (%) | 0 (0%) | 5 (7%) | 0 (0%) | 5 (6%) | ||
| CCA with PSC | 0 (0%) | 17 (23%) | <0.01 | 0 (0%) | 17 (21%) | 0.06 |
| Intrahepatic | - | 1 (6%) | - | 1 (6%) | ||
| Perihilar | - | 15 (88%) | - | 15 (88%) | ||
| Distal | - | 1 (6%) | - | 1 (6%) | ||
| Cirrhosis | 1 (7%) | 8 (11%) | 0.65 | 0 (0%) | 9 (11%) | 0.18 |
| AST, U/L, median [IQR] | 76 [43–130] | 60 [38–84] | 0.17 | 75 [37–167] | 61 [39–89] | 0.69 |
| ALT, U/L, median [IQR] | 48 [24–110] | 57 [31–111] | 0.70 | 69 [23–133] | 56 [30–107] | 0.55 |
| ALP, U/L, median [IQR] | 337 [130–424] | 241 [143–400] | 0.95 | 354 [160–580] | 239 [137–390] | 0.73 |
| Total Bilirubin, mg/dL, median [IQR] | 1.1 [0.4–2.6] | 1.2 [0.6–4.8] | 0.69 | 1.9 [0.8–2.9] | 1.1 [0.6–3.7] | <0.01 |
| Median Survival, months | 4.8 | 26.9 | <0.01 | 4.8 | 19.6 | <0.01 |
| Death, n (%) | 11 (73%) | 30 (41%) | 0.02 | 6 (75%) | 35 (44%) | 0.09 |
SD: Standard deviation
IQR: Interquartile range
iCCA: intrahepatic cholangiocarcinoma; pCCA: perihilar cholangiocarcinoma; dCCA: distal cholangiocarcinoma
CTC and tumor characteristics and treatment
CTC were associated with CCA tumor extent (Table 2). Patients with CTC ≥2 had larger mean tumor size than patients with CTC<2 (9.8 vs. 4.6 cm, P<0.01). Higher proportions of patients with CTC ≥2 had multinodular disease (73 vs. 30%, P<0.01), bilobar disease (80 vs. 38%, P<0.01), loco-regional lymph node invasion (73 vs. 36%, P<0.01), and distant extrahepatic metastasis (47 vs. 16%, P=0.02). There was a trend towards association of CTC ≥2 or ≥5 with the AJCC/UICC TNM staging system. Median CA19-9 was significantly higher in patients with CTC ≥2 than in patients with CTC <2 (579 vs. 112 U/mL, P=0.04). Similar results were noted when comparisons were made between patients with CTC ≥5 and CTC <5.
Table 2.
Tumor Characteristics and Treatment
| CTC ≥2 (N=15) | CTC <2 (N=73) | P | CTC ≥5 (N=8) | CTC <5 (N=80) | P | |
|---|---|---|---|---|---|---|
| Largest tumor size, cm, mean ± SD | 9.8 ± 4.5 | 4.6 ± 3.9 | <0.01 | 10 ± 5.0 | 4.7 ± 4.1 | 0.02 |
| Multinodular tumor, n (%) | 11 (73%) | 22 (30%) | <0.01 | 6 (75%) | 27 (34%) | 0.02 |
| Bilobar disease, n (%) | 12 (80%) | 28 (38%) | <0.01 | 7 (88%) | 33 (41%) | <0.01 |
| AJCC/UICC T staging | 2 [2–2] | 2 [1–3] | 0.63 | 2 [2–3] | 2 [1–3] | 0.80 |
| Loco-regional lymph node invasion, n (%) | 11 (73%) | 26 (36%) | <0.01 | 5 (63%) | 32 (40%) | 0.22 |
| Distant extrahepatic metastasis, n (%) | 7 (47%) | 12 (16%) | 0.02 | 4 (50%) | 15 (19%) | 0.06 |
| AJCC/UICC TNM staging | 0.13 | 0.11 | ||||
| 1–2 | 3 (20%) | 29 (40%) | 1 (13%) | 31 (39%) | ||
| 3–4 | 12 (80%) | 44 (60%) | 7 (87%) | 49 (61%) | ||
| CA19-9 U/mL, median [IQR] | 579 [49–5336] | 112 [39–562] | 0.04 | 3510 [835–8667] | 112 [37–564] | <0.01 |
| CA 19-9>100 U/mL, n (%) | 11 (73%) | 39 (56%) | 0.20 | 7 (88%) | 43 (56%) | 0.06 |
| Treatment | 0.07 | 0.04 | ||||
| Surgical resection/OLT | 1 (7%) | 25 (34%) | 0 (0%) | 26 (33%) | ||
| * Locoregional treatment | 3 (20%) | 16 (22%) | 1 (12%) | 18 (22%) | ||
| Systemic chemotherapy | 8 (53%) | 27 (37%) | 5 (63%) | 30 (38%) | ||
| Best supportive care | 3 (20%) | 5 (7%) | 2 (25%) | 6 (7%) |
SD: Standard deviation
IQR: Interquartile range
OLT: orthotopic liver transplantation
AJCC/UICC; American Joint Committee on Cancer/Union for International Cancer Control
Locoregional treatment: TARE (n=9), TACE (n=2), and external beam radiation (n=8)
The sensitivity of CTC ≥1 in detecting metastatic disease was 53%. The sensitivity decreased to 37% and 21% with cutoffs of CTC ≥2, and CTC ≥5, respectively, suggesting that CTC alone may not be a highly-sensitive biomarker for detecting metastatic disease.
Among patients with CTC <2, 25 (34%) patients underwent surgical resection (n=15) or OLT (n=10) while only 5 (7%) patients were treated with best supportive care. On the other hand, only one (7%) patient with CTC ≥2 underwent surgical resection and no patients with CTC ≥2 received OLT. Three (20%) patients with CTC ≥2 were treated with best supportive care. Similar results were noted when comparisons were made between patients with CTC ≥5 and CTC <5 (Table 2).
Factors associated with overall survival
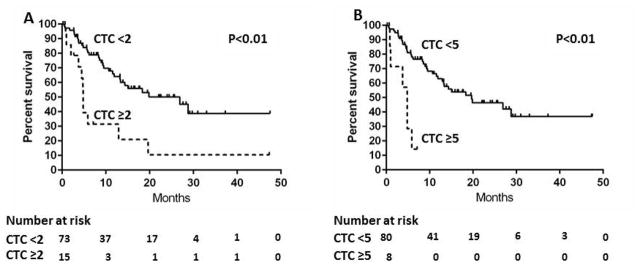
The median follow up was 18 months and 41 of the 88 (46.6%) patients died (Table 1). The median survivals were 5 and 27 months in patients with CTC ≥2 and CTC <2, respectively (P<0.01) (Figure 1A). The presence of CTC ≥2 was associated with poorer survival in univariate analysis (Hazard Ratio [HR] 3.1; 95% confidence interval [CI] 1.5–6.1; P<0.01) (Table 3). The median survivals were 5 and 20 months in patients with CTC ≥5 and CTC <5, respectively (P<0.01) (Figure 1B). The presence of CTC ≥5 was associated with poorer survival in univariate analysis (HR 5.4; 95%CI 1.9–13.0; P<0.01). (Table 3) Demographic features including age per 10 years (HR 1.3; 95%CI 1.0–1.6; P<0.01) and male sex (HR 2.1; 95%CI 1.1–4.5; P=0.02) were associated with poorer overall survival. Other tumor related factors, including multinodular disease (HR 2.1; 95%CI 1.1–3.8; P=0.02), bilobar disease (HR 2.4; 95%CI 1.3–4.6; P<0.01), loco-regional lymph node invasion (HR 2.9; 95%CI 1.5–5.6; P<0.01), CA-19-9 >100 U/mL (HR 3.7; 95%CI 1.8–8.2; P<0.01) and distant extrahepatic metastasis (HR 6.0; 95% CI 2.9–12.6; P<0.01) were also significantly associated with poor overall survival. There was a trend towards association of AJCC/UICC T staging with shorter overall survival (HR 1.4; 95%CI 1.0–1.9; P=0.07). Tumor subtype (iCCA vs. pCCA or dCCA) was not significantly associated with overall survival. Further, neither PSC nor cirrhosis was significantly associated with overall survival by univariate analysis. In multivariate analysis, the independent predictors of overall survival were distant extrahepatic metastasis (HR 6.4; 95%CI 2.8–14.5; P<0.01), CA 19-9 (HR 2.8; 95%CI 1.3–6.4; P<0.01), CTC ≥2 (HR 2.5; 95%CI 1.1–5.4; P=0.02), and age (HR 1.4; 95%CI 1.1–1.7; P<0.01) (Model 1). When CTC ≥5 was substituted for CTC ≥2 in the multivariate models, CTC ≥5 was a stronger independent predictor of overall survival (HR 4.1; 95%CI 1.4–10.8; P=0.01) (Model 2).
Figure 1.
Kaplan-Meier overall survival estimates according to CTC in patients with CCA
A: CTC ≥2 vs. CTC <2
B: CTC ≥5 vs. CTC <5
Table 3.
Factors affecting overall survival in patients with CCA
| Univariate | Multivariate Model 1 (CTC ≥2) | Multivariate Model 2 (CTC ≥5) | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| CTC ≥2 | 3.1 (1.5–6.1) | <0.01 | 2.5 (1.1–5.4) | 0.02 | ||
| CTC ≥5 | 5.4 (1.9–13.0) | <0.01 | 4.1 (1.4–10.8) | 0.01 | ||
| Age (10 year) | 1.3 (1.0–1.6) | <0.01 | 1.4 (1.1–1.7) | <0.01 | 1.4 (1.1–1.7) | <0.01 |
| AJCC/UICC T staging | 1.4 (1.0–1.9) | 0.07 | ||||
| Lymph node invasion | 2.9 (1.5–5.6) | <0.01 | - | - | ||
| Distant Metastasis | 6.0 (2.9–12.6) | <0.01 | 6.4 (2.8–14.5) | <0.01 | 7.4 (3.2–16.8) | <0.01 |
| Bilobar disease | 2.4 (1.3–4.6) | <0.01 | ||||
| Largest tumor size | 1.1 (1.0–1.1) | 0.14 | ||||
| Multinodular tumor | 2.1 (1.1–3.8) | 0.02 | ||||
| CA19-9 >100 U/mL | 3.7 (1.8–8.2) | <0.01 | 2.8 (1.3–6.4) | <0.01 | 2.7 (1.2–6.1) | 0.01 |
| Sex (male) | 2.1 (1.1–4.5) | 0.02 | ||||
| Race (White) | 0.4 (0.2–1.4) | 0.13 | ||||
| Tumor type | 0.45 | 0.84 | 0.99 | |||
| iCCA (ref) | - | - | ||||
| pCCA/dCCA | 0.8 (0.4–1.3) | 1.1 (0.6–2.1) | 1.0 (0.5–1.9) | |||
| PSC | 0.6 (0.2–1.4) | 0.25 | ||||
| Cirrhosis | 0.7 (0.2–1.8) | 0.52 | ||||
Model 1 includes CTC ≥2, Model 2 includes CTC ≥5 as a predictor of overall survival
iCCA: intrahepatic cholangiocarcinoma; pCCA: perihilar cholangiocarcinoma; dCCA: distal cholangiocarcinoma AJCC/UICC; American Joint Committee on Cancer/Union for International Cancer Control
Subgroup analyses
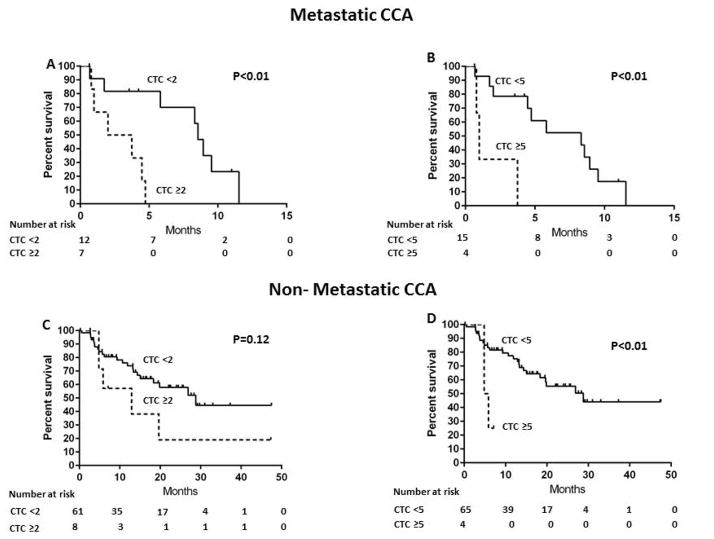
The associations between CTC and overall survival were examined in subgroups of patients with or without extrahepatic metastasis. Among patients with metastasis, the median survivals were 2 and 8 months in patients with CTC ≥2 and CTC <2, respectively (P<0.01) (Figure 2A). CTC ≥2 was associated with shorter overall survival compared to CTC <2 (HR 8.2; 95%CI 1.8–57.5; P<0.01). Similarly, the median survivals were 1 and 8 months in patients with CTC ≥5 and CTC <5, respectively (P<0.01) (Figure 2B) and CTC ≥5 was associated with shorter overall survival compared to CTC <5 (HR 7.7; 95%CI 1.4–42.9; P=0.02). Among patients without metastasis, the median survivals were 13 and 28 months in patients with CTC ≥2 and CTC <2, respectively (P=0.12) (Figure 2C); patients with CTC ≥2 had shorter survival, but the difference did not reach statistical significance, most likely due to the small number of patients without metastatic disease who had CTC (HR 2.1; 95%CI 0.7–5.2; P=0.16). The median survivals were 5 and 29 months in patients with CTC ≥5 and CTC <5, respectively (P=0.02) (Figure 2D); there was a trend towards association of CTC ≥5 with shorter overall survival compared to CTC <5 (HR 4.3; 95%CI 1.0–13.8; P=0.06)
Figure 2.
Kaplan-Meier overall survival estimates according to CTC in patients with metastatic/non-metastatic CCA
A: CTC ≥2 vs. CTC <2, Metastatic CCA
B: CTC ≥5 vs. CTC <5, Metastatic CCA
C: CTC ≥2 vs. CTC <2, Non-metastatic CCA
D: CTC ≥5 vs. CTC <5, Non-metastatic CCA
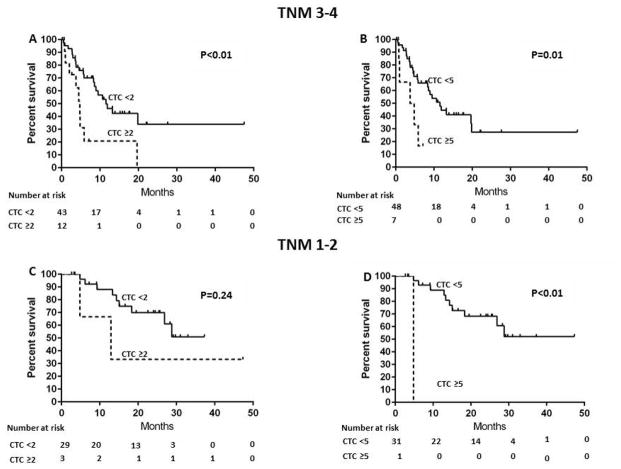
Similar results were obtained when the analyses were performed in the subgroups of patients with early stage (AJCC/UICC TNM stage 1–2) or advanced stage (AJCC/UICC TNM stage 3–4) CCA. Among patients with AJCC/UICC TNM stage 3–4, the median survivals were 5 and 12 months in patients with CTC ≥2 and CTC <2, respectively (P<0.01) (Figure 3A). CTC ≥2 was associated with shorter overall survival compared to CTC <2 (HR 3.0; 95%CI 1.3–6.6; P=0.01). Similarly, the median survivals were 4 and 12 months in patients with CTC ≥5 and CTC <5, respectively (P=0.01) (Figure 3B) and CTC ≥5 was associated with shorter overall survival compared to CTC <5 (HR 3.3; 95% CI 1.1–8.6; P=0.04). Among patients with AJCC/UICC TNM stage 1–2, the median survivals were >37 and 13 months in patients with CTC ≥2 and CTC <2, respectively (HR 2.5; 95%CI 0.4–9.7; p=0.30)(Figure 3C). The median survivals were 5 and >47 months in patients with CTC ≥5 and CTC <5, respectively (P<0.01) (Figure 3D); there was an association of CTC 5 with shorter overall survival compared to CTC <5 (HR 28; 95%CI 1.1–707.4; P=0.04)
Figure 3.
Kaplan-Meier overall survival estimates according to CTC in patients with AJCC/UICC TNM stage of 1–2/3–4 CCA
A: CTC ≥2 vs. CTC <2, TNM stage of 3–4 CCA
B: CTC ≥5 vs. CTC <5, TNM stage of 3–4 CCA
C: CTC ≥2 vs. CTC <2, TNM stage of 1–2 CCA
D: CTC ≥5 vs. CTC <5, TNM stage of 1–2 CCA
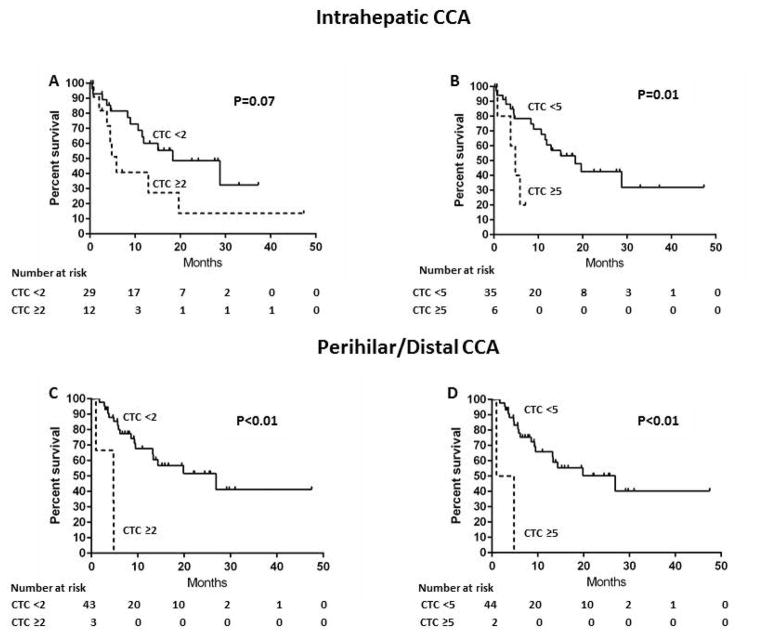
Lastly, the association between CTC and overall survival was examined in the subgroups of patients with iCCA (n=41) vs. pCCA/dCCA (n=47). Among patients with intrahepatic CCA, the median survivals were 6 and 18 months in patients with CTC ≥2 and CTC <2, respectively (P=0.07) (Figure 4A). There was a trend towards an association of CTC ≥2 with shorter overall survival compared to CTC<2 (HR 2.3; 95%CI, 0.9–5.4; P=0.09). The median survivals were 5 and 18 months in patients with CTC ≥5 and CTC <5, respectively (P=0.01) (Figure 4B). There was an association of CTC ≥5 with shorter overall survival compared to CTC <5 (HR 4.2; 95%CI 1.1–14.1; P=0.04). Among patients with pCCA/dCCA, the median survivals were 5 and 27 months in patients with CTC 2 and CTC <2, respectively (P<0.01) (Figure 4C), and CTC 2 was associated with shorter overall survival compared to CTC <2 (HR 10.5; 95%CI 2.2–40.1; P<0.01). In this group, the median survivals were 3 and 27 months in patients with CTC ≥5 and CTC <5, respectively (P<0.01) (Figure 4D). CTC ≥5 was also associated with shorter overall survival compared to CTC <5 (HR 10.2; 95%CI 1.5–42.3; P=0.02).
Figure 4.
Kaplan-Meier overall survival estimates according to CTC in patients with intrahepatic/perihilar-distal CCA
A: CTC ≥2 vs. CTC <2, Intrahepatic CCA
B: CTC ≥5 vs. CTC <5, Intrahepatic CCA
C: CTC ≥2 vs. CTC <2, Perihilar-distal CCA
D: CTC ≥5 vs. CTC <5, Perihilar-distal CCA
Clinical characteristics of patients with elevated CTC
The clinical characteristics of patients with CTC ≥2 are described in Table 4. CTC values ranged from 2 to 34. About half (53%) of patients with CTC ≥2 received treatment with chemotherapy, 20% received transarterial radioembolization, and 20% received best supportive care.
Table 4.
Patients with CTC ≥2 per 7.5 mL blood
| Case | # of CTC in 7.5 mL blood | Type | Age | Size (cm) | Bilobar | Multi nodular | N | M | Treatment | Survival status at last follow up | Survival duration (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | iCCA | 79 | 13 | + | − | − | − | Chemotherapy | Dead | 13 |
| 2 | 2 | pCCA | 60 | 1 | − | − | + | + | Chemotherapy | Dead | 5 |
| 3 | 3 | iCCA | 56 | 10 | + | + | + | − | TARE + Chemotherapy | Dead | 20 |
| 4 | 3 | iCCA | 63 | 10 | + | + | + | + | Best Supportive Care | Dead | 2 |
| 5 | 3 | iCCA | 55 | 8 | − | + | − | − | Surgical Resection | Alive | 47 |
| 6 | 3 | iCCA | 63 | 15 | + | + | + | − | TARE | Alive | 3 |
| 7 | 4 | iCCA | 60 | 10 | + | + | + | + | Chemotherapy | Dead | 4 |
| 8 | 5 | pCCA | 75 | 13 | + | + | − | − | Best Supportive Care | Dead | 5 |
| 9 | 8 | iCCA | 65 | 12 | + | + | + | − | Chemotherapy | Dead | 5 |
| 10 | 8 | pCCA | 75 | 4 | + | + | − | + | Chemotherapy | Dead | 1 |
| 11 | 8 | iCCA | 75 | 3 | + | + | + | + | Best Supportive Care | Dead | 1 |
| 12 | 12 | iCCA | 32 | 6 | − | − | + | + | Chemotherapy | Dead | 4 |
| 13 | 25 | iCCA | 61 | 12 | + | − | + | − | TARE | Alive | 7 |
| 14 | 25 | iCCA | 40 | 17 | + | + | + | + | Chemotherapy | Alive | 1 |
| 15 | 34 | iCCA | 76 | 14 | + | + | − | − | Chemotherapy | Dead | 6 |
iCCA: intrahepatic cholangiocarcinoma; pCCA: perihilar cholangiocarcinoma
N: nodal metastases; M: distant extrahepatic metastases
TARE: transarterial radioembolization
A few illustrative examples highlight the association of CTC with more aggressive disease. One subject (#5) was treated by surgical resection. Three CTC were detected in her blood one week prior to surgery. At surgery, she did not have any lymph node or extrahepatic metastasis and the surgical margin was negative for tumor, but microscopic venous invasion was noted. She was staged as T2N0M0 and started on adjuvant chemotherapy with gemcitabine and cisplatin. She developed recurrent metastatic disease in the liver and lungs 8 months after the surgery. Two patients (#1 and #2) who both had two CTC detected preoperatively were originally thought to have resectable disease, but were found to have unresectable and/or metastatic tumors at surgery and offered chemotherapy.
Discussion
In this single referral center based prospective study, we found CTC ≥2 in 15 (17%) and CTC ≥5 in 8 (9%) patients with CCA. CTC ≥2 or 5 were associated with more extensive tumor burden, represented by larger tumor size, multinodular disease, bilobar disease, lymph node involvement, and metastatic disease. The median survival was significantly shorter in patients with CTC ≥2 or 5. CTC ≥2 or 5 were associated with poorer survival in univariate and multivariate analysis: Patients with CTC ≥2 had a 2.5 fold-increased risk of earlier death compared to patients with CTC <2 (HR 2.5; 95%CI 1.1–5.4; P=0.02). Similarly, patients with CTC ≥5 had a 4.1 fold-increased risk of earlier death compared to patients with CTC <5 (HR 4.1; 95%CI 1.4–10.8; P=0.01). Additional analysis showed that CTC was associated with a much higher risk for death in the subgroups of patients with pCCA/dCCA (HR 10.5; 95%CI 2.2–40.1; P<0.01 for CTC ≥2 and HR 10.2; 95%CI 1.5–42.3; P=0.02 for CTC ≥5), advanced stage CCA (AJCC/UICC TNM stage of 3–4) (HR 3.0; 95%CI 1.3–6.6; P=0.01 for CTC ≥2, and HR 3.3; 95%CI 1.1–8.6; P=0.04 for CTC ≥5), and metastatic CCA (HR 8.2; 95% CI 1.8–57.5; P<0.01 for CTC ≥2 and HR 7.7; 95% CI 1.4–42.9; P=0.02 for CTC ≥5).
In a large study of blood samples from 344 healthy and nonmalignant disease subjects and 964 metastatic cancer subjects, less than 1% of the subjects without cancer (n=1) had a positive CTC ≥2.4 This was the rationale for setting the cut off at 2 or more CTC in the current study. Various thresholds have been used in studies examining the predictive performance of CTC in metastatic breast and metastatic prostate cancer (≥5 CTC/7.5mL blood)6, 8, 11 versus metastatic colorectal cancer (≥3 CTC/7.5 mL blood).9 There is also a precedent for the use of CTC thresholds of ≥1 or ≥2 in studies of nonmetastatic breast cancer and bladder cancer,5, 7, 10 although it is notable that at least one study7 collected three 8–10 mL tubes of peripheral blood in order to detect these rare cells. In order to compare the prognostic performance of CTC at different CTC cutoffs, we elected to show results with cutoffs of both CTC ≥5 and CTC ≥2.
CTC detection rates in previous studies were highest in patients with prostate cancer (57%) followed by breast (37%), ovary (37%), colon (30%), and lung (20%).4 The detection rate of CTC also appears to correlate with the frequency and intensity of EpCAM expression in individual tumors.15 Previous studies have investigated the expression of EpCAM in CCA tissues.12 More than 90% of CCAs showed positive staining for EpCAM, while the surrounding normal hepatocytes had negative staining. This parallels the situation in breast cancer and prostate cancer and supports the use of EpCAM as a target for the detection of CTC in patients with CCA. Despite the expected correlation between EpCAM expression in the tumor tissues and the ability to isolate related CTC in the peripheral blood, however, it is understood that CTC vary in the degree of EpCAM expression such that some cells may be missed by the CellSearch technology. Toward that end, other methodologies that do not rely on EpCAM for capture are available and will be explored, including the AccuCyte Enrichment Technology (RareCyte; Seattle, WA), which achieves isolation by density gradient centrifugation.16
The prognostic significance of CTC has been extensively described for breast cancer. A landmark study on CTC measured before and after a new line of treatment in 177 metastatic breast cancer patients showed that CTC ≥5 per 7.5 mL blood at baseline was associated with poor progression-free survival (2.7 vs. 7.0 months; P<0.01) and overall survival (10.1 vs. >18.0 months; P <0.01).6 CTC enumeration has also been shown to have prognostic utility in patients with nonmetastatic breast cancer.5 A large German study with 2,026 patients with early breast cancer showed that CTC ≥1 is an independent predictor of poor disease-free survival, breast cancer-specific survival, and overall survival; the worst prognosis was found in patients with CTC ≥5.7 Similar results have been shown for metastatic colon, prostate and other cancers.9,4, 11
The prognostic significance of CTC has also been evaluated in hepatocellular carcinoma (HCC). One study of 59 HCC patients showed that one or more CTC were detectable in 31% of patients.17 There was a strong correlation between CTC ≥1 and tumor stage. In addition, CTC ≥1 was associated with shorter overall survival in univariate analysis (P=0.02). Another study of 123 HCC patients undergoing surgical resection showed that 41% had CTC ≥2 preoperatively.18 Preoperative CTC ≥2 predicted the risk of recurrent disease (HR 5.2; 95%CI 2.7–10.2; P<0.01). Among patients who had preoperative CTC ≥2, patients who had persistent CTC ≥2 after surgery had a higher risk of recurrent disease compared to patients who had postoperative CTC <2 (P<0.01).
A previous pilot study has also shown that CTC are detectable in patients with CCA.13 Due to the small sample size in that study, the effect of CTC on overall survival in patients with CCA could not be addressed. Our study has now confirmed that CTC are detectable in patients with CCA and shown that CTC is an independent predictor of survival in patients with CCA.
Our study has several limitations. This is a single referral center study, hence the results of the current study may not be generalizable to all patients with CCA. The modest sample size also limited the robustness of our statistical analysis. For example, there were trends that patients with CTC ≥2 had poor overall survival in the subgroups of patients without metastatic disease and intrahepatic CCA without statistical significance. In addition, multivariate analysis was not performed in the subgroup analysis due to the limited sample size. Nonetheless, this study determined the prognostic value of CTC in the largest number of patients with CCA examined thus far. Inclusion of a heterogenous group of patients in terms of CCA subtype classification (iCCA, pCCA, dCCA), tumor extent (metastatic vs. non-metastatic), and treatment (curative surgical vs. non-curative palliative treatment vs. best supportive care) are both a strength and a limitation of the study. The heterogeneity allowed us to assess the influence of a variety of patient and tumor characteristics on outcomes. On the other hand, it will be important to perform additional validation studies focused on the specific subtypes of patients, including iCCA, pCCA and dCCA and also to validate our initial observations of possible correlations of CTC with poor overall survival in different clinical subgroups of CCA patients. Patients in our study did not have serial blood draws to determine trends in CTC titers, thus our study was not able to demonstrate whether trends in CTC are predictive of overall survival.
It is currently unknown whether patients with PSC have circulating epithelial non-tumoral cells, which may lead to false positive results in the CellSearch System. It is reassuring that none of the 17 patients with PSC-related CCA in our study had CTC ≥2; further, fourteen of the 17 patients with PSC-related CCA had no detectable CTC. This suggests that PSC per se is not associated with high false positive rates due to the presence of non-tumoral epithelial cells in the blood, making false positive detection of CTC in patients with PSC less likely. Before implementing this technique in routine practice, particularly in patients with PSC, more rigorous investigation of the specificity of the test is required.
Lastly, CTC detection by Veridex depends on EpCAM expression on the cell surface, thus the number of CTC identified by this study could be an underestimate. Future studies should address other complementary, label-free technologies for CTC enumeration and molecular analysis, as well as explore the use of a cell-free DNA liquid biopsy based approach to potentially improve sensitivity and specificity for prognosis, treatment monitoring and disease stratification in CCA.
CTC enumeration requires real time analyses on prospectively collected blood specimens. With the number of patients enrolled in the current study, we were able to demonstrate that CTC is an independent predictor of survival after adjusting for other known predictors of survival, including extrahepatic metastasis and age, and CA-19-9.19 The results of the current study may have clinical relevance. CTC could be useful in patients with metastatic disease to determine who should receive palliative chemotherapy vs. best supportive care: for example, in CCA patients with distant metastases, the median survival of patients with CTC ≥2 or ≥5 were only 2 and 1 month, respectively. In addition, CTC may serve as a predictor of recurrent disease in patients who underwent curative surgical resection or as a predictor of unresectable disease in the preoperative setting.
In summary, CTC was found to be associated with tumor extent and was shown to be an independent predictor of overall survival in patients with CCA. This study suggests that CTC can serve as a valuable tumor biomarker in CCA. Validation of these finding, including the prognostic role of CTC in specific subgroups of patients seen in different clinical settings: metastatic vs. non-metastatic CCA, iCCA vs. pCCA vs. dCCA; or curative surgery/OLT vs. palliative loco-regional treatment or chemotherapy, defining the best cutoff of CTC and the role of serial CTC trends in predicting overall and/or progression free survival should be further investigated.
Acknowledgments
Fundings: This publication was supported by Grant Number T32 DK07198 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (to JY) and CA100882, CA128633, and CA165076 from the National Cancer Institute (NCI) and The Cholangiocarcinoma Foundation (to LRR). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviation
- CCA
cholangiocarcinoma
- CI
confidence interval
- CTC
circulating tumor cells
- dCCA
distal cholangiocarcinoma
- EpCAM
epithelial cell adhesion molecule
- HCC
hepatocellular carcinoma
- HR
Hazard ratio
- iCCA
intrahepatic cholangiocarcinoma
- OLT
orthotopic liver transplantation
- pCCA
perihilar cholangiocarcinoma
Footnotes
Conflict of Interest:
Nothing to disclose except that Minetta C. Liu receives research funding from Veridex/Janssen; all funds are provided to the institution with no personal compensation.
References
- 1.Yang JD, Kim B, Sanderson SO, Sauver JS, Yawn BP, Larson JJ, Therneau TM, Roberts LR, Gores GJ, Kim WR. Biliary tract cancers in Olmsted County, Minnesota, 1976–2008. The American journal of gastroenterology. 2012;107:1256–62. doi: 10.1038/ajg.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:14608–13. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alunni-Fabbroni M, Sandri MT. Circulating tumour cells in clinical practice: Methods of detection and possible characterization. Methods. 2010;50:289–97. doi: 10.1016/j.ymeth.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 4.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:6897–904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 5.Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, Bedrosian I, Kuerer HM, Krishnamurthy S. Circulating tumour cells in non-metastatic breast cancer: a prospective study. The Lancet Oncology. 2012;13:688–95. doi: 10.1016/S1470-2045(12)70209-7. [DOI] [PubMed] [Google Scholar]
- 6.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. The New England journal of medicine. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 7.Rack B, Schindlbeck C, Juckstock J, Andergassen U, Hepp P, Zwingers T, Friedl TW, Lorenz R, Tesch H, Fasching PA, Fehm T, Schneeweiss A, Lichtenegger W, Beckmann MW, Friese K, Pantel K, Janni W. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. Journal of the National Cancer Institute. 2014:106. doi: 10.1093/jnci/dju066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu MC, Shields PG, Warren RD, Cohen P, Wilkinson M, Ottaviano YL, Rao SB, Eng-Wong J, Seillier-Moiseiwitsch F, Noone AM, Isaacs C. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:5153–9. doi: 10.1200/JCO.2008.20.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3213–21. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 10.Rink M, Chun FK, Minner S, Friedrich M, Mauermann O, Heinzer H, Huland H, Fisch M, Pantel K, Riethdorf S. Detection of circulating tumour cells in peripheral blood of patients with advanced non-metastatic bladder cancer. BJU international. 2011;107:1668–75. doi: 10.1111/j.1464-410X.2010.09562.x. [DOI] [PubMed] [Google Scholar]
- 11.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 12.de Boer CJ, van Krieken JH, Janssen-van Rhijn CM, Litvinov SV. Expression of Ep-CAM in normal, regenerating, metaplastic, and neoplastic liver. The Journal of pathology. 1999;188:201–6. doi: 10.1002/(SICI)1096-9896(199906)188:2<201::AID-PATH339>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Al Ustwani O, Iancu D, Yacoub R, Iyer R. Detection of circulating tumor cells in cancers of biliary origin. Journal of gastrointestinal oncology. 2012;3:97–104. doi: 10.3978/j.issn.2078-6891.2011.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.AJCC Cancer Staging Manual. 7. Springer; 2010. [Google Scholar]
- 15.Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, Dirnhofer S. Frequent EpCam protein expression in human carcinomas. Human pathology. 2004;35:122–8. doi: 10.1016/j.humpath.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Campton DE, Ramirez AB, Nordberg JJ, Drovetto N, Clein AC, Varshavskaya P, Friemel BH, Quarre S, Breman A, Dorschner M, Blau S, Blau CA, Sabath DE, Stilwell JL, Kaldjian EP. High-recovery visual identification and single-cell retrieval of circulating tumor cells for genomic analysis using a dual-technology platform integrated with automated immunofluorescence staining. BMC cancer. 2015;15:360. doi: 10.1186/s12885-015-1383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulze K, Gasch C, Staufer K, Nashan B, Lohse AW, Pantel K, Riethdorf S, Wege H. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. International journal of cancer Journal international du cancer. 2013;133:2165–71. doi: 10.1002/ijc.28230. [DOI] [PubMed] [Google Scholar]
- 18.Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu SJ, Shi RY, Hu B, Zhou J, Fan J. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013;57:1458–68. doi: 10.1002/hep.26151. [DOI] [PubMed] [Google Scholar]
- 19.Chaiteerakij R, Harmsen WS, Marrero CR, Aboelsoud MM, Ndzengue A, Kaiya J, Therneau TM, Sanchez W, Gores GJ, Roberts LR. A new clinically based staging system for perihilar cholangiocarcinoma. The American journal of gastroenterology. 2014;109:1881–90. doi: 10.1038/ajg.2014.327. [DOI] [PMC free article] [PubMed] [Google Scholar]