Abstract
Sulfiredoxin (SRXN1/Srx) is a multifunction enzyme with a primary antioxidant role of reducing the overoxidized inactive form of peroxiredoxins (Prxs). The function and mechanisms of Srx in cancer development are not well understood. Here, Srx is preferentially expressed in human colorectal cancer (CRC) cells but not in normal colon epithelial cells. Loss-of-function studies demonstrate that knockdown of Srx in poorly differentiated CRC cells not only leads to the inhibition of colony formation and cell invasion in vitro, but also reduces tumor xenograft growth and represses metastasis to distal organs in a mouse orthotopic implantation model. Notably, exactly opposite effects were observed in gain-of-function experiments when Srx was ectopically expressed in well-differentiated CRC cells. Mechanistically, expression of Srx enhances the activation of mitogen activated protein kinase (MAPK) signaling through increasing the C-terminal tyrosine phosphorylation levels of epidermal growth factor receptor (EGFR). This function of Srx is mediated through its inhibition of EGFR acetylation at K1037, a novel post-translational modification of EGFR in human CRC cells identified by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) proteomic analysis. Furthermore, abolishment of K1037 acetylation in human CRC cells by site-specific mutagenesis leads to sustained activation of EGFR-MAPK signaling. Combined, these data reveal that Srx promotes CRC cell invasion and metastasis through a novel mechanism of enhancing EGFR signaling.
Implications
Sulfiredoxin is a critical oncogenic protein that can be used as a molecular target to develop therapeutics for patients with metastatic colorectal cancer.
Keywords: Sulfiredoxin, Antioxidant, Tumor Invasion and Metastasis, Cell Signaling, Molecular Therapeutics
Introduction
Sulfiredoxin (Srx), or neoplastic progression 3, was initially identified as a preferentially expressed gene of unknown function in transformation sensitive mouse epithelial JB6P+ cells (1). It is now well documented that the primary function of Srx is to restore and repair hyperoxidized peroxiredoxins (Prxs) (2, 3), in particular, Prx I ~ IV. Mechanistically, Srx reduces the sulfinic acid form of overoxidized Prxs back to active peroxidases through hydrolysis of ATP, leading to the formation of intermediates including a phosphoryl sulfinyl anhydride and a covalent thiosulfinate (4–7). With this repairing mechanism, expression of Srx significantly increases cells’ capability of surviving through oxidative stress induced cell death. In addition, Srx shares significant sequence and structural similarity with a bacterial DNA-binding protein, Par B, and thus it may function as a nuclease that utilizes the single or double-stranded DNAs as substrates (8). Other function of Srx has also been reported, including the removal of glutathionylated moieties that are found in protein phosphatase and Prx II (9, 10), maintenance of adrenal corticosterone production (11), and protection of neurons against chemical induced toxicity (12). Compared with those well-understood biochemical function, the significance of Srx in the pathogenesis of human disease, including cancer, has not been fully explored.
Colorectal cancer (CRC) is the third most common cancer with more than a million new patients diagnosed each year worldwide, and it is the second leading cause of cancer death in both men and women (13). The 5-year survival rate of stage IV CRC patient is only 6% according to a recent report from World Health Organization. One of the major causes of high mortality in CRC patients attributes to cancer metastasis to distant organs including liver and lung. The epidermal growth factor receptor (EGFR) signaling pathway plays an essential role in cell transformation, tumor growth and progression of many types of cancer including CRC (14). Binding of ligands such as EGF leads to the autophosphorylation of EGFR c-terminal tyrosine residues, which collectively serve as docking sites that bind to intracellular adaptor proteins and thus activates downstream RAS-RAF-MAPK kinase cascade (15). Interestingly, abnormal activation of EGFR signaling in CRC is mainly due to increased copy number of the EGFR gene, and mutations of EGFR are rarely found in CRC (16). Monoclonal antibodies such as cetuximab and panitumumab against the extracellular domain of EGFR have been used in clinic to treat metastatic CRC with promising response. However, a significant portion of patients does not respond to anti-EGFR therapy (17, 18). Therefore, further understanding of EGFR signaling in CRC pathogenesis is of critical value for targeted therapy.
In our previous studies, we’ve demonstrated that Srx is preferentially expressed in tumor specimens of CRC patients and its levels are positively correlated with patients’ clinic stages, suggesting that Srx may have an oncogenic role in CRC development (19). We’ve also demonstrated that genomic depletion of Srx renders mice resistant to azoxymethane and dextran sulfate sodium induced colon carcinogenesis (19). In this study our goal is to determine the functional significance of Srx in human colon cancer cell invasion and metastasis and to understand molecular mechanisms by which Srx contributes to CRC pathogenesis. We report here that Srx promotes CRC cell invasion and metastasis through a novel mechanism of enhancing EGFR signaling.
Materials and Methods
Cell lines, plasmids, antibodies and chemicals
HEK293 was obtained from ATCC. Human colon normal cell line NCM460 was obtained from Incell Corporation (San Antonio, TX). Authenticated human colon cancer cell lines including SW640, RKO, HT29, HCT116 and Geo were obtained from the Cancer Cell Line Repository at Frederick National Laboratory for Cancer Research. All experiments were performed using cells within 10 passages from the original source. MISSION® ShRNA pLKO.1 based ShRNAs (Sigma-Aldrich, St. Louis, MO), including an empty vector control (ShV), a non-target ShRNA control (ShNT) and specific ShRNAs were used for knockdown experiments. Flag-EGFR expression plasmid was made by cloning human EGFR coding region into the HindIII/NotI site of p3XFlag plasmid and mutants were made using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). All plasmid constructs were confirmed by DNA sequencing and fusion protein expression confirmed by western blotting.
Primary antibodies used include rabbit anti-Srx (Proteintech), rabbit anti-Prx I (Abcam), rabbit anti-PrxSO3 (Abcam), mouse anti-β-actin (Sigma-Aldrich), mouse anti-Prx III, anti-pERK, anti-ERK, anti-pMEK, anti-MEK, and anti-pEGFR (Thr669, Tyr845, Tyr992, Tyr1068, Tyr1086, Tyr1148, Tyr1173) (cell signaling); anti-EGFR (NeoMarkers). Recombinant human EGF and PD98059 (MEK1/2 inhibitor) were commercially obtained (Sigma-Aldrich).
Western blot, immunoprecipitation, cell transfection, lentiviral particle production, infection and establishment of stable cell lines
Western blot and immunoprecipitation were performed as previously reported (20). Experiments of transient transfection of ShRNA were processed using Lipofectamine 2000 (Invitrogen) following manufacturer’s protocol. Strictly controlled ShRNA-based knockdown experiments were designed according to previous suggestions (21). Lentiviral particles and stable cells were established and maintained in puromycin containing medium as previously reported (22).
Cell proliferation, viability, soft-agar colony formation, transwell matrigel invasion, human protein profiler Phospho-RTK array and orthotopic implantation
Cell proliferation and viability were performed using XTT kit (Roche). Colony formation and matrigel invasion were performed as previously reported (22). Phospho-RTK arrays were performed using commercial kit (R&D Systems). Orthotopic implantation was performed as previously reported (23) using severe combined immunodeficiency (SCID) female mice at 5-week old (Toconic, Hudson, NY). The protocol for mouse orthotopic implantation was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Kentucky (UK). All animal procedures were conducted following the Policy on Humane Care and Use of Laboratory Animals, and Guidelines of the Animal Care and Laboratory Animal Welfare (NIH).
Liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) analysis and identification of EGFR post-translational modifications
Cells were lysed in RIPA buffer and endogenously expressed EGFR was immunoprecipitated by using monoclonal anti-EGFR antibody and resolved on a 8% SDS-polyacrylamide gel. After coomassie staining, EGFR bands were excised and subjected to in-gel trypsin digestion. LC-MS/MS analysis was performed as previously reported (24, 25).
Statistical analysis
Quantitative data from at least three replicates were presented as means ± standard deviation (x̄ ± sd). Data were analyzed by t test using GraphPad Prism (Version 5.04) or Microsoft Excel (Version 2010). For calculation of the p value, parameters of a two-tailed, 95% confidence interval were used for all analysis. A p value of less than 0.05 is considered statistically significant.
Results
Srx is preferentially expressed in cells derived from human colorectal carcinomas
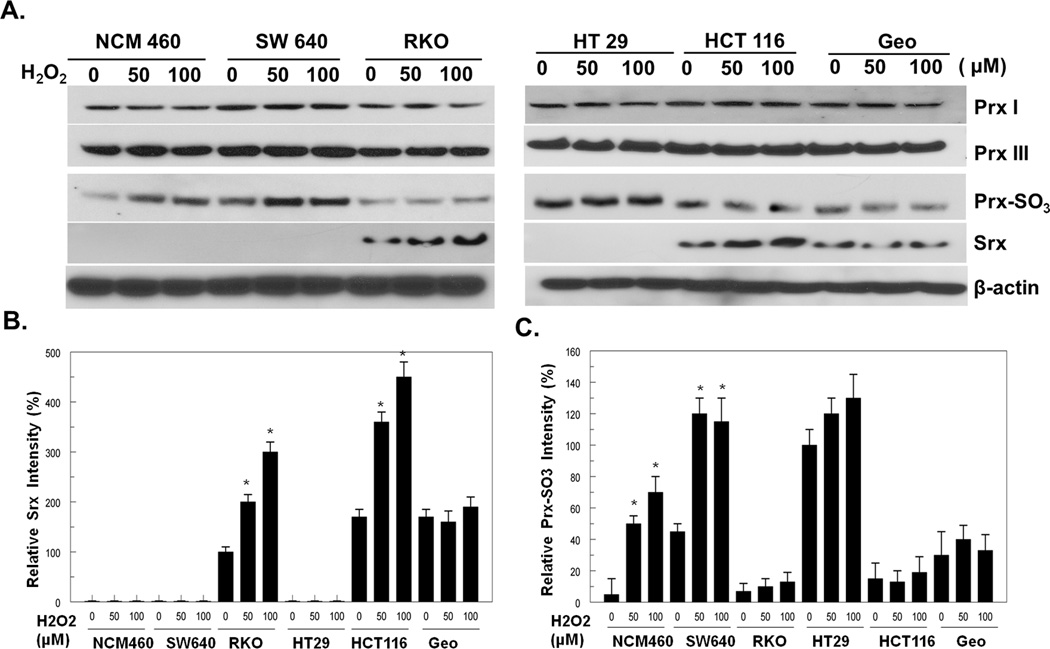
Previous studies have demonstrated that Srx is induced by oxidative stress. To understand the significance of Srx in human CRC development, firstly we asked whether Srx is endogenously expressed in human CRC cells, and whether its levels are regulated by oxidative stress. A total of six cell lines, including one derived from human normal colon epithelium and five CRC cell lines established from patients with colon carcinomas, were examined for Srx expression with or without treatment of hydrogen peroxide (H2O2). We found that Srx is not detected in cells derived from normal colon epithelium (NCM460) or cells derived from well-differentiated colorectal carcinomas (SW640 and HT29). Interestingly, Srx is expressed in RKO and GEO cells at moderate levels, and is highly expressed in poorly differentiated, aggressive HCT116 cells. In contrast, the levels of Prxs, such as Prx I and Prx III, are equivalent in all cell lines (Fig. 1A). In the presence of H2O2, the levels of Srx in RKO and HCT cells are further induced (Fig. 1A and B). It has been well documented that oxidative stress induced by H2O2 leads to the hyperoxidation of Prxs, which can be detected by a specific antibody recognizing exclusively the overoxidized cysteine residues (Prx-SO3) (2, 3). Therefore, the function integrity of Srx can be evaluated by investigating its ability to reduce the levels of Prx-SO3. In consistence, we found that endogenously expressed Srx significantly attenuates the levels of overoxidized Prxs induced by oxidative stress in RKO and HCT116 cells, whereas significant increases of overoxidized Prxs were observed in cells without Srx expression (Fig. 1C). Taken together, these data indicate that functionally active Srx is preferentially expressed in poorly differentiated CRC cell lines including RKO, HCT116 and Geo cells.
Fig. 1.
Srx is preferentially expressed in human colorectal cancer derived RKO, HCT116 and Geo cells. (A) Representative western blot of Prxs, Srx and overoxidized Prxs (Prx-SO3) expressed in colon normal (NCM460) or colorectal cancer derived cell lines. (B,C) Quantitative analysis of Srx (B) and hyperoxidized Prxs (C) from three independent experiments. *Compared with control cells without H2O2 treatment, p<0.05 (n = 3).
Knockdown of Srx represses anchorage independent colony formation and matrigel invasion in vitro but does not affect cell growth and proliferation under adherent conditions
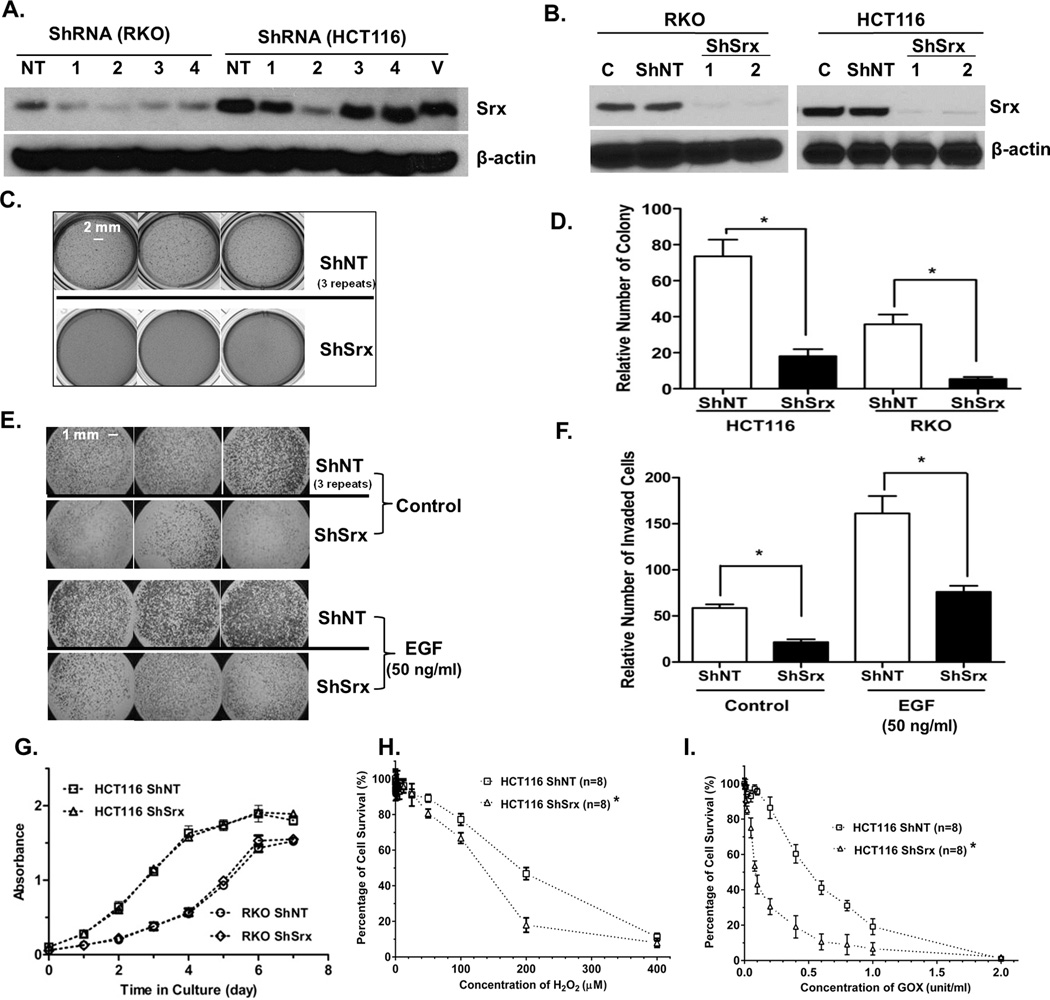
Lentivirus mediated stable ShRNA knockdown was used for loss-of-function studies. We designed a total of four ShRNA constructs, each with a unique hairpin sequence to target a distinctive coding region of the Srx mRNA. The efficiency of these constructs to knockdown protein expression of endogenous Srx was evaluated by Western blotting in transient transfection experiments. We found that ShRNA1 and 2 were more efficient in depleting the endogenously expressed Srx (Fig. 2A). Next, lentiviral particles containing either ShRNA1 or ShRNA2 were used to make stable knockdown of Srx in RKO and HCT116 cells. In both cell lines, the stable knockdown efficiency reaches more than 90% and there are no noticeable differences between cells expressing ShRNA1 and ShRNA2 (Fig. 2B). By comparing the phenotypical features of these stable cells with control cells expressing a non-target ShRNA (ShNT cells), we found that knockdown of Srx inhibits anchorage independent colony formation in soft agar in both cell lines (Fig. 2C and D). Moreover, knockdown of Srx in HCT 116 cells and RKO cells also significantly represses the basal number as well as serum or EGF-induced number of cells invaded through matrigel (Fig. 2E and F). We also performed a dose response of EGF treatment using HCT116 cells. Knockdown of Srx inhibits a wide range of EGF-induced cell invasion, with the most significant inhibition observed when EGF is equal or less than 50 ng/ml (Fig. S2).
Fig. 2.
Knockdown of Srx in CRC cells inhibits colony formation in soft agar and reduces cells’ ability to invade through matrigel, but has no significant effect on cell proliferation. (A) Testing the efficiency of different ShRNAs to knockdown endogenous Srx in RKO and HCT116 cells in transiently transfection experiments. NT, a non-targeting ShRNA; V, vector plasmid; 1–4, ShRNAs targeting different regions of Srx transcript. (B) CRC cells with stable knockdown of Srx by lentiviral expression of ShRNA1 or ShRNA2. (C) Representative triplicate images of colony formation in soft agar. (D) Quantitative analysis of data in (C). (E) Representative triplicate images of matrigel invasion. (F) Quantitative analysis of data in (E). (G–I) Quantitative analysis of cell growth and proliferation in culture (G) and cell survival under oxidative stress conditions induced either by exogenous H2O2 (H) or treatment with glucose oxidase (I). *Compared with ShNT, p<0.05 (n = 6).
To investigate whether above inhibition is due to reduced cell growth, we performed XTT assays to evaluate the effect of Srx knockdown on cell proliferation. In HCT116 and RKO cell lines, we did not find significant differences in cell proliferation between control and Srx knockdown cells (Fig. 1G). We then tested whether knockdown of Srx affects cells’ ability to survive through oxidative stress. By determining cell viability in the presence of H2O2, we found that the IC50 of control HCT116 to H2O2 is around 200 µM, however, the IC50 of Srx knockdown cells is decreased to 120 µM (Fig. 1H). H2O2 is also one of the metabolites produced in the process of cellular energy production, for example, through consumption of glucose by cellular glucose oxidase (GOX) (20). The IC50 of in control HCT116 cells to GOX is approximately 0.6 unit/ml, and it is decreased by 6-fold (to about 0.1 unit/ml) in Srx knockdown cells (Fig. 1I). Similar findings were also observed in Geo cells. Therefore, we demonstrate that knockdown of Srx in CRC cells does not affect the rate of cell proliferation, but increases their sensitivity to oxidative stress induced cell death. Taken together, these data suggest that Srx is required for the anchorage independent colony formation and invasion of human CRC cells but is not critical for cell growth and proliferation under adherent conditions in culture.
Knockdown of Srx reduces tumor xenograft growth and represses metastasis in vivo
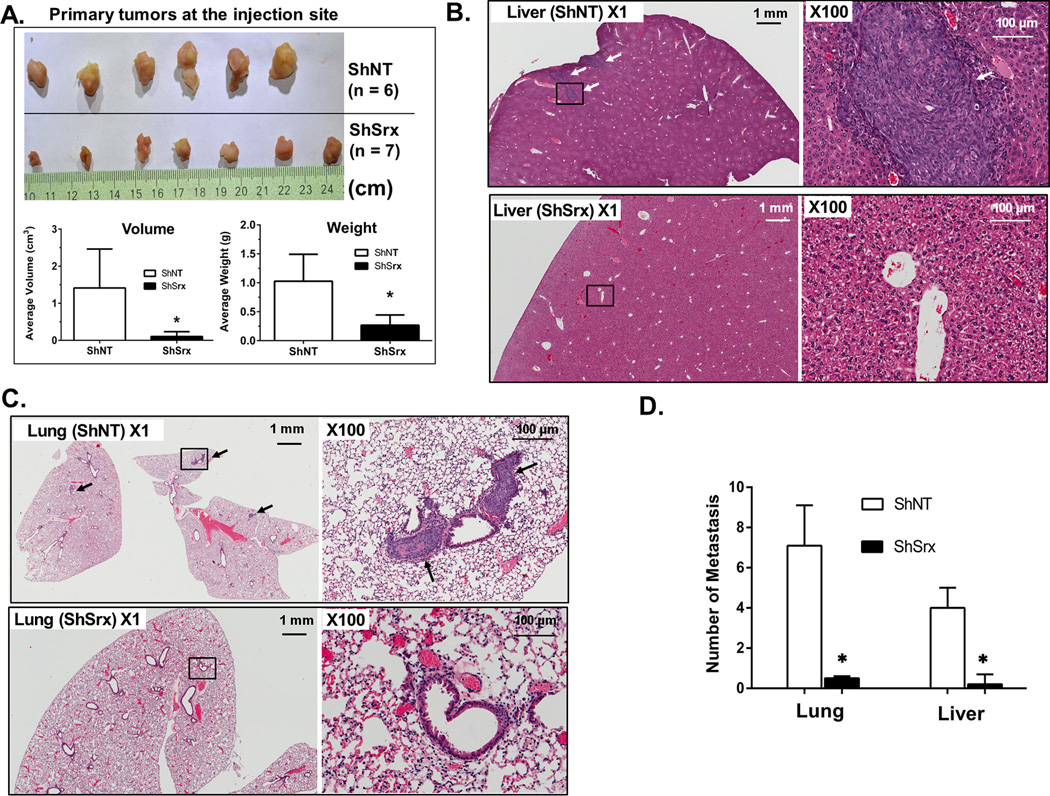
To study the effect of Srx on tumor growth and metastasis in vivo, HCT116 ShNT or ShSrx cells were injected into the cecal wall of SCID mice using an orthotopic implantation method (Fig. S1A). This model mimics CRC metastasis in a similar organ environment that closely replicates what occurred in human patients. By the end of the 8th week after injection, mice were euthanized and tissues were collected. Firstly, we extracted primary tumors at the injection site from all mice. Compared with control, the average volume and weight of primary tumors from mice injected with HCT116 ShSrx cells are significantly smaller or less (Fig. 3A), which is consistent with their reduced colony formation in soft agar. We also examined tumor metastases formed in secondary organs including mouse lung and liver. Tumor metastases with a diameter equal or larger than 1 mm were found in lung and liver of all mice receiving HCT116 ShNT cells (6 out of 6 mice bearing lung or liver metastasis visible to naked eye, 100%) (Fig. S1B). Microscopically, multiple metastases were found in the liver (Fig. 3B) and lung (Fig. 3C) of mice receiving HCT116 ShNT cells. In contrast, visible tumor nodule was a rare event in mice receiving HCT116 ShSrx cells, occurring in only 1 out of 7 mice and the differences are significant (Fig. 3D). Therefore, these data suggest that knockdown of Srx reduces the ability of HCT116 cells to grow as tumor xenografts and also inhibits their capability of establishing metastasis in immunodeficient mice.
Fig. 3.
Knockdown of Srx in HCT116 cells reduces tumor growth and metastasis in vivo in a mouse orthotopic implantation model. (A) Colon tumors at primary injection site and quantitative analysis of tumor averages in volume and weight. (B–C) Microscopic tumor metastasis found in the liver (B) and lung (C) of mice injected with HCT116-ShNT cells but not in mice injected with ShSrx cells. Arrow heads indicate tumor metastasis and black-square indicates the spot zoomed in. (D) Quantitative analysis of data from (B) and (C). *Compared with ShNT, p<0.05 (n1 = 6, n2 = 7).
Ectopic expression of Srx enhances CRC cell invasion in vitro and stimulates metastasis in vivo
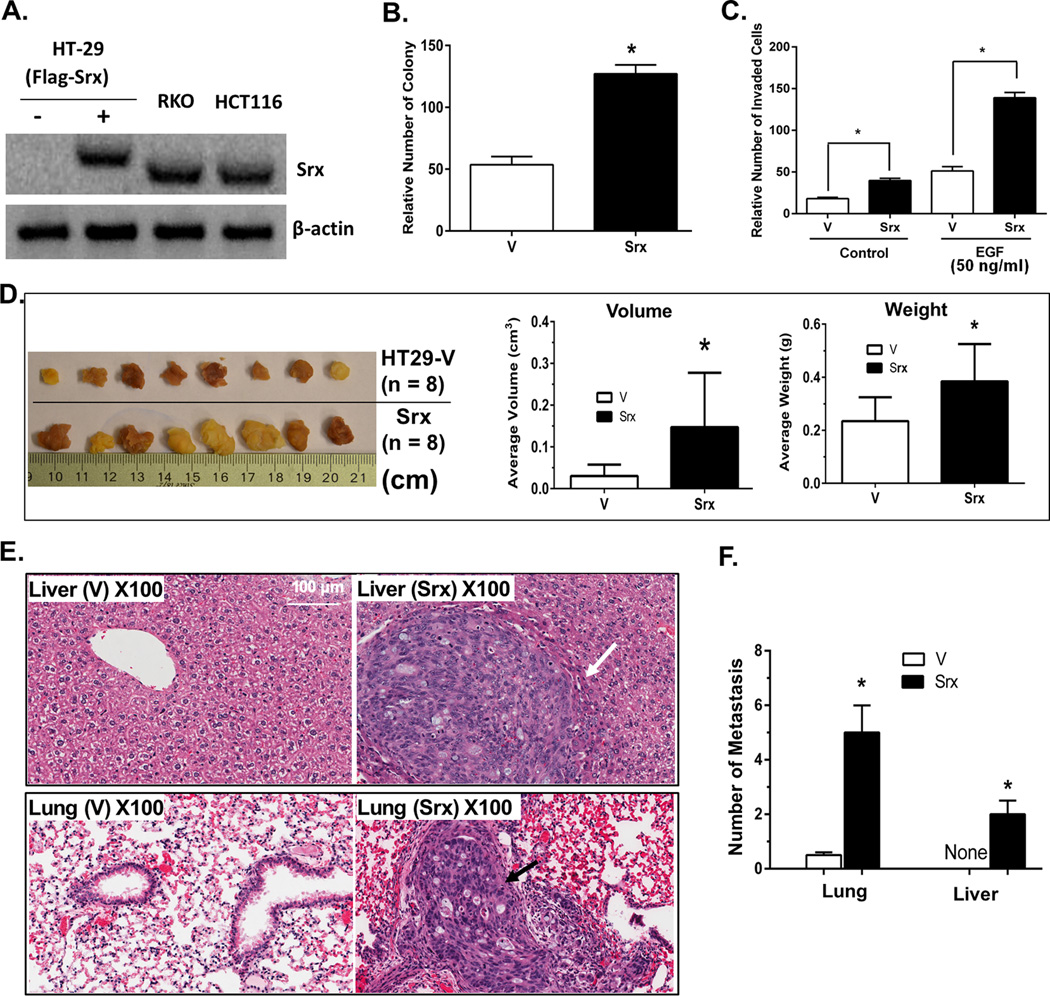
Next we tested whether ectopic expression of Srx in well-differentiated, non-Srx expressing cells increases their abilities of cell invasion and metastasis. Using the lentiviral infection strategy, Flag-Srx was stably expressed in HT-29 cells (HT29-FlagSrx) at levels that are comparable to those endogenously expressed in RKO or HCT116 cells (Fig. 4A). Previously we have demonstrated that addition of the Flag-tag does not change the oxidoreductase activity of Srx (20). Compared with control, ectopic expression of Srx in HT-29 cells leads to significant increase of colony formation in soft agar (Fig. 4B), and enhances the basal as well as serum or EGF-induced cell invasion (Fig. 4C). After being injected into the cecum of immunodeficient mice, the average volume and weight of primary tumors at the injection site in mice receiving HT29-FlagSrx cells are significantly larger/heavier than those injected with control HT29 cells (Fig. 4D). Visible tumors were not found on the surface of lung and liver in all mice, however, average numbers of microscopic metastases in the lung and liver of mice injected with HT29-FlagSrx cells are significantly higher than control (Fig. 4E and 4F). These data suggest that ectopic expression of Srx in well-differentiated colorectal cancer cells enhances their ability to grow as tumor xenografts and also increases their capability of establishing metastasis in immunodeficient mice.
Fig. 4.
Ectopic expression of Flag-Srx in HT29 cells promotes cell invasion in vitro and stimulates tumor growth & metastasis in vivo in a mouse orthotopic implantation model. (A) Stable expression of Flag-Srx in HT29 cells at levels equivalent to Srx endogenously expressed in HCT116 or RKO cells. (B–C) Compared with control cells expressing empty vector (V), expression of Flag-Srx in HT29 cells (Srx) stimulates colony formation in soft agar (B) and matrigel invasion (C). (D) Colon tumors at primary injection site and quantitative analysis of tumor averages in volume and weight. (E) Microscopic tumor metastases are not found in the liver and lung of mice injected with control HT29 cells, but are present in mice injected with HT29-FlagSrx cells. Arrow heads indicate microscopic tumor metastasis. (F) Quantitative analysis of data from (E). *Compared with vector control, p<0.05 (n = 8).
Knockdown of Srx attenuates MAPK activation through inhibiting the C-terminal tyrosine phosphorylation of EGFR
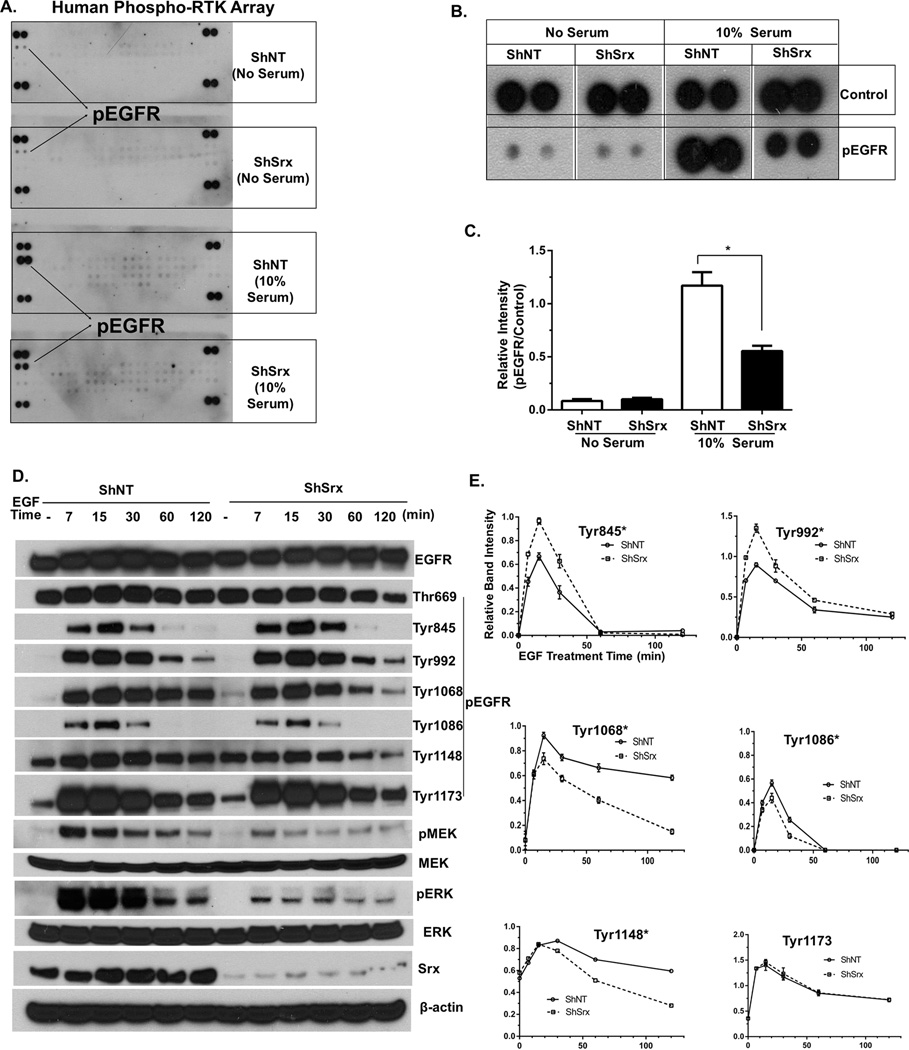
To understand molecular mechanisms by which Srx inhibits human CRC cell invasion and metastasis, we asked whether Srx affects the activation of receptor tyrosine kinases (RTKs), which are downstream of growth factor-mediated signaling pathways. We performed human protein profiler phospho-RTK arrays to compare the global activation levels of 49 different RTKs in HCT116-ShNT and ShSrx cells with the presence or absence of serum stimulation using phospho-specific antibodies. In this assay, each membrane detects the levels of pre-defined phospho-RTKs in duplicates, along with negative and positive controls (Fig. S3). In the absence of serum, RTKs in both ShNT and ShSrx cells are barely active. In response to serum stimulation, a strong induction of EGFR phosphorylation and mild activation of several other RTKs are observed in ShNT cells (Fig. 5A). Interestingly, the levels of EGFR activation in ShSrx cells are significantly reduced (Fig. 5B and 5C). Therefore, these data indicate that knockdown of Srx in HCT116 cells leads to reduced activation of EGFR but has no significant effect on other RTKs.
Fig. 5.
Knockdown of Srx attenuates MAPK signaling through the inhibition of the C-terminal tyrosine phosphorylation of EGFR. (A–C) Proteome Profiler Human Phospho-RTK array: original blots (A), EGFR phosphorylation (B) and its quantitative analysis (C). (D) Representative western blot of EGF-induced activation of MAPK cascade in HCT116 ShNT and ShSrx cells. (E) Quantitative analysis of the levels of phosphorylated tyrosine residues in (D). *Compared with ShNT cells, p<0.05 (n = 3).
EGFR mainly mediates the activation of MAPK cascade; therefore we compared the MAPK signaling between HCT116-ShNT and ShSrx cells under growth factor treatment. In ShNT cells, EGF strongly induces the activation/phosphorylation of p42/p44 (ERK) in a time dependent manner. The activation peaks at 15 minutes (min) after stimulation and sustains up to 60 min before gradually diminished (Fig. 5D). In ShSrx cells, a similar pattern of time-dependent activation of ERK is observed, however, both the basal as well as EGF-induced activation of ERK are significantly reduced (Fig. 5D). Moreover, the levels of activated MEK, the kinase upstream of ERK, were also significantly reduced in ShSrx cells compared with those of control cells (Fig. 5D). The activated sites of EGFR and their levels were further examined by phospho- and site-specific antibodies to individual serine, threonine or tyrosine residue. We found that there are significant decrease in the levels of phosphorylation in ShSrx cells at the C-terminal region of EGFR, which include Tyrosine residues 1068, 1086 and 1148 (Fig. 5D and E). Similar observation is also found in RKO cells, indicating that knockdown of Srx reduces the C-terminal tyrosine phosphorylation of EGFR (Fig. S4). Interestingly, we also found an increase of phosphorylation on Tyr845 and 992 in Srx depleting cells, which may be counterintuitive to the reduced phosphorylation of other tyrosine residues. Most importantly, the net consequence of these phosphorylation changes of EGFR on downstream signaling is the inhibition of MEK and ERK activation (Fig. 5D and S4). Taken together, these data suggest that knockdown of Srx attenuates MAPK signaling through inhibiting the C-terminal tyrosine phosphorylation of EGFR.
Ectopic expression of Srx enhances the C-terminal tyrosine phosphorylation of EGFR and activates downstream MAPK signaling
To further confirm that Srx has a specific effect on EGFR activation and MAPK signaling in CRC cells, we compared the levels of activation in HT29 control cells and HT29-FlagSrx cells. As shown in Fig. S5, there are significantly increased phosphorylation of Tyr1068, 1086 and 1148, and enhanced activation of downstream ERKs in HT29-FlagSrx cells. Therefore, our data suggest that expression of Srx enhances EGFR phosphorylation and stimulates downstream MAPK signaling, whereas knockdown of Srx has an exactly opposite effect.
Expression of Srx reduces EGFR acetylation specifically at K1037 to enhance receptor phosphorylation and to activate MAPK signaling
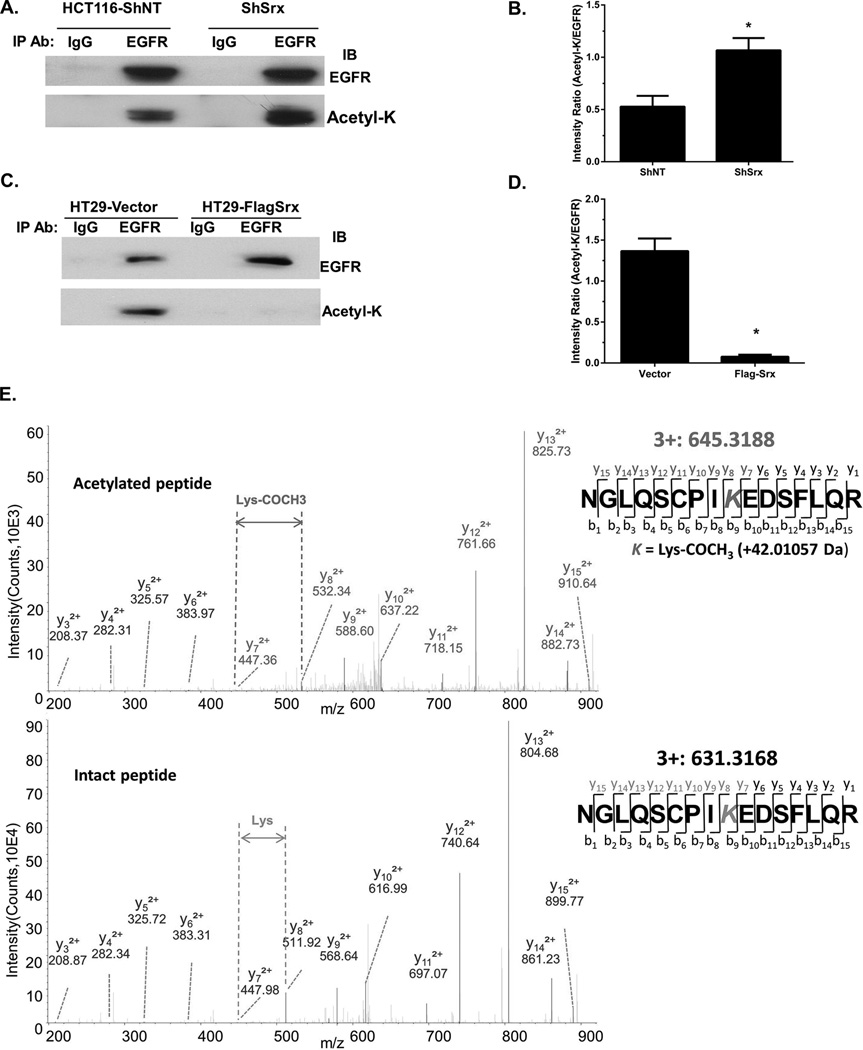
A variety of post-translational modifications of EGFR, including oxidation, methylation, acetylation and ubiquitination may affect receptor activation and downstream signaling. Since we did not observe differences in the total levels of EGFR, we tested whether Srx affects the post-translational modifications of EGFR. EGFR was pull down using immunoprecipitation and cells with or without Srx expression, the levels of EGFR modifications were examined by Western blotting using specific antibodies. We identified that the levels of lysine-acetylated EGFR in HCT116 ShSrx cells were significantly higher than those of control cells (Fig. 6A and B), whereas its levels in HT29-FlagSrx cells were significantly lower than control cells (Fig. 6C and D). These data suggest that the levels of Srx are negatively correlated with the levels of lysine acetylation in EGFR. There are a total of 66 lysine residues in human EGFR. We performed LC-ESI-MS/MS analysis to further determine the identities of acetylated lysine residue(s) that may be affected by Srx in CRC cells. Endogenous EGFR enriched from HT29 control cells and HCT116-ShSrx cells (Fig. S6A) were subjected to MS analysis. We discovered that only K203 and K1037 of EGFR were acetylated in both cell lines (Fig. 6E and Fig. S6B).
Fig. 6.
Knockdown of Srx stimulates lysine acetylation in EGFR specifically on K1037 as identified by LC-ESI-MS/MS. (A) Knockdown of Srx in HCT116 cells increases the total levels of lysine acetylation in EGFR. (B) Quantitative analysis of data in (A). (C) Ectopic expression of FlagSrx in HT-29 cells decreases the levels of lysine acetylation in EGFR. (D) Quantitative analysis of data in (C). (E) Identification of K1037 acetylation by MS in HCT116 ShSrx and HT29 cells. The upper panel shows a MS-spectrum from peptide containing acetylated K1037 and the bottom panel shows a MS-spectrum from non-acetylated intact peptide. *Compared with ShNT or cells expressing empty vector, p<0.05 (n = 3).
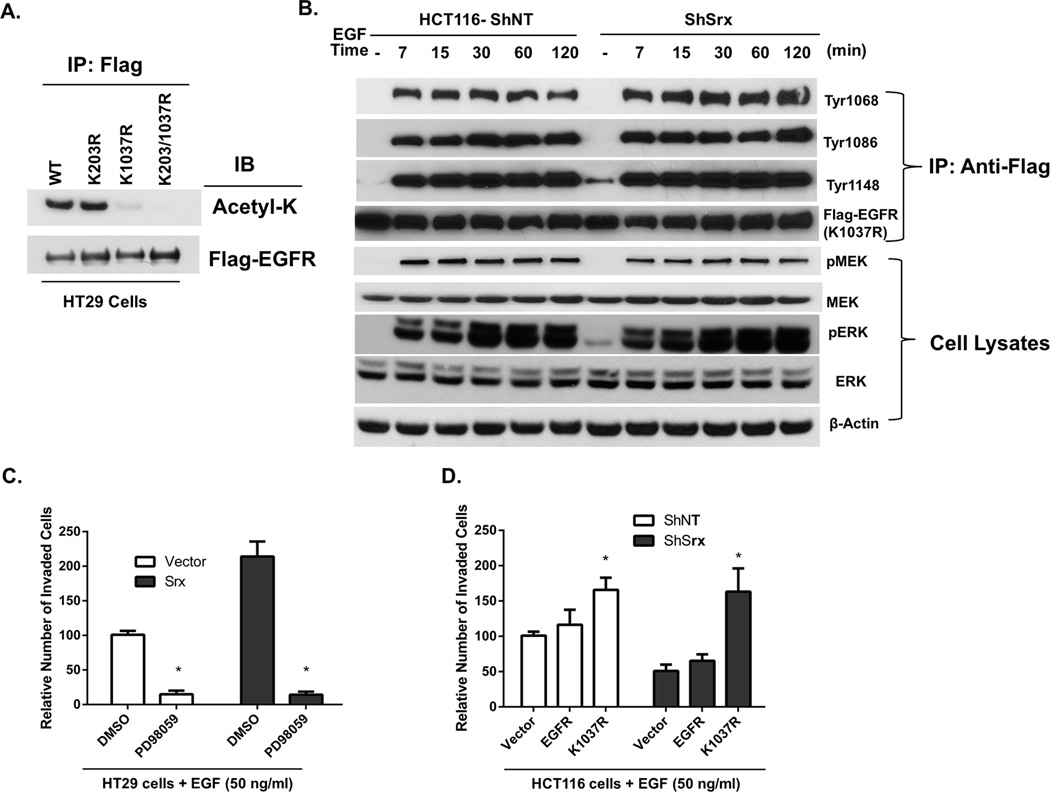
Unacetylated lysine is structurally similar to arginine (R) that cannot be acetylated, thus K to R replacement is frequently used as a loss-of-function mutation in protein acetylation studies (26). To further determine K203/K1037 contribution to the acetylation signal that observed in HT29 and HCT116-ShSrx cells, we made plasmids expressing a Flag tagged EGFR or mutants with either K203/K1037 or both mutated to R by site-specific mutagenesis. After transfection of these plasmids into HT29 cells, the acetylation levels of Flag-EGFR and mutants were examined. Compared with wildtype, the levels of lysine acetylation in K203R mutant did not change, while the levels of lysine acetylation in K1037R mutant (or double mutant) were completely lost (Fig. 7A). These data indicate that K1037 is the major site of EGFR lysine acetylation that subjects to a negative regulation by Srx in CRC cells.
Fig. 7.
Expression of K1037R mutant EGFR leads to prolonged EGFR phosphorylation and sustained MAPK signaling in human CRC cells. (A) Mutation of K1037 (but not K203) to arginine (R) abolishes acetylated lysine signal in EGFR of HT29 cells. (B) Expression of K1037R mutant EGFR in HCT116 ShNT or ShSrx cells leads to prolonged EGFR tyrosine phosphorylation and sustained MAPK activation. (C) Inhibition of MAPK cascade by a MEK inhibitor (PD98059) abolishes Srx-mediated cell invasion in HT29 cells. (D) Expression of K1037R EGFR mutant (but not the wildtype) rescues the inhibition of cell invasion in HCT116 ShSrx cells.
Next we tested whether a wildtype EGFR or a K1037R mutant can rescue the phenotype of Srx depleting cells. Transfection of a wildtype EGFR into HCT116 control and ShSrx cells result in a similar pattern of EGFR phosphorylation and MAPK activation as seen in Fig. 5D. However, transfection of K1037R mutant into HCT116 ShNT and ShSrx cells leads to robust, sustained phosphorylation of the C-terminal tyrosine residues and constitutive activation of downstream MEK and ERK phosphorylation, indicating a complete rescue of the MAPK signaling in Srx deficient cells (Fig. 7B). We further tested whether Srx-induced cell invasion is dependent on the activation of MAPK signaling. Inhibition of MEK activity by a known chemical inhibitor, PD98059, completely inhibits EGF-induced cell invasion (Fig. 7C). Moreover, expression of K1037R mutant (but not the wildtype EGFR) completely rescues cell invasion in Srx knockdown cells (Fig. 7D). Taken together, these data demonstrate that the acetylation of K1037 is negatively associated with EGFR phosphorylation and inhibits downstream MAPK activation. Therefore, by reducing the levels of K1037 acetylation through a mechanism yet to be determined, expression of Srx enhances the C-terminal phosphorylation of EGFR and activates downstream MAPK signaling.
Discussion
A novel function of Srx is to promote CRC invasion and metastasis through enhancing the EGFR-MAPK signaling
In this report we demonstrate that knockdown of Srx increases CRC cells’ susceptibility to oxidative stress but absence of Srx has no significant effect on cell growth and proliferation in culture. In consistence, we found that the levels of overoxidized Prxs in CRC cells are inversely correlated with the levels of Srx. This function of Srx is further substantiated in other studies. For example, fibroblasts derived from Srx null mouse embryos are more sensitive to oxidative injury and apoptosis (27), most likely resulting from increased levels of cellular ROS and accumulation of overoxidized Prxs (27, 28). Similar mechanism has also been documented in a variety of species including plants and vertebrates (29, 30). Compared with its function in redox regulation, the significance of Srx in intracellular signal transduction is less studied. A novel function of Srx, which is to enhance the EGFR-MAPK signaling cascade through decreasing the levels of receptor acetylation on a particular lysine residue of EGFR, is identified in our study. Inhibition of the EGFR-MAPK signaling by Srx enforces a profound consequence of inhibiting tumor xenograft growth as well as abolishing metastasis in the orthotopic mouse model of human CRC development.
The mechanism of Srx to enhance EGFR-MAPK signaling is mediated through a novel post-translational modification of EGFR acetylation on K1037
The significant effect of Srx to enhance EGF and serum induced cell invasion prompts us to study whether the activation of RTKs plays a role in this process. After comparing the activation of multiple RTK pathways, we demonstrate that the phosphorylation of EGFR is the only RTK that is affected by the status of Srx expression in CRC cells. It is well documented that EGFR is overexpressed in multiple types of human cancer. In particular, overexpression of EGFR is significantly associated with CRC invasion, angiogenesis, metastasis and its levels are also reversely correlated with patient prognosis (31). In clinic, anti-EGFR antibodies, such as cetuximab and panitumumab, are used as promising chemotherapeutic agents for the treatment of metastatic CRC in patients (32).
EGFR belongs to the family of receptor tyrosine kinases. Through binding to a number of ligands including EGF, EGFR plays a pivotal role in the activation of downstream oncogenic signaling pathways to promote cell proliferation, cell cycle progression, cell migration, invasion and metastasis. Among all post-translational modifications of EGFR, the first and foremost is phosphorylation. EGFR contains domains of extracellular ligand binding, transmembrane, intracellular kinase and a cytoplasmic tail. Binding of EGF to EGFR induces receptor dimerization, stimulates intracellular kinase activity and leads to the auto- and trans-phosphorylation of different tyrosine residues including Y845, 992, 1045, 1068, 1086, 1148 and 1173 (33–35). The phosphorylation of these residues serves as docking sites for adaptor proteins to form a stable complex and also provides a binding surface for downstream substrate proteins. Patterns of differential tyrosine phosphorylation lead to distinctive activation of downstream signal pathways. Specifically, phosphorylation of Y845 leads to the activation of Stat signaling cascade (36, 37), while phosphorylation of Y992, 1068, 1086, and 1148 results in the activation of MAPK and Akt signaling through interacting with scaffold protein such as Grb2 and Shc (38, 39). On the other hand, phosphorylation of Y1045 recruits Cbl, an E3 ubiquitin ligase, to induce receptor ubiquitination, recycling, trafficking and degradation (40). In our study, firstly we identified that the overall tyrosine phosphorylation levels of EGFR are reduced in Srx depleting cells by RTK profiling. To determine the identities of tyrosine residues that are responsible for this reduction, we examined the phosphorylation levels of individual tyrosine residue, and found that the levels of phosphorylated Y1068, 1086 and 1148 are reduced. Although the levels of phosphorylation on Y845 and 992 are slightly increased, further examination of the net consequence of these changes reveals a significant inhibition of downstream MEK and ERK activation in Srx-depleting cells. On the hand, it’s also possible that there are distinctive mechanisms that regulate the phosphorylation of EGFR on different tyrosine residues. In this study we have focused on the understanding of how the absence of Srx leads to the reduced phosphorylation on Y1086, 1086 and 1148. In the future, it may be of interest to further understand the mechanism by which Srx regulates the phosphorylation of Y845 and 992, and whether such effect is causally related with the change of redox balance of the acetylation status of EGFR.
In addition to phosphorylation, EGFR is also subjected to other modifications such as oxidation, methylation and acetylation. These modifications are interconnected with receptor phosphorylation through a conformational change and thus play significant roles in the regulation of downstream MAPK signaling. In human skin cancer cells, Cys797 of EGFR was identified as a redox sensitive residue that undergoes oxidation in response to EGF stimulation, and such oxidation enhances its tyrosine kinase activity and downstream MAPK signaling (41, 42). In human breast cancer cells, Arg1175 of EGFR is methylated by PRMT5 and this methylation enhances downstream MAPK activation through promoting the phosphorylation of Tyr1173 (43). Also in breast cancer cells, acetylation of K684, K836 and K843 in EGFR affects its receptor tyrosine kinase activity and contributes to cancer cell resistance to histone deacetylase inhibitors (44). Apparently, specific site(s) of EGFR to be acetylated is likely dependent on cell context, as acetylation of K1155, K1158 and K1164 is identified in endothelial cells (45). However, acetylation at these sites does not involve in the activation of MAPK cascade but instead contributes to receptor internalization and activation of protein kinase B/AKT pathway (45). Other forms of post-translational modifications of EGFR include glycosylation, ubiquitination, neddylation and sumoylation, and these modifications mainly affect receptor stability, endocytosis, trafficking and shuttling (45, 46).
In our study we did not observe significant changes at EGFR protein levels associated with the levels of Srx in CRC cells, therefore we focused on studying whether Srx affects the C-terminal phosphorylation of EGFR through a mechanism of regulating post-translational modifications by oxidation, methylation or acetylation. By MS analysis, we examined potential changes on such modifications. We identified that K203 and K1037 of EGFR are subjected to acetylation in human CRC cells and their levels are negatively correlated with the levels of Srx expression. These are completely novel identifications and there are no documented reports of EGFR acetylation on K203 or K1037 in the literature. K203 is located in the ligand binding domain of human EGFR and K1037 in the intracellular dimerization domain. With further examination we demonstrated that only K1037 acetylation is resulted from the downregulation of Srx and contributes to the repression of downstream MAPK signaling. Similar to previous reports (47), it is likely that the acetylation of K1037 causes a conformational change on EGFR intracellular dimerization domain and thus leads to the reduced trans-phosphorylation of its C-terminal tyrosine residues. Further functional experiments on the significance of K1037 acetylation may be important to understand such change. Nevertheless, we reveal a novel pathway of Srx to enhance the EGFR-MAPK signaling in human CRC cells through a negative regulation of receptor acetylation on K1037.
Mechanisms by which Srx reduces the levels of K1037 acetylation of EGFR remain to be determined
In our study we report that Srx plays a critical oncogenic role to promote cell invasion and metastasis, which is at least partially due to its stimulation of the EGFR-MAPK pathway. We further reveal that there is a causal relationship between Srx expression, EGFR deacetylation and MAPK activation. How the expression of Srx leads to the deacetylation of EGFR still remains to be studied in the future. Previous studies in the literature may shed some lights on how a coordinated regulatory model of EGFR acetylation may be involved. As demonstrated in skin cancer A431 cells, EGFR is acetylated by cAMP response element-binding protein (CBP), a nuclear protein with protein acetyltransferase activity that is also found in cytosol (44). Moreover, a cytosolic deacetylase, histone deacetylase 6 (HDAC6), has been found to interact with EGFR (48). Therefore, in the future it is of interest to study whether the effect of Srx to reduce EGFR acetylation on K1037 is mediated by an increase of HDACs activity or a decrease of CBP function. Nevertheless, due to its critical importance in CRC cell invasion and metastasis, our efforts of investigating the novel mechanism of Srx in promoting EGFR-MAPK signaling, not only provides in-depth understanding of the regulatory network of EGFR signaling but also indicates potentially therapeutic value for the treatment of metastatic CRC in patients.
Supplementary Material
Acknowledgements
The authors thank Dr. Hsin-Sheng Yang and his lab members for help on experimental techniques and project comments.
Footnotes
This work was partially supported by a start-up funding from the University of Kentucky Markey Cancer Center (to QW), a Pathway to Independence grant (R00CA149144-04 to QW) from National Cancer Institute and a pilot project (to QW) of the National Institute of General Medical Sciences COBRE grant (5P20GM103486-10). We acknowledge the University of Kentucky Proteomics Core that is partially supported by grants from the National Cancer Institute (P30CA177558) and the National Institute of General Medical Sciences (P20GM103486) from the National Institutes of Health. The LC-MS/MS equipment was acquired using a National Center for Research Resources High-End Instrumentation grant (1S10RR029127 to HZ).
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Sun Y, Hegamyer G, Colburn NH. Molecular cloning of five messenger RNAs differentially expressed in preneoplastic or neoplastic JB6 mouse epidermal cells: one is homologous to human tissue inhibitor of metalloproteinases-3. Cancer Res. 1994;54:1139–1144. [PubMed] [Google Scholar]
- 2.Biteau B, Labarre J, Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 3.Chang TS, Jeong W, Woo HA, Lee SM, Park S, Rhee SG. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J Biol Chem. 2004;279:50994–51001. doi: 10.1074/jbc.M409482200. [DOI] [PubMed] [Google Scholar]
- 4.Roussel X, Boukhenouna S, Rahuel-Clermont S, Branlant G. The rate-limiting step of sulfiredoxin is associated with the transfer of the gamma-phosphate of ATP to the sulfinic acid of overoxidized typical 2-Cys peroxiredoxins. FEBS Lett. 585:574–578. doi: 10.1016/j.febslet.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Jonsson TJ, Johnson LC, Lowther WT. Structure of the sulphiredoxin-peroxiredoxin complex reveals an essential repair embrace. Nature. 2008;451:98–101. doi: 10.1038/nature06415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roussel X, Bechade G, Kriznik A, Van Dorsselaer A, Sanglier-Cianferani S, Branlant G, et al. Evidence for the formation of a covalent thiosulfinate intermediate with peroxiredoxin in the catalytic mechanism of sulfiredoxin. J Biol Chem. 2008;283:22371–22382. doi: 10.1074/jbc.M800493200. [DOI] [PubMed] [Google Scholar]
- 7.Jonsson TJ, Johnson LC, Lowther WT. Protein engineering of the quaternary sulfiredoxin.peroxiredoxin enzyme.substrate complex reveals the molecular basis for cysteine sulfinic acid phosphorylation. J Biol Chem. 2009;284:33305–33310. doi: 10.1074/jbc.M109.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi YH, Kim SY, Jung IJ, Shin MR, Jung YJ, Park JH, et al. Dual functions of Arabidopsis sulfiredoxin: acting as a redox-dependent sulfinic acid reductase and as a redox-independent nuclease enzyme. FEBS Lett. 2012;586:3493–3499. doi: 10.1016/j.febslet.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Findlay VJ, Townsend DM, Morris TE, Fraser JP, He L, Tew KD. A novel role for human sulfiredoxin in the reversal of glutathionylation. Cancer Res. 2006;66:6800–6806. doi: 10.1158/0008-5472.CAN-06-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JW, Mieyal JJ, Rhee SG, Chock PB. Deglutathionylation of 2-Cys peroxiredoxin is specifically catalyzed by sulfiredoxin. J Biol Chem. 2009;284:23364–23374. doi: 10.1074/jbc.M109.021394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kil IS, Lee SK, Ryu KW, Woo HA, Hu MC, Bae SH, et al. Feedback control of adrenal steroidogenesis via H2O2-dependent, reversible inactivation of peroxiredoxin III in mitochondria. Molecular cell. 2012;46:584–594. doi: 10.1016/j.molcel.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 12.Wu CL, Yin JH, Hwang CS, Chen SD, Yang DY, Yang DI. c-Jun-dependent sulfiredoxin induction mediates BDNF protection against mitochondrial inhibition in rat cortical neurons. Neurobiology of disease. 2012;46:450–462. doi: 10.1016/j.nbd.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: a cancer journal for clinicians. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 14.Lowery FJ, Yu D. Growth factor signaling in metastasis: current understanding and future opportunities. Cancer metastasis reviews. 2012;31:479–491. doi: 10.1007/s10555-012-9380-x. [DOI] [PubMed] [Google Scholar]
- 15.Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77:400–410. doi: 10.1159/000279388. [DOI] [PubMed] [Google Scholar]
- 16.Moroni M, Veronese S, Benvenuti S, Marrapese G, Sartore-Bianchi A, Di Nicolantonio F, et al. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. The Lancet Oncology. 2005;6:279–286. doi: 10.1016/S1470-2045(05)70102-9. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. The New England journal of medicine. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 18.Saltz LB, Meropol NJ, Loehrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:1201–1208. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 19.Wei Q, Jiang H, Baker A, Dodge LK, Gerard M, Young MR, et al. Loss of sulfiredoxin renders mice resistant to azoxymethane/dextran sulfate sodium-induced colon carcinogenesis. Carcinogenesis. 2013;34:1403–1410. doi: 10.1093/carcin/bgt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei Q, Jiang H, Xiao Z, Baker A, Young MR, Veenstra TD, et al. Sulfiredoxin-Peroxiredoxin IV axis promotes human lung cancer progression through modulation of specific phosphokinase signaling. Proc Natl Acad Sci U S A. 2011;108:7004–7009. doi: 10.1073/pnas.1013012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. Rational siRNA design for RNA interference. Nat Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- 22.Jiang H, Wu L, Mishra M, Chawsheen HA, Wei Q. Expression of peroxiredoxin 1 and 4 promotes human lung cancer malignancy. American journal of cancer research. 2014;4:445–460. [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng W, Leong X, Engleman E. Orthotopic mouse model of colorectal cancer. Journal of visualized experiments : JoVE. 2007:484. doi: 10.3791/484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi J, Wang Y, Zeng L, Wu Y, Deng J, Zhang Q, et al. Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer cell. 2014;25:210–225. doi: 10.1016/j.ccr.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, Gal J, Chen J, Zhu H. Self-assembled FUS binds active chromatin and regulates gene transcription. Proc Natl Acad Sci U S A. 2014;111:17809–17814. doi: 10.1073/pnas.1414004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamieniarz K, Schneider R. Tools to tackle protein acetylation. Chemistry & biology. 2009;16:1027–1029. doi: 10.1016/j.chembiol.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Baek JY, Han SH, Sung SH, Lee HE, Kim YM, Noh YH, et al. Sulfiredoxin Protein Is Critical for Redox Balance and Survival of Cells Exposed to Low Steady-state Levels of H2O2. J Biol Chem. 287:81–89. doi: 10.1074/jbc.M111.316711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Planson AG, Palais G, Abbas K, Gerard M, Couvelard L, Delaunay A, et al. Sulfiredoxin protects mice from lipopolysaccharide-induced endotoxic shock. Antioxid Redox Signal. 2011;14:2071–2080. doi: 10.1089/ars.2010.3552. [DOI] [PubMed] [Google Scholar]
- 29.Bozonet SM, Findlay VJ, Day AM, Cameron J, Veal EA, Morgan BA. Oxidation of a eukaryotic 2-Cys peroxiredoxin is a molecular switch controlling the transcriptional response to increasing levels of hydrogen peroxide. J Biol Chem. 2005;280:23319–23327. doi: 10.1074/jbc.M502757200. [DOI] [PubMed] [Google Scholar]
- 30.Rey P, Becuwe N, Barrault MB, Rumeau D, Havaux M, Biteau B, et al. The Arabidopsis thaliana sulfiredoxin is a plastidic cysteine-sulfinic acid reductase involved in the photooxidative stress response. Plant J. 2007;49:505–514. doi: 10.1111/j.1365-313X.2006.02969.x. [DOI] [PubMed] [Google Scholar]
- 31.Repetto L, Gianni W, Agliano AM, Gazzaniga P. Impact of EGFR expression on colorectal cancer patient prognosis and survival: a response. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2005;16:1557. doi: 10.1093/annonc/mdi263. [DOI] [PubMed] [Google Scholar]
- 32.Sotelo Lezama MJ, Sastre Valera J, Diaz-Rubio Garcia E. Impact of cetuximab in current treatment of metastatic colorectal cancer. Expert opinion on biological therapy. 2014;14:387–399. doi: 10.1517/14712598.2014.883376. [DOI] [PubMed] [Google Scholar]
- 33.Jura N, Endres NF, Engel K, Deindl S, Das R, Lamers MH, et al. Mechanism for activation of the EGF receptor catalytic domain by the juxtamembrane segment. Cell. 2009;137:1293–1307. doi: 10.1016/j.cell.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honegger AM, Kris RM, Ullrich A, Schlessinger J. Evidence that autophosphorylation of solubilized receptors for epidermal growth factor is mediated by intermolecular cross-phosphorylation. Proc Natl Acad Sci U S A. 1989;86:925–929. doi: 10.1073/pnas.86.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein P, Mattoon D, Lemmon MA, Schlessinger J. A structure-based model for ligand binding and dimerization of EGF receptors. Proc Natl Acad Sci U S A. 2004;101:929–934. doi: 10.1073/pnas.0307285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato K, Nagao T, Iwasaki T, Nishihira Y, Fukami Y. Src-dependent phosphorylation of the EGF receptor Tyr-845 mediates Stat-p21waf1 pathway in A431 cells. Genes to cells : devoted to molecular & cellular mechanisms. 2003;8:995–1003. doi: 10.1046/j.1356-9597.2003.00691.x. [DOI] [PubMed] [Google Scholar]
- 37.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999;274:8335–8343. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 38.Zwick E, Hackel PO, Prenzel N, Ullrich A. The EGF receptor as central transducer of heterologous signalling systems. Trends in pharmacological sciences. 1999;20:408–412. doi: 10.1016/s0165-6147(99)01373-5. [DOI] [PubMed] [Google Scholar]
- 39.Chung I, Akita R, Vandlen R, Toomre D, Schlessinger J, Mellman I. Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature. 2010;464:783–787. doi: 10.1038/nature08827. [DOI] [PubMed] [Google Scholar]
- 40.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Experimental cell research. 2009;315:683–696. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 41.Truong TH, Carroll KS. Redox regulation of epidermal growth factor receptor signaling through cysteine oxidation. Biochemistry. 2012;51:9954–9965. doi: 10.1021/bi301441e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paulsen CE, Truong TH, Garcia FJ, Homann A, Gupta V, Leonard SE, et al. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nature chemical biology. 2012;8:57–64. doi: 10.1038/nchembio.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsu JM, Chen CT, Chou CK, Kuo HP, Li LY, Lin CY, et al. Crosstalk between Arg 1175 methylation and Tyr 1173 phosphorylation negatively modulates EGFR-mediated ERK activation. Nature cell biology. 2011;13:174–181. doi: 10.1038/ncb2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song H, Li CW, Labaff AM, Lim SO, Li LY, Kan SF, et al. Acetylation of EGF receptor contributes to tumor cell resistance to histone deacetylase inhibitors. Biochemical and biophysical research communications. 2011;404:68–73. doi: 10.1016/j.bbrc.2010.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goh LK, Huang F, Kim W, Gygi S, Sorkin A. Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. The Journal of cell biology. 2010;189:871–883. doi: 10.1083/jcb.201001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tong J, Taylor P, Moran MF. Proteomic analysis of the epidermal growth factor receptor (EGFR) interactome and post-translational modifications associated with receptor endocytosis in response to EGF and stress. Molecular & cellular proteomics : MCP. 2014;13:1644–1658. doi: 10.1074/mcp.M114.038596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dawson JP, Berger MB, Lin CC, Schlessinger J, Lemmon MA, Ferguson KM. Epidermal growth factor receptor dimerization and activation require ligand-induced conformational changes in the dimer interface. Molecular and cellular biology. 2005;25:7734–7742. doi: 10.1128/MCB.25.17.7734-7742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deribe YL, Wild P, Chandrashaker A, Curak J, Schmidt MH, Kalaidzidis Y, et al. Regulation of epidermal growth factor receptor trafficking by lysine deacetylase HDAC6. Science signaling. 2009;2:ra84. doi: 10.1126/scisignal.2000576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.