Abstract
We previously reported that selective ablation of certain γδ T cell subsets rather than removal of all γδ T cells, strongly affects serum antibody levels in non-immunized mice. This type of manipulation also changed T cells including residual γδ T cells, revealing some interdependence of γδ T cell populations. For example, in mice lacking Vγ4+ and Vγ6+ γδ T cells (B6.TCR-Vγ4−/−/6−/−), we observed expanded Vγ1+ cells, which changed in composition and activation and produced more IL-4 upon stimulation in vitro, increased IL-4 production by αβ T cells as well as spontaneous germinal center formation in the spleen, elevated serum Ig and autoantibodies. We therefore examined B cell populations in this and other γδ-deficient mouse strains. Whereas immature bone marrow B cells remained largely unchanged, peripheral B cells underwent several changes. Specifically, transitional and mature B cells in the spleen of B6.TCR-Vγ4−/−/6−/− mice and other peripheral B cell populations were diminished, most of all splenic marginal zone (MZ) B cells. However, relative frequencies and absolute numbers of antibody-producing cells, and serum levels of antibodies, IL-4 and BAFF, were increased. Cell transfers confirmed that these changes are directly dependent on the altered γδ T cells in this strain, and their enhanced potential of producing IL-4. Further evidence suggests the possibility of direct interactions between γδ T cells and B cells in the splenic MZ. Together, these data demonstrate the capability of γδ T cells of modulating size and productivity of pre-immune peripheral B cell populations.
Introduction
B cell differentiation from immature precursors to antibody producing plasma cells comprises numerous stages (1, 2). Development begins in the bone marrow with common lymphocyte precursors (CLP), and progresses to immature sIgMpos B cells, which migrate via the blood stream to the spleen. Here, new arrivals differentiate through transitional stages into mature B cells, including B2 follicular (FO), B2 marginal zone (MZ) and B1 B cells. Particularly in the serous cavities, B1 B cells are further divided into B1a and B1b B cells, which differ from one another by their expression of CD5, developmental requirements and functional roles (3, 4). Some mature B cells recirculate to the bone marrow (5, 6). B cell development is controlled by specific transcription factors (2). Further differentiation also depends on tonic BCR signaling, which is critical for incorporating immature B cells into the peripheral B cell pool (7), as well as on several additional factors and their interplay, including BAFF and NF-kB2 (8, 9). B cells can develop in the absence of IL-4 (10), but when it is present, this cytokine affects B cell development in bone marrow and periphery (11–13), and it enhances CD23 and MHCII expression (11, 14, 15), and suppresses CD5 expression by B cells (16). During B cell differentiation, B cell tolerance is established at several distinct checkpoints, including one in the bone marrow (central tolerance, BCR selection and editing) (17, 18), another during transition (more BCR selection and competition for BAFF) (17, 19), and a third during antigen activation in the germinal center, where B cells undergo somatic mutation as well as positive and negative selection (20, 21).
In contrast to the well-studied role of T cells as B cell helpers during the immune response and the differentiation of mature B cells into specific antibody-producing cells or memory cells (22–29), their role during pre-immune B cell development is unclear. On the other hand, NKT have been implicated in peripheral B cell homeostasis, especially regarding MZ B cells (30), and recent studies of hematopoietic transplantation in humans and humanized mice indicate that αβ T cells play such a role in the setting of transplantation (31, 32). However, studies in mouse strains with impaired TCR signaling suggested that γδ T cells influence antibody production already in non-immunized mice (33–35).
Subsets of murine γδ T cells as defined by their expression of different TCR-Vγ genes develop sequentially in the thymus during ontogeny (36, 37), and segregate to different organs and tissues (38, 39). Vγ1+ and Vγ4+ cells co-localize in the spleen, where they form comparatively large populations, but they are also present in other lymphoid tissues as well as in the lung and the dermal layer of the skin (40, 41). Comparison of these cells in thymus and spleen revealed different gene expression profiles (42, 43), and functional assays showed that they tend to play opposite roles during certain immune responses (44, 45). In particular, some Vγ1+ cells can produce large amounts of IL-4 whereas Vγ4+ cells have the capability of producing IL-17 (39, 46, 47). In addition, studies of the role of γδ T cells in a tumor model and during West Nile virus infection produced an indication of reciprocal regulatory interactions between these two γδ subsets during the immune response (48, 49), and we recently found in untreated mice genetically deficient in two γδ T cell subsets including Vγ4+ cells (B6.TCR-Vγ4−/−/6−/−) that the splenic Vγ1+ cell population was substantially altered: In this mouse strain, Vγ1+ cells were expanded, changed in composition, showed signs of activation and produced more IL-4 upon in vitro stimulation (50). Vγ6+ cells are not present in the spleen of untreated mice but they co-localize with Vγ4+ cells in skin and lung (40, 41, 51), and they are also found in tongue and female reproductive tract (38). However, at the present time, we have no indication of interactions between Vγ1+ and Vγ6+ cells.
Mindful of the functional differences between γδ T cell subsets and their ability to cross-regulate each other, we hypothesized that changes in γδ T cell composition might have effects on other immune cells and the immune responses. Our recent study examining mouse strains with genetic deficiencies in distinct γδ T cell subsets (52–54) validates this assumption with regard to serum Ig levels in non-immunized mice (50). Specifically, we found that mice deficient in Vγ1+ cells (B6.TCR-Vγ1−/−) generally had diminished antibody levels (with the exception of IgE), whereas B6.TCR-Vγ4−/−/6−/− mice had increased antibody levels (with the exception of IgG3 and IgA). This mouse strain also developed autoantibodies. The net-effect of γδ T cells assessed in mice deficient in all γδ T cells (B6.TCR-δ−/−) was neutral (for IgM, IgG3, IgG2c and IgA) or enhancing (for IgG1, IgG2b, and IgE). Several of the effects on the antibodies in γδ-deficient mice could be linked to changes in IL-4 production (50). Furthermore, B6.TCR-Vγ4−/−/6−/− mice displayed changes in granulocytes (50) likely to be associated with increased levels of IgE in this mouse strain (55).
Having observed such profound effect of γδ T cell composition on serum antibodies in non-immunized mice, and on IL-4 production (50), we wondered at which stage(s) in B cell development γδ T cells might intervene to effect changes in circulating antibodies. Here we report that γδ T cells begin to shape pre-immune B cell populations during the transitional stage in the spleen, eventually affecting all major populations of mature B cells. Additional data suggest that splenic γδ T cells modulate peripheral B cell populations in part through direct interactions with B cells that migrate through or reside within the MZ.
Materials and Methods
Mice
C57BL/6 mice and γδ T cell-deficient mice of the same genetic background (B6.TCR-δ−/−) were originally obtained from The Jackson Laboratory and bred at NJH. TCR-Vγ4−/−/Vγ6−/− mice were a gift from Dr. K. Ikuta (Kyoto University, Kyoto, Japan), were then backcrossed onto the C57BL/6 genetic background, and re-established after 11 backcross generations. B6.TCR-Vγ1−/− mice were a gift from Dr. Simon Carding (Norwich Med. Sch., Norwich, UK) and distributed by Dr. C. Wayne Smith (Baylor College of Medicine, Houston, TX). B6.TCR-Vγ1tg mice were a gift from Dr. Pablo Pereira (Inst. Pasteur, Paris, France. B6.IL-4−/− mice (C57BL/6-Il4tm1Nnt/J) were obtained from JAX (Bar Harbor, ME) and were a gift from Dr. P. Marrack at NJH. Double knockout (KO) mice were generated by crossing the corresponding mutant strains and selecting double KO mice in the F2 generation. These mice (TCR-Vγ4−/−/Vγ6−/−/IL-4−/−) were then bred as new homozygous strain. All mice were cared for at National Jewish Health (NJH) (Denver, CO), following guidelines for normal and immune deficient animals, and all experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee.
Flow cytometric analysis
Cells obtained from single cell suspensions (2×105/well) were stained in 96 well plates (Falcon; BD Biosciences, Franklin Lakes, NJ) for the cell surface markers shown in the figures/tables, using the specific mAbs and derivatized reagents listed in Table 1. CD93pos cells were detected using mAb AA4.1. Live cells were always gated based on forward and side scatter characteristics (lymphocyte gate), and unless indicated otherwise, forward scatter height and amplitude, and side scatter width and amplitude (to exclude or specifically include cellular conjugates), as well as expression of various B- or T cell markers (Table 1). All samples were analyzed on a LSRII flow cytometer, counting a minimum of 25,000 events per gated region, and the data were processed using FlowJo 9.5.2 software (FlowJo LLC, Ashland, OR).
Table 1.
Anti-mouse antibodies and secondary reagents used in flow cytometry
| Antibody | Clone | Dyes/Conjugates | Source |
|---|---|---|---|
| Anti-CD3ɛ | 145-2C11 | PE-Cy7 | Biolegend |
| Anti-CD5 | 53–7.3 | PE | BD Bioscience |
| Anti-B220 | RA3-6B2 | APC-Cy7 | Biolegend |
| Anti-CD19 | 1D3 | APC/ Biotin | eBioscience |
| Anti-CD22 | OX97 | FITC | Biolegend |
| Anti-CD23 | B3B4 | PE-Cy7 | eBioscience |
| Anti-CD25 | PC61 | PE | BD Bioscience |
| Anti-CD32 | 24G2 | FITC | In house |
| Anti-CD38 | 90 | PE | eBioscience |
| Anti-CD44 | 1M7 | PE | BD Bioscience |
| Anti-CD62L | MEL-14 | PE | BD Bioscience |
| Anti-CD69 | H1.2F3 | PE | BD Bioscience |
| Anti-CD80 | 16-10A1 | PE | Biolegend |
| Anti-CD86 | GL-1 | PE | Biolegend |
| Anti-CD93 | AA4.1 | APC | eBioscience |
| Anti-CD40L | MR1 | PE | BD Bioscience |
| Anti-ICOS | 15F9 | PE | BD Bioscience |
| Anti-MHC II | M5/114 | PE | eBioscience |
| Anti-IgKappa | 187.1 | FITC | Southern Biotech |
| Anti-IgM | B7.6 | FITC/DyLight 488 | In house |
| Anti-NK1.1 | PKH136 | APC | eBioscience |
| Anti-TCR-β | H57-597 | PE-Cy5 | BD Bioscience |
| Anti-TCR-δ | eBioGL3 | APC | eBioscience |
| Anti-TCR-δ | GL3 | FITC/Biotin | In house |
| Anti-TCR-Vγ1 | 2.11 | ||
| Anti-TCR-Vγ4 | UC3 | ||
| PNA | FITC | Vector Laboratories | |
| Streptavidin | FITC | eBioscience | |
| Streptavidin | PE-Cy5 | ||
| Streptavidin | APC | ||
| Streptavidin | eFluor 450 |
List of staining reagents
Nomenclature
Throughout this article, we use the nomenclature for murine TCR-Vγ genes introduced by Heilig and Tonegawa (56).
T cell purification
Suspensions of splenocytes were prepared by mechanical dispersion, treated with Gey’s solution for lysis of red blood cells, and passed through nylon wool columns to obtain T lymphocyte-enriched cell preparations, as previously described (57). Enriched cells were then incubated with biotinylated anti TCR antibodies (mAb GL3, anti TCR-δ or mAb 2.11, anti TCR-Vγ1) for 15 min at 4°C, washed and incubated with streptavidin-conjugated magnetic beads (Streptavidin Microbeads; Miltenyi Biotec, Bergisch Gladbach, Germany) for 15 min at 4°C, and passed through magnetic columns to purify total γδ T cells, as previously described in detail (58). This produced cell populations containing >85% viable γδ T cells as determined by dye exclusion and staining with specific anti TCR mAbs. These cells were used for cell transfer and co-culture experiments. CD8+ and CD8− Vγ1 subpopulations were sorted using a Sony/iCyt Synergy fluorescence activated cell sorter based on their distinctive phenotypes (CD3+TCR-β−TCR-δ+TCR-Vγ1+CD8+ and CD3+TCR-β−TCR-δ+TCR-Vγ1+CD8−, respectively).
ELISPOT assay for Ig-producing B cells
High protein-binding microlon ELISA plates were coated with either 3 µg/ml polyclonal goat anti-mouse Ig(H+L) or goat anti-mouse IgG1 (Southern Biotechnology Associates) in 1× PBS overnight at 4°C. Plates were blocked with ICTM for 30 min at room temperature. Splenocytes (5.0 × 105) were added to the first well of a row and titrated in serial 2-fold dilutions in ICTM. After 7 hrs, plates were washed three times with 0.05% Triton X-100 in 1x PBS. Biotinylated goat anti-mouse detecting antibodies were then applied at 0.5 ng/ml in blocking buffer and allowed to incubate overnight at 4°C. Biotinylated anti-IgKappa and anti-IgG were paired with goat anti-mouse Ig(H+L) coating antibody to determine total Ig- and IgG-producing cells, respectively. For detecting IgG1-producing cells, biotinylated anti-IgG was used to pair with anti-IgG1 coating antibody. Plates were washed in 1× PBS, and streptavidin-alkaline phosphatase (Biolegend) was applied at a dilution of 1:2000 in blocking buffer. After washing, plates were developed in 100 mM Tris-HCl (pH 9.5), 100 mM NaCl, and 10 mM MgCl2 with 1 mg/ml 5-bromo-4-chloro-3-indolyl phosphate (Pierce) for 2 hrs at 37°C. Plates were scanned into TIFF images for blinded counting.
Adoptive transfer of γδ T cells
For in vivo cell transfer, magnetic bead-purified cells were washed in PBS, re-suspended to a concentration of 2.5×107 cells/ml in PBS, and 5×106 cells/mouse were injected in 200 µl PBS via the tail vein of the transfer recipient.
Co-culture of B cells and γδ T cells
For co-culture experiments, MZ B-rich B cells were purified by labeling splenocytes from B6.TCR-Vγ1−/− mice with anti CD43-conjugated beads, followed by magnetic separation. The flow through was collected and contained >90% viable B220+CD43− B cells. These purified B cells at 2×106 per ml in culture medium were incubated with or without the addition of total Vγ1pos γδ T cells (1×106 cells/ml), or with CD8pos or CD8neg fractions of Vγ1pos cells (0.5×106 cells/ml). Cells were collected after 60 hours of cell culture, stained with the indicated antibodies, and analyzed by flow cytometry.
In vivo labeling of spleen cells
We followed the protocol described by Barral et al. (59), with minor modifications. Briefly, mice were injected via the tail vein with an antibody specific for the pan-lymphocytic marker CD45 (mAb clone 104, anti CD45.2 conjugated with PE or Pacific Blue), at 2 µg antibody mouse in 200 µl PBS, euthanized after 20 min of in vivo incubation, and single cell suspensions of spleen cells were prepared after first perfusing the spleens with PBS to wash out unbound antibodies. Splenocytes were then stained with specific antibodies to identify lymphocyte subsets as indicated in the figures, and analyzed cytofluorimetrically.
Statistical analysis
Data are presented as means +/− SD. The unpaired t test was used for two group comparisons, and ANOVA was used for analysis of differences in three or more groups. Statistically significant levels are indicated as follows: NS, not significant, * p < 0.05, ** p < 0.01, *** p < 0.001.
Results
Altered B cells in the peritoneal cavity, lymph nodes and blood; no change of immature B cells in bone marrow
We previously reported that non-immunized mice deficient in individual γδ T cell subsets have changed levels of serum immunoglobulins, and one strain developed autoantibodies (50). The latter strain, which is deficient in Vγ4pos and Vγ6pos γδ T cells (B6.TCR-Vγ4−/−/6−/−), has much elevated serum levels of IgE and IL-4, and T cells (both αβ T cells and residual γδ T cells) that secrete larger quantities of this cytokine (50). IL-4, originally termed B cell stimulatory factor-1, acts on resting B cells, drives their maturation (60) and, when over-expressed, can promote autoimmunity and allergic-like inflammatory disease (61). These observations, which uncovered an altered regulatory environment for B cells in partially γδ-deficient mice, led us to examine the B cells themselves.
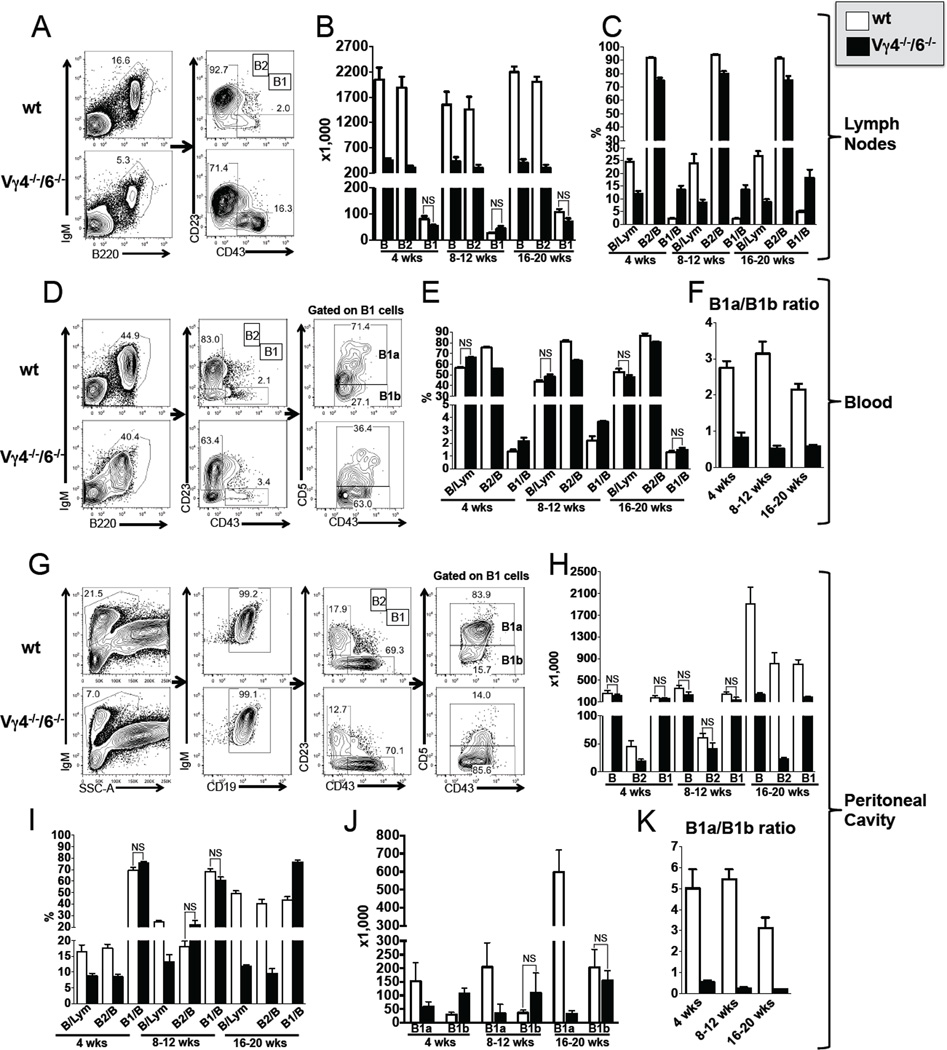
Comparing wt and B6.TCR-Vγ4−/−6−/− adult peripheral B cell populations, we found several differences in lymph nodes, blood and peritoneal cavity (Figure 1, Table 2). Total B cells (IgMposB220pos) in the lymph nodes of all, and peritoneal cavity of the older mutant mice were dramatically decreased, mainly because of decreases in B2 B cells (IgMposB220posCD23posCD43neg). This was unexpected given that IL-4 (50) and BAFF (not shown), which are elevated in these mice, promote B cell growth (8, 60). Even more surprising, the removal of IL-4 in B6.TCR-Vγ4−/−/6−/−/IL-4−/− mice restored peripheral B cell numbers (Table 2). Although numbers of B1 B cells (IgMposB220posCD23negCD43pos) were not substantially changed, their phenotype was altered, in blood and peritoneal cavity (Figure 1, Table 2). B1 B cells have been divided into two subsets based on the expression of the inhibitory receptor CD5 (16). In B6.TCR-Vγ4−/−/6−/− mice, cells expressing CD5 at high levels (B1a B cells) were much diminished in numbers and relative frequency whereas cells expressing CD5 at low levels (B1b B cells) were increased. Given that IL-4 inhibits CD5 expression (16), this change was predictable. Moreover, the restored composition of B1 B cells in B6.TCR-Vγ4−/−/6−/−/IL-4−/− mice confirmed the inhibitory role of IL-4 (Table 2). The changes were quite stable and seen in mice between the ages of 4 and 20 weeks (Figure 1F,K). Subsequently, we also examined mice deficient in Vγ1pos γδ T cells (B6.TCR-Vγ1pos) and in all γδ T cells (B6.TCR-δ−/−)(Table 2). In lymph nodes and peritoneal cavity, B6.TCR-δ−/− mice had normal or somewhat enlarged B cell populations, including both B2 and B1 B cells, with a normal B1a/B1b B cell ratio (peritoneal cavity), and similar results were obtained with B6.TCR-Vγ1pos mice although peritoneal B1a B cells were increased here, presumably due to the retention of IL-4-suppressive (Vγ4pos) and absence of IL-4-producing (Vγ1pos) γδ T cells in these mice (50). In sum, the data show that the particular γδ deficiency in B6.TCR-Vγ4−/−/6−/− mice has a large effect on peripheral B cells whereas the absence of Vγ1pos γδ T cells, or of all γδ T cells, affects peripheral B cell populations more subtly (but see distinct effects with splenic B cells, below).
Figure 1. Influence of γδ T cells on peripheral mature B cell populations.
(A–K) Comparison of B cell populations in female C57BL/6 (B6) and B6.TCR-Vγ4−/−/Vγ6−/− (Vγ4−/−/6−/−) mice. Total B cells, B1 and B2 B cells, and B1a and B1b B cells were identified using the indicated markers. Panels A, D and G show representative staining profiles of individual 8 wks old mice. (A–C) Lymph node B cells: Panels B and C show total numbers and relative frequencies of inguinal lymph node B cells (both sides pooled), respectively, comparing mice of different ages. The frequency of total B cells was calculated relative to total lymphocytes, and the frequencies of B1 and B2 B cells relative to total B cells. (D–F) Blood B cells: Panels E and F show relative frequencies (total B, B1 and B2) and B1a/B1b ratios, respectively, comparing mice of different ages. (G–K) Peritoneal cavity B cells: Panels H and I show total numbers and relative frequencies, respectively, comparing mice of different ages. Panels J and K show total numbers of B1a and B1b cells and their ratio, respectively, comparing mice of different ages. n ≥ 4 mice per group. For clarity, only comparisons where no significant differences were found are marked (NS), all others are significant at a P value of <0.05 or less.
Table 2.
Peripheral B cells in genetically γδ T cell-deficient mice
| Percentage (Absolute cell #) |
B6 (n=8) |
δ−/− (n=4) |
Vγ1−/− (n=5) |
Vγ4−/−/6−/− (n=8) |
Vγ4−/−/6−/−/IL4−/− (n=7) |
|
|---|---|---|---|---|---|---|
| Lymph node | ||||||
| B/Lym (%) | 23.9±9.8 | 24.9±0.9 | 15.0±2.2 | 8.5±3.0§ | 27.5±7.9 | |
| (B cells (x106)) | (1.9±0.7) | (2.9±0.3)* | (1.6±0.5) | (0.4±0.2)¶ | (2.8±1.0) | |
| B2 B/B (%) | 94.0±1.6 | 92.9±0.9 | 62.3±6.2¶ | 79.9±4.5¶ | 91.5±2.3 | |
| (B2 B cells (x106)) | (1.7±0.6) | (2.7±0.3)* | (1.0±0.4)¶ | (0.3±0.1)¶ | (2.6±0.8) | |
| B1 B/B (%) | 2.1±0.6 | 3.0±0.4 | 6.3±1.9¶ | 13.5±4.8¶ | 3.3±0.8 | |
| (B1 B cells (x103)) | (54.7±39.6) | (92.9±21.6) | (100.2±47.5) | (43.8±22.5) | (97.2±52.2) | |
| Peritoneal cavity | ||||||
| B/Live (%) | 25.1±1.6 | 21.4±4.0 | 32.0±7.5* | 13.3±5.3¶ | 26.0±9.5 | |
| (B cells (x103)) | (394.0±148.6) | (530.2±31.8*) | (1027.5±465.3¶) | (227.2±153.3) | (566.7±243.8) | |
| B2 B/B (%) | 18.5±4.1 | 20.7±7.0 | 8.2±2.4¶ | 21.9±7.3 | 21.8±9.1 | |
| (B2 B cells (x103)) | (57.4±17.6) | (54.3±4.8) | (84.6±40.2) | (41.0±25.9) | (122.5±87.4) | |
| B1 B/B (%) | 67.5±5.6 | 66.2±7.6 | 70.8±2.7 | 60.8±6.8 | 57.8±17.9 | |
| (B1 B cells (x103)) | (269.5±112.2) | (346.5±31.2) | (727.4±334.2§) | (143.7±104.84) | (384.1±176.0) | |
| B1a B/B1 B (%) | 83.5±2.3 | 83.8±2.8 | 88.0±1.8 | 20.5±7.1¶ | 74.8±6.1* | |
| (B1a B cells (x103)) | (226.7±98.0) | (290.9±35.7) | (642.8±305.9§) | (34.4±32.9¶) | (273.9±137.3) | |
| B1b B/B1 B (%) | 16.2±2.3 | 15.9±2.7 | 11.5±1.7 | 79.1±6.8¶ | 25.0±6.2* | |
| (B1b B cells (x103)) | (42.0±14.7) | (54.3±4.8) | (81.5±31.6*) | (109.0±73.8*) | (109.8±57.4*) | |
| B1a/B1b | 5.3±0.9 | 5.4±1.0 | 7.8±1.4§ | 0.3±0.1¶ | 3.2±1.1§ | |
| Blood | ||||||
| B/Lym (%) | 50.9±10.4 | 66.9±4.1* | ND | 48.1±5.3 | 68.1±11.0 | |
| B2 B/B (%) | 83.1±3.2 | 85.3±1.9 | ND | 63.1±2.1¶ | 89.6±2.3 | |
| B1 B/B (%) | 2.2±0.6 | 2.0±0.4 | ND | 3.7±0.2¶ | 1.4±0.5 | |
| B1a B/B1 B (%) | 73.3±6.5 | 70.5±7.1 | ND | 33.6±7.0¶ | 67.8±3.5 | |
| B1b B/B1 B (%) | 26.0±6.6 | 29.5±7.1 | ND | 65.4±7.0¶ | 32.3±3.5 | |
| B1a/B1b | 3.0±0.9 | 2.5±0.8 | ND | 0.5±0.2¶ | 2.1±0.3 | |
| Spleen | ||||||
| Total B cells | ||||||
| B/Lym (%) | 58.0±3.0 | 52.5±8.7 | 38.3±10.6§ | 29.3±1.9¶ | 52.0±2.7* | |
| (B cells) | (45.3±10.2) | (40.1±8.7) | (41.1±9.8) | (11.2±2.4)¶ | (45.6±21.3) | |
| Mature B cells | ||||||
| Mature B/Lym (%) | 46.8±2.0 | 43.9±9.7 | 32.3±8.8§ | 25.7±1.8¶ | 46.9±4.5 | |
| (Mature B cells) | (36.3±6.7) | (33.6±9.3) | (34.6±8.2) | (9.7±1.8)¶ | (41.4±20.8) | |
| MZB/Mature B (%) | 4.3±0.8 | 3.8±1.1 | 9.5±1.2¶ | 0.6±0.2¶ | 4.7±1.8 | |
| (MZB) | (1.6±0.4) | (1.3±0.6) | (3.3±0.9)¶ | (0.1±0.0) ¶ | (1.7±0.2) | |
| FOB/Mature B (%) | 84.0±1.4 | 81.2±3.6 | 74.9±9.2* | 62.7±6.5¶ | 80.0±2.2 | |
| (FOB) | (30.5±5.9) | (27.4±8.1) | (25.9±7.2) | (6.1±1.5)¶ | (32.9±15.8) | |
| NewB/Mature B (%) | 8.3±1.3 | 11.2±4.4 | 10.5±4.0 | 32.2±8.9¶ | 11.4±3.6 | |
| (NewB) | (3.0±0.6) | (3.6±0.9) | (4.8±3.3) | (3.1±0.9) | (5.3±4.3) | |
| Immature B cells | ||||||
| Immature B/Lym (%) | 9.5±1.1 | 8.6±2.4 | 6.1±2.2§ | 3.9±0.4¶ | 6.1±0.9¶ | |
| (Immature B) | (8.1±1.2) | (3.4±0.9) | (2.6±1.3) | (2.1±1.1)¶ | (5.7±1.0)* | |
| T1/Imm (%) | 27.4±1.2 | 25.4±1.0 | 27.6±0.9 | 48.0±1.2¶ | 25.8±3.3¶ | |
| (T1) | (2.1±0.2) | (1.5±0.3) | (2.0±0.3) | (1.0±0.1)¶ | (1.6±0.5) | |
| T2+T3/Imm (%) | 59.9±1.5 | 59.8±1.6 | 61.1±1.2 | 33.4±1.1¶ | 55.9±3.5 | |
| (T2+T3) | (5.0±0.8) | (3.3±0.6)§ | (4.3±0.5) | (0.7±0.1)¶ | (3.0±0.5) | |
| B2 cells | ||||||
| B2/B (%) | 76.9±3.0 | 78.9±0.4 | 32.5±2.8¶ | 58.7±5.2¶ | 80.2±0.5 | |
| (B2) | (36.5±10.4) | (31.6±8.0) | (13.2±3.6)¶ | (5.9±1.1)¶ | (37.8±18.3) | |
| B1 cells | ||||||
| B1/B (%) | 2.1±0.3 | 2.6±0.9 | 2.8±0.7 | 10.8±3.3¶ | 3.2±1.9 | |
| (B1) | (0.8±0.2) | (1.2±0.6) | (1.1±0.4) | (0.9±0.3) | (1.3±1.2) | |
p<0.05
p<0.01
p<0.001; ND, not determined
Peripheral B cells in various γδ T cell knockout mice. B cells in lymph nodes, peritoneal cavity, blood and spleen were identified based on their surface phenotype as described in Figures 1 and 3, and enumerated in individual mice. Absolute numbers (in parentheses) and relative frequencies are shown. n = 4–8 mice per group, p values, as indicated in the table.
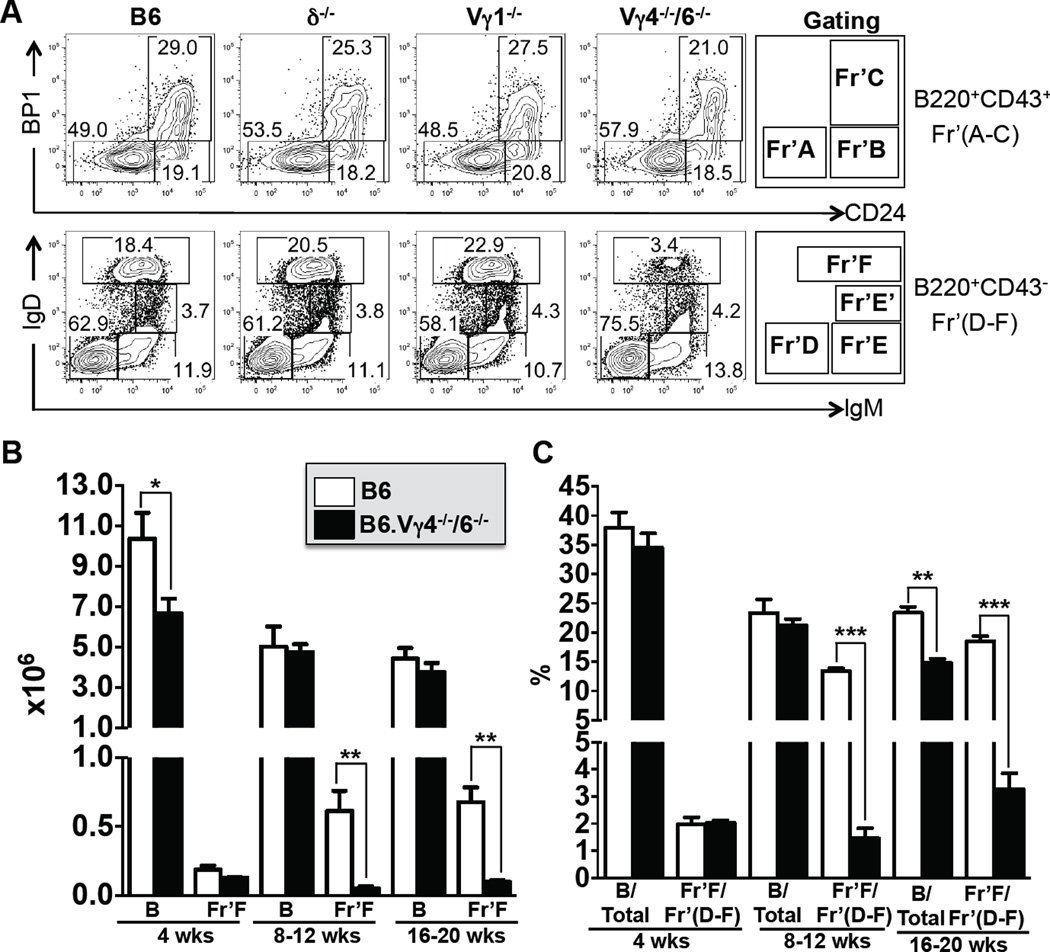
We next examined immature B cells in the bone marrow. Comparing bone marrow B cells from wt, B6.TCR-δ−/−, B6.TCR-Vγ1−/− and B6.TCR-Vγ4−/−6−/− mice, we hardly found any difference in immature B cell-types (Figure 2A, Table 3), including two developmentally late fractions of immature bone marrow B cells (Fractions E, E’) that give rise to some of the mature B cells in bone marrow as well as bone marrow emigrants (5). Only one fraction (Fraction F), which represents the mature IgDpos B cell population in bone marrow, was drastically reduced in B6.TCR-Vγ4−/−6−/− mice, both in absolute numbers and relative frequency (Figure 2A,B,C and Table 3). However, this fraction consists to a large extent of recirculating peripheral B cells (5, 6). To determine whether halted maturation in bone marrow or diminished recirculation are responsible for the loss of mature bone marrow B cells in B6.TCR-Vγ4−/−6−/− mice, we examined bone marrow B cells at several ages, with the older mice having a larger peripheral B cell compartment and increased potential for recirculation (Figure 2B, C). At 4 wks of age, fraction F mature bone marrow B cells in wt and B6.TCR-Vγ4−/−6−/− mice were essentially the same, both in absolute numbers and relative frequency, whereas between 8–20 wks of age, mature bone marrow B cells mice increased substantially in wt mice but not in B6.TCR-Vγ4−/−6−/− mice. This result is consistent with the interpretation that fewer returning peripheral B cells account for the smaller number of mature B cells in the bone marrow of B6.TCR-Vγ4−/−6−/− mice, and that B cell development during the bone marrow stages is unaffected by γδ T cells.
Figure 2. Immature B cells in bone marrow are not affected by γδ T cells.
(A) Comparison of bone marrow B cell populations in 8 wks old female C57BL/6 (B6), B6.TCR-δ−/− (δ−/−), B6.TCR-Vγ1−/− (Vγ1−/−) and B6.TCR-Vγ4−/−/Vγ6−/− (Vγ4−/−/6−/−) mice, representative examples. Bone marrow immature and mature B cells (Hardy fractions A-F) were identified using the indicated markers whereby fractions A-C were derived from B220posCD43pos and fractions D-F from B220posCD43neg cells. Fraction F represents mature B cells within the bone marrow. (B) Comparison of total and mature bone marrow B cell populations (fraction F) in female B6 and Vγ4−/−/6−/− mice at ages 4–20 wks in absolute numbers/mouse (left femur plus tibia) and (C) in frequency relative to total live cells or fractions D-F, respectively. For panels B and C, n = 4–8 mice per group. Only significant differences between wt and the γδ-deficient mice are marked. *P<0.05, **P<0.01, ***P<0.001
Table 3.
B cells in bone marrow from genetically γδ T cell-deficient mice
| Percentage % (Absolute cell # x106) |
B6 (n=13) |
δ−/− (n=6) |
Vγ1−/− (n=5) |
Vγ4−/−/6−/− (n=4) |
Vγ4−/−/6−/−/IL4−/− (n=5) |
|---|---|---|---|---|---|
| B cells/live cells | 29.0±3.9 | 29.7±4.0 | 23.5±3.6* | 24.4±1.7§ | 27.7±3.6 |
| (B cells) | (6.4±2.1) | (6.7±2.2) | (4.6±1.7) | (4.9±0.9) | (6.7±2.1) |
| Fr’A/(A–C) | 51.0±5.1 | 47.3±6.0 | 40.5±7.0* | 56.1±2.2 | 53.5±7.0 |
| (Fr’A) | (0.4±0.1) | (0.4±0.1) | (0.3±0.1)§ | (0.5±0.2) | (0.3±0.1) |
| Fr’B/(A–C) | 19.3±1.6 | 20.2±3.0 | 30.4±9.3 | 18.2±5.1 | 18.4±4.0 |
| (Fr’B) | (0.2±0.1) | (0.2±0.0) | (0.2±0.2) | (0.2±0.0) | (0.1±0.0) |
| Fr’C/(A–C) | 26.2±4.3 | 28.7±3.6 | 24.2±3.6 | 25.5±3.5 | 23.6±5.0 |
| (Fr’C) | (0.2±0.1) | (0.2±0.0) | (0.2±0.1) | (0.3±0.1) | (0.1±0.0) |
| Fr’D/(D–F) | 59.5±4.1 | 59.7 ±3.8 | 47.8±5.5§ | 72.4±9.8* | 64.4±4.7 |
| (Fr’D) | (3.3±1.2) | (4.0±1.1) | (1.9±0.9)* | (3.1±0.6) | (3.8±1.2) |
| Fr’E/(D–F) | 15.9±2.4 | 14.1±2.0 | 15.5±1.6 | 17.7±8.0 | 14.5±2.5 |
| (Fr’E) | (0.9±0.3) | (1.1±0.3) | (0.6±0.3) | (0.7±0.3) | (0.9±0.3) |
| Fr’E’/(D–F) | 4.2±0.6 | 4.1±0.6 | 5.1±0.3§ | 4.1±0.9 | 3.4±1.1 |
| (Fr’E’) | (0.2±0.1) | (0.2±0.1) | (0.2±0.1) | (0.2±0.0)* | (0.2±0.1) |
| Fr’F/(D–F) | 16.3±4.7 | 18.6±2.4 | 20.9±6.4 | 2.1±1.5¶ | 14.4±1.8 |
| (Fr’F) | (0.9±0.4) | (1.1±0.3)* | (0.8±0.3) | (0.1±0.1)¶ | (0.8±0.2) |
p<0.05
p<0.01
p<0.001
Bone marrow B cells in various γδ T cell knockout mice. B cell types were identified based on their surface phenotype as in Figure 2. Following a scheme first described by Hardy et al. (92), we divided B220posCD43pos early stage bone marrow B cells into developmentally consecutive fractions A-C based on their expression of CD24 and BP1. These cells represent pro-B and early pre-B cells. Later stage B220posCD43neg B cells, which include those that returned from the periphery, were divided into increasingly mature fractions D (pre-B cells), E and F (B cells), based on their expression of IgM and IgD. Cells contained within these fractions were enumerated in individual mice. Absolute numbers (in parentheses) and relative frequencies are shown. n = 4–13 mice per group, p values, as indicated in the table.
Changed mature and transitional B cells in the spleen
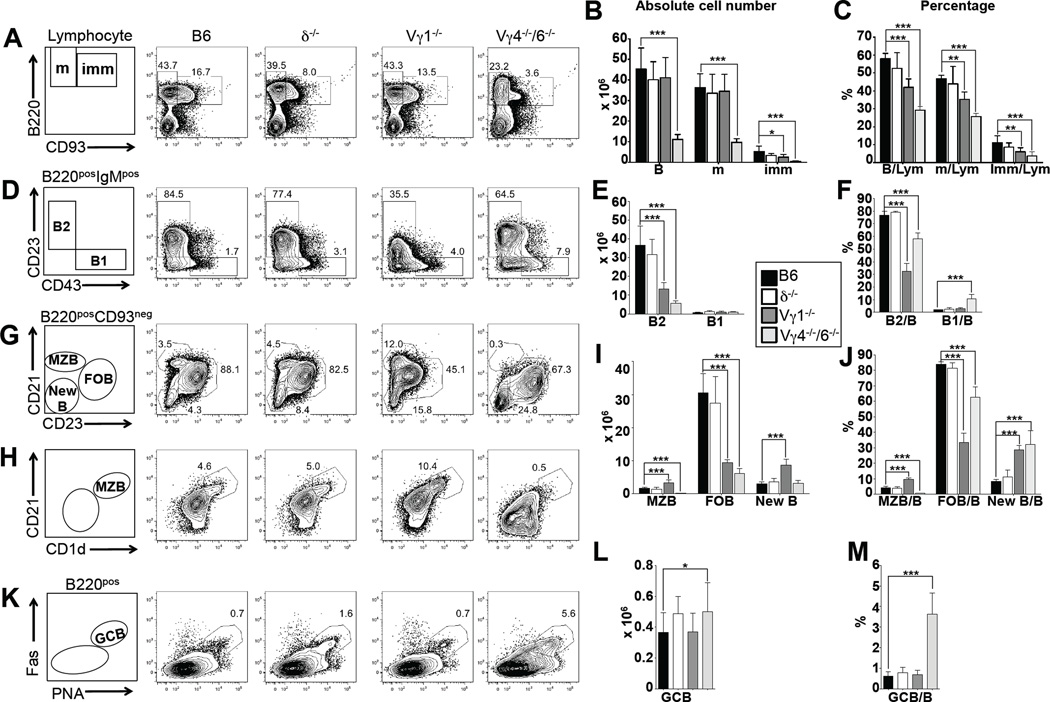
Having found substantial changes among mature peripheral but not immature bone marrow B cells, we proceeded to examine the intermediate stages of B cell development in the spleen (Figure 3, Table 2). We divided B220pos splenic B cells into mature CD93neg and immature CD93pos cells (Figure 3A), and further subdivided the mature B cells into follicular B cells (FOB, CD23posCD21int), marginal zone B cells (MZB, CD23negCD21hi or CD1dhiCD21hi) and “new” B cells (New, CD23negCD21neg) (Figure 3G, I, J, H) (62). We also identified B1 B cells in the spleen among B220posIgMpos cells based on their CD23negCD43pos phenotype (Figure 3D, E, F), and germinal center B cells (GCB) based on their distinctive CD38negFasposPNAhi phenotype (50) and (Figure 3K, L, M). Comparing the same panel of mice as before for these splenic B cell populations, we found that mice lacking all γδ T cells (B6.TCR-δ−/−) had nearly unaltered B cell populations. In contrast, mature and immature splenic B cell populations in B6.TCR-Vγ4−/−6−/− mice were diminished in numbers and relative frequencies (Figure 3A, B, C), and specifically B2 B cells. FO B cells were much diminished but MZ B cells were nearly wiped out (Figure 3G, H, I, J). In contrast, numbers of B1 B (Figure 3B, E, F) were relatively stable, and GC B cells (Figure 3K, L, M) in these mice were relatively increased. Again, removing IL-4 (B6.TCR-Vγ4−/−/6−/−/IL-4−/− mice) reversed all of these changes (Table 2) suggesting that the elevated IL-4 levels in B6.TCR-Vγ4−/−6−/− mice are responsible for the changes in their splenic B cells. B6.TCR-Vγ1−/− mice, on the other hand, showed little changes in numbers of mature and immature splenic B cells, although their MZ and “new” B cells were significantly increased. Apparent decreases in FO B cells in these mice seem to be merely a function of the lower CD23 expression, and a different gating strategy for FO B cells (based on CD21 and IgM expression) revealed normal FO B cell numbers (supplemental Figure 1). Nevertheless, the low CD23 expression in B6.TCR-Vγ1−/− mice is a distinctive trait (supplemental Figure 4), likely to have functional consequences in the IgE responses. CD23 is positively regulated by IL-4 (14), and was diminished in B6.TCR-Vγ4−/−/6−/− mice by ablation of IL-4 (supplemental Figure 4). Furthermore, it was diminished in wt mice by treatment with anti Vγ1 mAbs (supplemental Figure 4), partially restored in cultured splenic B cells of B6.TCR-Vγ1−/− mice by adding IL-4 in vitro (supplemental Figure 4), and much induced in B cells of B6.TCR-δ−/− mice following transfer of Vγ1pos cells from B6.TCR-Vγ4−/−/6−/− mice (supplemental Figure 4). Another distinctive trait of B6.TCR-Vγ1−/− mice is their enlarged population of MZ B cells (Figure 3G,H,I,J, supplemental Figure 1), in direct contrast to the diminished MZ B cells in B6.TCR-Vγ4−/−6−/− mice. The mere absence of Vγ1pos γδ T cells in B6.TCR-Vγ1−/− mice does not account for these traits because Vγ1pos cells are also missing in B6.TCR-δ−/− mice, which have normal CD23 expression and numbers of MZ B cells. Instead, they again probably reflect a changed function of the γδ T cells that remain in B6.TCR-Vγ1−/− mice mice. In sum, the data revealed that mature B cells in the spleen are sensitive to the influence of γδ T cells, and that much of this influence depends on IL-4.
Figure 3. Influence of γδ T cells on splenic B cell populations.
(A–M) Comparison of B cell populations in 8 wks old female C57BL/6 (B6) (black columns), B6.TCR-δ−/− (δ−/−) (open columns), B6.TCR-Vγ1−/− (Vγ1−/−) (dark grey columns) and B6.TCR-Vγ4−/−/Vγ6−/− (Vγ4−/−/6−/−) (light grey columns) mice. Mature and immature B cells, B1 and B2 B cells, marginal zone B cells (MZB), follicular B cells (FOB), “new” B cells (New B) and germinal center B cells (GCB) were identified using the indicated markers. Panels A, D, G, H, and K show representative staining profiles of individual mice. (A–C) Relative frequencies (compared to total B cells) and absolute numbers of mature (m) and immature (imm) B cells. Panels B and C also show numbers and frequencies (compared to total splenic lymphocytes) of total B cells. (D–F) Relative frequencies (compared to total B cells) and absolute numbers of B1 and B2 cells. (G–J) Relative frequencies (compared to total B cells) and absolute numbers of marginal zone B cells (MZB), follicular B cells (FOB) and “new” B cells (New B) B1. Panels G and H show two different ways of identifying MZB, based on expression of CD21 in combination with CD23 or CD1d. The counts of MZB in panels I and J are based on the method shown in panel H. (K–M) Relative frequencies (compared to total B cells) and absolute numbers of germinal center B cells (GCB). n = 5–8 mice per group. For visibility, only significant differences between wt and the γδ-deficient mice are marked. *P<0.05, **P<0.01, ***P<0.001
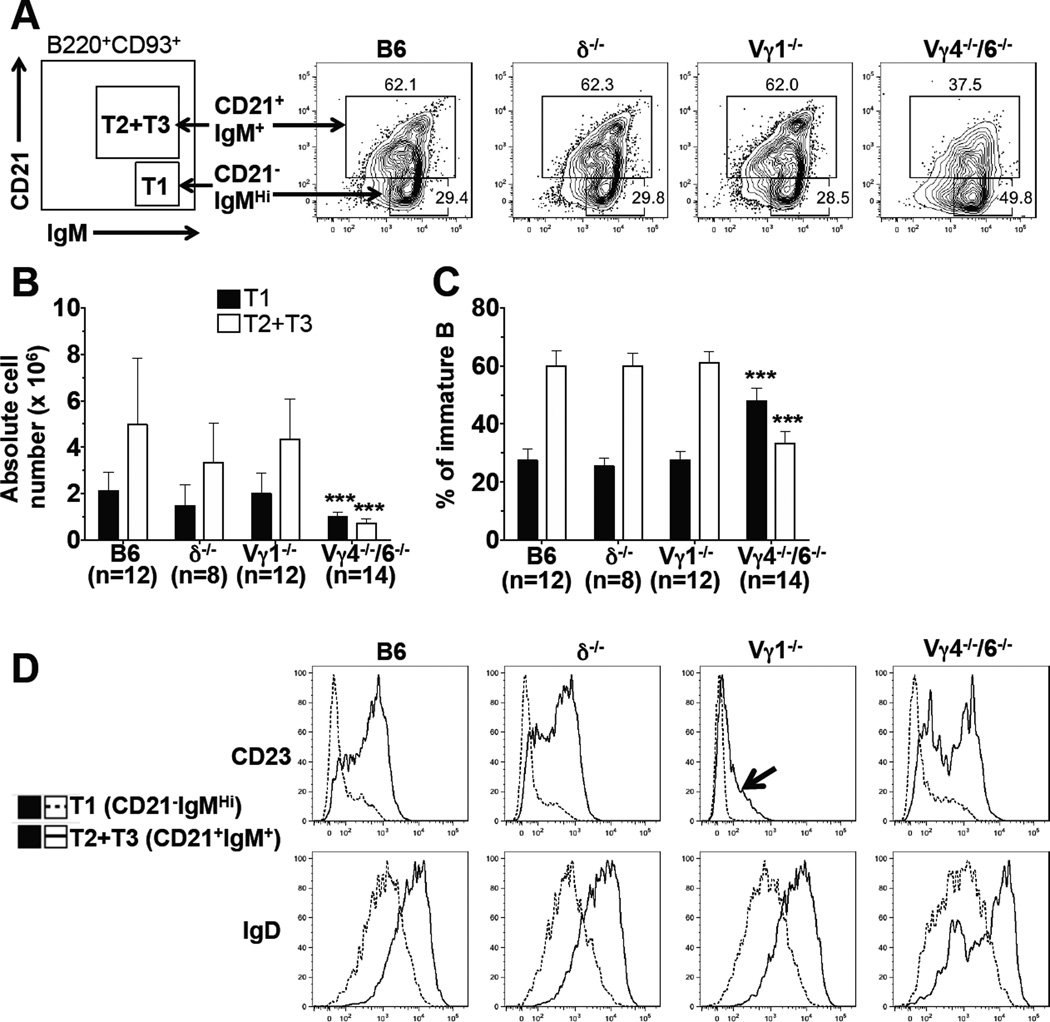
B cells newly arrived in the spleen can be divided into discrete transitional stages (T1-T3), distinguished by their surface phenotype and functional capability (17, 63). We identified immature B cells in the spleen of the test panel mice as IgMposB220posCD93pos cells, and further divided these cells into T1 (IgMhiCD21neg/lo) and T2 plus T3 (IgMposCD21pos) transitional subsets (Figure 4A). Furthermore, we analyzed both subsets for their expression of CD23 and IgD. At 8 wks of age, B6.TCR-δ−/− mice and B6.TCR-Vγ1−/− mice had nearly unchanged numbers of transitional B cells (Figure 4B), and relative frequencies of the transitional subsets were normal as well (Figure 4C). In contrast, B6.TCR-Vγ4−/−6−/− mice produced significantly fewer T1 and T2 plus T3 B cells (Figure 4B,C). Removal of IL-4 (B6.TCR-Vγ4−/−/6−/−/IL-4−/− mice) partially restored immature B cells, including T1 and T2 plus T3 transitional subsets (Table 2), suggesting that the diminished transitional B cell compartment in B6.TCR-Vγ4−/−6−/− mice is a indirect consequence of the deregulated IL-4 production in these animals (see below). CD23 and IgD expression revealed further differences between the transitional B cells of the test panel mice (Figure 4D). Hence, in contrast to immature bone marrow B cells, transitional B cells in the spleen were already altered, and thus might represent the earliest stage in B cell development affected by γδ T cells.
Figure 4. Genetic deficiency in γδ T cells alters transitional B cells in the spleen.
Comparison of transitional B cell populations in the spleens of 8 wks old female C57BL/6 (B6), B6.TCR-δ−/− (δ−/−), B6.TCR-Vγ1−/− (Vγ1−/−) and B6.TCR-Vγ4−/−/Vγ6−/− (Vγ4−/−/6−/−) mice. (A) Immature IgMposB220posCD93pos B cells in the spleen were further subdivided into CD21negIgMhi (T1) and CD21posIgMpos (T2 plus T3) B cells. The test panel mice were also compared for CD23 and IgD expression in the two subsets of transitional B cells. Representative examples are shown. (B) Absolute numbers of transitional B cells/spleen in 8 wks old mice and (C) relative frequencies. (D) Expression of CD23 and IgD in transitional B cells of wt and γδ-deficient mice. Profiles representative of at least three independent staining experiments are shown. For panels b and c, n = 8–14 mice per group. Significant differences between wt and the γδ-deficient mice are marked. ***P<0.001
Increased numbers of antibody-producing B cells in the spleen of B6.TCR-Vγ4−/−6−/− mice
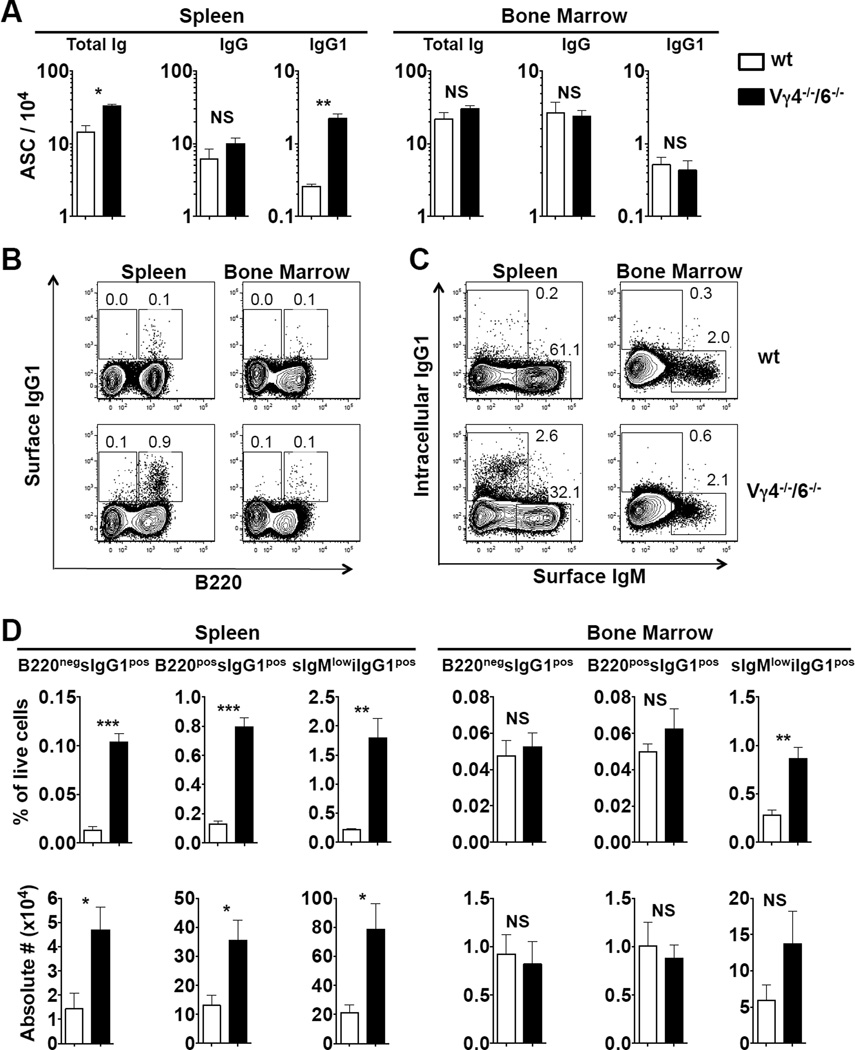
Untreated B6.TCR-Vγ4−/−6−/− mice have increased levels of circulating antibodies (50) but decreased numbers of mature B cells (this study). Both of these changes are IL-4-dependent. These mice also exhibit spontaneous germinal center formation in the spleen (50). Therefore, we compared numbers of antibody-producing B cells in this partially γδ-deficient strain and untreated wt mice (Figure 5). Total Ig producing cells in the spleen of 8–12 wks old B6.TCR-Vγ4−/−6−/− mice were increased > 2fold compared to wt mice, and IgG1-secreting cells > 7fold (Figure 5A). In contrast, there were no significant increased of antibody-producing B cells in bone marrow. IgG1 surface-positive B cells (B220neg and B220pos) were substantially increased but not in bone marrow (Figure 5B, D), and IgG1-secreting plasma cells (surface IgMlow, intracellular IgG1pos) were increased in the spleen as well (Figure 5C, D). Numbers of such cells in bone marrow were low and difficult to quantitate. Taken together, these data document increased numbers of antibody-producing B cells in the spleen but not in the bone marrow of B6.TCR-Vγ4−/−6−/− mice, and suggest that the increased levels of circulating antibodies in this mutant strain are a result of this cellular change.
Figure 5. Antibody-producing cells (ASCs) are increased in the spleen of Vγ4−/−/6−/− mice.
(A) Total Ig-, IgG-, and IgG1-producing cells determined by ELISPOT assay were enumerated in spleen and bone marrow of female C57BL/6 (wt) and B6.TCR-Vγ4−/−/Vγ6−/− (Vγ4−/−/6−/−) mice. n = 4 per group. (B–C) Representative FACS plots showing surface IgG1-expressing cells (sIgG1pos cells, panel B) and intracellular IgG1-expressing cells (iIgG1pos cells, panel C) in spleen and bone marrow of above mice. Panel D shows relative frequencies and total numbers in spleen and bone marrow of mice indicated in (A–C). n = 4 mice per group. NS, not significant, *P<0.5, **P<0.01, ***P<0.001
γδ T cells themselves shape splenic B cell populations
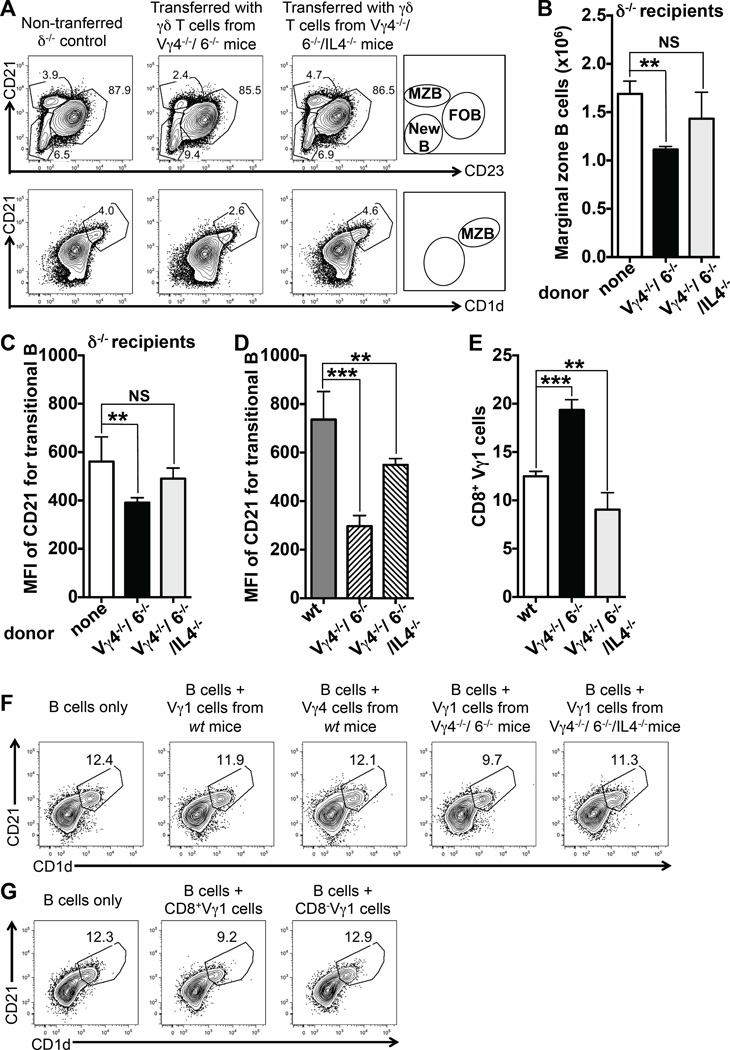
To address the question of whether γδ T cells themselves modulate the B cell populations, we took advantage of our earlier observation that residual γδ T cells in B6.TCR-Vγ4−/−6−/− mice, which mostly belong to the Vγ1pos subset, are changed (50). Such changes include higher relative frequencies and absolute numbers of IL-4-competent γδ T cells (50), altered TCR-Vδ expression among Vγ1pos γδ T cells (50) as well as a higher frequency of CD8 expression among these cells when IL-4 is present (see Figure 6E), suggesting IL-4-driven Tc2-like differentiation. We conducted cell transfer experiments with these changed cells, in a manner as previously described (55). Adoptive transfer of B6.TCR-Vγ4−/−6−/− -derived splenic γδ T cells into B6.TCR-δ−/− mice transiently restored splenic Vγ1pos cells in the cell transfer recipients, albeit only up to about 10% of the population size in wt mice (supplemental Figure 2). Still, the transferred γδ T cells selectively reduced MZ B cells in the transfer recipients (Figure 6A,B), replicating the trend seen in non-manipulated B6.TCR-Vγ4−/−6−/− mice (Figure 3). The similar cell transfer experiment shown in supplemental Figure 2 further extends this finding: here the transferred γδ T cells, which again reduced splenic MZB cells, did so despite the presence of recipient γδ T cells (recipient: B6.TCR-Vγ1−/−), which were unable to prevent this effect. When we examined transitional B cells in the B6.TCR-δ−/− cell transfer recipients, we found a reduction in CD21 expression (Figure 6C), also replicating the situation in B6.TCR-Vγ4−/−6−/− mice (Figure 6D). In all experiments, transferred γδ T cells derived from B6.TCR-Vγ4−/−/6−/−/IL-4−/− mice failed to induce these changes in B cells (Figure 6 and supplemental Figure 2), emphasizing the importance of IL-4 in the functional differentiation of the transferred γδ T cells. The dual effect of the transferred γδ T cells on transitional B cells, which must pass through the MZ (17), and on MZ B cells, which shuttle between follicles and marginal zone (64), is consistent with interactions between γδ T cells and B cells inside the splenic MZ.
Figure 6. Transferred residual γδ T cells alter splenic B cells in vivo and co-cultured residual γδ T cells selectively diminish MZ B cells in vitro.
(A) B6.TCR-δ−/− mice (δ−/−) were transferred with purified splenic γδ T cells from B6.TCR-Vγ4−/−/Vγ6−/− (Vγ4−/−/6−/−) or B6.TCR-Vγ4−/−/Vγ6−/−/IL-4−/− (Vγ4−/−/6−/−/IL-4−/−) mice. 10 days after the cell transfer, B cell populations in the spleen were compared as detailed for Figure 2, using the indicated markers. Data of one representative experiment are shown. (B) Effect of transferred residual γδ T cells on absolute numbers of MZB cells in the spleen of δ−/− mice, n = 4 mice per group. (C) Effect of transferred residual γδ T cells on CD21 expression in the spleen of δ−/− mice, n = 4 mice per group. (D) CD21 expression by transitional B cells in untreated wt, Vγ4−/−/6−/− and Vγ4−/−/6−/−/IL-4−/−, n = 4 mice per group. *P<0.05, **P<0.01, ***P<0.001 (E) The CD8pos fraction of Vγ1pos γδ T cells in the spleen: Relative frequencies of CD8pos γδ T cells within the splenic Vγ1pos subset of C57BL/6 (wt), B6.TCR-Vγ4−/−/6−/− (Vγ4−/−/6−/−) and B6.TCR-Vγ4−/−/Vγ6−/−/IL-4−/− (Vγ4−/−/6−/−/IL-4−/−) mice. n = 5 mice per group, **P<0.01, ***P<0.001 (F) CD43-negative MZ B cell-rich splenic B cells from B6-TCR-Vγ1−/− (Vγ1−/−) mice were cultured for 60 hrs alone or in the presence of splenic Vγ1pos γδ T cells from different mouse strains, and subsequently stained to identify MZ B cells. Only Vγ1pos cells from B6.TCR-Vγ4−/−/Vγ6−/− (Vγ4−/−/6−/−) mice substantially diminished MZ B cells. (G) Same B cells as in panel a were cultured alone or in the presence of CD8pos or CD8neg fractions of Vγ1pos cells from Vγ4−/−/6−/− mice, and subsequently stained to identify MZ B cells. Only Vγ1pos cells expressing CD8 diminished MZ B cells. Data panels F and G are representative of several similar experiments.
In addition, we tested for a possible effect of purified Vγ1pos γδ T cells on MZ B cells in vitro. After a culture period of 60 hrs without any added stimuli or growth factors, enriched CD43neg splenic B cells from B6.TCR-Vγ1−/− mice contained ~ 12% MZ B cells (Figure 6F). Co-culturing them with Vγ1pos γδ T cells from B6.TCR-Vγ4−/−/6−/− mice selectively diminished MZ B cells, similarly to the cell transfer experiments in vivo and consistent with the trend in B6.TCR-Vγ4−/−/6−/− mice, whereas γδ T cells from wt mice (either Vγ1pos or Vγ4pos), or from B6.TCR-Vγ4−/−/6−/−/IL-4−/− mice, failed to mediate this effect. Furthermore, to address the potential significance of the enlarged IL-4-dependent CD8pos subpopulation among Vγ1pos cells in B6.TCR-Vγ4−/−/6−/− mice, we compared purified CD8pos and CD8neg sub-fractions of Vγ1pos cells in B6.TCR-Vγ4−/−/6−/− mice for their effect on MZ B cells in the co-cultures (Figure 6G), and found that only the CD8pos Vγ1pos γδ T cells diminished MZ B cells. The effect in vitro - albeit consistent in its selectivity for MZ B cells with the effect in vivo – was comparatively small, which might reflect the importance of co-localization of γδ T cells and MZ B cells in the MZ in vivo. In sum, the combined results of the cell transfer and co-culture experiments suggest that the altered B6.TCR-Vγ4−/−/6−/− γδ T cells themselves are responsible for the changes of peripheral B cells in this strain.
γδ T-B cell interactions in the spleen may be facilitated by co-localization
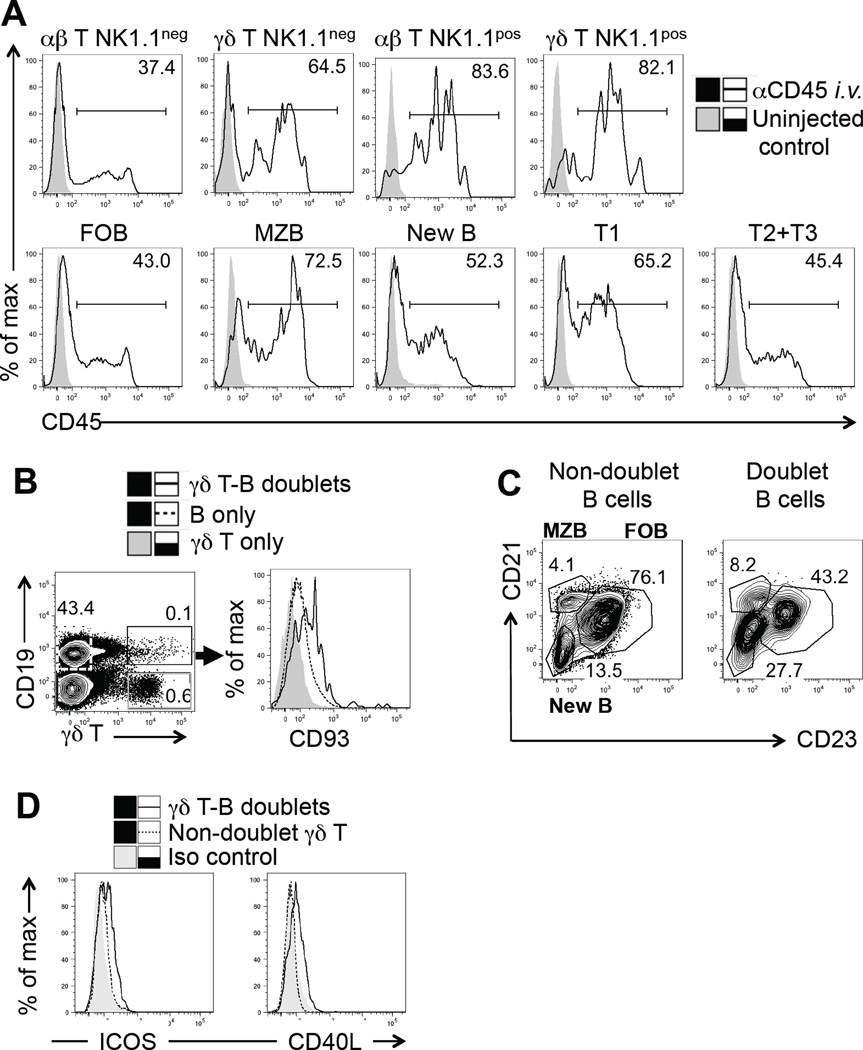
The compartments of the spleen differ in their accessibility to the circulation (65). Recently arrived immature B cells and MZ B cells migrate through or reside within the MZ, a splenic compartment far more accessible to the circulation than the follicles or the peri-arteriolar sheath (PALS) (59, 66). Because of technical difficulties in localizing splenic γδ T cells in wt mice by immunohistochemical methods (67), we instead assessed the exposure of splenic γδ T cells to the circulation. We i.v. injected antibodies specific for the ubiquitous leucocyte marker CD45, and compared labeling levels of the splenic lymphocyte-types at a fixed time point after the injection (Figure 7A). The broad range of labeling intensity in all cell populations examined likely reflects positional differences of individual cells within a given population. However, overall, the percentage of labeled γδ T cells in normal C57BL/6 mice was higher than that of αβ T cells, indicating that splenic γδ T cells tend to be more exposed to the circulation than αβ T cells. The percentage of labeled NK1.1pos γδ T cells was higher still, and similar to that of NKT cells. This finding in mice is consistent with histological studies in several other species placing γδ T cells in the red pulp and MZ of the spleen (68–72). Among immature B cells, T1 B cells were more exposed than T2 plus T3 B cells, as would be expected (17), and MZ B cells were most exposed among mature B cells, consistent with the literature (59). Although we found some differences in CD45 expression of the splenic cell types examined (based on in vitro antibody staining, see supplemental Figure 3B), the differential labeling in vivo did not correlate with these differences, rather reflecting differential circulation exposure than differential CD45 expression. Thus, circulation exposure assessed here and histological findings of others place γδ T cells, early transitional (T1) B cells and MZ B together inside the MZ. Secondly, we examined γδ T-B cell conjugates in fresh splenocyte preparations. CD93pos immature B cells (Figure 7B) and MZ B cells (Figure 7C) were enriched in the conjugates, consistent with the notion of encounters and contact between splenic γδ T cells and B cells that pass through (T1 B cells) or reside within the MZ (MZ B cells). In turn, the γδ T cells making these contacts with B cells seemed to be “aware” of them as they expressed the activation markers CD40L and ICOS at higher levels than non-conjugated γδ T cells (Figure 7D).
Figure 7. Localization of γδ T cells and immature B cells in the spleen, propensity to form T-B-conjugates and activation state of γδ T cells in the conjugates.
(A) 8 wks old female C57BL/6 mice were injected i.v. with dye-conjugated antibodies specific for CD45, splenocytes were stained in vitro for subset-specific markers after a 20 min in vivo labeling period, and analyzed cytofluorimetrically. One example representative of six similar experiments is shown. (B) γδ T-B cell conjugates among splenocytes from 8 wks old female C57BL/6 mice were identified based on their simultaneous expression of γδ T cell (TCR-δ) and B cell markers (CD19), and B cells in the conjugates compared with non-conjugated B cells for their expression of CD93, a marker of immature B cells or (C) for their expression of CD23 and CD21 in order to differentiate “new”, MZ and FO B cells. Individual examples representative of four similar experiments are shown. (D) γδ T cells in conjugates with splenic B cells and non-conjugated γδ T cells were compared for their expression of CD40L and ICOS. One experiment representative of four using 8–12 wks old female C57BL/6 mice is shown.
Discussion
Overall, the current study shows that γδ T cells are capable of modulating pre-immune peripheral B cells populations. This work was inspired by our preceding study indicating that γδ T cells strongly affect Ig serum levels and autoantibody development in non-immunized mice (50). Both studies take advantage of three connected observations, namely first that a correlation exists between TCR-Vγ expression by subsets of murine γδ T cells and their function (73); second, that such subsets in isolation tend to have a larger effect on the immune responses than γδ T cells as a whole (74); and third, that the absence of a subset can lead to functional changes in the remaining γδ T cells (50). These features enable the manipulation of γδ T cell function in vivo, using TCR-Vγ knockout as approach. Having employed this approach to reveal the γδ-influence on antibody production and self-tolerance (50, 55), we now demonstrate effects on B cell homeostasis and get a first glimpse at underlying mechanisms.
In particular, we found in the current study that mice deficient in two γδ T cell subsets (B6.TCR-Vγ4−/−/6−/−), which have normal numbers of immature bone marrow B cells, nevertheless have much reduced numbers of total peripheral B cells. This occurs in the presence of elevated levels of IL-4 (50) and BAFF (data not shown), both of which would be expected to support B cell growth (8, 60). Indeed, antibody producing B cells are increased, consistent with the increased levels of serum Ig (50). The loss of peripheral B cells seems to take place during development in the spleen because levels of immature B cells in the spleen and derived mature B cell populations were all affected whereas immature B cells in bone marrow were not. Among mature splenic B cell populations, those that reside in or repeatedly shuttle in and out of the MZ - the MZ B cells (64) – were diminished most. Immature B cells, which also have to pass through the MZ on their way to the white pulp (75, 76), were hit as well. Combined, these observations implicate the splenic MZ as a critical site of the γδ-influence on peripheral B cells.
The splenic MZ is a portal for cells in transit from the blood stream to the white pulp (65). It also contains various types of resident cells that depend on each other for their localization and function (77). Besides reticular fibroblasts, these include marginal sinus lining cells as well as several distinct myeloid and lymphoid cell-types. Splenic γδ T cells have been localized in the MZ as well, and within the red pulp, in humans, cattle, sheep, camels and birds (68–72). We previously reported that splenic γδ T cells in mice acquire blood-borne antigen (78), consistent with a similar localization of these γδ T cells. The data of the current study further support this notion: (i) splenic γδ T cells in normal mice were stained well by i.v. injected antibodies, indicating an exposure to the circulation similar to that of early transitional B cells and MZ B cells, (ii) splenic γδ T cells in αβ T cell-deficient mice, where γδ T cells move into the circulation-inaccessible PALS (67), were no longer well-stained by i.v. injected antibodies (data not shown), (iii) immature B cells and MZ B cells were enriched in splenic γδ T – B cell conjugates, and (iv) γδ T cells affected MZ B cells far more than the other mature B cell types. Due to their precursor progeny-relationship, the still substantial but smaller effect of the γδ T cells on other mature B cells likely is a consequence of their interaction with transitional B cells, which must pass through the MZ as well (17).
The cell transfer and co-culture data, and the data co-localizing B cells and γδ T cells in the MZ all suggest that action by splenic γδ T cells themselves, either direct or indirect, is responsible for the diminished peripheral B cells in B6.TCR-Vγ4−/−/6−/− mice. Splenic γδ T cells in these mice are mainly Vγ1pos, and they are also altered in composition and function (50), partly under the influence of IL-4, which drives expansions of both NKT-like (50) and CD8posTc2-like (79–81) Vγ1pos cells (this study). Indeed, adoptively transferred γδ T cells from the spleen of B6.TCR-Vγ4−/−/6−/− mice selectively diminished MZ B cells in the recipient mice. The transferred γδ T cells also lowered CD21 expression in transitional B cells, consistent with the changed phenotype of these B cells in B6.TCR-Vγ4−/−/6−/− mice. Both of these results support the notion of γδ T- B cell interactions in the MZ, but do not rule out such interactions elsewhere. The transfer experiments also underscore the role of IL-4 because γδ T cells obtained from B6.TCR-Vγ4−/−/6−/−/IL-4−/− mice failed to affect the B cells. Normal levels of IL-4 seem to be sufficient for some action of Vγ1pos γδ T cells in the spleen because wt C57BL/6 mice had significantly fewer MZ B cells than did B6.TCR-Vγ1−/− mice. However, in B6.TCR-Vγ4−/−/6−/− mice with their hyperplastic Vγ1pos γδ T cell population and increased IL-4 production, the effect is exacerbated, leading to substantial reductions in mature splenic B cells and a near disappearance of MZ B cells. Even co-cultured B6.TCR-Vγ4−/−/6−/− -derived Vγ1pos γδ T cells in vitro reproducibly diminished MZ B cells more than other B cells, although the effect was comparatively small. Both direct and indirect mechanisms might contribute to these γδ-dependent changes in peripheral B cells and serum Ig levels. The elevated IL-4 in B6.TCR-Vγ4−/−/6−/− mice potentially could drive premature switching of immature bone marrow B cells (82) but we did not see substantial changes here. The loss of mature splenic B cells in B6.TCR-Vγ4−/−/6−/− mice could be the result of accelerated maturation and differentiation into plasma cells, consistent with the increased numbers of antibody producing cells in the spleen of these mice (this study) as well as the elevated levels of circulating antibodies (50). This mechanism seems to be IL-4-dependent because the changes in peripheral B cells are absent when IL-4 is missing. The preferential loss of MZ B cells likely is due to the greater propensity of these cells to form plasma cells (83). In addition, their co-localization with γδ T cells in the spleen might make these B cells more available for the γδ influence. As well, MZ B cells might be more likely to interact with γδ T cells due to their differential expression of ligands for the γδ TCR such as the molecules CD1d and T-22 (84–86). Finally, we cannot exclude a role of changed microbiota (87) in the γδ-deficient mice but this seems less probable under conditions of transient reconstitution.
Although the massive loss of peripheral B cells in B6.TCR-Vγ4−/−/6−/− mice was the most noticeable γδ-effect, several phenotypic changes in B cells can be ascribed to the influence of γδ T cells as well, such as the much diminished expression of the IgE receptor CD23 in B6.TCR-Vγ1−/− mice, the diminished expression of the inhibitory receptor CD5 and FcγRIIB in B6.TCR-Vγ4−/−/6−/− mice, or the increased expression of MHCII and IL-4Rα in B6.TCR-Vγ4−/−/6−/− mice. All of the listed changes appear to be mediated or at least indirectly connected to γδ-dependent IL-4 (11, 16, 50) and, in the case of B6.TCR-Vγ4−/−/6−/− mice, might be exacerbated by the loss of peripheral B cells, which likely contributes to the increase in available serum IL-4 (50) and BAFF (not shown) in these mice.
The findings of this study raise several new questions. For example, γδ T cells in the MZ might be a differentiated and functionally specialized population similarly to the other specialized residents of this site (65), and they might function as part of a cellular network in the MZ. As already mentioned, it appears that MZ γδ T cells also participate in the monitoring of blood-borne antigens (78). Furthermore, our data suggest that the changes in the peripheral B cells of B6.TCR-Vγ4−/−/6−/− mice are directly connected to the elevated serum Ig and the development of autoantibodies in these mice (50). By extension, the observations described here predict that changes in γδ T cell populations due to natural causes (26, 33, 88) might similarly affect how peripheral B cells differentiate, how much Ig is produced, and whether or not autoantibodies develop. Consistently, impaired TCR signaling, which causes changes in γδ T cell populations has already been associated with increased IgE production (33, 34, 89). Likewise, changes in size and composition of γδ T cell populations, which have been found in hematopoietic transplantation (90) and in HIV infection (91), might affect T and B cell functions, B cell reconstitution and humoral immune competence.
Supplementary Material
Acknowledgments
We thank Drs. Roberta Pelanda, Raul Torres and Philippa Marrack and our reviewers for insight, advice and critical discussion of the data, Dr. C. Wayne Smith for providing mice, and Shirley Sobus and Joshua Loomis for expert help with flow cytometry and microscopy.
This work was supported by the National Institutes of Health grants R21 AI095765 to W.K.B., and R21 AI097962 and RO1 EY021199 to R.L.O.
Abbreviations used in this article
- CLP
common lymphocyte precursor
- BCR
B cell antigen receptor
- TCR
T cell antigen receptor
- FOB
follicular B cells
- MZB
marginal zone B cells
- SLE
systemic lupus erythematosus
- IL-4
interleukin 4
- BAFF
B cell activating factor
Footnotes
Author Contributions
Y.H. and W.K.B planned the studies, and H.H L.J.W, J.C.C. and R.L.O. helped with the study design. Y.H. performed most of the experiments, R.A.H., K.A. and A.G. performed some experiments, T.O.D., G.K., A.G., T.L.C. and M.K.A. helped with experiments and provided technical support, S.R.C. and K.I. provided γδ T cell-deficient mice, and R.L.O. generated several backcrossed mouse-strains. W.K.B. and Y.H. prepared the manuscript. H.H., L.J.W. and R.L.O. read and critically evaluated the manuscript.
References
- 1.Hardy RR, Hayakawa K, Parks DR, Herzenberg LA, Herzenberg LA. Murine B cell differentiation lineages. Journal of Experimental Medicine. 1984;159:1169–1188. doi: 10.1084/jem.159.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36:13–21. doi: 10.1016/j.immuni.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allman D, Pillai S. Peripheral B cell subsets. Current Opinion in Immunology. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Cariappa A, Chase C, Liu H, Russell P, Pillai S. Naive recirculating B cells mature simultaneously in the spleen and bone marrow. Blood. 2007;109:2339–2345. doi: 10.1182/blood-2006-05-021089. [DOI] [PubMed] [Google Scholar]
- 6.Pillai S, Cariappa A. The bone marrow perisinusoidal niche for recirculating B cells and the positive selection of bone marrow-derived B lymphocytes. Immunol Cell Biol. 2009;87:16–19. doi: 10.1038/icb.2008.89. [DOI] [PubMed] [Google Scholar]
- 7.Rowland SL, Tuttle K, Torres RM, Pelanda R. Antigen and cytokine receptor signals guide the development of the naive mature B cell repertoire. Immunol. Res. 2013;55:231–240. doi: 10.1007/s12026-012-8366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackay F, Schneider P, Rennert P, Browning JL. BAFF and APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 9.Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunological Reviews. 2010;237:205–225. doi: 10.1111/j.1600-065X.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 11.Lombard-Platet S, Meyer V, Ceredig R. Both IFN-gamma and IL-4 induce MHC class II expression at the surface of mouse pro-B cells. Dev. Immunol. 1997;5:115–120. doi: 10.1155/1997/76506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erhardt I, Lischke A, Sebald W, Friedrich K. Constitutive association of JAK1 and STAY5 in pro-B cells is dissolved by interleukin-4-induced tyrosine phosphorylation of both proteins. FEBS Lett. 1998;439:71–74. doi: 10.1016/s0014-5793(98)01341-6. [DOI] [PubMed] [Google Scholar]
- 13.Hunt AE, Williams LM, Lali FV, Foxwell BM. IL-4 regulation of p38 MAPK signalling is dependent on cell type. Cytokine. 2002;18:295–303. doi: 10.1006/cyto.2002.1043. [DOI] [PubMed] [Google Scholar]
- 14.Visan I, Goller M, Berberich I, Kneitz C, Tony HP. Pax-5 is a key regulator of the B cell-restricted expression of the CD23a isoform. European Journal of Immunology. 2003;33:1163–1173. doi: 10.1002/eji.200323747. [DOI] [PubMed] [Google Scholar]
- 15.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu. Rev. Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 16.Hidaka T, Kitani A, Hara M, Harigai M, Suzuki K, Kawaguchi Y, Ishizuka T, Kawagoe M, Nakamura K. Il-4 down-regulates the surface expression of CD5 on B cells and inhibits spontaneous immunoglobulin and IgM-rheumatoid factor production in patients with rheumatoid arthritis. Clin. Exp. Immunol. 1992;89:223–229. doi: 10.1111/j.1365-2249.1992.tb06936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tussiwand R, Bosco N, Ceredig R, Rolink AG. Tolerance checkpoints in B-cell development: Johnny B good. European Journal of Immunology. 2009;39:2317–2324. doi: 10.1002/eji.200939633. [DOI] [PubMed] [Google Scholar]
- 18.Hertz M, Nemazee D. Receptor editing and commitment in B lymphocytes. Curr. Opin. Immunol. 1998;10:208–213. doi: 10.1016/s0952-7915(98)80250-1. [DOI] [PubMed] [Google Scholar]
- 19.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 20.Scholz JL, Cancro MP. Resolve, revise, and relax: the 3 Rs of B cell repertoire adjustment. Immunology Letters. 2012;143:2–8. doi: 10.1016/j.imlet.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou YR, Diamond B. Fate determination of mature autoreactive B cells. Adv. Immunol. 2013;118:1–36. doi: 10.1016/B978-0-12-407708-9.00001-7. [DOI] [PubMed] [Google Scholar]
- 22.Reinhardt RL, Liang H-E, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nature Immunology. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe N, Ikuta K, Fagarasan S, Yazumi S, Chiba T, Honjo T. Migration and differentiation of autoreactive B-1 cells induced by activated γ/δ T cells in antierythrocyte immunoglobulin transgenic mice. J. Exp. Medicine. 2000;192:1577–1586. doi: 10.1084/jem.192.11.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen L, Pao W, Wong FS, Peng Q, Craft J, Zheng B, Kelsoe G, Dianda L, Owen MJ, Hayday AC. Germinal center formation, immunoglobulin class switching, and autoantibody production driven by “non α/β” T cells. Journal of Experimental Medicine. 1996;183:2271–2282. [Google Scholar]
- 25.Fischer K, Collins H, Taniguchi M, Kaufmann SHE, Schaible UE. IL-4 and T cells are required for the generation of IgG1 isotype antibodies against cardiolipin. J. Immunol. 2002;168:2689–2694. doi: 10.4049/jimmunol.168.6.2689. [DOI] [PubMed] [Google Scholar]
- 26.Ehl S, Schwarz K, Enders A, Duffner U, Pannicke U, Kuhr J, Mascart F, Schmitt-Graeff A, Niemeyer C, Fisch P. A variant of SCID with specific immune responses and predominance of gammadelta T cells. J. Clin. Invest. 2005;115:3140–3148. doi: 10.1172/JCI25221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gil J, Busto EM, Garcillan B, Chean C, Garcia-Rodriguez MC, Diaz-Alderete A, Navarro J, Reine J, Mencia A, Gurbindo D, Belendez C, Gordillo I, Duchniewicz M, Hohne K, Garcia-Sanchez F, Fernandez-Cruz E, Lopez-Granados E, Schamel WWA, Moreno-Pelayo MA, Recio MJ, Regueiro JR. A leaky mutation in CD3D differentially affects alphabeta and gammadelta T cells and leads to a Talphabeta-Tgammadelta+B+NK+ human SCID. J. Clin. Invest. 2011;121:3872–3876. doi: 10.1172/JCI44254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russano AM, Agea E, Corazzi L, Postle AD, De Libero G, Porcelli SA, De Benedictis F, Spinozzi F. Recognition of pollen-derived phosphatidyl-ethanolamine by human CD1d–restricted gamma delta T cells. J. Allergy Clin. Immunol. 2006;117:1178–1184. doi: 10.1016/j.jaci.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Caccamo N, Battistini L, Bonneville M, Poccia F, Fournie JJ, Meraviglia S, Borsellino G, Kroczek RA, La Mendola C, Scotet E, Dieli F, Salerno A. CXCR5 identifies a subset of Vgamma9Vdelta2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. J. Immunol. 2006;177:5290–5295. doi: 10.4049/jimmunol.177.8.5290. [DOI] [PubMed] [Google Scholar]
- 30.Zietara N, Lyszkiewicz M, Krueger A, Weiss S. ICOS-dependent stimulation of NKT cells by marginal zone B cells. European Journal of Immunology. 2011;41:3125–3134. doi: 10.1002/eji.201041092. [DOI] [PubMed] [Google Scholar]
- 31.Lang J, Kelly M, Freed BM, McCarter MD, Kedl RM, Torres RM, Pelanda R. Studies of lymphocyte reconstitution in a humanized mouse model reveal a requirement of T cells for human B cell maturation. J.Immunol. 2013;190:2090–2101. doi: 10.4049/jimmunol.1202810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiesa R, Gilmour K, Qasim W, Adams S, Worth AJ, Zhan h, Montiel-Equihua CA, Derniame S, Cale C, Rao K, Hiwarkar P, Hough R, Saudemont A, Fahrenkrog CS, Goulden N, Amrolia PJ, Veys P. Omission of in vivo T-cell depletion promotes rapid expansion of naive CD4+ cord blood lymphocytes and restores adaptive immunity within 2 months after unrelated cord blood transplant. Br J Haematol. 2012;156:656–666. doi: 10.1111/j.1365-2141.2011.08994.x. [DOI] [PubMed] [Google Scholar]
- 33.Felices M, Yin CC, Kosaka Y, Kang J, Berg LJ. Tec kinase Itk in gammadelta T cells is pivotal for controlling IgE production in vivo. Proc. Natl. Acad. Sci. (USA) 2009;106:8308–8313. doi: 10.1073/pnas.0808459106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qi Q, Xia M, Hu J, Hicks E, Iyer A, Xiong N, August A. Enhanced development of CD4+ gammadelta T cells in the absence of Itk results in elevated IgE production. Blood. 2009;114:564–571. doi: 10.1182/blood-2008-12-196345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sullivan SA, Zhu M, Bao S, Lewis CA, Ou-Yang CW, Zhang W. The role of LAT_PLCgamma1 interaction in the gammadelta T cell development and homeostasis. J Immunol. 2014 Feb 12; doi: 10.4049/jimmunol.1302493. [Epub ahead of print], PMID 24523509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Havran W, Allison JP. Developmentally ordered appearance of thymocytes expressing different T cell antigen receptors. Nature. 1988;335:443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 37.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nature Reviews Immunology. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 38.Haas W, Pereira P, Tonegawa S. Gamma/delta T cells. Annu. Rev. Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 39.Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nature Reviews Immunology. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 40.Wands JM, Roark CL, Aydintug MK, Jin N, Hahn Y-S, Cook L, Yin X, Dalporto J, Lahn M, Hyde DM, Gelfand EW, Mason DY, O’Brien RL, Born WK. Distribution and leukocyte contacts of gd T cells in the lung. J. Leukocyte Biology. 2005;78:1086–1096. doi: 10.1189/jlb.0505244. [DOI] [PubMed] [Google Scholar]
- 41.O’Brien RL, Born WK. Dermal gammadelta T cells - What have we learned? Cellular Immunology. 2015;296:62–69. doi: 10.1016/j.cellimm.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narayan K, Sylvia KE, Malhotra N, Yin CC, Martens G, Vallerskog T, Kornfeld H, Xiong N, Cohen NR, Brenner MB, Berg LJ, Kang J, Consortium TIGP. Intrathymic programming of effector fates in three molecularly distinct gammadelta T cell subtypes. Nature Immunol. 2012 doi: 10.1038/ni.2247. published online April 1, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong P, Zhang S, Cai M, Kang N, Hu Y, Cui L, Zhang J, He W. Global characterization of differential gene expression profiles in mouse Vgamma1+ and Vgamma4+ gammadelta T cells. PLOS One. 2014;9:e112964. doi: 10.1371/journal.pone.0112964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huber SA, Graveline D, Newell MK, Born WK, O’Brien RL. Vγ1+ T cells suppress and Vγ4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J. Immunology. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- 45.Born WK, Yin Z, Hahn YS, Sun D, O’Brien RL. Analysis of gamma delta T cell funcions in the mouse. J. Immunol. 2010;184:4055–4061. doi: 10.4049/jimmunol.0903679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerber DJ, Azuara V, Levraud J-P, Huang SY, Lembezat M-P, Pereira P. IL-4-producing γδ T cells that express a very restricted TCR repertoire are preferentially localized in liver and spleen. J. Immunol. 1999;163:3076–3082. [PubMed] [Google Scholar]
- 47.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O’Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, iL-17-producing gammadelta T cells. J.Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hao J, Dong S, Xia S, He W, Jia H, Zhang S, Wei J, O’Brien RL, Born WK, Wu Z, Wang P, Han J, Hong Z, Zhao L, Yin Z. Regulatory role of Vgamma1 gammadelta T cells in tumor immunity through Il-4 production. J. Immunol. 2011;187:4979–4986. doi: 10.4049/jimmunol.1101389. [DOI] [PubMed] [Google Scholar]
- 49.Welte T, Aronson J, Gong B, Rachamallu A, Mendell N, Tesh R, Paessler S, Born WK, O’Brien RL, Wang T. Vgamma4+ T cells regulate host immune responses to West Nile virus infection. FEMS Immunol. Med. Microbiol. 2011;63:183–192. doi: 10.1111/j.1574-695X.2011.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang Y, Heiser RA, Detanico TO, Getahun A, Kirchenbaum GA, Casper TL, Aydintug MK, Carding SR, Ikuta K, Huang H, Cambier JC, Wysocki LJ, O’Brien RL, Born WK. Gammadelta T cells affect IL-4 production and B cell tolerance. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E39–E48. doi: 10.1073/pnas.1415107111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayes SM, Sirr A, Jacob S, G-K S, Augustin A. Role of IL-7 in the shaping of the pulmonary γδ T cell repertoire. J. Immunol. 1996;156:2723–2729. [PubMed] [Google Scholar]
- 52.Sunaga S, Maki K, Komagata Y, Miyazaki J-I, Ikuta K. Developmentally ordered V-J recombination in mouse T cell receptor γ locus is not perturbed by targeted deletion of the Vγ4 gene. J. Immunol. 1997;158:4223–4228. [PubMed] [Google Scholar]
- 53.Egan CE, Dalton JE, Andrew EM, Smith JE, Gubbels MJ, Striepen B, Carding SR. A requirement for the Vgamma1+ subset of peripheral gammadelta T cells in the control of the systemic growth of Toxoplasma gondii and infection-induced pathology. J. Immunol. 2005;175:8191–8199. doi: 10.4049/jimmunol.175.12.8191. [DOI] [PubMed] [Google Scholar]
- 54.Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, Clarke AR, Hooper ML, Farr A, Tonegawa S. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 55.Huang Y, Jin N, Roark CL, Aydintug MK, Wands JM, Huang H, O’Brien RL, Born WK. The influence of IgE-enhancing and IgE-suppressive gammadelta T cells changes with exposure to inhaled ovalbumin. J.Immunol. 2009;183:849–855. doi: 10.4049/jimmunol.0804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- 57.Hahn Y-S, Taube C, Jin N, Sharp L, Wands JM, Kemal Aydintug M, Lahn M, Huber SA, O’Brien RL, Gelfand EW, Born WK. Different potentials of γδ T cell subsets in regulating airway responsiveness: Vγ1+ cells, but not Vγ4+ cells, promote airway hyperreactivity, TH2 cytokines, and airway inflammation. J.Immunol. 2004;172:2894–2902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 58.Jin N, Taube C, Sharp L, Hahn Y-S, Yin X, Wands JM, Roark CL, O’Brien RL, Gelfand EW, Born WK. Mismatched antigen prepares gd T cells for suppression of airway hyperresponsiveness. J. Immunol. 2005;174:2671–2679. doi: 10.4049/jimmunol.174.5.2671. [DOI] [PubMed] [Google Scholar]
- 59.Barral P, Sanchez-Nino MD, van Rooijen N, Cerundolo V, Batista FD. The location of splenic NKT cells favours their rapid activation by blood-borne antigen. The EMBO Journal. 2012;31:2378–2390. doi: 10.1038/emboj.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paul WE, Ohara J. B-cell stimulatory factor-1/Interleukin 4. Annu Rev Immunol. 1987;5:429–459. doi: 10.1146/annurev.iy.05.040187.002241. [DOI] [PubMed] [Google Scholar]
- 61.Tepper RI, Levinson DA, Stanger BZ, Campos-Torres J, Abbas AK, Leder P. IL-4 induces allergic-like inflammatory disease and alters T cell development in transgenic mice. Cell. 1990;62:457–467. doi: 10.1016/0092-8674(90)90011-3. [DOI] [PubMed] [Google Scholar]
- 62.Cassani B, Poliani PL, Marrella V, Schena F, Sauer AV, Ravanini M, Strina D, Busse CE, Regenass S, Wardemann H, Martini A, Facchetti F, van der Burg M, Rolink AG, Vezzoni P, Grassi F, Traggiai E, Villa A. Homeostatic expansion of autoreactive immunoglobulin-secreting cells in the Rag2 mouse model of Omenn syndrome. Journal of Experimental Medicine. 2010;207:1525–1540. doi: 10.1084/jem.20091928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monroe JG, Dorshkind K. Fate decisions regulating bone marrow and peripheral B lymphocyte development. Adv. Immunol. 2007;95:1–50. doi: 10.1016/S0065-2776(07)95001-4. [DOI] [PubMed] [Google Scholar]
- 64.Arnon TI, Horton RM, Grigorova IL, Cyster JL. Visualization of splenic marginal zone B-cell shuttling and follicular B-cell egress. Nature. 2012 doi: 10.1038/nature11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mebius RE, Kraal G. Structure and function of the spleen. Nature Reviews Immunology. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 66.Barral P, Sanchez-Nino MD, van Rooijen N, Cerundolo V, Batista FD. Corrigendum: The location of splenic NKT cells favours their rapid activation by blood-borne antigen. EMBO Journal. 2012;31:3029–3030. doi: 10.1038/emboj.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cook L, Miyahara N, Jin N, Wands JM, Taube C, Roark CL, Potter TA, Gelfand EW, O’Brien RL, Born WK. Evidence that CD8+ dendritic cells enable the development of gammadelta T cells that modulate airway hyperresponsiveness. J. Immunol. 2008;181:309–319. doi: 10.4049/jimmunol.181.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zidan M, Schuberth HJ, Pabst R. Immunohistology of the splenic compartments of the one humped camel (Camelus dromedarius) Vet. Immunol. Immunopathol. 2000;74:17–29. doi: 10.1016/s0165-2427(00)00153-7. [DOI] [PubMed] [Google Scholar]
- 69.Clevers H, MacHugh ND, Bensaid A, Dunlap S, Baldwin CL, Kaushal A, Iams K, Howard CJ, Morrison WI. Identification of a bovine surface antigen uniquely expressed on CD4−CD8− T cell receptor γ/δ+ T lymphocytes. European Journal of Immunology. 1990;20:809–817. doi: 10.1002/eji.1830200415. [DOI] [PubMed] [Google Scholar]
- 70.Howard C, Naessens J. General summary of workshop findings for cattle. Vet. Immunol. Immunopathol. 1993;39:25–48. doi: 10.1016/0165-2427(93)90161-v. [DOI] [PubMed] [Google Scholar]
- 71.Bucy RP, Chen C-LH, Cihak J, Losch U, Cooper MD. Avian T cells expressing gamma/delta receptors localize in the splenic sinusoids and the intestinal epithelium. J Immunol. 1988;141:2200–2205. [PubMed] [Google Scholar]
- 72.Bucy RP, Chen C-LH, Cooper MD. Tissue localization and CD8 accessory molecule expression of T γδ cells in humans. J Immunol. 1989;142:3045–3049. [PubMed] [Google Scholar]
- 73.O’Brien RL, Roark CL, Jin N, Aydintug MK, French JD, Chain JL, Wands JM, Johnston M, Born WK. Gammadelta T cell receptors: functional correlations. Immunol. Rev. 2007;215:77–88. doi: 10.1111/j.1600-065X.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 74.Born WK, Huang Y, Jin N, Huang H, O’Brien RL. Balanced approach of gammadelta T cells to type 2 immunity. Immunology and Cell biology. 2010;88:269–274. doi: 10.1038/icb.2009.105. [DOI] [PubMed] [Google Scholar]
- 75.Tussiwand R, Rauch M, Fluck LA, Rolink AG. BAFF-R expression correlates with positive selection of immature B cells. European Journal of Immunology. 2012;42:206–216. doi: 10.1002/eji.201141957. [DOI] [PubMed] [Google Scholar]
- 76.Chung JB, Silverman M, Monroe JG. Transitional B cells: step by step towards immune competence. Trends in Immunol. 2003;24:342–348. doi: 10.1016/s1471-4906(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 77.Mebius RE, Nolte MA, Kraal G. Development and function of the splenic marginal zone. Critical Reviews in Immunology. 2004;24:449–464. doi: 10.1615/critrevimmunol.v24.i6.40. [DOI] [PubMed] [Google Scholar]
- 78.Huang Y, Aydintug MK, Loomis J, Macleod MK, McKee AS, Kirchenbaum G, Jakubzick CV, Kedl RM, Sun D, Jacobelli J, O’Brien RL, Born WK. Antigen-Specific Regulation of IgE Antibodies by Non-Antigen-Specific gammadelta T Cells. J Immunol. 2013;190:913–921. doi: 10.4049/jimmunol.1202230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma H, Ke Y, Li Q, Kapp JA. Bovine and human insulin activate CD8+ autoreactive CTL expressing both type 1 and type 2 cytokines in C57BL/6 mice. J. Immunol. 2000;164:86–92. doi: 10.4049/jimmunol.164.1.86. [DOI] [PubMed] [Google Scholar]
- 80.Hiraoka S, Takeuchi N, Bian Y, Nakahara H, Kogo M, Dunussi-Joannopoulos K, Wolf S, Ono S, Fujiwara H. B7.2-Ig fusion proteins enhance IL-4-dependent differentiation of tumor-sensitized CD8+ cytotoxic T lymphocyte precursors. Int. Immunol. 2005;17:1071–1079. doi: 10.1093/intimm/dxh288. [DOI] [PubMed] [Google Scholar]
- 81.Woodland DL, Dutton RW. Heterogeneity of CD4+ and CD8+ T cells. Current Opinion in Immunology. 2003;15:336–342. doi: 10.1016/s0952-7915(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 82.Weseman DR, Magee JM, Boboila C, Calado DP, Gallagher MP, Portuguese AJ, Manis JP, Zhou X, Recher M, Rajewsky K, Notarangelo LD, Alt FW. Immature B cells preferentially switch to IgE with increased direct Smu to Sepsilon recombination. J. Exp. Med. 2011 doi: 10.1084/jem.20111155. ???: ??? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat. Rev. Immunol. 2013;13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nature Reviews Immunology. 2009;9:767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 85.Dieude M, Striegl H, Tyznik AJ, Wang J, Behar SM, Piccirillo CA, Levine JS, Zajonc DM, Rauch J. Cardiolipin binds to CD1d and stimulates CD1d–restricted gammadelta T cells in the normal murine repertoire. J. Immunol. 2011;186:4771–4781. doi: 10.4049/jimmunol.1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chien Y-H, Jores R, Crowley MP. Recognition by γ/δ T cells. Annu. Rev. Immunol. 1996;14:511–532. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- 87.Wesemann DR, Portuguese AJ, Meyers RM, Gallagher MP, Cluff-Jones K, Magee JM, Panchakshari RA, Rodig SJ, Kepler TB, Alt FW. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature. 2013 doi: 10.1038/nature12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bank I, Marcu-Malina V. Quantitative peripheral blood perturbations of gammadelta T cells in human disease and their clinical implications. Clinic Rev Allerg Immunol. 2013 doi: 10.1007/s12016-013-8391-x. [DOI] [PubMed] [Google Scholar]
- 89.Nunez-Cruz S, Aguado E, Richelme S, Chetaille B, Mura AM, Richelme M, Pouyet L, Jouvin-Marche E, Xerri L, Malissen B, Malissen M. LAT regulates gammadelta T cell homeostasis and differentiation. Nature Immunology. 2003;4:999–1008. doi: 10.1038/ni977. [DOI] [PubMed] [Google Scholar]
- 90.Kelly KA, O’Brien R, Born W. Reconstitution of SCID mice with haemopoietic precursors: a detailed analysis of γδ T-cell reconstitution. Immunology. 1997;91:65–72. doi: 10.1046/j.1365-2567.1997.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pauza CD, Poonia B, Li H, Cairo C, Chaudhry S. GammaDelta T cells in HIV disease: past, present and future. Frontiers in Immunology. 2015;5:1–11. doi: 10.3389/fimmu.2014.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. Journal of Experimental Medicine. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.